Highlights
-
•
Outpatient IV antibiotics can be safely given to individuals who inject drugs in a monitored outpatient setting.
-
•
Administering outpatient IV antibiotics to individuals who inject drugs reduces their hospital length of stay and improves their perception of care.
-
•
Long acting buprenorphine (Sublocade) and tamper resistant clamps (Neuma Clamp) are useful adjuncts in the outpatient management of individuals with serious infection and substance/opioid use disorder.
-
•
Daily safety and compliance checks in the infusion center are key to safely administering outpatient IV antibiotics to individuals who inject drugs.
Keywords: Intravenous drug use, outpatient antibiotic therapy, line protection clamp, buprenorphine, medication for opioid use disorder
Abstract
What is STOP OUD?
The STOP OUD project is an observational study on the use of long-acting buprenorphine (Sublocade) and a Tamper resistant PICC clamp for Outpatient IV antibiotic administration in Patients with serious infections and Opioid Use Disorder (STOP OUD).
Background
The US opioid crisis is driving up serious infections related to intravenous drug use. These infections require prolonged courses of antibiotics, often resulting in lengthy hospital stays. Extended hospitalizations for monitored parenteral antibiotics for patients with opioid use disorder are challenging for patients, reduce bed capacity, and are associated with significant cost. This observational study reviews the administration of intravenous (IV) antibiotics in a monitored outpatient setting using long-acting injectable buprenorphine (Sublocade, Indivior Inc., North Chesterfield, VA) and a tamper resistant clamp in patients with opioid use disorder .
Methods
Long-acting buprenorphine and a tamper resistant clamp were used to treat patients with serious infections and opioid use disorder as outpatients.
Results
Hospital days avoided were 30-days per STOP OUD project participant. Eleven of thirteen STOP OUD project participants completed their antibiotic courses as prescribed, there was no evidence of peripherally inserted central catheter (PICC) tampering, and they rated their care as a mean of 4.9/5 (SD 0.4). Institutional savings per STOP OUD patient was $33,000. Outpatient infusion costs were $9,300 for a net savings of $23,700 per STOP OUD project participant. Infections resolved in all participants.
Conclusions
The STOP OUD project reduced hospital length of stay for patients with opioid use disorder and serious infections, and had a favorable financial impact.
Introduction
More than 2 million people in the United States are estimated to have opioid use disorder (OUD), often resulting from prescription opioids for pain. Pennsylvania has been severely affected by the crisis and has the third-highest rate of drug overdose deaths in the US (Hedegaard et al., 2018). Individuals with substance use disorder (SUD) often inject drugs, which can lead to serious infections. Caring for patients with OUD and serious infections is complex. Infections like endocarditis and osteomyelitis require 4 – 6-week courses of antibiotics resulting in extended hospital stays (Schranz et al., A. 2020). Patients with OUD and serious infection often experience poor clinical outcomes (Bearnot et al., Bearnot et al., 2019).
In patients without a history of SUD, outpatient antibiotic therapy (OPAT) through a peripherally inserted central catheter (PICC) is routinely offered. OPAT is safe, cost effective, and improves patient satisfaction, allowing patients to return home sooner (Fanucchi et al., Fanucchi et al., 2020, Buehrle et al., 2017). Patients with intravenous drug use (IVDU) are not offered OPAT due to concerns regarding misuse of their intravenous access for nonprescription drug administration (Fanucchi et al., Fanucchi et al., 2019). A retrospective cohort study in a single tertiary care center revealed that access to oral antibiotic therapy in patients with OUD with invasive infections is beneficial if IV antibiotic therapy cannot be completed; however, further research is warranted before implementing this observation into clinical practice (Marks et al., 2020).
Medications such as buprenorphine or methadone are effective in treating patients with OUD (Bell et al., Bell and Strang, 2020, Coffa et al., Coffa and Snyder, 2019, McElrath et al., K. 2018). Buprenorphine diminishes dependency on opioids, increases safety in cases of overdose, and lowers the potential for opioid misuse (Substance Abuse and Mental Health Services Administration, 2021). A study including 20 participants with infections who received combined OPAT and medications for opioid use disorder (MOUD) showed a significant reduction in hospital length of stay (Fanucchi et al., Fanucchi et al., 2020). A retrospective study revealed decreased mortality in patients receiving MOUD post hospitalization for IVDU associated endocarditis in the month MOUD was used (Kimmel et al., 2020). Several small pilot studies looked at the prospect of OPAT in patients with OUD admitted with severe injection-related infections but are limited either by sample size or retrospective study design (Fanucchi et al., Fanucchi et al., 2020).
