Abstract
While immunoassays are pivotal to medical diagnosis and bioanalytical chemistry, current landscape of public health has catalyzed an important shift in the requirements of immunoassays that demand innovative solutions. For example, rapid, label-free, and low-cost screening of a given analyte is required to inform the best countermeasures to combat infectious diseases in a timely manner. Yet, current design of immunoassays cannot accommodate such requirements as constraint by accumulative challenges, such as repeated incubation and washing, and the need of two types of antibodies in the sandwich format. To provide a potential solution, herein, we report a plasmonic Raman immunoassay with single-antibody, multivariate regression, and shift-of-peak strategies, coined as the PRISM assay, for serum biomarkers detection. The PRISM assay relies on Raman reporter-antibody conjugates to capture analytes on a plasmonic substrate. The ensuing nanomechanical perturbations to vibration of Raman reporters induce subtle but characteristic spectral changes that encode rich information related to the captured analytes. By fusing Raman spectroscopy and chemometric analysis, we have developed both Raman frequency shift- and multivariate regression models for sensitive detection of biomarkers. The PRISM assay is expected to find a wide range of applications in clinical diagnosis, food safety surveillance, and environmental monitoring.
Keywords: Immunoassays, SERS, Frequency Shift, Biomarkers, Multivariate regression
Graphical Abstract
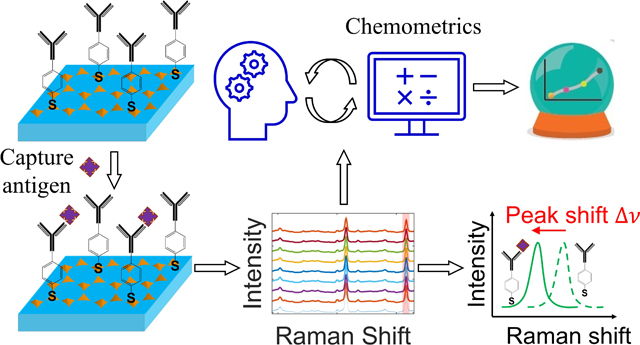
Leveraging nanomechanical perturbations in Raman spectro-immunoassays, we have designed a versatile serum biomarker detection platform. For macromolecules, nanomechanical perturbations can induce explicitly observable SERS frequency shift; for small molecules, they are manifested as subtle, but reproducible, spectral variations that can be captured by chemometric analysis. Collectively, the newly reported detection platform allows detection of a wide range of biomarkers spanning the spectrum of molecular weights in various sample matrices.
1. Introduction
Immunoassays have become the backbone of medical diagnosis and bioanalytical chemistry over the past several decades owing to their wide spectrum of applicability and superior analytical performance.1–4 Underpinning immunoassays is the formation of immune complex through paratope-epitope binding between a labelled antibody and an antigen. By measuring the transduced signal that linearly correlates with the labelled complex, immunoassays provide a powerful approach for identification and quantification of various analytes, including proteins, peptides, drugs, hormones, and pathogens, in a wide range of biological matrices, such as blood (serum or plasma), urine, saliva, cell lysates, tissue, and culture media.5–13 Among them, enzyme-linked immunosorbent assay and chemiluminescence immunoassays stand out because of their high sensitivity and specificity, wide detection range, good safety, and long service life.14–16 Yet, repeated incubation and washing steps, signal fluctuations of intensity-based transducing mechanism, and the prevailing sandwich format that requires two types of antibodies, have imposed accumulative challenges to the current design of immunoassays.17–18
Recently, advances in plasmon-enhanced spectroscopical methods have galvanized efforts to develop plasmon-enhanced spectro-immunoassays, as exemplified by the surface-enhanced Raman scattering (SERS) immunoassays.19–22 SERS capitalizes on the intense local electromagnetic field (EM) to amplify the intrinsically weak Raman scattering.23 As the SERS enhancement correlates with the fourth power of the local EM field enhancement, the performance of SERS-based immunoassays hinges on the structured EM field strength for a given plasmonic substrate. Indeed, such an exquisite correlation has allowed SERS-based immunoassays to be made highly sensitive by rationally engineering plasmonic nanostructures to maximize local EM fields.24–26 Nevertheless, the single peak intensity-based transducers exhibit intrinsic signal fluctuations owing to the SERS uncertainty principle, as the adsorption, desorption, diffusion, and reorientation of Raman reporters, and background scattering from biological matrices, can all affect the interplay between molecules and the plasmonic substrate.27–30 These unmet challenges highlight the need for engineering innovative design of SERS biosensing mechanisms to accommodate the shifting landscape.
