Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of the worldwide coronavirus (COVID-19) pandemic, has infected an estimated 525 million people with over 6 million deaths. Although COVID-19 is primarily a respiratory disease, an escalating number of neurologic symptoms have been reported in humans. Some neurologic symptoms, such as loss of smell or taste, are mild. However, other symptoms, such as meningoencephalitis or stroke, are potentially fatal. Along with surveys and postmortem evaluations on humans, scientists worked with several animal species to try to elucidate the causes of neurologic symptoms. Neurologic sequelae remain challenging to study due to the complexity of the nervous system and difficulties in identification and quantification of neurologic signs. We reviewed animal models used in the study of neurologic COVID-19, specifically research in mice, hamsters, ferrets, and nonhuman primates. We summarized findings on the presence and pathologic effects of SARS-CoV-2 on the nervous system. Given the need to increase understanding of COVID-19 and its effects on the nervous system, scientists must strive to obtain new information from animals to reduce mortality and morbidity with neurologic complications in humans.
Abbreviations and Acronyms: ACE2, angiotensin-converting enzyme 2; BBB, blood brain barrier; dpi, days post infection; hACE2, human angiotensin-converting enzyme 2; IHC, immunohistochemistry; K18-hACE2, keratin 18 humanized angiotensin-converting enzyme 2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease of 2019
Introduction
Since its identification in late 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spread rapidly to become a global pandemic, with 83 million coronavirus disease 19 (COVID-19) cases and over 1 million deaths in the United States as of May 29, 2022.11 Although SARS-CoV-2 predominantly affects the respiratory system, neurologic manifestations such as smell and taste dysfunction (ageusia/hypogeusia [complete or partial loss of taste] and anosmia/hyposmia [complete or partial loss of smell]), headaches, dizziness, seizures, delirium, altered mental status, neuropsychiatric disorders, ataxia, and strokes have also been documented (Figure 1).13,36,51,69,70 In case series and cohort studies, clinical signs related to the nervous system are reported in 14% to 57% of hospitalized patients with COVID-19.36,51,69,70 According to a global study on neurologic manifestations of COVID-19, 80% of hospitalized patients reported at least one neurologic symptom, with headache (37%) as the most common.14 Among nonhospitalized COVID-19 patients with mild illness, neurologic and psychiatric symptoms are also common, with 55% reporting fatigue, 52% loss of smell, 45% loss of taste, and 44% headache.69 In addition, olfactory and gustatory dysfunction are experienced by 5% to 88% of patients, depending on the study.28,47,51,70,81 Other peripheral nervous system manifestations include vision impairment, peripheral neuropathies, Guillain-Barré syndrome, and dysautonomia.14,51,70 If SARS-CoV-2 infects the medulla oblongata, brainstem dysfunction may contribute to both respiratory and heart failure in patients.55 Overall, neurologic clinical signs may indicate SARS-CoV-2 neurotropism, and clinical signs can persist well beyond clearance of initial infection. A global meta-analysis and systematic review revealed that 34% of nonhospitalized patients experience neurologic COVID symptoms beyond 3 mo after infection.11 Consequently, animal models are necessary to study and establish treatments for neurologic manifestations of COVID.
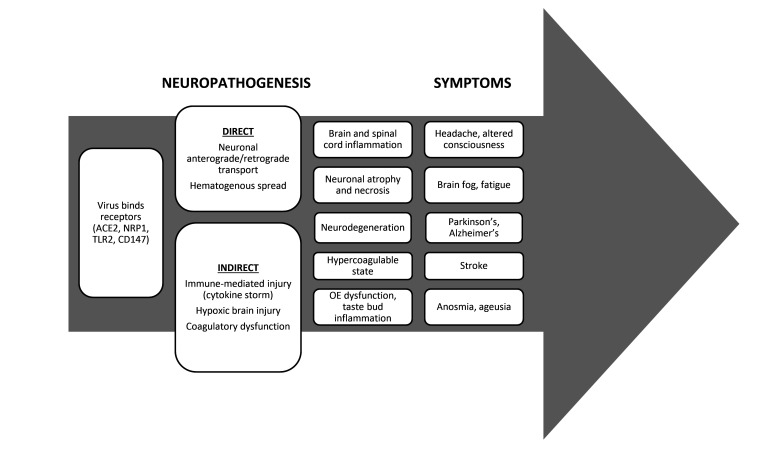
Figure 1.
Overview of hypothesized COVID-19 mechanisms of neuropathogenesis and symptoms observed in humans. Abbreviations, ACE2: angiotensin-converting enzyme 2, NRP1: neuropilin 1, TLR2: toll-like receptor 2, OE: olfactory epithelium.
Pathogenic Effects and Potential Mechanisms of Neurologic COVID-19
Despite the prevalence of neurologic signs, a clear pathophysiologic mechanism has not yet been elucidated for the betacoronavirus SARS-CoV-2. The route of central nervous system (CNS) invasion and viral distribution are fundamental to the diagnosis, prognosis, and treatment of neurologic COVID-19. SARS-CoV-2 binds various receptors including angiotensin-converting enzyme 2 (ACE2), neuropilin 1 (NRP1), toll-like receptor 2 (TLR2), CD147, and others (Figure 1).24,85,102 The viral surface spike glycoprotein binds to receptors to facilitate viral entry into host cells.24 ACE2 receptor expression occurs in all endothelia, especially in the CNS vasculature and on the sustentacular cells of the olfactory system.3 Olfactory receptor neurons rarely express ACE2.7,8 Because ACE2 expression is low overall in the CNS and olfactory neurons, neurologic symptoms are unlikely to be caused by viral ACE2-binding alone.85
Possible mechanisms contributing to COVID-19 neuropathogenesis include direct viral infection of brain tissue or indirect effects on the brain (Figure 1). Direct invasion could occur through olfactory nerves, a damaged blood-brain barrier (BBB), or infiltration of infected leukocytes.27 After oronasal exposure, the virus is hypothesized to invade the olfactory epithelium, which contains supporting sustentacular cells and olfactory sensory neurons.18 The virus may then travel retrogradely from olfactory sensory neurons to the olfactory bulb.18 Other possible routes include retrograde transport through the vagus nerve from the lungs or gastrointestinal tract.24 Indirect mechanisms of neuropathology include hypoxia, immune-mediated damage caused by cytokine storms, and coagulation dysfunction.27 Human studies have revealed a substantial role for inflammation in the pathogenesis of COVID-19 disease.1,6,24,27,32 Acute cerebral vascular disease reflects the hypercoagulable state recognized in severe COVID-19 cases.27 Potential mechanisms of stroke include a prothrombotic state, hyperinflammatory response, cardiomyopathy, vasculitis, and endothelial injury from direct viral invasion.27 A prothrombotic state may be related to an increase in adhesion molecule levels with elevated cytokines and reduced fibrinolysis.27 In humans, a direct link between brain inflammation and the presence of SARS-CoV-2 RNA has not yet been established.29 Consequently, controlled infection studies in animals are critical. Multiple animal models have been used to study the mechanisms and routes of nervous system infection of COVID-19. We reviewed experimental animal models used to study nervous system involvement in COVID-19 in mice, hamsters, ferrets, and nonhuman primates (NHPs; Figure 2). The different animal models enable studies of the neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2 during the course of infection. Models allow evaluation of the effects of virus dose, signalment, and comorbidities on the pathogenesis in a controlled manner. Significant species differences in the course of neurologic disease require researchers to choose the model that is most suitable for specific research questions.
