Highlights
-
•
Prospective longitudinal (by menstrual cycle phase) smoking cue neuroimaging study.
-
•
Women were imaged in 3 phases of the menstrual cycle during smoking cue exposure.
-
•
Hormone status was biochemically confirmed.
-
•
Ventral striatal responses to smoking cues were greatest during the late follicular phase.
-
•
Ventral striatal responses to smoking cues were least during the early follicular phase.
Keywords: Menstrual Cycle, Ventral Striatum, Cue reactivity, Women, Smoking, Cigarettes, Estradiol, Progesterone
Abstract
Background
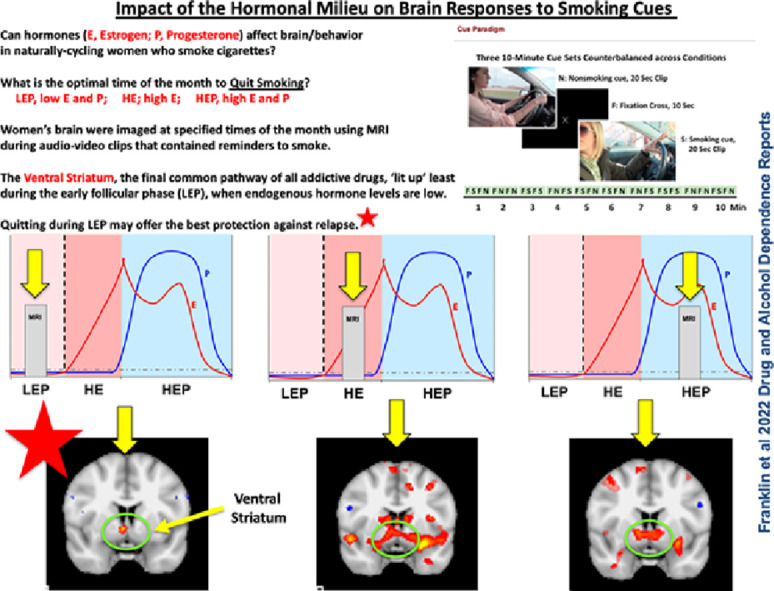
The female sex hormones estradiol (E) and progesterone (P) galvanize the ventral striatal reward pathway. E elevates ventral striatal dopamine and accelerates drug-cued reinstatement, while P has opposing ‘protective’ effects on drug-related behavior. We hypothesize that women may exhibit greater ventral striatal responses to smoking cues (SCs) during the late follicular phase of the menstrual cycle (MC) when E is high and unimpeded by P, and reduced responses during the late luteal phase when P is high.
Methods
To test our hypothesis, 24 naturally cycling cigarette-dependent women completed functional magnetic resonance (fMRI) sessions over the course of 3 MCs at select time points to reflect the early follicular (low E and P; LEP, control condition), late follicular (high E, low P; HE) and mid-luteal (high E, high P; HEP) MC phases. During fMRI sessions (counterbalanced by phase), women were exposed to a SC versus nonSC audio-visual clip. Ovulation was verified for each MC, and hormone levels were acquired prior to sessions.
Results
Contrasts within conditions showed that ventral striatal brain responses to SCs versus nonSCs were negligible during LEP and greater during HE (p=0.009) and HP (p=0.016). Contrasts across conditions showed that HE and HEP had greater responses than LEP (p=0.005), and HE had greater responses than HEP (p=0.049).
Conclusions
Results support and extend our retrospective cross-sectional study of the influence of the hormonal milieu on SC reactivity. Results are clinically relevant as they may guide novel, hormonally-informed and immediately translatable treatment strategies that can potentially reduce relapse in naturally cycling women.
Graphical abstract
1. Introduction
Several convincing lines of research indicate that female gonadal sex hormones galvanize the ventral striatal reward pathway (Carroll et al., 2009; Di Paolo et al., 1985; Dluzen and Anderson, 1997; Lynch et al., 2002; Thompson and Moss, 1994; Yoest et al., 2018), a key component of motivational circuitry and reward-related behavior (Kalivas and Volkow, 2005; Yoest et al., 2018). Estradiol (E) and progesterone (P) are the two major ovarian hormones that influence female brain function and behavior (Becker et al., 2002). In preclinical studies examining the properties underlying motivated ‘addictive’ brain and behavioral responses, E elevates ventral striatal dopamine and accelerates both drug-cued and drug-primed reinstatement, an animal model of relapse, while P has opposing ‘protective’ effects on drug-related behavior (Becker and Hu, 2008; Di Paolo et al., 1985; Dluzen and Anderson, 1997; Lynch, 2008). For example, self-administration of nicotine, an animal model of drug-seeking, is positively correlated with the E to P ratio and inversely correlated with P (Lynch, 2009). Clinical research examining the influence of hormones on addiction processes is generally supportive of the animal literature (Becker et al., 2017; Fattore et al., 2008; Wetherill et al., 2016). Research has shown that exogenously administered P decreases the positive subjective effects of combustible cigarette smoking and associated craving (Sofuoglu et al., 2001), reduces smoking intensity as measured by puff volume (Harrison et al., 2020), and higher within-person P levels predict reductions in the number of cigarettes smoked per day (Baker et al., 2020). These studies and others (DeVito et al., 2014; Mello, 2010; Schiller et al., 2012) suggest that P, of which levels are greatest relative to E during the mid-luteal phase of the menstrual cycle, may offer some protection over drug-seeking behavior and drug use.
