Highlights
-
•
While a number of pharmacological and behavioral treatments exist for alcohol use disorder (AUD), the therapeutic effect of these interventions are relatively modest.
-
•
Repetitive transcranial magnetic stimulation (rTMS) or transcranial direct current stimulation (tDCS) may provide new approaches to better treat alcohol craving.
-
•
There is evidence for a positive effect of rTMS, but not tDCS, on craving in AUD.
Keywords: Repetitive transcranial magnetic stimulation, Transcranial direct current stimulation, Alcohol craving, Alcohol use disorder, Neuromodulation
Abstract
Introduction
While several pharmacological and behavioral treatments are available for alcohol use disorder (AUD), they may not be effective for all patients. The aim of this systematic review and meta-analysis was to evaluate the efficacy and safety of rTMS and tDCS for craving in AUD.
Methods
EMBASE, Cochrane Library, PsycINFO, and PubMed databases were searched for original, peer-reviewed research articles in the English language published between January 2000 and January 2022. Randomized controlled trials (RCTs) reporting changes in alcohol craving among patients with AUD were selected. Random-effects meta-analysis was employed to pool data.
Results
Changes in alcohol craving were extracted from 15 RCTs. Six studies assessed the efficacy of rTMS while nine studies examined tDCS. Results demonstrated that in comparison to sham stimulation, active rTMS to the DLPFC yields small but significant reductions in alcohol craving (standardized mean difference [SMD] = -0.27, p = .03). However, DLPFC stimulation via tDCS was not superior to sham stimulation in producing changes in alcohol craving (SMD = -0.08, p = .59).
Conclusions
Our meta-analysis suggests that rTMS may be superior to tDCS in reducing alcohol craving in patients with AUD. However, additional research is needed to identify optimal stimulation parameters for both non-invasive neuromodulatory techniques in AUD.
1. Introduction
From 1990 to 2017, global alcohol consumption increased 70% (Manthey et al., 2019). Alcohol use is one of the leading causes of preventable deaths worldwide, contributing to over 3 million deaths annually (World Health Organization, 2019). Alcohol use disorder (AUD) is a chronic, relapsing disorder characterized by problematic alcohol use that heightens the risk of developing cardiovascular diseases, and over 200 other medical conditions including cancers of the hepatic, digestive, and cardiovascular systems (Iranpour and Nakhaee, 2019).
There have been significant advances in our understanding of neural mechanisms underlying AUD (Koob, 2016). However, treatment options for this disorder are limited to behavioral and pharmacological approaches, which are moderately effective, as approximately 50% of patients undergoing these treatments relapse within their first year (Oudejans et al., 2012). Craving, which refers to the intense desire or urge to use a given substance, is an important clinical feature of AUD. Reducing alcohol craving has been proposed as a meaningful goal in treatment, since it is a strong proximate predictor for relapse and is a major contributor to the maintenance of AUD (Pombo et al., 2016; Tiffany and Wray, 2012).
Preclinical and clinical studies have linked AUD with abnormal functioning of dopaminergic tracts of the mesocorticolimbic pathway, which includes the ventral tegmental area (VTA), striatum, nucleus accumbens (NAcc), and prefrontal cortex (Wilson, 2015). Indeed, studies employing positron emission tomography (PET) report decreased ventral striatal D2 receptor binding and reduced dopamine release in patients with AUD. Further, the downregulation of these receptors correlate with lifetime alcohol use as well as relapse risk (Heinz et al., 2009). Besides the dopamine deficiency hypothesis, AUD is also characterized by structural and functional alterations within prefrontal regions, including the dorsolateral prefrontal cortex (DLPFC). The DLPFC governs higher-order cognitive functions that modulate goal-directed and self-regulation behaviors (Koob, 2016). In patients with AUD, reduced DLPFC functioning correlates with impaired performance on cognitive tasks, including tests evaluating inhibitory control and reinforcement learning (Li et al., 2009; Park et al., 2010). Furthermore, other research has indicated that disrupted functional coupling between the DLPFC and ventral striatum predicts levels of alcohol craving and impairments in decision-making among patients with AUD (Park et al., 2010).
Subsequently, enhancing DLPFC activity may reduce alcohol craving through two neural mechanisms. First, increased DLPFC activity leads to increased dopamine release in mesolimbic structures, including the caudate nucleus (Strafella et al., 2001), which may remediate the dopamine dysfunction present in AUD. Rodent models of AUD have demonstrated that enhanced dopamine availability in limbic structures leads to reduced alcohol consumption and craving (Solanki et al., 2020). Second, the DLPFC has been strongly implicated in inhibitory control of drug-seeking behaviors. Thus, it is possible that stimulation of this region can lead to improved executive functioning and a reduced risk of cue-induced relapse. In practice, these outcomes may be achieved with novel non-invasive neuromodulation, including repetitive transcranial magnetic stimulation (rTMS) and transcranial direct cranial stimulation (tDCS).
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive neuromodulatory technique that can stimulate or inhibit shallow brain regions [∼2 cm into cortex; (Barr, 2014)] by projecting a fluctuating magnetic field onto the scalp through a copper wire coil (Lefaucheur et al., 2020). Generally, low frequency (<5 Hz) stimulation will inhibit neuronal activity, while high frequency (> 5 Hz) stimulation will facilitate neuronal activity (Pascual-Leone et al., 1998; Speer et al., 2000). Transcranial direct current stimulation (tDCS) is an additional non-invasive neuromodulatory technique that places oppositely charged electrodes (i.e., anode and cathode) on the scalp to deliver a weak, direct electrical current to specific brain regions. Anodal stimulation enhances cortical excitability, while cathodal stimulation produces an opposite effect (Jacobson et al., 2012). Both brain stimulation techniques have been used to treat a variety of neurological and psychiatric illnesses (Berlim et al., 2013; Nitsche et al., 2009), including substance use disorders (Coles et al., 2018). The therapeutic effects of excitatory DLPFC stimulation using rTMS or tDCS also support the dopaminergic deficiency hypothesis, as increased dopamine release in limbic structures have been obtained post-stimulation of the DLPFC (Fonteneau et al., 2018; Strafella et al., 2001). However, the few studies investigating DLPFC stimulation via rTMS or tDCS on craving in AUD have yielded inconsistent findings (Jansen et al., 2019; Wietschorke et al., 2016), which may be attributed to heterogeneity in methodology. We have identified two recent meta-analyses investigating the effects of rTMS on craving in substance use disorders, including tobacco, alcohol, and illicit substances (Maiti et al., 2017; Zhang et al., 2019). However, both reviews did not examine each substance independently, and could not conclude whether rTMS was effective for reducing alcohol craving in AUD. More recently, Mostafavi et al. (2020) conducted a meta-analysis examining changes in alcohol craving for both rTMS and tDCS. However, optimal stimulation parameters were not explored via subgroup analyses or meta-regressions, and there was heterogeneity in the brain region explored. Further, none of the aforementioned meta-analyses analyzed the safety and tolerability of these modalities. In light of these findings, we conducted a systematic review and meta-analysis investigating the effects of non-invasive neuromodulation targeting the DLPFC on craving in AUD to integrate the evidence and to determine if certain parameters are associated with stronger effects on craving. We further analyzed the safety and tolerability of these methods.