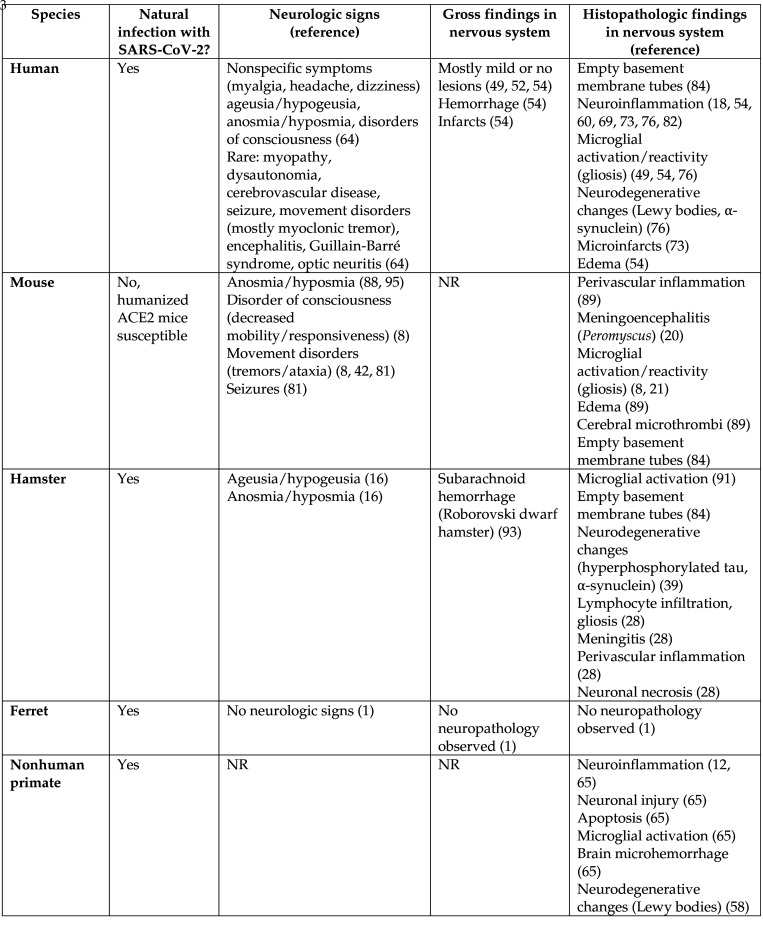
Figure 2.
Chart comparing animal models of COVID to the human condition, and relevant neurologic findings. Abbreviations: NR, none reported.
Neurologic COVID-19 in Humans
While the majority of patients with COVID-19 experience mild or moderate respiratory disease, extrapulmonary manifestations like neurologic symptoms are increasingly being recognized.14,27,36,47,51,70,75,81 The most common neurologic manifestations include alterations in taste and smell, headache, altered mental status, and neuropsychiatric disorders.27 Neurologic manifestations are more common in adults, yet are also seen in children, especially those with multisystem inflammatory syndrome.31 Symptoms in children include headache, altered mental status, seizures, stroke, anosmia, and ageusia.31 Sex differences also exist: females report anosmia, dysgeusia, weakness, and myalgia more frequently than males.61 The risk of ischemic stroke among COVID-19 patients is approximately 5%, which is higher than the risk for hemorrhagic stroke.64 In utero infection does not result in neurodevelopmental differences at 6 mo, but infants born during the pandemic scored lower on gross and fine motor assessments, possibly due to external maternal stressors.77 Some infected humans display symptoms resembling those of neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease.62,85 Brain fog and long COVID-19 syndrome are terms coined to describe long-term cognitive effects that humans may experience after SARS-CoV-2 infection.32 Long COVID-19 can affect 34% of nonhospitalized patients and 54% of hospitalized patients.12 Hypoxemia, systemic inflammation, coagulopathy, and neuroinvasion have been implicated in hospitalized patients who develop encephalopathy; in the postinfectious state, autoimmune mechanisms may also contribute to long COVID.32 Along with studies in animals, human autopsy research enhances our understanding of COVID-19 neuropathogenesis and its link to the onset of neurologic signs.
Like the variety of neurologic signs associated with COVID-19 infection, autopsy findings in the nervous system also vary (Figures 1 and 2). In a review of 142 human autopsies in fatal COVID-19, 65% of the brain examinations had no significant gross findings while 83% had microscopic changes.57 Most studies found no or mild neuropathologic lesions.52,57,79 When lesions were present, hemorrhage was a common gross finding, ranging in size from petechiae to large cerebral or cerebellar hemorrhages.57 In several studies, pathologists reported cerebral infarcts or acute hypoxic ischemic damage.20,57 Other brain findings included neuronal atrophy and necrosis, cerebral edema, and meningeal congestion.20,57,79 Brains of infected patients had higher numbers of empty basement membrane tubes.94 Scientists associated these so-called string vessels with endothelial cell death, BBB disruption, and brain ischemia.94 In addition, inflammation was present in the brain to varying degrees.20,52,66,80,85,92 Autopsy studies revealed infiltration of macrophages, CD8+ T lymphocytes in perivascular regions, and widespread brain microglial activation.52,57,85 T cell lymphocytic infiltrates occurred in 57% of brains without concurrent vasculitis or meningoencephalitis.57 In another study, extensive brain inflammation primarily developed in the olfactory bulbs and medulla oblongata.75 Acute and chronic inflammation in the olfactory epithelium were present in 10% of cases.57
Along with macroscopic and microscopic examinations, the presence of SARS-CoV-2 RNA and protein in brain was evaluated, with mixed results. The virus was present at low levels in 6 brain sections from 5 patients79 and was absent in the brains of 18 patients.48 Another study found SARS-CoV-2 RNA in 53% of 40 autopsied brains, with 8 (20%) positive for both SARS-CoV-2 RNA and protein.52 However, 8 patients that were untested or tested negative for brain viral RNA had viral proteins detected by immunohistochemistry (IHC) in the medulla oblongata.52 Viral RNA was found in regions of microischemic infarcts, suggesting the possibility of neuroinvasion-associated ischemia and blood vessel changes.79,80 In a review of COVID-19 autopsies, viral RNA was detected in 10% to 82% of brains, depending on the study.57 Viral RNA was present in cranial nerves, neurons, glia, ependyma, blood vessel endothelium, or isolated cells of the brainstem.52,57,79 Virus was also sporadically observed in olfactory epithelia and olfactory bulbs.18,55 Despite reports of viral RNA in the nervous system, IHC to detect viral proteins in the brain or olfactory epithelium was negative in many human cases.41,52,75,79 Viral spike protein was detected in cortical neurons and cerebrovascular endothelium of 3 ventilated ICU patients.80 Detection of SARS-CoV-2 RNA in cerebrospinal fluid (CSF) is uncommon but has been reported.25,38,50 Further studies in humans are essential to understanding the link between neurologic signs, pathology, and viral presence.
