Abstract
In the field of preclinical radiotherapy, many new developments were driven by technical innovations. To make research of different groups comparable in that context and reliable, high quality has to be maintained. Therefore, standardized protocols and programs should be used. Here we present a guideline for a comprehensive and efficient quality assurance program for an image-guided small animal irradiation system, which is meant to test all the involved subsystems (imaging, treatment planning, and the irradiation system in terms of geometric accuracy and dosimetric aspects) as well as the complete procedure (end-to-end test) in a time efficient way. The suggestions are developed on a Small Animal Radiation Research Platform (SARRP) from Xstrahl (Xstrahl Ltd., Camberley, UK) and are presented together with proposed frequencies (from monthly to yearly) and experiences on the duration of each test. All output and energy related measurements showed stable results within small variation. Also, the motorized parts (couch, gantry) and other geometrical alignments were very stable. For the checks of the imaging system, the results are highly dependent on the chosen protocol and differ according to the settings. We received nevertheless stable and comparably good results for our mainly used protocol. All investigated aspects of treatment planning were exactly fulfilled and also the end-to-end test showed satisfying values. The mean overall time we needed for our checks to have a well monitored machine is less than two hours per month.
Keywords: Small animal irradiator, Quality assurance, SARRP, Image-guided radiotherapy, Preclinical radiotherapy
1. Introduction
Most of the new developments in the field of radiation therapy over the last few decades were driven by technical innovations, e.g. new therapeutic machines with higher precision, or new irradiation techniques and treatment planning tools [1]. Some years ago, the preclinical research part of radiation therapy lagged behind in technical solutions, but is now able to catch up since commercial image-guided small animal irradiators are available on the market and also a number of in-house developments exist (an overview is available in [2]). Through the ongoing technical advances for these machines, the methods in preclinical research can now approximate the methods from clinical irradiations [3]. Therefore, the significance and strength of research in the preclinical field will gain. To make research of different groups comparable and reliable, particularly if high precision is sought, a high standard in quality, particularly in dose output, has to be maintained. Therefore, standardized protocols and calibrated equipment traceable to a primary standard should be used [2], [4] and a precise commissioning is needed [5]. In addition, a comparison of quality characteristics between different systems or systems at different institutions is essential [6], [7]. To facilitate comparability of studies within the same as well as between different institutions, a precise and clear reporting of quality characteristics is required [4], [8]. This includes also the comparability and transferability of preclinical research with and to clinical radiotherapy.
A further important task is to ensure that each single system behaves constantly (not only with regard to failures, see [9]) and reliably after commissioning, even over a long period of time (years). Therefore, comprehensive quality assurance (QA) programs have to be established and run routinely/regularly (similar to QA programs for human radiotherapy). There are suggestions for simple QA programs [10], for geometric QA [11], for specific phantoms [12], and for image quality [6]. To our knowledge, there is neither a published comprehensive QA program that covers all involved subsystems, namely imaging, treatment planning, and the irradiation system (in terms of geometric accuracy and dosimetric aspects), nor any guideline to create such a comprehensive program. Our program is meant to test all the mentioned subsystems as well as the complete procedure (end-to-end test) and can act as a guideline for other users of small animal irradiation systems for a complete QA. In comparison to other literature this work presents a comprehensive program that can be used as standalone guideline. As time on the systems as well as time of researchers is narrow, we tried to develop a program for measurements to be done in a time efficient way that is capable to be run periodically, ideally by medical physicists or other well trained personnel.
At our institution, a Small Animal Radiation Research Platform (SARRP) from Xstrahl (Xstrahl Ltd., Camberley, UK) is frequently used by biologists and physicians for preclinical research. For this system, we were in need of a QA program that ensures quality and does not block the machine for a long time. Here we present a guideline for a comprehensive and efficient QA program for image-guided small animal irradiators, designed and tested on a SARRP.
In this work we do not focus on a complete and seamless description of the setup and the implementation of single measurements in every detail. Instead, we will mainly concentrate on a more general description of the needed aspects for a complete and comprehensive quality assurance, to potentially be used as a guideline. Other users of SARRP systems can possibly adopt more of the practical specific measurements of this work than users of other systems, who may need to adapt some of the tests presented here to their own system, but will also benefit from the general outline of the comprehensive program.
In Section 2, we will present the used equipment and explain the different QA tests. Information on limits for the tests and associated recommendations will also be given in Section 2. Our suggestions for their frequency and our experience on the duration of each test will be reported in Section 3. In addition, we will present numerical results of our checks on our system. However, the main focus of this work is the formulation of the comprehensive program and not to act as a reference of numerical data, that will differ between systems.
2. Material and methods
The tests described below were implemented at our SARRP, a fully integrated small animal irradiation device, equipped with cone beam computed tomography (CBCT) for image-guidance. The gantry holds the dual-focus 225 kV X-ray tube (type NDI-225-22 from Varian, Varian Medical Systems, Palo Alto, CA, USA) with focal spot sizes of 1.0 and 5.5 mm according to EN12543 (or 0.4 and 3.0 mm according to IEC) and can be rotated by 360°. The motorized couch can also be rotated and translated [13], [14]. The open field can be collimated with a series of fixed collimators as well as with a variable collimator (here, the manual non-motorized version was used) with a light indicator field. All mentioned field sizes refer to the isocenter (not to the mechanical size of the collimator).
Where possible, several different tests were combined into the same set-up in order to simplify and shorten the workflow of the tests. Due to the broad variety of influencing factors, this is only partly possible.
All measurements indicated with ‘treatment beam’ used 220 kV, 13 mA, the broad focus, and a filter of 0.15 mm Cu. All measurements indicated with ‘imaging beam’ used 60 kV, 0.8 mA, the fine focus, and 1 mm Al as filter.
As stated above, the program is developed on a SARRP system. Nevertheless, the checks in Sections 2.1, 2.3, 2.4, 2.9, 2.12 and maybe in 2.5 could qualify to be transferred to other machines without further adaptations. The other remaining checks may need some more modifications, but can be used as a guideline to set up a comprehensive and complete QA program.