Our project adds to the existing literature by using long-acting injectable buprenorphine (Sublocade) to ensure MOUD compliance. Patients with OUD start by using full agonist opioids such as oxycodone or heroin. When they present to the hospital, they are generally experiencing withdrawal symptoms. Buprenorphine is administered sublingually to alleviate the symptoms of withdrawal. After seven days of sublingual buprenorphine and if they are tolerating the medication well, the patient can be offered long-acting injectable buprenorphine. One 300 mg shot provides thirty days of protection from withdrawal and opioid overdose. This extended duration of action ensures compliance with MOUD therapy during OPAT.
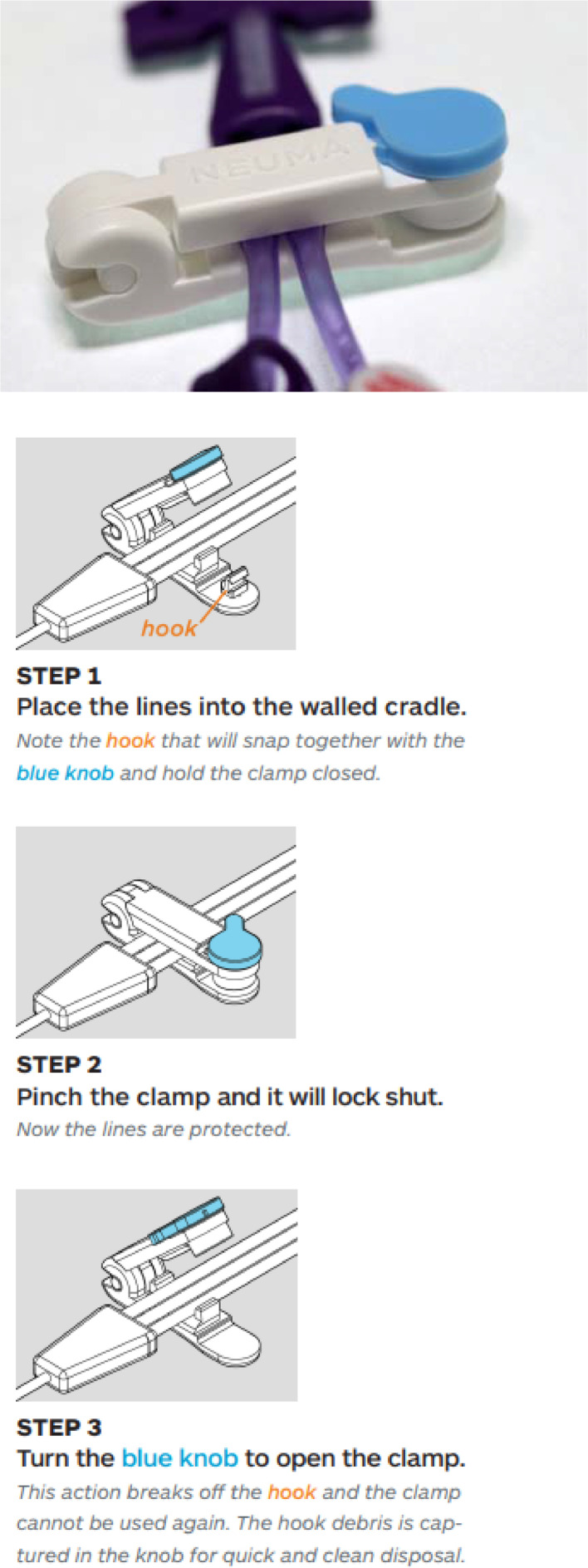
We also used a tamper resistant PICC clamp (Neuma Innovations, Burlingame CA, 2018, Fig. 1). Placing PICC lines in individuals with OUD raises the concern that opioids will be injected into the PICC line, leading to infection or overdose. We use a lockable plastic clamp which can be placed on the PICC line to deter tampering. It is a single use product which is broken when removed and a new one is applied following the infusion. PICC line tampering is immediately recognized if the clamp is broken or missing.
