Abstract
The advantageous depth dose profile of ion beams together with state of the art beam delivery and treatment planning systems allow for highly conformal tumor treatments in patients. First treatments date back to 1954 at the Lawrence Berkeley Laboratory (LBL) and in Europe, ion beam therapy started in the mid-1990s at the Paul-Scherrer Institute (PSI) with protons and at the Helmholtz Center for Heavy Ion Research (GSI) with carbon ions, followed by the Heidelberg Ion Therapy Center (HIT) in Heidelberg. This review describes the historical development of ion beam therapy in Germany based on the pioneering work at LBL and in the context of simultaneous developments in other countries as well as recent developments.
Keywords: Light ion beam therapy, Carbon ion therapy, Relative biological effectiveness, Beam scanning
1. Introduction
Ion beam therapy originates from the Lawrence Berkeley Laboratory (LBL), where Robert Wilson investigated range and energy loss of protons for shielding considerations of the new 150 MeV cyclotron. He found the unusual dose profiles with a maximum of energy deposition at the end of range, the so-called Bragg maximum and realized immediately the advantage of ion beams for radiotherapy. The phenomenon of the Bragg peak is named after William Henry Bragg, who first described this phenomenon in low energy alpha particles in 1903.
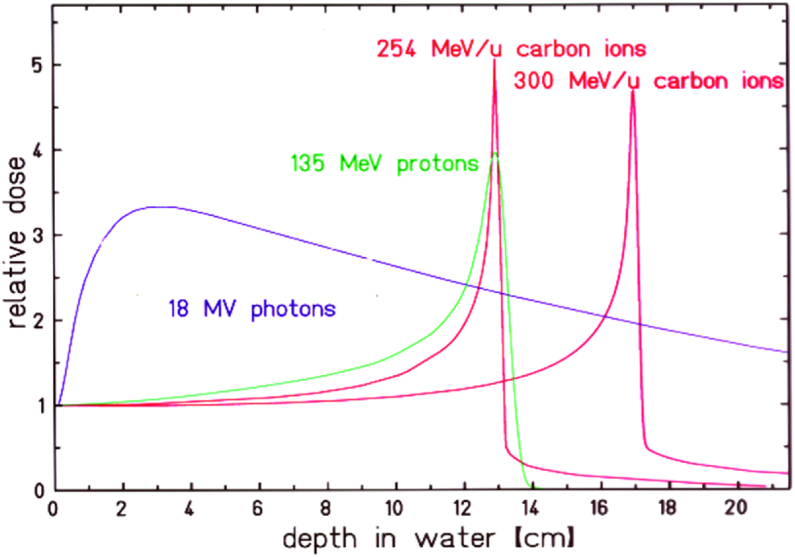
In his pioneering paper [1], Wilson outlined the advantageous properties of proton and carbon ion beams: the inverse depth dose profile with the high dose maximum at the end of the range and the small lateral and range scattering. While lateral scattering of protons leads to a penumbra, which in some cases is worse, than a high energy X-ray beam, the penumbra of a carbon beam remains always smaller than for an X-ray or proton beam. In Figure 1, typical depth dose curves are shown for X-rays, protons and carbon ions. Wilson also described the basic methods to spread the small primary and mono-energetic beam over an extended target volume using scattering materials and apertures.
Figure 1.
Comparison of the relative depth dose distributions of proton and carbon ions with18 MV photons as function of penetration depth. For the ion beams the energy deposition increases with penetration depth to the so-called Bragg maximum at the end of range. Changing the ion's energy, the position of the Bragg-maximum can be shifted in depth and the target volume can be covered with the high Bragg-peak dose (image reprinted from [2]).
Although this early publication mentioned all the essentials for ion beam therapy, it took nearly a decade before the first patient could be treated at LBL with protons and deuterons in 1954 and with Helium in 1957 [3]. Finally, heavier ions, mostly Neon were used for patient treatment focusing on head and neck cancers [4]. In 1957 proton therapy has also been established at the Svedberg Laboratory in Uppsala (Sweden) [5] and in 1961 at the Harvard cyclotron laboratory in Boston (US) [6]. In 1990, the first clinical proton facility opened in Loma Linda (US) [7]. After the decommissioning of the Berkeley accelerators in 1992, radiotherapy with carbon ions was strongly promoted by the National Institute of Radiological Science NIRS, Chiba (Japan), where a clinical facility was opened in 1994 [8].
2. Ion beam radiotherapy at the Lawrence Berkeley Laboratory (LBL)
2.1. Technical developments
In the beginning of ion beam therapy at LBL, two basic questions had to be solved: the shaping of the primary ion-beams to irradiate an extended target volume in the most conformal way and the choice of the optimal atomic number of the projectiles concerning physical and radiobiological properties. Both questions are complex and partially interrelated and depend on state of the art accelerator technology and computer control systems [9].
To answer to the first question, the passive beam delivery techniques were developed (Figure 2), because an active beam modulation by scanning the beam in an intensity-controlled way over the target volume was technically not feasible due to the limited computer power and the low accelerator flexibility, where a fast energy variation from pulse to pulse was not possible.
Figure 2.
Beam shaping of a pristine pencil beam (entering from the left) to an extended target volume (spread out Bragg peak, SOBP) by passive devices. The devices are schematically shown in the middle and their effects on the beam are shown above and below, respectively, for the lateral and longitudinal enlargement. Scattering by a multi-step device of heavy and light atomic material broadens the beam to a non-Gaussian shape with flat top. A range modulator is used to extend the Bragg peak longitudinally and produce the requested flat depth profile. With the range shifter the complete profile is shifted to the tumor depth. An adaption to the maximum contours of the tumor volume is achieved laterally by patient specific collimators and in depth by compensators. Using the passive technique, the resulting SOBP can only be tailored to the distal edge of the target volume at the expense of a corresponding high dose area in the proximal normal tissue (reprinted from [10]).
Therefore, the depth modulation was achieved with ridge filters where the different thicknesses of the absorber material modulated the ion's energy and consequently its range. The peak of the ridges causes the largest energy loss which corresponds to the proximal end, while the thin material in ridge valleys reduces the energy only minimal and produces the distal parts. The superposition of energies has to be designed with different weights to achieve a well-defined depth profile. Generally, the highest energy has the largest weight as the most deeply-seated parts of the target are only irradiated with this respective energy, while beams of all energies contribute to the shallower parts of the target volume. The design of the ridges is the most critical part of passive beam shaping because the shape of the flanks of the ridges is transferred to the slope of the flat top, the so-called spread out Bragg peak (SOBP). In each ridge filter, a certain longitudinal spread is built-in resulting in a specific superposition of the individual energies. Different technical solutions like linear or spiral ridge filters or fast rotating propellers etc. have been designed and used. In the clinical practice from a set of some 30 ridge filters the most appropriate one has to be selected to accommodate for the different tumor widths in depth. For protons these filters are designed according the absorbed dose in the Bragg-curve, for the heavier ions the variation of the relative biological effectiveness (RBE) in depth has to be included too.
At the LBL accelerator, a limited set of a few fixed energies was routinely available, the intermediate energies had to be adapted by range shifters which consisted of a digital set of sheets of light material. Finally, compensators were being used to spare critical structures at the distal end. These compensators modulate the range at the distal end on the expense of the proximal side, where the high dose region extends more into normal tissue, as shown in Figure 2.
To achieve a lateral coverage of the target, the primary beam is spread with scattering devices consisting of low and high atomic material in order to produce non-Gaussian profiles i.e. broad distributions with a flat top. Using only one scattering system the resulting lateral profile would be Gaussian and only the inner 10% could be used. In this case, the outer part has to be dumped in absorbers and causes a great loss of the primary intensity and a high neutron background. Therefore, complex multi-step and multi-material scattering systems have been developed to produce lateral distributions having a flat top. But also, magnetic systems of two perpendicular magnets were used for the lateral deflection [11]. With these magnets, different beam patterns were produced depending on the currents in the magnets like doughnuts, zig-zag and Lissajous-patterns including raster figures. However, the magnetic deflections only substituted the scattering foils and none of these patterns yielded a 3D conformal dose distribution in the target. In addition, these magnet deflections made the system sensitive against beam fluctuations. In order to wash out beam fluctuations, these patterns had to be applied rapidly and many times within the same fraction (multi-painting).
Finally, patient-specific collimators confine the lateral dose distribution to the outer contours of the target volume similar to conventional photon therapy. In Figure 2, a typical system with all components and their influence on the beam shape is shown, details of these techniques are outlined in [9].
For light ion therapy, the situation becomes more complex than for protons because the RBE is not expected to be constant throughout the depth modulation. In general, RBE is largest in the distal part of the extended Bragg maximum, where the ions with highest energies stop. Therefore, the absorbed dose has to be modulated to compensate the increase in RBE [12]. But RBE, depends also on many parameters such as particle energy and atomic number and cellular repair and dose response parameters. Therefore, a ridge filter system designed for resistant tumor does not produce a homogenous effect over the SOBP for sensitive tumors. In addition, the RBE values measured in an in vitro system cannot be directly transferred to therapy. RBE-values obtained in in vitro experiments are larger than those found by clinical experience (details are given in [12]). A typical modulated Bragg peak for carbon ion RT is shown in Figure 3.
Figure 3.
Absorbed dose distribution (black) for a depth modulated beam, leading to a constant RBE-weighted dose of 3 Gy in the target (red line). In this case, the RBE was calculated for cells with an α/β ratio of 2 Gy (Source: GSI).
For practical applications of the passive range modulation, an approximation of the RBE dependence with penetration depth is used to design a universal set of range modulators and the influence of other parameters is empirically compensated for instance by opposing fields [13].
At the early times at LBL, the passive or semi-passive systems were the optimal solutions for beam application using the BEVALAC for proton and the BEVATRON for heavy ion therapy.1 These techniques have been improved by the later constructed proton and ion machines and are still in use in most particle therapies today.
