Abstract
Introduction
This retrospective claims database study examined the prevalence of mortality and morbidity among adults with type 2 diabetes (T2D) and obesity.
Methods
The study used deidentified data from 2007 to 2021 from the Optum® Market Clarity Dataset. A cohort of adults with T2D and obesity were identified, and age- and sex-adjusted prevalence rates were calculated for mortality, a composite cardiovascular outcome (CCO), a composite microvascular outcome (CMO), and other complications. Results were examined overall and by obesity class (class 1, class 2, and class 3).
Results
For the 15,970 adults included in the study, the prevalence of CCO and CMO after 5 years was 15.3% and 60.7%, respectively. The 5-year prevalence of mortality was 10.9%. There were statistically significant differences in prevalence rates by obesity class, with obesity class 3 associated with higher rates of morbidity and mortality compared to obesity classes 1 or 2. Specifically, after 5 years, the prevalence of mortality was 9.4%, 10.3% and 13.6% for obese classes 1, 2 and 3, respectively (P < 0.05 between class 3 and class 2 or 1). Similarly, For obesity classes 1, 2 and 3, the 5-year prevalence of CCO was 13.0%, 14.5% and 18.4% and the rates for CMO were 58.0%, 57.9% and 64.8%, respectively (both P < 0.05 between class 3 and class 2 or 1). Regarding other complications, differences in the prevalence of atherosclerotic cardiovascular disease (ASCVD) and obstructive sleep apnea (OSA) were statistically significantly higher with increasing obesity class.
Conclusions
The results indicate that for a cohort of adults with T2D and obesity, obesity class 3 is associated with significantly higher mortality and morbidity, including CCO, CMO, ASCVD and OSA. These findings suggest that treatment which reduces obesity among individuals with T2D may have significant health benefits, although additional studies are needed to confirm the results.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-023-01384-7.
Keywords: Complications, Morbidity, Mortality, Obesity, Prevalence, Type 2 diabetes
Key Summary Points
| Why Carry out this study? |
| In the USA, the rates of both obesity and diabetes are high and still growing. |
| This study examines the prevalence of morbidity and mortality among adults with both type 2 diabetes (T2D) and obesity, both overall and by obesity class. |
| What was Learned from the Study? |
| The results indicate that for a cohort of adults with T2D and obesity, obesity class 3 (body mass index [BMI] ≥ 40 kg/m2) is associated with significantly higher mortality as well as significantly higher prevalence of cardiovascular and microvascular complications. |
| Statistically significant increases in the prevalence of atherosclerotic cardiovascular disease and obstructive sleep apnea were associated with higher obesity classes. |
Introduction
Among the population of all US adults, an estimated 42% have obesity [1], and 13.2–14.0% have type 2 diabetes (T2D) [2, 3]. Furthermore, research has shown that, among individuals with T2D in the USA, the proportion of individuals with obesity is rising [4]. The costs of obesity and diabetes are staggering. In the USA in 2016, the medical costs of obesity among adults totaled $260.6 billion in aggregate, while the mean per-person medical costs among those with obesity were double those of normal-weight adults ($5010 vs. $2504) [5]. In 2017, the costs of diabetes in the USA came to an estimated $327 billion, which included $237 billion in direct medical expenses and $90 billion in reduced productivity [6].
Previous research has shown both obesity and T2D to be associated with disease and premature death. For instance, obesity has been found to be associated with comorbid obstructive sleep apnea (OSA) [7, 8], as well as with T2D, hypertension, hyperlipidemia, osteoarthritis and coronary artery disease [8]. T2D has been correlated with overweight/obesity, as well as with hypertension, hyperlipidemia, chronic kidney disease and cardiovascular disease [9]. Both obesity and T2D have been shown to be major risk factors for cardiovascular disease [10–12]. Together, diabetes and obesity have been shown to increase mortality risk by sevenfold [13, 14]. In addition, research has shown that among individuals with T2D, those with comorbid obesity were significantly more likely to have comorbid nephropathy, neuropathy, cardiovascular disease, peripheral vascular disease and metabolic disease [15].
Given the foregoing research, we hypothesized that a US population diagnosed with concomitant obesity and T2D would have a large burden of comorbidity and mortality. In addition, we theorized that the rate of illness and death would increase as body mass index (BMI) rose. To test these hypotheses, we constructed a retrospective study using data from US health insurance claims and electronic medical records (EMRs). The aims of the study were to: (1) observe the 5-year prevalence and incidence of cardiovascular, microvascular and other comorbidities and mortality in a population of American adults with concomitant obesity and T2D, and (2) compare the prevalence of death and comorbidity across obesity categories in this same population.
Methods
The retrospective cohort analyses were conducted using patient health information from the US-based Optum® Market Clarity Dataset, a source of insurance claims, EMRs and outpatient laboratory results. The linked data were fully deidentified and compliant with the Health Insurance Portability and Accountability Act (HIPAA). For this study, the dataset supplied longitudinal information on patient demographics, coverage eligibility, diagnostic and procedure codes, outpatient prescription fills, laboratory test results, mortality and observational data, such as BMI and blood pressure. Data for this study covered the period from 1 January 2007 through to 30 June 2021. Given the use of retrospective and deidentified data, ethics committee approval was not required. Permission was granted by Optum® for use of the data, and all research was conducted in accordance with the 1964 Declaration of Helsinki [16].
