Abstract
Introduction
Patients with ankylosing spondylitis (AS) have significant unmet treatment needs, despite advancements in biologic therapies. This study evaluated the impact of upadacitinib on clinically meaningful improvement in patient-reported outcomes (PROs) assessing disease activity, pain, fatigue, function, health-related quality of life (HRQoL), and work productivity in patients with AS with inadequate responses or intolerance to biologic disease-modifying antirheumatic drugs (bDMARD-IR).
Methods
Patients enrolled in the phase 3 SELECT-AXIS 2 AS bDMARD-IR study received blinded once-daily oral upadacitinib 15 mg or placebo for 14 weeks. The percentage of patients achieving improvements ≥ minimum clinically important differences (MCID) at week 14 were compared between treatment groups for disease activity (Bath Ankylosing Spondylitis Disease Activity Index, BASDAI), patient global assessment of disease activity (PtGA), total and nocturnal back pain, fatigue (Functional Assessment of Chronic Illness Therapy-Fatigue, FACIT-F), physical function (Bath Ankylosing Spondylitis Functional Index, BASFI), HRQoL (Assessment of SpondyloArthritis international Society Health Index [ASAS HI], Ankylosing Spondylitis Quality of Life [ASQoL], Short form-36 [SF-36] physical [PCS] and mental [MCS] component summary scores), and work productivity (Work Productivity and Activity Impairment [WPAI] Questionnaire). Mean changes from baseline through week 14 in fatigue and HRQoL were compared between treatment groups.
Results
A total of 420 patients with active AS who were bDMARD-IR were included. A higher proportion of patients reported MCIDs at week 14 across all PROs with upadacitinib compared with placebo (nominal p ≤ 0.05). Greater improvements in mean change from baseline through week 14 were reported with upadacitinib compared with placebo across FACIT-F, HRQoL, and WPAI, with improvements differentiated as early as week 1 for ASAS HI, ASQoL and SF-36 PCS and week 4 for SF-36 MCS.
Conclusions
Upadacitinib 15 mg demonstrated rapid and clinically meaningful improvements in disease activity, pain, FACIT-F, function, HRQoL, and WPAI among bDMARD-IR patients with active AS.
Trial Registry
Clinical Registration number: NCT04169373, SELECT-AXIS 2.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-023-00536-2.
Keywords: Ankylosing spondylitis, Axial spondyloarthritis, Patient-reported outcomes, Health-related quality of life, Upadacitinib
Key Summary Points
| Why carry out this study? |
| In addition to reduced spinal mobility, impaired function, and presence of inflammation, patients with ankylosing spondylitis (AS) suffer from chronic back pain, fatigue, and reduced health-related quality of life (HRQoL). |
| Despite recent advancements in biologic therapies, there remains a significant, unmet need for the treatment of AS, highlighted by the high proportion of patients who do not achieve an adequate response to biologic disease-modifying antirheumatic drugs (bDMARDs). |
| This study evaluated the effect of upadacitinib versus placebo on response rates and clinically meaningful improvements in patient-reported outcomes (PROs) in patients with AS in SELECT-AXIS 2. |
| What has been learned from the study? |
| A higher proportion of patients reported clinically meaningful improvements at week 14 across PROs with upadacitinib 15 mg compared with placebo: BASDAI (73.9 vs. 47.8%), PtGA (82.9 vs. 63.2%), total back pain (80.1 vs. 65.1%), nocturnal back pain (82.9 vs. 65.1%), FACIT-F (57.7 vs. 43.0%), BASFI (74.4 vs. 53.6%), ASAS HI (46.4 vs. 23.0%), ASQoL (60.3 vs. 35.6%), SF-36 PCS (70.9 vs. 54.1%), and WPAI activity impairment (58.5 vs. 31.0%), nominal p ≤ 0.001. |
| Improvements in mean change in fatigue, disease-specific and general HRQoL patient-reported measures from baseline were seen as early as week 1 or 2 and continued through week 14 in bDMARD-IR patients with active AS who received upadacitinib 15 mg compared with placebo (ASQoL, ASAS HI and SF-36 PCS and MCS; p < 0.05). |
Introduction
Axial spondyloarthritis (axSpA) is a chronic inflammatory disease that primarily involves the spine and sacroiliac joints [1]. Patients with axSpA can be differentiated by radiographic findings as having either non-radiographic axial spondyloarthritis (nr-axSpA), or ankylosing spondylitis (AS), also known as radiographic axial spondyloarthritis [2, 3].
Patients with AS experience inflammatory back pain and other symptoms leading to impaired function and reduced spinal mobility, contributing to a high burden of disease as demonstrated by diminished health-related quality of life (HRQoL) [4]. Furthermore, fatigue and impaired function, experienced by patients with AS have been associated with a detrimental impact on work productivity [4–6]. A high disease burden as demonstrated by poor patient-reported outcome (PROs) scores at the start of treatment may be a predictor of poor treatment response and retention of first tumor necrosis factor (TNF) inhibitor treatment [7].