While intensity-based optical transducers are prone to signal interference, the discovery of SERS frequency shift of antibody-conjugated Raman reporters after binding with analytes provides an innovative signal transducing mechanism that can be potentially used to overcome this problem.31 As previously demonstrated,31 the SERS frequency shift results from mechanical perturbations to the vibrational modes of Raman reporters after analytes capturing. Even though this mechanism depends on a characteristic SERS peak that is amenable to deformation-induced frequency shift, it is independent of the peak intensity, and thus unaffected by intensity fluctuations. Such a distinct frequency shift-based transducer relies only on a single antibody-antigen pair and has been harnessed for detection of proteins,32–34 circulating tumor DNA,35 and serum microRNA.34, 36 We have recently leveraged the SERS frequency shift combined with the intensity change to create a dual-modal spectro-immunoassy for detecting small molecules.37 Notably, it relied on a two-step reaction with its sensing performance augmented by the synergistic plasmonic coupling between gold star nanoprobes and the underlying plasmonic substrate.
To significantly simplify the immunoassay design and broaden its applicability, we hypothesize that mechanical perturbations to molecular vibrations, and thus the Raman vibrational modes, render additional subtle global spectral changes, which could complement the explicitly observable frequency shift for a given peak as an additional transducing mechanism. These subtle and often visually indistinguishable spectral variations could encode rich information related to the captured analytes and can be unveiled using chemometric analysis. Deciphering these spectral variations and establishing their correlation with the analyte concentrations can further empower and extend the SERS approach while fundamentally simplifying the immunoassay design and lowering costs for detection of a wider range of analytes spanning the spectrum of molecular weights.
To that aim, in this study, we have developed a plasmonic Raman immunoassay with single-antibody, multivariate regression, and shift-of-peak strategies, which we coin as the PRISM assay, for detection of biomarkers, as schematically shown in Fig. 1. The PRISM assay was built on a gold nanopyramid array-based plasmonic substrate. The substrate was first functionalized with a Raman reporter, 4-mercaptobenzoic acid (MBA). The MBA-functionalized substrate can produce a SERS spectrum amenable to deformation-induced spectral changes. Capture antibody was subsequently functionalized via the carboxylic group of MBA using the carbodiimide crosslinker chemistry. By pipetting samples onto the substrate and incubating them at 37°C for 20 min, analytes would be captured through immunoreaction with the MBA-conjugated antibody, instead of relying on a second antibody that is a critical component of the prevailing sandwich immunoassays. As the captured analytes can impose a nanomechanical deformation on the antibody-conjugated MBA molecules, and the extent to which the MBA molecules would be deformed is influenced by the number and molecular weight of captured analytes, we thus took advantage of such unique nanomechanical perturbations to establish a correlation between the deformation-induced spectral changes and the analyte concentration. We envision that for macromolecules, they can impose a robust nanomechanical deformation to MBA molecules and induce a visually discernable SERS frequency shift. For small molecules, we reason, although they can also nanomechanically perturb the vibrational modes of MBA molecules, they cannot induce a consistently detectable SERS frequency shift, as observed based on our experimental measurements. Nevertheless, those vibrational perturbations would be manifested as subtle, but reproducible, spectral variations that can be captured by chemometric analysis.
Figure 1. Principle of the PRISM assay.
(a) Functionalization of the gold nanopyramid array plasmonic substrate and subsequent analyte detection. (b) Schematic symbols (left) and antigen capturing process. (c) The captured antigens in (b) transduce a spectral signal that can be analyzed by the shift of the peak from the Raman reporters MBA (left) and/or a chemometric method (right).
2. Results and discussion
2. 1. Chemometric analysis
In this study, we aim to generalize the single-antibody approach and extend it for detection of biomarkers irrespective of the molecular weight or sample matrix by analyzing the spectral variations through a combination of chemometric analysis and plasmon-enhanced Raman spectroscopy. Specifically, the chemometric method involved a three-step process (Fig. 1c, right), including feeding input spectra, outlier rejection by robust principal component analysis (ROB-PCA), and partial least square (PLS) analysis in conjunction with significant multivariate correlation (sMC) algorithm for wavenumber reduction (Fig. S1). Following variable reduction by the sMC method, two types of PLS analysis were performed for predication of the analyte concentration (Fig. S2). The first type is the leave-one-spectrum-out PLS analysis, where one spectrum was used for testing and the rest for training the regression model. The second type is the training and testing split of 60:40 PLS analysis, in which 40% of spectra for a given concentration was used for testing and the remaining 60% for training the model. Given the more stringent conditions imposed on the training and testing split PLS analysis, we focused on this approach in the main text while including the leave-one-spectrum-out PLS analysis in the supporting information. Ultimately, the performance of chemometric analysis hinges on the correlation between the predicted analyte concentration and the true value.