To be included in the study, individuals were required to have both a recorded BMI indicating obesity (BMI ≥ 30 kg/m2) [17] and a recorded hemoglobin A1c (HbA1c) test result indicating diabetes (HbA1c ≥ 6.5%) [18]. The date of first overlap between such BMI and HbA1c results was identified as the index date. Individuals were also required to have had T2D, based upon receipt of at least one diagnosis of T2D and no diagnosis of type 1 diabetes at any time from 1 year prior to the index date (the pre-period) through 5 years after the index date (the post-period). Individuals were excluded from the study if at any time from the start of the pre-period through to the end of the post-period their medical claims revealed that they had received: (1) a diagnosis of pregnancy, (2) a prescription fill for a chronic weight loss medication or (3) a weight loss procedure. Individuals were required to have been aged ≥ 18 years at the index date and continuously insured from the start of the pre-period to either the end of the post-period or their date of death, whichever came first. Figure 1 illustrates how each of the inclusion and exclusion criteria affected the sample size.
Fig. 1.
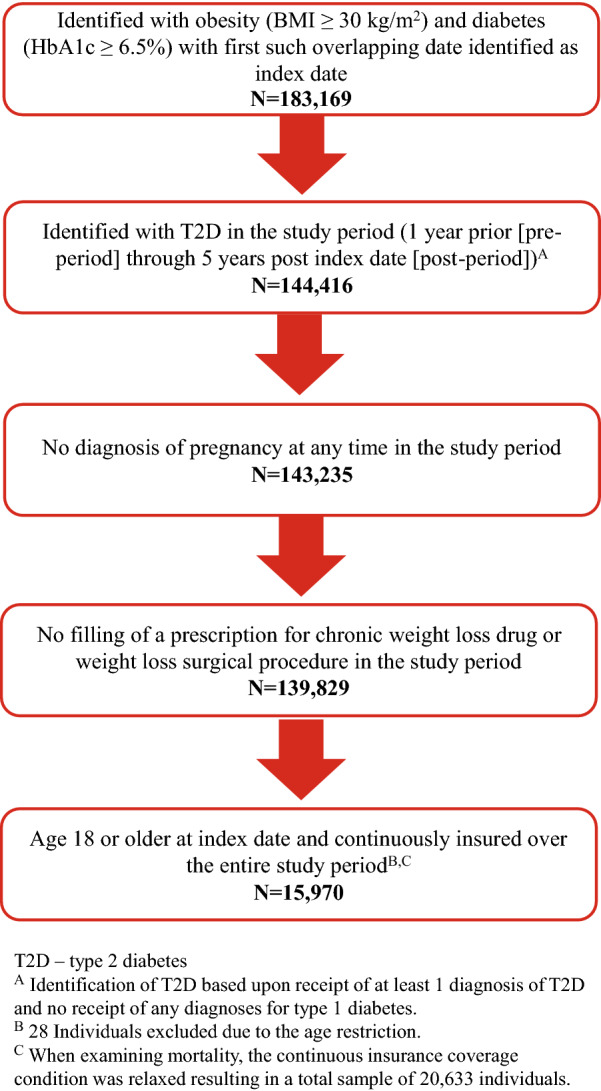
Flow chart of inclusion–exclusion criteria and sample size. BMI Body mass index, HbA1c glycated hemoglobin, T2D type 2 diabetes
For each 365-day period from 1 year prior to the index date (the baseline) through to 5 years post index date, the study examined the prevalence and incidence of comorbidities and mortality. All rates were adjusted for age and sex based upon the 2019 US Census Bureau data [19]. Consistent with prior research which has utilized claims and EMR data [20], the “lookback period” was defined as a fixed time period of the 1 year prior to the index date, and prevalence was defined as the number of patients identified with the complication of interest over the year(s) of interest post index date or the lookback period divided by the number of patients included in the study. Similarly, incidence was defined as the number of individuals who did not have the complication of interest in the lookback time who are subsequently observed to develop the disease in the post index year of interest divided by the number of patients included in the study. Outcomes of interest included mortality, a composite cardiovascular outcome (CCO) and a composite microvascular outcome (CMO). The CCO comprised: (1) acute care visits (hospitalization or emergency room) with an accompanying diagnosis of heart failure, (2) myocardial infarctions and/or (3) strokes. The CMO comprised diagnoses of (1) nephropathy, (2) neuropathy and/or (3) retinopathy. The study also examined the prevalence of diagnoses of atherosclerotic cardiovascular disease (ASCVD), atrial fibrillation, liver cirrhosis, nonalcoholic steatohepatitis/nonalcoholic fatty liver disease (NASH/NAFLD) and OSA.
All outcomes were examined for the population as a whole. Consistent with prior research which has examined obesity using categorical variables [12, 21, 22], the analyses also examined results for subgroups defined by obesity class, where class 1 obesity was defined as 30 kg/m2 < BMI < 35 kg/m2; class 2 was defined as 35 kg/m2 < BMI < 40 kg/m2; class 3 was defined as BMI ≥ 40 kg/m2 [17]. The analyses were primarily descriptive in nature. For descriptive statistics, means and standard deviations were reported for continuous variables, and frequencies and percentages were reported for categorical variables. Differences in continuous variables were examined using t-tests, and differences in categorical variables were reported using Chi-square statistics. Trends over time were examined using generalized estimating equations (GEE) tests, and differences in prevalence rates between obesity classes were examined based upon their 95% confidence intervals (CIs). All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA), and a P value < 0.05 was considered, a priori, to be statistically significant.
