Highlights
-
•
Intranasal oxytocin treatment is safe and feasible with cocaine use disorder patients in a community clinic setting.
-
•
Intranasal oxytocin may increase the odds of abstinence from cocaine after some weeks of treatment.
-
•
Intranasal oxytocin treatment was associated with a greater drop-out rate.
Keywords: Intranasal, Oxytocin, Cocaine, Stress
Abstract
Background
Oxytocin (OT) treatment in drug addiction studies have suggested potential therapeutic benefits. There is a paucity of clinical trial studies of oxytocin in cocaine use disorders.
Method
This was a 6-week randomized, double-blind, outpatient clinical trial study investigating the effect of daily Intranasal Oxytocin (24 IU) on cocaine use by cocaine use disorder patients. After a 7-day inpatient abstinence induction stage, patients were randomized to intranasal oxytocin or intranasal placebo. During the outpatient phase, cocaine use disorder patients were required to present themselves to the research staff 3 times a week for witnessed randomized medication administration, to provide a urine sample for qualitative toxicology, and complete mandatory assessments, including the Time-Line-Follow Back. For the interim days, patients were given an “at-home” bottle that was weighed at each clinic visit to monitor compliance.
Results
Neither administration of Intranasal placebo (n = 11) or Oxytocin (n = 15) induced at least 3 weeks of continuous abstinence. However, from week 3, the odds of weekly abstinence increased from 4.61 (95% CI = 1.05, 20.3) to 15.0 (CI = 1.18, 190.2) by week 6 for the Intranasal Oxytocin group (t = 2.12, p = 0.037), though there was no significant group difference overall in the odds of abstinence over time (F1,69 = 1.73, p = 0.19). More patients on Intranasal Oxytocin dropped out (p = 0.0005).
Conclusions
Intranasal Oxytocin increased the odds of weekly abstinence in Cocaine patients after 2 weeks compared to PBO, but was associated with a higher dropout rate. (ClinicalTrials.gov 02,255,357, 10/2014)
1. Introduction
Cocaine addiction affected nearly 1.5 million people in the USA in 2014 (NIDA, 2020). Addiction to cocaine is related to behavioral conditioning and physiological adaptations in brain neural systems that become dysregulated to the point of being non-functional without the drug itself (Raby et al., 2008). This dysregulation is thought to underlie the transition from abuse to addiction and its relapsing nature (Koob and Kreek, 2007).
The gravity of cocaine addiction is compounded by the lack of FDA-approved medicinal treatment to support the effect of behavioral treatment. Most research efforts have focused on monoamine, serotonergic, GABAergic, or glutaminergic agents (Shorter and Kosten, 2011; Johnson et al., 2013; Kampangkaew et al., 2019) and stress reactivity (Fox and Sinha, 2014; Schluger et al., 2001; Kablinger et al., 2012). Focus on regulation of stress reactivity in addiction (Koob and Kreek, 2007) and on the loss of social norms among drug users (McGregor et al., 2008; Young et al., 2011), has generated interest in oxytocin due to its purported role in these traits and regulation of stress.
Oxytocin was one of the first characterized neuropeptides (Du Vigneaud et al., 1954). It was isolated from the Supraoptic and Paraventricular nuclei of the hypothalamus where it is produced, and from the neurohypophysis, from which it is secreted in the peripheral circulation (Martin, 2012) to regulate autonomic functions such as lactation. Since then, oxytocin and its respective receptor, has been detected in the central nervous system beyond the hypothalamus (Buijs, 1978; Tribollet, 1992; Leong et al., 2017) and the effects of oxytocin has been studied on an array of physiological and behavioral manifestations (Lee and Weerts, 2016; Aguilera and Rabadan-Diehl, 2000).
Research reports suggest a role for oxytocin in addiction treatment (Carson et al., 2013; Sarnyai, 2014). In animal models oxytocin administration is reported to attenuate cue-induced cocaine seeking (Bentzley and Aston-Jones, 2014; Morales-Rivera et al., 2014), cocaine stereotyped behavior (Carson et al., 2013; Leong et al., 2016) and cocaine self-administration (Zhou et al., 2014). In human studies, it has been reported to reduce cravings in cannabis users (McRae-Clarke et al., 2013), stress reactivity (Moeni et al., 2019), and state anger in cocaine users, a predictor of relapse (Lee et al., 2014). One pilot clinical trial of Intranasal Oxytocin reported decreased cocaine craving and use in methadone-maintained patients (Stauffer et al., 2016). Furthermore, recurrent cocaine use leads to lower endogenous oxytocin levels (Light et al., 2004; Lin et al., 2015), to deplete oxytocin stores in the hypothalamus and amygdala (Sarnyai et al., 1992; Sivukhina et al., 2006) and increasing oxytocin receptors at these sites (Liberzon and Young, 1997). Consequently, cocaine abuse may create a hormonal environment sensitive to exogenous oxytocin. As a small polypeptide, oxytocin can be administered by an Intranasal (IN) route that delivers it to CNS areas where the pathology of cocaine addiction is thought to arise (Banks, 2008; Lee et al., 2020).