Fig. 1.
The Neuma Clamp. Neuma innovations LLC. 2018. The Neuma PICC and central line protection clamp introduction and frequently asked questions. www.neumainnovations.com. Accessed 11/19/2021.
This project reports on the feasibility of administering OPAT to patients with severe infections and OUD using long-acting buprenorphine and a tamper resistant clamp.
Methods
This was a multicenter observational report. Patients with a history of SUD/OUD use as defined by DSM-5 criteria admitted to the hospital with serious infections requiring prolonged (> 10 days) parenteral antibiotic therapy were considered for the study. Education was delivered across service lines and hospitals regarding the STOP OUD program. Referrals were made by hospitalists, infectious disease specialists, and case managers. Participants were given the option of continued inpatient care or outpatient STOP OUD care. The participants had to be willing to have a PICC line placed, sign a controlled substance agreement, have a stable living arrangement, and be able and willing to travel to the outpatient infusion center daily. Participants needed to have weekly lab work and to have expressed a desire to stop using recreational drugs. Insurance which covered treatment, testing, and follow-up was required and participants could not be incarcerated.
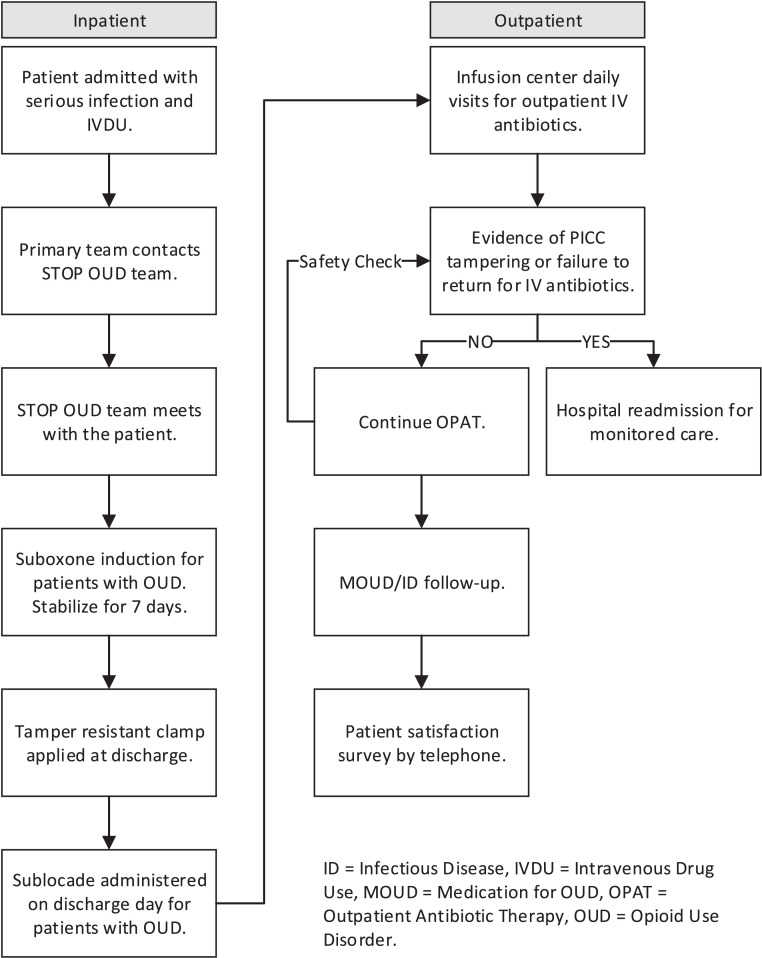
The STOP OUD process was followed (Fig. 2)
Fig. 2.
STOP OUD Process.