Results
Table 1 presents patient characteristics for the overall cohort and for each obesity class. The average age of individuals included in the study was 62.4 years, with the majority of participants identified as female (51.0%), Caucasian (77.5%) and Non-Hispanic (85.8%), and to be residing in the Midwest (45.1%) or Southern (28.9%) regions of the USA. Examination of characteristics across the obesity classes revealed that the higher obesity class was statistically significantly associated with younger age and with a higher proportion of individuals identified as African American, and with a lower proportion of males and individuals identified as Hispanic (all P < 0.05). Furthermore, the higher obesity class was associated with a statistically significantly lower percentage of individuals who resided in the Northeast or West and with more participants who resided in the Southern or Midwest regions (P < 0.05).
Table 1.
Patient characteristics
| Patient characteristics | All patients | By obesity classa | ||
|---|---|---|---|---|
| Obese class 1 | Obese class 2 | Obese class 3 | ||
| Sample size | 15,970 | 7513 | 4635 | 3822 |
| Age (years), mean ± SD | 62.4 ± 11.7 | 64.7 ± 11.3 | 61.8 ± 11.6 | 58.7 ± 11.4 |
| Sex, n (%) | ||||
| Female | 8138 (51.0) | 3397 (45.2) | 2415 (52.1) | 2326 (60.9) |
| Male | 7829 (49.0) | 4113 (54.7) | 2220 (47.9) | 1496 (39.1) |
| Unknown | 3 (0.0) | 3 (0.0) | 0 (0.0) | 0 (0.0) |
| Race, n (%) | ||||
| African American | 2303 (14.4) | 1048 (13.9) | 649 (14.0) | 606 (15.9) |
| Asian | 207 (1.3) | 149 (2.0) | 43 (0.9) | 15 (0.4) |
| Caucasian | 12,378 (77.5) | 5761 (76.7) | 3639 (78.5) | 2978 (77.9) |
| Other/unknown | 1082 (6.8) | 555 (7.4) | 304 (6.6) | 223 (5.8) |
| Ethnicity, n (%) | ||||
| Hispanic | 1141 (7.1) | 606 (8.1) | 309 (6.7) | 226 (5.9) |
| Non-Hispanic | 13,710 (85.8) | 6349 (84.5) | 3998 (86.3) | 3363 (88.0) |
| Unknown | 1119 (7.0) | 558 (7.4) | 328 (7.1) | 233 (6.1) |
| Region, n (%) | ||||
| Midwest | 7202 (45.1) | 3258 (43.4) | 2100 (45.3) | 1844 (48.2) |
| Northeast | 1874 (11.7) | 991 (13.2) | 525 (11.3) | 358 (9.4) |
| South | 4610 (28.9) | 2088 (27.8) | 1365 (29.4) | 1157 (30.3) |
| West | 1814 (11.4) | 941 (12.5) | 525 (11.3) | 348 (9.1) |
| Other/unknown | 470 (2.9) | 235 (3.1) | 120 (2.6) | 115 (3.0) |
All differences in patient characteristics by obesity class are statistically significant (P < 0.0001)
SD Standard deviation
aClass 1 obesity: 30 kg/m2 < body mass index (BMI) < 35 kg/m2. Class 2 obesity: 35 kg/m2 < BMI < 40 kg/m2. Class 3 obesity: BMI ≥ 40 kg/m2
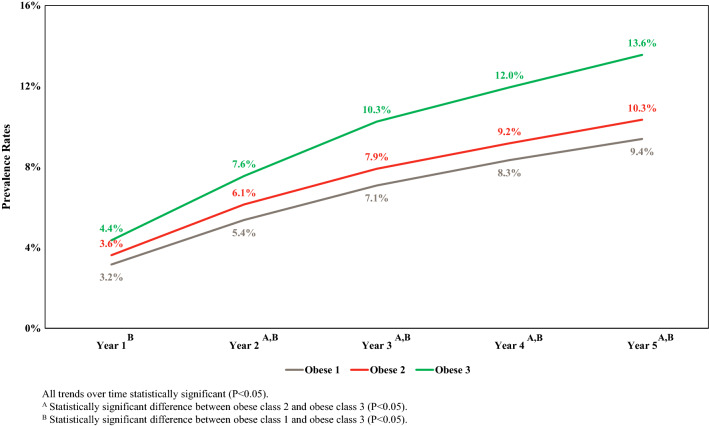
Figure 2 illustrates and compares the prevalence of mortality by obesity class, adjusted for age and sex, for each of the 5 years of study. After 5 years, the overall prevalence of mortality was 11.0% (95% CI 10.5–11.3%), and the rates for obesity class 1, 2 and 3 were 9.4%, 10.3% and 13.6%, respectively. As Fig. 2 shows, higher obesity class was associated with higher mortality each year, although the differences between obesity class 1 and 2 were not statistically significant and differences between class 2 and 3 did not reach statistical significance in the first year post index date. The prevalence of mortality rose faster for obese class 3 than for the other classes.
Fig. 2.