2.1. Dose output
The dose output test for the treatment beam was performed with a Semiflex Ion Chamber (type 31013) from PTW (PTW-Freiburg, Freiburg, Germany). This dosimeter has a sensitive volume of 0.3 cm3 and is meant for the use in photon beams in the energy range starting at 140 kV with an calibration factor ND,w from PTW, that is traceable to the primary standard of the Physikalisch Technische Bundesanstalt (PTB), Braunschweig, Germany. It was connected to a PTW UNIDOS webline electrometer for high voltage supply and readout. All dose output measurements were done in a slab phantom with the size of 20 × 20 × 6 cm3 consisting of several solid water slabs from Gammex (Gammex, Middleton, WI, USA). The measurements were performed according or similar to TRS398 with correction factors for temperature and air pressure as well as for the beam quality. The chamber was placed in the isocenter in 2 cm depth, which means a source to surface distance of 33 cm. The use of this ion chamber and its capabilities for such applications were described in [15], [16]. Beam-on time for this test was 30 s, whereas the measurement was started beforehand and ended afterwards to include the ramp-up and -down effects. The test is meant to check the constancy of the absolute dose, that was measured with the same setup during the acceptance test after installing the machine. Therefore, it is not crucial which dosimetry protocol is followed, but it is nevertheless important to use the same setup and procedure each time. We used an irradiation without additional collimation, meaning the removable collimator with the nozzle was absent during this dose output test. If the smallest collimators (for SARRP it is 0.5 and 1 mm) are used for experiments, the output stability should additionally be tested for these field sizes. The reason is that any change in the alignment of the collimator means a change in the output of these small fields.
2.2. Output constancy of imaging beam
The set-up for output constancy check of the imaging beam is the same as described above in ‘dose output’. Due to the lower energy of the imaging beam, the chamber is not calibrated for absolute dose, but can still be used to check the constancy of the output.
2.3. Long-time irradiations
To check the stability of the treatment beam output over a prolonged irradiation time, we did a measurement over 20 min in the same set-up as for ‘dose output’. The PTW electrometer statistics was used to record 40 measurements each lasting 10 s, followed by a 20 s pause.
2.4. Energy constancy check
A constancy check for beam energy could be done by measuring the half value layer (HVL) of the relevant beam. The subsequently described check is a similar test, but in a different setup and geometry than a regular HVL measurement. Our test uses the same set-up as described in ‘dose output’ to have a fast and easy check without much modification of the before mentioned setup. For beam collimation, we used the variable collimator with a field size of 5 × 4 cm2. Here, sheets of Cu (for the treatment beam) or Al (for the imaging beam) were hold 2 cm above the slab phantom by two PMMA slabs that were placed outside of the field (Figure 1). First, the dose without additional sheets of Cu or Al was measured to have a reference value for each beam. We then used the mentioned material to reduce the measured dose to the half (and quarter) of the reference values. The material was varied in steps of 0.025 mm in the case of Cu and in steps of 0.1 mm in the case of Al to find the exact values.
Figure 1.
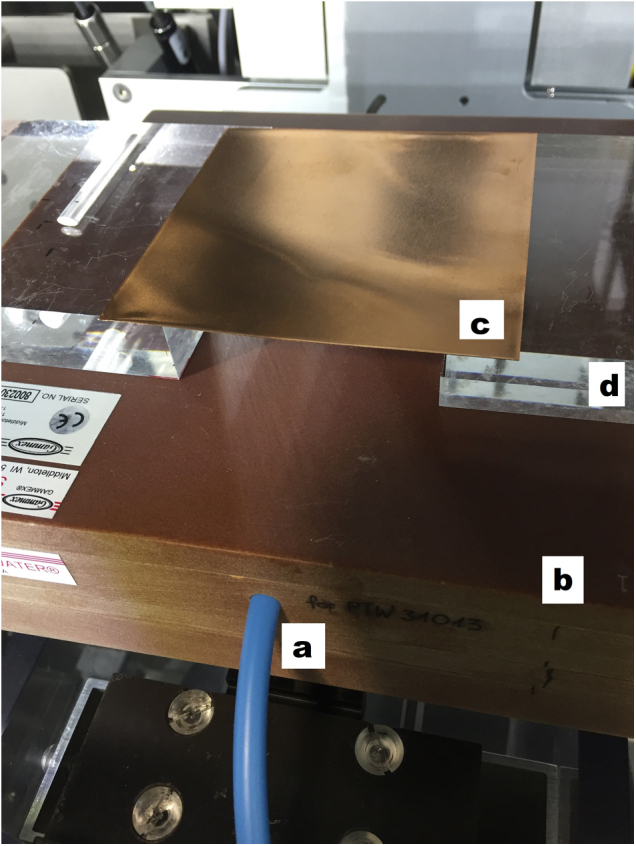
Set-up of the energy constancy check. The ion chamber with its blue cable (a) is positioned in the isocenter of the machine in a depth of 2 cm in the solid water slab phantom (b). On top of the phantom a Cu sheet (c) is brought into the beam to measure the absorption of the material. This sheet is hold in position by PMMA slabs (d).
2.5. Isocenter, laser and movement check
A metal marker (BB or similar) is placed several mm next to the isocenter. After performing and registering a CBCT (in the SARRP inherent software MuriSlice) with the marker in the isocenter, the couch with the marker will be moved to the new position presenting the marker in the isocenter. A visual check of laser alignment as well as checks with all imaging modalities (in our case CBCT with flat panel and rotating couch, a flat panel double exposure with a small (e.g. 3 × 3 mm2) collimator, and images from 0° and 90° (also double exposures) with the portal camera and couch at 0° with an appropriate collimator (e.g. 3 × 3 mm2)) can be used to verify the location of the marker in the isocenter. The deviation was measured on the images. Larger movements of gantry and couch are checked for the correct direction and absolute value. Needed tools for a fast check are (in addition to the BB) a ruler (for translations) and a square, or other appropriate tools like a level or for a more precise check the detector of the system. For all possible translations and rotations (after the starting point has been recorded), a definite value is triggered via the user interface to initiate the movement and afterwards the movement is verified by measurements (with the ruler or if possible with the detector for more precision). In addition, the stability of the isocenter can be checked for a full couch rotation. Therefore, the position (pixels on the image) of the BB is checked on the X-ray images (raw projections) taken during a CBCT.