Based on the preclinical and clinical research briefly outlined above, we hypothesize that women may experience nicotine, cigarettes, and smoking reminders as more rewarding when E is high and unimpeded by P. Possible consequences are relapse in those who have quit or greater difficulty during a quit attempt (Franklin and Allen, 2009). Thus, in Franklin et al. (2015), we retrospectively grouped women by self-reported MC phase and utilized a functional magnetic resonance imaging (fMRI) smoking cue paradigm to examine the influence of MC phase on neural responses to smoking cues. Women were categorized as luteal (the 14 days prior to the first day of menses) or follicular (the remaining days of the MC) (Reed and Carr, 2000). We observed that the follicular phase of the MC, when E is high and unopposed by P, is associated with greater reward-related neural responses to smoking cues than during the luteal phase (Franklin et al., 2015). However, our study was significantly limited in that it was cross-sectional, and MC phase was not biochemically verified. Thus, we designed the current fMRI study to prospectively test our hypothesis in a new cohort of naturally cycling women. We aimed to link biochemically verified hormonal status with reward-related responses to smoking cues in a longitudinal counterbalanced design. Because of its role in addictive motivated behavior, we focused on the ventral striatum. Specifically, we hypothesized that (1) women will exhibit greater reward-related responses to appetitive smoking cues during the late follicular phase (high E) compared to the early follicular phase (low E and P), and (2) women will exhibit reduced reward-related responses during the mid-luteal phase (high P, high E) compared to the early follicular phase.
The World Health Organization identifies cigarette smoking as the leading cause of 6 out of 8 of the top diseases that lead to premature death (World Health Organization, 2011). Compared to men, it is well-documented that the health burden of smoking is higher for women (Huxley and Woodward, 2011; Stabile and Siegfried, 2003), and smoking cessation is more difficult to attain (Smith et al., 2016). Approximately 35% of women of childbearing age do not use contraception, and another 9% use a condom (Center for Disease Control, 2018), suggesting that approximately 44% of women of childbearing age experience a naturally-occurring MC. Thus, information provided by the current study is clinically relevant, may generalize to other substance use disorders, and may guide novel, hormonally-informed and immediately translatable treatment strategies with the potential to reduce relapse, thereby aiding in our quest to improve smoking cessation rates for all.
2. Methods
2.1. Design overview
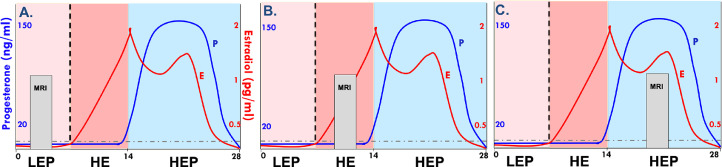
The current longitudinal, within-subjects neuroimaging study examined the influence of biochemically-verified ovarian hormone fluctuations on the ventral striatal response to smoking cues (SCs) in naturally cycling women who smoke combustible cigarettes over 3-4 menstrual cycles. Participants completed at least 3 hormone biochemical verification visits and 3 fMRI sessions over the course of the study. As previously described (Wetherill et al., 2021) and shown in Fig. 1, a typical 28-day menstrual cycle (MC) has three major hormonal phases. MRIs were scheduled once per MC at carefully selected time points to reflect the early follicular (low E and P; LEP), late follicular (high E, low P; HE) and mid-luteal (high E, high P; HEP) phases. LEP served as the control condition as hormonal influences are negligible during this time of the MC. Ovulation was verified for each MC, and hormone levels were acquired prior to fMRI sessions to ensure proper MC phase assignment.
Fig. 1.
Three consecutive menstrual cycles (MCs) are shown in A., B., and C. The X axis denotes days, 1 – 28. The Y axis denotes hormone levels. E, estradiol levels are shown in red and P, progesterone levels are shown in blue. Light pink background signifies the early follicular phase (low E and P, LEP), dark pink signifies the late follicular phase (high E and low P, HE), and blue signifies the mid-lutual phase (high E and high P, HEP). Magnetic resonance imaging (MRI) occurred at strategic intervals over the course of the three MCs (one MRI per month) to capture the greatest variability in E and P (counterbalanced across participants).
2.2. Recruitment
The study was conducted at the Center for Studies of Addiction at the University of Pennsylvania, Philadelphia, PA, USA. All procedures were approved by the University of Pennsylvania's Institutional Review Board and were conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent.
