Abstract
Introduction
Most patients significantly benefit from cochlear implantation (CI). However, speech understanding varies widely, with a small proportion of patients demonstrating limited audiometric outcomes. While there are well‐documented determinants of poor performance, there remains a cohort of patients that do not meet expected outcomes. Preoperative prognostication is desirable to manage expectations, ensure value of the intervention, and reduce risk. The objective of the study is to evaluate variables found within a single CI center's most limited functioning cohort following implantation.
Methods
A retrospective review of a single CI program's cohort of (344 ears) patients implanted between 2011 and 2018 whose 1‐year postimplantation AzBio scores fall 2 SDs below the mean was performed. Exclusion criteria includes skullbase pathology, pre/peri‐lingual deafness, cochlear anatomic abnormalities, English as an additional language, and limited electrode insertion depth. Overall, 26 patients were identified.
Results
The study population's postimplantation net benefit AzBio score is 18% compared to the entire program's 47% (p < 0.05). This group is older (71.8 vs. 59.0 years, p < 0.05) with a longer duration of hearing loss (26.4 vs. 18.0 years, p < 0.05) and with a lower preoperative AzBio score [14% lower (p < 0.05)]. A host of medical conditions were identified in the subpopulation, with a trend towards significance in those suffering from either malignancy or cardiac condition. Escalating comorbid status was associated with worse performance (p < 0.05).
Conclusion
Within a cohort of limited‐performing CI users, benefit tended to decrease with escalating number of comorbid conditions. This information may serve to inform preoperative patient counseling.
Level of evidence
Level IV (evidence from a case control study).
Keywords: audiology, cochlear implants, sensorineural hearing loss
Many patients receive benefit from cochlear implant surgery, however a cohort of patients remain that do not perform as expected with age and duration of hearing loss the primary risk factors.

1. INTRODUCTION
Cochlear implantation (CI) is a common treatment for severe‐to‐profound hearing loss that involves direct electrical stimulation of the auditory nerve. It is quite successful in partial hearing restoration. However, differences in functional outcomes are common. 1 , 2 , 3 Speech understanding varies and a small proportion of recipients perform poorly on formal outcome measures. 4 , 5 While there are well‐documented determinants of poor performance, a better understanding of these factors can facilitate preoperative prognostication of outcome and patient counseling.
A number of variables are well appreciated to be associated with more limited functional outcomes. Age at implantation and duration of hearing loss are correlated with lower postoperative hearing scores. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 However, while an association with age is illustrated, there are mixed outcomes and causation may be multifaceted. 6 , 10 , 16 , 17 The issue is complex as age is associated with changes in cognition, reductions in neural plasticity, limited social activity, and comorbid status. 18 , 19 A similarly complicated arrangement is seen with disparate etiologies of hearing loss, cochlear nerve diameter, and electrode insertion dynamics. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18
In general, the incidence of medical issues increases with age. The effect of comorbidities on CI outcomes has not been well delineated. One study found that older patients undergoing CI tended to have more comorbidities, however, did not differentiate audiometric outcomes based on health. 18 Outcomes specifically comparing comorbid burden to audiometric outcomes and quality‐of‐life (QOL) measures were not addressed. Furthermore, the import of disparate types of medical conditions on audiometric outcomes is not clear.
QOL has been shown to improve in both younger and older CI patients, although studies used varied QOL instruments. 20 , 21 , 22 , 23 , 24 Progressive hearing loss in adults is associated with reduced QOL due to communication barriers and decreased speech recognition. 25 , 26 There is some evidence that speech understanding increases postoperatively and patients report significant improvement, however, outcomes were influenced by factors, such as educational level, co‐existing depression, and preoperative working memory. 20 , 21 , 23
The objective of the study is to determine and evaluate variables that may assist in forecasting outcomes and communicating expectations in patients that are anticipated to have challenged postoperative speech discrimination following CI (older age, longer duration of deafness, and modest preoperative function).