We present here the results of a preliminary 6-week double-blind, randomized, placebo-controlled outpatient clinical trial that assessed the effect of daily Intranasal Oxytocin on abstinence from cocaine. We hypothesized: 1) Intranasal Oxytocin would be a safe and feasible treatment for patients seeking treatment for cocaine use disorder; 2) Intranasal Oxytocin would reduce cocaine use compared to placebo. The primary clinical outcome was at least 3 weeks of continued abstinence after abstinence induction and the main secondary outcome was weekly cocaine use during the 6-week trial.
2. Material and methods
2.1. Study patients
Patients were recruited by local advertising. The study was conducted at the STARS clinic, a research clinic of the Division on Substance Use at the New York State Psychiatric Institute (NYSPI) and Columbia University. It was approved by the NYSPI Institutional Review Board (IRB 6093).
Medical screening included a history and physical examination, urine toxicology to verify cocaine use, pregnancy test for women, an electrocardiogram, a comprehensive metabolic panel, and complete blood count. We used the MINI International Neuropsychiatric Interview to confirm the DSM-IV diagnosis of cocaine dependence (Lecrubier et al., 1997). To verify the integrity of the olfactory system in intranasal cocaine users, potential patients were administered the Picture Smell Identification Test (PSIT; Suzuki et al., 2004). All patients passed the PSIT. Study inclusion criteria required that cocaine use disorder patients were: 1) between the age of 18–60; 2) met DSM-IV criteria for current cocaine dependence, and were seeking treatment; 3) displayed at least one cocaine-positive urine toxicology during screening; 4) used cocaine weekly, at least 4 days in the past thirty days; 5) no current or history of hyponatremia (Na ≤ 135 meq/L, exclusion criteria); 6) for female patients, a negative pregnancy test and written assurance of the use of a contraceptive during the study. Patients voluntarily signed a written consent form describing all aspects of the study. The study was conducted from December 2014 through May of 2016.
2.2. Study design
After a 7-day inpatient abstinence induction stage, cocaine use disorder patients were randomized to Intranasal Oxytocin (IN OT 24 IU) or Intranasal Placebo (IN PBO) according to a stratified permuted blocks algorithm that was unknown to the investigators until data analysis could commence. There we no demographic differences between medication groups. The patients were asked to attend the clinic three times a week. At each of these visits, they completed self-reports: Cocaine craving scale (Weiss et al., 1997), the Perceived Stress Scale (Cohen et al., 1983), and the Clinician Global Inventory (CGI-self; Guy, 1976). With a member of the research staff, they completed the Time Line Follow Back (Robinson et al., 2012) to document cocaine use, underwent compliance monitoring (see below), supplied urine toxicology (qualitative by urine dip-stick) and self-administered the randomized medication. At one of these visits, they met with the principal investigator to review progress in the study, undergo compliance enhancement therapy, and complete clinician-based ratings: CGI-observer (Guy, 1976), Hamilton Depression scale, 25 items (Williams, 1988).
2.3. Training for in administration
All cocaine use disorder patients were trained by staff to self-administer IN solutions for the clinical trial (Born et al., 2002; Dhuria et al., 2010). Two pre-packaged insufflator bottles of either oxytocin or placebo solutions were prepared for each participant, one designated as the “at home” bottle for use outside the clinic, and one the “clinic” bottle for use at the clinic. Each bottle was weighed prior to use. Twenty-four IU of oxytocin required three insufflations per nostrils, similarly for placebo. For use at home, Patients were instructed to administer 3 insufflations or “puffs” in each nostril daily upon awakening. Based on animal and human studies, Intranasal Oxytocin enters the brain and reach its peak pharmacodynamics concentration within 30 min of insufflation (Lee et al., 2020; Paloyelis et al., 2016).
2.4. Compliance monitoring
Compliance was monitored in two ways: (1) witnessed self-administration of the randomized medication at the time of clinic visits from a bottle designated as the “clinic bottle”, and (2) single-blinded weighing of the “at home bottle” at each study visit. For (1), compliance was computed as the percentage of visits where IN administration of the medication was actually witnessed. For (2), the expected mass (cc) of the “at home bottle” was compared to the weighed mass by plotting the mass at each visit. Deviation of the actual curve above the expected curve was an indication of under-compliance. The area under the curve (AUC) was computed for the expected and actual curves, and the ratio of the expected to actual AUC was computed for each participant and summarized using means and standard deviations.
2.5. Compensation
Patients could accumulate vouchers for completing all study procedures (bringing “at-home bottle”, assessments, study visits). If they did, each study visit added $5. Each consecutive week with full study compliance added an extra $20. We covered transportation costs ($5). With all study procedures completed, patients could earn $300.
2.6. Data analysis
2.6.1. Clinical trial outcomes
Cocaine use was determined using urine toxicology and TLFB self-reports. In a given week, cocaine use was rated positive if the urine toxicology test was positive, regardless of the self-report, or if the self-report was positive regardless of the urine toxicology test. Cocaine use was rated negative if both the urine toxicology test and the self-report were negative, if the self-report was negative and the urine toxicology test was missing, or if the urine toxicology was negative and the self-report was missing. Cocaine use status was rated as missing if both the urine toxicology test and self-report were missing. To assess the veracity of our results, analyses were repeated where missing cocaine use status was considered positive for cocaine use.
