Abstract
Amide compounds are important organic compounds, which play an important role in biomedical chemistry, materials science, life science, and other fields. The synthesis of α-CF3 amides, especially compounds containing 3-(trifluoromethyl)-1,3,4,5-tetrahydro-2H-benzo[b][1,4]diazepine-2-one, has long been a challenge due to the tensile properties and instability of the rings. Here, we report an example of palladium-catalyzed carbonylation of CF3-containing olefin to form α-CF3 acrylamide. By controlling the ligands, we can get different amide compounds as products. This method has good substrate adaptability and functional group tolerance.
Introduction
Fluorine-containing compounds are of considerable interest because of their superior physicochemical properties in materials chemistry and their favorable pharmacokinetic properties in medicinal chemistry.1 Along with fluorine-containing compounds, α-CF3 amides are an important class of organic compounds that play a key role in biomedical chemistry, materials science, life science, and other areas.2 In addition, α-CF3 amides can serve as versatile precursors for the synthesis of α-trifluoromethylated carboxylic acids,3 α-trifluoromethylated alcohols,4 and amines.5 However, due to their high propensity for β-fluoride elimination, which is triggered by strong metal–fluorine interactions,6 limited examples have been reported thus far regarding the synthesis of their trifluoromethylated derivatives.7−9 In particular, only one example of the synthesis of seven-membered rings containing α-CF3 amides has been reported till date.10
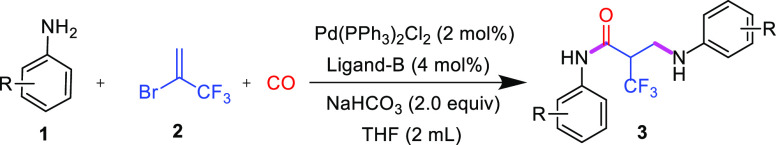
2-Bromo-3,3,3-trifluoro-1-propene is environmentally friendly (atmospheric greenhouse effect, GWP = 0; atmospheric ozone depletion value, ODP = 0) and used in many organic syntheses, such as the synthesis of α-(trifluoromethyl)styrenes,11 trifluoromethylated vinyl boron reagent,12 ethyl 3,3,3-trifluoropropionate,13 difluoromethyl-substituted 2,3-dihydrobenzoheteroles,14 trifluoroacrylic acid,15 and 3-trifluoromethylpyrazole.16 Developing methods to efficiently convert the compound into fluorine-containing fine chemicals remains a very meaningful area for future research. To develop a simpler and more efficient method to synthesize α-CF3 amides and as part of our continued interest in the area of trifluoromethylation17 and carbonylation,18 here, we envisaged a one-step sequential synthetic strategy that involved the direct carbonylation of anilines with an inexpensive and available trifluoromethylated olefin that could yield the desired α-CF3 amides in a controlled manner (Scheme 1).
Scheme 1. Palladium-Catalyzed Carbonylation of Anilines.
Results and Discussion
We chose p-methoxyaniline 1a and 2 as the substrates for the model reaction. PdCl2 (2 mol %) was used as the catalyst, PCy3 (4 mol %) was used as the ligand, and carbon monoxide (8 atm) was used to react in 1,4-dioxane at 100 °C for 12 h. Disappointingly, we obtained target compound 3a in a poor yield (6%) (Table 1, entry 1). Therefore, we performed a large number of experiments to optimize the conditions for this reaction. First, we screened palladium sources and examined two different palladium catalysts. As a result, we found that Pd(PPh3)2Cl2 was the best palladium source (Table 1, entries 2–3). Phosphine ligands are the focus of our investigation. We examined a total of eight phosphine ligands herein, including monodentate and bidentate ligands. We found that ligand B had the best effect on this reaction, resulting in a reaction yield of 72% (Table 1, entries 4–11). Next, we examined the reaction solvents. We investigated solvents with different polarities and found that tetrahydrofuran (THF) was the best solvent for this reaction and that the reaction yield could be increased to as high as 84% (Table 1, entries 12–15). We also examined other types of bases, and we observed that NaHCO3 was the best base for this reaction (Table 1, entries 16–18). Thus, the following conditions were determined to be optimal for the reaction: Pd(PPh3)2Cl2 as the catalyst, phosphine ligand B, NaHCO3 as the reaction base, and THF as the solvent. The reaction was performed at 100 °C for 12 h.