Physically and mentally healthy females aged 18 to 45 years (age cutoff is to prevent the inclusion of peri-menopausal females) were recruited using flyers, advertisements, and social media platforms (e.g., Facebook). Eligible participants smoked ≥ 5 cigarettes/day and smoked ≥ 1 year prior to study start and practiced a medically acceptable, nonhormonal method of birth control (i.e., diaphragm or condom, nonhormonal intrauterine device, or abstinence). Exclusion criteria included the use of nicotine products other than cigarettes; currently attempting to quit smoking; a positive urine drug screen for drugs other than nicotine or cannabis; a current DSM-5 Axis I diagnosis (other than nicotine use disorder, mild or moderate cannabis use disorder, and mild or moderate alcohol use disorder) as determined by a psychological examination by a trained clinician using the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998); a diagnosis of endometriosis, polycystic ovarian syndrome, or premenstrual dysphoric disorder; use of a hormonal contraceptive during the 2 months prior to screening; pregnancy, lactating, or a desire to become pregnant over the next 4 months; irregular or missing menses during the 3 months prior to screening; irregular MC length (outside of a 23-32 day window) as determined by a physical examination and structured medical history performed by a nurse practitioner; and any MRI contraindications (Dill, 2008). Ninety women successfully consented via telephone interviews. Of those, 43 were not enrolled. Ten women were lost to follow up, and 4 lost interest. The remaining 29 were excluded during the baseline physical and psychological visit because of the following reasons: 11, psychological comorbidities; 7, physical health issues; 1, irregular MC length; 2, taking medications that affect brain homeostasis; 1, receiving treatment for substance use disorder; 1, positive drug screen; 1, pregnant; 2, MRI incompatible; and 3, negative cotinine. Forty-seven women were enrolled in the study, of which 15 were withdrawn for the following reasons: 1, health issue; 6 noncompliant with study procedures; 1, irregular ovulation; 1, irregular hormone levels; 1, irregular MC length; 1, positive pregnancy test; and 4, lost interest or lost to follow up. Of the 32 remaining participants, 2 quit smoking during the study, and 6 had hormone levels on one or more of the fMRI session days that did not correspond to the correct MC phase. This resulted in a final sample size of 24 participants that completed 3 fMRI sessions during the 3 phases of the MC under investigation.
2.3. Procedures
Following informed consent and screening, eligible participants participated in a baseline examination scheduled during their LEP phase to verify the self-report of MC status, record baseline serum hormone levels, and conduct a pregnancy test. During this visit, research staff instructed participants on the appropriate use of an ovulation predictor kit and advised participants on topical and/or oral medications to avoid throughout the study that could interfere with hormone assays. Participants were then counterbalanced to one of 3 conditions (LEP; corresponding to 2-5 days after the onset of menses, HE; corresponding to 7-14 days after the onset of menses or HEP; corresponding to 5-7 days after ovulation). Given that MC length is variable across women (Lenton et al., 1983), these timeframes were adjusted based on each woman's natural MC (Wetherill et al., 2021). After verifying ovulation, biochemical verification of hormonal status and MRI visits were scheduled and completed. MRI visits were scheduled to occur once per month to allow for a wash-out period (average length of time between visits was (mean ± SEMs) 28.8 ± 3.50.
MRI test days began at the same time of day for all participants to reduce interference from diurnal hormonal fluctuations (Bao et al., 2003). Participants provided a urine sample for drug, pregnancy, and cotinine testing and a saliva sample for hormonal assay assessment. Participants smoked a cigarette approximately 90 minutes prior to scanning. The scanning session included a 5-minute resting-state blood oxygen level-dependent (BOLD) scan, a 5-minute resting-state pseudo-continuous arterial spin-label perfusion scan, and a 10 min SC task during BOLD data acquisition, the focus of the current study. To assess self-reported craving, withdrawal, and mood throughout the scanning session, participants completed the Within Session Rating Scale (WSRS), a brief 9-item measure designed to measure state-related mood, cigarette craving, and interest in task-related stimuli (formerly the Craving and Withdrawal Questionnaire) (Childress et al., 1999; Franklin et al., 2015, 2007; Wetherill et al., 2013).
2.4. Biochemical measurements
2.4.1. Hormone measurements
The blood (serum) samples acquired to verify hormone status were assayed by the Penn Fertility Care Endocrine Laboratory (same or next day). Test Day saliva samples were stored in a (-) 86°C freezer and shipped in bulk to Zava Research Testing (CLIA-certified, #38D0960950). Evidence of ovulation was tracked using the Ovulation Predictor Kit manufactured by ClinicalGuard®. Pregnancy was assessed using FDA-approved urine pregnancy test strips that measure the presence β-human chorionic gonadotropin.
2.4.2. Nicotine measurements
Expired carbon monoxide (CO), an indicator of recent smoking behavior, was measured using a calibrated CO gas-monitoring device manufactured by MicroDirect®. At the consent visit, a rapid qualitative nicotine test was used to detect cotinine. At subsequent visits, a quantitative cotinine screen was used to assess cigarette smoking. Samples were assayed at the Veteran's Affairs Medical Center Pathology and Laboratory Medical Services, Philadelphia, PA.
2.5. Smoking cue and nonsmoking cue stimuli and cue task
The smoking and nonsmoking cues for this task were explicitly designed for the demographic of the current study and are described in detail and illustrated in our proof of concept study (Wetherill et al., 2021). Briefly, the appetitive SCs include people 20-40 years of age and of various ethnic-racial identities engaged in activities such as driving, conversing with others, strolling and engaging in behavior indicative of ‘enjoyment’ while smoking cigarettes. Comparator nonSCs include similar but different actors engaged in similar activities while explicitly not smoking. The 10-minute cue paradigm consists of 20 10-second fixation cross screens (a black screen with an X at its center) interspersed with 10 of each 20-second SCs and nonSCs in a fixed order. No other cue types were presented. Stimuli were presented using E-prime (version 3.0, Psychology Software Tools Inc.).
A different but similarly valenced cue set was presented at each MRI session to control for possible habituation effects. MRI scheduling and cue sets were counterbalanced by MC phase to control for order effects.