2.2. Clinical trials at LBL
Although starting with proton therapy, the clinical treatments at LBL were focusing on heavier ions. Radiobiological research had shown, that the heavier ions could potentially overcome a major problem in radiotherapy: the radio-resistance of hypoxic cells in a tumor, which typically require 2–3 times higher dose for inactivation as compared to oxygenated tumor-cells (see details in [12]). Cell experiments revealed that carbon ions reduced this effect by a factor of 2 and heavier ions such as Argon could suppress this resistance even more. Therefore, Argon ions were favored in the beginning but led to long-term sequelae because RBE is high also in the entrance region and leads to side effects in the normal tissue. Lower Z ions reduce these side effects and exhibit a more favorable RBE-profile. After some Silicon treatments, Neon ions were used for nearly all of the 440 tumor patients treated at LBL [4], [5], [14].
Besides the Neon treatments there was also a pituitary gland treatment program running, using mainly Helium beams since 1957 [15], [16].
Clinical indications focused on tumors in the brain and skull because of the possibility of immobilizing these patients by external fixation, esp. since at LBL patients were treated in a seated, upright position (Figure 4). To verify treatment position, a vertical CT-system was installed, that allowed imaging in this position. The upright treatment position allowed for flexible beam portals, as the chair could be rotated, while the horizontal beam was fixed. In Figure 5, an experimental treatment plan for base of skull tumor is shown for Helium ions.
Figure 4.
Treatment positioning at LBL facility: The patient was fixed in front of the beam exit window at the left side. Also, the compensator, consisting of many individual PMMA rods is visible (Source: imaging archive of the Lawrence Berkeley National Laboratory, © 2010–2019 The Regents of the University of California, Lawrence Berkeley National Laboratory).
Figure 5.
Treatment plan with five fixed horizontal fields showing a relatively large high-intermediate dose area in the normal tissue which limits the target dose (Source: imaging archive of the Lawrence Berkeley National Laboratory, © 2010–2019 The Regents of the University of California, Lawrence Berkeley National Laboratory).
3. The transition phase 1980–1997
The very first exploratory treatments at LBL paved the way for new irradiation modalities for protons and heavier ions but also pions, intensity modulated photons and even neutrons experienced a renaissance. The passive beam adaption developed first at LBL was then used and improved also by the other particle therapy centers.
Parallel to the new ion beam therapy, conventional photon therapy became more sophisticated using the steadily improving application of computers in planning and tomography and flexible beam collimation by the use of multi-leaf collimators. In addition, neutron therapy had shown improved tumor control especially for otherwise radio-resistant tumors. But neutron therapy was finally terminated because of severe late side-effects. To improve the efficiency in ion beam therapy and to decrease the neutron background produced in scattering and modulating systems, a so-called homogenous scanning or wobbling system has been introduced.[9] These techniques have been also transferred successfully, e.g. to the Japanese facilities.
In parallel to the work with light ions, the use of negative Pi-Mesons was also pursued in laboratories in Canada, US and Switzerland.[17], [18], [19] Since pions are produced as secondary particles from a high energy proton beam (at PSI e.g. with Ep = 590 MeV) impinging on a metal target, the efficient use of the low intensity pion beam was crucial. Therefore, a sophisticated beam delivery system has been developed at PSI in Switzerland: the Piotron [20]. The Piotron collected pions in a large 360° toroidal magnet in which the patient was inserted and treated with a first version of a mechanical beam scanning technique. The high intensity area inside the Piotron had a symmetrical beam spot of approximately 3 cm in diameter. To irradiate an extended tumor, the patient enclosed by a water bag was moved in 3 dimensions around the position of the fixed beam spot in a raster-like pattern, turning off the beam during the 3-D-translation of the patient. Since the effectiveness of pion therapy was not considered convincing, pion therapy was terminated also at the other pion treatment facilities at Stanford, Vancouver and in Switzerland and charged particle therapy was focused on protons and light ions [21].
The transition time was also characterized by the submission of many proposals for ion beam therapy facilities to the governments of several countries, such as LIBRA and Venus at Berkeley (US), MARIA in Alberta (Canada), EULIMA at Nice (France), DKFZ in Heidelberg (Germany) and many more. Although, most of these proposals were very elaborated and realistic, they failed because the initial investment of 50–100 Mio $ was too large for a single institute. However, the basic advantage of ion beam therapy, the inverse dose profile and increased biological effectiveness remained attractive for physicists and radiation oncologists as a tool to improve tumor treatment.
There have also been some very successful new initiatives in Japan, in USA and in Europe: At the National Institute of Radiological Science (NIRS) in Japan, a large double ring synchrotron HIMAC originally designed for nuclear research was converted in a very powerful therapy system, which could accelerate all ions up to Argon with sufficient range in tissue. It is in clinical use since 1994 and more than 14,000 patients have been treated here with carbon ions.
In Europe, the first multinational feasibility study for a European Light Ion Medical Accelerator (EULIMA) started in 1989 at the Institute Lacassagne in Nice (France), financed by the European authorities in Brussels in cooperation with CERN. The project brought together the expertise from different laboratories to study accelerator options such as superconducting cyclotrons or normal synchrotrons, the optimal beam delivery to the patient using beam scanning and the production and use of radioactive beams for simultaneous treatment and imaging. In addition, the radiobiological properties of carbon ions were compared to heavier ions. Finally, socioeconomic studies on possible patient recruitments in the different countries were performed to demonstrate the necessity of a few therapy units all over Europe [22].
Although the EULIMA project in Nice was terminated after 2 years for political reasons, the project had significant impact on the European radiotherapy community and their interaction with accelerator laboratories like CERN in Geneva (Suisse) and GSI in Darmstadt (Germany), where studies on technical problems like beam scanning and gantry design could be financed. At GSI, these studies were the start of an ion beam therapy activity mainly focusing on the development of a beam scanning prototype. At CERN in strong interaction with the Italian TERA foundation and the Austrian MedAustron initiative, a Proton-Ion Medical Machine Study (PIMMS) was started to develop an optimal design of a therapy synchrotron producing ion beams with long and smooth extractions, suitable for beam scanning. PIMMS collaborated also with GSI and Onkologie 2000, a Czech project [23].
Later, the PIMMS design served as basis of two projects realized in Europe: the National Center of Oncological Hadrontherapy (CNAO) in Pavia (Italy) and the Austrian project MedAustron in Wiener Neustadt.
In 1991, CNAO started with the basic memorandum to INFN, the Italian nuclear physics society on Hadron-therapy, evaluating beams like neutrons, pions, protons and heavier ions, which are Hadrons according to their nuclear structure [24].
In 1992, the TERA foundation was installed at Novara (Italy) to raise funds and to gather staff needed for the center. As a result, CNAO was realized as a proton and carbon ion facility using the PIMMS-design for the synchrotron and the GSI-injector for acceleration of protons and carbon ions to 3 treatment areas with horizontally and vertically scanned beams.
In 1989, after the end of the cold war, a concept for an international research center, Austron, came up in Austria, where a neutron spallation source should be operated, driven by a large rapid cycling synchrotron. Using extremely short proton pulses, intense neutron pulses should be produced where intermittent proton pulses should be also used for tumor therapy. Because of compatibility problems between the short pulses needed for the neutron production and longer pulses required for proton therapy, the design of an additional small synchrotron was proposed in a separate MedAustron project, which was finally realized while the larger Austron project was not funded. With the financial assistance of the city of Wiener Neustadt, a detailed MedAustron feasibility study was initiated in 1996, combining ion beam therapy with the possibility of basic physics research using the concept of the PIMMS-accelerator design. MedAustron was finally completed after difficult financing and tendering negotiations and started medical treatment with protons in 2017 and carbon ions in 2019.
The feasibility studies EULIMA and PIMMS and the early started, but delayed realized projects MedAustron and CNAO demonstrated that for a general acceptance of ion beam therapy, an integrated project was required that developed new techniques in beam scanning, dosimetry and treatment planning, but also demonstrated the clinical feasibility of patient treatments. Such a pilot project was initiated in 1993 at GSI and started patient treatment by the end of 1997.
4. The heavy ion therapy project at GSI
In 1988, a first proposal “Construction of an experimental heavy ion therapy at GSI Darmstadt” was submitted by three cooperating institutes, the University Medical Centre and German Cancer Research Centre, DKFZ (both Heidelberg) and GSI (Darmstadt) in cooperation with the FZD Rossendorf-Dresden to the German federal government but was not funded. This first proposal was followed by further attempts to obtain project funding from national and international sources for basic biology research and technical developments like the prototype of a beam scanning system and prototypes of the Positron emission tomography (PET) system, which was designed to measure the activation induced in a patient during therapy (see Section 4.5). At that time, GSI did not want to be involved in a large therapy project as it was feared, that a therapy program would be too successful and could overrule the nuclear and atomic program at GSI.
This view changed completely in spring 1993 when a new GSI director supported the project, giving it the highest priority in all technical, physical and radiobiological aspects, wherever GSI had experience. Independent from the preparation of a new proposal to the government, the construction of a medical treatment facility started immediately in May 1993.
-
•
The intention was to construct a high-technology heavy ion therapy facility based on
-
•
Intensity controlled raster-scanning as active beam delivery system
-
•
Fast energy variation by the accelerator
-
•
Individual beam spot treatment planning
-
•
Inclusion of RBE modelling in treatment planning to allow for a more flexible variation of RBE within different tissues at different dose levels
-
•
Online beam monitoring based on PET-measuring the auto-activation of the primary carbon beam
4.1. The principles of raster-scanning
The main innovation of the GSI project was the intensity controlled scanning beam application. Active beam scanning is much more flexible in spatial dose conformation and RBE adaption. In addition, the beam is much cleaner because it avoids the beam contamination in front of the patient by passive modulators like energy absorbers, apertures or compensators and beam monitors were reduced to a minimum thickness. For the range variation, the beam energy could be varied within seconds by the synchrotron accelerator in 255 steps between 85–430 MeV/u (with 0.5% resolution). Thus, the range of the carbon ions could be adapted in steps of 2 mm to the extension of the target volume in up to 60 equidistant slices (Figure 6). Each slice was covered by a net of pixels according to the lateral outline. For each pixel the intensity, i.e. the number of carbon ions was calculated in treatment planning, taking into account the pre-irradiation by the deeper layers as well as the variation in the relative biological effectiveness RBE. When this pre-calculated particle number was reached in one pixel, the beam was moved magnetically to the next pixel. The distance from one to the next pixel as well as the distance between the raster-lines was chosen to be less than 1/3 of the beam diameter, typical values were 1–3 mm raster step size and a beam width of 4–10 mm at full width half-maximum (fwhm). The same strategy of overlapping beam profiles was applied for the distance of the raster lines and the stacking of the various energy planes. This strategy of overlapping beam profiles produced a very stable dose-coverage even when the position of a single pixel deviated from the pre-calculated position because the energy deposition of many beam positions contributed to the local dose in each spot.