2. METHODS
A retrospective analysis of a group of adult patients who gained the least benefit after CI in a single program's cohort of patients implanted between 2011 to 2018 was performed. A total of 344 ears were implanted during this time period. Inclusion criteria included those whose postimplantation AzBio speech perception scores at 1‐year fell two standard deviations below the mean. Exclusion criteria included any skullbase pathology, pre/peri‐lingual deafness, cochlear anatomic abnormalities, English as an additional language prohibiting accurate speech scores, and limited electrode insertion depth. Approval was received from the Research Ethics Board at University of Manitoba prior to any study activity (HS18623 (H2015:209)).
Peri‐operative data collection included patient questionnaires, imaging characteristics, and surgical findings. Patient self‐account of health status and review of an inclusive provincial electronic record were collected. Postoperative speech recognition scores are measured annually, employing the AzBio speech perception test. Outcomes were compared to the general cohort of 344 patients. Statistical analysis was conducted using two‐tailed T‐test.
Secondary outcomes include quality of life questionnaires from the International Outcome Inventory for Cochlear Implants (IOI‐CI) routinely collected from patients. Quality of life measures were analyzed using Mann–Whitney Test.
3. RESULTS
A total of 31 patients were identified and 26 patients were ultimately included in analysis. One patient with Neurofibromatosis type 2 and four prelingually deafened patients were removed from the study. There were four patients deceased at time of analysis.
The study cohort's average preoperative AzBio score is 12%, the postoperative scores at 1 year are 30%, with a net benefit of 18%. In the general cohort, the preoperative AzBio score is 27%, the postoperative score at 1 year is 74% and net benefit is 47% (Table 1). Preoperative AzBio scores are 14% lower (p < 0.05) than the general population. The postimplantation net benefit AzBio score at one year was also significantly different (47% compared to 18%, p < 0.05).
TABLE 1.
Patient word scores found in the general cohort and study subpopulation
| Study subgroup (n = 26) | General cohort (n = 344) | p‐value | |
|---|---|---|---|
| Pre‐Op AzBio score | 12% (±14%) | 27% (±20%) | <0.05 |
| Post‐Op AzBio score | 30% (±12%) | 74% (±17%) | <0.05 |
| Net Benefit AzBio score | 18% (±12%) | 47% (±18%) | <0.05 |
| Age at implantation (years) | 71.2 (±12.7) | 59.0 (±15.9) | <0.05 |
| Duration of hearing loss (years) | 26.4 (±13.9) | 18.7 (±16.5) | <0.05 |
The average age at implantation of the study subgroup was significantly older (71.2 vs. 59.0 years, p < 0.05) than the general CI population (Table 1). Duration of hearing loss in the study subgroup was significantly longer (26.4 vs. 18.0 years, p < 0.05). The ratio of male: female in the study subgroup was 10:16. Males had a mean post‐op AzBio score of 25% and net benefit of 11% compared to females, with a mean AzBio score of 31% and net benefit of 21%. There were no significant differences.
The presence of comorbid conditions was reviewed. The type of condition did not significantly influence postoperative function (Table 2). A nonsignificant difference is identified in the net benefit AzBio scores in individuals with either cancer or cardiac condition (Table 2).
TABLE 2.
A comparison of AzBio scores by comorbid condition
| Pre‐Op AzBio score | Post‐Op AzBio score | Net benefit AzBio score | |
|---|---|---|---|
| Study subgroup (n = 26) | 12% (±14%) | 30% (±12%) | 18% (±12%) |
| No comorbidities (n = 7) | 5% (±7%) | 32% (±13%) | 27% (±9%) |
| Cardiac condition (n = 9) | 15% (±18%) | 25% (±13%) | 10% (±14%) |
| Otologic condition (n = 7) | 15% (±17%) | 28% (±16%) | 13% (±10%) |
| Neurologic condition (n = 6) | 11% (±21%) | 25% (±13%) | 14% (±17%) |
| Autoimmune condition (n = 9) | 10% (±12%) | 31% (±8%) | 20% (±8%) |
| Cancer (n = 4) | 27% (± 23%) | 38% (± 6%) | 11% (± 20%) |
| Renal condition (n = 3) | 0% | 21% (±18%) | 21% (±18%) |
An inverse relationship is illustrated when contrasting audiometric outcomes and number of comorbid conditions (Figure 1). Differences were found between nonmorbid and those with one comorbidity, and those with three or more comorbidities (p < 0.05). Having two conditions illustrated a trend to worse function without statistical significance.