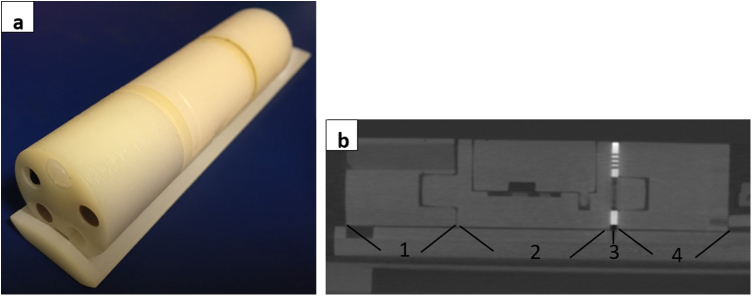
2.6. Image quality check
To validate the quality of the CBCT a dedicated imaging phantom is scanned. Here we use the Xstrahl imaging phantom (MuriQA, 88 mm in length, diameter of 24 mm, 4 sections, see Figure 2 and [17]) to check for CT number constancy (with five inserts with different material densities for air, lung, fat, tissue, and bone), resolution, geometric accuracy, signal uniformity, noise, and signal to noise ratio (SNR). The selected imaging protocol (imaging protocol here means machine setup in terms of kV, mA, frames per second, number of projections, resolution, voxel size) should be the same that is used regularly for mouse imaging. If there are several different protocols in use, all of them may be checked. The most frequently used imaging protocol in our institution is the imaging beam with 1506 projections, 1.4°/s rotation speed of the couch, and 6 frames per second. The reconstruction is done with 0.115 × 0.115 × 0.115 mm3 voxels, and a reconstructed volume of 286 × 433 × 433 voxels. In addition, a protocol with 360 projections, 6°/s, 6 frames per second with equal reconstruction parameters was investigated as it is also frequently used in our institution. For the analysis of the resolution, the phantom has a specific section with rectangular arrays of holes of different diameters (1, 0.75, and 0.5 mm). For each scan we evaluate for which arrays the holes can clearly be separated. In the geometric section, the diameter of two circles with a set of holes can be measured as well as the length of the whole section. The length of the geometric section is obtained from the number of slices in which this section is visible multiplied by the nominal slice thickness. This helps to check the geometric accuracy of the reconstructed image. To evaluate the signal uniformity (and noise), we proceeded like in [6]. We calculated the signal intensity difference between four peripheral volumes of interest (VOIs) and a central VOI in the uniform area. The image noise was then calculated as the average of the standard deviation within the five VOIs. SNR measurement was done in the area with high density as well as in the uniformity section. The image data from the SARRP CBCT are given in proprietary CT numbers, not in well-known HU values. Moreover, the CT numbers may fluctuate from scan to scan for identical material, dependent on further material in the particular scan as the image is scaled to the whole range of available CT numbers in each scan. To make the measurements comparable we transformed the CT numbers from our CT scans to HU values. Therefore, a small tank with water was scanned together with the phantom (which does not contain water or water equivalent material). In an in-house software we calculated the HU values for each scan according to the formula
where CTMat means the CT number of the material (Mat) under consideration coming from the scan, and and CTAir are the CT numbers of water and air coming from the scan. The resulting HUMat stands for the new calculated value in Hounsfield units for the respective material.
Figure 2.
Imaging phantom. The imaging phantom from Xstrahl with its different sections is used for several image quality checks including signal to noise ratio (SNR) and geometrical measurements. (a) Picture of the phantom. (b) Reconstructed sagittal plane from a CBCT scan of the phantom (1: CT number section, 2: section used for end-to-end test, 3: resolution section, 4: uniformity and geometric section).
2.7. Dose calculation
The treatment planning system (TPS) is also checked for consistency. For a scan (same scan data set each time) all parameters like segmentation, isocenter placement, and calculation presets have to be set in the same way each time. Then the needed irradiation time for a certain dose is calculated. In our case we used a 5 × 5 mm2 field size for a fixed beam as well as for an arc (gantry rotation −178° to +178°) to calculate the irradiation time for 10 Gy in the planning system MuriPlan (Xstrahl). In addition, we calculated the time for a static beam with a field size of 40 × 20 mm2 (also 10 Gy) with the variable collimator. We used a dose of 10 Gy to have a treatment time significantly more than 100 s to recognize also small variations. With this check we minimize the risk to have unrecognized changes in the TPS settings including the basic input data and to thereby receive wrong results. A TPS not configured for clinical use (like this one) may be prone to or less protected from such changes (that are sometimes even part of ongoing research projects).
2.8. Light field check
To check the light field versus the irradiation field is a well-known test from clinical treatment machines. The intent is to determine whether the light field indicates the true irradiation field and can therefore be used to align patients or in this case animals for irradiation. At our SARRP, a light field is only available with the variable collimator. To check the concordance, we placed a radiochromic film (Gafchromic EBT3, Ashland, USA) on the treatment couch in or near the isocenter, adjusted the collimator to the desired field size (40 × 80 mm2), marked the light field and the film orientation, and irradiated the film with the treatment beam. Afterwards, the film can easily be evaluated visually by just comparing the marks with the darkened area on the film. This check can be combined with the following one (profiles, field size, and homogeneity) using the same film irradiation also for evaluation of the next check.
2.9. Profiles, field size, and homogeneity
As beam profiles, field size, and homogeneity are important parameters for a precise irradiation, these have to be checked regularly. For fixed collimators, we assume that the collimator itself will not change and therefore does not need QA for the field size. Mechanical changes at the collimator mount (close to the tube) would also affect the variable collimator using the same mount, therefore a check using the variable collimator is sufficient to detect this (and also any other changes for the variable collimator). If very small field sizes (e.g. 1 or 0.5 mm) are used, these should be checked additionally, as already very small changes could have an impact. Most changes in profiles or homogeneity are mainly expected for large field sizes, so we checked a field size of 40 × 80 mm2 of the variable collimator. We chose the film method and therefore put a radiochromic film (Gafchromic EBT3) on the treatment couch in the isocenter to irradiate it with the treatment beam. The irradiated film was visually inspected and scanned using an Epson Perfection V700 Photo scanner (EPSON Deutschland GmbH, Meerbusch, Germany) in color at 120 dpi in transmission mode. Only the red channel was evaluated without using any image filter. For the film analysis we created a calibration curve on the SARRP with the same batch of film beforehand and used the same ion chamber as mentioned in part ‘dose output’. The homogeneity index (HI) was calculated according to HI = (D10% − D90%)/D50% in the inner of the field. In the formula the dose D10% means that 10% of all points reach at least that dose, D90% defines that 90% of all points reach at least that dose, and D50% specifies the dose that is at least reached by 50% of all points. As this check uses the same equipment and the same irradiation field as the check before (light field check), both checks can be combined.
2.10. Overall alignment test
For an overall check of the alignment of the couch and the gantry, the so-called sandwich test as suggested by the manufacturer can be used, which has its name from the sandwich-like setup. Three radiochromic films are placed between slabs of solid water (minimum thickness of 5 mm of each slab). The middle film needs a 0.5 mm hole in the center. The test intends to check the position of treatment isocenter as well as the correct couch rotation together with correct gantry rotation. The slab phantom is centered on the isocenter with the middle film just in the isocenter. This is done by performing a CBCT and shifting the couch for the necessary distance. During irradiation with the 0.5 mm collimator, the couch is rotated isocentrically and the gantry stays at several different angles. These gantry angles have to be chosen in the manner that (on the upper and lower film) different circles arise during irradiation. For each gantry angle (be aware of possible collisions), only a part of a full rotation is performed. The missing part can be completed from a different gantry angle (e.g. opposite angle). We used 30°, 45°, 60°, −60°, −150°, 135°, such that at least one beam from each quadrant is used. After irradiation, the partial rings from different but associated angles should complete each other and show a ring with the same radius. The film in the middle should only be darkened in the center. The other two films can be overlaid during evaluation and the rings are checked for same size on both films.