Treatment goals are to improve patient’s HRQoL, function, and social involvement by controlling inflammation and other disease symptoms [8–10]. The American College of Rheumatology (ACR), the Spondylitis Association of America (SAA), the Spondyloarthritis Research and Treatment Network (SPARTAN) recommend non-steroidal, anti-inflammatory drugs (NSAIDs) as first-line treatment for AS, followed by biologic disease-modifying antirheumatic drugs (bDMARDs) such as TNF inhibitors (the first bDMARDs available for AS) [9]. Although the current practice is to start a TNF inhibitor or IL-17 inhibitor, the updated Assessment of SpondyloArthritis International Society (ASAS), and European League Against Rheumatism (EULAR) treatment recommendations have expanded this to also now recommend that a Janus kinase (JAK) inhibitor should be considered in patients with persistently high disease activity despite conventional therapy [8, 10]. Additionally, the updated guidance recommends switching to another biologic DMARD (TNF inhibitor or IL-17 inhibitor) or JAK inhibitor if the first biologic DMARD fails [10].
Despite recent advancements in treatments, there remains an unmet need in the treatment of AS with current therapies [11, 12]. Only 40–50% of patients achieve ASAS 40 response across different biologic studies in AS [13, 14]. In addition, approximately a third of patients do not achieve Ankylosing Spondylitis Disease Activity Score (ASDAS) low disease activity, and 65% do not achieve ASDAS inactive disease after 1 year of bDMARD therapy. In addition, there are a lack of oral agents to treat AS beyond NSAIDs, as all current biologics are injectable or infused.
Inhibition of JAK-mediated pathways may be a promising approach for the treatment of patients with AS [15, 16]. Upadacitinib, an oral and reversible JAK inhibitor, was efficacious and well tolerated in a phase 2/3 study (SELECT-AXIS 1) in patients with active AS who had an inadequate response (IR) or contraindication to NSAIDs compared with placebo. In SELECT-AXIS 2, upadacitinib has shown efficacy in patients with previous exposure to bDMARDs, including TNF inhibitors, and for the first time, also in patients with an IR to IL-17 inhibitors [17]. This analysis of the SELECT-AXIS 2 trial expands on the previously reported efficacy of upadacitinib and evaluates clinically meaningful improvements in patient-reported disease activity, pain, fatigue, function, HRQoL, and work productivity in patients with AS who had an IR to bDMARD therapy (bDMARD-IR) treated with upadacitinib 15 mg versus placebo.
Methods
Study Design and Participants
Details of the full study design and primary results of the global, multicenter, phase 3 clinical trial, SELECT-AXIS 2 (NCT04169373) have previously been published [17]. Here we report detailed 14-week PRO results in patients with active AS who were bDMARD-IR. Briefly, this was a 14-week randomized, double-blind parallel-group, and placebo-controlled period followed by a 90-week open-label extension period. Patients were randomized 1:1 to receive upadacitinib 15 mg once daily (QD) (N = 211) or placebo QD (N = 209) for 14 weeks.
Eligible patients were ≥ 18 years of age with a clinical diagnosis of AS that fulfilled the modified New York criteria [18], with increased disease activity at study entry based on BASDAI and total back pain scores of 4 or more (numerical rating scale 0–10). Radiographic sacroiliitis (modified New York criteria) during the screening period was checked in central reading by two readers and an adjudicator if there were any discrepancies. Patients were bDMARD-IR, defined as prior exposure to one or two bDMARDs (TNF inhibitor and/or IL-17 inhibitor) with lack of efficacy or intolerance. Prior exposure to a second bDMARD was permitted for no more than 30% of patients; lack of efficacy to one bDMARD and intolerance to another was permitted, but patients could not have lack of efficacy to two bDMARDs [17].
This study was conducted in compliance with the protocol, International Conference on Harmonisation (ICH) guidelines, local regulations and guidelines governing clinical study conduct, and the ethical principles of the Declaration of Helsinki. As per Good Clinical Practice, the protocol and consent were approved by an ethics committee or institutional review board at all study sites. All patients provided informed consent before study participation.
Outcomes
This analysis assessed multiple PROs from baseline to 14 weeks across the key domains of disease activity, pain, fatigue, function, HRQoL, and work productivity to provide a patient perspective on the benefits of upadacitinib to complement clinical measurements.
Clinically meaningful improvements in PROs were assessed by reporting the proportion of patients with improvements ≥ minimal clinically important difference (MCID) at week 14. MCIDs were defined as ≥ 1.1-point decrease for BASDAI [19], ≥ 1-point decrease for Patient Global Assessment of Disease Activity (PtGA), total and nocturnal back pain, [20] ≥ 4-point increase for Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) [21], ≥ 0.6-point decrease for Bath Ankylosing Spondylitis Functional Index (BASFI) [19], and ≥ 3-point decrease for Assessment of SpondyloArthritis international Society Health Index (ASAS HI) [22], and Ankylosing Spondylitis Quality of Life (ASQoL) [23]. Short form-36 (SF-36), consisting of a Physical Component Summary (PCS) and a Mental Component Summary (MCS) score, was used to assess general HRQoL (MCID for PCS and MCS: ≥ 2.5-point increase) [24]. Work Productivity and Activity Impairment Questionnaire (WPAI) included the domains of absenteeism (work missed; MCID not available), presenteeism (impairment at work/reduced work effectiveness; MCID: ≥ 20-point decrease), overall work impairment (productivity loss; MCID: ≥ 15-point decrease), and activity impairment (MCID: ≥ 20-point decrease) [25]. The percentage of patients achieving MCID was not ranked, and was evaluated post hoc with nominal p values reported.