Table 1. Optimization of the Reaction Conditionsa.
| entry | catalyst | ligand | base | solvent | yield (%)b |
|---|---|---|---|---|---|
| 1 | PdCl2 | PCy3 | NaHCO3 | 1,4-dioxane | 6 |
| 2 | Pd(OAc)2 | PCy3 | NaHCO3 | 1,4-dioxane | 29 |
| 3 | Pd(PPh3)2Cl2 | PCy3 | NaHCO3 | 1,4-dioxane | 45 |
| 4 | Pd(PPh3)2Cl2 | S-Phos | NaHCO3 | 1,4-dioxane | 36 |
| 5 | Pd(PPh3)2Cl2 | Ru-Phos | NaHCO3 | 1,4-dioxane | 31 |
| 6 | Pd(PPh3)2Cl2 | X-Phos | NaHCO3 | 1,4-dioxane | 26 |
| 7 | Pd(PPh3)2Cl2 | Dave-Phos | NaHCO3 | 1,4-dioxane | 19 |
| 8 | Pd(PPh3)2Cl2 | CyJohnPhos | NaHCO3 | 1,4-dioxane | 23 |
| 9 | Pd(PPh3)2Cl2 | A | NaHCO3 | 1,4-dioxane | 51 |
| 10 | Pd(PPh3)2Cl2 | B | NaHCO3 | 1,4-dioxane | 72 |
| 11 | Pd(PPh3)2Cl2 | C | NaHCO3 | 1,4-dioxane | 58 |
| 12 | Pd(PPh3)2Cl2 | D | NaHCO3 | 1,4-dioxane | 16 |
| 13 | Pd(PPh3)2Cl2 | B | NaHCO3 | MeCN | 22 |
| 14 | Pd(PPh3)2Cl2 | B | NaHCO3 | THF | 84 |
| 15 | Pd(PPh3)2Cl2 | B | NaHCO3 | MePh | 23 |
| 16 | Pd(PPh3)2Cl2 | B | NaHCO3 | DMF | 35 |
| 17 | Pd(PPh3)2Cl2 | B | Na2CO3 | THF | 74 |
| 18 | Pd(PPh3)2Cl2 | B | KHCO3 | THF | 81 |
| 19 | Pd(PPh3)2Cl2 | B | K2CO3 | THF | 76 |
To investigate the universality of this method, we performed a substrate extension experiment. Most of the aniline derivatives could be well converted into the corresponding acrylamides (Table 2). Especially for aniline with para-electron-rich substituents, this conversion process proceeded very smoothly, and the target compounds were obtained in good to excellent yields (Table 2, entries 3b–3g). Aniline substrates containing an electron-deficient substituent at the para position could also be ideally converted into the desired target compound under the optimized reaction conditions (Table 2, entries 3h–3k). Next, we investigated aniline-containing meta-substituents. To our satisfaction, anilines containing meta-rich substituents could also generate the corresponding acrylamides in moderate yields (Table 2, entry 3l). Third, we performed experiments on anilines containing substituents at the ortho position. It was also pleasing that these anilines could promote this conversion in good yields, regardless of whether electron-deficient or electron-rich substituents were in the ortho position (Table 2, entries 3m–3o). We also investigated disubstituted anilines. It was found that they were also smoothly converted into the corresponding products, regardless of whether the substituents were present in the 3 and 5, 3 and 4 positions (Table 2, entries 3p–3r). In addition, we explored 1-naphthylamine as a substrate. To our delight, it was also converted into the corresponding amide in a moderate yield (Table 2, entry 3s). Finally, we applied this method to pyrazine substrates. Gratifyingly, these substrates were also converted into the corresponding products in moderate yields (Table 2, entries 3t–3u).
Table 2. Palladium-Catalyzed Carbonylation to α-CF3 Amidesa.
Reaction conditions: aniline (1) (1.0 mmol), 2 (2.0 mmol), Pd(PPh3)2Cl2 (2 mol %), ligand-B (4 mol %), NaHCO3 (2.0 equiv), CO (8 atm), THF (2.0 mL), 100 °C, 12 h.