Study population
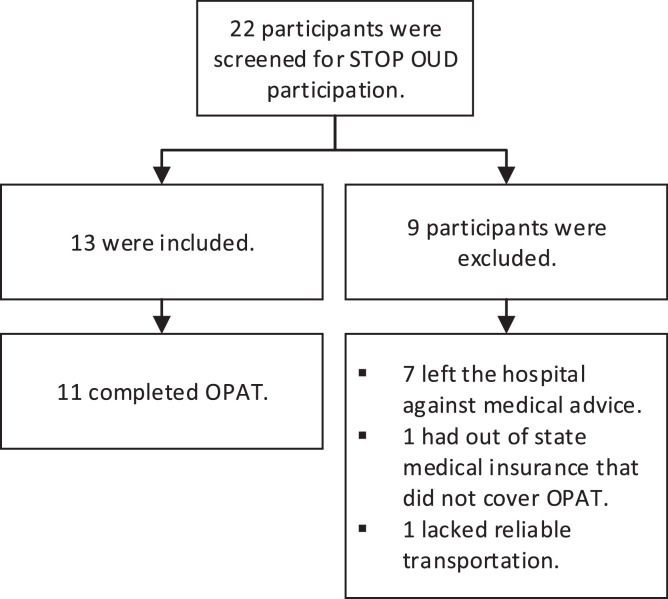
Twenty-two patients were referred to the STOP OUD team between May of 2020 and November of 2021 (Fig. 3). Of the thirteen who were included (Table 1), two failed to complete their courses of IV antibiotics. Once noncompliance was noted, they were contacted and readmission was offered and declined in both cases. The PICC lines were removed by home care nurses. Five were readmitted due to medical complications in their care. The first was a PICC-associated DVT which was found on the last day of IV antibiotic therapy. The participant with worsening valvular heart disease was readmitted after completion of OPAT. The participant with fever was readmitted after completion of OPAT. The participant with pneumonia was readmitted for four days during the course of IV antibiotic therapy. The participant with a UTI was admitted for ten days during IV antibiotic therapy.
Fig. 3.
STOP OUD Participants.
Table 1.
STOP OUD included subject characteristics.
| Pt # | Age | Gender | Race | Hosp LOS | Hosp days avoided | Organism | Antibiotic | Infection | Substance use history | PICC related adverse events | Course completed? | Infection resolved? | Readmission |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 26 | M | Caucasian | 8 | 34 | MSSA | Ceftriaxone | Bacteremia, spinal abscess, diskitis | Amphetamine | None | Y | Y | None |
| 2 | 57 | M | Black | 14 | 27 | MSSA | Daptomycin | Bacteremia, septic arthritis | Heroin | Arm DVT | Y | Y | Treatment for DVT |
| 3 | 36 | F | Caucasian | 7 | 51 | MSSA | Ceftriaxone | Bacteremia, diskitis, spinal osteomyelitis | Heroin | None | Y | Y | None |
| 4 | 43 | M | Puerto Rican | 12 | 37 | MSSA | Ceftriaxone | Bacteremia, pneumonia | Heroin | None | Y | Y | None |
| 5 | 60 | M | Caucasian | 18 | 28 | MSSA | Ceftriaxone | Bacteremia, prosthetic joint infection | Heroin | None | Y | Y | Fever |
| 6 | 26 | M | Caucasian | 6 | 21 | MSSA | Ceftriaxone | Osteomyelitis | Heroin | None | N | Y | None |
| 7 | 26 | F | Caucasian | 38 | 22 | MSSA | Ceftarolin, daptomycin, doxycycline | Bacteremia, infective endocarditis | Heroin | None | Y | Y | Worsening valvular heart disease |
| 8 | 34 | M | Caucasian | 9 | 28 | Strep viridans | Ceftriaxone | Hand abscess, tenosynovitis | IV cocaine | None | Y | Y | Pneumonia |
| 9 | 45 | F | Caucasian | 5 | 45 | MRSA, MRCNS | Daptomycin | Hand infection | Heroin | None | Y | Y | UTI |
| 10 | 34 | F | Caucasian | 5 | 9 | None | Ceftriaxone | Hand tenosynovitis | Heroin | None | Y | Y | None |
| 11 | 29 | M | Caucasian | 16 | 31 | MRSA | Daptomycin | Infective endocarditis | Heroin | None | Y | Y | None |
| 12 | 35 | M | Caucasian | 5 | 37 | Beta strep | Ceftriaxone | Prosthetic hip infection | Heroin | None | N | Y | None |
| 13 | 26 | F | Hispanic | 8 | 21 | MRSA | Daptomycin | Infective endocarditis, epidural abscess | Heroin | None | Y | Y | None |
| Ave. | 30 |
MSSA = methicillin sensitive Staphylococcus aureus, MRSA = methicillin resistant Staphylococcus aureus, MRCNS = methicillin resistant coagulase negative Staphylococcus, M = male, F = female, UTI = urinary tract infection, DVT = deep venous thrombosis, PICC = peripherally inserted central catheter, IV = intravenous.