FIGURE 1.
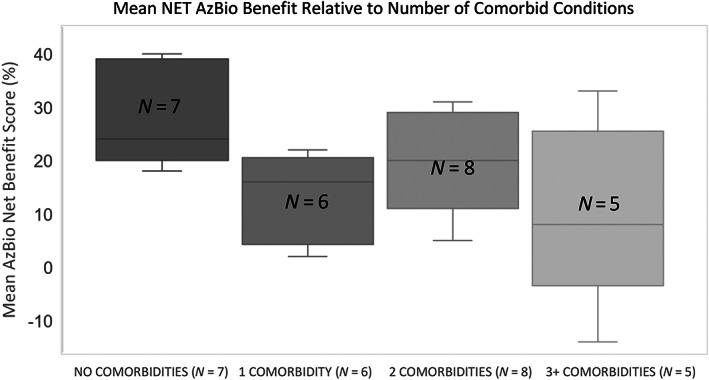
Contrasting the number of comorbid conditions to postoperative Net AzBio score
The average responses to the QOL survey from the study cohort did not differ from the general cohorts (Figure 2).
FIGURE 2.
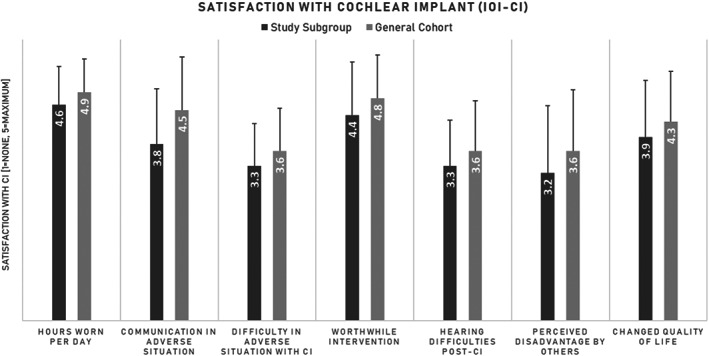
Population satisfaction with cochlear implantation compared to the entire cohort
4. DISCUSSION
This study investigated factors associated with limited outcomes in a cohort of patients who performed 2 SDs below the average postoperative AzBio speech perception score. The determination to examine only those 2 SDs from the mean performance was arbitrary, but with the intent to address those with the most limited functional outcomes.
4.1. Comorbidity
The occurrence of multimorbidity increases with age and prevalence ranges from 55% to 98% in persons 60 years and older. 19 The majority of the study cohort, 19 out of 26 participants, had one or more comorbidities. This study found that the greater the number of comorbid conditions tended to correlate with more limited performance. This was independent of age. This is not unanticipated. Neurologic function, and ischemia are both a correlate for cogitation. With increasing comorbidities come polypharmacy, heightened psychological stress, and decreased quality of life, all of which are factors found to have association with lower implant audiological measures. 18 , 25 , 27 However, there is also developing literature illustrating that hearing rehabilitation has a very positive impact on validated cognitive instruments. 28 , 29 , 30
Type of comorbid conditions (cardiac, otologic, neurologic, rheumatologic, renal, and cancer) was not a significant determinant of function; however, patients with cardiac conditions and cancer trended to worse outcomes.
An explanation of outcomes of patients with cardiac conditions is likely owing to vascular health and the determinants and associations with vascular health, with central demyelination, neuronal density, and recruitment following implantation. 31 The discrepancy found in patients with historic cancer could relate to iatrogenic inner ear damage (skullbase radiation or ototoxic exposures). 32 , 33 However, all treatment modalities for cancer impact central function with possible deleterious effects on postimplantation speech intelligibility.
It was not possible to correct for age in reviewing the implications of associated health conditions in this subpopulation as the cohort size was relatively small. Furthermore, a significant caveat is that it was not possible to differentiate the degree or significance of any given illness. As an illustration, the import of paroxysms of SVT on a patients' microangiopathic health is likely different from having previously undergone a five‐vessel bypass graft following years of ischemia.