The 5-year age- and sex-adjusted prevalence of morality by obesity class. Obes1 Class 1 obesity: 30 kg/m2 < body mass index (BMI) < 35 kg/m2, Obes2 class 2 obesity: 35 kg/m2 < BMI < 40 kg/m2. Obes3 class 3 obesity: BMI ≥ 40 kg/m2
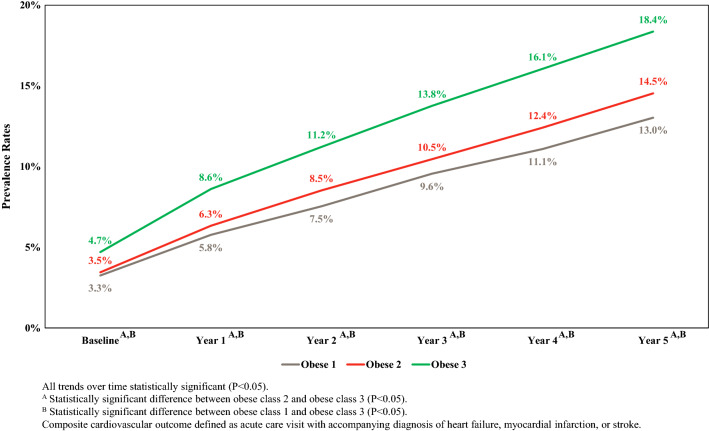
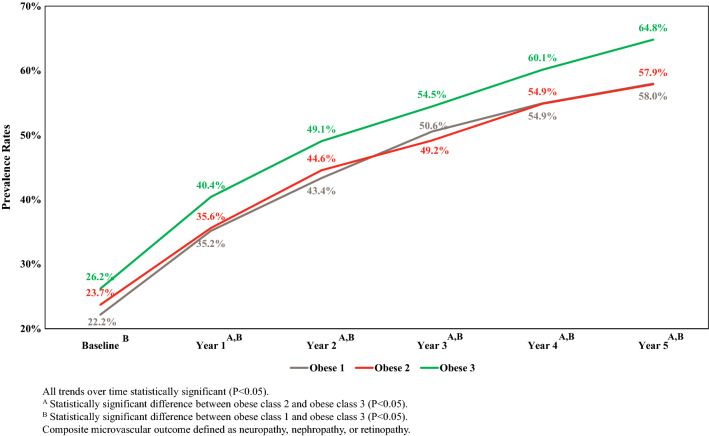
Figures 3 and 4 illustrate the baseline and 5-year prevalence, adjusted for age and sex, of the CCO and CMO, respectively. As expected, both types of composite outcomes were highly prevalent. Specifically, the overall 5-year prevalence of the CCO was 15.3% (95% CI 14.7–15.8%) and 60.7% (59.9–61.4%) for the composite microvascular outcome. In addition, higher obesity class was associated with a higher prevalence of each type of composite outcome. For example, after 5 years, the prevalence of the CCO for obesity class 1, 2 and 3 was 13.0% (95% CI 12.3–13.8%), 14.5% (95% CI 13.5–15.6%) and 18.4% (95% CI 17.1–19.6%), respectively. Similarly, the prevalence after 5 years of the CMO for obesity class 1, 2 and 3 was 58.0% (95% CI 56.9–59.1%), 57.9 (95% CI 56.5–59.3%) and 64.8% (95% CI 63.3–66.3%), respectively. For both the CCO and CMO, no statistically significant difference existed at any time period when the prevalence of outcomes for obesity class 1 was compared to that for obesity class 2. However, the prevalence of both outcomes for individuals with obesity class 3 was statistically significantly higher compared to the prevalence for individuals with obesity class 1 or obesity class 2 for the CMO for years 1 through 5 and for the CMO for all years.
Fig. 3.
The 5-year age- and sex-adjusted prevalence of composite cardiovascular outcome by obesity class
Fig. 4.
The 5-year age- and sex-adjusted prevalence of composite microvascular outcome by obesity class
Table 2 illustrates the 5-year prevalence rates, adjusted for age and sex, of the components of both types of composite comorbidities as well as the additional complications for the cohort overall and for each obesity class. The prevalence rates for every year in the study are included in Electronic Supplementary Material (ESM) Table 1. Taken together, these results show that statistically significant differences in the CCO were driven by statistically significant differences in acute care visits for heart failure. These tables also illustrate that differences in the CMO were consistent with statistically significant differences in nephropathy and neuropathy. Generally, patients identified with obesity class 3 had significantly higher rates of neuropathy compared to individuals identified with obesity class 1 or 2 and had a significantly higher prevalence of nephropathy compared to those in class 1.
Table 2.