2. Methods
2.1. Search strategy
The study was submitted to the PROSPERO international database of prospectively registered systematic reviews in July 2021 (PROSPERO number: CRD42021257664). Using EMBASE, Cochrane Library, PsycINFO, and PubMed databases, original, peer-reviewed research articles were searched for based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Fig. 1) (Moher et al., 2009). Articles available online in the English language between 2000 through January 2022 were considered. Details of the search string can be found in Supplementary Table 1.
Fig. 1.
PRISMA flow diagram.
2.2. Inclusion and exclusion criteria
Using the PICOS framework (Schardt et al., 2007), the populations, interventions, comparisons, outcomes, and study designs of interest were defined a priori. Studies were included in this review if they satisfied the following criteria—population (P): studies recruiting participants with alcohol use disorder or alcohol dependence; intervention (I): intervention employing either tDCS or rTMS on the DLPFC; comparison (C): studies including either sham rTMS, sham tDCS, or a control group receiving no intervention; and outcomes (O): studies investigating alcohol craving as either the primary or secondary outcome via a validated or objective measurement tool (e.g., scores from the Obsessive Compulsive Drinking Scale [OCDS]); and study design (S): studies employing either a parallel (between-subject) or cross-over (within-subject) randomized controlled trial (RCT).
The exclusion criteria for this review are as follows; (1) studies recruiting participants without alcohol use disorder (e.g., heavy drinkers); (2) other literature reviews, meta-analyses, dissertations, abstracts, conference presentations, and case studies; (3) studies lacking a well-defined control group and (4) studies employing tDCS or rTMS to brain regions other than the DLPFC.
2.3. Selection of articles
Two authors (M.S. and N.Sto.) independently screened each extracted title and abstract to determine eligibility for full-text review. The full-text of the screened studies were subsequently reviewed by three authors (M.S., N.Sto., and N.Say.). Any disagreements were resolved by consensus and discussion between all authors.
2.4. Data extraction
For each included study, two of the authors (M.S and N.Sto), extracted author information, sample characteristics, study design, stimulation parameters, and outcome variables. The primary outcome in the present study was defined as the relative changes in alcohol craving scores post-stimulation. Secondary outcomes included participant attrition and the presence or absence of adverse events associated with rTMS and tDCS, including headaches, irritation at stimulation site (e.g., burning, pain, itching, or numbness), fatigue, difficulty concentrating, insomnia, and nausea. Corresponding authors were contacted if data could not be extracted in a usable form from the original publication.
2.5. Risk of Bias
The Cochrane Risk-of-Bias Tool (RoB-2) assessed the quality of included RCTs (Corbett et al., 2014). Low risk of bias resulted if at least five of the individual domains were considered of low risk and no domain indicated a high risk of bias. Moderate risk of bias results if 2-3 individual domains are considered of moderate concern, or one domain was considered high risk. Finally, high risk of bias resulted if at least four domains were considered of moderate risk or if two or more individual domains were considered of high risk.
2.6. Data analysis
Quantitative data were pooled using Review Manager 5.3. A random-effects model was implemented, consistent with the underlying assumption of variability across individual study samples. We utilized standardized mean difference (SMD; Hedge's g) with 95% confidence intervals (CI's) to calculate the effect size of changes in alcohol craving due to non-invasive neuromodulation of the DLPFC (p < .05, two-tailed). Our models combined data from studies reporting end-point alcohol craving scores and changes in alcohol craving from pre- to post-stimulation. The effect size of dichotomous variables, including reports of adverse events and dropout rates, were summarized by odds ratios (OR). Subgroup analyses were conducted to evaluate the effects of different stimulation parameters (e.g., stimulation intensity, number of sessions, and brain laterality) against sham stimulation for both rTMS and tDCS. Heterogeneity was estimated using the I2 statistic, where an I2 of <40% was considered low heterogeneity, 40–60% moderate heterogeneity, and >60% high heterogeneity (Fletcher, 2007). Publication bias was assessed by visual inspection of the funnel plot generated in RevMan.
Exploratory meta-regression models were performed using STATA 16 (Statistics/Data Analysis http://www.stata.com) to determine the association between rTMS stimulation paramters such as motor threshold (MT%) and number of pulses with alcohol craving scores.
3. Results
3.1. Included study characteristics and quality assessment
After removing 280 duplicates, 279 titles and abstracts were reviewed (Fig. 1; PRISMA Diagram). From preliminary examination of titles and abstracts, 55 relevant articles were identified for full-text screening. Of these, 17 studies met inclusion criteria for this review. Across subjects, 488 were randomized to either active rTMS or tDCS, and 467 were randomized to the respective sham group. Eight of the included RCTs utilized rTMS (Addolorato et al., 2017; Del Felice et al., 2016; Herremans et al., 2012, 2013; Höppner et al., 2011; Jansen et al., 2019; Mishra et al., 2010; Raikwar et al., 2020), while nine studies employed tDCS (Boggio et al., 2008; da Silva et al., 2013; den Uyl et al., 2018, 2017; Holla et al., 2020; Klauss et al., 2018, 2014; Nakamura-Palacios et al., 2012; Wietschorke et al., 2016).
Three studies employed a cross-over design (Boggio et al., 2008; Herremans et al., 2013; Nakamura-Palacios et al., 2012), and studies significantly varied in length of intervention and follow-up period (Table 1). Total number of sessions ranged from 1 to 12. Although every study utilised a self-report questionnaire or scale to ascertain subjects’ alcohol craving levels, there was considerable heterogeneity in the exact instrument employed. Questionnaires and scales assessing alcohol craving included the Obsessive-Compulsive Drinking Scale (OCDS), the Alcohol Urge Questionnaire (AUQ), Visual Analogue Scale (VAS), Penn Alcohol Craving Scale (PACS), and the Alcohol Craving Questionnaire (ACQ).
Table 1.