Patients were excluded if they were unable to meet inclusion criteria (Table 2). All participants were offered drug and alcohol support services. None of the patients were lost to follow-up.
Table 2.
STOP OUD excluded subject characteristics.
| Pt # | Reason excluded | Organism | Infection | Substance use history | Language | Gender | Race | SO | GI |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Homeless, left against medical advice | MRSA | Bacteremia | Heroin | English | Male | Black | NA | NA |
| 2 | Out of state insurance not covering OPAT | MRSA | Bacteremia, lower extremity cellulitis | Heroin | English | Female | Caucasian | Straight | Cisgender |
| 3 | Left against medical advice | MRSA | Bacteremia, empyema | Heroin | English | Male | Caucasian | NA | NA |
| 4 | Missed OPAT, readmitted | MRSA | Bacteremia | Heroin | English | Female | Caucasian | Lesbian | Cisgender |
| 5 | Left against medical advice | Staph epi and propioni-bacterium | Osteomyelitis shoulder | Heroin | English | Male | Caucasian | NA | NA |
| 6 | Left against medical advice | Polymicrobial | Osteomyelitis | Heroin | English | Male | Black | NA | NA |
| 7 | Left against medical advice | MSSA | Endocarditis | Heroin | English | Female | Caucasian | NA | NA |
| 8 | Left against medical advice | MSSA | Endocarditis | Heroin | English | Female | Caucasian | Straight | Cisgender |
| 9 | Left against medical advice | Strep mitis | Endocarditis | Heroin | English | Male | None | Straight | Cisgender |
MRSA = methicillin resistant Staphylococcus aureus, MSSA = methicillin sensitive Staphylococcus aureus, OPAT = outpatient antibiotic therapy.
Intervention adherence
Eleven of thirteen participants completed OPAT. The STOP OUD project was designed to detect non-compliance. Participants traveled to the infusion center for daily IV antibiotic doses. In two cases, participant noncompliance was promptly detected and reported. There were no cases of PICC line tampering.
Statistical methods
Descriptive statistics were used to summarize the characteristics of the patients. Categorical variables were reported as number and percent, and numerical variables were reported as mean and range. Hospital days avoided were calculated by subtracting the participants’ hospital length of stay from the total duration of antibiotic administration. This projection was based on the number of days required to complete the course of IV antibiotics according to the Infectious Disease Society of America guidelines.
The cost per day was provided by the hospital finance department. The cost per day multiplied by hospital days avoided produces the savings per STOP OUD project participant. The national average hospital length of stay in 2016 was approximately 4.5 days with an average revenue per admission of $11,700 (Freeman et al., Freeman et al., 2018). Estimated additional revenue per STOP OUD project participant is hospital days avoided divided by average length of stay multiplied by the average revenue per admission. The total estimated financial variance per STOP OUD project participant is the savings per patient plus the additional revenue. Outpatient costs were provided by the hospital finance department. Outpatient costs included those related to the infusion center pharmacy receipts (not charges). The monetary impact per STOP OUD project participant was projected based on system financial data. A 5-point Likert scale was used for the patient satisfaction survey (Fig. 4).
Fig. 4.
All analysis was done in Microsoft Excel
Ethical considerations
Participants were informed of the project design. The project director coordinated with the providers involved in the participant's care. Participation in the STOP OUD project was not limited by race, sex, sexual orientation, or gender identity. Participant safety and compliance was monitored throughout therapy during daily infusion center visits.