Non-parametric Wilcoxon rank-sum tests were used to examine differences in the maximum number of consecutive cocaine abstinent weeks between the two medication groups.
Weekly cocaine abstinence was analyzed using longitudinal logistic mixed effect models with random intercept and slope with an autoregressive correlation structure to account for within-subject repeated measures. The fixed effects of baseline self-reported using days (30 days prior to study entry), medication group (IN OT 24 IU daily vs. PBO), week (1–7), and medication group by week interaction was included in the model. If the interaction was not significant, it was removed from the final model.
Subjective ratings of cocaine cravings (24-hour, place-induced, person/event-induced, stress-induced, medication-induced), Perceived Stress Scale, cocaine dependence (CGI severity and improvement), and depression (HAM-D 25 items) were also analyzed using longitudinal mixed effect models with random intercept and slope with an autoregressive correlation structure to account for within-subject measures. The fixed effects of medication group, week, and medication group by week interaction were included in the model. When available, baseline subjective ratings were adjusted as a covariate in the model. Main effects models were analyzed if the interaction was not significant. All analyses were conducted using SAS®. All tests were tested as two-sided with levels of significance of 5%.
3. Results
3.1. Sample characteristics
Fig. 1 presents the Consort distribution of patients. Table 1 shows the demographic and clinical characteristics of the 26 cocaine use disorder patients by treatment group. The majority of the sample was male (73.1%), and had on average 12.7 years (SD = 1.2) of education. Among the patients, the average age of first cocaine use was 21.4 (SD = 5.6) and 25.2 (SD = 6.9) for regular cocaine use. Baseline average days of use for the 30 days prior to study entry for the entire sample was 11.1 (SD = 5.7).
Fig. 1.
Consort diagram.
Table 1.
Demographic characteristics at baseline by treatment group (n = 26).
| IN OT | IN PBO | Overall | ||||
|---|---|---|---|---|---|---|
| (n = 15) |
(n = 11) |
(n = 26) |
||||
| Characteristic | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) |
| or% | or% | or% | ||||
| Demographics | ||||||
| Age | 15 | 50.1 (5.3) | 11 | 50.4 (5.9) | 26 | 50.2 (5.4) |
| Sex | ||||||
| Male | 10 | 66.7% | 9 | 81.8% | 19 | 73.1% |
| Female | 5 | 33.3% | 2 | 18.2% | 7 | 26.9% |
| Race | ||||||
| African American | 14 | 93.3% | 10 | 90.9% | 24 | 92.3% |
| White | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Asian | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Hispanic/Latino | 1 | 6.7% | 1 | 9.1% | 2 | 7.7% |
| Years of Education | 13 | 12.6 (1.0) | 10 | 12.7 (1.5) | 23 | 12.7 (1.2) |
| Annual Income | ||||||
| <$20K | 1 | 6.7% | 3 | 27.3% | 4 | 15.4% |
| $20K-$30K | 2 | 13.3% | 2 | 18.2% | 4 | 15.4% |
| >$30K | 1 | 6.7% | 0 | 0.0% | 1 | 3.9% |
| N/A | 11 | 73.3% | 6 | 54.6% | 17 | 65.4% |
| Days of Cocaine Use for the 30 days prior to study entry | 15 | 11.1 (5.8) | 11 | 11.2 (5.8) | 26 | 11.1 (5.7) |
| Age of Substance Use | ||||||
| Age of 1st Cocaine Use | 11 | 21.2 (5.7) | 10 | 21.7 (5.9) | 21 | 21.4 (5.6) |
| Age of Regular Cocaine Use | 9 | 23.8 (5.7) | 5 | 27.8 (8.7) | 14 | 25.2 (6.9) |
| Age of 1st Crack Cocaine Use | 12 | 28.0 (10.0) | 9 | 27.0 (6.0) | 21 | 27.0 (8.0) |
| Age of Regular Crack Cocaine Use | 11 | 30.0 (9.0) | 9 | 28.0 (6.0) | 20 | 29.0 (7.0) |
| Age of 1st Marijuana Use | 10 | 15.2 (3.6) | 11 | 16.6 (3.7) | 21 | 16.0 (3.6) |
| Age of Regular Marijuana Use | 7 | 15.7 (4.0) | 3 | 16.3 (1.5) | 10 | 15.9 (3.4) |
| Age of 1st Alcohol Use | 12 | 15.8 (3.8) | 11 | 14.7 (2.9) | 23 | 15.3 (3.4) |
| Age of Regular Alcohol Use | 8 | 18.0 (4.7) | 8 | 19.4 (4.7) | 16 | 18.7 (4.6) |
| Age of 1st Nicotine Use | 9 | 15.9 (2.8) | 10 | 16.0 (2.3) | 19 | 16.0 (2.5) |
| Age of Regular Nicotine Use | 8 | 16.5 (4.3) | 9 | 17.3 (2.5) | 17 | 16.9 (3.4) |
3.2. Compliance estimates
The compliance with witnessed clinic administration was 93% +/- 6% due to occasional missed visits. Compliance with the “at home bottle”, assessed by the average ratio of the expected to actual area under the curve was 1.15 (SD = 0.13) or approximately 85% (Fig 2).
Fig. 2.