2.11. Arc check
While the system irradiates a standard arc, the dose is measured in a phantom and the time for the rotation is recorded. The check intends to ensure the right dose application (that is stopped after a certain run time) with the right angular distribution. We measured in a cylindrical PMMA phantom (radius 1.4 cm, length 4 cm, see Figure 3) with the before mentioned ion chamber and the same corrections (see ‘dose output’) in the isocenter. For the arc from −178° to +178° with a rotation and irradiation time of 76 s we used a 10 × 40 mm2 field of the variable collimator to ensure dose in the complete sensitive volume of the chamber.
Figure 3.

Cylindrical phantom. The PMMA phantom is used for the arc check. An ion chamber can be inserted to measure the dose in the center of the phantom.
2.12. End-to-end test
By performing an end-to-end test, the whole process of imaging, planning, and irradiating as a whole is checked. All relevant steps are done as similar as possible to the real workflow during animal irradiation. We used the Xstrahl imaging phantom (described in ‘image quality check’) and mounted a calibrated radiochromic film (Gafchromic EBT3) inside the phantom. For absolute measurements, the films were calibrated using the calibration procedure according to [18]. After performing the CBCT, the scan was imported to MuriPlan and the phantom was segmented and planned for treatment. In run A (with film A) we planned one 5 × 5 mm2 static field (fixed collimator) with a gantry angle of 0° (to be perpendicular to the isocentric film) and a dose of 2 Gy in the isocenter. After this, run B (with film B) used the same setup with one arc from −178° to +178° (5 × 5 mm2 field size with the fixed collimator; planned dose: 3 Gy). After the irradiations, the accurate positioning of the beam in relation to the phantom was visually checked on the films. The films were then scanned and analyzed for field size and absolute dose. Another film (run C) was put into the phantom and was just CBCT-scanned with the imaging beam to evaluate the dose contribution from a CBCT scan.
2.13. Tolerance limits and recommended actions
In terms of acceptable results of measurements every center will have its own tolerance limits. These limits depend on regulations and experience, but may also vary for different experiments on the systems (e.g. a system used for high precision irradiation in the brain of mice may require different geometrical accuracy than a system used only whole body irradiation). In our institution we adapted the clinical requirements from human radiotherapy to the pre-clinical needs and in particular scaled it to the size of the specimen.
The limit for absolute dose measurements in our clinic is ±3%, which seems also applicable for the preclinical setting. Measurements 2.1–2.3, 2.7, and 2.11 are handled according to that specification in our institution. If a dose limit is exceeded, the reason for the deviation should be eliminated or, if not possible, the experiments should be reviewed for the new magnitude of deviation or dose (possibly dependent whether the dose output is out of limit, but stable, or whether the dose output is unstable and fluctuating in a larger range).
Limits in geometry related measurements (like in 2.5 and 2.8) should be scaled to the size of the experiments done on the machine and its requirements, e.g. used field sizes, safety margins, etc.. It should be possible to be more precise than 1 mm, which is the typical limit in our clinical setting. To measure more accurate than 0.2 mm is sometimes very challenging and may be simply not achievable. Measurement 2.10 should also result in an accuracy in the order of the beforementioned. If a geometrical limit is reached or exceeded, the geometrical alignment has to be improved, e.g. by a new calibration involving a service engineer from the manufacturer. Measurement 2.12, however, will need a combination of dose and geometrical limits.
3. Results
In this section we report typical results and the required times for all checks, and make suggestions for their frequencies. All of the suggested checks were done in our department at least for several months, some for several years (e.g. dose output and energy constancy check).
3.1. Frequencies and duration
The above-mentioned checks have to be performed regularly, but in different frequencies. Table 1 summarizes our suggestion for their repetition frequency. We suggest a monthly check of the dose output as this is one of the most important parameters to reach an exact dose in the experiment. The imaging dose is less important but comes with nearly no cost as it is the same setup. A quarterly repetition is needed in our opinion for the prolonged irradiation to check for the constancy of the beam even for longer irradiations. The energy constancy has also a big impact on the correct dose distribution in the experiments, so we suggest to do this check also quarterly. For the isocenter, laser and movement checks a quarterly repetition seems reasonable as the influence on the results of experiments is noticeable and the effort for this checks is manageable. We do not think monthly checks are needed for these checks as the isocenter will not change rapidly unless there is a mechanical event (e.g. a collision, which will be noticed), the lasers are usually not used for precise setup and an unrecognized change in movement behavior is not expected without intended intervention. Semi-annual repetitions for the following checks seem sufficient: the image quality check (the impact on most experiments is relatively small and big changes could possibly be recognized by the user), TPS dose calculation (has a big impact, but errors are unlikely to happen), light field check (could have an impact if used for setup), profiles/field size/homogeneity (could have a noticeable impact on experiments but are unlikely to happen without mechanical changes on the system), and the overall alignment test (is very time consuming, but will reliably disclose deviations). Arc test and end-to-end test are time consuming and are meant to check several aspects and their interplay. As most of the aspects are checked separately, these checks can be performed once a year. The individual setup or known problems on a specific machine may require more frequent checks. Also, after maintenance, new calibrations or repairs some of the checks may and should be repeated extraordinarily. The monthly check (dose output) requires about 20 min to perform (without machine warmup procedures but including the analysis of the results and the output constancy of the imaging beam). For all of the quarterly checks (long-time irradiations, energy constancy check, isocenter and laser-check, and movement check) together, about 90 min are needed on the machine. Semi-annual checks (image quality check, TPS dose calculation, light field check, profiles, field size, and homogeneity check, and overall alignment test) may require about 180 min including the time-consuming alignment test, which requires about 120 min. These times (180 and 120 min) will greatly vary due to experience and are also strongly dependent on the specific protocol. However, for SARRP machines the overall alignment test is typically being done by the service engineer during planned maintenance anyway. Evaluation of the semi-annual tests may take about 90 min. Performing the annual tests (arc test and end-to-end test) may take roughly 2 h, including roughly 1 hour for evaluation. Altogether this means a yearly time effort of roughly 21 h (measurements and evaluation) or a mean time of less than 2 h (105 min) per month. This effort should be acceptable in order to have a well monitored machine.