2. 2. Fabrication and characterization of the plasmonic substrate
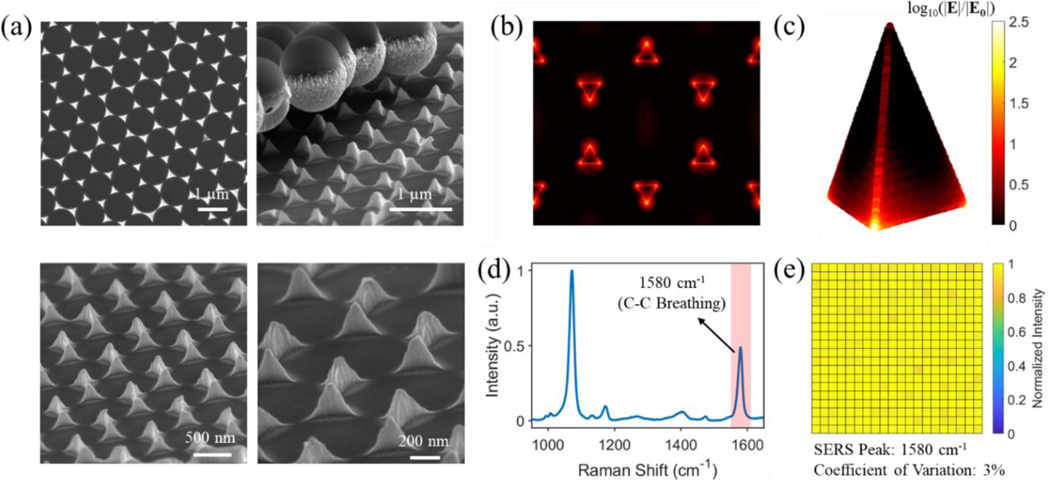
The plasmonic substrate used is the gold nanopyramid array. As established in our previous study, it can be readily fabricated using nanosphere lithography (Fig. S3) while possessing superior SERS performance.37 Representative SEM images and reflection spectrum of the fabricated gold nanopyramid arrays were respectively shown in Fig. 2a and Fig. S4. The pyramidal geometry featured sharp edges and vertices that are desired for SERS enhancement. Finite-difference time-domain (FDTD) simulations confirmed these “edge and vertex hotspots” with intense electric field enhancement (Fig. 2b–c). The homogeneity of SERS signals on the MBA-functionalized substrate was corroborated by Raman mapping over an area of 0.5 mm × 0.5 mm with a total of 21 × 21 spectra collected. The reconstructed intensity heat map using the 1580 cm−1 peak (Fig. 2d) showed a marginal coefficient of variation of about 3%. It is also noted that the 1580 cm−1 peak originates from the C-C breathing mode. As this peak is amenable to nanomechanical deformation-induced SERS frequency shift, it was exploited as an explicit spectral feature for thyroid stimulating hormone (TSH) detection in buffer solution.
Figure 2. Characterizations of the fabricated gold nanopyramid array.
(a) SEM images, (b) and (c) FDTD-calculated electric field enhancement of the gold nanopyramid array with vertical polarization. The electric field enhancement with horizontal polarization is shown in Fig. S5. (d) Measured SERS spectra of MBA functionalized onto the substrate. (e) Raman mapping over an area of 0.5 mm × 0.5 mm with a pixel size about 24 μm and a total of 21 × 21 spectra using the 1580 cm−1 peak in (d) on the substrate.
2. 3. Detection of TSH in buffer and goat serum
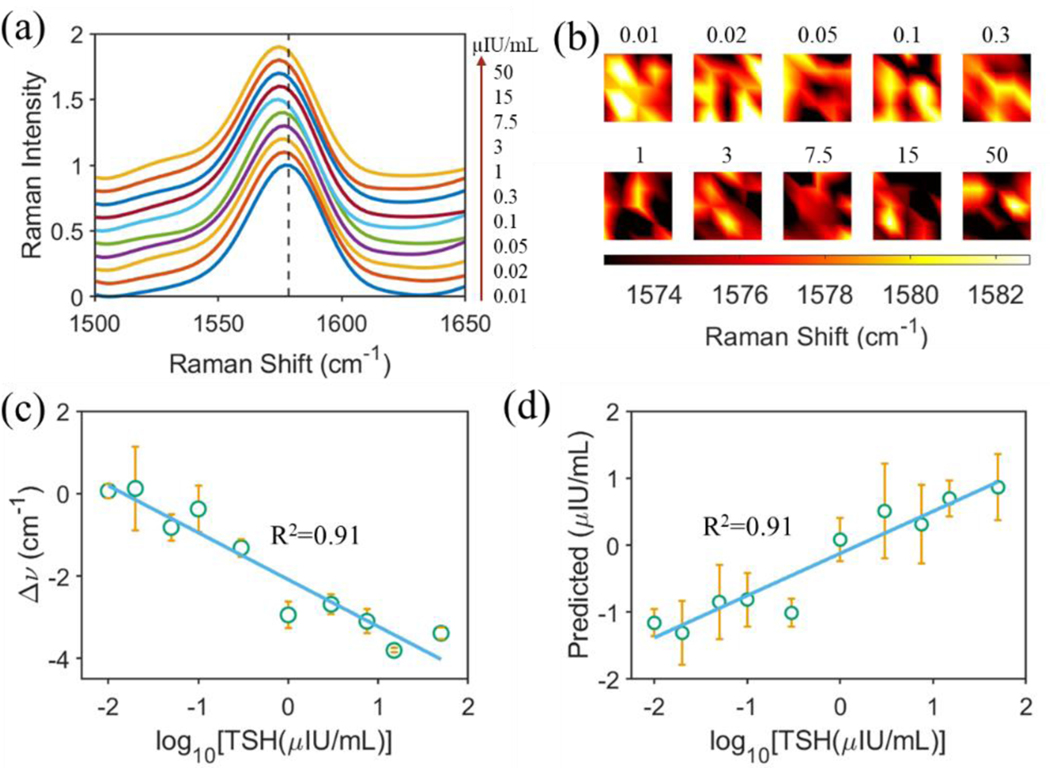
Using TSH as a model macromolecule, which has a molecular weight of 28 – 30 kDa, we first provided a proof-of-concept study of the PRISM assay. Following the protocol in Fig. 1a, TSH calibrator in buffer solution (provided by Beckman Coulter Inc.) was pipetted onto the gold nanopyramid array substrate functionalized with TSH antibody-MBA conjugates. After incubation and subsequent removal of nonspecifically adsorbed TSH antigens and protein aggregates using Access Wash Buffer, the substrate was subjected to Raman mapping over a 20 μm × 20 μm area with 5 × 5 spectra collected under a 785 nm laser