Abstract
Background
Pharyngocutaneous fistula (PCF) and salivary leaks are well known complications of head and neck surgery. The medical management of PCF has included the use of octreotide without a well‐defined understanding of its therapeutic mechanism. We hypothesized that octreotide induces alterations in the saliva proteome and that these alterations may provide insight into the mechanism of action underlying improved PCF healing. We undertook an exploratory pilot study in healthy controls that involved collecting saliva before and after a subcutaneous injection of octreotide and performing proteomic analysis to determine the effects of octreotide.
Methods
Four healthy adult participants provided saliva samples before and after subcutaneous injection of octreotide. A mass‐spectrometry based workflow optimized for the quantitative proteomic analysis of biofluids was then employed to analyze changes in salivary protein abundance after octreotide administration.
Results
There were 3076 human, 332 Streptococcus mitis, 102 G. haemolyans, and 42 Granulicatella adiacens protein groups quantified in saliva samples. A paired statistical analysis was performed using the generalized linear model (glm) function in edgeR. There were and ~300 proteins that had a p < .05 between the pre‐ and post‐octreotide groups ~50 proteins with an FDR‐corrected p < .05 between pre‐ and post‐groups. These results were visualized using a volcano plot after filtering on proteins quantified by 2 more or unique precursors. Both human and bacterial proteins were among the proteins altered by octreotide treatment. Notably, four isoforms of the human cystatins, belonging to a family of cysteine proteases, that had significantly lower abundance after treatment.
Conclusion
This pilot study demonstrated octreotide‐induced downregulation of cystatins. By downregulation of cystatins in the saliva, there is decreased inhibition of cysteine proteases such as Cathepsin S. This results in increased cysteine protease activity that has been linked to enhanced angiogenic response, cell proliferation and migration that have resulted in improved wound healing. These insights provide first steps at furthering our understanding of octreotide's effects on saliva and reports of improved PCF healing.
Keywords: fistula, head and neck cancer, proteomics, wound healing
Octreotide has been used historically in the medical management of salivary fistulas without a clear understanding of its therapeutic mechanism. In this investigation, we gave octreotide to healthy volunteers and examined changes in the saliva proteome to better understand the effects of octreotide on the proteomic environment and thus potentially identify potential mechanisms of action for octreotide in the healing of fistula.

1. INTRODUCTION
Pharyngocutaneous fistulas (PCF) and salivary leaks are well known complications of head and neck surgery, occurring in up to 25% cases involving pharyngeal and laryngeal defects 1 , 2 , 3 with the highest rates in those patients with prior radiation, chemotherapy, malnutrition, infection, and hypothyroidism. 4 Management of these complications can be quite challenging for both patient and surgeon. Conservative management and observation success rates have been shown to be as high as 65%, decreasing to 30–40% in previously irradiated patients. 5 Approximately 40% of those failing conservative therapies ultimately require surgical management, with even higher rates of surgical intervention in those with prior radiation. 6 Beyond the medical and surgical implications, these complications often necessitate hospital re‐admissions, prolong length of stay following surgery, and increase healthcare costs.
Saliva's effects on wound healing have been a subject of study for many years, particularly based on the rapid healing of oral mucosa in comparison to skin wounds, much of which has been attributed to the presence of saliva and its proteins and peptides. 7 , 8 , 9 In contrast, the role of saliva in wound healing in Head and Neck Cancer is less understood—as those afflicted with PCF experience delayed healing. 6 , 10
The management of PCF has included the use of the octreotide, the first FDA approved synthetic analog of somatostatin. Somatostatin analogs have a number of physiologic effects, inhibiting both endocrine and exocrine secretions including growth hormone, prolactin, thyrotropin and amylase, as well as the reduced absorption of glucose, fat, and amino acids. 11 However, its use in the setting of salivary fistulas has not been well studied. There have been anecdotal and case reports supporting the use of octreotide in the setting of PCF 12 , 13 that have justified a placebo controlled clinical investigation of octreotide in PCF closure in Israel. 14
We hypothesized that octreotide induces alterations in the saliva proteome, and these alterations may provide insight into the mechanism of action underlying improved PCF healing. We undertook an exploratory pilot study in healthy controls that involved collecting saliva before and after a subcutaneous injection of octreotide. Using a mass spectrometry‐based workflow optimized for proteomic analysis of biofluids, 15 , 16 we report these novel observations in salivary proteome alterations after octreotide administration.