Mean change in PROs from baseline through 14 weeks were also assessed for fatigue (FACIT-F), HRQoL (ASAS HI, ASQoL, SF-36 MCS and PCS), and work productivity (WPAI). Mean change from baseline to 14 weeks for BASDAI, PtGA, total and nocturnal back pain, and BASFI have been published previously [17].
BASDAI, PtGA, total and nocturnal back pain, BASFI, ASAS HI, ASQoL, and SF-36 PCS and MCS were assessed at baseline and from week 1; FACIT-F was assessed at baseline and from week 2; WPAI was assessed at baseline and week 14. The WPAI domains of absenteeism, presenteeism, and overall work impairment were assessed in patients who were employed at baseline, while activity impairment was assessed in all patients.
Statistical Analyses
The full analysis set included all randomized patients who received at least one dose of the study drug and was used for all efficacy and baseline analyses. Patients were included in the analysis based on the treatment group as randomized.
Demographic and baseline characteristics were collected at the baseline visit of the study and summarized for the full analysis set by the treatment group. Categorical variables were summarized by the number and percentage of patients, and continuous variables were summarized with mean and standard deviation (SD).
MCID analyses were summarized at week 14, reporting the number and percentage of patients with improvements in PRO ≥ MCID. p values for the test of the difference between the proportion of patients achieving MCID were made between upadacitinib 15 mg and the placebo group constructed based on the multiple imputation inference. A non-responder imputation was applied incorporating multiple imputations (NRI-MI) to handle missing data due to COVID-19. Patients who prematurely discontinued the study drug were considered non-responders for all subsequent visits after discontinuation. In addition, any patients with missing values for the PRO assessed at a specific visit were treated as non-responders for that visit to calculate the MCID.
Least squares (LS) mean change from baseline to week 14 was assessed; 95% confidence intervals and nominal p values were based on mixed-effects repeated measures model (MMRM) analysis, including stratification factors of treatment, visit, and treatment-by-visit interaction as fixed factors and baseline value as a covariate. Stratification factor high-sensitivity C-reactive protein (hsCRP) level (≤ upper limit of normal [ULN] vs. > ULN) was also included in the model. All observed patient data are included in the MMRM. For WPAI domains, LS mean change, 95% CI, and nominal p value for each visit were based on analysis of covariance (ANCOVA), including treatment and main stratification factors MRI and screening hsCRP status as fixed factors and baseline value as covariate.
NNT analyses are reported, defined as the number of patients who need to be treated to achieve one additional “responder” on PRO of interest versus placebo. NNTs per MCID response were calculated for upadacitinib versus placebo and compared using the Cochran–Mantel–Haenszel test (CMH). NNTs for each PRO with an MCID were calculated as follows: 1/MCID response rate in the treatment group – MCID response rate in the placebo group.
MCID and NNT analyses were repeated in a post hoc subgroup analysis of patients with prior exposure ≥ 1 TNF inhibitor (TNFi-IR) at baseline to identify whether results are consistent compared with the total population (which included IL-17 IR).
Results
Study Population
A total of 420 bDMARD-IR patients with active AS were included in this analysis and each was randomly assigned to either placebo (N = 209) or upadacitinib 15 mg QD (N = 211). Several key demographic and baseline disease characteristics describing the population were previously reported: 74% were male, mean age of 42.4 years (SD 7.5), with a mean disease (symptom) duration of 12.8 years (SD 9.2) [17]. Additional demographics, disease characteristics, and PRO scores at baseline reported here were balanced across study arms. Baseline PRO scores indicate that these patients have a high burden of disease (Table 1).