Characteristics of included studies.
| (Author, Year) | Sample | Study Design | tDCS or rTMS Features | Brain Target | Total Sessions | Alcohol Craving Outcome | Alcohol Craving Results |
|---|---|---|---|---|---|---|---|
| rTMS | |||||||
| Addolorato et al. (2017) | 14 AUD patients, aged 39 −64 (85.71% men) | 4-week, randomized, parallel groups, sham-controlled, double-blind, pilot study | Deep rTMS and sham, H-coil, 10 Hz, 1000 pulses, 100% MT | Bilateral DLPFC | 12 (3/week) | OCDS | Craving did not significantly change from pre-rTMS to post-rTMS in either the active or sham group |
| Del Felice et al. (2016) | 17 AUD patients, aged 18 – 65 (76.47% men) | 2-week, randomized, parallel groups, sham-controlled, double blind, study | High-frequency rTMS and sham, figure-8 coil, 10 Hz, 1000 pulses, 100% MT | Left DLPFC | 4 (2/week) | VAS | Craving did not significantly change from pre-rTMS to post-rTMS in either the active or sham group |
| Herremans et al. (2012) | 31 AUD detoxified inpatients, aged 18–65 (67.74% men) | One randomized single-blind, parallel groups, sham-controlled, study with a 3-day follow-up | High frequency rTMS and sham, figure-8 coil, 20 Hz, 1560 pulses, 110% MT | Right DLPFC | 1 | OCDS | Craving did not significantly change from pre-rTMS to post-rTMS in either the active or sham group |
| Herremans et al. (2013) | 29 detoxified AUD patients, aged 18–65 (65.51% men) | One randomized, single blind, sham (placebo)-controlled, crossover study with a 7-day follow-up | High frequency rTMS and sham, figure-8 coil, 20 hz, 1560 pulses, 110% MT | Right DLPFC | 1 | OCDS | Craving did not significantly change from pre-rTMS to post-rTMS in either the active or sham group |
| Höppner et al. (2011) | 19 female detoxified AUD patients, aged 18 – 65 (0% men) | 10-day, randomized single-blind, parallel groups, sham-controlled, study | High frequency rTMS and sham, figure-8 coil, 20 Hz, 1000 pulses, 90% MT | Left DLPFC | 10 (daily sessions) | OCDS | Craving significantly decreased from pre- to post-intervention in both the active and sham group |
| Jansen et al. (2019) | 39 recently detoxed AUD patients, aged 18 – 65 (66.67% men) | One randomized single-blind, parallel groups, sham-controlled, study | High-frequency rTMS and sham, figure-8 coil, 10 Hz, 1000 pulses, 110% MT | Right DLPFC | 1 | AUQ | Craving did not significantly change from pre-rTMS to post-rTMS in either the active or sham group |
| Mishra et al. (2010) | 45 male AUD patients, aged 18–60 (100% men) | 10-day, randomized, parallel groups, single-blind, shame-controlled, study with a 1-month follow-up | Active and sham rTMS, figure-8 coil, 10 Hz, 1000 pulses, 110% MT | Right DLPFC | 10 sessions (daily) | ACQ | In comparison to sham, active rTMS led to significant decreases in alcohol craving post-intervention |
| Raikwar et al. (2020) | 60 male AUD inpatients, aged 25 – 56 (100% men) | 10-day,single-blind, randomoized, sham-controlled, parallel groups, study with a 14-day follow-up | Active and sham rTMS, figure-8 coil, 10 Hz, 800 pulses, 120% MT | Left DLPFC | 10 (daily sessions) | ACQ | Craving did not significantly change from pre-rTMS to post-rTMS in either the active or sham group |
| tDCS | |||||||
| Boggio et al. (2008) | 13 AUD patients, aged 30 – 55 (84.61% men) | Single-session, randomized, sham-controlled double blind, crossover study | Anodal left/cathodal right, cathodal left/ anodal right, and sham tDCS, 2 mA, 20mins | Bilateral DLPFC | 1 | AUQ | In comparison to sham stimulation, stimulation in either anodal left/cathodal right or anodal right/cathodal decreased alcohol craving upon exposure to alcohol cues |
| Da Silva et al. (2013) | 13 AUD patients, aged 18–75 (100% men) | 5-week, randomized, parallel groups, sham-controlled double-blind study | Anodal left and sham tDCS, 2 mA, 20mins | Left DLPFC | 5 (1/week) | OCDS | Craving significantly decreased from pre- to post-intervention only in the active tDCS group |
| den Uyl et al. (2017) | 91 AUD inpatients, aged 18–65 (67.03% men) | 1-week, double-blind, sham-controlled, parallel groups (active rTMS and cognitive bias modification (CBM) vs. sham rTMS and CBM vs. active rTMS), study with a 3- and 12-month follow-up | Anodal left tDCS and sham, 2 mA, 20mins | Left DLPFC | 4 (4/week) | PACS | Participants in all three groups demonstrated a significant reduction in alcohol craving from pre- to post-intervention |
| den Uyl et al. (2018) | 83 AUD inpatients, aged 18–65 (72.29% men) | 2-by-2 double-blind factorial design (controlvs. real Attentional Bias Modification (ABM) and sham vs. active tDCS) parallel groups study with a 12-month follow-up | Anodal left tDCS and sham, 2 mA, 20mins | Left DLPFC | 4 (4/week) | PACS | Participants in all four groups demonstrated a significant reduction in alcohol craving from pre- to post-intervention |
| Holla et al. (2020) | 24 male AUD patients, aged 18 – 65 (100% men) | 5-day, randomized, sham-controlled, parallel groups study with follow-ups at Day 7, 14, 30, 60, and 90 post-intervention | Left cathodal/right anodal and sham tDCS, 2 mA, 35cm2, 20mins | Bilateral DLPFC | 5 (daily sessions) | ACQ | There was no significant effect of active or sham tDCS in reducing craving from pre- to post-intervention or at follow-up |
| Klauss et al. (2014) | 33 AUD patients, aged 18–75 (96.96% men) | 1-week, parallel groups, randomized, sham-controlled, single-blinded study with 4-weekly and 5-monthly follow-ups | Left cathodal/right anodal and sham tDCS, 2 mA, 35cm2, 13mins | Bilateral DLPFC | 5 (daily sessions) | OCDS | Craving did not significantly change from pre-tDCS to post-tDCS in either the active or sham group |
| Klauss et al. (2018) | 45 AUD patients, aged 18 – 65 (82.2% men) | 20-day, parallel groups, randomized, double-blind, sham-controlled, clinical trial, with 3-weekly and a 3-month follow-up | Left cathodal/right anodal and sham tDCS, 2 mA, 35cm2. 20mins | Bilateral DLPFC | 10 (1 session/2 days) | OCDS | There was a significant decrease in alcohol craving immediately after the intervention and at the one-week follow-up for both active and sham groups. However, the decrease in alcohol craving was significantly greater in the active tDCS group. |
| Nakamura-Palacios et al. (2012) | 49 AUD patients, aged 18 – 75 (91.50% men) | 14-day, randomised, single-blind, sham-controlled, crossoverstudy | Left anodal and sham tDCS, 1 mA, 35cm2, 10mins | Left DLPFC | 2 (1/week) | OCDS | Craving did not significantly change from pre-tDCS to post-tDCS in either the active or sham group |
| Wietschorke et al. (2016) | 30 AUD patients, aged 18–65 (63.33% men) | Randomized, double-blind, shame-controlled, parallel-group, single-session study | Right anodal/left cathodal and sham tDCS, 2 mA, 35cm2, 20mins | Bilateral DLPFC | 1 | VAS | Only active tDCS led to a small, significant decrease in alcohol cravings from pre- to post- stimulation |
List of Abbreviations: rTMS: repetitive transcranial magnetic stimulation, tDCS: transcranial direct current stimulation, AUD: alcohol use disorder, DLPFC: dorsolateral prefrontal cortex, ABM: attentional bias modification, CBM: cognitive bias modification, OCDS: Obsessive-Compulsive Drinking Scale, AUQ: Alcohol Urge Questionnaire, VAS: Visual Analogue Scale, PACS: Penn Alcohol Craving Scale, and ACQ: Alcohol Craving Questionnaire.