The project was observational in design and was exempted by the Institutional Review Board of UPMC, Central Pennsylvania Region.
Results
Mean hospital days avoided by the STOP OUD project were thirty. Each hospital day costs the health system approximately $1,100, leading to institutional savings of $33,000 per participant. Assuming an average length of stay of 4.5 days, 6.6 additional admissions could be accommodated in the vacated bed. Assuming average hospital revenue per admission of $11,700 (Liang et al., 2020), an estimated $78,371 per participant could be generated, with an estimated favorable variance of $111,371 per STOP OUD project participant. Participants rated their care highly at a mean of 4.9/5 (SD 0.4).
Outpatient costs ranged from $170 - $200 per day for the infusion center and $100 - $150 per day for pharmacy, drug, and misc. costs. The total cost per patient per day for outpatient infusions was $270 - $350, or approx. $9,300 for a 30-day course of care.
Inpatient savings (approx. $1,100/day) less outpatient costs (approx. $310/day) yields net savings of $790 per patient per day, or $23,700 per 30-day course of care.
Discussion
An increasing number of patients with OUD are hospitalized with severe, injection-related infections amidst the opioid epidemic (Kim et al., 2020). Prolonged hospitalizations increase health costs, consume healthcare resources, and are burdensome for patients. Our hospital system's standard of care prior to implementation of the STOP OUD project was for individuals with OUD/SUD and serious infections to remain in the hospital for monitored care through the completion of their antibiotic course. The average length of stay for this group in 2019 was 41.6 days. Inpatient monitored care has its proponents (Tice et al., 2004, Rapoport et al., 2017). Outpatient parenteral antibiotic therapy has been found to be more cost-effective than inpatient treatment (Beieler, 2016). Following initiation of long-acting injectable buprenorphine, participants enrolled in the STOP OUD project were discharged after approximately 2 weeks, or approximately 4 weeks sooner than was previously observed for a similar patient population.
Several pilot studies have been published which have described outpatient IV antibiotic use in patients with OUD. A recent review has similarly shown successful completion of outpatient IV antibiotic therapy in patients with OUD. No deaths were reported using this approach (Suzuki et al., Suzuki et al., 2018). Our study adds to this research through the successful use of long-acting injectable buprenorphine, a tamper resistant clamp, and monitored outpatient antibiotic infusions.
Keeping this patient population in the hospital can be a challenge. While against medical advice (AMA) discharges comprise 2% of US discharges (Alfandre et al., 2017), 32% of patients in our study group left the hospital prematurely. This occurred despite the availability of buprenorphine. The use of MOUD, a tamper resistant clamp, and a shortened hospital length of stay all work together to support this vulnerable patient population to complete their inpatient stay and safely return home to complete treatment.
Heroin was the most common type of injection drug encountered in our project. Other injectable drugs such as cocaine and methamphetamine were used. These individuals were not treated with buprenorphine. Since there is no medication which protects patients from injectable cocaine or methamphetamine, these patients may be at higher risk of drug relapse following hospital discharge. While the two patients in our study successfully completed OPAT, further study is warranted to verify the safety of this approach.
Administering IV antibiotics in an infusion center has advantages as well as limitations. The advantage is the safety and compliance check. If the participant fails to present for a dose or if the clamp is missing, it is known immediately. The disadvantages with this approach are that antibiotics can only be given once daily and participants must have transportation to the infusion center.
Building a STOP OUD program requires multidisciplinary collaboration. Addiction medicine, infectious disease, IV therapy, infusion services, pharmacy, nursing, case management, and inpatient providers all play a role. We started STOP OUD programs at each of our system hospitals. The key elements of our STOP OUD program include inpatient buprenorphine induction, long-acting injectable buprenorphine administration, a supply of tamper resistant clamps, 7-day per week availability of outpatient infusion services, MOUD and infectious disease follow up, and a path to readmission for noncompliance or safety concerns. The wholesale price of long-acting buprenorphine 300 mg injections is $1,394/dose (Lexicomp, 2021), a cost which is offset by savings seen through a reduced hospital length of stay.