Table 1.
Baseline disease characteristics and PROs
| Placebo (N = 209) | Upadacitinib 15 mg QD (N = 211) | |
|---|---|---|
| Male, n (%) | 158 (75.6) | 153 (72.5) |
| Age (years) | 42.2 (11.8) | 42.6 (12.4) |
| HLA-B27 positive, n (%) | 168 (81.2) | 180 (85.3) |
| Time (years) since AS diagnosis | 7.5 (7.5) | 7.9 (7.5) |
| Time (years) since AS symptoms | 12.6 (9.3) | 12.9 (9.1) |
| Baseline medication use | ||
| NSAID, n (%) | 163 (78.0) | 163 (77.3) |
| Oral corticosteroids, n (%) | 18 (8.6) | 27 (12.8) |
| csDMARDs, n (%) | 62 (29.7) | 68 (32.2) |
| Prior bDMARD use | ||
| One or more TNFi, n (%) | 172 (82.3) | 173 (82.0) |
| One or more IL-17i, n (%) | 25 (12.0) | 30 (14.2) |
| One TNFi and one IL-17i, n (%) | 11 (5.3) | 8 (3.8) |
| Presence of enthesitis (MASES > 0) | 162 (77.9) | 148 (70.1) |
| Tender joint count (TJC68) | 4.4 (6.1) | 4.6 (7.4) |
| Swollen joint count (SJC66) | 1.8 (4.1) | 1.5 (3.1) |
| hsCRP at screening, mg/l | 14.5 (17.8) | 15.8 (17.7) |
| hsCRP > ULNa at screening, n (%) | 163 (78.0) | 165 (78.2) |
| MRI spine SPARCC score | 8.8 (12.5) | 10.7 (15.4) |
| MRI-SI joints SPARCC score | 5.6 (10.6) | 5.0 (10.8) |
| BASDAI (score 0–10) | 6.8 (1.3) | 6.8 (1.3) |
| PtGA (0–10) | 7.4 (1.3) | 7.5 (1.4) |
| Total back pain (NRS score 0–10) | 7.4 (1.4) | 7.5 (1.5) |
| Nocturnal back pain (NRS score 0–10) | 7.2 (1.5) | 7.1 (1.8) |
| FACIT-F (0–52) | 28.3 (9.7) | 27.5 (9.6) |
| BASFI (0–10) | 6.2 (1.9) | 6.3 (2.0) |
| Total back pain (NRS score 0–10) | 7.4 (1.4) | 7.5 (1.5) |
| ASAS HI (0–17) | 8.9 (3.7) | 9.4 (3.5) |
| ASQoL (0–18) | 11.5 (4.4) | 11.6 (4.4) |
| SF-36 PCS (0–100) | 34.6 (7.5) | 34.0 (6.7) |
| SF-36 MCS (0–100) | 45.8 (10.9) | 44.8 (11.1) |
| WPAI—absenteeism (0–100)b | 13.2 (26.4) | 11.1 (21.3) |
| WPAI—presenteeism (0–100)b | 52.9 (20.91) | 55.4 (23.7) |
| WPAI—overall work impairment (0–100)b | 57.9 (24.0) | 59.2 (25.3) |
| WPAI—activity impairment (0–100)b | 60.2 (20.7) | 62.0 (21.9) |
Results are reported as mean (SD) unless otherwise specified
AS ankylosing spondylitis, ASAS HI Assessment of SpondyloArthritis international Society Health Index, ASQoL Ankylosing Spondylitis Quality of Life, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BASFI Bath Ankylosing Spondylitis Functional Index, csDMARDs conventional synthetic disease-modifying antirheumatic drugs, FACIT-F Functional Assessment of Chronic Illness Therapy-Fatigue, HLA human leukocyte antigen, hsCRP high-sensitivity C-reactive protein, IL-17i interleukin-17 inhibitor, MASES Maastricht Ankylosing Spondylitis Enthesitis Score, MCS mental component summary, MRI magnetic resonance imaging, NSAIDs non-steroidal anti-inflammatory drugs, PCS physical component summary, PROs patient-reported outcomes, PtGA Patient Global Assessment of Disease Activity, QD once daily, SD standard deviation, SF-36 36-Item Short Form Health Survey, SI sacroiliac, SJC swollen joint count, SPARCC Spondyloarthritis Research Consortium of Canada, TJC tender joint count, TNFi tumor necrosis factor inhibitor, ULN upper limit of normal, WPAI Work Productivity and Activity Impairment
aULN = 2.87 mg/l
bIncludes patients currently employed; PBO n = 137 (65.7%), UPA n = 132 (63.8%)
Outcomes
Clinically Meaningful Improvements in PROs
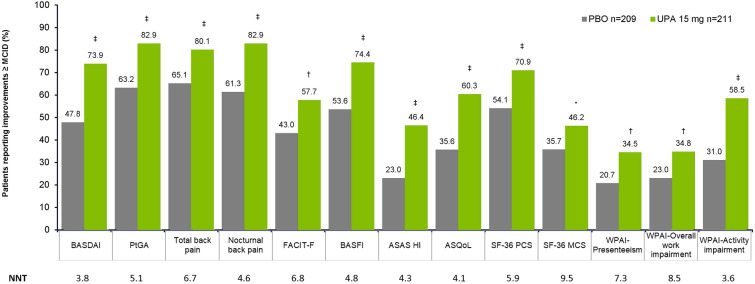
A higher proportion of patients reported clinically meaningful improvements at week 14 across all PROs with upadacitinib 15 mg compared with placebo: BASDAI (73.9 vs. 47.8%), PtGA (82.9 vs. 63.2%), total back pain (80.1 vs. 65.1%), nocturnal back pain (82.9 vs. 61.3%), FACIT-F (57.7 vs. 43.0%), BASFI (74.4 vs. 53.6%), ASAS HI (46.4 vs. 23.0%), ASQoL (60.3 vs. 35.6%), SF-36 PCS (70.9 vs. 54.1%), and WPAI activity impairment (57.5 vs. 31.0%), nominal p ≤ 0.001; WPAI presenteeism (34.5 vs. 20.7%), and WPAI overall work impairment (34.8 vs. 23.0%), nominal p ≤ 0.01; and SF-36 MCS (46.2 vs. 35.7%), nominal p < 0.05 (Fig. 1). The NNTs were < 10, ranging from 3.6 to 9.5 across all PROs at week 14 for upadacitinib 15 mg versus placebo (Fig. 1).
Fig. 1.