Isolated yield.
To further explore the substrate universality of this reaction, we explored substrates for the formation of acrylamides by synthetic route B (Table 3). First, anilines could be converted into the corresponding acrylamides, regardless of whether they contained electron-rich substituents in the ortho- or para-position (Table 3, entries 4a–4d). Second, we also tried an indole-substituted amine and found that it could also be converted into the corresponding acrylamide product in good yields (Table 3, entry 4e). Finally, we also investigated biologically active substrates. Favorably, they were converted into the desired acrylamides (Table 3, entries 4f–4g).
Table 3. Palladium-Catalyzed Carbonylation to Acrylamidesa.
Reaction conditions: aniline (1.0 mmol), 2 (2.0 mmol), Pd(PPh3)4(2 mol %), NaHCO3 (2.0 equiv), THF (4.0 mL), CO (8 atm), 100 °C, 12 h.
Isolated yield.
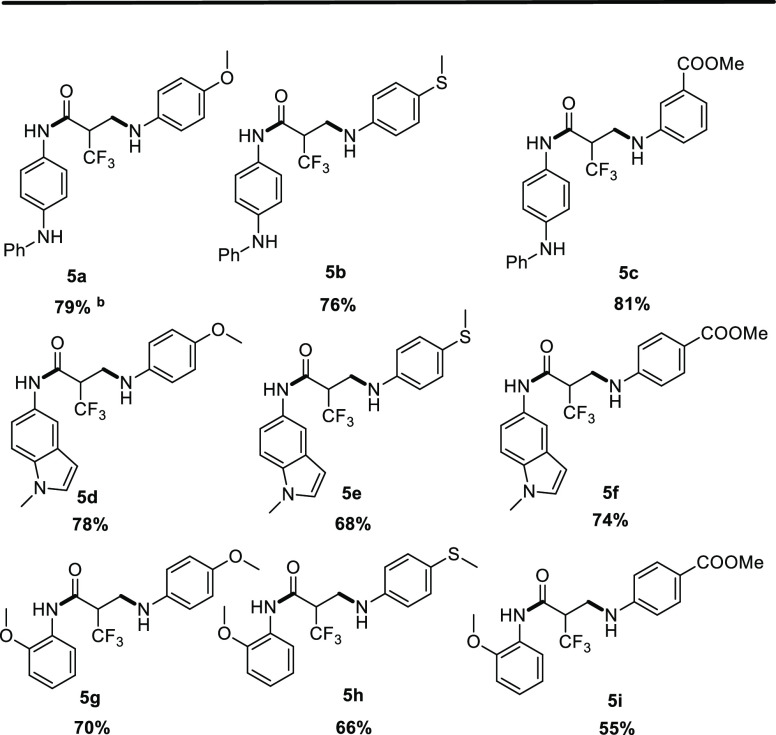
Next, we conducted a substrate expansion test for reaction path C. We found that when N-phenyl acrylamide was used as a substrate, it underwent intermolecular Michael addition reactions with different types of aromatic amines. The reaction only needed to be carried out in THF at 80 °C for 5 h (Table 4, 5a–5c). We tried the same reaction with indole-acrylamine as the substrate. Gratifyingly, the results obtained were the same as the previous results; namely, three different types of aromatic amines underwent Michael addition reactions well, and the isolated yields were all above moderate (Table 4, 5d–5f). Finally, we used ortho-methoxyacrylamide with a sterically hindered group as the substrate. Under the same conditions, the intermolecular Michael addition still proceeded very smoothly, and the isolated yield of the product was above 50% (Table 4, 5g–5i). The realization of this conversion pathway provides a good method for the future synthesis of trifluoromethylated propanamides with different substituents.
Table 4. Substrate Scope of Anilines for Michael Additiona.
Reaction conditions: acrylamide (4) (1.0 mmol), aniline (1) (1.0 mmol), THF (4.0 mL), 80 °C, 5 h.
Isolated yield.