2. MATERIALS AND METHODS
This pilot study was approved by the Duke University Institutional Review Board. Healthy, adult participants provided a sample of saliva 5 min after rinsing their mouth with tap water and a 2 h fast. The subjects were then given 100 μg octreotide in 1 cm3 saline (Novartis) via subcutaneous injection. A second sample of saliva was then collected after 45–55 min. This interval was selected because peak plasma concentrations have been found to occur 0.4 h after dosing. 17 A total of four subjects were recruited, each providing a set of “pre” and “post” samples. Upon thawing, samples were probe sonicated and diluted 1:1 with sodium dodecyl sulfate (SDS) buffer followed by an additional round of sonication and a protein assay. The concentration and volume of samples from subject 1 were insufficient based on our target of >100 μl saliva and >100 μg of protein per sample (Table S1), so we proceeded with trypsin digestion from the three remaining subject samples using a commercial suspension‐trapping (S‐trap) device. The remaining samples were adequate.
2.1. Sample preparation
Samples were frozen at −80°C and processed in a single batch. Samples were thawed and sonicated, and 100 μl of each sample was diluted 1:1 with 5% SDS in 50 mM triethylammonium bicarbonate, pH 8.5 (TEAB) followed by additional probe sonication and brief heating at 80°C for 5 min. One hundred micrograms of each sample was adjusted to 75 μl with 5% SDS/TEAB. Samples were reduced by addition of 7.5 μl of 110 mM dithiothreitol and heated at 80°C for 15 min. After cooling, samples were alkylated by addition of 7.5 μl of 250 mM iodoacetamide and incubation in the dark for 30 min. Finally, 9 μl of 12% phosphoric acid was added followed by 600 μl of 90% (v/v) methanol/100 mM TEAB, and the samples were processed using S‐traps micro devices (Protifi). Samples were digested with 7 μg of Sequencing‐Grade Modified Trypsin (Promega) at 47°C for 2 h. Eluted peptides were lyophilized and reconstituted in 33 μl of 1/2/97 (v/v/v) trifluoroacetic acid/acetonitrile/water with incubation in a bath sonicator, followed by centrifugation. A study pool quality control (SPQC) sample was prepared by mixing equal quantities of all samples.
2.2. Quantitative LC–MS/MS using data‐independent acquisition
Saliva proteomes were analyzed using microflow liquid chromatography (1 mm internal diameter × 100 mm length; 100 μl/min flow rate and 60 min gradient), post‐column infusion of DMSO, 18 and data‐independent acquisition (DIA) using a staggered, overlapping window configuration. 19 , 20 To compensate for the narrow peak widths at these higher flow rates, the DIA method used 16 m/z windows (8 m/z effective after deconvolution). Based on total ion current (Figure S1), slightly less than half of recovered peptides (15 μl out of 33 μl) were analyzed in singlicate in a block‐randomized run order (Table S1). A SPQC sample, containing an equal mixture of each sample, were analyzed in triplicate at the beginning, middle and end of the queue.