The 5-year age- and sex-adjusted prevalence rates
| Outcome | All individuals | Obese class 1 | Obese class 2 | Obese class 3 |
|---|---|---|---|---|
| 5-Year prevalence rate (95% CI) | ||||
| Individual components of the cardiovascular outcome | ||||
| Acute care visit with accompanying diagnosis of heart failure | 8.4 (7.9–8.8) | 6.1a,c (5.6–6.7) | 7.7a,b (6.9–8.5) | 12.4b,c (11.4–13.5) |
| Myocardial infarction | 4.4 (4.0–4.7) | 3.9 (3.5–4.4) | 4.7 (4.1–5.3) | 4.4 (3.7–5.0) |
| Stroke | 7.5 (7.1–7.9) | 7.0 (6.4–7.6) | 6.7 (6.0–7.4) | 7.6 (6.7–8.4) |
| Individual components of the microvascular outcome | ||||
| Neuropathy | 39.5 (38.8–40.3) | 34.7c (33.7–35.8) | 36.3b (34.9–37.7) | 45.2b,c (43.6–46.8) |
| Nephropathy | 30.0 (29.3–30.7) | 29.2c (28.2–30.2) | 29.7 (28.4–31.1) | 32.3c (30.8–33.8) |
| Retinopathy | 23.5 (22.9–24.2) | 23.5 (22.5–24.4) | 23.5 (22.3–24.7) | 24.0 (22.7–25.4) |
| Secondary outcomes | ||||
| ASCVD | 81.9 (81.3–82.5) | 77.1a,c (76.1–78.0) | 81.5a,b (80.4–82.6) | 85.5b,c (84.3–86.6) |
| Atrial fibrillation | 9.2 (8.8–9.7) | 7.7c (7.1–8.3) | 8.7b (7.9–9.5) | 12.7b,c (11.7–13.8) |
| Liver cirrhosis | 24.3 (23.6–24.9) | 20.2c (19.3–21.1) | 21.9b (20.7–23.1) | 28.7b,c (27.3–30.2) |
| NASH/NAFLD | 20.9 (20.2–21.5) | 17.0a,c (16.2–17.9) | 20.5a,b (19.3–21.6) | 23.2b,c (21.8–24.5) |
| Obstructive sleep apnea | 32.1 (31.4–32.9) | 19.4a,c (18.5–20.3) | 31.2a,b (29.9–32.5) | 47.2b,c (45.7–48.8) |
ASCVD Atherosclerotic cardiovascular disease, CI confidence interval, eGFR estimated glomerular filtration rate, NAFLD non-alcoholic fatty liver disease, NASH non-alcoholic steatohepatitis
aStatistically significant difference between obese class 1 and obese class 2 (P < 0.05)
bStatistically significant difference between obese class 2 and obese class 3 (P < 0.05)
cStatistically significant difference between obese class 1 and obese class 3 (P < 0.05)
Results from Table 2 and ESM Table 1 showed that, in general, higher obesity class was associated with higher prevalence of comorbidities. In addition, these results illustrate some statistically significant differences in prevalence when comparison was based upon obesity class. Specifically, for all years, OSA was found to be statistically significantly higher as obesity class increased, and the same result was found for ASCVD in years 1–5 post index date. The results for atrial fibrillation, liver cirrhosis and NASH/NAFLD showed that in years 1–5 post index date, individuals with obesity class 3 had statistically significantly higher prevalences of these comorbidities relative to individuals with obesity class 1 or 2. Incidence rates for all outcomes for years 1–5 for all patients and by obesity class are provided in ESM Table 2.
Discussion
This retrospective, observational study measured the 5-year prevalence of mortality and morbidity in a population of US adults with concomitant obesity and T2D. Consistent with our original hypothesis, this population had a large burden of death and disease, with all comorbidities and mortality increasing significantly over the 5-year post index period. The 5-year prevalence of mortality (10.9%) for individuals with T2D and obesity was more than 11-fold higher than the 0.9% rate previously estimated over the time period from 2010 to 2017 for the average US adult age 55 to 64 years [23]. Regarding the degree of change in prevalences over the study period, mortality almost tripled, cardiovascular events quadrupled and microvascular events more than doubled. Consistent with previous research [21], those in the heaviest BMI category (class 3) in our study had the worst outcomes, with statistically significantly higher prevalences of mortality, cardiovascular events and microvascular events relative to those in the lower BMI categories.
Mortality
Previous studies on the association between BMI and mortality have also examined the relationship between obesity and mortality. Consistent with the current findings, the ACCORD trial revealed that individuals with T2D and class 3 obesity had the highest risk, relative to those with T2D and lower BMI categories, of all-cause mortality, cardiac death and non-cardiac death [21]. Similarly, a retrospective study of Iranian individuals with T2D found no statistically significant difference in the all-cause mortality rate when comparing individuals with obesity class 1 and obesity class 2, while individuals with obesity class 3 had statistically significantly higher mortality compared to individuals in either of the lower obesity classes [24]. In contrast, data from the ORIGIN trial revealed that for individuals with obesity class 1, 2 or 3, the unadjusted all-cause mortality rate declined as obesity class increased, although there was no statistically significant difference in the adjusted mortality rate between the three classes [25].
Cardiovascular Composite Outcome
Given that cardiovascular disease is the most common cause of death and illness among individuals with diabetes [26], our finding that obesity class 3 is associated with a statistically significantly higher prevalence of the CCO highlights the fact that individuals in this weight category constitute a unique treatment group. This finding is consistent with research showing that, even relative to other obesity classes, extreme (“morbid”) obesity is associated with worse myocardial outcomes, including a higher prevalence of eccentric left ventricular hypertrophy, an increased risk of arrhythmia leading to sudden death and decreased parasympathetic tone leading to an increased mean heart rate [27]. Likewise, other research has shown that the lifetime risk of cardiovascular events rises as BMI increases from obesity class 1 to 3 [12]. Obesity has been shown to increase the risk of cardiovascular disease directly by altering the structure and function of heart tissues and possibly indirectly by increasing the risk of related comorbidities, such as dyslipidemia and hypertension [27, 28].