Due to structural instability, N-hetero seven-membered cyclic amides are difficult to synthesize. We found that under our reaction conditions D, benzene-1,2-diamine can undergo an intramolecular cycloaddition reaction, thereby forming an acrylamide 3-(trifluoromethyl)-1,3,4,5-tetrahydro-2H-benzo[b][1,4]diazepin-2-one. In the same way, we also optimized the synthesis route considerably and finally obtained an ideal synthesis process. Here, we examined the substrate universality of this synthetic method. Benzene-1,2-diamine containing various substituents could be well converted into 3-(trifluoromethyl)-1,3,4,5-tetrahydro-2H-benzo[b][1,4]diazepin-2-one (Table 5). Substituted benzene-1,2-diamine, o-resistant diamine, and benzene-1,2-diamine containing a double substituent at the 3 and 4 positions all resulted in a single benzoic seven-membered cyclic acrylamide in good yields (Table 5, 6a–6e). For benzene-1,2-diamine containing a single substituent at the 3 or 4 position, both electron-deficient and electron-rich substituted benzene-1,2-diamine could be converted into the corresponding acrylamides in moderate yields (Table 5, 6f–6h). The disadvantage is that all these products exist as mixtures, and basically all are obtained in a ratio of 1:1 to 1:2. However, this method provided a new technique to synthesize 3-(trifluoromethyl)-1,3,4,5-tetrahydro-2H-benzo[b][1,4]diazepin-2-one.
Table 5. Synthesis of 3-(Trifluoromethyl)-1,3,4,5-tetrahydro-2H-benzo[b][1,4]diazepin-2-onea.
Reaction conditions: benzene-1,2-diamine (1.0 mmol), 2 (2.0 mmol), Pd(PPh3)2Cl2 (2 mol %), ligand B (4 mol %), NaH2PO4 (2.0 equiv), THF (2.0 mL), 100 °C, 12 h.
Isolated yield.
To examine the amplification effect of this reaction, we performed a scale-up experiment. The reaction yield decreased only slightly, which shows that this method has good scale-up potential (Scheme 2). The actual picture of the product is also displayed.
Scheme 2. Gram-Scale Experiment.
A possible mechanism for the palladium-catalyzed carbonylation of aniline is described in Scheme 3. Initially, the reaction starts with the reduction of Pd(II) to Pd(0) by the ligand, and then an oxidative addition occurs between vinyl bromide and Pd(0), yielding intermediate A. After coordination to form B and insertion with CO, acyl palladium C is formed;19 then, aniline attacks intermediate C to form Compound D. Finally, intermediate D undergoes reductive elimination to afford amide E and Pd(0). Intermediate E undergoes a Michael addition under different reaction conditions and produces compounds F and G.
Scheme 3. Reaction Mechanism.
Conclusions
In summary, we developed a new strategy for the facile synthesis ofα-CF3 acrylamides via a Pd(0)-catalyzed fluorinated carbonylation reaction. Importantly, this conversion process exhibit excellent regioselectivity and chemoselectivity, and the reaction has good compatibility with substrate functional groups. Furthermore, this process does not require the addition of any metal additives and appears to be a simple and efficient method. We expect that the discovery of this reaction will play an important role in the synthesis of α-CF3 amides.
Experimental Section
The reaction was carried out in an autoclave containing a 5.0 mL glass reaction tube, and Pd(PPh3)2Cl2 (0.02 mmol), ligand B (0.04 mmol), aniline (1.0 mmol), NaHCO3 (2.0 mmol), THF (2.0 mL), and 2-bromo-3,3,3-trifluoro-1-propene (2.0 mmol) were added to the tube. The tube was placed in the autoclave. Once sealed, the autoclave was purged three times with CO, then pressurized to 8 atm at room temperature, and heated in an oil bath at 100 °C for 12 h. After the reaction, the autoclave was then cooled to room temperature and vented to discharge CO. The crude product was purified by column chromatography on silica gel using a mixture of ethyl acetate and petroleum ether as the eluent to give the following compounds.
Acknowledgments
We are grateful for the financial support from the National Natural Science Foundation of China (GZ-1645 and 21902126), the Shaanxi Province Natural Science Basic Research Program (2021JLM-30 and 2022GY-195), the Basic Research Project of Natural Science of Shaanxi Province (2019JQ-546), and the Doctoral Scientific Research Foundation of Xi’an Polytechnic University (107020336 and 107020403).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c08206.