Thirteen microliters of the SPQC sample and 15 μl of individual samples were analyzed by microflow‐liquid chromatography hyphenated with tandem mass spectrometry (LC–MS/MS) using an ACQUITY UPLC (Waters) interfaced to an Exploris 480 high resolution tandem mass spectrometer (Thermo). After direct injection, peptides were separated on a 1 mm × 10 mm 1.7 μm CSH C18 column (Waters) using a flow rate of 100 μl/min, a column temperature of 55°C and a gradient using 0.1% (v/v) formic acid (FA) in H2O (mobile phase A) and 0.1% (v/v) FA in acetonitrile (mobile phase B) at 100 μl/min as follows: 0–60 min, 3–28% B; 60–60.5 min, 28–90% B; 60.5–62.5 min, 90% B; 62.5–63 min, 90–3% B; 63–67 min, 3% B. A zero dead volume Peek tee (Thermo) was used post‐column to introduce a solution of 50% (v/v) dimethyl sulfoxide/acetonitrile at 6 μl/min. The LC was interfaced to the MS with an Optamax NG ion source under heated electrospray ionization conditions with following tune parameters: sheath gas, 32; aux gas, 5; spray voltage, 3.5 kV; capillary temperature, 275°C; aux gas heater temp, 125°C.
The DIA analysis used a staggered, overlapping window method 20 with a 60,000 resolution precursor ion (MS1) scan from 390 to 1020 m/z, AGC target of 1000% and maximum injection time (IT) of 60 ms and RF lens of 40%; data was collected in centroid mode. MS/MS was performed using targeted (tMS2) method with default charge state = 3, 15,000 resolution, automatic gain control (AGC) target of 1000%, maximum IT of 22 ms, and a normalized collision energe (NCE) of 30; data was collected in centroid mode. The DIA windows were generated using EncyclopeDIA (https://bitbucket.org/searleb/encyclopedia/) 19 with a mass range of 400–1000 and 77 × 16 m/z windows, with a 36 window cycle. The MS cycle time was 1.6 s, and the total injection‐to‐injection time was 67 min.
2.3. Quantitative analysis of data‐independent acquisition data
Raw MS data was demultiplexed and converted to *.htrms format using HTRMS converter (Biognosys) and processed in Spectronaut 15.5 (Biognosys). A spectral library was built using Direct‐DIA searches of all individual files. Searches used a Swissprot database with homo sapiens taxonomy (downloaded on 10/16/20) and appended with a concatemer containing single amino acid variant peptides, as well as porcine trypsin. Protein sequences from Streptococcus mitis, Gamella haemolysans, and Granulicatella adiacens were also downloaded from Uniprot and appended to generate a combined database with 25,944 entries. Search settings included N‐terminal semi‐tryptic specificity, up to 2 missed cleavages, fixed carbamidomethyl(Cys) and variable acetyl(protein‐N‐terminus) and oxidation(Met) modifications.
For DIA analysis, default extraction, calibration, identification, and protein inference settings were used. iRT profiling was utilized to quantify precursors that did meet a q‐value cut‐off of <0.01 in a particular run. Protein quantification was performed at MS2 level using the MaxLFQ algorithm, 20 , 21 q‐value percentile 0.2 settings (all precursors that passed a q‐value in at least 20% of runs were included) with run‐wise imputing, and local normalization 22 using precursors that met a q‐value in all runs (q‐value complete).
2.4. Statistical analysis
Statistical analysis used the generalized linear model (glm) function in edgeR, 23 , 24 and data was visualized using ggVolcanoR (https://ggvolcanor.erc.monash.edu/). 25
3. RESULTS
3.1. Quantitative proteomics of saliva from healthy controls treated with octreotide
We achieved high proteome coverage of the saliva proteome (~3000) and high precision in a 1‐h, microflow liquid chromatography run. Database searching and quantification of de‐multiplexed data was performed using Spectronaut. Based on previous studies of the saliva microbiome, 26 we appended sequences for S. mitis (STRMT), G. haemolysans (9BACL) and G. adiacens (9LACT). Along with the six subject samples and three additional quality control samples (which were prepared by pooling equal quantities of the six samples), there were 3550 total protein groups quantified from 36,547 unique peptide precursors, with 3076 human protein groups, 332 S. mitis protein groups, 102 G. haemolyans protein groups, and 42 G. adiacens protein groups (Tables S2 and S3). Analytical precision was good, as measured by the percent coefficient of variation (%CV) of the three SPQC runs versus individuals, a mean %CV of 10% versus 33%, respectively (Table S3). Hierarchical clustering suggested that individual biological variability was the major determinant of the saliva proteome, as sample clustered by subject rather than treatment (Figure 1).