Fewer reports of COVID-19 neuropathology exist as compared with general pathology reports because of the initial focus on the respiratory system, a longer formalin fixation time of 2 to 3 wk for the brain, and concerns over aerosolization with brain removal.57 Autopsy results can be challenging to interpret due to comorbidities. Pathologists described neurodegeneration as possible evidence of prior brain disease, making a postmortem link between COVID-19 and neurodegenerative diseases challenging.57 However, a preliminary analysis of SARS-CoV-2 patient brains revealed Lewy bodies, accumulations of phosphorylated α-synuclein, suggesting a possible link to Parkinson’s disease.85 Treatments for COVID-19 infection can also confound autopsy results. Ventilation can promote cerebral microbleeds, and steroids can modulate immune responses. Available data has an inherent bias because only the most severe, fatal cases are examined by autopsy. Studying neurologic disease in human COVID-19 patients provides several challenges: only a subset of the patient population has neuroinvasion and/or neurologic signs, time-course studies of neuroinvasion are unethical, technology to directly sample CNS tissues is invasive, and direct neuroinvasion of the brain is hard to distinguish from systemic viremia. Therefore, along with the ongoing evaluation of human neurologic tissue, animal studies are necessary to control the various factors involved in viral pathogenesis and outcome.
Mouse Models of Neurologic COVID-19
Several previous review articles have discussed the use of mice to study the immunopathogenesis, viral transmission, diagnosis, and therapeutics of the betacoronavirus SARS-CoV-2.9,44,90,104 Mice (Mus musculus) are naturally susceptible to another betacoronavirus called mouse hepatitis virus (MHV).16 Many betacoronaviruses, including MHV, manifest neuroinvasive tendencies through different mechanisms.3 MHV binds to target cells through a murine carcinoembryonic antigen-related adhesion molecule.95 Mice can be infected by certain variants of SARS-CoV-2,60,82 but the ACE2 receptor, the cellular receptor for SARS-CoC-2, has some differences in mice and humans.93 Several approaches have been used to increase mouse susceptibility to SARS-CoV-2 infection. The study of Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) and later Middle Eastern Respiratory Syndrome coronavirus (MERS-CoV) led to the generation of genetically engineered mice that express human ACE2 under different promoters, thus modeling human infection and disease in mice. One study of this type generated transgenic mice that express human ACE2 under the cytokeratin 18 promoter (K18-hACE2); these mice develop a rapid lethal infection after intranasal inoculation of SARS-CoV.53 The virus initially attacks the upper airway epithelia and eventually spreads to the brain.53 The original study on hACE2 mice under regulation by the mouse Ace2 promoter confirmed the pathogen responsible for COVID-19.5 Another transgenic mouse model for SARS-CoV-2 was HFH4-hACE2 in C3B6 mice; in this model, hACE2 expression is driven by a lung ciliated epithelial cell-specific hepatocyte nuclear factor-3/forkhead homologue 4 (HFH4) promoter.39 Due to concerns that SARS-CoV-2 could infect natural wildlife populations, scientists conducted studies in deer mice (Peromyscus maniculatus).22
Mice infected with COVID-19 exhibit a variety of neurologic clinical signs. Except for the Omicron variant, K18-hACE2 mice develop fatal disease associated with lethal neurodissemination after intranasal inoculation of SARS-CoV-2.10,29,39,45,59,65,80,96,108 In contrast, several other mouse models of SARS-CoV-2 (adeno associated virus [AAV]–mediated expression of hACE2 and hACE2 knock-in mice) display low to no lethality.5,23,35,65,80 The route of infection is important. Aerosol exposure of K18-hACE2 mice did not lead to fatal viral neuroinvasion whereas intranasal exposure led to fatal encephalitis.26 Intragastric administration of SARS-CoV-2 caused lung injury without invasion to the CNS.84 As in humans, mice can develop anosmia at 2 to 3 d after aerosol or intranasal inoculation.26,100,108 The social scent discrimination test assesses hyposmia/anosmia in mice.26 A larger proportion of of infected female mice developed anosmia occurred as compared with infected male mice, as is the case in women compared with men with COVID-19.61,108 Pretreatment with convalescent plasma prevented lethal disease, but not profound anosmia.108 After intranasal inoculation, 6-wk-old, K18-hACE2 mice displayed neurologic symptoms, such as tremors and ataxic gait, and all mice died by 6 days after inoculation.45 A recent study reported ataxia, tremors, decreased mobility/responsiveness, and decreased urine voiding in intranasally infected K18-hACE2 mice.10 Two distinct clinical phenotypes were reported in HFH4-hACE2 transgenic mice after intranasal inoculation, one characterized by neurologic symptoms and death by 6 days postinoculation (dpi) and the other by no clinical signs and survival.39 In another study of intranasally-infected hACE2 mice, neurologic signs from 5 to 7 dpi consisted of trembling and seizures.91 Intranasally inoculated deer mice (Peromyscus maniculatus) had viral meningoencephalitis without observed clinical signs.22
Viral expression was examined in the nervous system to study the aforementioned neurologic clinical signs. Brain infection after intranasal challenge depended on the type of promoter used to control expression of hACE2.4 Viral RNA was found in the brain of k18-hACE2 and HFH4-hACE2 mice at various acute time points after inoculation, based mostly on PCR.29,39,45,59,65,80,96,101,108 Conversely, mice expressing hACE2 under the control of the endogenous or exogenous murine ACE2 promoter or the cytomegalovirus (CMV) promoter only occasionally had virus in the brain.5,35,65,80,100,109 Intranasal inoculation of SARS-CoV-2 in K18-hACE2 mice was associated with viral neurodissemination and lethality independent of inoculation dose.10 The virus was first detected at the olfactory bulb at 4 dpi without evidence of viremia, suggesting axonal retrograde migration via the olfactory neuroepithelium as the entry point.10 Other studies further supported viral expression in both the brain and olfactory bulb.45,100,108 Intranasal infection of SARS-CoV-2 in K18-hACE2 mice induced encephalitis, with viral particles found in the nasal turbinate, eye, and olfactory bulb.45 SARS-CoV-2 inoculation of HFH4-hACE2 mice led to virus detection primarily in the lung, but also in the brain, heart, and eyes by RT-PCR.39 Overall, the high level of viral presence in the K18-hACE2 mouse brain conflicts with the occasional focal detection of the virus in human olfactory bulbs and brains.