1H NMR (13C NMR and 19F NMR) spectra for all compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Kirsch P.Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]; b Mikami K.; Itoh Y.; Yamanaka M. Fluorinated Carbonyl and Olefinic Compounds: Basic Character and Asymmetric Catalytic Reactions. Chem. Rev. 2004, 104, 1–16. 10.1021/cr030685w. [DOI] [PubMed] [Google Scholar]; c Begue J. P.; Bonnet-Delpon D.. Bioorganic and Medicinal Chemistry of Fluorine; Wiley: Hoboken, NJ, 2008. [Google Scholar]; d Hagmann W. K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369. 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]; e Ojima I.Fluorine in Medicinal Chemistry and Chemical Biology; Wiley-Blackwell: Chichester, U.K., 2009. [Google Scholar]; f Salwiczek M.; Nyakatura E. K.; Gerling U. I. M.; Ye S.; Koksch B. Fluorinated amino acids: compatibility with native protein structures and effects on protein-protein interactions. Chem. Soc. Rev. 2012, 41, 2135–2171. 10.1039/c1cs15241f. [DOI] [PubMed] [Google Scholar]
- a Foster R. W.; Lenz E. N.; Simpkins N. S.; Stead D. Organocatalytic Stereoconvergent Synthesis of α-CF3 Amides: Triketopiperazines and Their Heterocyclic Metamorphosis. Chem.—Eur. J. 2017, 23, 8810–8813. 10.1002/chem.201701548. [DOI] [PubMed] [Google Scholar]; b Saito A.; Kumagai N.; Shibasaki M. Cu/Pd Synergistic Dual Catalysis: Asymmetric α-Allylation of an α-CF3 Amide. Angew. Chem., Int. Ed. 2017, 56, 5551–5555. 10.1002/anie.201702113. [DOI] [PubMed] [Google Scholar]; c Yin L.; Brewitz L.; Kumagai N.; Shibasaki M. Catalytic Generation of α-CF3 Enolate: Direct Catalytic Asymmetric Mannich-Type Reaction of α-CF3 Amide. J. Am. Chem. Soc. 2014, 136, 17958–17961. 10.1021/ja511458k. [DOI] [PubMed] [Google Scholar]; d Matsuzawa A.; Noda H.; Kumagai N.; Shibasaki M. Direct Catalytic Asymmetric Aldol Addition of an α-CF3 Amide to Arylglyoxal Hydrates. J. Org. Chem. 2017, 82, 8304–8308. 10.1021/acs.joc.7b01381. [DOI] [PubMed] [Google Scholar]; e Brewitz L.; Arteaga F.; Yin L.; Alagiri K.; Kumagai N.; Shibasaki M. Direct Catalytic Asymmetric Mannich-Type Reaction of α- and β-Fluorinated Amides. J. Am. Chem. Soc. 2015, 137, 15929–15939. 10.1021/jacs.5b11064. [DOI] [PubMed] [Google Scholar]
- Ma J.-A.; Cahard D. Asymmetric Fluorination, Trifluoromethylation, and Perfluoroalkylation Reactions. Chem. Rev. 2004, 104, 6119–6146. 10.1021/cr030143e. [DOI] [PubMed] [Google Scholar]
- Shimizu M.; Hiyama T. Modern Synthetic Methods for Fluorine-Substituted Target Molecules. Angew. Chem., Int. Ed. 2005, 44, 214–231. 10.1002/anie.200460441. [DOI] [PubMed] [Google Scholar]
- Schlosser M. CF3-Bearing Aromatic and Heterocyclic Building Blocks. Angew. Chem., Int. Ed. 2006, 45, 5432–5446. 10.1002/anie.200600449. [DOI] [PubMed] [Google Scholar]
- O’Hagan D. Understanding organofluorine chemistry. An introduction to the C-F bond. Chem. Soc. Rev. 2008, 37, 308–319. 10.1039/b711844a. [DOI] [PubMed] [Google Scholar]