FIGURE 1.
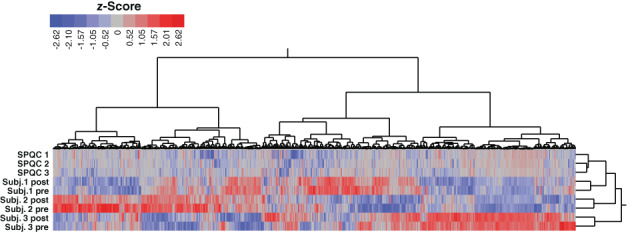
Heatmap of protein group expression. Z‐scored abundances were exported from Spectronaut, and two‐dimensional hierarchical clustering was performed in JMP Pro 16 using Ward's method. Legend shows color scale of z‐score values
A paired statistical analysis was performed using the generalized linear model (glm) function in edgeR. 23 , 24 There were and ~300 proteins that had a p < .05 between the pre‐ and post‐octreotide groups ~50 proteins with an FDR‐corrected p < .05 between pre‐ and post‐groups (Table S4). These results were visualized using a volcano plot after filtering on proteins quantified by 2 more or unique precursors (Figure 2). Both human and bacterial proteins were among the proteins altered by octreotide treatment. Notably, four isoforms of the human cystatins, belonging to a family of cysteine proteases, had significantly lower abundance after treatment (Figure 3). Based on clustering (Figure 1 and Table S4), histatin‐1 and (HIS1_HUMAN) and proline‐rich protein 27 (PRR27_HUMAN), two other significantly downregulated proteins, had a similar expression pattern as three of the cystatins (CYTS; CYTD and CYTN).
FIGURE 2.
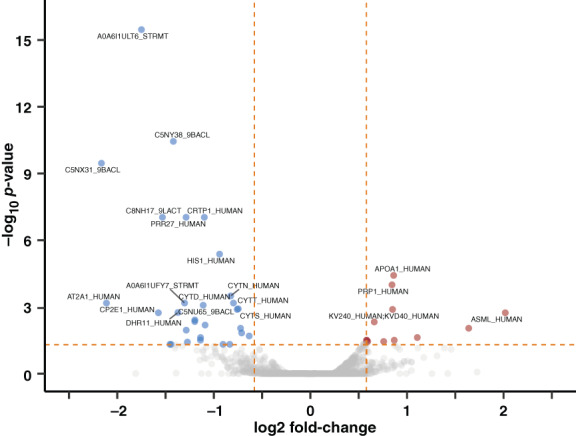
Volcano plot of proteins with differential abundance in saliva post‐ versus pre‐octreotide w/ p‐values. Statistical analysis used the generalized linear model (glm) function in edgeR. Log2FC and p‐value data was filtered by proteins quantified by more than one precursor and visualized using ggVolcanoR. 24 The top 20 proteins with absolute log2FC >0.58 and FDR‐corrected p‐value <.05 were labeled as significant
FIGURE 3.