2.6. Analyses
2.6.1. MRI acquisition, preprocessing, and analyses
As in our previous publications (Regier et al., 2022; Wetherill et al., 2014a), T2*-weighted BOLD images were acquired on a Siemens MAGNETOM 3.0 Tesla scanner (Siemens AG, Erlangen, Germany). Parameters used for the single-shot gradient echo (GRE) echo planar imaging (EPI) sequence were: field of view (FOV) = 192, matrix 64 × 64, repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 80°. Images were slice time corrected, realigned and unwarped, co-registered to the structural MRI image (T1-weighted 3-dimensional high resolution magnetization-prepared rapid acquisition with gradient echo; MPRAGE); FOV = 240, matrix 192 × 256, TR = 1820 ms, TE = 3.5 ms, flip angle = 9°, voxel size = 0.9 × 0.9 × 1 mm), normalized to the Montreal Neurological Institute (MNI) standard space, and smoothed using an 8-mm full-width at half-maximum Gaussian kernel. Individual first-level (within-subject) analyses were conducted using a general linear model to measure relationships between event-related BOLD signals and regressors encoding experimental conditions (e.g., SCs versus nonSCs). Experimental conditions and canonical hemodynamic response functions were convolved to create regressors. Motion estimates made during motion correction were added as control factors. Analyses were conducted using Statistical Parametric Mapping software (SPM12, Wellcome Department of Cognitive Neurology, London, UK) within a MATLAB environment (MATLAB 2019a; The Mathworks, Inc., Natick, Massachusetts, USA).
2.6.2. Behavioral data analyses
Statistical significance tests used an alpha of .05. Continuous variables were summarized by calculating means and SEMs.
2.6.3. Hormonal analyses
One-way repeated-measures ANOVAs were used to analyze saliva ovarian hormone concentrations. Post-hoc pairwise comparisons were made using t-tests (Bonferroni corrected).
3. Results
3.1. Participants
Participants were 24 naturally cycling women with an average age of (means ± SEMs) 30.9 ± 1.3 and 14.8 ± 0.3 years of education. Participants smoked 11.1 ± 1.1 cigarettes per day for 13.6 ± 1.3 years, providing a pack year score of 7.9 ± 1.3. Cigarette dependence was moderate as measured by the Fagerstrom Test for Cigarette Dependence (4.0 ± 0.3) (Fagerstrom, 2012). One participant met criteria for a current DSM-5 Axis I diagnosis of mild alcohol use disorder, and 1 participant met criteria for mild cannabis use disorder. Four women self-identified as non-Hispanic Black and twenty as non-Hispanic White. Participant's MC length was normalized to the average length of all participants (M=28.6 days, SEM=0.42) by dividing the day of the participant's MC, wherein hormonal assays were acquired, by the length of that month's MC, multiplied by the average MC length of all participants (Wetherill et al., 2021). Normalized MC phase day on the day of MRI was LEP, 4.4 ± 0.42; HE, 13.2 ± 0.34 and HEP, 22.7 ± 0.49.
3.2. Hormones
The mean fluctuations in ovarian hormones corresponded well with the literature (Becker et al., 2005). A one-way repeated measures ANOVA showed a significant effect of MC phase on saliva E and P concentrations, F(1, 21) = 12.48, p = 0.002 and 155.54, p < 0.001, respectively. Post-hoc pairwise comparisons using t-tests with Bonferroni correction indicated that saliva E levels were significantly higher during HE (means (0.96 0.04 pg/ml) compared to LEP (0.42 0.04 pg/ml; p < 0.001) and not different between HE and HEP (0.89 0.06 pg/ml; p > 0.05). Saliva E levels were significantly higher during HEP than during LEP (p < 0.001). Saliva P concentrations during LEP and HE were low and steady (19.60 3.22 and 18.37 2.51 ng/ml, respectively) and were highest during HEP (115.21 7.47 ng/ml) compared to LEP (p < 0.001) and HE (p < 0.001).
3.3. Craving
Craving scores were derived from item 3 of the WSRS, “On a scale from 1 to 7 with 1 being the least and 7 being the greatest, how much do you desire a cigarette right now?” acquired at baseline, and before and after the cue task (See Table 1). There were no significant differences between conditions in the most relevant craving score when measuring cue-induced craving (baseline cue-exposure score subtracted from the post-cue exposure score).
Table 1.
Craving scores (means ± SEM) were derived from responses to item 3 of the Within Session Rating Scale (WSRS), “On a scale from 1 to 7 with 1 being the least and 7 being the most, how much do you desire a cigarette right now?” HE, high estradiol/low progesterone condition (late follicular phase); HEP, high estradiol/high progesterone condition (mid-luteal phase); LEP, low estradiol/low progesterone condition (early follicular phase).