4.2. Age, duration of hearing loss and preoperative function
Similar to other work, this study found all three variables to be associated with worse overall function. The study population experienced a longer duration of hearing loss, a finding that is echoed in previous studies. 1 , 2 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 The cohort is also significantly older than the general cohort. It is assumed that the degenerative process of aging (multifaceted impact) and lengthier auditory deprivation leads to a negative correlation with postoperative hearing outcomes. This is perhaps through auditory nerve fiber atrophy, damage to hair cells and stria vascularis, or reduced plasticity of the auditory pathways or auditory cortex. 26 , 34 , 35
In addition, the definition of duration of deafness is not consistent across reports, ranging from age at implantation subtracted from reported onset of deafness, to reported years of hearing loss. 1 , 2 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 In this study, duration of hearing loss is measured via patient‐reported years.
The subgroup entered into surgery with less hearing as evidenced by lower preoperative AzBio scores compared to the general population. This variable is likely highly associated with age at implantation and duration of deafness. Within the confines of this study, it was not possible to analyze as an isolated variable.
4.3. Quality of Life
QOL did not significantly differ between the general CI cohort and the study population. In general, the study population with unexpected outcomes tended to have similarly improved QOL as those with normative performance. This is arguably equally important for patients to audiometric outcomes. A recent study found that patients reported improved QOL following CI which did not necessarily correlate with popular clinical measures of speech recognition. 24
This feature is humbling and reminds implant centers of the need to be patient‐centric in decision making.
There are several study limitations. The small sample size may have profound implications when generalizing the impact of comorbid conditions on function. Furthermore, the cohort size did not have power to allow for multivariate analysis, nor adjustments for age at implantation or duration of deafness. Other confounding factors (i.e., hearing aid fit, neurocognition, psychological factors) would likely have influence, however, sufficient data was not available. Future work should attempt to obtain more granular detail regarding individual conditions. It would be interesting to assess for medical conditions in the broader CI database for a similar finding of more limited objective function with the increasing number of comorbid conditions.
5. CONCLUSION
Within a population of limited performing CI users, objective benefit declined with more comorbid health. This information should inform preoperative patient counseling.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
We would like to acknowledge the Central Speech and Hearing Clinic in Winnipeg, Manitoba.
Lee E, Pisa J, Hochman J. Comorbidity associated with worse outcomes in a population of limited cochlear implant performers. Laryngoscope Investigative Otolaryngology. 2023;8(1):230‐235. doi: 10.1002/lio2.985
REFERENCES
- 1. Summerfield AQ, Marshall DH. Preoperative predictors of outcomes from cochlear implantation in adults: performance and quality of life. Ann Otol Rhinol Laryngol Suppl. 1995;104(9):105‐108. [PubMed] [Google Scholar]
- 2. Savvas E, Heslinga K, Sundermann B, et al. Prognostic factors in cochlear implantation in adults: determining central process integrity. Am J Otolaryngol. 2020;41(3):102435. doi: 10.1016/j.amjoto.2020.102435 [DOI] [PubMed] [Google Scholar]
- 3. Gantz BJ, Woodworth GG, Knutson JF, Abbas PJ, Tyler RS. Multivariate predictors of audiological success with multichannel cochlear implants. Ann Otol Rhinol Laryngol. 1993;102(12):909‐916. [DOI] [PubMed] [Google Scholar]