excitation. The process was repeated to account for all the TSH calibrators from the lowest to the highest concentration. The averaged SERS spectra were displayed in Fig. 3a and were offset for clarity. The SERS peak at around 1580 cm−1 exhibited a gradual frequency shift to the small wavenumber side with an increase of the TSH concentration. The TSH concentration-dependent frequency shift was also observed in the heat map that was reconstructed using the SERS peak wavenumber of the collected 5 × 5 spectra (Fig. 3b). It was revealed that the relative SERS frequency shift for the 1580 cm−1 peak strongly correlated with the logarithmic concentration of TSH with a R2 of 0.91, as shown in Fig. 3c. The relative SERS frequency shift was defined as , where is the peak wavenumber for TSH calibrator with a concentration of and is that for calibrator matrix with a zero TSH concentration.
Figure 3. Detection of TSH calibrators.
(a) Measured SERS spectra using the PRISM assay with an increase of TSH concentration as specified in the figure. Each spectrum was averaged from 5 × 5 spectra collected over 20 μm × 20 μm area using Raman mapping. (b) Mapping of the SERS peak wavenumber at 1580 cm−1 with various TSH concentrations as specified. (c) Regression analysis based on the shift of the SERS peak about 1580 cm−1. (d) Correlation between predicted and true TSH concentrations in log10 scale.
With the SERS peak frequency shift as one of spectral manifestations of MBA molecules under the TSH antigen-induced structural nanomechanical deformation, we also turned to chemometric analysis to uncover both these explicitly observable and those hidden spectral changes to create an analytical model for prediction of TSH concentrations. We started by differentiating and removing spectra outliers using the ROB-PCA method. This was followed by variable reduction using the sMC algorithm. With these rationally processed datasets, we employed the training and testing split of 60:40 PLS analysis to predict the TSH concentration. The strong correlation with a R2 of 0.91 between the predicted and the true TSH concentrations was not only consistent with the SERS frequency shift-based analysis, but also validated the chemometric method we have developed in conjunction with plasmon-enhanced Raman spectroscopy. Additionally, the leave-one-spectrum-out PLS analysis for TSH prediction was shown in Fig. S6, which gave a similarly strong correlation between the predicted and true concentrations.