Illustrative “At Home” bottle compliance curve for 2 Cocaine use disorder patients in Phase 2 pilot clinical trial.
3.3. Clinical trial outcomes
Twenty-six cocaine patients were randomized to 6 weeks of IN OT (n = 15) or PBO (n = 11, Fig. 1). The median (interquartile range) and range of the maximum number of consecutive weeks of abstinence was 1.0 (IQR: 1.0 to 1.0; range: 0 to 6) for the OT group and 1.0 (IQR: 1.0 to 2.0; range: 0 to 5) for the PBO group. The Wilcoxon rank-sum test for the effect of treatment arm on the maximum number of consecutive abstinent weeks was not significant (W = 144.5, p = 0.67).
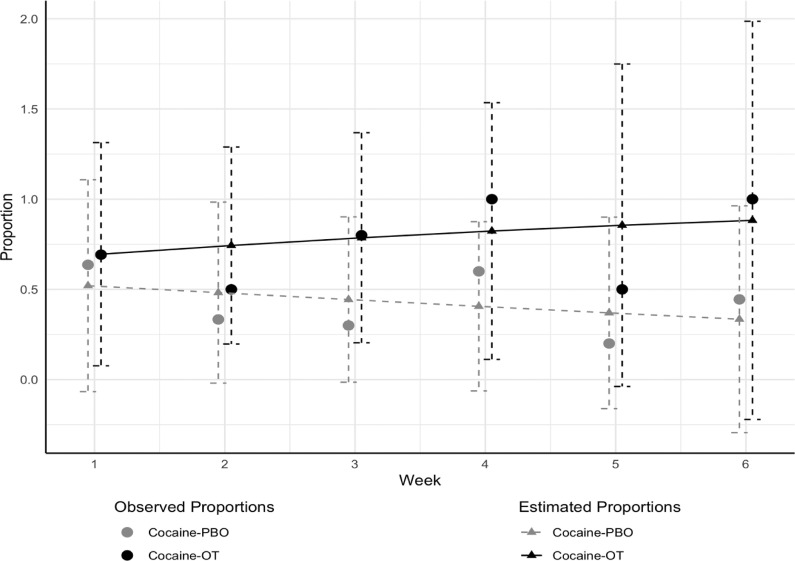
Fig. 3 shows the observed proportions and model estimated proportions of cocaine abstinence as determined by urine toxicology and TLFB self-reports. The odds of cocaine abstinence were higher in the OT group starting at week 3, reaching 15.0 (95% confidence interval = 1.18 to 190.2) by week 6 (t(69) = 2.12, p = 0.037). However, the overall medication group by time interaction across all 6 weeks was not significant (F1,69 = 1.73, p = 0.19). After removing the interaction, the difference between the overall effect of medication (OT vs. PBO) over all 6 weeks reached trend-level significance (F1,70 = 3.42, p = 0.07). There were no meaningful differences in results when analyses were repeated treating missing cocaine use status as positive for cocaine use.
Fig. 3.
Observed and model estimated proportions of cocaine abstinence during Phase 2 pilot clinical trial for Cocaine use disorder patients randomized to IN OT vs. IN PBO, with +1 standard error bars.
All subjective rating outcomes of cocaine cravings, perceived stress, cocaine dependence, and depression did not show a significant medication group by time interaction effect. Stress-induced cravings trended towards signifcance between medication groups (F = 3.31, P = 0.076, df = 43). After removing the non-significant group by time interaction, the main effect of time was significant for Person/things induced craving (b (se) = −0.25(0.11), p = 0.03) and for the CGI Observer Cocaine Dependence severity (b (se) = −0.38(0.06), p = 0.0001), suggesting that with each week in the study, Person/things induced cravings and cocaine dependence severity scores improved in both the IN OT and placebo group.
Retention during the 6 weeks of Phase 2 differed between the two randomized groups. Thirteen out of 26 patients did not complete the 6 weeks (50%); of these, 11 (85%) belonged to the OT group, 2 (15%) belonged to the PBO group. Seven of these 11 were abstinent at the time of drop-out for at least one week. Of the 13 who dropped out, 8 did so after week 1. The proportion of drop out was significantly different (χ2 = 12.3, DF = 1, p = 0.0005; Fig. 4).
Fig. 4.
Time to drop out by medication group (IN OT vs. IN PBO) among Cocaine use disorder patients who were randomized (IN OT n = 15; IN PBO n = 11) in Phase 2 pilot clinical trial.
Table 2 shows the frequency of reported side effects. There were no significant differences in the rates of reported side effects between the two medication groups.
Table 2.