Figure 6.
Principles of the raster-scan system: the target volume is dissected into layers of equal particle ranges, which are covered by a net of individual beam positions. For each energy the beam is guided magnetically over the individual beam positions Reprinted from [25]). The raster scan technique at GSI was the first 3D application of carbon ions using an intensity controlled pencil beam scanning. But the idea of active beam scanning instead of passive beam application was virulent since 1980 when at NIRS used a 70 MeV proton beam to produce an irregular figure of 12 cm diameter with single beam spots of 1 cm in diameter. This experiment showed that a homogenous dose coverage of better 2% could be achieved. But this spot scanning technique was not extended to clinical relevant energies in 3D and was not used in clinical routine [26], similar to a scanning system proposed in Berkeley [8].
At the PSI, Villigen, following the strategy of the Piotron, a hybrid scanning system for protons was completed in 1996, where the beam was scanned in one dimension and the patient was moved in the second dimension while the energy was changed by absorbers for the penetration variation (3rd dimension). Only after the construction of the second gantry 2013 beam-scanning was extended to 2 dimensions at PSI.
Also, at NIRS beam scanning was introduced into clinical application when the new treatment facility was completed in 2004. But before these upgrades at PSI and NIRS, the GSI raster-scan system was the first system for a routine pencil beam treatment. This innovation was triggered at GSI by a previous development, the installation of a micro-beam facility for material research. There, single ions of 1.4 MeV/u were magnetically deflected in micrometre steps over an area of less than 1 mm2. With this technique it was possible to produce 2D patterns of given density distributions. The reproduced photo of H. Helmholtz in Figure 7 was the starting point at GSI to transfer this technology from single ions in micro dimensions to therapy-beams applying the same technique as developed for the micro beam facility but extended in 3 dimensions [11], [27].
Figure 7.
Left: Image of H. Helmholtz reproduced with 1.4 MeV/u C-ions at the micro-lithography facility at the GSI. Each spot corresponds to a single ion hit in a nuclear track detector. The low intensity beam was moved from one to the next pixel without turning of the beam. The image size was 0.3 mm × 0.5 mm (courtesy of B. Fischer, GSI Darmstadt). Right: Reproduction the famous photo of A. Einstein using a 430 MeV/u C-beam on a X-ray film of 15 cm × 18 cm. The beam of 1.7 mm FWHM in diameter contained 1.5 × 1010 ions in total and was moved using the raster-scan technology, developed on the basisof micro-beam technology (reprinted from [28]).
This type of active beam delivery requests a perfect adaption of the accelerator in energy variation but also a rather smooth beam extraction with a preselected beam intensity.2
4.2. Accelerator and beam delivery
Although the technical achievements, described above, were necessary for a successful operation of the new therapy unit, other novel solutions had to be developed, like e.g. a fast scanner control system, an overall quality control system, treatment planning, dose verification and finally the accurate modelling of the RBE in a mixed radiation field and as well as its validation.
A major challenge is the fast beam control system, i.e. monitors in front of the patient that guarantee the precise position and the particle fluence at each pixel: for the high granularity of several 10,000 pixels in the scanned area it turned out that a position sensitive ionization chamber having a spatial resolution smaller than 2 mm was not feasible. Therefore, the functions of the monitor system were separated: for the position measurement, wire chambers were installed and ionization chambers for the intensity measurement, which were read out in 120 and 10 μs, respectively. This separation of functions was possible because it was not expected to have two beams at two different locations at the same time. The speed of the control system is one of the limiting factors of the overall treatment time and consequently for the number of patients that can be treated per year.
The main parameters of the GSI therapy beam delivery system, were a maximum field size of 20 cm by 20 cm, variable beam width of 4–10 mm (fwhm) and a variable intensity between 106 and 108 ions/spill. For beam extraction, a slow flat top extraction over several seconds was used. With a cycle time of 5 s and an energy switching time of around 60 s, irradiation times of less than 5 min for 100 cm3 volume could be achieved.
For the irradiation of an extended target of 50,000 raster points, these features would result in a minimum irradiation time of approx. 5 s. In practice, the irradiation times were much longer, as several intensity measurements have to be performed at each scan spot and additional time is needed for the data transfer between the different control systems (accelerator and beam delivery), as well as for switching the beam energy (at the end of the extraction cycle, all magnets had to be ramped up into saturation to overcome hysteresis effects). As the beam intensity had to be kept constant in a single-energy layer, a strong intensity modulation (more than a factor of 10) requires the use of a lower intensity to accurately control the number of particles at the low intensity spots. Altogether, typical exposure times range between 2 and 6 min for a target volume of 20,000–50,000 pixels (corresponding to 50–100 ml) with an absorbed dose of around 1 Gy, but the delivery time depends also on the geometry of the target volume.
The time needed to treat one fraction of about 10 min in average plays an important role for the estimation of the number of patients that could be treated at such a heavy ion therapy unit and consequently for the costs for each completed patient treatment of e.g. 20 fractions.
It was essential for the success of the GSI therapy project that all the different components for beam monitoring and application were developed and integrated in a unique control system, which moreover could be operated by the clinical staff in almost the same way as a conventional treatment device.
4.3. Treatment planning
Another crucial point was the development of an appropriate treatment planning system (TPS) dealing with the problem of dose conformation for scanned beam delivery and including an RBE model. Since no commercial treatment planning system (TPS) for scanned ion beams was available, a new TPS was developed by combining existing components, developed at GSI and DKFZ. A research TPS for 3D conformal treatment planning, developed at DKFZ, provided the clinical functionality of image registration and segmentation and allowed for definition of treatment plan parameters and assessment of dose distributions using standard dose metrics and various possibilities to display the resulting dose. The system was called Virtual Radiotherapy System (VIRTUOS) and was one of the first 3D planning systems worldwide [29], [30]. It was interfaced to a dose engine for calculation and optimization of dose for carbon ions, which was developed at GSI (the so-called Temporary Raster Planning, or TRP, later called Treatment Planning for Particles, TRiP [31]). The combination was used for clinical treatment planning at the DKFZ [32].
Clinical implementation of this TPS required some further steps: (i) setting up a lookup table to calibrate ion ranges based on CT-data [33], [34], (ii) measuring and validating the data base of the pencil beam library for absorbed dose calculation [35], and (iii) implementing an RBE-model and input data for calculation the RBE-weighted dose distribution [36].
For RBE-modelling, the Local Effect Model was developed at GSI [37] and benchmarked against in vitro [38] as well as in vivo data [39], [40]. Because of the strong and non-linear dependence of RBE on physical parameters like particle energy, dose and ion type, RBE has to be calculated during each iteration of the optimization process based on the details of the radiation field at each position in the treatment field (the latter is affected by the fragmentation processes of the carbon beam in tissue). This combination of algorithms for treatment planning has been used for all patients treated at GSI, and although the LEM was further developed (resulting in version LEM IV) [41], only the initial version (LEM I) has been employed during the project [12].
The majority of patients has been treated with single field uniform dose (SFUD) optimization, in which the fields of the treatment plan are optimized independently of each other [32]. As an intermediate step, so-called wedged fields have been introduced, introducing a defined gradient in the single treatment fields [42]. This was already a first form of intensity modulated radiotherapy with ions (without the simultaneous optimization of fields) as only the sum of all fields yielded a homogeneous biological effect. It is interesting to note, that due to the limited computer resources, simultaneous optimization of several treatment fields (so-called multiple field optimization, MFO) was not feasible until around 2005. Only then a full intensity-modulated ion beam therapy of all contributing pencil beams could be obtained and the full potential of biologically optimized ion beam therapy could be used clinically. The first patients that were treated in 2005 with this novel technique were patients with skull base tumors, which benefitted significantly by the additional dose sparing of the brain stem using the IMPT technique.
4.4. Dosimetry and quality assurance
At the beginning of the project, accurate and traceable ionization chamber (IC) dosimetry for carbon ions was not well-developed and existing protocols referred to passive beam shaping rather than scanning techniques [43]. Differences for scanned beams arise especially for the monitor calibration, which has to be performed in the entrance region of monoenergetic beams of different energies [44]. For this purpose, a code of practice for ion chamber dosimetry for carbon ions was established and the uncertainty budget was specified [45]. Later-on, these methods became an part of international standards [46], [47], as well as of the German regulation [48].
To guarantee accurate and reliable patient treatments, a comprehensive quality assurance (QA) program was developed by DKFZ [49], [50], [51], [52]. This included (i) measurements of the geometrical accuracy of patient positioning and setup verification by imaging, (ii) validation of range and dose calculation in homogeneous and inhomogeneous phantoms, and (iii) extensive tests of the monitor and beam delivery system. While the basic concepts of such tests were known from photon therapy, they had to be adapted for ion beam radiotherapy. For each functional performance characteristics, a test description was formally documented and test frequencies and intervention thresholds were defined. These tests were used for acceptance testing at the beginning of each beam time and were regularly repeated as constancy tests.
Patient-specific QA included a number of consistency tests of the treatment plans as well as the dosimetric verification the treatment plan. For this, each treatment field was measured in a water-phantom using a stack of 24 pinpoint ICs and the measured doses were compared with those from the dose distribution recalculated to the geometry of the water-phantom [51], [53].
As result of these developments, the first comprehensive QA program was established for carbon ions, and many of the methods were taken over by the Heidelberg Ion-Beam Therapy Center (HIT) and other ion beam therapy facilities [54], [55], [56].
4.5. Online-PET monitoring
The most innovative system for quality control at the GSI pilot project was the development and installation of an in-beam Positron Emission Tomography (PET) system to trace the carbon ion beam inside the patient [57]. Clinically, PET scanners are normally used to measure organ functions after the injection of specific metabolic tracer labeled with positron emitting isotopes. At LBL, the possibility of using a radioactive neon beam has been pioneered to measure the range in patients [58], but this technique has not been used routinely in patients. At the GSI pilot project, PET in situ measurements of the stopping carbon beam were performed for the first time within clinical routine. During irradiation, a small fraction of the primary carbon 12 beam undergoes nuclear fragmentation yielding the lighter carbon-10 and -11 isotopes, which are positron emitters. These positron-emitters decay only after stopping because of their long half-life times of 20 min and 19 s, respectively. As their stopping point correlates with Bragg maximum of the primary beam, the PET-image may be used to check the range of the carbon ions (Figure 8). For the measurement, a PET camera was shifted over the treated area of the patient and the location of the positron decay inside the patient was recorded without extra exposure to the patient [59].