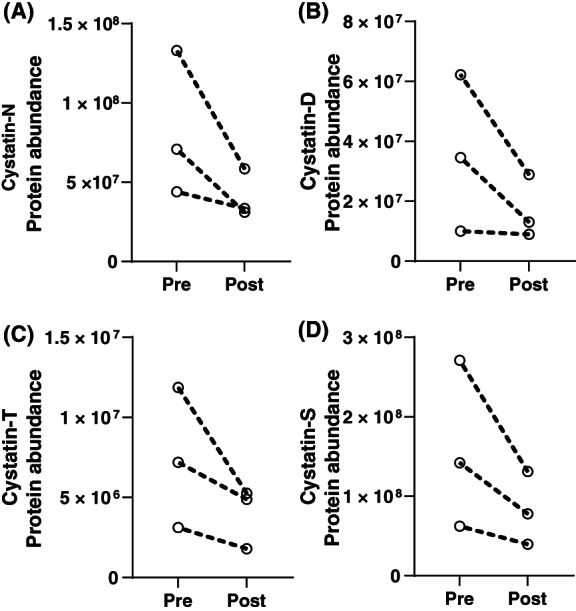
Human cystatin proteins with differential abundance. Protein abundances were plotted for the three subjects, with intraindividual pre‐ and post‐octreotide values separated by a dotted line. Cystatin proteins which were significantly lower after octreotide treatment included (A) cystatin‐SN (CYTN_HUMAN), (B) cystatin‐D (CYTD_HUMAN), (C) cystatin‐SA (CYTT_HUMAN), and (D) cystatin‐S (CYTS_HUMAN). Data were plotted in GraphPad Prism
Rank protein abundance in saliva protein was estimated using the average MaxLFQ values of three SPQC samples (Figure 4A). Bacterial proteins were absent in the top two orders of magnitude, and the affected cystatin proteins were estimated to be among the 200 most abundant proteins (Figure 4A) but not within the top 20 (Figure 4B). Note that a similar analysis by Grassl et al. 27 excluded keratins due the fact they are potential contaminants. However, levels of cytokeratins exhibited low intra‐individual variability, and high inter‐subject variability, suggesting that they are endogenous to saliva and not laboratory contaminants.
FIGURE 4.
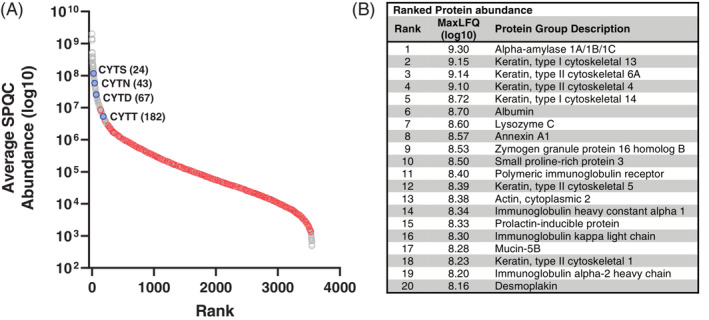
Ranked protein abundance in saliva. (A) Average protein abundances in SPQC runs (log10 MaxLFQ values) were plotted for all quantified proteins. Gray, human proteins; red, Streptococcus mitis proteins; blue, cystatin proteins that were significantly lower after octreotide treatment. (B) Top 20 proteins based on rank abundance (excluding porcine trypsin; Table S4)
4. DISCUSSION
This pilot study is the first to report octreotide induced salivary proteome alterations using mass spectrometry‐based proteomics. Furthermore, our findings provide a potential mechanism the role of octreotide in wound healing. Microflow liquid chromatography coupled to DIA quantified a comparable number of saliva proteins as compared to a recent nanoflowLC analysis 27 in approximately one‐half the total instrument cycle time. One class of proteins significantly downregulated were cystatins.
Cystatins belong to a superfamily of ancestrally related proteins, most of which are inhibitors of cysteine proteinases. The levels of salivary cystatins have been examined in the oral health literature, which has found a significant range in cystatin abundance among periodontally healthy individuals. 28 , 29 Cysteine proteinases include a number of cathepsins found in the oral environment. For example, cathepsin S is expressed in both microvascular endothelial cells as well as periodontal ligament cells. Shi and colleagues developed both in vivo and in vitro models demonstrated that deficiency in cathepsin S impairs microvascular angiogenesis in the context of wound healing. 30 Furthermore, Memmert et al. utilized an in vitro wound healing assay to show that cathepsin S significantly enhanced wound healing rates by increasing cell proliferation, migration, and wound closure. These authors further postulate that the role of cathepsins may be inhibited by periodontal flora in impairing wound healing. 31
The downregulation of cystatins in the saliva allows for increased expression of cysteine proteases such as Cathepsin S. This results in increased cysteine protease activity that has been linked to enhanced angiogenic response, cell proliferation and migration that have resulted in improved wound healing. These insights provide first steps at furthering our understanding of octreotide's effects on saliva and reports of improved PCF healing.