- Ma J.-A.; Cahard D. Update 1 of: Asymmetric Fluorination, Trifluoromethylation, and Perfluoroalkylation Reactions. Chem. Rev. 2008, 108, PR1–PR43. 10.1021/cr800221v. [DOI] [PubMed] [Google Scholar]
- a Pitts C. R.; Lectka T. Chemical Synthesis of β-Lactams: Asymmetric Catalysis and Other Recent Advances. Chem. Rev. 2014, 114, 7930–7953. 10.1021/cr4005549. [DOI] [PubMed] [Google Scholar]; b Szostak M.; Aubé J. Chemistry of Bridged Lactams and Related Heterocycles. Chem. Rev. 2013, 113, 5701–5765. 10.1021/cr4000144. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Worthington R. J.; Melander C. Overcoming Resistance to β-Lactam Antibiotics. J. Org. Chem. 2013, 78, 4207–4213. 10.1021/jo400236f. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Lall M. S.; Tao Y.; Arcari J. T.; Boyles D.; Brown M. F.; Damon D. B.; Lilley S. C.; Mitton-Fry M. J.; Starr J.; Stewart A. M.; Sun J. Process Development for the Synthesis of Monocyclic β-Lactam Core 17. Org. Process. Res. Dev. 2018, 22, 212–218. 10.1021/acs.oprd.7b00359. [DOI] [Google Scholar]; e Piotti M. E.; Alper H. Inversion of Stereochemistry in the Co2(CO)8-Catalyzed Carbonylation of Aziridines to β-Lactams. The First Synthesis of Highly Strained trans-Bicyclic β-Lactams. J. Am. Chem. Soc. 1996, 118, 111–116. 10.1021/ja9531586. [DOI] [Google Scholar]; f Deyrup J. A.; Clough S. C. New route to .beta.-lactams. J. Am. Chem. Soc. 1969, 91, 4590–4591. 10.1021/ja01044a070. [DOI] [Google Scholar]; g Kaneko T. Possible biomimetic synthesis of .beta.-lactams. J. Am. Chem. Soc. 1985, 107, 5490–5492. 10.1021/ja00305a026. [DOI] [Google Scholar]; h Alper H.; Perera C. P.; Ahmed F. R. A novel synthesis of .beta.-lactams. J. Am. Chem. Soc. 1981, 103, 1289–1291. 10.1021/ja00395a082. [DOI] [Google Scholar]; i Yada A.; Okajima S.; Murakami M. Palladium-Catalyzed Intramolecular Insertion of Alkenes into the Carbon-Nitrogen Bond of β-Lactams. J. Am. Chem. Soc. 2015, 137, 8708–8711. 10.1021/jacs.5b05308. [DOI] [PubMed] [Google Scholar]; j Lo M. M. C.; Fu G. C. Cu(I)/Bis(azaferrocene)-Catalyzed Enantioselective Synthesis of β-Lactams via Couplings of Alkynes with Nitrones. J. Am. Chem. Soc. 2002, 124, 4572–4573. 10.1021/ja025833z. [DOI] [PubMed] [Google Scholar]; k Dailler D.; Rocaboy R.; Baudoin O. Synthesis of β-Lactams by Palladium(0)-Catalyzed C(sp3)–H Carbamoylation. Angew Chem. Int. Ed. 2017, 56, 7218–7222. 10.1002/anie.201703109. [DOI] [PubMed] [Google Scholar]; l Shu T.; Zhao L.; Li S.; Chen X. −Y.; von Essen C.; Rissanen K.; Enders D. Asymmetric Synthesis of Spirocyclic β-Lactams through Copper-Catalyzed Kinugasa/Michael Domino Reactions. Angew. Chem., Int. Ed. 2018, 57, 10985–10988. 10.1002/anie.201806931. [DOI] [PubMed] [Google Scholar]; m Li W.; Liu C.; Zhang H.; Ye K.; Zhang G.; Zhang W.; Duan Z.; You S.; Lei A. Palladium-Catalyzed Oxidative Carbonylation ofN-Allylamines for the Synthesis of β-Lactams. Angew. Chem., Int. Ed. 2014, 53, 2443–2446. 10.1002/anie.201309081. [DOI] [PubMed] [Google Scholar]