Proportion of patients reporting improvements ≥ MCID and NNTs in PROs at week 14 (NRI-MI). *p < 0.05, †p ≤ 0.01, and ‡p ≤ 0.001 versus placebo. p values nominal. NRI-MI is non-responder imputation (NRI) incorporating multiple imputation (MI) to handle missing data due to COVID-19. MCID definitions: BASDAI, ≥ 1.1-point decrease; PtGA, total back pain and nocturnal back pain, ≥ 1-point decrease; FACIT-F, ≥ 4-point increase; BASFI, ≥ 0.6-point increase; ASAS HI and ASQoL, ≥ 3 points decrease;SF-36 PCS and MCS, ≥ 2.5-point increase; WPAI presenteeism and activity impairment, ≥ 20-point decrease; WPAI overall work impairment, ≥ 15-point decrease; WPAI absenteeism, MCID not available. ASAS HI Assessment of SpondyloArthritis international Society Health Index, ASQoL Ankylosing Spondylitis Quality of Life, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BASFI Bath Ankylosing Spondylitis Functional Index, FACIT-F Functional Assessment of Chronic Illness Therapy-Fatigue, MCID minimal clinically important difference, MCS mental component summary, NNT number needed to treat, NRI non-responder imputation, PBO placebo, PCS physical component summary, PROs patient-reported outcomes, PtGA Patient Global Assessment of Disease Activity, SF-36 36-Item Short Form Health Survey, UPA upadacitinib, WPAI Work Productivity and Activity Impairment
Improvements from Baseline in PROs
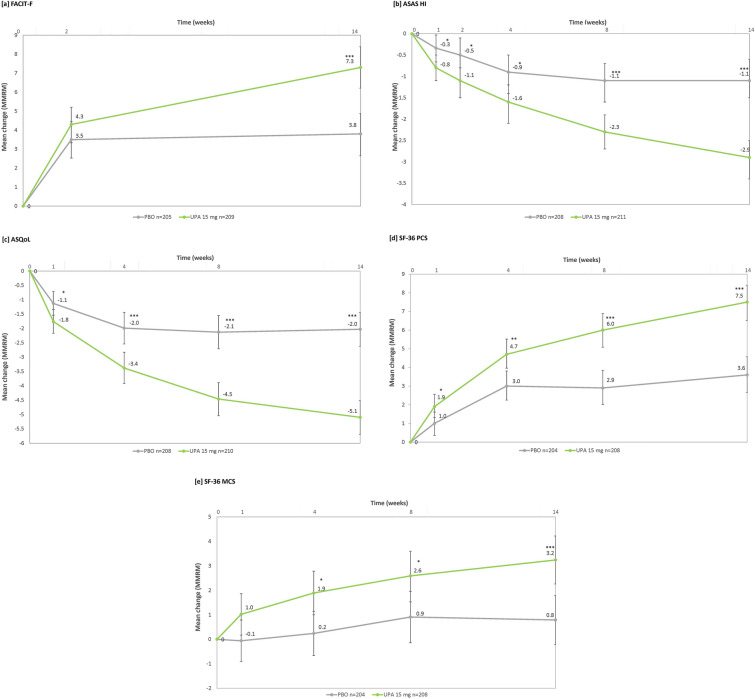
Improvements from baseline with upadacitinib 15 mg versus placebo were differentiated as early as week 1 for ASAS HI, ASQoL and SF-36 PCS, and week 4 for SF-36 MCS (Fig. 2), and these continued through week 14. Improvements from baseline for FACIT-F were not differentiated between upadacitinib and placebo at early visits (e.g., week 2), but were evident by week 14 (Fig. 2).
Fig. 2.
Change from baseline through week 14 in a FACIT-F, b ASAS HI, c ASQoL, d SF-36 PCS, e SF-36 MCS (MMRM). MMRM analysis included treatment, visit and treatment-by-visit interaction as fixed factors and baseline value as covariate. Stratification factor hsCRP level (≤ ULN vs. > ULN) were also included in the model. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001. p values nominal except for ASAS HI and ASQoL at week 14, which were ranked endpoints in the multiplicity-controlled analysis. ASAS HI Assessment of SpondyloArthritis international Society Health Index, ASQoL Ankylosing Spondylitis Quality of Life, CI confidence interval, FACIT-F Functional Assessment of Chronic Illness Therapy-Fatigue, hsCRP high-sensitivity C-reactive protein, MCS mental component summary, MMRM mixed effect model for repeated measures, PBO placebo, PCS physical component summary, SF-36 36-Item Short Form Health Survey, ULN upper limit of normal, UPA upadacitinib
Improvement in mean change from baseline to week 14 across WPAI domains of presenteeism, overall work impairment, and activity impairment were greater with upadacitinib 15 mg compared with placebo; however, improvement in absenteeism was similar between the treatment groups (Fig. 3).
Fig. 3.
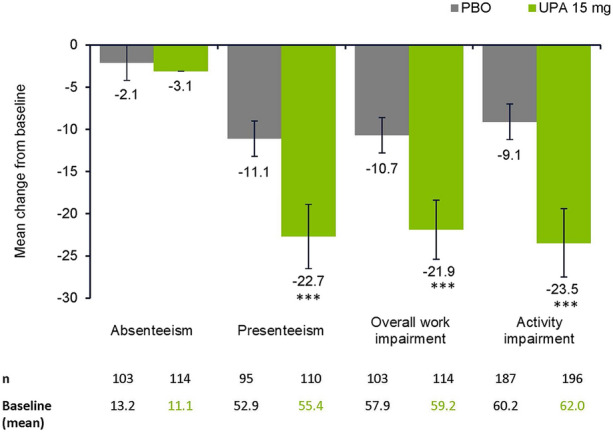
Mean change (95% CI) from baseline in PRO scores at week 14 in WPAI. WPAI: change from baseline at week 14 and nominal p value are based on ANCOVA model including treatment and main stratification factors MRI and screening hsCRP status (MRI+/hsCRP > ULN, MRI+/hsCRP ≤ ULN, and MRI−/hsCRP > ULN) as fixed factors and baseline value as covariate. ***p ≤ 0.001. ANCOVA analysis of covariance, hsCRP high-sensitivity C-reactive protein, PBO placebo, ULN upper limit of normal, UPA upadacitinib, WPAI Work Productivity and Activity Impairment
TNFi-IR Subgroup
The TNFi-IR post hoc subgroup analysis included 364 patients who had prior exposure to one or more TNFi (N = 183 for placebo and N = 181 for upadacitinib). Consistent with the full analysis set that included IL-17 inhibitor exposed patients, prior exposure to ≥ 1 TNF inhibitor at baseline also saw a notably higher proportion of patients achieving clinically meaningful improvements in PROs with upadacitinib 15 mg treatment at week 14 compared with placebo (Figure S1). NNTs at week 14 ranged from 3.8 to 9.8 with upadacitinib 15 mg compared with placebo.