Figure 8.
The dose distribution of a stopping carbon beam (dashed line) is compared to the β+ activity (red) induced by the carbon ions (reproduced from [57], [60]).
The reconstructed activity distribution was then compared with the respective distribution simulated by Monte Carlo methods based on the fluence pattern of the treatment field and the patient CT-based patient model [61]. Deviations between both distributions reveal useful information on potential errors in beam delivery and patient setup. The PET-method was shown to be especially sensitive to range errors [62]. PET measurements were performed daily for each field and detected discrepancies lead to further analysis and in some cases to a re-planning of the patient [60].
Although using the novel raster-scan technique and the biology-based treatment planning system, the treatments at GSI were established in a rather short time: The novel online PET-technique provided a tool to inspect the location of the delivered dose distribution only a few minutes after dose delivery. In addition, the leading role of the East-German research center in Dresden opened the possibility to participate in the funding program of the government after the German reunion in 1989.
4.6. Commissioning phase
In the very short time between May 1993 and December 1997, all the innovations for the therapy pilot project were developed and installed at GSI. This included novel dosimetry procedures and all safety features for accelerator control and for the subsequent beam monitoring in front of and in the scanning system
After the main technical developments were completed, commissioning of the experimental carbon ion irradiation system was started in 1997 employing the previously established QA protocols and based on the results, governmental approval was applied for. As this was the first ion beam facility in Germany, expert opinions based on the QA documents and the commissioning results were requested by the authorities. In December 1997, approval was obtained and subsequently, the first two patients were irradiated. However, it was the common understanding from the beginning, to operate the pilot therapy at GSI for a maximum time of 5 years. Only if a succeeding project would start in time, GSI would operate the pilot project until the new project would become operational.
4.7. Clinical trials
The first 2 patients suffering from brain tumours were treated with a boost of 5 fractions in the week starting December 13, 1997. After the first two patients, there was an accelerator shut down of 6 months which allowed to evaluate whether there were any unexpected effects mainly because of the high local doses during beam scanning. Since this was not the case, treatments continued on a regular basis with three beam times of 4 weeks per year and 20–25 patients per beam time resulting in 434 patients over the 10 years of clinical operation [63]. Each beam time started with acceptance testing of the treatment system over 4–5 days and in parallel, treatment plans were prepared. As most patients received 20 fractions, all had to start treatments within few days. Additionally, patients receiving only 6 fractions combined with a photon treatment were included during the beam time. Patient treatments continued until 2008, before HIT started clinical operation in 2009.
Main indications at GSI were skull base chordoma and chondrosarcoma (treated with typically 60–66 Gy RBE weighted dose in 20 Fx), adenoidcystic carcinoma, sacral chordoma and advanced prostate carcinoma (all treated with a boost of typically 18 Gy RBE-weighted dose in 6 Fx after conventional radiotherapy). All treatments were optimized with LEM I using an α/β-ratio of 2 Gy [12]. Clinical trials for chordoma [64], chondrosarcoma [65] and adenoid carcinoma [66] showed excellent tumour control rates and in case of adenoid carcinomas, these were higher than in a comparable photon collective [66]. Even after the end of the project, patient follow-up continued resulting in 5y- and 10y-local control rates of 72%/54% for chordoma [67], 88%/88% for chondrosarcoma [68], and 59.6%/42.2% for adenoidcystic carcinoma [69], respectively. As a result of these clinical trials, safety and effectiveness of carbon ion treatments have been shown and chordoma, chondrosarcoma and adenoidcystic carcinoma were established as standard indications for carbon ion therapy, including reimbursement by the health insurances.
5. The Heidelberg Ion-Beam Therapy Center
In summer 1998, the second treatment block at GSI started and after demonstrating clinical feasibility of carbon ion treatments with the raster-scan technique, a project proposal for funding of a clinical facility in Heidelberg was submitted to the Federal Ministry of Research and Science during the official inauguration ceremony of the GSI pilot project This proposal was reviewed and positively evaluated by the German Science Council (Wissenschaftsrat der Bundesregierung) in May 2001.3 Consequently, the University and University Medical Center continued and detailed the planning of the facility. The Science Council finally approved the plans in May 2003 and recommended to fund this project of the University Medical Center Heidelberg with 50% of the total costs of 119 M€. Construction works started in the same year.
At May 14th 2004, the foundation ceremony took place on the Heidelberg university campus and the building was completed with a roofing-ceremony in June 2005. The building comprises three levels and has a footprint of around 5000 square meters. A unique feature of the facility is the integration into the oncological services on the Heidelberg campus including the university medical centre, the German Cancer Research Centre and the National Centre for Tumour diseases (NCT).
5.1. Technology of the HIT facility
The Heidelberg Ion Beam Therapy Centre at Heidelberg University Medical Centre, HIT uses a dedicated synchrotron designed by GSI for medical applications [70], [71].
It is feasible to accelerate protons and carbon ions with ranges up to 35 cm (corresponding to a kinetic energy of 430 MeV/u for carbon ions and 220 MeV for clinically used protons) and HIT started the commissioning in 2005 with two ion sources for protons and carbon ions. The ions are injected into a compact linac structure, which accelerates ions up to around 6 MeV/u. The linac, built by Siemens, is based on a design of the University of Frankfurt. The particles are then injected into the synchrotron and accelerated to the necessary energies before they are extracted and transported to three treatment rooms (two horizontal fixed beams and a gantry) and an experimental room, all equipped with the raster scanning beam delivery manufactured by Siemens based on the prototype developed at GSI. The accelerator control system features the same technology of virtual machines, allowing to select a predefined set of beam parameters defined by ion type, energy, intensity and beam-width. The energy switching of the machine together with the beam scanning, allows for a 3D conformal treatment without any additional passive beam shaping elements (similar to the GSI system).
In 2007, also the setup of the world's first isocentric gantry for light ions beams started. The gantry is a normal conducting device and probably the biggest medical device worldwide: it has a diameter of 12 m, a length of 25 m and an overall weight of approximately 660 t. A sketch of the overall building and beamlines is shown in Figure 9.
Figure 9.
Layout of the Heidelberg Ion Beam Therapy facility (HIT) at the University Clinic Heidelberg. The facility is equipped with three ion sources (only 2 are shown here on the left side), a linac injector (left side), a synchrotron (upper left), two fixed horizontal treatment beams (central part) and an isocentric gantry (right side). The beam continues on the upper right side to an experimental area (not shown here), The facility offers beams of protons, carbon, helium and oxygen ions and uses beam scanning delivery only (Image: Heidelberg University Clinic).
By November 2009, the commissioning of one of the horizontal beam lines was completed and patient treatments started. By 2012, all three horizontal beamlines as well as the gantry were commissioned and the overall patient number exceeded 1000, with more than 550 patients treated in the last year.
In the following years, a third ion source for Helium ions was added and Helium beams have been commissioned for future clinical applications by 2017. Also, Oxygen beams are available (from the carbon source) for experimental purposes.
HIT has been approved in 2009 as an in-house product of Heidelberg University Medical Centre according to the German medical product law. The main components of the system are the building (built by Heidelberg University), the accelerator (designed by GSI), all patient related technology including the patient positioning and imaging devices, treatment control and delivery system and the treatment planning system (built by Siemens) and some auxiliary systems (technical equipment, patient positioning devices, etc. built by other manufacturers).
A novelty of the HIT facility are the robotic patient positioning and imaging devices, which allow for a highly accurate and largely automated setup of the patient. The imaging systems are based on a Siemens cone beam CT system, which is similarly used for surgical operating rooms. It allows acquisition of X-rays under various projections and in principle also cone-beam CT images. The latter functionality, was however, not integrated into the control systems and is therefore not used routinely. Since adequate 3D imaging is a key for controlling inter-fractional motion of organs, an in-room CT scanner on rails was recently installed in one of the horizontal treatment rooms (Aero CT by Brainlab). The scanner can be retracted over the beam nozzle, after images have been acquired. In the gantry room, an optical surface monitoring system (Align RT, by VisionRT) is currently under commissioning.
The treatment planning system (Siemens Syngo Planning RT) is following the concepts of the prototype used for the GSI pilot project, but allowed for an integration into the clinical information systems, as well as for a significantly improved workflow and speed of dose calculations. More recently, also a novel planning system from Raysearch, Sweden has been installed for proton as well as for carbon treatment planning. This system is the basis for the planning of Helium beam treatments, which recently started at HIT.
The facility has also an offline PET CT which can be used in critical cases. But the PET signal is much smaller than the signal at the original online PET at the GSI pilot project. This fainting is mainly due to the problem of wash-out of the activity in the body during the transfer time and a reason for extensive biological modelling. Nevertheless, it has been shown, that at the information gained may be analysed retrospectively to allow for an assessment of the overall range accuracy at a level of a few millimetres [72]. To arrive with higher activity and consequently better signals from PET activation images, a large research project, funded by EU, was recently launched at GSI to investigate the direct use of radioactive beams for treatment [73].
5.2. Clinical trials and standard treatments
By autumn 2021, more than 7000 patients have been treated in a series of clinical trials as well as standard treatment procedures, where approximately 50% of the patients were treated with protons and 50% with carbon ions. The standard procedures included tumours, which were accepted for liquidation by the insurance companies already in 2005, like skull base tumours and adenoid cystic carcinoma.
As can be seen from Table 1, the majority of clinical trials at HIT is still in the recruiting phase. The main reason for this is that due to numerous selection criteria and the relatively large number of patients required for statistically significant results, this recruitment phase may last for many years. Even when recruitment is finalized, typically a five-year follow-up period is needed to arrive with clinically meaningful results. It is therefore still too early to make definitive statements about the clinical results from the patients treated at HIT.
Table 1.