Two of the eight trials administering rTMS were excluded from quantitative analyses due to unreported data and are discussed qualitatively (Del Felice et al., 2016; Höppner et al., 2011). Similarly, one rTMS study (Del Felice et al., 2016) and four tDCS studies (den Uyl et al., 2018, 2017; Nakamura-Palacios et al., 2016; Wietschorke et al., 2016) were not included in the meta-analysis assessing adverse events. Finally, two rTMS studies (Jansen et al., 2019; Raikwar et al., 2020) and two tDCS studies (Boggio et al., 2008; Nakamura-Palacios et al., 2012) were excluded from the quantitative analyses examining attrition due to unavailable information.
A summary of the risk of bias assessments are reported in Supplementary Fig. 1. Overall, eight RCTs were deemed low risk of bias, seven demonstrated moderate risk of bias, and two trials indicated high risk of bias. Upon visual inspection of the funnel plot for alcohol craving, no substantial publication bias was identified (Supplementary Fig. 2).
3.2. rTMS study characteristics
The eight included rTMS studies varied substantially in stimulation protocols utilized. Three of the RCTs employed a single-session protocol (Herremans et al., 2012, 2013; Jansen et al., 2019), while the number of sessions across prospective trials ranged from 10 to 12 (mean = 10.67). One study stimulated the DLPFC bilaterally (Addolorato et al., 2017), three studies stimulated the left DLPFC (Del Felice et al., 2016; Höppner et al., 2011; Raikwar et al., 2020), and four studies stimulated the right DLPFC (Herremans et al., 2012, 2013; Jansen et al., 2019; Mishra et al., 2010). High-frequency (>5) rTMS was employed in all studies, with three studies administering 20 Hz (Herremans et al., 2012, 2013; Höppner et al., 2011), while the remaining trials deployed 10 Hz of rTMS (Addolorato et al., 2017; Del Felice et al., 2016; Jansen et al., 2019; Mishra et al., 2010; Raikwar et al., 2020).
3.3. tDCS study characteristics
Nine studies employing tDCS were eligible, including two, randomized, single-blind, controlled, trials (Klauss et al., 2014; Nakamura-Palacios et al., 2012) and seven double-blind, randomized, controlled trials (Boggio et al., 2008; da Silva et al., 2013; den Uyl et al., 2018, 2017; Holla et al., 2020; Klauss et al., 2018; Wietschorke et al., 2016). The DLPFC was bilaterally stimulated in five studies (Boggio et al., 2008; Holla et al., 2020; Klauss et al., 2018, 2014; Wietschorke et al., 2016), while four studies targeted the left DLPFC (da Silva et al., 2013; den Uyl et al., 2018, 2017; Nakamura-Palacios et al., 2012) using anodal left electrode stimulation. Intensity of tDCS ranged from 1 to 2 mA, while active sessions and follow-up periods ranged from 1 to 10 sessions and 3–12 months, respectively.
3.4. Meta-Analysis of included trials
3.4.1. Effects of rTMS on alcohol craving
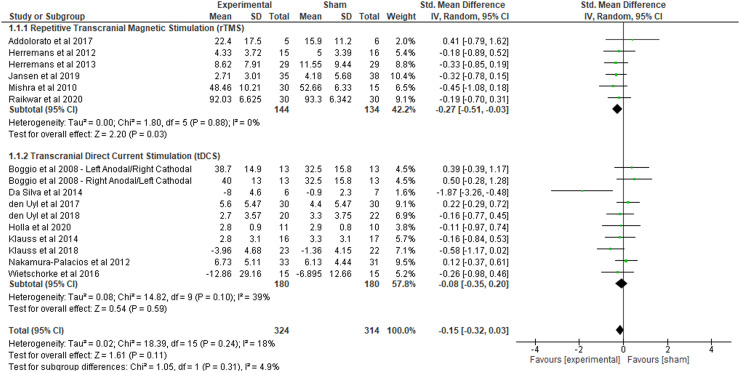
A total of six trials were combined in a meta-analysis to determine the effects of DLPFC stimulation via rTMS on alcohol craving (polled n = 278; Fig. 2). The meta-analysis revealed that, in comparison to sham stimulation, active rTMS yields small but significant reductions in craving among individuals with AUD (SMD = −0.27, 95% CI: −0.51 to −0.03, p = .03, I2 = 0%). Since only one of the six included trials bilaterally stimulated the DLPFC, we were unable to quantitatively evaluate the standardized mean difference for this parameter. However, the subgroup analysis for unilateral DLPFC stimulation yielded a significant mean effect size (SMD = −0.30, 95% CI: −0.54 to −0.05, p = .02, I2 = 0%) (Table 2). Concerning stimulation intensity, our subgroup analysis did not reveal a significant standardized mean difference for studies utilizing 10 Hz of stimulation (SMD = −0.26, 95% CI: −0.55 to 0.03, p=.08, I2 = 0%) or 20 Hz of stimulation (SMD = −0.28, 95% CI: −0.70 to 0.14, p = .29, I2 = 0%) against sham controls. Similarly, the subgroup analysis for number of sessions did not reveal any significant differences between single session (SMD = −0.30, 95% CI: −0.61 to 0.01, p = .06, I2 = 0%) or multi-session rTMS (SMD = −0.23, 95% CI: −0.60 to 0.15, p = .24, I2 = 0%) against sham.
Fig. 2.
Changes in alcohol craving in repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS): results of the meta-analysis.
Table 2.
Changes in alcohol craving in repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS): Results of the subgroup analyses .
| Stimulation Parameter | n | SMD (95% CI) | I2 | p-value* |
|---|---|---|---|---|
| rTMS Stimulation Intensity | ||||
| 10 Hz | 4 | −0.26 (−0.55 - 0.03) | 0% | .08 |
| 20 Hz | 2 | −0.28 (−0.70 - 0.14) | 0% | .29 |
| rTMS Number of Sessions | ||||
| Single Session | 3 | −0.30 (−0.61 - 0.01) | 0% | .06 |
| Multiple Sessions | 3 | −0.23 (−0.60 - 0.15) | 0% | .24 |
| rTMS Laterality of Stimulation | ||||
| Unilateral | 5 | −0.30 (0.54 - −0.05) | 0% | .02 |
| Bilateral | 1 | – | – | – |
| tDCS Stimulation Intensity | ||||
| 1 mA | 1 | – | – | – |
| 2 mA | 9 | −0.12 (−0.43 - 0.20) | 44% | .48 |
| tDCS Number of Sessions | ||||
| Single Session | 3 | −0.38 (−0.91 - 0.06) | 0% | .09 |
| Multiple Sessions | 7 | −0.18 (−0.52 - 0.16) | 45% | .29 |
| tDCS Laterality of Stimulation | ||||
| Unilateral | 4 | −0.14 (−0.68 - 0.39) | 63% | .60 |
| Bilateral | 6 | −0.35 (−0.65 - −0.02) | 0% | .02 |
*all analyses are comparing active against sham stimulation.