Discussion
This study demonstrates that a higher proportion of bDMARD-IR patients with active AS achieved clinically meaningful improvements at week 14 in patient-reported disease activity, pain, fatigue, function, HRQoL, and work productivity with upadacitinib 15 mg compared with placebo. Rapid improvements from the patients’ perspective were observed, with improvements from baseline in PROs evaluating HRQoL seen as early as week 1 (ASQoL, ASAS HI, and SF-36-PCS) and week 2 (SF-36-MCS) with upadacitinib treatment, that were sustained through week 14. Improvement in fatigue (FACIT-F) was observed by week 14, indicating that improvement in fatigue may take longer to emerge, consistent with other studies [26]. Additionally, NNTs were below the clinically meaningful threshold of 10 for all PROs at week 14, further substantiating the efficacy of upadacitinib [27]. This study supports the increasing body of evidence demonstrating that JAK inhibitors are an effective oral therapy for the treatment of active AS, including for patients with an IR to bDMARDs [14].
These findings are in line with the SELECT-AXIS 1 study, which previously demonstrated significant improvement in a bDMARD-naïve AS population in key ranked secondary endpoints of function and consistent improvements in HRQoL (ASAS HI and ASQoL), with upadacitinib versus placebo [28]. The current study in bDMARD-IR patients provides further evidence supporting upadacitinib as a promising treatment option for this treatment-refractory population, particularly given the large number of patients with AS who do not achieve desired treatment goals on current biologic therapies [13, 29].
Furthermore, real-world evidence suggests that over two-thirds of patients with AS who were treated with TNF inhibitors either discontinued or switched to a second TNF inhibitor within 2 years, highlighting the unmet need in this population [29, 30]. In a post hoc analysis, the subgroup of TNFi-IR patients showed consistent results with the full analysis set, indicating consistent improvements in PROs for patients with past use of TNF inhibitors.
There is a high burden of disease for patients with AS, with wide-ranging negative impacts across physical function and wellbeing, detrimental impact on HRQoL, loss of work productivity, and limits to daily activities [31]. Normalizing the patient’s function, HRQoL, and ability to perform daily activities is an important treatment goal, highlighting the importance of improvements across a range of PROs from disease activity and function (such as BASDAI, PtGA, and BASFI), to fatigue (FACIT-F), general HRQoL (e.g. ASAS HI), and work productivity (WPAI) in the current study. Improvements observed with PROs have been linked with clinical disease activity in patients with AS, which is critical given this high burden.
Improvements in work productivity are also encouraging as this is aligned with the recommendations from ASAS-EULAR stating that work productivity loss should be taken into account in treatment decisions, as it is associated with indirect costs of economic burden in AS [8]. It has been reported that almost one in six (14.4%) patients with axSpA on biologic therapy experienced axSpA-related job loss and poor work outcomes, such as presenteeism, were associated with poorer scores for PROs [32].
Limitations of the study include the fact that the results may not be generalizable further than the trial population given that patients enrolled in clinical trials meet strict eligibility criteria and may differ from patients with AS in the general population. The limited sample size may also result in imprecise estimates that may limit the ability to make causal inferences. Additionally, the outcome data are provided only up to 14 weeks, therefore long-term follow-up is needed to confirm the durability of PRO improvements. Concomitant use of steroids or NSAIDs was allowed during the study period, which may have contributed to the high MCID response rates seen across both the placebo and UPA treatment groups for some outcomes e.g., PtGA and total back pain. However, higher proportions of patients achieved clinically meaningful improvements with UPA compared to placebo. This study used phase 3 clinical trial data, ensuring that patients are closely monitored and that the outcomes of interest are well-measured. The randomized study design implemented mitigates bias that may arise due to unobservable differences between cohorts. This study demonstrated improvements seen across a broad range of PROs previously not measured within other studies of JAK inhibitors. Another key strength of this study is the clinically meaningful improvement across outcomes in TNFi-IR patients observed with upadacitinib versus placebo, supporting the current evidence base in patients with AS refractory to biological therapy.
Conclusions
In bDMARD-IR patients with active AS, upadacitinib 15 mg demonstrated clinically meaningful improvements in disease activity, pain, fatigue, function, HRQoL, and work productivity at week 14 compared with placebo. Rapid improvements from baseline were seen as early as week 1 for disease-specific and general HRQoL patient-reported measures. These data support findings from previous clinical trials evaluating the efficacy of upadacitinib in AS by further substantiating the benefit of upadacitinib in this biologic refractory population.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This work was supported by AbbVie, including the Rapid Service and Open Access fees. AbbVie sponsored the study, participated in the interpretation of data, review, and approval of the manuscript. All authors contributed to the development of the manuscript and maintained control over the final content. No honoraria or payments were made for authorship.