Side effects reported by Cocaine Use Patients in Phase 2 by medication group.
| Side effect or Qualitative Comment | IN OT (n = 15) |
IN PBO (n = 11) |
p-valuea | ||
|---|---|---|---|---|---|
| n | % of Patients Reporting | n | % of Patients Reporting | ||
| Craving for Cocaine | 3 | 20 | 0 | 0 | 0.239 |
| Craving for Medication | 1 | 7 | 0 | 0 | 1.000 |
| Sore Throat | 1 | 7 | 0 | 0 | 1.000 |
| Insomnia | 1 | 7 | 0 | 0 | 1.000 |
| Rhinorrhea | 1 | 7 | 1 | 9 | 1.000 |
| Nasal Irritation | 1 | 7 | 1 | 9 | 1.000 |
| Unpleasant smell | 1 | 7 | 0 | 0 | 1.000 |
| Tiredness | 2 | 13 | 1 | 9 | 1.000 |
| Nausea | 1 | 7 | 0 | 0 | 1.000 |
| Feeling Up | 1 | 7 | 0 | 0 | 1.000 |
| Increased Energy | 1 | 7 | 0 | 0 | 1.000 |
| Feels like Water | 0 | 0 | 1 | 9 | 0.423 |
| Tingling in Fingers | 1 | 7 | 0 | 0 | 1.000 |
| No Effect | 0 | 0 | 1 | 9 | 0.423 |
| No Cravings | 1 | 7 | 0 | 0 | 1.000 |
| Headache | 1 | 7 | 2 | 18 | 0.556 |
| Feel Calm | 3 | 20 | 1 | 9 | 0.614 |
Results from Fisher's exact test.
4. Discussion
This preliminary study demonstrates: 1) Intranasal administration of oxytocin in patients seeking treatment for cocaine use disorder is feasible, simple to execute, and safe; 2) Intranasal Oxytocin can be administered in an outpatient setting with good compliance; 3) Intranasal Oxytocin may increase the odds of abstinence from cocaine after some latency period of approximately 2 weeks; 4) Intranasal Oxytocin was associated with more dropout over a 6 week trial compared to placebo. No serious adverse events occurred. Patients mentioned several effects or side effects, of which the most commonly reported for Intranasal Oxytocin were feeling calm (20%), craving for cocaine (20%) and tiredness (13%). The most commonly reported side effect for IN Placebo was headache (13%).
In this pilot clinical trial, we could not demonstrate that Intranasal Oxytocin induced at least three weeks of continuous abstinence. However, there is some suggestion that Intranasal Oxytocin increases the odds of abstinence, reflecting results from another clinical trial (Stauffer et al., 2016) in methadone-maintained patients. However, this result is cautioned by the paradox of a disproportionately high rate of drop out amongst patients assigned to Intranasal Oxytocin, which is contrary to the data presented by Stauffer & colleagues (2016). Of note, this study was carried out in a population that is required to be present daily in most instances to receive methadone. Aside from chance given the small sample size, the higher drop-out rate in the oxytocin group may imply that the effect of oxytocin on factors that govern behavior in cocaine use disorder is not unitary, but multifaceted.
Although the overall effect of intranasal oxytocin on abstinence was not significant, the odds of weekly abstinence in the Intranasal Oxytocin group began to reach significance at week 3 (Fig. 3), by which time the drop-out rate in the oxytocin group had reached nearly 75%, and only about 10% in the placebo group (Fig. 4). If odds of abstinence alone drove retention rate, the placebo group should have been more affected by drop-out outcomes. Another possibility, given the indigence of many of our patients, was that some patients may have sought respite and solace in the inpatient abstinence phase, perhaps motivating early drop out during the outpatient phase. This factor, if impactful, should have affected both medication groups, however it did not. Hence, the possibility that in the early phase of outpatient treatment acute effects of oxytocin were at play must be considered. Of these, the most concerning would pertain to the possibility that it may increase craving for, and use of cocaine. In light of this, sensitivity analyses conducted in which missing weeks were treated as cocaine positive weeks, rather than simply missing data, did not show any significant differences in effects of oxytocin on odds of cocaine abstinence at week 6.
McGregor et al. (2008) suggested that oxytocin may cause reinforcing effects in drug-dependent patients but also restraining effects on drug seeking behavior that may manifest at a later point. The possibility of oxytocin having reinforcing effects has been reported: In non-addicted adults, Dolan & colleagues (2020) reported that after receiving 40 IU of intranasal oxytocin, subjects in a laboratory investigation reported more drug-liking for oxytocin than placebo, and a significant correlation between the difference in drug liking and lowest price purchasing compared to placebo. Lee et al. (2014) reported the results of a human laboratory study of cocaine use disorder patients mandated to a 6 month inpatient treatment following incarceration. In this report, Intranasal Oxytocin (24 IU as in this study) provoked increased urges to use cocaine and increased cocaine cue reactivity, but also reduced state anger, a factor in drug use relapse (Fox et al., 2008). This highlights that the effects of oxytocin in cocaine use disorder may be complex and differentiated either in time or specificity; with early effects heightening the risk of use, and remedial effects on use emerging later.
Insel (2003) proposed that sustained motivated behavior could involve the interaction of oxytocin and dopamine reward pathways, which are significantly affected by cocaine use. Oxytocin neurons in the Paraventricular Nucleus of the Hypothalamus innervate mesolimbic dopamine neurons (Melis and Argiola, 2003) and increase the activity of these neurons. Activation of these yet dysregulated dopaminergic pathways could mimic the effect of cocaine and could promote conditioned cocaine seeking behavior (Volkow et al., 2006). Taken together, these findings create a theoretical framework by which one may understand the high and early drop-out rate among patients assigned to oxytocin in this study.