Microvascular Composite Outcome
Generally consistent with the high prevalence of microvascular complications in our population with obesity and T2D, a recent observational study by Callaghan et al. of 138 individuals with obesity and 46 “lean controls” found a prevalence of neuropathy of 2.2% for the controls versus 7.1% for those with obesity and normoglycemia and 40.8% for those with obesity and diabetes [29]. Callaghan et al.’s findings suggest that neuropathy is independently associated with obesity and far more likely to be present when obesity co-occurs with diabetes. Given that Callaghan et al. [29] focused on individuals with BMI ≥ 35 kg/m2, the association between neuropathy and obesity may have been even stronger if they had used the standard BMI ≥ 30 kg/m2 threshold.
Our finding that individuals with obesity class 3 had a statistically significantly higher prevalence of nephropathy relative to those in class 1 is notable given that renal disease is a major cause of morbidity and mortality among those with either T2D or obesity. For instance, diabetes has been established as the most common cause of end-stage renal disease worldwide, and kidney dysfunction has been shown to be associated with obesity independent of diabetes [30]. In addition, research has shown that diabetic nephropathy is becoming an increasingly important cause of morbidity and mortality worldwide as the prevalence of T2D increases, largely due to the increase in the prevalence of obesity [30].
Other Complications
In addition to the outcomes discussed above, this study also examined the prevalence of ASCVD, atrial fibrillation, cirrhosis of the liver, NASH/NAFLD and OSA. The findings revealed that the frequency of all of these diagnoses generally increased as the BMI rose. Moreover, for each of these complications of interest, statistically significant differences among obesity classes existed after 5 years.
ASCVD and Atrial Fibrillation
Prior research has found that having overweight or obese is associated with a higher risk of cardiovascular events among individuals with T2D [31]. Consistent with that research and with the composite cardiovascular findings from our study, the prevalence of ASCVD rose with each higher strata of BMI, and the prevalence of atrial fibrillation remained consistently higher for those with class 3 obesity. Previous literature has identified ASCVD as the primary physical cost related to obesity, making the case that obesity leads to ASCVD by increasing key risk factors, including high cholesterol, high blood pressure and hyperglycemia, as well as “emerging” risk factors, including atherogenic lipid levels, insulin resistance, chronic inflammation and a tendency toward blood clotting [32]. Likewise, obesity has been shown to be a major, independent risk factor for the cause and progression of atrial fibrillation due to the associations between obesity and inflammation, fat deposition around the heart, fibrosis of the atrial myocardium, dysfunction of the nervous system concomitant with sleep apnea and excess visceral fat with its impact on the body’s electrical signaling [33].
NASH/NAFLD and Liver Cirrhosis
In our study, each higher BMI strata was associated with a greater prevalence of NASH/NAFLD, consistent with previous literature indicating a strong association between obesity and NAFLD [34], as well as with research which has shown that weight loss of ≥ 7% can mitigate or resolve NASH [35]. Our analyses also looked at liver cirrhosis and found that it was more prevalent among those with obesity class 3, in agreement with research which has shown that liver cirrhosis may progress from NAFLD and NASH [34, 36, 37]. In particular, up to 10% of NASH cases are estimated to progress to cirrhosis [34]. Given that liver cirrhosis is the 12th leading cause of death in the USA and is often asymptomatic at onset [38], our findings suggest that liver function should be monitored closely among individuals with T2D and obesity in general and with class 3 obesity in particular.
Obstructive Sleep Apnea
Nearly one-third (32.1%) of the present study population had OSA after 5 years, consistent with the previously well-documented link between obesity and OSA [39]. In addition, the prevalence of OSA increased as obesity class rose, in alignment with the findings of a study of a UK population of 276,600 individuals who had obesity but not necessarily T2D [22]. The prevalence of OSA was more than fivefold higher in this US population relative to the 5.45% prevalence found in a study in that UK study [22]. This difference in results may be because OSA may be underdiagnosed in the UK [22], and there is a lower prevalence of obesity in the UK relative to the USA [40]. Furthermore, the difference in results may be because our population had concomitant T2D, given the previously established association between glucose intolerance, insulin resistance and OSA [41].
Strengths and Limitations
The strengths of the present study include the large sample size, the use of standard BMI categories, the recent source data and the 5-year observation period. However, as with any research, this study also had limitations. One limitation was a risk of selection bias due to the requirement that patients have at least one recorded HbA1c measurement and one recorded BMI measurement, a requirement which excluded a fairly large proportion of individuals with claims in the database. Second, diabetes type and comorbid conditions were identified based solely on diagnostic codes, a method which is less rigorous than more formal assessments to identify comorbid conditions. Third, the focus on individuals who were relatively well insured may limit applicability to other populations. Fourth, while the study period extended through the beginning of the Covid-19 pandemic, the analyses did not formally examine possible effects of the pandemic. Fifth, while the analyses presented age- and sex-adjusted prevalence rates, it did not attempt to adjust for other factors, such as race, socioeconomic status, medication use or duration of diabetes, all of which also may affect outcomes. Furthermore, the analyses focused on annual prevalence rates and, as such, paid less attention to incidence rates and did not examine less aggregated time periods. In addition, the study focused on the relationship between obesity classes and outcomes but did not examine the development and severity of obesity over time for the patient population. Finally, the study examined prevalence rates only and did not attempt to examine causality.