Table 1.
Suggested frequencies of QA procedures.
| QA procedures | Frequency |
|---|---|
| Dose output | Monthly |
| Output constancy of imaging beam | Monthly |
| Long-time irradiations | Quarterly |
| Energy constancy check | Quarterly |
| Isocenter and laser check | Quarterly |
| Movement check | Quarterly |
| Image quality check | Semi-annual |
| TPS dose calculation | Semi-annual |
| Light field check | Semi-annual |
| Profiles, field size, and homogeneity | Semi-annual |
| Overall alignment test | Semi-annual |
| Arc test | Yearly |
| End-to-end test | Yearly |
3.2. Dose output, output constancy of imaging beam, and long-time irradiations
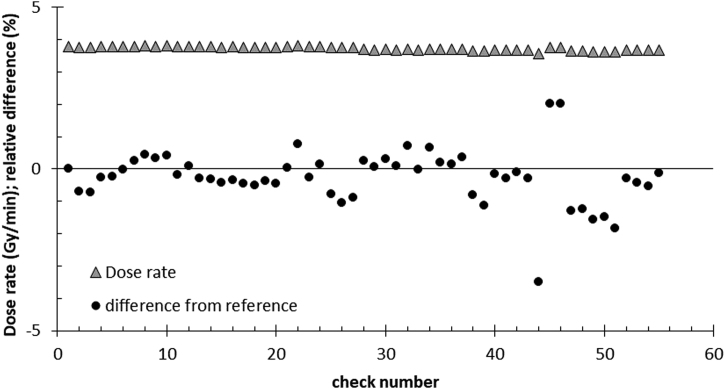
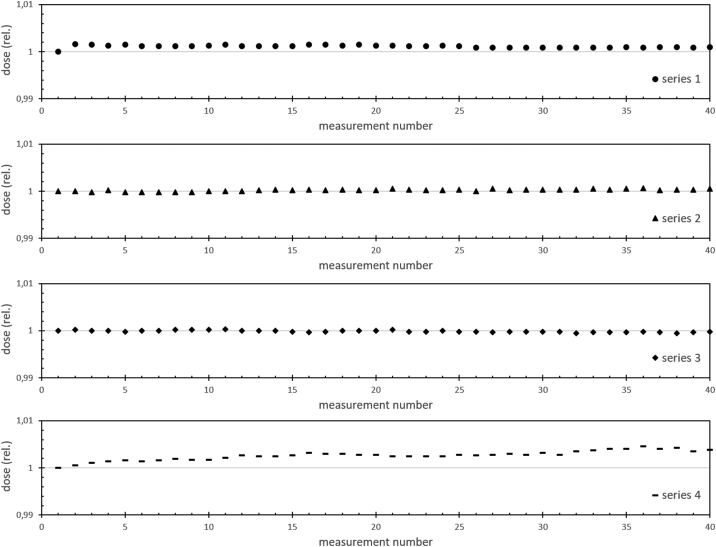
Our measurements prove a stable output of the treatment beam. The dose rate (shown as triangle in Figure 4) in our setup (about 3.8 Gy/min) differs less than 1% from the reference in 90% of all 55 shown measurements of the dose output. The measurements of the imaging beam in the same setup have a slightly higher variation of about 3%. The prolonged irradiations (see Figure 5) with the treatment beam (long-time irradiation) show a very good constancy within each measurement series over 20 min with a Min/Max–ratio of more than 0.995.
Figure 4.
Dose output. The triangles show the measured dose rate of the treatment beam in Gy/min within the water slab phantom in a depth of 2 cm (similar to setup in Figure 1). The circles in the figure show the difference from the reference dose in %. The 55 checks were performed over several years.
Figure 5.
Long-time irradiations. Shown are four series of 40 measurements each. The stability of the measured dose over the 20 min period of measurements are observed. Every 30 s a new measurement over 10 s is shown.
3.3. Energy constancy check
The half value of the reference output for the treatment beam in our measurement setup is reached with 0.725 (±0.025) mm Cu, with additional 1.625 (±0.1) mm Cu the quarter of the reference value is reached. For the imaging beam, 2.15 (±0.05) mm Al and additional 3.05 (±0.1) mm Al are needed to reach the half and quarter value in our case. Both beams were measured six times over several months and each time the half value was reached with the same thickness of material. In comparison, a change of the treatment beam voltage from 220 to 210 kV changed the needed material thickness already for about 0.05 and 0.175 mm for the half and quarter value, respectively. A change of the voltage of the image beam of 5 kV resulted in about 0.2 mm different material thickness to reach the half value and more than 0.4 mm to reach the quarter value. This implies that this check is sensitive to energy changes in the mentioned range.
3.4. Isocenter, laser, and movement check
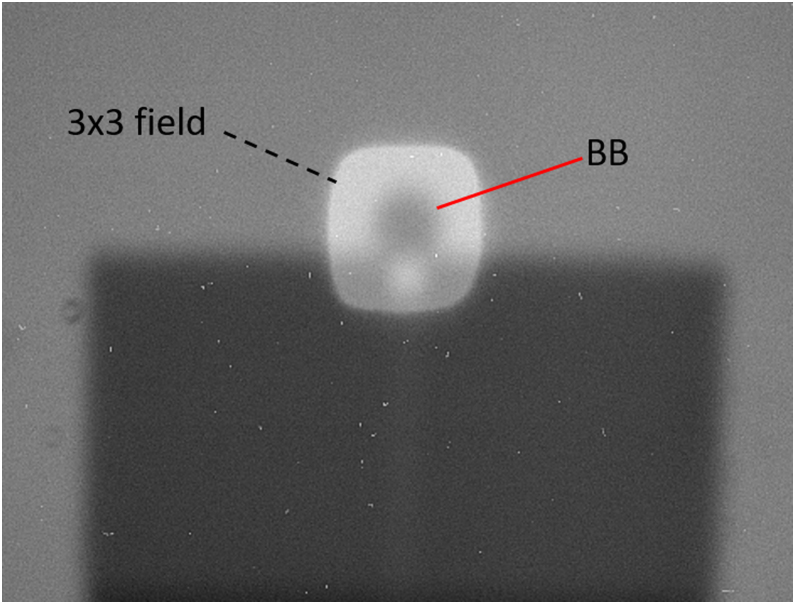
After shifting the marker to the isocenter of the CBCT scan, the lasers were centered on the marker with a deviation of less than 1 mm (as the dimension of the lasers is roughly 1 mm we can hardly be more precise than 1 mm). Also, the verification images (double exposures on flat panel and on portal camera, see Figure 7) result in visually good coincidence with the CBCT isocenter in our measurements whereas the results varied for 0.5 (±0.2) mm. All checked larger movements of the motorized couch agreed in direction as well as in the desired magnitude, which we measured by a ruler (within ±0.5 mm) as the high precision for small movements was already checked in the isocenter check. The rotation of the couch was centered to the isocenter and did not move out by more than 0.5 mm from the center, whereas a theoretical precision of about 0.2 mm could be reached on our system if the X-ray images are used.