Medical Writing, Editorial, and Other Assistance
Medical writing assistance was provided by Sarah Hodgkinson PhD, of Fishawack Facilitate Ltd., part of Fishawack Health, and was funded by AbbVie Inc., North Chicago, IL.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Substantial contributions to conception and design: All authors. Acquisition of data and data Analysis: Christopher D. Saffore. Interpretation of data: All authors. Involved in drafting or revising critically for important intellectual content: All authors. All authors approved the final version of the article, including the authorship list.
Disclosures
Victoria Navarro-Compán: has served as a speaker, consultant, and/or instructor for: AbbVie, Eli Lilly and Company, Galapagos, Janssen, Moonlake, Novartis, Pfizer, and UCB Pharma; and has received grant and/or research support from AbbVie and Novartis. Xenofon Baraliakos: has received grant and research support and consultancy fees from AbbVie, Amgen, Chugai, Galapagos, Hexal, Lilly, MSD, Novartis, Pfizer and UCB. Marina Magrey: has received research/grant support from AbbVie and UCB, and has received consultancy fees from AbbVie, Eli Lilly, Janssen, Novartis, Pfizer Inc, and UCB. Andrew Östör: has served as a consultant and/or on advisory boards for AbbVie, BMS, Roche, Janssen, Lilly, Novartis, Pfizer, UCB, Gilead, and Paradigm. Christopher D. Saffore, Manish Mittal, In-Ho Song, Fabiana Ganz and Jayne Stigler are employees of AbbVie and may own AbbVie stock. Atul Deodhar: Consulting, Advisory Boards: AbbVie, Amgen, Aurinia, Bristol Myers Squibb, Celgene, Eli Lilly, Glaxo Smith & Kline, Janssen, Moonlake, Novartis, Pfizer, UCB. Research Grants: AbbVie, Bristol Myers Squibb, Celgene, Eli Lilly, Glaxo Smith & Kline, Janssen, Novartis, Pfizer, UCB.
Compliance with Ethics Guidelines
The SELECT-AXIS 2 study was conducted according to the International Conference on Harmonisation guidelines and the principles of the Declaration of Helsinki. All patients provided written informed consent. The trial protocol was approved by independent ethics committees and institutional review boards.
Data Availability
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g., protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
References
- 1.Navarro-Compán V, Sepriano A, El-Zorkany B, van der Heijde D. Axial spondyloarthritis. Ann Rheum Dis. 2021;80:1511–1521. doi: 10.1136/annrheumdis-2021-221035. [DOI] [PubMed] [Google Scholar]
- 2.Baraliakos X, Braun J. Non-radiographic axial spondyloarthritis and ankylosing spondylitis: what are the similarities and differences? RMD Open. 2015;1:e000053. doi: 10.1136/rmdopen-2015-000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–783. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 4.Deodhar A. Understanding axial spondyloarthritis: a primer for managed care. Am J Manang Care. 2019;25:S319–S330. [Google Scholar]
- 5.Macfarlane GJ, Rotariu O, Jones GT, Pathan E, Dean LE. Determining factors related to poor quality of life in patients with axial spondyloarthritis: results from the British Society for Rheumatology Biologics Register (BSRBR-AS) Ann Rheum Dis. 2020;79:202–208. doi: 10.1136/annrheumdis-2019-216143. [DOI] [PubMed] [Google Scholar]
- 6.Haywood KL, Garratt AM, Dawes PT. Patient-assessed health in ankylosing spondylitis: a structured review. Rheumatology (Oxford) 2005;44:577–586. doi: 10.1093/rheumatology/keh549. [DOI] [PubMed] [Google Scholar]
- 7.Krabbe S, Glintborg B, Østergaard M, Hetland ML. Extremely poor patient-reported outcomes are associated with lack of clinical response and decreased retention rate of tumour necrosis factor inhibitor treatment in patients with axial spondyloarthritis. Scand J Rheumatol. 2019;48:128–132. doi: 10.1080/03009742.2018.1481225. [DOI] [PubMed] [Google Scholar]
- 8.van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978–991. doi: 10.1136/annrheumdis-2016-210770. [DOI] [PubMed] [Google Scholar]
- 9.Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Care Res. 2019;71:1285–1299. doi: 10.1002/acr.24025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2022 doi: 10.1136/ard-2022-223296:ard-2022-223296. [DOI] [PubMed] [Google Scholar]
- 11.Fragoulis GE, Siebert S. Treatment strategies in axial spondyloarthritis: what, when and how? Rheumatology (Oxford) 2020;59:iv79–iv89. doi: 10.1093/rheumatology/keaa435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro-Compán V, Plasencia-Rodríguez C, de Miguel E, et al. Switching biological disease-modifying antirheumatic drugs in patients with axial spondyloarthritis: results from a systematic literature review. RMD Open. 2017;3:e000524. doi: 10.1136/rmdopen-2017-000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conti F, Ceccarelli F, Marocchi E, et al. Switching tumour necrosis factor alpha antagonists in patients with ankylosing spondylitis and psoriatic arthritis: an observational study over a 5-year period. Ann Rheum Dis. 2007;66:1393–1397. doi: 10.1136/ard.2007.073569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deodhar A, Poddubnyy D, Pacheco-Tena C, et al. Efficacy and safety of ixekizumab in the treatment of radiographic axial spondyloarthritis: sixteen-week results from a phase III randomized, double-blind, placebo-controlled trial in patients with prior inadequate