Conclusion
In this US population of adults with concomitant T2D and obesity, higher categories of obesity in general and obesity class 3 in particular were associated with higher prevalence of mortality, cardiovascular events, microvascular diagnoses, ASCVD, atrial fibrillation, cirrhosis of the liver, NASH/NAFLD and OSA. Given the rapid increase in the prevalence of mortality and morbidity over 5 years among a population with consistent health insurance, the results of this study indicate an urgent need to aggressively treat people with obesity and T2D. While additional research is needed to confirm the results, the findings of this study suggest that treatments which help to reduce weight for adults with T2D, especially those who are obese class 3, may have significant health benefits.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Funding for this study, including the Rapid Service Fee, was provided by Eli Lilly and Company.
Medical Writing and/or Editorial Assistance
The authors would like to thank Patricia Nespor for her assistance in the writing of the manuscript. Ms. Nespor was compensated by HealthMetrics Outcomes Research for her assistance.
Author Contributions
Kristina S Boye conceptualized the study and was responsible for funding acquisition. Maureen J Lage conducted the analyses and drafted the manuscript. All authors (Kristina S Boye, Janet H Ford, Maureen J Lage, Kendra A Terrell and Vivian T Thieu) were responsible for study design and review and editing of the manuscript. All authors read and approved the final manuscript.
Disclosures
The authors Kristina Boye, Kendra Terrell and Vivian Thieu are employees and shareholders of Eli Lilly and Company and conducted this research as part of their employment. Janet Ford is currently employed at Agios Pharmaceutical and is a past employee/current shareholder of Eli Lilly and Company. Janet Ford conducted this research as part of her employment at Lilly. Maureen Lage was compensated by Eli Lilly and Company for her work on this research.
Compliance with Ethics Guidelines
Given the use of retrospective and deidentified data, ethics committee approval was not required. Permission was granted by Optum® for use of the data and all research was conducted in accordance with the 1964 Declaration of Helsinki.
Data Availability
The Optum® data used for this study are available via licensing agreement.
References
- 1.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. 2020. Atlanta: Centers for Disease Control and Prevention; 2020. https://www.cdc.gov/nchs/products/databriefs/db360.htm. Accessed 8 Jun 2022.
- 2.Centers for Disease Control and Prevention. Diabetes: diabetes fast facts. 2021. Atlanta: Centers for Disease Control and Prevention. https://www.cdc.gov/diabetes/basics/quick-facts.html. Accessed 8 Jun 2022.
- 3.Centers for Disease Control and Prevention. Diabetes: prevalence of both diagnosed and undiagnosed diabetes. 2021. Atlanta: Centers for Disease Control and Prevention; 2021.. https://www.cdc.gov/diabetes/data/statistics-report/diagnosed-undiagnosed-diabetes.html. Accessed 23 May 2023.
- 4.Boye KS, Lage MJ, Shinde S, Thieu V, Bae JP. Trends in HbA1c and body mass index among individuals with type 2 diabetes: evidence from a US database 2012–2019. Diabetes Ther. 2021;12:2077–2087. doi: 10.1007/s13300-021-01084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cawley J, Biener A, Meyerhoefer C, et al. Direct medical costs of obesity in the United States and the most populous states. J Manag Care Spec Pharm. 2021;27:354–366. doi: 10.18553/jmcp.2021.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association Economic costs of diabetes in the US in 2017. Diabetes Care. 2018;41(5):917–928. doi: 10.2337/dci18-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jehan S, Zizi F, Pandi-Perumal SR, et al. Obstructive sleep apnea and obesity: implications for public health. Sleep Med Disord. 2017;1(4):00019. [PMC free article] [PubMed] [Google Scholar]
- 8.Liu N, Birstler J, Venkatesh M, Hanrahan L, Chen G, Funk L. Obesity and BMI cut points for associated comorbidities: electronic health record study. J Med Internet Res. 2021;23(8):e24017. doi: 10.2196/24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iglay K, Hannachi H, Howie PJ, et al. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin. 2016;32(7):1243–1252. doi: 10.1185/03007995.2016.1168291. [DOI] [PubMed] [Google Scholar]
- 10.Abdul-Ghani M, DeFronzo RA, Del Prato S, Chilton R, Singh R, Ryder REJ. Cardiovascular disease and type 2 diabetes: has the dawn of a new era arrived? Diabetes Care. 2017;40(7):813–820. doi: 10.2337/dc16-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Heart disease: prevent heart disease. 2020.. Atlanta: Centers for Disease Control and Prevention. https://www.cdc.gov/heartdisease/prevention.htm. Accessed 9 Jun 2022.
- 12.Khan SS, Ning H, Wilkins JT, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3(4):280–287. doi: 10.1001/jamacardio.2018.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leitner DR, Frühbeck G, Yumuk V, et al. Obesity and type 2 diabetes: two diseases with a need for combined treatment strategies—EASO can lead the way. Obes Facts. 2017;10(5):483–492. doi: 10.1159/000480525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oldridge NB, Stump TE, Nothwehr FK, Clark DO. Prevalence and outcomes of comorbid metabolic and cardiovascular conditions in middle- and older-age adults. J Clin Epidemiol. 2001;54(9):928–934. doi: 10.1016/S0895-4356(01)00350-X. [DOI] [PubMed] [Google Scholar]
- 15.Boye KS, Lage MJ, Terrell K. Healthcare outcomes for patients with type 2 diabetes with and without comorbid obesity. J Diabetes Complications. 2020;34(12):107730. doi: 10.1016/j.jdiacomp.2020.107730. [DOI] [PubMed] [Google Scholar]
- 16.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Defining adult overweight and obesity: defining adult overweight and obesity. 2021, Atlanta: Centers for Disease Control and Prevention. https://www.cdc.gov/obesity/adult/defining.html. Accessed 18 Mar 2022.