For those patients who continued beyond week 3, the anti-stress properties of Oxytocin may have become more significant (Dabrowska et al., 2011), and possibly may have led to a reduced probability of use. Stress is a factor known to clinicians to cause relapse among cocaine use disorder patients. Sinha et al. (2006) demonstrated that stress induced cravings were predictive of relapse and were coincident with increased activity of the Hypothalamo-Pituitary-Adrenal axis. Oxytocin has a well-documented inhibitory effect on this endocrine axis (Legros, 2001; Windle et al., 2004) and central stress mechanisms based in the Amygdala (Zhou et al., 2010). Furthermore, oxytocin and other neuropeptides can display gradually evolving, latent effects on neural activity (Theodosis, 2002). These reports relating to neurobiological effects of oxytocin can constitute a hypothetical construct for comprehending the later emerging remedial effect on odds of abstinence observed in this study.
This study is limited by the small sample size, and perhaps by an insufficient duration of the clinical trial that may have allowed more significant effects to emerge. In addition, since the intranasal route is a typical one for cocaine users, it may have served as a conditioned cue for craving and drug-seeking (O'Brien et al., 1992), especially if Intranasal Oxytocin has a rewarding effect.
Further research should focus on the effect of Intranasal Oxytocin, particularly whether it is experienced as cocaine-like or triggers cravings; seek larger sample size and longer clinical trial duration; and more strenuous follow-up in drop cases to document cause and outcome. Other potential directions could involve coupling Intranasal Oxytocin to a behavioral treatment aimed at sustaining motivation such as Motivational Interviewing (Bowen et al., 2009), implement a longer abstinence induction phase, or use high value vouchers contingent on abstinence (Higgins et al., 2007), especially in the early phase of treatment. Nonetheless, this and other reports suggest that intranasal oxytocin is safe to use and feasible to implement, and that it offers some promise in the treatment of cocaine use disorder, provided that the complexity of its effects are accounted for in the design of future studies.
Declaration of Competing Interest
None.
Acknowledgments
Acknowledgments
The authors thank NIDA and our Project Officer Dr. Aidan Hampson for their support. The Principal investigator (WNR) wishes to acknowledge Drs. Leo P Renaud MD PhD FRCP FRS, Ruud Buijs PhD, Charles Bourque PhD, Maurice Manning MD, and Eliane Tribollet PhD for their help in conceptualizing this study
Funding Source
This study was supported by a R21 award from the National Institute of Drug Abuse (NIDA) DA035461 and Supplement DA035461–02S1. NIDA did not have a role in the study design, collection, analysis, and interpretation of the data, nor in the writing of this manuscript or the decision by the authors to submit this report for publication.
Author disclosures
NIDA: Nothing to Declare.
Dr. Wilfrid Noël Raby, PhD MD: Principal Investigator of this study. Dr. Raby conceptualized the study, applied for the R21 that funded the study, conducted data collection, supervised the integrity of the study and of the data collected, and wrote the manuscript. The PI has no conflict of interest to declare.
Matthew Heller, BSc MSc: Worked as a research assistant in the conduction of the study and reviewed all the collected data for accuracy. No conflict of interest to declare.
Demetrios Milliaressis, BSc, MSc, PhD (Honorary): Worked as a research assistant in the conduction, reviewed all the collected data for accuracy, and participated in the editing of the manuscript. No conflict of interest to declare.
C. Jean Choi MSc: Performed the data analysis for the study and participated in the final editing of the manuscript. No conflict of interest to declare.
Cale Basaraba, MPH: Participated in the data analysis. No conflict of interest to declare.
Martina Pavlicova PhD: Oversaw the initial design of the study and the data analysis. No conflict of interest to declare.
Dan Alshuler, MS: Participated in the data analysis. No conflict of interest to declare.
Frances R. Levin MD: Provided mentorship and guidance to the PI, and participated in the editing of the final manuscript. No conflict of interest to declare.
Sarah Church PhD: Provided material support to the study. No conflict of interest to declare.
Edward V. Nunes MD: Provided mentorship and support during all phases of this study, and participated in the editing of the final manuscript.