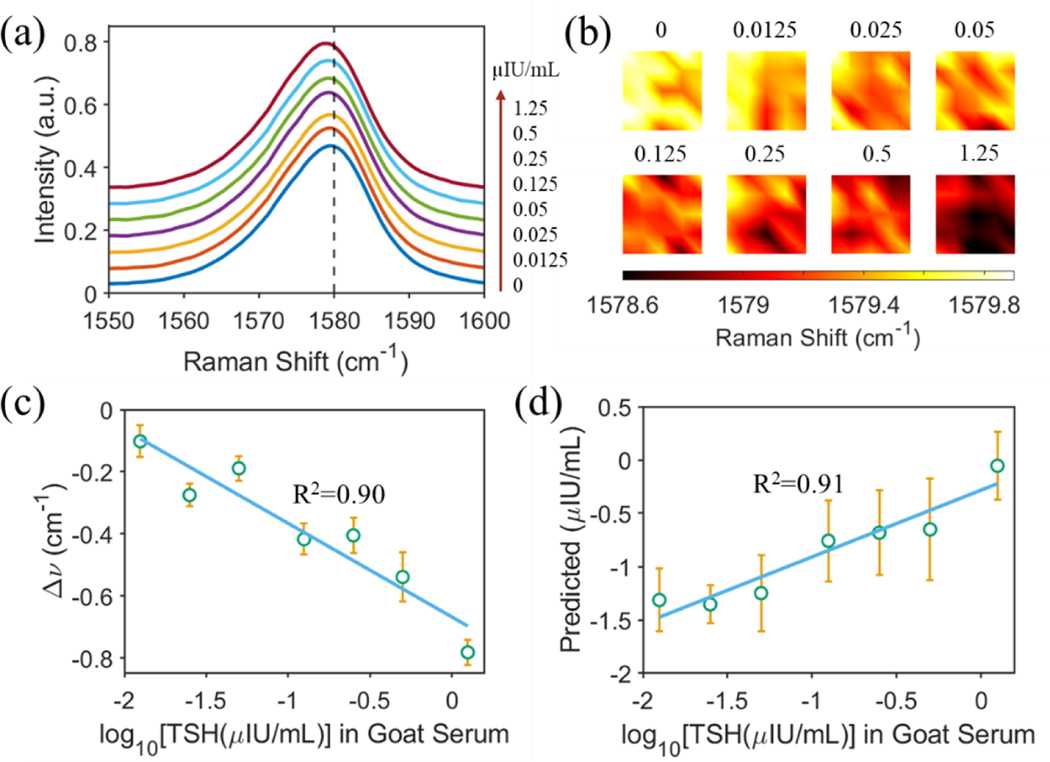
Success in the proof-of-concept demonstration of the PRISM assay for TSH detection in buffer solution allowed us to extend the approach for detecting TSH in goat serum. Given the complexity and potential interference of the serum matrix on the SERS spectra, the goat serum matrix was diluted using the TSH calibrator matrix with a ratio of 1:3. A robust SERS frequency shift was observed (Fig. 4a–b) in response to the increasing TSH concentration in the diluted goat serum matrix, which is similar to the case of detecting TSH in buffer solution (Fig. 3a). Regression analysis confirmed the strong correlation between the relative SERS frequency shift and the logarithmic concentration of TSH with a R2 of 0.90 (Fig. 4c). Implementation of the training and testing split of 60:40 PLS analysis returned an excellent prediction of the TSH concentration with a R2 of 0.91 (Fig. 4d). The leave-one-spectrum-out PLS analysis was shown in Fig. S7, displaying a similarly strong prediction power.
Figure 4. Detection of TSH in diluted goat serum.
(a) Measured SERS spectra using the PRISM assay with an increase of TSH concentration as specified in the figure. Each spectrum was averaged from 5 × 5 spectra collected over a 20 μm × 20 μm area using Raman mapping with a 785 nm excitation laser wavelength. (b) Mapping of the SERS peak wavenumber at 1580 cm−1 with various TSH concentrations as specified. (c) Regression analysis based on the shift of the SERS peak about 1580 cm−1. (d) Correlation between the predicted and the true TSH concentrations in log10 scale. The goat serum matrix was diluted using the TSH calibrator matrix with a ratio of 1:3.
2. 4. Detection of small molecules
With the chemometric method satisfactorily tackling the serum matrix, the PRISM assay was further expanded for detection of small molecules in serum matrix. Free thyroxine (T4) and testosterone with a molecular weight of 777 Daltons and 228 Daltons were selected as a pair of model small molecules. While free T4, along with TSH, is an important biomarker for diagnosis of thyroid diseases and monitoring of therapeutical effects,38 a low level of testosterone has been associated with variations in non-cardiovascular biomarkers in above middle-aged men39 and even mortality based on most observational studies40. Owing to their small molecular weights, no appreciated SERS frequency shifts were observed after they were captured onto the antibody-MBA conjugates-functionalized gold nanopyramid array substrate (Fig. 5a, c). Nevertheless, these hidden spectral changes resulting from nanomechanical perturbations to the vibrational modes of MBA molecules still allowed a strong correlation to be established based on the training and testing split of 60:40 PLS analysis between the predicted and their true concentrations (Fig. 5b, d). The leave-one-spectrum-out PLS analysis for both small molecules were shown in Fig. S8, where a similarly strong correlation was established.
Figure 5. Detection of small molecules free T4 (upper panel) and testosterone (lower panel).