Currently ongoing clinical studies. These include comparative studies of proton vs. carbon RT for established indications, like skull base chordoma and chondrosarcoma, adenoidcystic carcinoma, prostate carcinoma, hepatocellular carcinoma, pancreatic and rectal carcinoma, pediatric osteosarcoma and some other tumour entities.
| Tumor type or region | Trial name and indication | Type of study | Status | Ref. |
|---|---|---|---|---|
| Chordoma and sarcoma | Chordoma of the skull base | p vs. C12 | Recruiting | [74] |
| ISAC (sacral chordoma) | p vs. C12 | Recruiting | [75] | |
| Chondrosarcoma of the skull base: | p vs. C12 | Recruiting | [76] | |
| OSCAR (inoperable osteosarcoma) | p/C12 (boost) | Recruiting | [77] | |
| RETRO-ION (retroperitoneal soft tissue sarcoma) | C-12 | Recruiting | [78]10 | |
| Head and neck | COSMIC (salivary glands ACC) | C12 (boost) | Published | [79] |
| ACCO (Adenoidcystic ca.) | C12 | Recruiting | See footnote 11 | |
| ACCEPT (Adeoidcystic carcinoma) RT + chemo (Erbitux) | C12 (boost) | Published | [80] | |
| TPF-C HIT (head & neck) | C12 (boost) | Closed | [81] | |
| IMRT HIT-SNT (sinu-nasal cancer) | C12 (boost) | Recruiting | [82] | |
| Brain | CLEOPATRA (prim. glioblastoma) | p vs. C12 (boost) | f/u phase | [83] |
| CINDERELLA (rec. gliobastoma) | C12 | f/u phase | [84] | |
| MARCIE (meningeoma grade 2) | C-12 boost | Recruiting | [85] | |
| Prostate | IPI (prostate cancer) | p vs. C12 | Published | [86], [87] |
| PROLOG (rec. prostate cancer) | p hypofx | f/u phase | See footnote 12 | |
| KOLOG (rec. prostate cancer) | C hypofx | f/u phase | See footnote 12 | |
| PAROS (prostate-Ca.) adjuvant RT | p hypofx vs. IMRT | Recruiting | [88] | |
| Gastro-intestinal region | PROMETHEUS (Hepatucellular Ca) | C12 hypofx. | Recruiting | [89] |
| PACK (pancreatic carcinoma) | C-12 | Recruiting | [90] | |
| PANDORA (rec. rectal carcinoma) | C12 | Recruiting | [91] | |
| Lung | INKA (inop. sulcus superior tumors, NSCLC) | C12 neoadj. | Recruiting | [92] |
| Gynecological tumors | APPROVE (cervical carcinoma) | p | Recruiting | See footnote 12 |
Exceptions are the COSMIC and ACCEPT trials for treatment of adenoid cystic carcinoma patients (ACC), as well as the comparative IPI trial for prostate cancer. In the COSMIC trial, an IMRT treatment with an integrated boost was compared to a combination of an IMRT treatment for the CTV with a Carbon ion boost applied to the GTV only. It could be demonstrated, that an increased local control can be achieved by applying a carbon ion boost and also that this increased local control is turned into an increase of overall survival, which amounted to 45% at 10 years as compared to 20% for the patients treated with IMRT alone (Jensen et al.). In this trial, no dose limiting acute toxicity was observed and late toxicity higher than grade 2 was observed in less than 5% of the cases. Following these encouraging results, the ACCEPT trials was exploring the feasibility to combine this treatment with concomitant chemotherapy using Cetuximab. The aim was to potentially further improve the overall survival, which is typically limited by distant metastases. The feasibility (as defined in the study) could, however, not be demonstrated, especially due to a high rate of grade 3 dermatitis (22%) and mucositis (48%). This rate is, however, comparable to existing results for combined radiotherapy and Cetuximab protocols [80]. This result clearly show the importance of clinical trials for the exploration of new treatment protocols, esp. when combining chemotherapy and radiation.
In the IPI trial, the feasibility of combining proton therapy for prostate with a proton boost was compared to the combination of protons with a carbon boost for localized tumors in a hypofractionated treatment (both 66 Gy (RBE) in fractions of 3.3 Gy (RBE)). The observed toxicities in both arms were comparable and acceptable [86], but did not favour a carbon boost as compared to protons alone.
In clinical application of light ion beams, the uncertainty connected to the RBE has to be considered and is still a major source of uncertainty. Uncertainties in RBE originate from the models used for calculating RBE, but also from the inherent uncertainty of the input parameters needed for a certain indication, mainly the α/β values for the tumor and normal tissue (see details in [12]). It is difficult to estimate these uncertainties, but the overall uncertainty of RBE are easily within 20–30%. Therefore, clinical trials are of special importance to gain confidence in the RBE-models and their parameters.4
5.3. Future developments
The higher ballistic accuracy of ion beam is a great advantage to concentrate more dose to the target volume and to spare the tumor-surrounding tissue as long as it is not moving. But breathing and heart beat leads to organ motion that may interfere with the scanning procedure and yield inhomogeneous dose distributions as well as spreading of a significant dose outside the planned target volume. For the compensation of target motion, different strategies have been proposed such as multiple repainting, which reduces dose inhomogeneities but increases the target volume, gating, where the tumour is irradiated only in a predefined position or fast 3D beam tracking [93], [94], [95], which would restore the congruence of irradiated and planned target volume but which has not been realized at HIT up to now. Clinically, however, only the gating techniques has been used so far.
Recently, the HIT facility has been commissioned for Helium beam treatments and a first patient was treated here in July 2021. Helium beams combine physical advantages from Carbon (significantly reduced lateral scattering as compared to protons) with radiobiological properties, which are less pronounced as compared to carbon ions [96]. From principle considerations, it is expected that also the secondary neutron background from Helium ion treatments should be strongly reduced. This makes Helium ions a potentially ideal ion for pediatric patients, but also for the increasing number of pregnant patients. In both cases the additional sparing of normal tissue due to the reduced lateral penumbra, but also the potentially reduced neutron background, may be beneficial. The new clinical availability of Helium ions will open interesting new lines for clinical research, which are also considered at other centres like NIRS and MD Anderson.
Another important line of development is the integration of improved anatomical imaging for organ motion management. For this purpose, the possibility of MR-guided ion beam therapy will be investigated [97], [98], [99]. Funded by the BMBF, the ARTEMIS project was just recently initiated (Adaptive RadioThErapy Mit IonenStrahlen). Within ARTEMIS, the concept of off-line MR-imaging and integration of MRI based plan adaption will be explored. In a second step, the potential of an in-room MRI combined with a robotic patient positioning system will be investigated. This system will include a patient capsule, which will allow rotating the patient within the MRI in a horizontal beam. With this study, the potential of a gantry-less, but MRI-based ion beam treatment will be explored.
6. More recent developments in ion beam therapy
After the initial exploration of light ion beam therapy using mostly neon ions in the US at the Lawrence Berkeley Laboratory, the development of carbon ion beam therapy was driven by Japanese researchers at the NIRS, where the first clinical light ion therapy center was established at NIRS (Chiba, Japan) with the Heavy Ion Medical ACcelerator (HIMAC). Since 1994, approximately 14,000 patients have been treated at NIRS using carbon beams with very good results. The very successful history of the Japanese proton and ion beam therapy facilities has been described previously and is beyond the scope of this article [100], [101]. Japan has continuously increased the number of carbon ion facilities and is operating today six carbon ion facilities (a seventh is under construction) and 18 proton facilities. While the first centers in Japan started with passive beam delivery, also beam scanning has been developed at NIRS and has been installed in the most recent facilities. There are also developments ongoing at NIRS, to reduce the size and costs of ion beam therapy facilities. This lead to the first superconducting gantry at NIRS, starting operation in 2016. This development will be continued with a second generation of superconducting gantries and synchrotron rings, based on superconducting magnet technology. Also in Europe, the number of light ion therapy centers is slowly increasing, with 2 facilities in operation in Germany (HIT and MIT, the Marburg Ion beam Therapy center at Marburg University Clinic) and two additional facilities in Austria (MedAustron, Wiener Neustadt) and Italy (Centro Nazionale di Adroterapia Oncologica, CNAO, Pavia). Two additional carbon ion therapy facilities were established in Lanzhou and Shanghai (China), the latter being based on a machine designed by Siemens and is the twin facility of the MIT at Marburg.
The MIT resulted from the interest of the Rhoen Klinikum AG (RKA), a private company in the novel field of precision radiotherapy. RKA had acquired the university clinics of Giessen and Marburg and invested for new techniques to become a center of excellence also for patient from outside Germany. Siemens had decided to sell complete heavy ions systems and MIT was the first together with the system in Shanghai and another one at the university of Kiel (Germany). The aim of these systems was to treat significantly more than 1000 patients per year to lower the cost per patient. The construction phase of MIT started in March 2007 and patient treatment was scheduled for 3 years later. But the number could not be reached and finally, RKA did not buy the system. At this time Siemens decided to terminate its involvement in particle therapy and later on also in conventional therapy. Siemens continued to use MIT as a test base to bring the Chinese system to the guaranteed performance. In 2015, MIT was sold to a joined venture of Heidelberg University and RKA. After insolvency, MIT became a part of the University Clinics of Marburg. The Kiel project NROCK was stopped and the operational system was dismantled and converted to a conventional radiotherapy unit while the Shanghai project was completed and became operational. With the acquisition of Varian Inc., one of the biggest vendors of conventional and proton therapy facilities, Siemens Healthineers returned to the market in 2020 as a supplier for radiation oncology treatment device equipment.
The MedAustron and CNAO facilities are an outcome of a CERN initiative that proposed a dedicated medical accelerator design (Proton Ion Medical Machine Study, PIMMS [102]). CNAO became operational in 2011 for protons and 2014 for carbon ions. In 2014, an additional proton center in Trento opened [103].
The MedAustron facility became operational for protons and carbon ions in 2017 and 2019, respectively and is operating one research room and 3 patient treatment areas including a proton gantry, a horizontal treatment area and an area with a combined horizontal and vertical beam. The facility is operating one research room and 3 patient treatment areas including a proton gantry, a horizontal treatment area and an area with a combined horizontal and vertical beam [104].