References
- Aguilera G., Rabadan-Diehl C. Vasopressinergic regulation of the hypothalamic-pituitary-adrenal axis: implication for stress adaptation. Regul. Peptides. 2000;96:23–29. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- Banks W.A. Delivery of peptides to the brain: emphasis on therapeutic developments. Biopolymers. 2008;90:589–594. doi: 10.1002/bip.20980. [DOI] [PubMed] [Google Scholar]
- Bentzley B.S.J.T., Aston-Jones G. Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc. Nat. Acad. Sci. USA. 2014;111(32):11822–11827. doi: 10.1073/pnas.1406324111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J. Sniffing neuropeptides: a transnasal approach to the human brain. Nature Neurosci. 2002;5(6):514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Bowen S. Mindfulness-based relapse prevention for substance use disorders: a pilot efficacy trial. Subst. Ab. 2009;30:295–305. doi: 10.1080/08897070903250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs R.M. Intra- and extra-hypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res. 1978;192:423–435. doi: 10.1007/BF00212323. [DOI] [PubMed] [Google Scholar]
- Carson D.S. A brief history of oxytocin and its role in modulating psychostimulant effects. J. Psychopharmacol. 2013;27(3):231–247. doi: 10.1177/0269881112473788. [DOI] [PubMed] [Google Scholar]
- Cohen S. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:386–396. [PubMed] [Google Scholar]
- Dabrowska J. Neuroanatomical evidence for reciprocal regulation of the corticotropin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: implications for balancing stress and affect. Psychoneuroendocrinol. 2011;36:1312–1326. doi: 10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhuria S.V. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J. Pharmaceutical Sci. 2010;99(4):1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- Dolan S.B. Potential for limited reinforcing and abuse-related subjective effects of intranasal oxytocin. J. Psychopharmacol. 2020;34(3):336–347. doi: 10.1177/0269881119867607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Vigneaud V. The synthesis of oxytocin. J. Am. Chem. Soc. 1954;76(12):3115–3121. [Google Scholar]
- Fox H. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacol. 2008;33:796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H., Sinha R. The role of guanfacine as a therapeutic agent to address stress-related pathophysiology in cocaine-dependent individuals. Adv. Pharmacol. 2014;69:217–265. doi: 10.1016/B978-0-12-420118-7.00006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. 1976. ECDU Assessment Manual for Psychopharmacology: revised (DHEW publication number ADM 76-338), Rockville, MD, US Department of Health, Education and Wellfare, Public Health Service, Alcohol, Drug Abuse and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs, pp: 534-537.
- Higgins S.T., Heil S.H., Dantona R., Donham R., Matthews M., Badger G.J. Effects of varying the monetary value of voucher-based incentives on abstinence during and following treatment among cocaine-dependent patients. Addiction. 2007;102(2):271–281. doi: 10.1111/j.1360-0443.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- Insel T. Is social attachment an addictive disorder? Physiol. Behav. 2003;79:351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Johnson B.A. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338–1346. doi: 10.1001/jamapsychiatry.2013.2295. [DOI] [PubMed] [Google Scholar]
- Kablinger A.S. Effects of the combination of metyrapone and oxazepam on cocaine cravings and cocaine taking: a double-blind, randomized, placebo-controlled pilot study. J. Psychopharmacol. 2012;26(7):973–981. doi: 10.1177/0269881111430745. [DOI] [PubMed] [Google Scholar]
- Kampangkaew J.P. Pharmacogenetic role of dopamine transporter (SLC6A3) variation on response to disulfiram treatment for cocaine addiction. Am. J. Addiction. 2019;28(4):311–317. doi: 10.1111/ajad.12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., Kreek M.J. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am. J. Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrubier Y. The MINI International Neuropsychiatric Interview. A short diagnostic structured interview: reliability and validity according to the CIDI. Eur. Psychiatry. 1997;12:224–231. [Google Scholar]
- Lee M.R. Complexity of oxytocin's effects in a chronic cocaine dependent population. Eur. Neuropsychopharmacol. 2014;24(9):1483–1491. doi: 10.1016/j.euroneuro.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.R., Weerts E.M. Oxytocin for the treatment of drug and alcohol use disorders. Behav. Pharmacol. 2016;27(8):640–648. doi: 10.1097/FBP.0000000000000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.R. Labeled oxytocin administered via the intranasal route reaches the brain in rhesus macaques. Nat Commun. 2020;11:2783–2793. doi: 10.1038/s41467-020-15942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros J.J. Inhibitory effects of oxytocin on corticotrope function in humans: are vasopressin and oxytocin ying-yang hormones? Psychoneuroendocrinol. 2001;26:649–655. doi: 10.1016/s0306-4530(01)00018-x. [DOI] [PubMed] [Google Scholar]
- Leong K. Oxytocin decreases cocaine taking, cocaine seeking, and locomotor activity in female rats. Exp. Clin. Psychopharmacology. 2016;24(1):55–64. doi: 10.1037/pha0000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong K. Oxytocin reduces cocaine cued fos activation in a regionally specific manner. Int. J. Neuropsychopharmacology. 2017;20(10):844–854. doi: 10.1093/ijnp/pyx058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I., Young E.A. Effects of stress and glucocorticoids on CNS oxytocin receptor binding. Psychoneuroendocrinol. 1997;22:411–422. doi: 10.1016/s0306-4530(97)00045-0. [DOI] [PubMed] [Google Scholar]
- Light K.C. Deficits in plasma oxytocin and increased negative affect, stress, and blood pressure in mothers with cocaine exposure during pregnancy. Addict. Behavior. 2004;29(8):1541–1564. doi: 10.1016/j.addbeh.2004.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.H. Association between blood level of plasma oxytocin and novelty seeking among methadone-maintained heroin users. Neuropsychobiology. 2015;71(2):65–69. doi: 10.1159/000371637. [DOI] [PubMed] [Google Scholar]
- Martin J.H. In: Neuroanatomy Text and Atlas. Martin J.H., editor. McGraw-Hill; New York: 2012. The hypothalamus and regulation of bodily function; pp. 355–383. editor. 4th ed. [Google Scholar]
- McGregor I.S. From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long term adverse consequences of drug abuse? British J. Pharmacol. 2008;154:358–368. doi: 10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark A. Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study. Psychopharmacology (Berl.) 2013;228:623–631. doi: 10.1007/s00213-013-3062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M.R., Argiolas A. Central oxytocinergic neurotransmission : a drug target for the therapy of psychogenic erectile dysfunction. Curr. Drug Targets. 2003;4:55–66. doi: 10.2174/1389450033347190. [DOI] [PubMed] [Google Scholar]
- Moeni M. The effects of oxytocin on withdrawal, craving, and stress response in heroin-dependent patients. A randomized, double blind clinical trial. Eur. Addict. Res. 2019;25:41–47. doi: 10.1159/000496194. [DOI] [PubMed] [Google Scholar]
- Morales-Rivera A. Anxiolytic effect of oxytocin in cue-induced cocaine seeking behavior in rats. Psychopharmacol. 2014;231(21):4145–4155. doi: 10.1007/s00213-014-3553-y. [DOI] [PubMed] [Google Scholar]
- NIDA . National Institute on Drug Abuse; 2020. What is the Scope of Cocaine Use in the United States? June 11 2020, [Google Scholar]
- O'Brien C.P. Classical conditioning in drug-dependent humans. Ann. N.Y. Acad. Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y. A spatiotemporal profile of in vivo cerebral blood flow changes following intranasal oxytocin in humans. Biol. Psychiatry. 2016;79:693–705. doi: 10.1016/j.biopsych.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Raby W.N., 2008. Pharmacological Treatment of Substance Abuse Disorders. In Tasman A, Kay J, Lieberman JA, First MD and Mai M. eds. Psychiatry, third edition, Chichester, England, John Wiley & Sons, 2390-2416.