The exact mechanism regulating SARS-CoV-2 neuroinvasion remains unclear. Studies show that ACE2 is involved in SARS-CoV-2 infection of neurons.80 However, in K18-hACE2 mice, ACE2 protein and mRNA levels are low in neurons despite viral presence.10 These findings suggest that ACE2 is likely not the sole host factor associated with neuroinvasion. SARS-CoV-2 localizes in vascular endothelial cells and crosses the BBB through disruption of cerebral basement membranes.107 Elevated inflammatory immune signatures also suggest that a proinflammatory immune response may contribute to neuropathogenesis as well as COVID-19 morbidity and mortality.22 The elevated inflammatory response could increase procoagulant factors and endothelial dysfunction, ultimately resulting in systemic embolism and ischemic stroke.86 Some mouse studies support retrograde axonal transport through the olfactory nerve as a possible route of neuroinvasion.10,26,108 In inoculated deer mice, the virus appeared to invade the brain via retrograde axonal transmission through the gustatory-olfactory-trigeminal pathway, with eventual compromise of the BBB.22 However, a previous study argues against the retrograde route of brain infection due to the timeline of viral expression after inoculation, conflicting findings in various studies, and limited methods of viral detection and localization.8 In summary, murine studies suggest a variety of methods of neuroinvasion.
In terms of pathologic changes, findings have consisted of vasculitis, encephalitis, and necrosis of varying degrees after intranasal inoculation with SARS-CoV-2. One study found vasculitis and denatured and necrotic neurons in the brains of infected K18-hACE2 mice.107 Another study reported spongiosis with neuronal degeneration and necrosis.10 In mAce2 Promoter-hACEs transgenic mice, vasculitis without encephalitis was observed in the brain.4 K18-hACE2 mice developed encephalitis, vasculitis, and meningitis by 5 dpi, with rare brain thromboses.29,45,65,96 Intranasal inoculation in K18-hACE2 mice led to accumulation of lymphocytes, monocytes, and eosinophils in the brain.26 Other pathologic changes reported in inoculated K18-hACE2 mice included lymphocytic perivascular cuffing, gliosis, edema, and rare cerebral microthrombi.101 The cerebral thrombi in mouse studies could model the ischemic strokes that occur in humans but a clear mechanism remains to be determined. AAV-hACE2 mice infected intranasally with a low dose developed a brain lesion with microglial reactivity selective to the white matter.23 Pathologic changes were also seen in the olfactory system. Suppurative rhinitis correlated with abundant intraepithelial SARS-CoV-2 protein and RNA.10 In deer mice, pathologic changes included fibrinosuppurative sinusitis with degeneration and inflammation of olfactory, ethmoidal, and maxillary nerves.22 Apart from intranasal inoculation, other routes of SARS-CoV-2 infection in mice have not been documented to cause neuropathology.
The use of genetically engineered mice to study the neuropathogenesis of COVID-19 disease has some limitations. Because hACE2 is not naturally expressed in mice, transgenic mice may express hACE2 in a very different cellular pattern than do humans.8 hACE2 expression can vary under the regulation of different promoters. The viral ability to invade the CNS may be affected by these factors. Consequently, mice display disproportionately high CNS infection rates, leading to fatal encephalitis, which rarely occurs in infected humans. Therefore, clinical data from transgenic mice should be interpreted cautiously. Nonetheless, mice have already been crucial in the understanding of COVID-19 neuropathogenesis and neuropathology.
Hamster Models of Neurologic COVID-19
Hamsters can be infected by various human respiratory viruses including human metapneumovirus, human parainfluenza virus 3, and influenza A virus, and they may support influenza transmission by contact or airborne routes.78 Clinical disease with SARS-CoV-2 depends on the hamster species and other experimental logistics. The golden Syrian hamster (Mesocricetus auratus) is a widely used experimental model, and supports replication of both the original SARS-CoV and SARS-CoV-2.78 Sequence alignment of the ACE2 protein among different animal species suggests that the spike protein of SARS-CoV-2 may interact more efficiently with the hamster ACE2 receptor than with the mouse ACE2 receptor.78 In addition to systemic and respiratory signs of COVID-19, hamsters develop the neurologic symptoms of ageusia/hypogeusia and anosmia/hyposmia. In addition to the more commonly used golden Syrian hamster, dwarf hamster species such as Phodopus roborovskii, P. campbelli, and P. sungorus are also susceptible to SARS-CoV-2. Specifically, the Roborovski dwarf hamster develops a rapid onset of fulminant clinical disease.88,105 Compared with wild type hamsters that naturally suppress infection and primarily show transient pathologic disturbances in the respiratory tract and nasal cavity, transgenic hamsters with a humanized ACE2 receptor (K18-hACE2) develop extensive damage to the CNS.30 As with the original SARS-CoV, in which clinical virulence depended on viral strain,67 different strains of SARS-CoV-2 also differ in virulence in hamsters.33
Like humans, hamsters infected with COVID-19 develop ageusia/hypogeusia and anosmia/hyposmia. In a sucrose preference test after intranasal inoculation, infected golden Syrian hamsters had no preference between sucrose water and unaltered water, while noninfected hamsters displayed a clear preference for sucrose-supplemented water.18 Infected hamsters also took longer to find hidden and buried food, and had a greater rate of failure to find food as compared with noninfected hamsters.18 However, motor function defects, as measured by the open field and painted footprint tests, were not detected.18 Unlike transgenic mice, hamsters have not been reported to display other neurologic signs, such as ataxia. Apart from intranasal inoculation, other routes of SARS-CoV-2 infection in hamsters have not been documented to cause neurologic clinical signs.
Viral expression was examined in the olfactory system to study the observed neurologic clinical signs. In the nasal cavity, the virus was detected in olfactory sensory neurons and sustentacular cells,18,106 as well as at the junction of the olfactory nerve and bulb.18 Viral RNA was detected in the vomeronasal organs and Steno’s gland ducts of K18-hACE2 hamsters.30 Some studies detected virus in olfactory bulbs of golden Syrian hamsters18 or K18-hACE2 hamsters,30 while others did not detect any virus.7,106 Similarly, some studies detected the virus in other parts of the brain of golden Syrian hamsters37,87 and K18-hACE2 hamsters,30 while others failed to detect virus.18 Roborovski dwarf hamsters had only a small number of copies of viral RNA detected in the brain compared with other organs, suggesting a lack of productive infection in the brain.88 In an ex vivo organotypic culture of hamster brainstem and cerebellum, transmission electron microscopy analysis showed that the virus is selective for neuronal subtypes: SARS-CoV-2 peripherally targeted granular neurons with a developed Golgi apparatus, but did not infect Purkinje cells.24 Viral genomic RNA was detected in K18-hACE2 hamster spinal cords from 3 to 5 dpi, but only in spinal cord neurons and not microglial cells.30 Conversely, viral antigen was detected in both neuronal and microglial cells in the brain.30
Pathologic changes have also been reported in hamsters. Intranasal and aerosol inoculation cause the highest degree of pathology, viral loads, and weight loss.63 In the nasal cavity of hamsters, SARS-CoV-2 causes loss of ciliation in the olfactory epithelium around 2 dpi, with regeneration at 14 dpi.7,18 Whether the loss of cilia is limited to just sustentacular cells7 or also occurs in olfactory sensory neurons18 is debatable. Other studies report a decrease in sustentacular cells and an increase in microglia and other immune cells,103 upregulation of select genes consistent with a proinflammatory environment,18 and downregulation of olfactory receptor genes.103 In the olfactory bulb, microglial activation42 and bulbar inflammation with upregulation of select genes18 were observed. Small cerebral vessels exhibited more empty basement membrane tubes, representing remnants of lost capillaries, which was consistent with findings in infected humans and mice.94 One study reported signature proteinopathies such as accumulation of hyperphosphorylated tau and α-synuclein in cortical neurons in the absence of overt inflammation and neurodegeneration, which contributed to progressive neuronal dusfunction.42 The Roborovski dwarf hamster had mild brain pathology with infiltration of lymphocytes and/or subarachnoid hemorrhage.105 K18-hACE2 hamsters had multifocal gliosis throughout the gray matter, meningitis, perivascular inflammation, and neuronal necrosis in the brainstem and spinal cord.30 Although hemorrhagic and ischemic infarcts have been reported in human COVID-19 patients,42 to our knowledge this has not been documented in hamsters. However, risk factors, including systemic inflammation and a hypercoagulable state,99 would likely predispose hamsters infected with SARS-CoV-2 to stroke. This possibility should be explored in the future.