- a Jakowiecki J.; Loska R.; Makosza M. Synthesis of α-Trifluoromethyl-β-lactams and Esters of β-Amino Acids via 1,3-Dipolar Cycloaddition of Nitrones to Fluoroalkenes. J. Org. Chem. 2008, 73, 5436–5441. 10.1021/jo800721w. [DOI] [PubMed] [Google Scholar]; b Wu X.; Zhao Y.; Ge H. Nickel-Catalyzed Site-Selective Amidation of Unactivated C(sp3)-H Bonds. Chem.—Eur. J. 2014, 20, 9530–9533. 10.1002/chem.201403356. [DOI] [PubMed] [Google Scholar]; c Wang Z.; Ni J.; Kuninobu Y.; Kanai M. Copper-Catalyzed Intramolecular C(sp3)·H and C(sp2)·H Amidation by Oxidative Cyclization. Angew. Chem., Int. Ed. 2014, 53, 3496–3499. 10.1002/anie.201311105. [DOI] [PubMed] [Google Scholar]; d Wu X.; Yang K.; Zhao Y.; Sun H.; Li G.; Ge H. Cobalt-catalysed site-selective intra- and intermolecular dehydrogenative amination of unactivated sp3 carbons. Nat. Commun. 2015, 6, 6462–6471. 10.1038/ncomms7462. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Haddad M.; Wakselman C. An efficient synthesis of 3-methyl-3-trifluoromethyl azetidin-2-one. J. Fluorine Chem. 1995, 73, 57–59. 10.1016/0022-1139(94)03198-9. [DOI] [Google Scholar]
- Kitazume T.; Ikeya T.; Murata K. Synthesis of optically active trifluorinated compounds: asymmetric Michael addition with hydrolytic enzymes. J. Chem. Soc., Chem. Commun. 1986, 1331–1333. 10.1039/c39860001331. [DOI] [Google Scholar]
- a Fujita T.; Konno N.; Watabe Y.; Ichitsuka T.; Nagaki A.; Yoshida J.; Ichikawa J. Flash generation and borylation of 1-(trifluoromethyl)vinyllithium toward synthesis of α-(trifluoromethyl)styrenes. J. Fluorine Chem. 2018, 207, 72–76. 10.1016/j.jfluchem.2018.01.004. [DOI] [Google Scholar]; b Zhou Q.; Bao Y.; Yan G. 2-Bromo-3,3,3-Trifluoropropene: A Versatile Reagent for the Synthesis of Fluorinated Compounds. Adv. Synth. Catal. 2022, 364, 1371–1387. 10.1002/adsc.202200023. [DOI] [Google Scholar]
- Phelan J. P.; Wiles R. J.; Lang S. B.; Kelly C. B.; Molander G. A. Rapid access to diverse, trifluoromethyl-substituted alkenes using complementary strategies. Chem. Sci. 2018, 9, 3215–3220. 10.1039/c7sc05420c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Inoue M.; Masahiro S.; Hajime M. Synthesis of ethyl 3,3,3-trifluoropropionate from 2-bromo-3,3,3-trifluoropropene. J. Fluorine Chem. 2014, 167, 135–138. 10.1016/j.jfluchem.2014.07.009. [DOI] [Google Scholar]; b Guo P.; Tao M.; Xu W. −W.; Wang A. −J.; Li W.; Yao Q.; Tong J.; He C. −Y. Synthesis of Secondary Trifluoromethylated Alkyl Bromides Using 2-Bromo-3,3,3-trifluoropropene as a Radical Acceptor. Org. Lett. 2022, 24, 2143–2148. 10.1021/acs.orglett.2c00425. [DOI] [PubMed] [Google Scholar]
- Fujita T.; Sanada S.; Chiba Y.; Sugiyama K.; Ichikawa J. Two-Step Synthesis of Difluoromethyl-Substituted 2,3-Dihydrobenzoheteroles. Org. Lett. 2014, 16, 1398–1401. 10.1021/ol5001582. [DOI] [PubMed] [Google Scholar]
- Evano G. Carboxylic Acids: Synthesis from organic halides. Sci. Synth. 2016, 20a, 137–172. [Google Scholar]