An additional two rTMS trials were identified; however, data were unextractable and, therefore, could not be included in the meta-analysis. One study obtained significant reductions in craving post-stimulation (Höppner et al., 2011), while a separate group obtained null findings (Del Felice et al., 2016). After stimulating the left DLPFC for ten consecutive days using high-frequency (20 Hz) rTMS, Höppner et al. (2011) found that in comparison to the sham group, patients receiving active rTMS reported a decrease in alcohol craving, in addition to improvements in cognition and depressive symptoms. In contrast, Del Felice et al. (2016) failed to find significant effects of rTMS on alcohol craving after administering four sessions over a two-week period. However, other clinical benefits emerged post-treatment, where only individuals receiving active stimulation significantly improved performance on an inhibitory control task and reported a reduction in depressive symptoms.
Meta-regression: Random effects meta-regression models for motor threshold (%) and number of pulses for rTMS revealed no significant association between these variables and alcohol craving scores (Supplementary Figs. 3 and 4). These are preliminary findings that must be interpreted cautiously given the limited number of studies included in the analysis (n = 6).
3.4.2. Effects of tDCS on alcohol craving
Ten comparisons from nine studies were included for the meta-analysis evaluating the effects of tDCS on alcohol craving (n = 360). One tDCS publication contained two active groups with differing anodal and cathodal placements; these two groups were reported independently in the comparison according to target site (Boggio et al., 2008). Compared to sham stimulation, there was no significant effect of active tDCS on alcohol craving (SMD = −0.08, 95% CI: −0.35 to 0.20, p = .59, I2 = 39%). However, the subgroup analysis revealed a significant standardized mean difference for bilateral DLPFC stimulation (SMD = −0.35, 95% CI: −0.65 to −0.06, p = .02, I2 = 0%) but not for unilateral DLPFC stimulation (SMD = −0.35, 95% CI: −0.68 to 0.39, p = .60, I2 = 63%) (Table 2). For number of sessions, the results of the subgroup analysis were nonsignificant for trials utilizing either a single-session (SMD = −0.38, 95% CI: −0.81 to 0.06, p = .09, I2 = 0%) or multi-session (SMD = −0.17,=8 95% CI: −0.52 to 0.16, p = .29, I2 = 45%) design. A subgroup analysis on stimulation intensity did not reveal any significant effects for active stimulation with 2 mA (SMD = −0.12, 95% CI: −0.43 to 0.20, p = .48, I2 = 44%). There was only one included study that utilized tDCS at 1 mA, so a meta-analysis could not be synthesized for this intensity level.
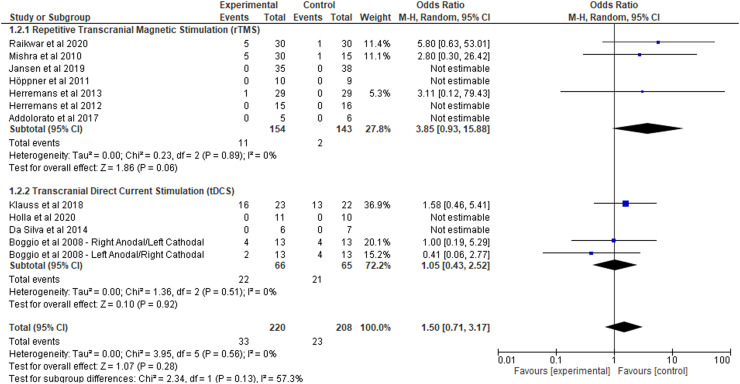
3.5. Adverse events
Seven rTMS studies (n = 197) and five tDCS studies (n = 131) reported on the presence or absence of adverse events following stimulation (Fig. 3). Three studies administering rTMS (n = 103) and three studies administering tDCS (n = 98) reported no adverse events in both the sham and active groups. The frequency and types of adverse events experienced in both the active and sham groups are reported in Table 3. Overall, adverse events were not significantly more likely to be reported by the active group in comparison to the sham group (OR = 1.50, 95% CI: 0.43 to 2.52, p = .92). Subgroup analyses further revealed that individuals receiving either active rTMS or tDCS were not significantly more likely at risk of experiencing adverse events than individuals receiving sham stimulation (p = .06, I2 = 0% vs. p = .92, I2 = 0%, respectively).
Fig. 3.
Adverse Events in repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS): Results of the meta-analysis.
Table 3.
Types and frequency of adverse events reported by participants.
| Number of Participants per Non-Invasive Neuromodulation Technique | ||||
|---|---|---|---|---|
| rTMS (N = 286) |
tDCS (N = 428) |
|||
| Type of Adverse Event | Active (n = 143) | Sham (n = 143) | Active (n = 220) | Sham (n = 208) |
| Headaches | 10 | 0 | 2 | 1 |
| Discomfort at Stimulation Side | 1 | 0 | 4 | 1 |
| Seizure | 0 | 1 | 0 | 0 |
| Heaviness of the Head | 0 | 1 | 0 | 0 |
| Tingling | 0 | 0 | 16 | 13 |
| Itchiness | 0 | 0 | 0 | 1 |
| Mood Changes | 0 | 0 | 0 | 1 |
| Total Number of Adverse Events | 11 | 2 | 22 | 17 |
3.6. Attrition
We conducted analyses only on publications that explicitly reported attrition for both the active and sham groups. Overall, risk of attrition was not greater in the active group when comparing against the sham group (OR = 1.01, CI: 0.46 to 2.21, p = .97) (Fig. 4). Similar findings were obtained when conducting subgroup analyses for rTMS (p = .95, I2 = 0%) and tDCS (p = .97, I2 = 0%).
Fig. 4.
Attrition in repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS): Results of the meta-analysis.
4. Discussion
This systematic review and meta-analysis included 17 randomized, sham-controlled studies to examine the effects of rTMS or tDCS over the DLPFC on alcohol craving. We demonstrated that in comparison to sham stimulation, participants receiving active rTMS reported significantly greater reductions in alcohol craving. However, the random effect meta-analysis revealed no significant, overall tDCS effect on craving in AUD. Nevertheless, we established that both non-invasive neuromodulation techniques are safe, and we did not detect significant differences in dropout rates between active and sham groups.