Measured SERS spectra using the PRISM assay for (a) free T4 and (c) testosterone. Each spectrum was averaged from 5 × 5 spectra collected over 20 μm × 20 μm area using Raman mapping. (d) Correlation between predicted and true concentrations in log10 scale for (b) free T4 and (d) testosterone.
Successful demonstration of the PRISM assay for detecting various biomolecules in different sample matrices validates the methods of leveraging nanomechanical perturbations in Raman spectroscopy to design versatile biomarker detection platform. Furthermore, in light of recent advances on point-of-care devices,41–44 and given the two-dimensional nature of the plasmonic substrates used, the PRISM assay holds great promise to be seamlessly integrated into either paper- or plastic-based lateral flow devices for field-deployable applications.
3. Conclusions
In conclusion, we have developed a general single-antibody plasmonic immunoassay for detection of serum biomarkers. By capitalizing on the plasmon-enhanced Raman spectroscopy and chemometric method, the PRISM assay displayed strong capabilities towards detecting both macromolecules and small molecules in complicated biological matrices. Underpinning the principle of the PRISM assay is the nanomechanical perturbation of captured analytes to the vibrational modes of the antibody-conjugated Raman reporters. These nanomechanical perturbations were manifested as explicitly observable SERS frequency shifts for macromolecules in buffer solution or diluted serum matrix. While no appreciated SERS frequency shifts was observed for small molecules, the PRISM assay was able to capture the hidden spectral changes based on chemometric analysis and allowed an accurate predication of the analyte concentration. This PRISM assay, relying on the single-antibody approach, is expected to hold great promise to simplify the current design of immunoassays, lower the costs, augment the performance by fusing plasmon-enhanced Raman spectroscopy and chemometrics. Success in detecting TSH in goat serum, and free T4 and testosterone in serum matrix, demonstrates the interference tolerance and potential of the PRISM assay for analyzing real samples. We envision the PRISM assay can find a wide spectrum of applications in rapid screening of infectious diseases, detection of protein-, DNA-, and microRNA-based biomarkers, and other bioanalytical domains, such as pharmaceutical analysis.
Supplementary Material
Acknowledgement
This research was supported by National Institute of General Medical Sciences (DP2GM128198), National Institute of Biomedical Imaging and Bioengineering (2-P41-EB015871-31), and by Beckman Coulter Inc.
Footnotes
Associated Contents
Declaration of competing interests
The authors declare the following competing financial interest(s): I.B, P.Z., and T.M. are inventors on the U.S. Patent Application No. 63/132,248 filed by Beckman Coulter, Inc., and Johns Hopkins University.
Reference
- 1.Li Y-F; Sun Y-M; Beier RC; Lei H-T; Gee S; Hammock BD; Wang H; Wang Z; Sun X; Shen Y-D; Yang J-Y; Xu Z-L, Immunochemical techniques for multianalyte analysis of chemical residues in food and the environment: A review. TrAC Trends in Analytical Chemistry 2017, 88, 25–40. [Google Scholar]
- 2.Dhillon RS; Kelly JD; Srikrishna D; Garry RF, Overlooking the importance of immunoassays. The Lancet Infectious Diseases 2016, 16 (10), 1109–1110. [DOI] [PubMed] [Google Scholar]
- 3.Theel ES; Carpenter AB; Binnicker MJ, Immunoassays for Diagnosis of Infectious Diseases. Manual of Clinical Microbiology 2015, 91–105. [Google Scholar]
- 4.Darwish IA, Immunoassay Methods and their Applications in Pharmaceutical Analysis: Basic Methodology and Recent Advances. Int J Biomed Sci 2006, 2 (3), 217–235. [PMC free article] [PubMed] [Google Scholar]
- 5.Ju H, Immunosensing for Detection of Protein Biomarkers. Elsevier.: 2017. [Google Scholar]
- 6.Zhou S; Lu X; Chen C; Sun D, An immunoassay method for quantitative detection of proteins using single antibodies. Analytical Biochemistry 2010, 400 (2), 213–218. [DOI] [PubMed] [Google Scholar]
- 7.Li Q; Bencherif SA; Su M, Edge-Enhanced Microwell Immunoassay for Highly Sensitive Protein Detection. Analytical Chemistry 2021, 93 (29), 10292–10300. [DOI] [PubMed] [Google Scholar]