| Phase | Baseline | Pre-Cue Exposure | Post-Cue Exposure | Post (-) Baseline |
|---|---|---|---|---|
| HE | 3.23 ± 0.31 | 3.82 ± 0.38 | 4.23 ± 0.38 | 1.00 ± 0.25 |
| HEP | 3.67 ± 0.35 | 4.08 ± 0.43 | 4.13 ± 0.41 | 0.46 ± 0.18 |
| LEP | 3.11 ± 0.33 | 3.67 ± 0.43 | 3.89 ± 0.46 | 0.78 ± 0.36 |
| p value | ||||
| HE vs HEP | 0.08 | |||
| HE vs LEP | 0.61 | |||
| HEP vs LEP | 0.40 |
3.4. Ventral striatal responses to SCs
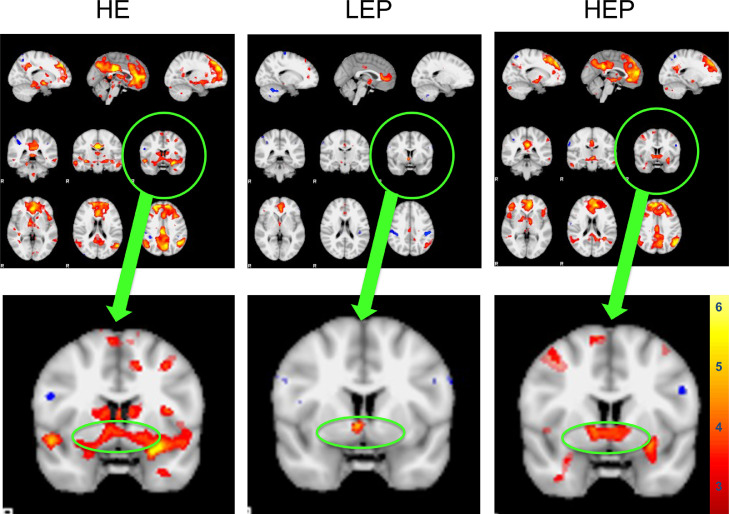
To test whether hormones affected the ventral striatal response to SCs versus nonSCs, small volume correction using a 4mm sphere centered on the left and right ventral striatum (x, y, z; in SPM 12 was applied. Contrasts within conditions showed brain responses to SCs versus nonSCs were not significantly different during LEP (p = 0.16) and were greater during HE (p = 0.009) and HP (p = 0.016), FWE corrected. Contrasts across conditions showed that HE and HEP had greater responses than LEP (p = 0.005), and HE had greater responses than HEP (p = 0.049), FWE corrected (see Fig. 2).
Fig. 2.
Neural responses to smoking cues (SCs) compared to nonsmoking cues (nonSCs) in the ventral striatum (VS) of women at 3 separate timepoints of the menstrual cycle; the late follicular phase, associated with high levels of estradiol (HE), the early follicular phase, associated with low levels of E and progesterone (LEP), and the mid-luteal phase, associated with high levels of E and P. Top panel: Representative fMRI sagittal (top), axial (middle), and coronal (bottom) brain slices analyzed using Statistical Parametric Mapping software. Bottom panel: Axial slice with VS circled. Small volume correction (4mm sphere) centered on the left and right VS (x, y, z; was applied. Contrasts within conditions showed brain responses to SCs versus nonSCs were not significantly different during LEP (p = 0.16) and were greater during HE (p = 0.009) and HP (p = 0.016). Contrasts across conditions showed that HE and HEP had greater responses than LEP (p = 0.005), and HE had greater responses than HEP (p = 0.049). FWE-corrected data are displayed neurologically (left is left). Greater brain responses are shown in red to yellow (strongest) hues.
4. Discussion
4.1. Overview
We tested the hypothesis that during HE, when E is elevated and unopposed by P, ventral striatal responses to SCs (vs nonSCs) would be greater than during LEP, when levels of both hormones are steady and low. We also tested whether, during HEP, when P levels are at their highest, ventral striatal responses to SCs would be more attenuated than during LEP. This study, during which women participated in 3 MRIs spaced over 3 MCs, timed to occur during LEP, HE and HEP, showed that responses to SCs were greatest during HE, still strong but somewhat less during HEP and nonexistent during LEP. Results suggest that E enhances vulnerability to drug cues and that while P may offer some protection against the powerful pull of cues, the least vulnerable time of the MC is when both E and P are low. The results of the study have the potential to improve our understanding of the influence of hormones on addictive processes and suggest that simply timing quit smoking attempts to occur during the early follicular phase could improve smoking cessation success.
These results support and extend our previously published study of the influence of the hormonal milieu on SC reactivity (Franklin et al., 2015), wherein we reported that the follicular phase was associated with greater brain responses to SCs compared to the luteal phase. In Franklin et al. (2015), we retrospectively grouped women by self-reported MC phase as a proxy for ovarian hormones, which were not acquired. Our design was, of necessity, cross-sectional: Women were grouped as luteal (the 14 days prior to the first day of menses) or follicular (the remaining days of the MC). Although sufficient information was acquired to identify MC phase with reasonable accuracy (first day of last menses, MC length, regularity, method of birth control), we were unable to verify whether ovulation occurred or to verify definitive hormonal status. With the current biochemically-verified within-subjects study design that pinpointed times of the MC wherein the hormonal milieu was the most dissimilar, we provide a more rigorous analysis of hormonal effects on brain response.