Figure 7.
Overlaid images of a BB. A 3 × 3 mm2 field and an open field on the portal camera are overlaid. The BB is at the isocenter, which is visible due to the overlay of the 3 × 3 mm2 field.
3.5. Image quality check
The check of the CT number constancy for true water in the scan showed deviations over time of the mean CT number in the volume of interest of up to 6% within one imaging protocol (1506 projections) and up to 10% if scans of different protocols were compared. As the dose calculation on the SARRP system is typically done according to a material segmentation without any influence of the exact density these deviations have no or only minor impact on the dose planning. After the transformation to HU, the values of different materials are very stable for scans with the same imaging protocol and vary typically in the range of ±1%. In the resolution section (see Figure 8) we could distinguish the holes down to the diameter of 0.5 mm with the chosen imaging protocols. The diameters of the circles with holes in the geometric section are 12 and 19 mm, as it should be according to the manufacturer of the phantom. We determined the length of the whole section to be 25.3 (±0.4) mm in different scans (number of slices multiplied by slice distance), whereas the physical measurement resulted in 25.6 mm. The nominal slice thickness was 0.115 mm. The measured diameter of the phantom is 24 mm as expected. Signal uniformity test resulted in our reference scan in 68 HU (mean of the four outer VOIs each compared to the central one). The image noise (in the uniformity section) was calculated to 36 HU whereas the SNR was 16. In the high-density area, the SNR was 23. With the mentioned checks we can ensure there is no trend of the measured parameters that could be overlooked by daily use.
Figure 8.
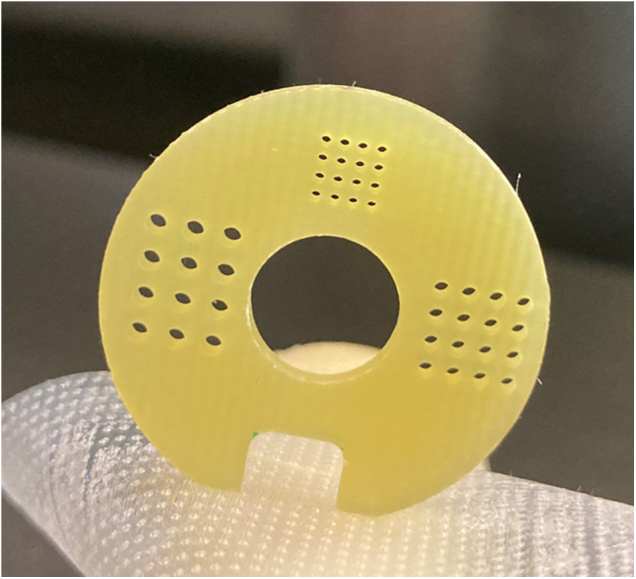
Resolution section of the Xstrahl imaging phantom. The three arrays have holes in the sizes of 1 mm (left), 0.75 mm (right), and 0.5 mm (upper).
3.6. Dose calculation
Consistency check of the TPS produced exactly the same treatment times for all field configurations each time. No variation in calculated treatment times was detected for 10 Gy in the isocenter of our TPS check phantom for the 5 × 5 mm2 fixed collimator and static beam as well as for the fixed collimator (5 × 5 mm2) arc (−178° to +178°), and for a 40 × 20 mm2 static field with the variable collimator.
3.7. Light field check
The light field versus irradiation field check already showed during commissioning of the variable collimator that in our configuration the light field does not exactly match the irradiation field. A shift of roughly 1 mm each in directions outward of the gantry and to the left related to the irradiation beam is observed. This shift is present since first measurement during commissioning (in 2014) and has to be considered if the light field is used for positioning. As the light field is usually not used for precise alignment in image-guided systems but rather for a rough alignment, a precision in the range of 0.5–1 mm is acceptable.
3.8. Profiles, field size, and homogeneity
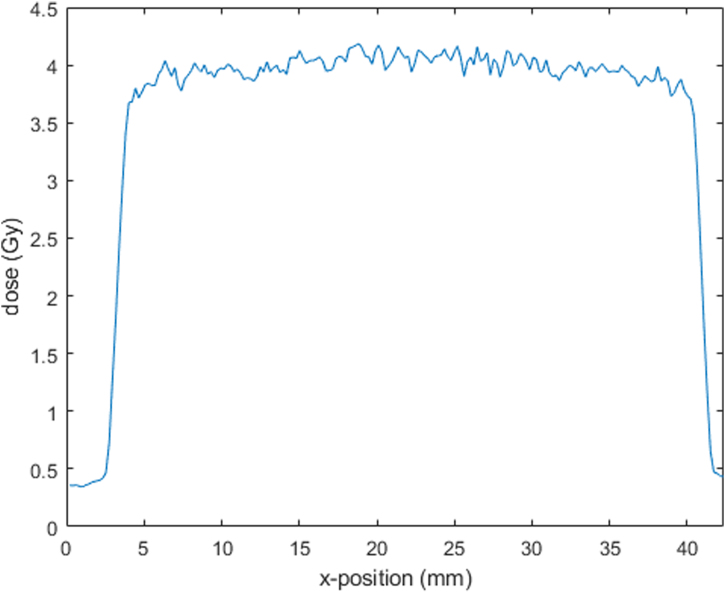
The field profiles were checked on the scanned film and compared with former records. They did not show any abnormalities. A relatively high variation in the high dose area (see Figure 6) is expected for film analysis, depending on the scan resolution. The shown film has a scan resolution of 120 dpi and is presented without any data smoothing nor a comparable statistical method. Due to the usage of the manual variable collimator, we got a noticeable variety in field sizes with a deviation of typically 1–2 mm to the desired field sizes. (Our shown film measurement (Figure 6) was 37.9 mm × 78.7 mm instead of 40 × 80 mm2.) This possible variety should be taken into account if the variable collimator is used in experiments. If a higher precision is needed, the field size could be adjusted with supporting tools (e.g. a plug-in in the collimator to adjust the field size that is removed before irradiation). The mean homogeneity index is 0.14.
Figure 6.
Beam profile in x-direction (40 mm) measured by radiochromic film, scanned with a resolution of 120 dpi and no image filtering. The shown beam profile has steep gradients on both sides of the field.