- Robinson S.M. Reliability of the TimeLine Follow-Back for cocaine, cannabis, and cigarette use. Psychol. Addict. Behav. 2012 doi: 10.1037/a0030992. doi: 10.1037/a0030992, accessed 05/30/2018. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z. Effects of cocaine on the contents of neurohypophysial hormones in the plasma and in different brain structures in rats. Neuropeptides. 1992;23:27–31. doi: 10.1016/0143-4179(92)90006-i. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z.K.G. Oxytocin in learning and addiction: from early discoveries to the present. Pharmacol., Biol. Behav. 2014;119:3–9. doi: 10.1016/j.pbb.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Schluger J.H. Altered HPA axis responsivity to metyrapone testing in methadone-maintained former heroin addicts with ongoing cocaine addiction. Neuropsychopharmacol. 2001;24(5):568–575. doi: 10.1016/S0893-133X(00)00222-0. [DOI] [PubMed] [Google Scholar]
- Shorter D., Kosten T. Novel pharmacotherapeutics for cocaine addiction. BMC Med. 2011;9:119–126. doi: 10.1186/1741-7015-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Stress-induced cocaine craving and hypothalamo-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch. Gen. Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sivukhina E.V. Effects of chronic alcohol disease on magnocellular and parvocellular hypothalamic neurons in men. Horm. Metab. Res. 2006;38:382–390. doi: 10.1055/s-2006-944522. [DOI] [PubMed] [Google Scholar]
- Stauffer C.S. A two-week pilot study of intranasal oxytocin for cocaine-dependent individuals receiving methadone maintenance treatment for opioid use disorder. Addict. Res. Theory. 2016;24(6):490–498. doi: 10.3109/16066359.2016.1173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y. Smell identification test as an indicator of cognitive impairment in Alzheimer's disease. Int J. Geriatric Psychiatry. 2004;19:727–733. doi: 10.1002/gps.1161. [DOI] [PubMed] [Google Scholar]
- Theodosis D.T. Oxytoxin-secreting neurons: a physiological model of morphological neuronal and glial plasticity in the adult hypothalamus. Front. Neuroendocrinol. 2002;23:101–135. doi: 10.1006/frne.2001.0226. [DOI] [PubMed] [Google Scholar]
- Tribollet E. 1992. Vasopressin and oxytocin receptors in the rat brain. In: Bjorklund A, Hokfelt T and Kuhar MJ (eds), Handbook of Chemical Neuroanatomy, vol 11: Neuropeptide receptors in the CNS, Elsevier, pp. 289-320.
- Volkow N.D. Cocaine cues and dopamine in the dorsal striatum: mechanism of craving in cocaine addiction. J. Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R.D. Early prediction of initiation of abstinence from cocaine: use of a craving questionnaire. Am. J. Addiction. 1997;6(3) [PubMed] [Google Scholar]
- Windle R.J. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal axis. J. Neurosci. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.B. A structured interview guide for the Hamilton Depression Rating Scale. Arch. Gen. Psychiatry. 1988;45(8) doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- Young K.A. The role of mesocorticolimbic dopamine in regulating interactions between drugs of abuse and social behavior. Neurosci. Biobehav. Rev. 2011;35:498–515. doi: 10.1016/j.neubiorev.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. Drug-induced and genetic alterations in stress-responsive systems: implications for specific addictive diseases. Brain Res. 2010;1314:235–252. doi: 10.1016/j.brainres.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L. Oxytocin reduces cocaine seeking and reverses chronic cocaine-induced changes in glutamate receptor function. Int. J. Neuropsychopharmacol. 2014;1:1–11. doi: 10.1093/ijnp/pyu009. [DOI] [PMC free article] [PubMed] [Google Scholar]