The findings of this study most likely only apply to naturally cycling women and likely do not translate to women taking exogenous hormones. Exogenous hormone treatment (i.e., oral contraceptives) generally consists of 3 weeks of medication that provides low and steady levels of both E and P, followed by a one-week wash-out period. The low levels of E and P prevent ovulation. Thus, there is no menstruation, per se, but during the wash-out period, women experience pseudo-menses (loss of blood). Menstruation is the cyclic, orderly sloughing of the uterine lining in response to complex interactions of multiple hormones including the major players, P and E, but also inhibin A, inhibin B, follicle-stimulating hormone, gonadotropin-releasing hormone, aromatase, and 3β-hydroxysteroid dehydrogenase to name just a few, produced by the brain (hypothalamus and pituitary gland) and the ovaries (Reed and Carr, 2000). Here we have focused on three timepoints within the MC that comprise hormonal milieus that are vastly different in E and P that may influence brain and behavioral responses to SCs. In support, Allen et al. 2019 conducted a review that included an examination of oral contraceptive use on subjective symptoms of smoking behavior, including craving, with inconclusive results (Allen et al., 2019).
4.2. Lessons learned
There are multiple ways in which hormone concentrations can be acquired, and the choice is driven by the prevailing hypotheses and other factors, such as availability of assays, subject burden, practicality and funding constraints. We began our study by acquiring saliva-based hormone measures for our MRI test day data. Unfortunately, the laboratory that conducted our hormone analyses uses a detection threshold of 0.5 - 2 pg/ml for E, which was insufficient to capture the granular variability in E that occurs over the MC. This prevented us from testing an initial goal, which was to use hormonal concentrations as continuous variables within the fMRI data, which would strengthen the scientific value of our study.
We discovered no difference in E levels between HE and HEP conditions (Wetherill et al., 2021). This was surprising given that the literature does not generally highlight the second peak in E that occurs during the mid-luteal phase of the MC, and representations of the MC suggest it is lower than the pre-ovulatory peak. Our focus on the mid-luteal phase, when luteal-E levels are high, might have reduced our ability to observe stronger protective effects of P. Attention to the early luteal phase, during which there is a drop in E following ovulation, while P continues to rise, may be an important focus for future studies. Alternatively, a more accurate value of hormonal levels (e.g., serum values) may aid in more specifically defining the roles of each of these major fluctuating hormones. This would also allow an examination of E and P ratios, which have been shown to be an important variable in hormone research (Harrison et al., 2020; Lynch, 2009; Schiller et al., 2012). Nonetheless, our hormone assay collection timepoints, which were acquired at the same time of day for each MRI to minimize diurnal hormonal fluctuations, and were acquired during carefully selected timepoints of the MC to maximize the potential to observe hormonal influences, allowed us to sufficiently test our primary hypotheses.
Notably, a link with smoking behavior (i.e., craving) was not established. There are at least 3 plausible contributors to this null finding. First, our sample size is small. The study was halted mid-way due to the global COVID pandemic, and restrictions on recruitment prevented us from collecting a sample size that would provide the power necessary to observe behavioral differences. Second, the study design was not optimal for examining drug cue-induced craving because drug cues and non-drug cues were interspersed, potentially distracting participants. A third reason relates to the secondary aim of the study. Based on our previous and replicated findings of the effect of dopamine transporter genotype on brain and behavioral smoking cue reactivity (Franklin et al., 2011, 2009; Wetherill et al., 2014b), our secondary aim was to test for an interaction between hormonal status and dopamine transporter (DAT) genotype, which may interject variability in overall results. Thus, exploratory analyses were conducted by parsing subjects by DAT genotype, resulting in extremely small sample sizes. However, analyses suggest a strong genetic influence and an interaction with the hormonal milieu on SC responses (see supplementary materials).
4.3. Strengths and limitations
The repeated measures counterbalanced design is a strength of the study. Another strength is using the natural and ideal comparator condition, LEP, which is associated with negligible levels of both E and P. Further, the within-subjects study design reduces the natural variance across individuals. Another strength of the study is our use of three similarly-valenced cue sets that were counterbalanced and presented over three temporally distinct MCs.
Notable limitations include potential confounds in internal and external validity. Due to an intensive study design and strict recruitment criteria, the results may suffer from selection bias and lack of generalizability to all naturally cycling women who smoke cigarettes.
4.4. Summary
This is the first study to accurately and precisely link ovarian hormonal status to the brain's response to drug cues, a primary relapse trigger. This within-subjects longitudinal study extends the current literature by showing that elevated levels of E, such as what is observed in the late follicular phase and during the mid-luteal phase, are associated with greater responses to SCs in a key region of the brain long studied for its role in motivated drug-seeking behavior, the ventral striatum (Kalivas and Volkow, 2005). It also shows that when E is unopposed by P, such as in the late follicular phase, brain responses to SCs are even greater in the ventral striatum. These results identify specific times of the MC when quitting smoking may be affected by ovarian hormones in naturally cycling women and pinpoint the LEP phase as the least SC-vulnerable time of the MC. Guided by our current knowledge base, LEP may be the ideal time of the month to quit smoking.
Contributors
TRF, Investigator, conceived and designed the study, and drafted the manuscript; NHS, Lead Imaging Technician, conducted the MRI sessions, contributed substantially to the manuscript draft, managed data quality; analyzed data; HK, Study Coordinator, conducted subject recruitment, and oversaw all aspects of subject participation, maintained study records; MM, Lab Manager, ensured all aspects of the study were conducted according to protocol, managed IRB interaction; oversaw study staff; KJ, Neuroimaging Specialist, was responsible for all imaging related aspects of the study (analyses, data quality, images); RRW, Investigator, participated in study conceptualization, data analyses, data interpretation. TRF and RRW shared the role of principle investigator of the study. All Contributors participated in weekly study meetings and read, edited and approved the final draft.