This study is limited by its small sample size due to the COVID‐19 restrictions that were imposed during the time of this study, as well as sample analysis‐related costs. We were unfortunately forced to exclude one subject due to insufficient volume and protein concentration, so while the microflowLC approach is useful for throughput and robustness, it may suffer from sensitivity and is not preferred in sample‐limited circumstances.
Additionally, somatostatin (and its exogenous form, octreotide) has been show have inhibit the secretion of both pancreatic and salivary amylase. 32 While the presence and quantity of amylase in drain fluid has been used in the diagnosis of fistulae (in not only Head and Neck surgery, but also abdominal surgery), there is limited literature describing a mechanism for amylase it fistula development. 11 It is certainly possible that amylase itself does have a role in wound healing and fistula development. There was no statistically significant effect of total salivary production following administration of octreotide though the small sample size limits this inference.
Another limitation of this study is that it does not evaluate the effects of drug in a diseased population; rather, we can only infer potential mechanisms of action from the changes to control subject. Nonetheless, the relative abundance of approximately 50 proteins (of >3500 quantified) were significantly altered after octreotide treatment, and cystatins appeared to be one of the major affected classes of host proteins.
In summary, this work represents the first to report octreotide induced alterations in saliva and a potential mechanism of action that could account for improved healing of PCF. The key finding is a significant decrease in cystatins that result in likely increased activity of cysteine proteinases, which has been associated with improved wound healing. These findings represent preliminary insights into a postulated mechanism for the therapeutic effects of octreotide. Further research in a larger cohort is needed to investigate this potential mechanism of action as it relates to octreotide use and PCF closure.
FUNDING INFORMATION
There is no funding to report for this submission.
CONFLICT OF INTEREST
The authors whose names are listed immediately below report the following details of affiliation or involvement in an organization or entity with a financial or non‐financial interest in the subject matter or materials discussed in this manuscript.
REFERENCES
- 1. Suh JD, Sercarz JA, Abemayor E, et al. Analysis of outcome and complications in 400 cases of microvascular head and neck reconstruction. Arch Otolaryngol Head Neck Surg. 2004;130(8):962‐966. doi: 10.1001/archotol.130.8.962 [DOI] [PubMed] [Google Scholar]
- 2. Singh B, Cordeiro PG, Santamaria E, Shaha AR, Pfister DG, Shah JP. Factors associated with complications in microvascular reconstruction of head and neck defects. Plast Reconstr Surg. 1999;103(2):403‐411. doi: 10.1097/00006534-199902000-00007 [DOI] [PubMed] [Google Scholar]
- 3. Azizzadeh B, Yafai S, Rawnsley JD, et al. Radial forearm free flap pharyngoesophageal reconstruction. Laryngoscope. 2001;111(5):807‐810. doi: 10.1097/00005537-200105000-00010 [DOI] [PubMed] [Google Scholar]
- 4. Bomeli SR, Desai SC, Johnson JT, Walvekar RR. Management of salivary flow in head and neck cancer patients—a systematic review. Oral Oncol. 2008;44(11):1000‐1008. doi: 10.1016/j.oraloncology.2008.02.007 [DOI] [PubMed] [Google Scholar]
- 5. Molteni G, Sacchetto A, Sacchetto L, Marchioni D. Optimal management of post‐laryngectomy pharyngo‐cutaneous fistula. Open Access Surgery. 2020;13:11‐25. doi: 10.2147/oas.S198038 [DOI] [Google Scholar]