An additional carbon ion facility is under construction in France and will be the first ion beam facility based on a cyclotron accelerator.5 Several new centers are already under construction in China, South Korea, Taiwan and Japan.6
In the USA, the Harvard University continued treating patients with protons and installed a second, synchrotron-based accelerator in 2020, to increase patient throughput. Since Loma Linda University Medical Center started with proton therapy in 1991, 39 additional proton facilities were installed up to now and 5 more are under construction. Recently, the construction of a Carbon facility is also considered from Mayo clinics in collaboration with Hitachi, Japan.7
Concerning the standardization of the clinical application of light ions, it should be noted, that recently, the International commission for radiation units and measurements (ICRU) has published a report on prescribing, recording and report light ion beam therapy (ICRU Report 93 [63]). This report is an important contribution to harmonize the clinical prescription, reporting and the application of the different radiobiological models used at different centers. Also, the international dosimetry protocol issued by the international atomic energy agency (IAEA), TRS-398, which includes proton and light ion beams is currently undergoing a revision and will contribute to a harmonization of dosimetry standards for light ions.8
7. Discussion
Heavy ion therapy originates from accelerator physics and was strongly promoted by nuclear physicists partially because as physicists they were convinced by the superiority of the physical properties of ion beams, partially because they needed a justification for new accelerators. This later fact explained failure of many proposals, not mentioned in this review. But the successful centers could consistently demonstrate improved clinical results and were a great stimulus for radiotherapy in general. The major advantage of ion beams, the inverse dose profile with a maximal dose at the end of range remained the strongest argument for both protons and heavier ions as this feature cannot be mimicked by any other radiation modality.[96], [105], [106] Because of the lower cost and smaller size, the number of proton facilities increased much faster and up to now, nearly 250,000 patients have been successfully treated with protons and nearly 40,000 patients received carbon ion treatments by the end of 2020.9
The remarkable clinical results of carbon ion therapy prompted the interest of many scientists and institutions. But the main obstacle for a fast distribution of this novel treatment approach was and is still the large investment in the order of hundred million Euro for a single heavy ion facility.
Japan was the first country to establish a clinical heavy ion facility at NIRS in Chiba. The clinical results motivated the construction of additional 6 facilities for heavy ion therapy together with 18 proton facilities in Japan as of November 2021 (see footnote 9). In Europe, the pilot project at GSI, Darmstadt was the pioneer for heavy ion therapy which provoked great enthusiasm and many projects were proposed in the following. This development was also supported by the fact that Siemens, at that time the leader in medical application industry, offered such systems as a commercial product. However, in 2012 when Siemens withdrew from heavy ion radiotherapy, the public opinion changed and only a few projects survived.
Today the promising clinical results generated a renaissance for ion beam therapy and the interest is again growing. This is even true for the US, where the Mayo clinics is planning to build a carbon therapy facility in Jacksonville, Florida, constructed by the Japanese company Hitachi. We hope that the excellent results of proton and carbon ion therapy, which still present a novel approach in many aspects, will create more interest for the construction of further ion beam facilities. These centers could provide a larger basis for multicentric clinical studies, which may then explore in more detail the benefits of light ions for cancer patients.
The consistent reporting of RBE-models and parameters used in therapy planning, which was introduced by ICRU 93 is an important contribution to make the clinical application more comparable between centers. This is an important ingredient to perform multi-centric clincal studies, which in turn are needed to arrive with conclusive clinical results.
Also, the question, which ion type is most beneficial for which type of tumor is still not clear and more studies comparing protons, carbon ion beams and in the future also helium ion beams will hopefully be conducted. The development of smaller machines for light ions, as it is currently approached by NIRS with the SUPERconducting Magnet INstalled Ion Medical Accelerator in Chiba (Super MINIMAC, or the Quantum Knife) may allow building facilities with significantly lower cost (below 50 M US$) by around 2030 [107] to address these questions.
Conflict of interest
None declared.
Footnotes
In the following the term heavy ions will be used, although, strictly, the term light ions for ions up to Neon (Z = 10) should be used according to the ICRU. In this sense, only treatments with silicon and argon, as performed in Berkeley should be called heavy ion therapy.
It should be stressed, that this type of intensity-controlled raster scanning intrinsically includes a modulation of the number of particles delivered to each position and may thus be regarded as a form of intensity-modulated radiotherapy (IMRT). IMRT, however, is usually understood as being connected to a simultaneous optimization of all fields contributing to a treatment plan, as explained in Section 4.2.
https://www.wissenschaftsrat.de/download/archiv/pm_1401.html (accessed November 4th 2021).
See www.clinicaltrials.gov with search items „Neoadjuvant Irradiation of Retroperitoneal Soft Tissue Sarcoma With Ions Retro-Ion (Retro-Ion)“ (accessed on November 4th 2021).
See www.clinicaltrials.gov Adenoid Cystic Carcinoma and Carbon Ion Only Irradiation (ACCO) (accessed November 4th 2021).
See https://www.klinikum.uni-heidelberg.de/kliniken-institute/interdisziplinaere-zentren/heidelberger-ionenstrahl-therapiezentrum-hit/fuer-aerzte/klinische-studien (accessed November 4th 2021).
In principle the same holds true for proton therapy, but here variations of RBE are of the same relative size, but smaller I absolute numbers and the present use of a fixed value of 1.1 eliminates the variations between patients and institutions.
https://www.france-hadron.fr/en/nodes/archade-caen.html (accessed November 4th 2021).
www.ptcog.ch (accessed November 4th 2021).
www.ptcog.ch/index.php/facilities-in-planning-stage (accessed November 4th 2021).
https://humanhealth.iaea.org/HHW/MedicalPhysics/Radiotherapy/Dosimetry/Proton_therapy/index.html (accessed November 4th 2021).
www.ptcog.ch (accessed November 4th 2021).
References
- 1.Wilson R.R. Radiological use of fast protons. Radiology. 1946;47(5):487–491. doi: 10.1148/47.5.487. [DOI] [PubMed] [Google Scholar]
- 2.Bethge K., Kreisler K.G.P., Walter G. Springer; Heidelberg: 2004. Hadron therapy in medical applications of nuclear physics. [Google Scholar]
- 3.Tobias C., et al. Pituitary irradiation with high-energy proton beams a preliminary report. Cancer Res. 1958;18(2):121–134. [PubMed] [Google Scholar]
- 4.Linstadt D.E., Castro J.R., Phillips T.L. Neon ion radiotherapy: results of the phase I/II clinical trial. Int J Radiat Oncol Biol Phys. 1991;20(4):761–769. doi: 10.1016/0360-3016(91)90020-5. [DOI] [PubMed] [Google Scholar]
- 5.Larsson B. Pre-therapeutic physical experiments with high energy protons. Br J Radiol. 1961;34(399):143–151. doi: 10.1259/0007-1285-34-399-143. [DOI] [PubMed] [Google Scholar]
- 6.Suit H.D., et al. Exploratory study of proton radiation-therapy using large field techniques and fractionated dose schedules. Cancer. 1975;35(6):1646–1657. doi: 10.1002/1097-0142(197506)35:6<1646::aid-cncr2820350626>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Slater J.M., et al. The proton treatment center at Loma-Linda-University-Medical-Center – rationale for and description of its development. Int J Radiat Oncol Biol Phys. 1992;22(2):383–389. doi: 10.1016/0360-3016(92)90058-p. [DOI] [PubMed] [Google Scholar]
- 8.Kanai T., et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44(1):201–210. doi: 10.1016/s0360-3016(98)00544-6. [DOI] [PubMed] [Google Scholar]
- 9.Chu W., Ludewigt B., Renner T. Instrumentation for treatment of cancer using proton and light-ion beams. Rev Sci Instrum. 1993;64(8):2055–2122. [Google Scholar]
- 10.Kraft G. In: Encyclopedia of nuclear physics and its application. Stock R., editor. Wiley VCH Weinheim; 2013. Cancer therapy with ion beams; pp. 527–596. [Google Scholar]
- 11.Haberer T., et al. Magnetic scanning system for heavy-ion therapy. Nucl Instrum Methods Phys Res Sect A Accel Spectrom Detect Assoc Equip. 1993;330(1–2):296–305. [Google Scholar]
- 12.Karger C.P., et al. The RBE in ion beam radiotherapy: in vivo studies and clinical application. Z Med Phys. 2021 doi: 10.1016/j.zemedi.2020.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Chen G.T., Castro J.R., Quivey J.M. Heavy charged particle radiotherapy. Annu Rev Biophys Bioeng. 1981;10:499–529. doi: 10.1146/annurev.bb.10.060181.002435. [DOI] [PubMed] [Google Scholar]
- 14.Castro J.R., et al. Treatment of cancer with heavy charged particles. Int J Radiat Oncol Biol Phys. 1982;8(12):2191–2198. doi: 10.1016/0360-3016(82)90569-7. [DOI] [PubMed] [Google Scholar]
- 15.Castro J.R., et al. 15 years experience with helium ion radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys. 1997;39(5):989–996. doi: 10.1016/s0360-3016(97)00494-x. [DOI] [PubMed] [Google Scholar]
- 16.Levy R.P., et al. Heavy-charged-particle radiosurgery of the pituitary gland: clinical results of 840 patients. Stereotact Funct Neurosurg. 1991;57(1–2):22–35. doi: 10.1159/000099553. [DOI] [PubMed] [Google Scholar]