Declaration of Competing Interest
Given her role as Editor-in-Chief, Teresa Franklin, PhD had no in-volvement in the peer-review of this article and has no access to infor-mation regarding its peer-review. Given her role as an Editorial Board Member, Reagan Wetherill, PhD had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Associate Editor, Sherry McKee, PhD.
Acknowledgments
This study was funded by the National Institute on Drug Abuse, R01DA040670.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dadr.2022.100119.
Appendix. Supplementary materials
References
- Allen A.M., Weinberger A.H., Wetherill R.R., Howe C.L., McKee S.A. Oral contraceptives and cigarette smoking: a review of the literature and future directions. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2019;21:592–601. doi: 10.1093/ntr/ntx258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N.L., Gray K.M., Ramakrishnan V., Tomko R.L., McClure E.A., Carpenter M.J., Saladin M.E. Increases in endogenous progesterone attenuate smoking in a cohort of nontreatment seeking women: an exploratory prospective study. Addict. Biol. 2020 doi: 10.1111/adb.12918. e12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao A., Liu R., van Someren E., Hofman M., Cao Y., Zhou J. Diurnal rhythm of free estradiol during the menstrual cycle. Eur. J. Endocrinol. 2003:227–232. doi: 10.1530/eje.0.1480227. [DOI] [PubMed] [Google Scholar]
- Becker J.B., Arnold A.P., Berkley K.J., Blaustein J.D., Eckel L.A., Hampson E., Herman J.P., Marts S., Sadee W., Steiner M., Taylor J., Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Becker J.B., Breedlove S.M., Crews D., McCarthy M.M. 2nd Ed. MIT Press, Bradford Books; Cambridge, MA: 2002. Behavioral Endocrinology; pp. 579–628. [Google Scholar]
- Becker J.B., Hu M. Sex differences in drug abuse. Front. Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.B., McClellan M.L., Reed B.G. Sex differences, gender and addiction. J. Neurosci. Res. 2017;95:136–147. doi: 10.1002/jnr.23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M.E., Anker J.J., Perry J.L. Modeling risk factors for nicotine and other drug abuse in the preclinical laboratory. Drug Alcohol Depend. 2009;104(Suppl 1):S70–S78. doi: 10.1016/j.drugalcdep.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control, 2018. Current contraceptive status among women aged 15–49: United States, 2015–2017 [WWW Document]. URL https://www.cdc.gov/nchs/products/databriefs/db327.htm (accessed 7.12.22).
- Childress A.R., Mozley P.D., McElgin W., Fitzgerald J., Reivich M., O'Brien C.P. Limbic activation during cue-induced cocaine craving. Am. J. Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito E.E., Herman A.I., Waters A.J., Valentine G.W., Sofuoglu M. Subjective, physiological, and cognitive responses to intravenous nicotine: effects of sex and menstrual cycle phase. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2014;39:1431–1440. doi: 10.1038/npp.2013.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo T., Rouillard C., Bédard P. 17β-estradiol at a physiological dose acutely increases dopamine turnover in rat brain. Eur. J. Pharmacol. 1985;117:197–203. doi: 10.1016/0014-2999(85)90604-1. [DOI] [PubMed] [Google Scholar]
- Dill T. Contraindications to magnetic resonance imaging: non-invasive imaging. Heart Br. Card. Soc. 2008;94:943–948. doi: 10.1136/hrt.2007.125039. [DOI] [PubMed] [Google Scholar]
- Dluzen D.E., Anderson L.I. Estrogen differentially modulates nicotine-evoked dopamine release from the striatum of male and female rats. Neurosci. Lett. 1997;230:140–142. doi: 10.1016/s0304-3940(97)00487-4. [DOI] [PubMed] [Google Scholar]
- Fagerstrom K. Determinants of tobacco use and renaming the FTND to the fagerstrom test for cigarette dependence. Nicotine Tob. Res. 2012;14:75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- Fattore L., Altea S., Fratta W. Sex differences in drug addiction: a review of animal and human studies. Womens Health. 2008;4:51–65. doi: 10.2217/17455057.4.1.51. [DOI] [PubMed] [Google Scholar]
- Franklin T.R., Allen S.S. Influence of menstrual cycle phase on smoking cessation treatment outcome: a hypothesis regarding the discordant findings in the literature. Addiction. 2009;104:1941–1942. doi: 10.1111/j.1360-0443.2009.02758.x. Abingdon Engl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T.R., Jagannathan K., Wetherill R.R., Johnson B., Kelly S., Langguth J., Mumma J., Childress A.R. Influence of menstrual cycle phase on neural and craving responses to appetitive smoking cues in naturally cycling females. Nicotine Tob. Res. 2015;17:390–397. doi: 10.1093/ntr/ntu183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T.R., Lohoff F.W., Wang Z., Sciortino N., Harper D., Li Y., Jens W., Cruz J., Kampman K., Ehrman R., Berrettini W., Detre J.A., O'Brien C.P., Childress A.R. DAT genotype modulates brain and behavioral responses elicited by cigarette cues. Neuropsychopharmacology. 2009;34:717–728. doi: 10.1038/npp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T.R., Wang Z., Li Y., Suh J.J., Goldman M., Lohoff F.W., Cruz J., Hazan R., Jens W., Detre J.A., Berrettini W., O'Brien C.P., Childress A.R. Dopamine transporter genotype modulation of neural responses to smoking cues: confirmation in a new cohort: DAT genotype effects on SCs. Addict. Biol. 2011;16:308–322. doi: 10.1111/j.1369-1600.2010.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T.R., Wang Z., Wang J., Sciortino N., Harper D., Li Y., Ehrman R., Kampman K., O'Brien C.P., Detre J.A., Childress A.R. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Harrison K., Petersen A., Tosun N., Crist K., Allen A.M., Allene S. Effect of exogenous progesterone administration on cigarette smoking-related symptomology in oral contraceptive users who smoke. Addict. Behav. 2020;102 doi: 10.1016/j.addbeh.2019.106148. [DOI] [PubMed] [Google Scholar]