Hamsters have an established history as a model of human respiratory viruses, and, when infected with SARS-CoV-2, they develop respiratory and neurologic signs that are consistent with those of humans. Hamsters show important clinical hallmarks such as anosmia, neurotropism, and vascular inflammation. Hamsters are an animal model of COVID-19 infection that closely mimics moderate disease in humans. In addition, the hamster ACE2 receptor binds tightly to the SARS-CoV-2 spike protein, making the hamster a valuable model for mechanistic studies of SARS-CoV-2 without the need to modify either the animal or the virus.17 As compared with other animal models such as transgenic mice expressing the human ACE2 receptor or NHPs, hamster studies may be more accessible and cost-effective.
Ferret Models of Neurologic COVID-19
Domestic ferrets (Mustela putorius furo) are susceptible to several different coronavirus infections including ferret systemic coronavirus, ferret enteric coronavirus, severe acute respiratory syndrome coronavirus 1, and COVID-19.83 Ferrets have several advantages as compared with other species for the study of COVID-19. Ferrets are naturally susceptible to SARS-CoV-2 without genetic modification, have lung physiology similar to that of humans, and are larger with a longer lifespan than rodents. Genetic variability in commercially available ferrets is higher than that of inbred mouse strains, which better reflects the human genetically diverse population. In contrast to hamsters, experimental infection of ferrets with SARS-CoV-2 typically results in absent or mild respiratory signs with limited evidence for effects on the nervous system.15,21,56,74,76,89 Unlike hamsters, ferrets have a low ACE2 binding score but are still susceptible to infection; therefore, ferret infections may use another mechanism.17 Clinical signs include fever, lethargy, ruffled fur, coughing, stertor, and inappetence,43,56,72,76 with no specific neurologic signs.2 Hemorrhagic or ischemic strokes have not been reported in ferrets, but high CCL8 levels in ferrets with SARS-CoV-2 infection could be associated with the marked cytokine surge that increases the risk for thrombotic events.6 Despite a paucity of neurologic signs, ferrets with SARS-CoV-2 infection provide insight into the virus’s effects on the nervous system.
Documentation of viral presence in the ferret brain suggests the possibility of CNS injury. The detection of tissue viral RNA varied but typically indicated respiratory tract localization with some systemic involvement. The levels of viral RNA in the upper respiratory tract peaked at 4 to 7 dpi,21,72 and viral RNA was also found in the brain at acute time points.2,21,56,74,89 In general, higher RNA levels were detected at earlier time points with a decrease over time.2,56,74,89 Brain areas evaluated differed among studies, with examples including the cerebrum, cerebellum, medulla oblongata, and olfactory bulb. Intranasal and intratracheal routes of inoculation both led to viral dissemination in the brain.89 However, ferrets with intranasal inoculation had higher brain viral RNA levels than did ferrets with intratracheal inoculation.89 Regardless of infection route, ferrets had no clinical signs or pathologic changes. Significantly, the presence of viral RNA in the brain is consistent with reports that SARS-CoV-2 can infect the CNS of humans.55,75,80 Moreover, the presence of viral RNA in ferret brains could reflect the symptoms described in humans. Localization in the olfactory bulb could explain the anosmia frequently experienced in human COVID-19 patients. Dissemination to the medulla oblongata could affect respiratory regulation.56 However, ferrets with viral RNA in the nervous system did not display observable COVID-19 neurologic signs or neuropathy.2 Therefore, the overall significance of viral RNA detection in ferret brains is unclear.
Despite the detection of viral RNA in the CNS in some ferret studies, neuroinvasion is not always replicable or verified with other methods. An analysis of ferret brains for viral RNA and virus titration in Vero E6 cells did not detect viral RNA or infectious virus.76 Low levels of viral RNA were found in the brain tissue of 2 ferrets21 and in another study of 12 ferrets,74 but virus isolation and detection of antigen by immunohistochemistry were not successful. Despite the presence of viral RNA in the nasal mucosa or medulla oblongata at 4 dpi, SARS-CoV-2 nucleoprotein antigen was not detected.15 Other studies used the 50% Tissue Culture Infectious Dose (TCID50) to quantify and assess the infectivity of the virus in cells.2,89 Although brain samples were positive by RT-PCR or viral subgenomic mRNA analysis, TCID50 revealed no infectious virus at any of the timepoints evaluated.2,89 Therefore, infectious virus production in the brain was either completely absent or occurred in the first few days. Alternatively, if the virus was present only in blood vessels or migrating immune cells, viral RNA detection may not be related to neuronal infection.8 Overall, inconsistent results in the central nervous system could be related to different methods; more studies are needed to explain these discrepancies.
Infection of olfactory neurons is also under debate in ferrets. In the nasal cavity, viral antigen and pathologic lesions are reported as either restricted to the respiratory mucosa or also impacting cells in the olfactory epithelium.2,15,21,74Antibodies for ACE2 were detected in both the respiratory and olfactory epithelium, which could suggest a mechanism for viral entry.46 Microscopy analysis of the nasal turbinates revealed viral protein and RNA in cells of the respiratory and olfactory mucosae, including olfactory neuronal cells.2,21 Antigen load was generally low in both the olfactory and respiratory epithelium.2 The presence of antigen-positive cells in the olfactory epithelium was not typically associated with a significant inflammatory response.2 In the olfactory bulb, some studies detected no viral RNA or antigen,21 whereas others did.2,56,89 Because infection of olfactory neurons and bulb may be related to anosmia experienced by some infected humans, future studies with ferrets should evaluate their sense of smell after SARS-CoV-2 infection.