The observed effect size for rTMS was comparable or greater than several pharmacological agents used to treat AUD (Blodgett et al., 2014; Maisel et al., 2013). For example, a meta-analysis of 64 RCTs utilizing pharmacotherapy for alcohol craving obtained a mean effect size of 0.14 (CI: 0.05–0.2) and 0.03 (CI: −0.04 - 0.1) for acamprosate and naltrexone, respectively (Maisel et al., 2013). Comparable effect sizes on craving have been obtained for baclofen (Rose and Jones, 2018) and topiramate (Blodgett et al., 2014). Such findings suggest that rTMS may be comparable to pharmacological treatments in reducing alcohol craving. Nevertheless, the clinical utility of rTMS for reducing alcohol consumption remains unclear, as few of the included studies investigated this outcome and optimal stimulation parameters have yet to be established.
Our tDCS findings are consistent with a previous meta-analysis that did not demonstrate active tDCS as superior to sham in producing changes in alcohol craving (Mostafavi et al., 2020). Rather, we observed a placebo effect in tDCS overall where individuals in both the active and sham group significantly reduced craving post-intervention. This may be attributed to a substantial number of the tDCS studies included in the meta-analysis had incorporated additional treatment options for sham and active groups, including behavioural therapies and pharmacotherapy (Boggio et al., 2008; den Uyl et al., 2018, 2017; Holla et al., 2020; Nakamura-Palacios et al., 2012). However, it is encouraging to note that bilateral stimulation of the DLPFC was associated with a significant reduction in alcohol craving in comparison to sham treatment, with an effect size comparable to current pharmacological treatments (Blodgett et al., 2014; Rose and Jones, 2018). Furthermore, tDCS is well-tolerated in AUD, as none of the studies reported unexpected or serious adverse events. Therefore, tDCS should still be considered as a potential treatment modality for alcohol craving, but further research is warranted to determine optimal stimulation parameters.
While our results are promising, several limitations need to be considered. Due to the small number of included studies, we were unable to conduct meaningful subgroup analyses and evaluate certain stimulation parameters against one another (e.g., 10 Hz vs. 20 Hz), which precluded us from investigating optimal stimulation parameters to treat alcohol craving. Further, while we did obtain interesting results within our subgroup analyses, including 10 Hz of rTMS and single-session of rTMS trending towards significance, our small sample size may have been underpowered to detect true effects, and more studies are required to ascertain the optimal stimulation parameters to treat craving in AUD. Similarily, the exploratory meta-regression analyses for rTMS stimulation paraterms were extremely preliminary as a minimum of 10 studies is typically required in order to draw meaningful conclusions from the resulting associations. An additional limitation involves the significant methodological heterogeneity across studies, including trial duration, stimulation intensity, and participant characteristics, may impact our findings. For example, concomitant medication use was not adequately addressed in the literature, despite past research demonstrating that certain psychotropic medications can interact with non-invasive neuromodulation techniques (Brunoni et al., 2013; Hunter et al., 2019). An additional concern is that the effects of tDCS and rTMS on craving were assessed only through self-report questionnaires and visual analogue scales, which are prone to socially desirable responding. Past research has illustrated that self-report measures of alcohol consumption lead to underreporting of use, and this effect may be stronger in heavy drinkers as opposed to light or moderate drinkers (Taylor et al., 2007). Subsequently, future research should corroborate self-report questionnaires with behavioral and psychophysiological measures of alcohol craving, as these measures have been found to predict treatment outcome (Drummond and Glautier, 1994). Moreover, the exact location of DLPFC stimulation is ambiguous in several studies. Six of the included trials relied upon a 5cm rule (Addolorato et al., 2017; Herremans et al., 2012, 2013; Höppner et al., 2011; Mishra et al., 2010; Raikwar et al., 2020), which maintains that the DLPFC is positioned 5 cm anterior from the abductor pollicis muscle (Pascual-Leone et al., 1996). However, this method does not consider variability in head size or shape, leading to inaccuracies in the localization of the DLPFC target point (Rusjan et al., 2010). In contrast, only one publication utilised a tailored approach to define the stimulation site for each individual via fMRI data obtained at baseline (Jansen et al., 2019). Finally, we only focused on studies stimulating the DLPFC, and trials targeting other brain regions were not assessed. While the DLPFC plays a critical role in the maintenance of a drug addiction (Koob, 2016), stimulating other brain regions, such as the ventromedial prefrontal cortex, may produce significant therapeutic effects (Ceccanti et al., 2015).
In conclusion, our meta-analysis demonstrated that rTMS produces significant reductions in alcohol craving, with effect sizes comparable to evidence-based pharmacotherapies. However, our understanding of the most efficient stimulation parameters, treatment schedules, and brain targets is limited for both non-invasive neuromodulatory techniques. Thus, there is a need for further randomized, double-blind, sham controlled trials with sufficient follow-up periods to determine the efficacy of rTMS and tDCS for AUD. Additionally, future studies should combine these techniques with neuroimaging to provide insights into treatment-related neurobiological changes, which may elucidate physiological mechanisms by which rTMS or tDCS improve alcohol craving. These studies may provide novel neuroscience-based methods for improving AUD outcomes.
Funding and disclosures
This work was supported in part by NIDA grant R21-DA-043949 and the CAMH Foundation (to Dr. George). The authors declare no competing interests.
CRediT authorship contribution statement
Maryam Sorkhou: Funding acquisition, Writing – original draft, Formal analysis, Visualization. Nicolette Stogios: Funding acquisition, Writing – original draft, Formal analysis, Visualization. Negar Sayrafizadeh: Formal analysis, Visualization. Margaret K. Hahn: Writing – review & editing. Sri Mahavir Agarwal: Formal analysis, Visualization, Writing – review & editing. Tony P. George: Funding acquisition, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
There are no conflicts of interest to report
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dadr.2022.100076.
Appendix. Supplementary materials
References
- Addolorato G., Antonelli M., Cocciolillo F., Vassallo G.A., Tarli C., Sestito L., Mirijello A., Ferrulli A., Pizzuto D.A., Camardese G. Deep transcranial magnetic stimulation of the dorsolateral prefrontal cortex in alcohol use disorder patients: effects on dopamine transporter availability and alcohol intake. Eur. Neuropsychopharmacol. 2017;27(5):450–461. doi: 10.1016/j.euroneuro.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Barr M.S., George T.P. Deep repetitive transcranial magnetic stimulation for smoking cessation: is going deeper better? (Commentary) Biol. Psychiatry. 2014;76:678–680. doi: 10.1016/j.biopsych.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Berlim M.T., Van den Eynde F., Daskalakis Z.J. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology. 2013;38(4):543–551. doi: 10.1038/npp.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blodgett J.C., Del Re A., Maisel N.C., Finney J.W. A meta-analysis of topiramate's effects for individuals with alcohol use disorders. Alcohol. Clin. Exp. Res. 2014;38(6):1481–1488. doi: 10.1111/acer.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio P.S., Sultani N., Fecteau S., Merabet L., Mecca T., Pascual-Leone A., Basaglia A., Fregni F. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend. 2008;92(1–3):55–60. doi: 10.1016/j.drugalcdep.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Brunoni A.R., Ferrucci R., Bortolomasi M., Scelzo E., Boggio P., Fregni F., Dell'Osso B., Giacopuzzi M., Altamura A., Priori A. Interactions between transcranial direct current stimulation (tDCS) and pharmacological interventions in the major depressive episode: findings from a naturalistic study. Eur. Psychiatry. 2013;28(6):356–361. doi: 10.1016/j.eurpsy.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Ceccanti M., Inghilleri M., Attilia M.L., Raccah R., Fiore M., Zangen A., Ceccanti M. Deep TMS on alcoholics: effects on cortisolemia and dopamine pathway modulation. A pilot study. Can. J. Physiol. Pharmacol. 2015;93(4):283–290. doi: 10.1139/cjpp-2014-0188. [DOI] [PubMed] [Google Scholar]
- Coles A.S., Kozak K., George T.P. A review of brain stimulation methods to treat substance use disorders. Am. J. Addict. 2018;27(2):71–91. doi: 10.1111/ajad.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett M.S., Higgins J.P., Woolacott N.F. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Res. Synth. Methods. 2014;5(1):79–85. doi: 10.1002/jrsm.1090. [DOI] [PubMed] [Google Scholar]
- da Silva M.C., Conti C.L., Klauss J., Alves L.G., do Nascimento Cavalcante H.M., Fregni F., Nitsche M.A., Nakamura-Palacios E.M. Behavioral effects of transcranial direct current stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence. J. Physiol. Paris. 2013;107(6):493–502. doi: 10.1016/j.jphysparis.2013.07.003. [DOI] [PubMed] [Google Scholar]; Epub 2013 Jul 25.