- Huxley R.R., Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet Lond. Engl. 2011;378:1297–1305. doi: 10.1016/S0140-6736(11)60781-2. [DOI] [PubMed] [Google Scholar]
- Kalivas P.W., Volkow N.D. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Lenton E.A., Lawrence G.F., Coleman R.A., Cooke I.D. Individual variation in gonadotrophin and steroid concentrations and in the lengths of the follicular and luteal phases in women with regular menstrual cycles. Clin. Reprod. Fertil. 1983;2:143–150. [PubMed] [Google Scholar]
- Lynch W.J. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol. Biochem. Behav. 2009;94:43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch W.J. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl.) 2008;197:237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch W.J., Roth M.E., Carroll M.E. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl.) 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Mello N.K. Hormones, nicotine, and cocaine: clinical studies. Horm. Behav. 2010;58:57–71. doi: 10.1016/j.yhbeh.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, B.G., Carr, B.R., 2000. The normal menstrual cycle and the control of ovulation, in: Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., Korbonits, M., McLachlan, R., Morley, J.E., New, M., Perreault, L., Purnell, J., Rebar, R., Singer, F., Trence, D.L., Vinik, A., Wilson, D.P. (Eds.), Endotext. MDText.com, Inc., South Dartmouth (MA).
- Regier P.S., Gawrysiak M.J., Jagannathan K., Childress A.R., Franklin T.R., Wetherill R.R. Trauma exposure among cannabis use disorder individuals was associated with a craving-correlated non-habituating amygdala response to aversive cues. Drug Alcohol Depend. Rep. 2022;100098 doi: 10.1016/j.dadr.2022.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller C.E., Saladin M.E., Gray K.M., Hartwell K.J., Carpenter M.J. Association between ovarian hormones and smoking behavior in women. Exp. Clin. Psychopharmacol. 2012;20:251–257. doi: 10.1037/a0027759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Smith P.H., Bessette A.J., Weinberger A.H., Sheffer C.E., McKee S.A. Sex/gender differences in smoking cessation: a review. Prev. Med. 2016;92:135–140. doi: 10.1016/j.ypmed.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu, Babb D.A., Hatsukami D.K. Progesterone treatment during the early follicular phase of the menstrual cycle: effects on smoking behavior in women. Pharmacol. Biochem. Behav. 2001;69:299–304. doi: 10.1016/s0091-3057(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Stabile L.P., Siegfried J.M. Sex and gender differences in lung cancer. J. Gend.Specif. Med. 2003;6:37–48. JGSM Off. J. Partnersh. Womens Health Columbia. [PubMed] [Google Scholar]
- Thompson T.L., Moss R.L. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J. Neurochem. 1994;62:1750–1756. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- Wetherill R.R., Childress A.R., Jagannathan K., Bender J., Young K.A., Suh J.J., O'Brien C.P., Franklin T.R. Neural responses to subliminally presented cannabis and other emotionally evocative cues in cannabis-dependent individuals. Psychopharmacology (Berl.) 2014;231:1397–1407. doi: 10.1007/s00213-013-3342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill R.R., Franklin T.R., Allen S.S. Ovarian hormones, menstrual cycle phase, and smoking: a review with recommendations for future studies. Curr. Addict. Rep. 2016;3:1–8. doi: 10.1007/s40429-016-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill R.R., Jagannathan K., Lohoff F.W., Ehrman R., O'Brien C.P., Childress A.R., Franklin T.R. Neural correlates of attentional bias for smoking cues: modulation by variance in the dopamine transporter gene. Addict. Biol. 2014;19:294–304. doi: 10.1111/j.1369-1600.2012.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill R.R., Spilka N.H., Maron M., Keyser H., Jagannathan K., Ely A.V., Franklin T.R. Influence of the natural hormonal milieu on brain and behavior in women who smoke cigarettes: Rationale and methodology. Contemp. Clin. Trials Commun. 2021;21 doi: 10.1016/j.conctc.2021.100738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill R.R., Young K.A., Jagannathan K., Shin J., O'Brien C.P., Childress A.R., Franklin T.R. The impact of sex on brain responses to smoking cues: a perfusion fMRI study. Biol. Sex Differ. 2013;4:9. doi: 10.1186/2042-6410-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2011. WHO | WHO report on the global tobacco epidemic 2011 [WWW Document]. WHO. URL http://www.who.int/tobacco/global_report/2011/en/(accessed 7.10.20).
- Yoest K.E., Quigley J.A., Becker J.B. Rapid effects of ovarian hormones in dorsal striatum and nucleus accumbens. Horm. Behav. 2018;104:119–129. doi: 10.1016/j.yhbeh.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.