The ferret is a suitable model for studying asymptomatic or mild human SARS-CoV-2 infection. Immunocompetent ferrets may not be suitable for studying severe COVID-19, but they can be used to model viral replication, immune responses, and asymptomatic infection after COVID-19 inoculation. Although ferrets do not develop stroke after SARS-CoV-2 inoculation, they have been used in models of late preterm hypoxic ischemic brain injuries.97 Many factors can influence the outcome of COVID-19 infection in ferrets including individual susceptibility, virus strain, infectious route, and infection dose. Virus was detected in ferret brain primarily by quantitative PCR, rather than immunocytochemistry, thus limiting the ability to determine the cellular source of viruses. Neuropathology has not been reported. Therefore, future studies are necessary to elucidate SARS-CoV-2 neuroinvasiveness in ferrets.
Models of Nonhuman Primate Neurologic COVID-19
Numerous studies have shown that several NHP species are susceptible to SARS-CoV-2 infection. COVID-19 research in NHPs has primarily used rhesus and cynomolgus macaques, with some studies in African green monkeys (AGM). Benefits of working with NHP models include their large size, genetic variability, close phylogenetic relationship with humans, and many physiologic parallels to humans during viral infections. All Old World primates are likely susceptible to SARS-CoV-2 infection due to their high ACE2 homology to humans.17 In general, NHPs exhibit minimal-to-moderate self-limiting clinical signs after SARS-CoV-2 inoculation. As in ferrets, no overt neurologic signs have been reported. Rhesus macaques displayed mild clinical disease similar to humans, with signs including fever, weight loss, occasional cough, and reduced activity and appetite.58 Disease is less pronounced in cynomolgus macaques.54 AGMs showed mild and varied clinical signs; decreased appetite was most common.98 In another study, infected rhesus macaques and AGMs demonstrated neuroinflammation, microhemorrhages with or without ischemic injury, brain hypoxia, and neuropathology that may contribute to long-term neurologic symptoms.71 Overall, studies show viral replication in the central nervous system with some reports of neuropathology.
NHP models provide a potential means to study neurotropism associated with SARS-CoV-2, but results vary between studies and between animals within individual studies. Studies on rhesus macaques, cynomolgus macaques, and AGMs detected viral RNA in several brain regions after viral inoculation.19,34,40,62,98 Inoculation route does not appear to be a major factor in viral presence in the brain; neuroinflammation was observed after conjunctival, intranasal, intracranial, aerosol, or multiple routes of inoculation.19,40,71 In one study, pathologic findings included neuronal death, glial hyperplasia, and edema in the olfactory bulb and cortex;40 microhemorrhages and abnormal neuronal morphology were found in another.71 Viral encephalitis seen in one subclinical macaque was confirmed with immunohistochemistry.14 However, other studies did not detect SARS-CoV-2 presence in the brain.14,19,49,58,68,71,73 SARS-CoV-2 viral antigen nucleoprotein was found in neurons, astrocytes, and microglia in a very small number of positive cells but electron microscopy failed to find viral particles in brain tissue sections.40 Subgenomic viral RNA analysis failed to detect active virus replication in brains, and antigen was not detectable by immunohistochemistry.62 Consequently, viral replication may be limited in the CNS; pathologic findings could be due to indirect mechanisms such as inflammatory cytokine upregulation. Differences in viral detection could be related to individual susceptibility, dose of SARS-CoV-2, or infection route.
Although the mechanism of CNS invasion is unclear, NHPs remain a highly relevant model for investigating COVID-19 neuropathogenesis. In rhesus macaques, SARS-CoV-2 invaded primarily via olfactory nerves to the olfactory bulb, followed by spread into the CNS and induction of neuroinflammation.40 However, neuronal transfer along olfactory nerves may not be the only pathway for viral penetration of the brain.8,40 Viral RNA appeared rapidly in CSF after nasal inoculation, providing a faster mode of infection than neuronal retrograde alone.8 In another study, multiple brain microhemorrhages without virus in blood vessels or parenchyma were most consistent with acute hypoxic injury, indicating a significant role for brain hypoxia in COVID-19 neuropathogenesis.71 Hypoxia, exaggerated immune responses, and direct virus injury to endothelial cells likely contributed to the observed microhemorrhages and ischemia.71 Furthermore, SARS-CoV-2 caused endothelial disruption and vascular thrombosis via upregulation of proinflammatory cytokines.1 The progression to vascular disease over time involved complement, macrophage, cytokine, and thrombosis cascades.1 Finally, a compromised BBB and immune cell infiltration may also be involved. One study supported a SARS-CoV-2-induced activated immune response in the brain at 2 to 5 wk after inoculation.62 Infiltrating T-cells in intraparenchymal brain tissues indicated BBB integrity may have been disrupted.62 One study reported histologic vascular changes in the brain, including endothelial cell hypertrophy, endotheliitis, and vascular inflammation, supporting an altered BBB.14 These changes were more prominent in hamsters.14 Overall, SARS-CoV-2 likely uses a variety of methods to infect the nervous system.
Along with neuropathogenesis, NHP models also help to explain human symptoms because viral presence in certain brain regions can be correlated with human symptoms. One study found viral protein in the medulla oblongata and hippocampus.40 Infection of the cardiorespiratory center in the medulla oblongata could contribute to death in severe cases,71 and another study reported irregular respiratory patterns in infected rhesus macaques under anesthesia.58 SARS-CoV-2 detection and histopathology in the hippocampus suggests that memory should be evaluated in COVID-19 patients,40 but memory has not yet been evaluated in NHPs. Intracellular Lewy bodies have been found in the midbrain of NHPs; these could be related to the development of Parkinson’s or Alzheimer’s disease in humans.62 Additional studies are necessary to determine if smell, taste, memory, and/or cognitive alternations occur in SARS-CoV-2-infected NHPs.
The susceptibility of NHPs to SARS-CoV-2 makes them useful preclinical models for studying SARS-CoV-2, and they are currently being used extensively for investigation of SARS-CoV-2 pathogenesis and for the development of interventions. NHPs may be ideal for understanding neuropathogenesis and potential long-term consequences of infection, even in the absence of severe respiratory disease.71 NHP studies with an extensive evaluation of the nervous system and correlated clinical signs are necessary to better understand the virus and develop effective therapies. A better understanding of SARS-CoV-2 neurotropism, the route of CNS invasion, and viral distribution are essential for the diagnosis, prognosis, and treatment of COVID-19.