- a Jiang B.; Xu Y.; Yang J. A facile synthesis of 3-trifluoromethylpyrazole and its derivatives. J. Fluorine Chem. 1994, 67, 83–85. 10.1016/0022-1139(93)02939-c. [DOI] [Google Scholar]; b Zeng H.; Fang X.; Yang Z.; Zhu C.; Jiang H. Regioselective Synthesis of 5-Trifluoromethylpyrazoles by [3 + 2] Cycloaddition of Nitrile Imines and 2-Bromo-3,3,3-trifluoropropene. J. Org. Chem. 2021, 86, 2810–2819. 10.1021/acs.joc.0c02765. [DOI] [PubMed] [Google Scholar]
- a Li Y.; Neumann H.; Beller M. Ruthenium-Catalyzed Site-Selective Trifluoromethylations and (Per)Fluoroalkylations of Anilines and Indoles. Chem.—Eur. J. 2020, 26, 6784–6788. 10.1002/chem.202001439. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Li Y.; Hao M.; Xia M.; Sun N.; Zhang C. −L.; Zhu W. −Q. Synthesis of 3-trifluoromethylated 1,3-butadienes via a Pd(0)-catalyzed fluorinated Heck reaction. React. Chem. Eng. 2020, 5, 961–966. 10.1039/d0re00093k. [DOI] [Google Scholar]; c Li Y.; Hao M.; Chang Y.; Wang W.; Sun N.; Zhu W.; Gao Z. Synthesis of 4-Trifluoromethylated 1, 3-Butadienes via Palladium Catalyzed Heck Reaction. Chin. J. Chem. 2021, 39, 2962–2966. 10.1002/cjoc.202100313. [DOI] [Google Scholar]; d Li Y.; Sun N.; Zhang C. −L.; Hao M. Base-Promoted Formylation and N -Difluoromethylation of Azaindoles with Ethyl Bromodifluoroacetate as a Carbon Source. Chin. J. Chem. 2021, 39, 1477–1482. 10.1002/cjoc.202100008. [DOI] [Google Scholar]
- Li Y.; Zhang C. −L.; Huang W. −H.; Sun N.; Hao M.; Neumann H.; Beller M. A general strategy for the synthesis of α-trifluoromethyl- and α-perfluoroalkyl-β-lactams via palladium-catalyzed carbonylation. Chem. Sci. 2021, 12, 10467–10473. 10.1039/d1sc02212a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For the reaction mechanisms see:; a Wu X. −F.; Neumann H.; Beller M. Palladium-catalyzed carbonylative coupling reactions between Ar-X and carbon nucleophiles. Chem. Soc. Rev. 2011, 40, 4986. 10.1039/c1cs15109f. [DOI] [PubMed] [Google Scholar]; b Wu X. −F.; Neumann H.; Beller M. Synthesis of Heterocycles via Palladium-Catalyzed Carbonylations. Chem. Rev. 2013, 113, 1–35. 10.1021/cr300100s. [DOI] [PubMed] [Google Scholar]; c Li Y.; Hu X. −F.; Wu X.-F. Non-noble metal-catalysed carbonylative transformations. Chem. Soc. Rev. 2018, 47, 172–194. 10.1039/c7cs00529f. [DOI] [PubMed] [Google Scholar]; d Peng J. −B.; Wu F. −P.; Wu X. −F. First-Row Transition-Metal-Catalyzed Carbonylative Transformations of Carbon Electrophiles. Chem. Rev. 2019, 119, 2090–2127. 10.1021/acs.chemrev.8b00068. [DOI] [PubMed] [Google Scholar]; e Zhang S.; Neumann H.; Beller M. Synthesis of α,β-unsaturated carbonyl compounds by carbonylation reactions. Chem. Soc. Rev. 2020, 49, 3187–3210. 10.1039/c9cs00615j. [DOI] [PubMed] [Google Scholar]; f Cheng R.; Zhao H. −Y.; Zhang S.; Zhang X. Nickel-Catalyzed Carbonylation of Secondary Trifluoromethylated, Difluoromethylated, and Nonfluorinated Aliphatic Electrophiles with Arylboronic Acids under 1 atm of CO. ACS Catal. 2020, 10, 36–42. 10.1021/acscatal.9b04038. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.