3.9. Overall alignment test
If the alignment of the machine is correct, (parts of) precise circles are visible on the upper and lower films, and only a dark dot in the center of the middle film (position of isocenter). In our case the so-called sandwich test was performed by service engineers from Xstrahl. The final tests after their calibrations always showed good results, which was a requirement for a successful hand-over of the machine.
3.10. Arc check
A rotation time of 76 (±1) s for the arc (−178° to +178° with a 10 × 40 mm2 field) was reproduced each time the measurement was repeated. Also, the measured dose in the cylindric phantom (3.2 Gy) was stable within ±3% over two years.
3.11. End-to-end test
Both runs (A and B) showed a precise positioning of the treatment beam in relation to the phantom structure that was verified by visual inspection of the darkened area on the film in the phantom. The evaluation of the film C that was just CBCT scanned with the same protocol showed a CT dose of 0.02 Gy. For the evaluation of the treatment dose of runs A and B we subtracted the value of film C from the particular film doses. The expected dose of 2 Gy in run A was nearly matched in the measurement with a mean dose of 2.04 Gy (+2%). Run B (expected dose: 3 Gy) showed a nearly perfect agreement with 3.01 Gy (+0.3%) mean dose in the plateau. The measured field size (the area that received at least 50% of the mentioned mean dose value) within the phantom for run A was 5.14 × 5.15 mm2 with the used 5 × 5 mm2 collimator (nominal field size at isocenter). Run B gave a measured field size of 5.19 mm in the arc plan (measurement in direction of the rotation axis).
4. Discussion
The present study introduces a comprehensive quality assurance program for a small animal irradiation unit, developed and tested on a SARRP. All relevant aspects from imaging, treatment planning, and irradiation, including output constancy, energy stability, geometrical aspects and an end-to-end test are addressed. For a reasonable use of such small animal irradiation units with the demand to produce comparable results, it is crucial to have a good QA of the constant quality of the machine in the time after commissioning.
The frequency of such checks and measurements can be orientated on the frequencies of clinical systems, on legal requirements, and on specific experience with the individual machine as well as the frequency of use and the type of experiments on the machine. We suggested repetition frequencies for the different checks that seem to be reasonable for our machine. If materials or tools (e.g. specific phantoms) may not be present in the particular surrounding, our program can still be a guideline on what should be done and be adapted to the specific requirements and conditions. An overview of different phantoms and materials can be found in [19].
Our results for the beam output (treatment beam and imaging beam) indicate that the machine is very stable also for a long beam on time (long-time irradiation). As a stable dose output is important for every single user and experiment (independent of the usage of the imaging system or the TPS), this has to be checked most frequently. The results in dose output were very similar to the study of [12], who found the dose stability within about 2% (we found about 1%). Also, the energy is very constant in our beams, which can be seen on the stable results for the energy constancy check. According to [7], a ‘15% change in HVL leads to a <1% change in PDD at 2 cm depth’. For our treatment beam (half value with 0.725 mm Cu) a change of roughly up to 0.1 mm for the first value could therefore be acceptable. In addition, we verified the sensibility of our measurements by intentional slightly changed energies and were able to detect reliably a 10 kV change. Profiles, homogeneity and field sizes should be checked regularly to monitor the constancy of the machine as well as potential application uncertainties.
To facilitate the setup of mice for irradiation it is helpful to have a precise laser system and important to check the light and treatment field concordance (if lasers and light field are used for positioning). In this work we demonstrated the consistency of the precise laser system. As the light field is usually not used for precise alignment in image-guided systems but rather for a rough alignment, a precision in the range of 0.5–1 mm is acceptable for that. We do not use the light field at all for our specimen experiments, but only the laser system. The isocenter and movement check is important especially if image-guidance is used for the treatment or high geometrical precision should be reached. Both checks gave satisfying results. Others [11] found that their system has a slightly different central axis for the fine and the broad focus of about 0.2 mm. Our check is not meant to find such small differences, but to verify the stability of the system over time. A possible deviation of the variable collimator of 1–2 mm could be a major issue for small field sizes. As usually fix collimators are used for the small field sizes and the variable collimator is mainly used for bigger field sizes, the deviation may be less problematic. Nevertheless, before using a specific field size of the variable collimator, it should be verified to be the right one (also in terms of precision) as it also affects the dose delivery. The overall alignment test as well as the arc check also monitor the interplay of the x-ray hardware with the motorized motion. Both tests produced consistently good results.
For dose calculation as well as for the image interpretation it is crucial to have a good image quality for the scans, which is being checked semi-annually in our QA program. Our image quality results agree well with results in the literature. We found the image noise to be 36 HU. Johnstone et al. [6] suggested a value of <55 HU should be reached. The SNR in the high density area of our QA phantom was 23. They also propose a tolerance level of >36 for a vial with the highest iodine concentration of 30 mg ml−1. As we did not use Iodine concentrations, this value is not easily comparable. The SNR of an Iodine concentration of 15 mg ml−1 in [6] reflects pretty much our SNR of 23 (whereupon there is a big variety between the 11 institutions of that multi-center comparison).
The evaluation of the checks of the geometrical section shows that the diameters of phantom and circles are as expected. The length of the geometric section (25.3 mm) is obtained from the number of slices multiplied by the nominal slice thickness. One main inaccuracy thereby is the manual definition of the beginning and the end of the section. Apart from that, the resulted length is in good accordance with the physical measurement on the phantom.
Dose calculation in the TPS was stable and also according to the end-to-end test very accurate. Cho et al. [20] found out that the superposition-convolution method of MuriPlan is consistent within 5% with Monte-Carlo calculations and film measurements, at least in the flat regions of the profile, which means the most interesting high dose area.
Finally, an end-to-end test is very important, not only to check the machine as a complete system, but also to monitor the whole workflow. In addition, it gives a good indication about the overall precision on that machine.
For a well-maintained machine, 1.5–2 h per month could be enough for our comprehensive and efficient QA program and should definitely be invested. With training and small adjustments, the time can also be reduced without compromising the quality. By repeating the presented program in the suggested manner, upcoming errors or arising inaccuracies may be discovered in time. If the first measurements (especially the reference measurements) and the adaptations (if necessary) of the program are done by a very experienced user, ideally a medical physicist, the execution of the repeated QA checks can be performed by a normal trained user. Nevertheless, supervision (in case of problems or irregularities) is needed and may improve the outcome.
5. Conclusion
We proposed a comprehensive and efficient QA program to ensure constant and reliable operation as well as comparable and accurate results in research. The checks include imaging, treatment planning, the irradiation system (geometry and dosimetry) as well as a complete end-to-end test. To perform all checks in the suggested repetition frequency, less than two hours per month could be enough to efficiently detect irregularities.