Conclusion
As cases and deaths continue to rise in the COVID-19 pandemic, research with animals will remain indispensable. The literature on neurologic COVID-19 is growing but the significance of brain infection with SARS-CoV-2 in humans and various animal models has not yet been delineated. Some researchers propose neuroinvasion may be acutely lethal as soon as the brainstem becomes infected and respiratory centers shut down.10,24,45,80 Others hypothesize that brain infection may have little consequence due to the lack of a correlation between disease severity and evidence of the virus in brains.52,79 Golden hamsters, ferrets, and nonhuman primates typically exhibit mild disease despite having virus in brains, whereas Roborovski dwarf hamsters and hACE2 mice consistently develop severe disease with brain infections. Alternatively, brain infection may increase the long-term risk for neurodegenerative diseases due to chronic inflammation, as supported by human, hamster, and NHP studies.42,62,85 Along with neurodegenerative COVID-19 models, models must be developed for long COVID-19 syndrome in order to learn more about pathophysiology and treatments. One study proposed nonhuman primates may be ideal for studying long COVID-19,71 but chronic studies are needed. Unfortunately, the vague neurologic symptoms associated with this syndrome, such as brain fog and lightheadedness, may be hard to duplicate and study in animals. In general, studies should evaluate inoculated animals for anosmia/ageusia, neuropsychiatric disorders, and mobility changes. Animal models for COVID-19 peripheral nervous system involvement should also be developed and evaluated. Unlike hACE2 mice, hamsters, and nonhuman primates, the neuropathology of ferrets appears dissimilar to histopathology findings in humans (Figure 2). Consequently, ferrets may not be an appropriate model for mechanistic studies of neuroinvasive COVID-19. Appropriate animal models of SARS-CoV-2 infection are needed to better understand the mechanisms of neuropathogenesis and develop treatments.
Acknowledgments
We would like to acknowledge all healthcare workers and essential staff caring for human patients and research animals during the COVID-19 pandemic, and patients and animals who contribute research to help understand neurologic disease.
References
- 1.Aid M, Busman-Sahay K, Vidal SJ, Maliga Z, Bondoc S, Starke C, Terry M, Jacobson CA, Wrijil L, Ducat S, Brook OR, Miller AD, Porto M, Pellegrini KL, Pino M, Hoang TN, Chandrashekar A, Patel S, Stephenson K, Bosinger SE, Andersen H, Lewis MG, Hecht JL, Sorger PK, Martinot AJ, Estes JD, Barouch DH. 2020. Vascular disease and thrombosis in SARS-CoV-2-infected rhesus macaques. Cell 183:1354–1366. 10.1016/j.cell.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Au GG, Marsh GA, McAuley AJ, Lowther S, Trinidad L, Edwards S, Todd S, Barr J, Bruce MP, Poole TB, Brown S, Layton R, Riddell S, Rowe B, Soldani E, Suen WW, Bergfeld J, Bingham J, Payne J, Durr PA, Drew TW, Vasan SS. 2022. Characterisation and natural progression of SARS-CoV-2 infection in ferrets. Sci Rep 12:5680. 10.1038/s41598-022-08431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakhtazad A, Garmabi B, Joghataei MT. 2021. Neurological manifestations of coronavirus infections, before and after COVID-19: A review of animal studies. J Neurovirol 27:864–884. 10.1007/s13365-021-01014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao L, Deng W, Gao H, Xiao C, Liu J, Xue J, Lv Q, Liu J, Yu P, Xu Y, Qi F, Qu Y, Li F, Xiang Z, Yu H, Gong S, Liu M, Wang G, Wang S, Song Z, Liu Y, Zhao W, Han Y, Zhao L, Liu X, Wei Q, Qin C. 2020. Lack of reinfection in rhesus macaques infected with SARS-CoV-2. bioRxiv:2020.2003.2013.990226. 10.1101/2020.03.13.990226 [DOI]
- 5.Bao L, Deng W, Huang B, Gao H, Liu J, Ren L, Wei Q, Yu P, Xu Y, Qi F, Qu Y, Li F, Lv Q, Wang W, Xue J, Gong S, Liu M, Wang G, Wang S, Song Z, Zhao L, Liu P, Zhao L, Ye F, Wang H, Zhou W, Zhu N, Zhen W, Yu H, Zhang X, Guo L, Chen L, Wang C, Wang Y, Wang X, Xiao Y, Sun Q, Liu H, Zhu F, Ma C, Yan L, Yang M, Han J, Xu W, Tan W, Peng X, Jin Q, Wu G, Qin C. 2020. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 583:830–833. 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 6.Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, Jordan TX, Oishi K, Panis M, Sachs D, Wang TT, Schwartz RE, Lim JK, Albrecht RA, tenOever BR. 2020. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181:1036–1045. 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryche B, St Albin A, Murri S, Lacôte S, Pulido C, Ar Gouilh M, Lesellier S, Servat A, Wasniewski M, Picard-Meyer E, Monchatre-Leroy E, Volmer R, Rampin O, Le Goffic R, Marianneau P, Meunier N. 2020. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav Immun 89:579–586. 10.1016/j.bbi.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butowt R, Meunier N, Bryche B, von Bartheld CS. 2021. The olfactory nerve is not a likely route to brain infection in COVID-19: A critical review of data from humans and animal models. Acta Neuropathol 141:809–822. 10.1007/s00401-021-02314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caldera-Crespo LA, Paidas MJ, Roy S, Schulman CI, Kenyon NS, Daunert S, Jayakumar AR. 2022. Experimental models of COVID-19. Front Cell Infect Microbiol 11:792584. 10.3389/fcimb.2021.792584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carossino M, Kenney D, O'Connell AK, Montanaro P, Tseng AE, Gertje HP, Grosz KA, Ericsson M, Huber BR, Kurnick SA, Subramaniam S, Kirkland TA, Walker JR, Francis KP, Klose AD, Paragas N, Bosmann M, Saeed M, Balasuriya UBR, Douam F, Crossland NA. 2022. Fatal neurodissemination and SARS-CoV-2 tropism in K18-hACE2 mice is only partially dependent on hACE2 expression. Viruses 14:535. 10.3390/v14030535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2022. [Internet]. COVID data tracker. [Cited 29 May 2022]. Available at: https://covid.cdc.gov/covid-data-tracker/#datatracker-home.