- 4. Arnoldner C, Lin VYW. Expanded selection criteria in adult cochlear implantation. Cochlear Implants Int. 2013;14(SUPPL. 4):9‐13. doi: 10.1179/1467010013Z.000000000123 [DOI] [PubMed] [Google Scholar]
- 5. Decady Y, Greenberg L. Health at a Glance: Ninety Years of Change in Life Expectancy. Vol 82. Statistics Canada catalogue ; 2014:624‐X. [Google Scholar]
- 6. Battmer RD, Gupta SP, Allum‐Mecklenburg DJ, Lenarz T. Factors influencing cochlear implant perceptual performance in 132 adults. Ann Otol Rhinol Laryngol Suppl. 1995;104(9):185‐187. [PubMed] [Google Scholar]
- 7. Kurz A, Grubenbecher M, Rak K, Hagen R, Kühn H. The impact of etiology and duration of deafness on speech perception outcomes in SSD patients. Eur Arch Oto‐Rhino‐Laryngology. 2019;276(12):3317‐3325. doi: 10.1007/s00405-019-05644-w [DOI] [PubMed] [Google Scholar]
- 8. Lee SY, Shim YJ, Han JH, et al. The molecular etiology of deafness and auditory performance in the postlingually deafened cochlear implantees. Sci Rep. 2020;10(1):1‐12. doi: 10.1038/s41598-020-62647-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Dijk JE, van Olphen AF, Langereis MC, Mens LHM, Brokx JPL, Smoorenburg GF. Predictors of cochlear implant performance. Int J Audiol. 1999;38(2):109‐116. doi: 10.3109/00206099909073010 [DOI] [PubMed] [Google Scholar]
- 10. Green K, Bhatt Y, Mawman D, et al. Predictors of audiological outcome following cochlear implantation in adults. Cochlear Implants Int. 2007;8(1):1‐11. doi: 10.1179/cim.2007.8.1.1 [DOI] [PubMed] [Google Scholar]
- 11. Lazard DS, Vincent C, Venail F, et al. Pre‐, per‐ and postoperative factors affecting performance of postlinguistically deaf adults using cochlear implants: a new conceptual model over time. PLoS One. 2012;7(11):1‐11. doi: 10.1371/journal.pone.0048739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holden LK, Finley CC, Firszt JB, et al. Factors affecting open‐set word recognition in adults with cochlear implants. Ear Hear. 2013;34(3):342‐360. doi: 10.1097/AUD.0b013e3182741aa7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beyea JA, McMullen KP, Harris MS, et al. Cochlear implants in adults: effects of age and duration of deafness on speech recognition. Otol Neurotol. 2016;37(9):1238‐1245. doi: 10.1097/MAO.0000000000001162 [DOI] [PubMed] [Google Scholar]
- 14. Chung J, Jang JH, Chang SO, Song JJ, Cho SW, Kim SY, Lee JH, Oh SH Does the width of the bony cochlear nerve canal predict the outcomes of cochlear implantation? Biomed Res Int 2018:5675848. doi: 10.1155/2018/5675848, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim H, Kang WS, Park HJ, et al. Cochlear implantation in postlingually deaf adults is time‐sensitive towards positive outcome: prediction using advanced machine learning techniques. Sci Rep. 2018;8(1):1‐9. doi: 10.1038/s41598-018-36404-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharma RK, Chen SY, Grisel J, Golub JS. Assessing cochlear implant performance in older adults using a single, universal outcome measure created with imputation in HERMES. Otol Neurotol. 2018;3:987‐994. doi: 10.1097/MAO.0000000000001907 [DOI] [PubMed] [Google Scholar]
- 17. Den Brandt AH, Van MG, Gilles A, et al. Auditory performances in older and younger adult cochlear implant recipients: use of the hearring registry. Otol Neurotol. 2019;40(8):E787‐E795. doi: 10.1097/MAO.0000000000002333 [DOI] [PubMed] [Google Scholar]
- 18. Wilkerson BJ, Porps SF, Babu SC. The impact of comorbidities in the aging population on Cochlear implant outcomes. Otol Neurotol. 2017;38(8):e285‐e288. doi: 10.1097/MAO.0000000000001501 [DOI] [PubMed] [Google Scholar]