Limitations
Given the small sample size in this study, generalizability to a larger population is limited. The patients needed to be motivated to seek substance use treatment. Despite the offer of buprenorphine, several screened patients declined. Urine toxicology testing was not a part of this project. While these results are promising, the safety of this approach needs to be confirmed with further study.
Outpatient MOUD follow up is important for patients with OUD. Some of our participants received either methadone or buprenorphine outside of our program. We elected to support our patients’ previously established support systems rather than compel them into our MOUD treatment program. The downside of this is that we did not control their outpatient MOUD follow up. We were able to follow our patients closely while they were receiving their antibiotics. However, once they completed antibiotic treatment, long-term follow up was limited.
Selection bias was not controlled in the study due to the observational study design.
Conclusion
This project adds to available knowledge that outpatient IV antibiotics can be given safely to patients with severe infection and OUD/SUD. Long-acting injectable buprenorphine and tamper resistant PICC clamps can add to the safety of treating OUD/SUD patients with severe infections outside the hospital. This project appeals to patients and health systems alike by satisfying the triple aim of improved patient experience, improved population health, and reduced cost (Berwick et al., 2008).
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
Funding
UPMC Central PA made this study possible. UPMC Central PA employed members of the medical staff, case management, IV therapy, and pharmacy managed this project.
Acknowledgements
We would like to thank Helen L. Houpt, MSLS, AHIP for her insightful editorial support and Yijin Wert, MS for her statistical support.
Main point
This observational study reports on the use of long acting buprenorphine and a tamper resistant clamp in the outpatient treatment of serious infections in patients who inject drugs.
Contributor Information
Thomas Pineo, Email: pineotz2@upmc.edu.
John D. Goldman, Email: goldmanjd@upmc.edu.
Greg Swartzentruber, Email: swartzentrubergs2@upmc.edu.
Hafiz Qurashi, Email: qurashih@upmc.edu.
Christina Dimech, Email: dimechct@upmc.edu.
References
- Alfandre D., Brenner J., Onukwugha E. Against Medical Advice Discharges. J. Hosp. Med. 2017;12(10):843–845. doi: 10.12788/jhm.2796. [DOI] [PubMed] [Google Scholar]
- Bearnot B., Mitton J.A., Hayden M., Park E.R. Experiences of care among individuals with opioid use disorder-associated endocarditis and their healthcare providers: Results from a qualitative study. J. Subst. Abuse Treat. 2019;102:16–22. doi: 10.1016/j.jsat.2019.04.008. [DOI] [PubMed] [Google Scholar]
- Beieler A.M., Dellit T.H., Chan J.D., Dhanireddy S., Enzian L.K., Stone T.J., Dwyer-O’Connor E., Lynch J.B. Successful implementation of outpatient parenteral antimicrobial therapy at a medical respite facility for homeless patients. J. Hosp. Med. 2016;11(8):531–535. doi: 10.1002/jhm.2597. [DOI] [PubMed] [Google Scholar]
- Bell J., Strang J. Medication treatment of opioid use disorder. Biol. Psychiatry. 2020;87(1):82–88. doi: 10.1016/j.biopsych.2019.06.020. [DOI] [PubMed] [Google Scholar]
- Berwick D.M., Nolan T.W., Whittington J. The triple aim: care, health, and cost. Health Aff. (Millwood) 2008;27(3):759–769. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]
- Buehrle D.J., Shields R.K., Shah N., Shoff C., Sheridan K. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infectious Diseases. 2017;4(3) doi: 10.1093/ofid/ofx102. ofx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffa D., Snyder H. Opioid use disorder: Medical treatment options. Am. Fam. Physician. 2019;100(7):416–425. [PubMed] [Google Scholar]
- Fanucchi L.C., Walsh S.L., Thornton A.C., Lofwall M.R. Integrated outpatient treatment of opioid use disorder and injection-related infections: A description of a new care model. Prev. Med. 2019;128 doi: 10.1016/j.ypmed.2019.105760. [DOI] [PubMed] [Google Scholar]