- 6. White HN, Golden B, Sweeny L, Carroll WR, Magnuson JS, Rosenthal EL. Assessment and incidence of salivary leak following laryngectomy. Laryngoscope. 2012;122(8):1796‐1799. doi: 10.1002/lary.23443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oudhoff MJ, Bolscher JGM, Nazmi K, et al. Histatins are the major wound‐closure stimulating factors in human saliva as identified in a cell culture assay. FASEB J. 2008;22(11):3805‐3812. doi: 10.1096/fj.08-112003 [DOI] [PubMed] [Google Scholar]
- 8. Engeland CG, Bosch JA, Cacioppo JT, Marucha PT. Mucosal wound healing: the roles of age and sex. Arch Surg. 2006;141(12):1193‐1197; discussion 1198. doi: 10.1001/archsurg.141.12.1193 [DOI] [PubMed] [Google Scholar]
- 9. Brand HS, Ligtenberg AJ, Veerman EC. Saliva and wound healing. Monogr Oral Sci. 2014;24:52‐60. doi: 10.1159/000358784 [DOI] [PubMed] [Google Scholar]
- 10. Gall AM, Sessions DG, Ogura JH. Complications following surgery for cancer of the larynx and hypopharynx. Cancer. 1977;39(2):624‐631. doi:10.1002/1097‐0142(197702)39:2<624::aid‐cncr2820390238>3.0.co;2‐7 [DOI] [PubMed] [Google Scholar]
- 11. Gomes‐Porras M, Cardenas‐Salas J, Alvarez‐Escola C. Somatostatin analogs in clinical practice: a review. Int J Mol Sci. 2020;21(5):117‐118. doi: 10.3390/ijms21051682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spinell C, Ricci E, Berti P, Miccoli P. Postoperative salivary fistula: therapeutic action of octreotide. Surgery. 1995;117(1):117‐118. doi: 10.1016/s0039-6060(05)80242-9 [DOI] [PubMed] [Google Scholar]
- 13. Gibson S, Strutt R, Chye R. Managing a malignant orocutaneous fistula: stem the tide with octreotide? Intern Med J. 2002;32:191‐192. doi: 10.1046/j.1444-0903.2001.00190.x [DOI] [PubMed] [Google Scholar]
- 14. Weinberger J. Otreotide vs. Placebo in Prevention of Salivary Fistulae After Post Radiation Salvage Surgery. NCT02437825. Accessed January 1, 2022. https://clinicaltrials.gov/ct2/show/NCT02437825
- 15. Schaller TH, Foster MW, Thompson JW, et al. Pharmacokinetic analysis of a novel human EGFRvIII:CD3 bispecific antibody in plasma and whole blood using a high‐resolution targeted mass spectrometry approach. J Proteome Res. 2019;18(8):3032‐3041. doi: 10.1021/acs.jproteome.9b00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kamyszek RW, Foster MW, Evans BA, et al. The effect of pathogen inactivation on cryoprecipitate: a functional and quantitative evaluation. Blood Transfus. 2020;18(6):454‐464. doi: 10.2450/2020.0077-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Novartis Pharmaceuticals Corporation .Sandostatin(octreotide acetate)[package insert]. U.S. Food and Drug Administration website. Accessed January 8, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/019667s058,021008s023lbl.pdf
- 18. Distler U, Lacki MK, Schumann S, Wanninger M, Tenzer S. Enhancing sensitivity of microflow‐based bottom‐up proteomics through Postcolumn solvent addition. Anal Chem. 2019;91(12):7510‐7515. doi: 10.1021/acs.analchem.9b00118 [DOI] [PubMed] [Google Scholar]
- 19. Searle BC, Pino LK, Egertson JD, et al. Chromatogram libraries improve peptide detection and quantification by data independent acquisition mass spectrometry. Nat Commun. 2018;9(1):5128. doi: 10.1038/s41467-018-07454-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pino LK, Just SC, MacCoss MJ, Searle BC. Acquiring and analyzing data independent acquisition proteomics experiments without spectrum libraries. Mol Cell Proteomics. 2020;19(7):1088‐1103. doi: 10.1074/mcp.P119.001913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cox J, Hein MY, Luber CA, Paron I, Nagaraj N, Mann M. Accurate proteome‐wide label‐free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cell Proteomics. 