- 19. Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430‐439. doi: 10.1016/j.arr.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 20. Völter C, Götze L, Haubitz I, Dazert S, Thomas JP. Benefits of cochlear implantation in middle‐aged and older adults. Clin Interv Aging. 2020;15:1555‐1568. doi: 10.2147/CIA.S255363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McRackan TR, Bauschard M, Hatch JL, et al. Meta‐analysis of quality‐of‐life improvement after cochlear implantation and associations with speech recognition abilities. Laryngoscope. 2018;128(4):982‐990. doi: 10.1002/lary.26738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Sousa AF, Couto MIV, Martinho‐Carvalho AC. Quality of life and cochlear implant: results in adults with postlingual hearing loss. Braz J Otorhinolaryngol. 2018;84(4):494‐499. doi: 10.1016/j.bjorl.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Issing C, Baumann U, Pantel J, Stöver T. Cochlear implant therapy improves the quality of life in older patients‐a prospective evaluation study. Otol Neurotol. 2020;41(9):1214‐1221. doi: 10.1097/MAO.0000000000002741 [DOI] [PubMed] [Google Scholar]
- 24. Andries E, Gilles A, Topsakal V, et al. Systematic review of quality of life assessments after cochlear implantation in older adults. Audiol Neurotol. 2021;26(2):61‐75. doi: 10.1159/000508433 [DOI] [PubMed] [Google Scholar]
- 25. Solheim J, Kværner KJ, Falkenberg ES. Daily life consequences of hearing loss in the elderly. Disabil Rehabil. 2011;33(23–24):2179‐2185. doi: 10.3109/09638288.2011.563815 [DOI] [PubMed] [Google Scholar]
- 26. Dalton D, Cruickshanks K, Klein B, Klein R, Wiley T, Nondahl D. The impact of hearing loss on the quality of life in adults. Gerontologist. 2003;43(5):661‐668. doi: 10.1093/geront/43.5.661 [DOI] [PubMed] [Google Scholar]
- 27. Knopke S, Häussler S, Gräbel S, et al. Age‐dependent psychological factors influencing the outcome of cochlear implantation in elderly patients. Otol Neurotol. 2019;40(4):e441‐e453. doi: 10.1097/MAO.0000000000002179 [DOI] [PubMed] [Google Scholar]
- 28. Van Dijkhuizen JN, Boermans PPBM, Briaire JJ, Frijns JHM. Intelligibility of the patient's speech predicts the likelihood of cochlear implant success in prelingually deaf adults. Ear Hear. 2016;37(5):e302‐e310. doi: 10.1097/AUD.0000000000000286 [DOI] [PubMed] [Google Scholar]
- 29. Moberly AC, Castellanos I, Mattingly JK. Neurocognitive factors contributing to cochlear implant candidacy. Otol Neurotol. 2018;39(10):e1010‐e1018. doi: 10.1097/MAO.0000000000002052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jayakody DMP, Friedland PL, Nel E, Martins RN, Atlas MD, Sohrabi HR. Impact of cochlear implantation on cognitive functions of older adults: pilot test results. Otol Neurotol. 2017;38(8):e289‐e295. doi: 10.1097/MAO.0000000000001502 [DOI] [PubMed] [Google Scholar]
- 31. Bainbridge KE, Hoffman HJ, Cowie CC. Diabetes and hearing impairment in the United States: audiometric evidence from the National Health and nutrition examination survey, 1999 to 2004. Ann Intern Med. 2008;149(1):1‐10. doi: 10.7326/0003-4819-149-1-200807010-00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jereczek‐Fossa BA, Zarowski A, Milani F, Orecchia R. Radiotherapy‐induced ear toxicity. Cancer Treat Rev. 2003;29(5):417‐430. doi: 10.1016/S0305-7372(03)00066-5 [DOI] [PubMed] [Google Scholar]
- 33. Wong KL, Song TT, Wee J, Fook‐Chong SMC, De YW. Sensorineural hearing loss after radiotherapy and chemoradiotherapy: a single, blinded, randomized study. J Clin Oncol. 2006;24(12):1904‐1909. doi: 10.1200/JCO.2005.05.0096 [DOI] [PubMed] [Google Scholar]
- 34. Suzuki T, Nomoto Y, Nakagawa T, et al. Age‐dependent degeneration of the stria vascularis in human cochleae. Laryngoscope. 2006;116(10):1846‐1850. doi: 10.1097/01.mlg.0000234940.33569.39 [DOI] [PubMed] [Google Scholar]
- 35. Keithley EM. Pathology and mechanisms of cochlear aging. J Neurosci Res. 2020;98(9):1674‐1684. doi: 10.1002/jnr.24439 [DOI] [PMC free article] [PubMed] [Google Scholar]