- Fanucchi L.C., Walsh S.L., Thornton A.C., Nuzzo P.A., Lofwall M.R. Outpatient parenteral antimicrobial therapy plus buprenorphine for opioid use disorder and severe injection-related infections. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2020;70(6):1226–1229. doi: 10.1093/cid/ciz654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman W.J., Weiss A.J., Heslin K.C. Overview of U.S. Hospital Stays in 2016: Variation by Geographic Region. Healthcare Cost and Utilization Project. 2018 https://www.hcup-us.ahrq.gov/reports/statbriefs/sb246-Geographic-Variation-Hospital-Stays.jsp. [PubMed] [Google Scholar]
- Hedegaard H., Miniño A.M., Warner M. Drug overdose deaths in the United States, 1999-2017. NCHS Data Brief. 2018;(329):1–8. November 2018. Accessed July 26, 2021. https://www.cdc.gov/nchs/products/databriefs/db329.htm. [PubMed] [Google Scholar]
- Kim J.H., Fine D.R., Li L., Kimmel S.D., Ngo L.H., Suzuki J., Price C.N., Ronan M.V., Herzig S.J. Disparities in United States hospitalizations for serious infections in patients with and without opioid use disorder: A nationwide observational study. PLoS Med. 2020;17(8) doi: 10.1371/journal.pmed.1003247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel S.D., Walley A.Y., Li Y., Linas B.P., Lodi S., Bernson D., Weiss R.D., Samet J.H., Larochelle M.R. Association of treatment with medications for opioid use disorder with mortality after hospitalization for injection drug use-associated infective endocarditis. JAMA Network Open. 2020;3(10) doi: 10.1001/jamanetworkopen.2020.16228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexicomp Buprenorphine: Drug Information. UpToDate. 2021 Retrieved July 29, 2021 from https://www.uptodate.com/contents/buprenorphine-drug-information?search=sublocade&source=panel_search_result&selectedTitle=1∼148&usage_type=panel&kp_tab=drug_general&display_rank=1#F5989198. [Google Scholar]
- Liang L., Moore B., Soni A. National inpatient hospital costs: The most expensive conditions by payer, 2017: Statistical Brief #261. In Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US) 2020 [PubMed] [Google Scholar]
- Marks L.R., Liang S.Y., Muthulingam D., Schwarz E.S., Liss D.B., Munigala S., Warren D.K., Durkin M.J. Evaluation of partial oral antibiotic treatment for persons who inject drugs and are hospitalized with invasive infections. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2020;71(10):e650–e656. doi: 10.1093/cid/ciaa365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath K., Joseph H. Medication-assisted treatment (MAT) for opioid addiction: Introduction to the special issue. Subst. Use Misuse. 2018;53(2):177–180. doi: 10.1080/10826084.2017.1404106. [DOI] [PubMed] [Google Scholar]
- Substance abuse and mental health services administration (SAMHSA) office of communications Buprenorphine. 2021 www.samhsa.gov/medication-assisted-treatment/medications-counseling-related-conditions/buprenorphine. Accessed May 11, 2021. [Google Scholar]
- Suzuki J., Johnson J., Montgomery M., Hayden M., Price C. Outpatient parenteral antimicrobial therapy among people who inject drugs: A review of the literature. Open Forum Infectious Diseases. 2018;5(9) doi: 10.1093/ofid/ofy194. ofy194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuma innovations LLC. 2018. The Neuma PICC and central line protection clamp introduction and frequently asked questions. www.neumainnovations.com. Accessed 11/19/2021.
- Rapoport A.B., Fischer L.S., Santibanez S., Beekmann S.E., Polgreen P.M., Rowley C.F. Infectious diseases physicians' perspectives regarding injection drug use and related infections, United States, 2017. Open Forum Infectious Diseases. 2018;5(7):ofy132. doi: 10.1093/ofid/ofy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schranz A., Barocas J.A. Infective endocarditis in persons who use drugs: Epidemiology, current management, and emerging treatments. Infect. Dis. Clin. North Am. 2020;34(3):479–493. doi: 10.1016/j.idc.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tice A.D., Rehm S.J., Dalovisio J.R., Bradley J.S., Martinelli L.P., Graham D.R., Gainer R.B., Kunkel M.J., Yancey R.W., Williams D.N. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2004;38(12):1651–1672. doi: 10.1086/420939. [DOI] [PubMed] [Google Scholar]