- Del Felice A., Bellamoli E., Formaggio E., Manganotti P., Masiero S., Cuoghi G., Rimondo C., Genetti B., Sperotto M., Corso F. Neurophysiological, psychological and behavioural correlates of rTMS treatment in alcohol dependence. Drug Alcohol Depend. 2016;158:147–153. doi: 10.1016/j.drugalcdep.2015.11.018. [DOI] [PubMed] [Google Scholar]
- den Uyl T.E., Gladwin T.E., Lindenmeyer J., Wiers R.W. Oct 2018-12-20). A clinical trial with combined transcranial direct current stimulation and attentional bias modification in alcohol-dependent patients. Alcohol. Clin. Exp. Res. 2018;42(10):1961–1969. doi: 10.1111/acer.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Uyl T.E., Gladwin T.E., Rinck M., Lindenmeyer J., Wiers R.W. A clinical trial with combined transcranial direct current stimulation and alcohol approach bias retraining. Addict. Biol. 2017;22(6):1632–1640. doi: 10.1111/adb.12463. [DOI] [PubMed] [Google Scholar]
- Drummond D.C., Glautier S. A controlled trial of cue exposure treatment in alcohol dependence. J. Consult. Clin. Psychol. 1994;62(4):809. doi: 10.1037//0022-006x.62.4.809. [DOI] [PubMed] [Google Scholar]
- Fletcher J. What is heterogeneity and is it important? BMJ. 2007;334(7584):94–96. doi: 10.1136/bmj.39057.406644.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonteneau C., Redoute J., Haesebaert F., Le Bars D., Costes N., Suaud-Chagny M.-.F., Brunelin J. Frontal transcranial direct current stimulation induces dopamine release in the ventral striatum in human. Cereb. Cortex. 2018;28(7):2636–2646. doi: 10.1093/cercor/bhy093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A., Beck A., Grüsser S.M., Grace A.A., Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict. Biol. 2009;14(1):108–118. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herremans S., Baeken C., Vanderbruggen N., Vanderhasselt M.-.A., Zeeuws D., Santermans L., De Raedt R. No influence of one right-sided prefrontal HF-rTMS session on alcohol craving in recently detoxified alcohol-dependent patients: results of a naturalistic study. Drug Alcohol Depend. 2012;120(1–3):209–213. doi: 10.1016/j.drugalcdep.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Herremans S., Vanderhasselt M.A., De Raedt R., Baeken C. Reduced intra-individual reaction time variability during a go–NoGo task in detoxified alcohol-dependent patients after one right-sided dorsolateral prefrontal HF-rTMS session. Alcohol Alcohol. 2013;48(5):552–557. doi: 10.1093/alcalc/agt054. [DOI] [PubMed] [Google Scholar]
- Holla B., Biswal J., Ramesh V., Shivakumar V., Bharath R.D., Benegal V., Venkatasubramanian G., Chand P.K., Murthy P. Effect of prefrontal tDCS on resting brain fMRI graph measures in Alcohol Use Disorders: a randomized, double-blind, sham-controlled study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2020;102 doi: 10.1016/j.pnpbp.2020.109950. [DOI] [PubMed] [Google Scholar]
- Höppner J., Broese T., Wendler L., Berger C., Thome J. Repetitive transcranial magnetic stimulation (rTMS) for treatment of alcohol dependence. World J. Biol. Psychiatry. 2011;12(sup1):57–62. doi: 10.3109/15622975.2011.598383. [DOI] [PubMed] [Google Scholar]
- Hunter A.M., Minzenberg M.J., Cook I.A., Krantz D.E., Levitt J.G., Rotstein N.M., Chawla S.A., Leuchter A.F. Concomitant medication use and clinical outcome of repetitive transcranial magnetic stimulation (rTMS) treatment of major depressive disorder. Brain Behav. 2019;9(5):e01275. doi: 10.1002/brb3.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranpour A., Nakhaee N. A review of alcohol-related harms: a recent update. Addict. Health. 2019;11(2):129. doi: 10.22122/ahj.v11i2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L., Koslowsky M., Lavidor M. tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp. Brain Res. 2012;216(1):1–10. doi: 10.1007/s00221-011-2891-9. [DOI] [PubMed] [Google Scholar]
- Jansen J.M., van den Heuvel O.A., van der Werf Y.D., de Wit S.J., Veltman D.J., van den Brink W., Goudriaan A.E. The effect of high-frequency repetitive transcranial magnetic stimulation on emotion processing, reappraisal, and craving in alcohol use disorder patients and healthy controls: a functional magnetic resonance imaging study. Front. Psychiatry. 2019;10(11) doi: 10.3389/fpsyt.2019.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauss J., Anders Q.S., Felippe L.V., Nitsche M.A., Nakamura-Palacios E.M. Multiple sessions of transcranial direct current stimulation (tDCS) reduced craving and relapses for alcohol use: a randomized placebo-controlled trial in alcohol use disorder. Front. Pharmacol. 2018;9:716. doi: 10.3389/fphar.2018.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauss J., Penido Pinheiro L.C., Silva Merlo B.L., Correia Santos G.d.A, Fregni F., Nitsche M.A, Miyuki Nakamura-Palacios E. A randomized controlled trial of targeted prefrontal cortex modulation with tDCS in patients with alcohol dependence. Int. J. Neuropsychopharmacolog. 2014;17(11):1793–1803. doi: 10.1017/S1461145714000984. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiat. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur J.P., Aleman A., Baeken C., Benninger D.H., Brunelin J., Di Lazzaro V., Filipović S.R., Grefkes C., Hasan A., Hummel F.C. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018) Clin. Neurophysiol. 2020;131(2):474–528. doi: 10.1016/j.clinph.2019.11.002. [DOI] [PubMed] [Google Scholar]
- Li C.S.R., Luo X., Yan P., Bergquist K., Sinha R. Altered impulse control in alcohol dependence: neural measures of stop signal performance. Alcohol. Clin. Exp. Res. 2009;33(4):740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel N.C., Blodgett J.C., Wilbourne P.L., Humphreys K., Finney J.W. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275–293. doi: 10.1111/j.1360-0443.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti R., Mishra B.R., Hota D. Effect of high-frequency transcranial magnetic stimulation on craving in substance use disorder: a meta-analysis. J. Neuropsychiatry Clin. Neurosci. 2017;29(2):160–171. doi: 10.1176/appi.neuropsych.16040065. [DOI] [PubMed] [Google Scholar]