2014;13(9):2513‐2526. doi: 10.1074/mcp.M113.031591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Callister SJ, Barry RC, Adkins JN, et al. Normalization approaches for removing systematic biases associated with mass spectrometry and label‐free proteomics. J Proteome Res. 2006;5(2):277‐286. doi: 10.1021/pr050300l [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139‐140. doi: 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA‐Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40(10):4288‐4297. doi: 10.1093/nar/gks042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mullan KA, Bramberger LM, Munday PR, et al. ggVolcanoR: a shiny app for customizable visualization of differential expression datasets. Comput Struct Biotechnol J. 2021;19:5735‐5740. doi: 10.1016/j.csbj.2021.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43(11):5721‐5732. doi: 10.1128/JCM.43.11.5721-5732.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grassl N, Kulak NA, Pichler G, et al. Ultra‐deep and quantitative saliva proteome reveals dynamics of the oral microbiome. Genome Med. 2016;8(1):44. doi: 10.1186/s13073-016-0293-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baron AC, Gansky SA, Ryder MI, Featherstone JDB. Cysteine protease inhibitory activity and levels of salivary cystatins in whole saliva of periodontally diseased patients. J Periodontal Res. 1999;34(8):437‐444. doi: 10.1111/j.1600-0765.1999.tb02279.x [DOI] [PubMed] [Google Scholar]
- 29. Henskens YM, van der Velden U, Veerman EC, Nieuw Amerongen AV. Protein, albumin and cystatin concentrations in saliva of healthy subjects and of patients with gingivitis or periodontitis. J Periodontal Res. 1993;28(1):43‐48. doi: 10.1111/j.1600-0765.1993.tb01049.x [DOI] [PubMed] [Google Scholar]
- 30. Shi G‐P, Sukhova GK, Kuzuya M, et al. Deficiency of the cysteine protease cathepsin S impairs microvessel growth. Circ Res. 2003;92(5):493‐500. doi: 10.1161/01.RES.0000060485.20318.96 [DOI] [PubMed] [Google Scholar]
- 31. Memmert S, Nokhbehsaim M, Damanaki A, et al. Role of cathepsin S In periodontal wound healing–an in vitro study on human PDL cells. BMC Oral Health. 2018;18(1):60. doi: 10.1186/s12903-018-0518-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. De Sol A, Cirocchi R, Di Patrizi MS, et al. The measurement of amylase in drain fluid for the detection of pancreatic fistula after gastric cancer surgery: an interim analysis. World J Surg Oncol. 2015;13(1):65. doi: 10.1186/s12957-014-0428-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
We would like to acknowledge the clinical research support of Amy Walker, Research Program Lead, and Victoria Eifert, Senior Clinical Research Coordinator. We also thank Robert Plumb (Waters Corp) for supplying the custom‐format ACQUITY Premier column used in this study.
Cohen J, Reed W, Foster MW, et al. Octreotide may improve pharyngocutaneous fistula healing through downregulation of cystatins: A pilot study. Laryngoscope Investigative Otolaryngology. 2023;8(1):113‐119. doi: 10.1002/lio2.962
Jonathan Cohen and William Reed are the co‐first authorship.
DATA AVAILABILITY STATEMENT
The raw mass spectrometry data, and data supplement, have been uploaded to the MassIVE repository (massive.ucsd.edu). and can be downloaded at ftp://massive.ucsd.edu/MSV000089053 or via ProteomeXchangeID (PXD032249).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
The raw mass spectrometry data, and data supplement, have been uploaded to the MassIVE repository (massive.ucsd.edu). and can be downloaded at ftp://massive.ucsd.edu/MSV000089053 or via ProteomeXchangeID (PXD032249).