- 17.von Essen C.F. The pi meson therapy program at SIN. J Can Assoc Radiol. 1980;31(1):19–25. [PubMed] [Google Scholar]
- 18.Elwood J.M. The design of clinical trials comparing pi-meson therapy with conventional radiotherapy. J Can Assoc Radiol. 1979;30(2):79–82. [PubMed] [Google Scholar]
- 19.Pistenma D.A., et al. Treatment planning for negative pi-meson radiation therapy: simultaneous multi-port irradiation with the Stanford Medical Pion Generator (SMPG) Int J Radiat Oncol Biol Phys. 1977;3:315–323. doi: 10.1016/0360-3016(77)90270-x. [DOI] [PubMed] [Google Scholar]
- 20.von Essen C.F., et al. The PIOTRON: initial performance, preparation and experience with pion therapy. Int J Radiat Oncol Biol Phys. 1982;8(9):1499–1509. doi: 10.1016/0360-3016(82)90609-5. [DOI] [PubMed] [Google Scholar]
- 21.Schmitt G., et al. Review of the SIN and Los Alamos Pion Trials. Radiat Res Suppl. 1985;8:S272–S278. [PubMed] [Google Scholar]
- 22.Mandrillon P., Ostojic R., Farley F., Rocher C., Susini A., Godot J.C., Ryckewaert G. Proceedings of the 1989 IEEE Particle Accelerator Conference. Accelerator Science and Technology; 1989. Advances of the feasibility study of the European Light Ion Medical Accelerator. [Google Scholar]
- 23.Bryant P.J. Progress of the Proton-Ion Medical Machine Study (PIMMS) Strahl Onkol Strahl Onkol. 1999;17(Suppl. II):1–4. doi: 10.1007/BF03038873. [DOI] [PubMed] [Google Scholar]
- 24.Amaldi U. Springer; 2012. From big bang to hadron therapy. [Google Scholar]
- 25.Kraft G. In: The physics of multiply and highly charged ions. Curell F.J., editor. Acad. Press Kluwer; 2003. Radiobiological effects of highly charged ions; pp. 150–196. [Google Scholar]
- 26.Kanai T., et al. Spot scanning system for proton radiotherapy. Med Phys. 1980;7(4):365–369. doi: 10.1118/1.594693. [DOI] [PubMed] [Google Scholar]
- 27.Fischer B. R.S., Heavy ion microlithography — a new tool to generate and investigate submicroscopic structures. Nucl Instr Methods. 1980;168(1–3):241–246. [Google Scholar]
- 28.Kraft G. The heavy ion therapy project at GSI. Phys Med. 1998;XIV:86–90. [Google Scholar]
- 29.Schlegel W., et al. Computer systems and mechanical tools for stereotactically guided conformation therapy with linear accelerators. Int J Radiat Oncol Biol. Phys. 1992;24(4):781–787. doi: 10.1016/0360-3016(92)90729-2. [DOI] [PubMed] [Google Scholar]
- 30.Bendl R., Höss A., Keller M., Preiser K., Pross J., Schlegel W. In: Medizinische Informatik, Proc. 38. Jahrestagung der GMDS 1993. Pöppel S.J., editor. MMV Medizinverlag: Lübeck; 1993. Virtuelle 3D-Simulation der Strahlentherapie mit VIRTUOS; pp. 241–244. [Google Scholar]
- 31.Krämer M., et al. Overview of recent advances in treatment planning for ion beam radiotherapy. Eur Phys J D. 2014;68:306. [Google Scholar]
- 32.Jäkel O., et al. Treatment planning for heavy ion radiotherapy: clinical implementation and application. Phys Med Biol. 2001;46(4):1101–1116. doi: 10.1088/0031-9155/46/4/314. [DOI] [PubMed] [Google Scholar]
- 33.Jäkel O., et al. Relation between carbon ion ranges and X-ray CT numbers. Med Phys. 2001;28(4):701–703. doi: 10.1118/1.1357455. [DOI] [PubMed] [Google Scholar]
- 34.Rietzel E., Schardt D., Haberer T. Range accuracy in carbon ion treatment planning based on CT-calibration with real tissue samples. Radiat Oncol. 2007;2:14. doi: 10.1186/1748-717X-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krämer M., et al. Treatment planning for heavy-ion radiotherapy: physical beam model and dose optimization. Phys Med Biol. 2000;45(11):3299–3317. doi: 10.1088/0031-9155/45/11/313. [DOI] [PubMed] [Google Scholar]
- 36.Krämer M., Scholz M. Treatment planning for heavy-ion radiotherapy: calculation and optimization of biologically effective dose. Phys Med Biol. 2000;45(11):3319–3330. doi: 10.1088/0031-9155/45/11/314. [DOI] [PubMed] [Google Scholar]
- 37.Scholz M., et al. Computation of cell survival in heavy ion beams for therapy. The model and its approximation. Radiat Environ Biophys. 1997;36(1):59–66. doi: 10.1007/s004110050055. [DOI] [PubMed] [Google Scholar]
- 38.Weyrather W.K., et al. RBE for carbon track-segment irradiation in cell lines of differing repair capacity. Int J Radiat Biol. 1999;75(11):1357–1364. doi: 10.1080/095530099139232. [DOI] [PubMed] [Google Scholar]
- 39.Debus J., et al. Radiation tolerance of the rat spinal cord after single and split doses of photons and carbon ions. Radiat Res. 2003;160(5):536–542. doi: 10.1667/3063. [DOI] [PubMed] [Google Scholar]
- 40.Karger C.P., et al. Radiation tolerance of the rat spinal cord after 6 and 18 fractions of photons and carbon ions: experimental results and clinical implications. Int J Radiat Oncol Biol Phys. 2006;66(5):1488–1497. doi: 10.1016/j.ijrobp.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 41.Elsässer T., et al. Quantification of the relative biological effectiveness for ion beam radiotherapy: direct experimental comparison of proton and carbon ion beams and a novel approach for treatment planning. Int J Radiat Oncol Biol Phys. 2010;78(4):1177–1183. doi: 10.1016/j.ijrobp.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 42.Krämer M., Jakel O. Biological dose optimization using ramp-like dose gradients in ion irradiation fields. Phys Med. 2005;21(3):107–111. doi: 10.1016/S1120-1797(05)80011-0. [DOI] [PubMed] [Google Scholar]
- 43.IAEA . International Atomic Energy Agency; Wien: 2000. Absorbed dose determination in external beam radiotherapy – an international code of practice for dosimetry based on standards of absorbed dose to water. Technical report series No. 398. [Google Scholar]
- 44.Jäkel O., et al. A calibration procedure for beam monitors in a scanned beam of heavy charged particles. Med Phys. 2004;31(5):1009–1013. doi: 10.1118/1.1689011. [DOI] [PubMed] [Google Scholar]
- 45.Hartmann G.H., et al. Determination of water absorbed dose in a carbon ion beam using thimble ionization chambers. Phys Med Biol. 1999;44(5):1193–1206. doi: 10.1088/0031-9155/44/5/008. [DOI] [PubMed] [Google Scholar]
- 46.Karger C.P., et al. Dosimetry for ion beam radiotherapy. Phys Med Biol. 2010;55(21):R193–R234. doi: 10.1088/0031-9155/55/21/R01. [DOI] [PubMed] [Google Scholar]
- 47.Palmans H., Vatnitsky S.M. Beam monitor calibration in scanned light-ion beams. Med Phys. 2016;43(11):5835. doi: 10.1118/1.4963808. [DOI] [PubMed] [Google Scholar]
- 48.DIN 6801-1:2016-06 . Deutsches Institut für Normung e.V.; Beuth Verlag, Berlin: 2016. Procedures of dosimetry with probe-type detectors for proton and ion radiation – part 1: ionization chambers. [Google Scholar]
- 49.Jäkel O., et al. Quality assurance for a treatment planning system in scanned ion beam therapy. Med Phys. 2000;27(7):1588–1600. doi: 10.1118/1.599025. [DOI] [PubMed] [Google Scholar]
- 50.Karger C.P., et al. Quality management of medical physics issues at the German heavy ion therapy project. Med Phys. 2000;27(4):725–736. doi: 10.1118/1.598935. [DOI] [PubMed] [Google Scholar]
- 51.Karger C.P., et al. Clinical dosimetry for heavy ion therapy. Z Med Phys. 2002;12(3):159–169. doi: 10.1016/s0939-3889(15)70463-0. [DOI] [PubMed] [Google Scholar]
- 52.Heeg P., et al. Quality assurance at the heavy-ion therapy facility at GSI. Strahlenther Onkol. 1999;175(Suppl. 2):36–38. doi: 10.1007/BF03038885. [DOI] [PubMed] [Google Scholar]
- 53.Karger C.P., Jäkel O., Hartmann G.H. A system for three-dimensional dosimetric verification of treatment plans in intensity-modulated radiotherapy with heavy ions. Med Phys. 1999;26(10):2125–2132. doi: 10.1118/1.598728. [DOI] [PubMed] [Google Scholar]
- 54.Jäkel O., et al. Methodology paper: a novel phantom setup for commissioning of scanned ion beam delivery and TPS. Radiat Oncol. 2019;14(1):77. doi: 10.1186/s13014-019-1281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mirandola A., et al. Dosimetric commissioning and quality assurance of scanned ion beams at the Italian National Center for Oncological Hadrontherapy. Med Phys. 2015;42(9):5287–5300. doi: 10.1118/1.4928397. [DOI] [PubMed] [Google Scholar]
- 56.Molinelli S., et al. Dosimetric accuracy assessment of a treatment plan verification system for scanned proton beam radiotherapy: one-year experimental results and Monte Carlo analysis of the involved uncertainties. Phys Med Biol. 2013;58(11):3837–3847. doi: 10.1088/0031-9155/58/11/3837. [DOI] [PubMed] [Google Scholar]
- 57.Enghardt W., et al. Limited-angle 3D reconstruction of PET images for dose localization in light ion tumour therapy. Phys Med Biol. 1992;37(3):791–798. doi: 10.1088/0031-9155/37/3/021. [DOI] [PubMed] [Google Scholar]
- 58.Llacer J. Positron emission medical measurements with accelerated radioactive ion beams. Nucl Sci Appl. 1988;3:111–131. [Google Scholar]
- 59.Kraft G. Tumor therapy with ion beams. Nucl Instrum Meth. 2000;A454:1–10. [Google Scholar]
- 60.Enghardt W., et al. Dose quantification from in-beam positron emission tomography. Radiother Oncol. 2004;73(Suppl. 2):S96–S98. doi: 10.1016/s0167-8140(04)80024-0. [DOI] [PubMed] [Google Scholar]
- 61.Pönisch F., et al. The modelling of positron emitter production and PET imaging during carbon ion therapy. Phys Med Biol. 2004;49(23):5217–5232. doi: 10.1088/0031-9155/49/23/002. [DOI] [PubMed] [Google Scholar]
- 62.Fiedler F., et al. On the effectiveness of ion range determination from in-beam PET data. Phys Med Biol. 2010;55(7):1989–1998. doi: 10.1088/0031-9155/55/7/013. [DOI] [PubMed] [Google Scholar]
- 63.ICRU Report 93: prescribing, recording, and reporting light ion beam therapy. J ICRU. 2016;16(1–2) [Google Scholar]
- 64.Schulz-Ertner D., et al. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int J Radiat Oncol Biol Phys. 2007;68(2):449–457. doi: 10.1016/j.ijrobp.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 65.Schulz-Ertner D., et al. Carbon ion radiotherapy of skull base chondrosarcomas. Int J Radiat Oncol Biol Phys. 2007;67(1):171–177. doi: 10.1016/j.ijrobp.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 66.Schulz-Ertner D., et al. Therapy strategies for locally advanced adenoid cystic carcinomas using modern radiation therapy techniques. Cancer. 2005;104(2):338–344. doi: 10.1002/cncr.21158. [DOI] [PubMed] [Google Scholar]