response to or intolerance of tumor necrosis factor inhibitors. Arthritis Rheumatol (Hoboken, NJ) 2019;71:599–611. doi: 10.1002/art.40753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deodhar A, Sliwinska-Stanczyk P, Xu H, et al. Tofacitinib for the treatment of ankylosing spondylitis: a phase III, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2021;80:1004–1013. doi: 10.1136/annrheumdis-2020-219601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navarro-Compán V, Wei JC, Van den Bosch F, et al. Effect of tofacitinib on pain, fatigue, health-related quality of life and work productivity in patients with active ankylosing spondylitis: results from a phase III, randomised, double-blind, placebo-controlled trial. RMD Open. 2022;8(2):e002253. doi: 10.1136/rmdopen-2022-002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Heijde D, Baraliakos X, Sieper J, et al. Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial. Ann Rheum Dis. 2022 doi: 10.1136/ard-2022-222608:annrheumdis-2022-222608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 19.Kviatkovsky MJ, Ramiro S, Landewé R, et al. The minimum clinically important improvement and patient-acceptable symptom state in the BASDAI and BASFI for patients with ankylosing spondylitis. J Rheumatol. 2016;43:1680–1686. doi: 10.3899/jrheum.151244. [DOI] [PubMed] [Google Scholar]
- 20.Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain (London, England) 2004;8:283–291. doi: 10.1016/j.ejpain.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Hewlett S, Dures E, Almeida C. Measures of fatigue: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ), Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF NRS) for severity, effect, and coping, Chalder Fatigue Questionnaire (CFQ), Checklist Individual Strength (CIS20R and CIS8R), Fatigue Severity Scale (FSS), Functional Assessment Chronic Illness Therapy (Fatigue) (FACIT-F), Multi-Dimensional Assessment of Fatigue (MAF), Multi-Dimensional Fatigue Inventory (MFI), Pediatric Quality Of Life (PedsQL) Multi-Dimensional Fatigue Scale, Profile of Fatigue (ProF), Short Form 36 Vitality Subscale (SF-36 VT), and Visual Analog Scales (VAS) Arthritis Care Res. 2011;63(Suppl 11):S263–S286. doi: 10.1002/acr.20579. [DOI] [PubMed] [Google Scholar]
- 22.Kiltz U, van der Heijde D, Boonen A, et al. Measurement properties of the ASAS Health Index: results of a global study in patients with axial and peripheral spondyloarthritis. Ann Rheum Dis. 2018;77:1311–1317. doi: 10.1136/annrheumdis-2017-212076. [DOI] [PubMed] [Google Scholar]
- 23.Richard N, Haroon N, Tomlinson GA, et al. FRI0208 Ankylosing spondylitis quality of life: defining minimal clinically important change. Ann Rheum Dis. 2018;77:645. [Google Scholar]
- 24.Strand V, Boers M, Idzerda L, et al. It's good to feel better but it's better to feel good and even better to feel good as soon as possible for as long as possible. Response criteria and the importance of change at OMERACT 10. J Rheumatol. 2011;38:1720–1727. doi: 10.3899/jrheum.110392. [DOI] [PubMed] [Google Scholar]
- 25.Tillett W, Lin CY, Zbrozek A, Sprabery AT, Birt J. A threshold of meaning for work disability improvement in psoriatic arthritis measured by the work productivity and Activity Impairment Questionnaire. Rheumatol Therapy. 2019;6:379–391. doi: 10.1007/s40744-019-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cella D, Lenderking WR, Chongpinitchai P, et al. Functional assessment of chronic illness therapy-fatigue is a reliable and valid measure in patients with active ankylosing spondylitis. J Patient-Rep Outcomes. 2022;6:100. doi: 10.1186/s41687-022-00508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siwek J, Newman DH. Introducing medicine by the numbers: a collaboration of The NNT group and AFP. Am Fam Physician. 2015;91:434–435. [PubMed] [Google Scholar]
- 28.van der Heijde D, Song IH, Pangan AL, et al. Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): a multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet (London, England) 2019;394:2108–2117. doi: 10.1016/S0140-6736(19)32534-6. [DOI] [PubMed] [Google Scholar]
- 29.Ørnbjerg LM, Brahe CH, Askling J, et al. Treatment response and drug retention rates in 24 195 biologic-naïve patients with axial spondyloarthritis initiating TNFi treatment: routine care data from 12 registries in the EuroSpA collaboration. Ann Rheum Dis. 2019;78:1536–1544. doi: 10.1136/annrheumdis-2019-215427. [DOI] [PubMed] [Google Scholar]
- 30.Hunter T, Schroeder K, Sandoval D, Deodhar A. Persistence, discontinuation, and switching patterns of newly initiated TNF inhibitor therapy in ankylosing spondylitis patients in the United States. Rheumatol Therapy. 2019;6:207–215. doi: 10.1007/s40744-019-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strand V, Singh JA. Patient burden of axial spondyloarthritis. JCR J Clin Rheumatol. 2017;23:383–391. doi: 10.1097/RHU.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nadin T, Wallis D, Holroyd CR, et al. Amongst patients taking biologic therapies for axial spondyloarthritis, which factors are associated with work non-participation? BMC Musculoskelet Disord. 2020;21:209. doi: 10.1186/s12891-020-03247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g., protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.