- Manthey J., Shield K.D., Rylett M., Hasan O.S., Probst C., Rehm J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet N. Am. Ed. 2019;393(10190):2493–2502. doi: 10.1016/S0140-6736(18)32744-2. [DOI] [PubMed] [Google Scholar]
- Mishra B.R., Nizamie S.H., Das B., Praharaj S.K. Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction. 2010;105(1):49–55. doi: 10.1111/j.1360-0443.2009.02777.x. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafavi S.A., Khaleghi A., Mohammadi M.R. Noninvasive brain stimulation in alcohol craving: a systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2020;101(12) doi: 10.1016/j.pnpbp.2020.109938. [DOI] [PubMed] [Google Scholar]
- Nakamura-Palacios E.M., de Almeida Benevides M.C., da Penha Zago-Gomes M., de Oliveira R.W.D., de Vasconcellos V.F., de Castro L.N.P., da Silva M.C., Ramos P.A., Fregni F. Auditory event-related potentials (P3) and cognitive changes induced by frontal direct current stimulation in alcoholics according to Lesch alcoholism typology. Int. J. Neuropsychopharmacolog. 2012;15(5):601–616. doi: 10.1017/S1461145711001040. [DOI] [PubMed] [Google Scholar]
- Nakamura-Palacios E.M., Lopes I.B.C., Souza R.A., Klauss J., Batista E.K., Conti C.L., Moscon J.A., de Souza R.S.M. Oct 2016-02-14). Ventral medial prefrontal cortex (vmPFC) as a target of the dorsolateral prefrontal modulation by transcranial direct current stimulation (tDCS) in drug addiction. J. Neural Transm. 2016;123(10):1179–1194. doi: 10.1007/s00702-016-1559-9. [DOI] [PubMed] [Google Scholar]
- Nitsche M.A., Boggio P.S., Fregni F., Pascual-Leone A. Treatment of depression with transcranial direct current stimulation (tDCS): a review. Exp. Neurol. 2009;219(1):14–19. doi: 10.1016/j.expneurol.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Oudejans S., Schippers G., Spits M., Stollenga M., van den Brink W. Five years of ROM in substance abuse treatment centres in the Netherlands. Tijdschr. Psychiatr. 2012;54(2):185–190. [PubMed] [Google Scholar]
- Park S.Q., Kahnt T., Beck A., Cohen M.X., Dolan R.J., Wrase J., Heinz A. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J. Neurosci. 2010;30(22):7749–7753. doi: 10.1523/JNEUROSCI.5587-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A., Rubio B., Pallardó F., Catalá M.D. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet N. Am. Ed. 1996;348(9022):233–237. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A., Tormos J.M., Keenan J., Tarazona F., Cañete C., Catalá M.D. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J. Clin. Neurophysiol. 1998;15(4):333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Pombo S., Figueira M.L., Walter H., Lesch O. Motivational factors and negative affectivity as predictors of alcohol craving. Psychiatry Res. 2016;243:53–60. doi: 10.1016/j.psychres.2016.02.064. [DOI] [PubMed] [Google Scholar]
- Raikwar S., Divinakumar K., Prakash J., Khan S.A., GuruPrakash K., Batham S. A sham-controlled trial of repetitive transcranial magnetic stimulation over left dorsolateral prefrontal cortex and its effects on craving in patients with alcohol dependence. Ind. Psychiatry J. 2020;29(2):245. doi: 10.4103/ipj.ipj_53_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose A.K., Jones A. Baclofen: its effectiveness in reducing harmful drinking, craving, and negative mood. A meta-analysis. Addiction. 2018;113(8):1396–1406. doi: 10.1111/add.14191. [DOI] [PubMed] [Google Scholar]
- Rusjan, P.M., Barr, M.S., Farzan, F., Arenovich, T., Maller, J.J., Fitzgerald, P.B., & Daskalakis, Z.J. (2010). Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation (1065-9471). [DOI] [PMC free article] [PubMed]
- Schardt C., Adams M.B., Owens T., Keitz S., Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007;7(1):1–6. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanki N., Abijo T., Galvao C., Darius P., Blum K., Gondré-Lewis M.C. Administration of a putative pro-dopamine regulator, a neuronutrient, mitigates alcohol intake in alcohol-preferring rats. Behav. Brain Res. 2020;385 doi: 10.1016/j.bbr.2020.112563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer A.M., Kimbrell T.A., Wassermann E.M., Repella J.D., Willis M.W., Herscovitch P., Post R.M. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol. Psychiatry. 2000;48(12):1133–1141. doi: 10.1016/s0006-3223(00)01065-9. [DOI] [PubMed] [Google Scholar]
- Strafella A.P., Paus T., Barrett J., Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J. Neurosci. 2001;21(15):RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. RC157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B., Rehm J., Patra J., Popova S., Baliunas D. Alcohol-attributable morbidity and resulting health care costs in Canada in 2002: recommendations for policy and prevention. J. Stud. Alcohol. Drugs. 2007;68(1):36–47. doi: 10.15288/jsad.2007.68.36. [DOI] [PubMed] [Google Scholar]
- Tiffany S.T., Wray J.M. The clinical significance of drug craving. Ann. N. Y. Acad. Sci. 2012;1248:1. doi: 10.1111/j.1749-6632.2011.06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietschorke K., Lippold J., Jacob C., Polak T., Herrmann M.J. Transcranial direct current stimulation of the prefrontal cortex reduces cue-reactivity in alcohol-dependent patients. J. Neural Transm. 2016;123(10):1173–1178. doi: 10.1007/s00702-016-1541-6. [DOI] [PubMed] [Google Scholar]
- Wilson S.J. The Wiley Handbook on The Cognitive Neuroscience of Addiction. 2015 [Google Scholar]
- Zhang J.J.Q., Fong K.N.K., Ouyang R.g., Siu A.M.H., Kranz G.S. Effects of repetitive transcranial magnetic stimulation (rTMS) on craving and substance consumption in patients with substance dependence: A systematic review and meta-analysis. Addiction. 2019;114(12):2137–2149. doi: 10.1111/add.14753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.