- 8.Brett GM; Chambers SJ; Huang L; Morgan MRA, Design and development of immunoassays for detection of proteins. Food Control 1999, 10 (6), 401–406. [Google Scholar]
- 9.Lim S-L; Ichinose H; Shinoda T; Ueda H, Noncompetitive Detection of Low Molecular Weight Peptides by Open Sandwich Immunoassay. Analytical Chemistry 2007, 79 (16), 6193–6200. [DOI] [PubMed] [Google Scholar]
- 10.Zhao X; Kottegoda S; Shippy SA, Solid-phase immunoassay detection of peptides from complex matrices without a separation. Analyst 2003, 128 (4), 357–362. [DOI] [PubMed] [Google Scholar]
- 11.Schütz H; Paine A; Erdmann F; Weiler G; Verhoff MA, Immunoassays for drug screening in urine. Forensic Science, Medicine, and Pathology 2006, 2 (2), 75–83. [DOI] [PubMed] [Google Scholar]
- 12.Moore C; Crouch D, Oral fluid for the detection of drugs of abuse using immunoassay and LC–MS/MS. Bioanalysis 2013, 5 (12), 1555–1569. [DOI] [PubMed] [Google Scholar]
- 13.Yang L; Deng W; Cheng C; Tan Y; Xie Q; Yao S, Fluorescent Immunoassay for the Detection of Pathogenic Bacteria at the Single-Cell Level Using Carbon Dots-Encapsulated Breakable Organosilica Nanocapsule as Labels. ACS Applied Materials & Interfaces 2018, 10 (4), 3441–3448. [DOI] [PubMed] [Google Scholar]
- 14.Butler JE, Enzyme-linked immunosorbent assay. Journal of immunoassay 2000, 21 (2–3), 165–209. [DOI] [PubMed] [Google Scholar]
- 15.Rissin DM; Kan CW; Campbell TG; Howes SC; Fournier DR; Song L; Piech T; Patel PP; Chang L; Rivnak AJ, Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nature biotechnology 2010, 28 (6), 595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao L; Sun L; Chu X, Chemiluminescence immunoassay. TrAC Trends in Analytical Chemistry 2009, 28 (4), 404–415. [Google Scholar]
- 17.Hoofnagle AN; Wener MH, The fundamental flaws of immunoassays and potential solutions using tandem mass spectrometry. Journal of Immunological Methods 2009, 347 (1), 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyd JM; Sadrzadeh SMH, Chapter 14 - Limitations of immunoassays for screening of drugs of abuse in urine: issues of false positive and false negative results. In Accurate Results in the Clinical Laboratory (Second Edition), Dasgupta A; Sepulveda JL, Eds. Elsevier: 2019; pp 233–242. [Google Scholar]
- 19.Li M; Kang JW; Sukumar S; Dasari RR; Barman I, Multiplexed detection of serological cancer markers with plasmon-enhanced Raman spectro-immunoassay. Chemical Science 2015, 6 (7), 3906–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu X; Ren W; Hu C; Liu C; Li Z, Plasmon-Enhanced Surface-Enhanced Raman Scattering Mapping Concentrated on a Single Bead for Ultrasensitive and Multiplexed Immunoassay. Analytical Chemistry 2020, 92 (18), 12387–12393. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z; Zong S; Wu L; Zhu D; Cui Y, SERS-Activated Platforms for Immunoassay: Probes, Encoding Methods, and Applications. Chemical Reviews 2017, 117 (12), 7910–7963. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira MJ; Cunha I; de Almeida MP; Calmeiro T; Fortunato E; Martins R; Pereira L; Byrne HJ; Pereira E; Águas H; Franco R, Reusable and highly sensitive SERS immunoassay utilizing gold nanostars and a cellulose hydrogel-based platform. Journal of Materials Chemistry B 2021, 9 (36), 7516–7529. [DOI] [PubMed] [Google Scholar]
- 23.Ding S-Y; You E-M; Tian Z-Q; Moskovits M, Electromagnetic theories of surface-enhanced Raman spectroscopy. Chemical Society Reviews 2017, 46 (13), 4042–4076. [DOI] [PubMed] [Google Scholar]
- 24.Choi H-K; Lee KS; Shin H-H; Koo J-J; Yeon GJ; Kim ZH, Single-Molecule Surface-Enhanced Raman Scattering as a Probe of Single-Molecule Surface Reactions: Promises and Current Challenges. Accounts of Chemical Research 2019, 52 (11), 3008–3017. [DOI] [PubMed] [Google Scholar]
- 25.Le Ru EC; Etchegoin PG, Single-Molecule Surface-Enhanced Raman Spectroscopy. Annual Review of Physical Chemistry 2012, 63 (1), 65–87. [DOI] [PubMed] [Google Scholar]