Declaration of interests
-
•
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors have no relevant conflicts of interest to disclose.
Contributor Information
Severin Kampfer, Email: Severin.Kampfer@mri.tum.de.
Manuela A. Duda, Email: Manuela.Duda@tum.de.
Sophie Dobiasch, Email: Sophie.Dobiasch@tum.de.
Stephanie E. Combs, Email: Stephanie.Combs@tum.de.
Jan J. Wilkens, Email: Wilkens@tum.de.
References
- 1.Verhaegen F., van Hoof S., Granton P.V., Trani D. A review of treatment planning for precision image-guided photon beam pre-clinical animal radiation studies. Zeit Med Phys. 2014;24:323–334. doi: 10.1016/j.zemedi.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Verhaegen F., Dubois L., Gianolini S., Hill M.A., Karger C.P., Lauber K., et al. ESTRO ACROP: technology for precision small animal radiotherapy research: optimal use and challenges. Radiother Oncol. 2018;126:471–478. doi: 10.1016/j.radonc.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Tillner F., Thute P., Bütof R., Krause M., Enghardt W. Pre-clinical research in small animals using radiotherapy technology – a bidirectional translational approach. Zeit Med Phys. 2014;24:335–351. doi: 10.1016/j.zemedi.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Desrosiers M., DeWerd L., Deye J., Lindsay P., Murphy M.K., Mitch M., et al. The importance of dosimetry standardization in radiobiology. J Res Natl Inst Stand Technol. 2013;118:403–418. doi: 10.6028/jres.118.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuess P., Bozsaky E., Hopfgartner J., Seifritz G., Dörr W., Georg D. Dosimetric challenges of small animal irradiation with a commercial X-ray unit. Zeit Med Phys. 2014;24:363–372. doi: 10.1016/j.zemedi.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Johnstone C.D., Lindsay P., Graves E.E., Wong E., Perez J.R., Poirier Y., et al. Multi-institutional MicroCT image comparison of image-guided small animal irradiators. Phys Med Biol. 2017;62:5760–5776. doi: 10.1088/1361-6560/aa76b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindsay P.E., Granton P.V., Gasparini A., Jelveh S., Clarkson R., van Hoof S., et al. Multi-institutional dosimetric and geometric commissioning of image-guided small animal irradiators. Med Phys. 2014;41:31714. doi: 10.1118/1.4866215. [DOI] [PubMed] [Google Scholar]
- 8.Draeger E., Sawant A., Johnstone C., Koger B., Becker S., Vujaskovic Z., et al. A dose of reality: how 20 years of incomplete physics and dosimetry reporting in radiobiology studies may have contributed to the reproducibility crisis. Int J Radiat Oncol Biol Phys. 2020;106:243–252. doi: 10.1016/j.ijrobp.2019.06.2545. [DOI] [PubMed] [Google Scholar]
- 9.Poirier Y., Johnstone C.D., Anvari A., Brodin N.P., Santos M.D., Bazalova-Carter M., et al. A failure modes and effects analysis quality management framework for image-guided small animal irradiators: a change in paradigm for radiation biology. Med Phys. 2020;47:2013–2022. doi: 10.1002/mp.14049. [DOI] [PubMed] [Google Scholar]
- 10.Brodin N.P., Guha C., Tomé W.A. Proposal for a simple and efficient monthly quality management program assessing the consistency of robotic image-guided small animal radiation systems. Health Phys. 2015;109:S190–S199. doi: 10.1097/HP.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anvari A., Poirier Y., Sawant A. A comprehensive geometric quality assurance framework for preclinical microirradiators. Med Phys. 2019;46:1840–1851. doi: 10.1002/MP.13387. [DOI] [PubMed] [Google Scholar]
- 12.Jermoumi M., Korideck H., Bhagwat M., Zygmanski P., Makrigiogos G.M., Berbeco R.I., et al. Comprehensive quality assurance phantom for the small animal radiation research platform (SARRP) Phys Med. 2015;31:529–535. doi: 10.1016/j.ejmp.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matinfar M., Gray O., Iordachita I., Kennedy C., Ford E., Wong J., et al. Small animal radiation research platform: imaging, mechanics, control and calibration. Med Image Comput Comput Assist Interv. 2007;10:926–934. doi: 10.1007/978-3-540-75759-7_112. [DOI] [PubMed] [Google Scholar]
- 14.Wong J., Armour E., Kazanzides P., Iordachita I., Tryggestad E., Deng H., et al. High-resolution, small animal radiation research platform with X-ray tomographic guidance capabilities. Int J Radiat Oncol Biol Phys. 2008;71:1591–1599. doi: 10.1016/j.ijrobp.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill R., Mo Z., Haque M., Baldock C. An evaluation of ionization chambers for the relative dosimetry of kilovoltage X-ray beams. Med Phys. 2009;36:3971–3981. doi: 10.1118/1.3183820. [DOI] [PubMed] [Google Scholar]
- 16.Kampfer S., Cho N., Combs S.E., Wilkens J.J. Dosimetric characterization of a single crystal diamond detector in X-ray beams for preclinical research. Z Med Phys. 2018;28:303–309. doi: 10.1016/j.zemedi.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Breitkreutz D.Y., Bialek S., Vojnovic B., Kavanagh A., Johnstone C.D., Rovner Z., et al. A 3D printed modular phantom for quality assurance of image-guided small animal irradiators: design, imaging experiments, and Monte Carlo simulations. Med Phys. 2019;46:2015–2024. doi: 10.1002/mp.13525. [DOI] [PubMed] [Google Scholar]
- 18.Reinhardt S., Hillbrand M., Wilkens J.J., Assmann W. Comparison of Gafchromic EBT2 and EBT3 films for clinical photon and proton beams. Med Phys. 2012;39:5257–5262. doi: 10.1118/1.4737890. [DOI] [PubMed] [Google Scholar]
- 19.Biglin E.R., Price G.J., Chadwick A.L., Aitkenhead A.H., Williams K.J., Kirkby K.J. Preclinical dosimetry: exploring the use of small animal phantoms. Radiat Oncol. 2019;14:134. doi: 10.1186/s13014-019-1343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho N., Tsiamas P., Velarde E., Tryggestad E., Jacques R., Berbeco R., et al. Validation of GPU-accelerated superposition-convolution dose computations for the Small Animal Radiation Research Platform. Med Phys. 2018;45:2252–2265. doi: 10.1002/mp.12862. [DOI] [PubMed] [Google Scholar]