- 18.American Diabetes Association. Understanding A1c: diagnosis. 1995. Arlington: American Diabetes Association; 1995. https://www.diabetes.org/diabetes/a1c/diagnosis. Accessed 4 Jul 2022.
- 19.US Census Bureau. Age and sex composition in the United States: 2019. 2019. Suitland: US Census Bureau. https://www.census.gov/data/tables/2019/demo/age-and-sex/2019-age-sex-composition.html. Accessed 4 Apr 2022.
- 20.Rassan JA, Bartels DB, Schneeweiss S, et al. Measuring prevalence and incidence of chronic conditions in claims and electronic health records databases. Clin Epidemiol. 2019;11:1–15. doi: 10.2147/CLEP.S181242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xing Z, Pei J, Huang J, Peng X, Chen P, Hu X. Relationship of obesity to adverse events among patients with mean 10-year history of type 2 diabetes mellitus: results of the ACCORD study. J Am Heart Assoc. 2018;7(22):e010512. doi: 10.1161/JAHA.118.010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erridge S, Moussa O, McIntyre C, et al. Obstructive sleep apnea in obese patients: a UK population analysis. Obes Surg. 2021;31(5):1986–1993. doi: 10.1007/s11695-020-05196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolf S, Schoomaker H. Life expectancy and mortality rates in the United States, 1959–2017. JAMA. 2019;322(20):1996–2016. doi: 10.1001/jama.2019.16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salehidoost R, Mansouri A, Amini M, Yamini SA, Aminorroaya A. Body mass index and the all-cause mortality rate in patients with type 2 diabetes. Acta Diabetol. 2018;55(6):569–577. doi: 10.1007/s00592-018-1126-y. [DOI] [PubMed] [Google Scholar]
- 25.Doehner W, Gerstein HC, Ried J, et al. Obesity and weight loss are inversely related to mortality and cardiovascular outcome in prediabetes and type 2 diabetes: data from the ORIGIN trial. Eur Heart J. 2020;41(28):2668–2677. doi: 10.1093/eurheartj/ehaa293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6(13):1246–1258. doi: 10.4239/wjd.v6.i13.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashraf MJ, Baweja P. Obesity: the “huge” problem in cardiovascular diseases. Mo Med. 2013;110(6):499–504. [PMC free article] [PubMed] [Google Scholar]
- 28.Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res. 2016;118(11):1723–1735. doi: 10.1161/CIRCRESAHA.115.306825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Callaghan BC, Reynolds E, Banerjee M, Chant E, Villegas-Umana E, Feldman EL. Central obesity is associated with neuropathy in the severely obese. Mayo Clin Proc. 2020;95(7):1342–1353. doi: 10.1016/j.mayocp.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maric C, Hall JE. Obesity, metabolic syndrome and diabetic nephropathy. Contrib Nephrol. 2011;170:28–35. doi: 10.1159/000324941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costanzo P, Cleland JG, Pellicori P, et al. The obesity paradox in type 2 diabetes mellitus: relationship of body mass index to prognosis: a cohort study. Ann Intern Med. 2015;162(8):610–618. doi: 10.7326/M14-1551. [DOI] [PubMed] [Google Scholar]
- 32.Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89(6):2595–2600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- 33.Vyas V, Lambiase P. Obesity and atrial fibrillation: epidemiology, pathophysiology and novel therapeutic opportunities. Arrhythm Electrophysiol Rev. 2019;8(1):28–36. doi: 10.15420/aer.2018.76.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma B, John S. Nonalcoholic Steatohepatitis (NASH). In: StatPearls. Treasure Island: StatPearls Publishing. 2022 [updated 2021 Sep 21]. https://www.ncbi.nlm.nih.gov/books/NBK470243. Accessed cited 17 Jun 2022.
- 35.Younossi ZM, Loomba R, Rinella ME, et al. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2018;68(1):361–371. doi: 10.1002/hep.29724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayo Clinic. Nonalcoholic fatty liver disease—symptoms and causes. Rochester: Mayo Clinic. 2021.. https://www.mayoclinic.org/diseases-conditions/nonalcoholic-fatty-liver-disease/symptoms-causes/syc-20354567. Accessed 17 Jun 2022.
- 37.Schiavo L, Busetto L, Cesaretti M, Zelber-Sagi S, Deutsch L, Iannelli A. Nutritional issues in patients with obesity and cirrhosis. World J Gastroenterol. 2018;24(30):3330–3346. doi: 10.3748/wjg.v24.i30.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith A, Baumgartner K, Bositis C. Cirrhosis: diagnosis and management. Am Fam Physician. 2019;100(12):759–770. [PubMed] [Google Scholar]
- 39.Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea. Chest. 2010;137(3):711–719. doi: 10.1378/chest.09-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.GBD 2015 Obesity Collaborators. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;77(1):13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kent BD, McNicholas WT, Ryan S. Insulin resistance, glucose intolerance and diabetes mellitus in obstructive sleep apnoea. J Thorac Dis. 2015;7(8):1343–1357. doi: 10.3978/j.issn.2072-1439.2015.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Optum® data used for this study are available via licensing agreement.