- 26.Mao P; Liu C; Favraud G; Chen Q; Han M; Fratalocchi A; Zhang S, Broadband single molecule SERS detection designed by warped optical spaces. Nature Communications 2018, 9 (1), 5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindquist NC; de Albuquerque CDL; Sobral-Filho RG; Paci I; Brolo AG, High-speed imaging of surface-enhanced Raman scattering fluctuations from individual nanoparticles. Nature Nanotechnology 2019, 14 (10), 981–987. [DOI] [PubMed] [Google Scholar]
- 28.Almehmadi LM; Curley SM; Tokranova NA; Tenenbaum SA; Lednev IK, Surface Enhanced Raman Spectroscopy for Single Molecule Protein Detection. Scientific Reports 2019, 9 (1), 12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Natan MJ, Concluding Remarks Surface enhanced Raman scattering. Faraday Discussions 2006, 132 (0), 321–328. [DOI] [PubMed] [Google Scholar]
- 30.Zong C; Xu M; Xu L-J; Wei T; Ma X; Zheng X-S; Hu R; Ren B, Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chemical Reviews 2018, 118 (10), 4946–4980. [DOI] [PubMed] [Google Scholar]
- 31.Kho KW; Dinish US; Kumar A; Olivo M, Frequency Shifts in SERS for Biosensing. ACS Nano 2012, 6 (6), 4892–4902. [DOI] [PubMed] [Google Scholar]
- 32.Guerrini L; Pazos E; Penas C; Vázquez ME; Mascareñas JL; Alvarez-Puebla RA, Highly Sensitive SERS Quantification of the Oncogenic Protein c-Jun in Cellular Extracts. Journal of the American Chemical Society 2013, 135 (28), 10314–10317. [DOI] [PubMed] [Google Scholar]
- 33.Ma H; Liu S; Zheng N; Liu Y; Han XX; He C; Lu H; Zhao B, Frequency Shifts in Surface-Enhanced Raman Spectroscopy-Based Immunoassays: Mechanistic Insights and Application in Protein Carbonylation Detection. Analytical Chemistry 2019, 91 (15), 9376–9381. [DOI] [PubMed] [Google Scholar]
- 34.Cheng L; Zhang Z; Zuo D; Zhu W; Zhang J; Zeng Q; Yang D; Li M; Zhao Y, Ultrasensitive Detection of Serum MicroRNA Using Branched DNA-Based SERS Platform Combining Simultaneous Detection of α-Fetoprotein for Early Diagnosis of Liver Cancer. ACS Applied Materials & Interfaces 2018, 10 (41), 34869–34877. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J; Dong Y; Zhu W; Xie D; Zhao Y; Yang D; Li M, Ultrasensitive Detection of Circulating Tumor DNA of Lung Cancer via an Enzymatically Amplified SERS-Based Frequency Shift Assay. ACS Applied Materials & Interfaces 2019, 11 (20), 18145–18152. [DOI] [PubMed] [Google Scholar]
- 36.Zhu W-F; Cheng L-X; Li M; Zuo D; Zhang N; Zhuang H-J; Xie D; Zeng Q-D; Hutchison JA; Zhao Y-L, Frequency Shift Raman-Based Sensing of Serum MicroRNAs for Early Diagnosis and Discrimination of Primary Liver Cancers. Analytical Chemistry 2018, 90 (17), 10144–10151. [DOI] [PubMed] [Google Scholar]
- 37.Zheng P; Wu L; Raj P; Mizutani T; Szabo M; Hanson WA; Barman I, A Dual-Modal Single-Antibody Plasmonic Spectro-Immunoassay for Detection of Small Molecules. Small 2022, 18 (18), 2200090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y; He D-H; Jiang S-N; Wang H-L; Xu X-H; Kong L-R, Biological variation of thyroid function biomarkers over 24 hours. Clinica Chimica Acta 2021, 523, 519–524. [DOI] [PubMed] [Google Scholar]
- 39.Samoszuk M; Morgentaler A; de Groot M; van Solinge W; Li Y; Adair F; Hoefer I; Haitjema S, Association of low testosterone with changes in non-cardiovascular biomarkers in adult men. International Journal of Impotence Research 2020, 32 (2), 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shores MM; Matsumoto AM, Testosterone, aging and survival: biomarker or deficiency. Current Opinion in Endocrinology, Diabetes and Obesity 2014, 21 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sim D; Brothers MC; Slocik JM; Islam AE; Maruyama B; Grigsby CC; Naik RR; Kim SS, Biomarkers and Detection Platforms for Human Health and Performance Monitoring: A Review. Advanced Science 2022, 9 (7), 2104426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin J; Wang W; Gao L; Yao SQ, Emerging biosensing and transducing techniques for potential applications in point-of-care diagnostics. Chemical Science 2022, 13 (10), 2857–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin C; Wu Z; Molinski JH; Zhou J; Ren Y; Zhang JXJ, Plasmonic nanosensors for point-of-care biomarker detection. Materials Today Bio 2022, 14, 100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mejía-Salazar JR; Oliveira ON, Plasmonic Biosensing. Chemical Reviews 2018, 118 (20), 10617–10625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.