- 67.Uhl M., et al. Highly effective treatment of skull base chordoma with carbon ion irradiation using a raster scan technique in 155 patients: first long-term results. Cancer. 2014;120(21):3410–3417. doi: 10.1002/cncr.28877. [DOI] [PubMed] [Google Scholar]
- 68.Uhl M., et al. High control rate in patients with chondrosarcoma of the skull base after carbon ion therapy: first report of long-term results. Cancer. 2014;120(10):1579–1585. doi: 10.1002/cncr.28606. [DOI] [PubMed] [Google Scholar]
- 69.Jensen A.D., et al. Combined intensity-modulated radiotherapy plus raster-scanned carbon ion boost for advanced adenoid cystic carcinoma of the head and neck results in superior locoregional control and overall survival. Cancer. 2015;121(17):3001–3009. doi: 10.1002/cncr.29443. [DOI] [PubMed] [Google Scholar]
- 70.Combs S.E., et al. Heidelberg Ion Therapy Center (HIT): initial clinical experience in the first 80 patients. Acta Oncol. 2010;49(7):1132–1140. doi: 10.3109/0284186X.2010.498432. [DOI] [PubMed] [Google Scholar]
- 71.Amaldi U.K.G. Recent applications of synchrotrons in cancer therapy with carbon ions. Europhys News. 2005;36(4):114–118. [Google Scholar]
- 72.Bauer J., et al. Implementation and initial clinical experience of offline PET/CT-based verification of scanned carbon ion treatment. Radiother Oncol. 2013;107(2):218–226. doi: 10.1016/j.radonc.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 73.Durante M.P.K. Radioactive beams in particle therapy: past presence and future. Front Phys. 2020;8 doi: 10.3389/fphy.2020.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nikoghosyan A.V., et al. Randomised trial of proton vs. carbon ion radiation therapy in patients with chordoma of the skull base, clinical phase III study HIT-1-Study. BMC Cancer. 2010;10:607. doi: 10.1186/1471-2407-10-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uhl M., et al. Randomized phase II trial of hypofractionated proton versus carbon ion radiation therapy in patients with sacrococcygeal chordoma-the ISAC trial protocol. Radiat Oncol. 2014;9:100. doi: 10.1186/1748-717X-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nikoghosyan A.V., et al. Randomised trial of proton vs. carbon ion radiation therapy in patients with low and intermediate grade chondrosarcoma of the skull base, clinical phase III study. BMC Cancer. 2010;10:606. doi: 10.1186/1471-2407-10-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blattmann C., et al. Non-randomized therapy trial to determine the safety and efficacy of heavy ion radiotherapy in patients with non-resectable osteosarcoma. BMC Cancer. 2010;10:96. doi: 10.1186/1471-2407-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seidensaal K., et al. Neoadjuvant irradiation of retroperitoneal soft tissue sarcoma with ions (Retro-Ion): study protocol for a randomized phase II pilot trial. Trials. 2021;22(1):134. doi: 10.1186/s13063-021-05069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jensen A.D., et al. COSMIC: a regimen of intensity modulated radiation therapy plus dose-escalated, raster-scanned carbon ion boost for malignant salivary gland tumors: results of the prospective phase 2 trial. Int J Radiat Oncol Biol Phys. 2015;93(1):37–46. doi: 10.1016/j.ijrobp.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 80.Adeberg S., et al. The phase 1/2 ACCEPT trial: concurrent cetuximab and intensity modulated radiation therapy with carbon ion boost for adenoid cystic carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2020;106(1):167–173. doi: 10.1016/j.ijrobp.2019.09.036. [DOI] [PubMed] [Google Scholar]
- 81.Jensen A.D., et al. Phase II study of induction chemotherapy with TPF followed by radioimmunotherapy with Cetuximab and intensity-modulated radiotherapy (IMRT) in combination with a carbon ion boost for locally advanced tumours of the oro-, hypopharynx and larynx--TPF-C-HIT. BMC Cancer. 2011;11:182. doi: 10.1186/1471-2407-11-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jensen A.D., et al. Treatment of malignant sinonasal tumours with intensity-modulated radiotherapy (IMRT) and carbon ion boost (C12) BMC Cancer. 2011;11:190. doi: 10.1186/1471-2407-11-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Combs S.E., et al. Randomized phase II study evaluating a carbon ion boost applied after combined radiochemotherapy with temozolomide versus a proton boost after radiochemotherapy with temozolomide in patients with primary glioblastoma: the CLEOPATRA trial. BMC Cancer. 2010;10:478. doi: 10.1186/1471-2407-10-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Combs S.E., et al. Randomised phase I/II study to evaluate carbon ion radiotherapy versus fractionated stereotactic radiotherapy in patients with recurrent or progressive gliomas: the CINDERELLA trial. BMC Cancer. 2010;10:533. doi: 10.1186/1471-2407-10-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Combs S.E., et al. Treatment of patients with atypical meningiomas Simpson grade 4 and 5 with a carbon ion boost in combination with postoperative photon radiotherapy: the MARCIE trial. BMC Cancer. 2010;10:615. doi: 10.1186/1471-2407-10-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Habl G., et al. Ion prostate irradiation (IPI) – a pilot study to establish the safety and feasibility of primary hypofractionated irradiation of the prostate with protons and carbon ions in a raster scan technique. BMC Cancer. 2014;14:202. doi: 10.1186/1471-2407-14-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Habl G., et al. Acute toxicity and quality of life in patients with prostate cancer treated with protons or carbon ions in a prospective randomized phase ii study – the IPI Trial. Int J Radiat Oncol Biol Phys. 2016;95(1):435–443. doi: 10.1016/j.ijrobp.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 88.Koerber S.A., et al. Prostate bed irradiation with alternative radio-oncological approaches (PAROS) – a prospective, multicenter and randomized phase III trial. Radiat Oncol. 2019;14(1):122. doi: 10.1186/s13014-019-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Combs S.E., et al. Phase i study evaluating the treatment of patients with hepatocellular carcinoma (HCC) with carbon ion radiotherapy: the PROMETHEUS-01 trial. BMC Cancer. 2011;11:67. doi: 10.1186/1471-2407-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liermann J., et al. Carbon ion radiotherapy as definitive treatment in non-metastasized pancreatic cancer: study protocol of the prospective phase II PACK-study. BMC Cancer. 2020;20(1):947. doi: 10.1186/s12885-020-07434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Combs S.E., et al. Phase I/II trial evaluating carbon ion radiotherapy for the treatment of recurrent rectal cancer: the PANDORA-01 trial. BMC Cancer. 2012;12:137. doi: 10.1186/1471-2407-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hauswald H., et al. Ion therapy within the trimodal management of superior sulcus tumors: the INKA trial. BMC Cancer. 2015;15:192. doi: 10.1186/s12885-015-1163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bert C., Herfarth K. Management of organ motion in scanned ion beam therapy. Radiat Oncol. 2017;12(1):170. doi: 10.1186/s13014-017-0911-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miki K., et al. Gated carbon-ion scanning treatment for pancreatic tumour with field specific target volume and organs at risk. Phys Med. 2016;32(12):1521–1528. doi: 10.1016/j.ejmp.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 95.Mori S., et al. Amplitude-based gated phase-controlled rescanning in carbon-ion scanning beam treatment planning under irregular breathing conditions using lung and liver 4DCTs. J Radiat Res. 2014;55(5):948–958. doi: 10.1093/jrr/rru032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jakel O. Physical advantages of particles: protons and light ions. Br J Radiol. 2020;93(1107) doi: 10.1259/bjr.20190428. p. 20190428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hoffmann A., et al. MR-guided proton therapy: a review and a preview. Radiat Oncol. 2020;15(1):129. doi: 10.1186/s13014-020-01571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burigo L.N., Oborn B.M. MRI-guided proton therapy planning: accounting for an inline MRI fringe field. Phys Med Biol. 2019;64(21):215015. doi: 10.1088/1361-6560/ab436a. [DOI] [PubMed] [Google Scholar]
- 99.Oborn B.M., et al. Future of medical physics: real-time MRI-guided proton therapy. Med Phys. 2017;44(8):e77–e90. doi: 10.1002/mp.12371. [DOI] [PubMed] [Google Scholar]
- 100.Mohamad O., Makishima H., Kamada T. Evolution of Carbon Ion Radiotherapy at the National Institute of Radiological Sciences in Japan. Cancers (Basel) 2018;10 doi: 10.3390/cancers10030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kamada T., et al. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol. 2015;16(2):e93–e100. doi: 10.1016/S1470-2045(14)70412-7. [DOI] [PubMed] [Google Scholar]
- 102.Bryant P.J. Progress of the Proton-Ion Medical Machine Study (PIMMS) Strahlenther Onkol. 1999;175(Suppl 2):1–4. doi: 10.1007/BF03038873. [DOI] [PubMed] [Google Scholar]
- 103.Amaldi U. The Italian hadrontherapy project CNAO. Phys Med. 2001;17(Suppl. 1):33–37. [PubMed] [Google Scholar]
- 104.Benedikt M., Wrulich A. MedAustron—project overview and status. Eur Phys J Plus. 2011;126(7) [Google Scholar]
- 105.Weber U.K.G. Comparison of carbon ions versus protons. Cancer J. 2009;4:325–332. doi: 10.1097/PPO.0b013e3181b01935. [DOI] [PubMed] [Google Scholar]
- 106.Suit H., et al. Proton vs carbon ion beams in the definitive radiation treatment of cancer patients. Radiother Oncol. 2010;95(1):3–22. doi: 10.1016/j.radonc.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 107.Mohamad O., Makishima H., Kamada T. Evolution of Carbon Ion Radiotherapy at the National Institute of Radiological Sciences in Japan. Cancers (Basel) 2018;10(3) doi: 10.3390/cancers10030066. [DOI] [PMC free article] [PubMed] [Google Scholar]