- 12.Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. 2022. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: A meta-analysis and systematic review. The Journal of Infectious Diseases . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou SH-Y, Beghi E, Helbok R, Moro E, Sampson J, Altamirano V, Mainali S, Bassetti C, Suarez JI, McNett M. 2021. Global incidence of neurological manifestations among patients hospitalized with COVID-19—A report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium. JAMA Netw Open 4:e2112131. 10.1001/jamanetworkopen.2021.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choudhary S, Kanevsky I, Yildiz S, Sellers RS, Swanson KA, Franks T, Rathnasinghe R, Munoz-Moreno R, Jangra S, Gonzalez O, Meade P, Coskran T, Qian J, Lanz TA, Johnson JG, Tierney CA, Smith JD, Tompkins K, Illenberger A, Corts P, Ciolino T, Dormitzer PR, Dick EJ, Jr, Shivanna V, Hall-Ursone S, Cole J, Kaushal D, Fontenot JA, Martinez-Romero C, McMahon M, Krammer F, Schotsaert M, García-Sastre A. 2022. Modeling SARS-CoV-2: Comparative pathology in rhesus macaque and golden Syrian hamster models. Toxicol Pathol 50:280–293. 10.1177/01926233211072767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciurkiewicz M, Armando F, Schreiner T, de Buhr N, Pilchová V, Krupp-Buzimikic V, Gabriel G, von Köckritz-Blickwede M, Baumgärtner W, Schulz C, Gerhauser I. 2022. Ferrets are valuable models for SARS-CoV-2 research. Vet Pathol 59:661–672. 10.1177/03009858211071012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Compton SR. 2021. Overview of coronaviruses in veterinary medicine. Comp Med 71:333–341. 10.30802/AALAS-CM-21-000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damas J, Hughes GM, Keough KC, Painter CA, Persky NS, Corbo M, Hiller M, Koepfli K-P, Pfenning AR, Zhao H, Genereux DP, Swofford R, Pollard KS, Ryder OA, Nweeia MT, Lindblad-Toh K, Teeling EC, Karlsson EK, Lewin HA. 2020. Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proc Natl Acad Sci USA 117:22311–22322. 10.1073/pnas.2010146117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Melo GD, Lazarini F, Levallois S, Hautefort C, Michel V, Larrous F, Verillaud B, Aparicio C, Wagner S, Gheusi G, Kergoat L, Kornobis E, Donati F, Cokelaer T, Hervochon R, Madec Y, Roze E, Salmon D, Bourhy H, Lecuit M, Lledo PM. 2021. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med 13:eabf8396. 10.1126/scitranslmed.abf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng W, Bao L, Gao H, Xiang Z, Qu Y, Song Z, Gong S, Liu J, Liu J, Yu P, Qi F, Xu Y, Li F, Xiao C, Lv Q, Xue J, Wei Q, Liu M, Wang G, Wang S, Yu H, Chen T, Liu X, Zhao W, Han Y, Qin C. 2020. Ocular conjunctival inoculation of SARS-CoV-2 can cause mild COVID-19 in rhesus macaques. Nat Commun 11:4400. 10.1038/s41467-020-18149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doyle MF. 2022. Central nervous system outcomes of COVID-19. Transl Res 241:41–51. 10.1016/j.trsl.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everett HE, Lean FZX, Byrne AMP, van Diemen PM, Rhodes S, James J, Mollett B, Coward VJ, Skinner P, Warren CJ, Bewley KR, Watson S, Hurley S, Ryan KA, Hall Y, Simmons H, Núñez A, Carroll MW, Brown IH, Brookes SM. 2021. Intranasal infection of ferrets with SARS-CoV-2 as a model for asymptomatic human infection. Viruses 13:113. 10.3390/v13010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fagre A, Lewis J, Eckley M, Zhan S, Rocha SM, Sexton NR, Burke B, Geiss B, Peersen O, Bass T, Kading R, Rovnak J, Ebel GD, Tjalkens RB, Aboellail T, Schountz T. 2020. SARS-CoV-2 infection, neuropathogenesis and transmission among deer mice: Implications for spillback to New World rodents. PLoS Pathog. 17:e1009585. 10.1371/journal.ppat.1009585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Castañeda A, Lu P, Geraghty AC, Song E, Lee MH, Wood J, O'Dea MR, Dutton S, Shamardani K, Nwangwu K, Mancusi R, Yalçın B, Taylor KR, Acosta-Alvarez L, Malacon K, Keough MB, Ni L, Woo PJ, Contreras-Esquivel D, Toland AMS, Gehlhausen JR, Klein J, Takahashi T, Silva J, Israelow B, Lucas C, Mao T, Peña-Hernández MA, Tabachnikova A, Homer RJ, Tabacof L, Tosto-Mancuso J, Breyman E, Kontorovich A, McCarthy D, Quezado M, Vogel H, Hefti MM, Perl DP, Liddelow S, Folkerth R, Putrino D, Nath A, Iwasaki A, Monje M. 2022. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 185:2452–2468. 10.1016/j.cell.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferren M, Favède V, Decimo D, Iampietro M, Lieberman NAP, Weickert J-L, Pelissier R, Mazelier M, Terrier O, Moscona A, Porotto M, Greninger AL, Messaddeq N, Horvat B, Mathieu C. 2021. Hamster organotypic modeling of SARS-CoV-2 lung and brainstem infection. Nat Commun 12:5809. 10.1038/s41467-021-26096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franco P, Bellesi Y, Nocent E, Strappa A, Galeano ML. 2021. Detección de SARS-CoV-2 en líquido cefalorraquídeo en un paciente pediátrico. Reporte de un caso. [SARS-CoV-2 Detection in cerebrospinal fluid in a pediatric patient. Case report] Arch Argent Pediatr 119:e58–e60. [Article in Spanish]. 10.5546/aap.2021.e58. [DOI] [PubMed] [Google Scholar]
- 26.Fumagalli V, Ravà M, Marotta D, Di Lucia P, Laura C, Sala E, Grillo M, Bono E, Giustini L, Perucchini C, Mainetti M, Sessa A, Garcia-Manteiga JM, Donnici L, Manganaro L, Delbue S, Broccoli V, De Francesco R, D’Adamo P, Kuka M, Guidotti LG, Iannacone M. 2022. Administration of aerosolized SARS-CoV-2 to K18-hACE2 mice uncouples respiratory infection from fatal neuroinvasion. Sci Immunol 7:eabl9929. 10.1126/sciimmunol.abl9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galea M, Agius M, Vassallo N. 2022. Neurological manifestations and pathogenic mechanisms of COVID-19. Neurol Res 44:571–582. 10.1080/01616412.2021.2024732. [DOI] [PubMed] [Google Scholar]
- 28.Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, Rusconi S, Gervasoni C, Ridolfo AL, Rizzardini G, Antinori S, Galli M. 2020. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: A cross-sectional study. Clin Infect Dis 71:889–890. 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golden JW, Cline CR, Zeng X, Garrison AR, Carey BD, Mucker EM, White LE, Shamblin JD, Brocato RL, Liu J, Babka AM, Rauch HB, Smith JM, Hollidge BS, Fitzpatrick C, Badger CV, Hooper JW. 2020. Human angiotensin-converting enzyme 2 transgenic mice infected with SARS-CoV-2 develop severe and fatal respiratory disease. JCI Insight 5:e142032. 10.1172/jci.insight.142032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golden JW, Li R, Cline CR, Zeng X, Mucker EM, Fuentes-Lao AJ, Spik KW, Williams JA, Twenhafel N, Davis N, Moore JL, Stevens S, Blue E, Garrison AR, Larson DD, Stewart R, Kunzler M, Liu Y, Wang Z, Hooper JW. 2022. Hamsters expressing human angiotensin-converting enzyme 2 develop severe disease following exposure to SARS-CoV-2. MBio 13:e0290621. 10.1128/mbio.02906-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Govil-Dalela T, Sivaswamy L. 2021. Neurological effects of COVID-19 in children. Pediatr Clin North Am 68:1081–1091. 10.1016/j.pcl.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham EL, Clark JR, Orban ZS, Lim PH, Szymanski AL, Taylor C, DiBiase RM, Jia DT, Balabanov R, Ho SU, Batra A, Liotta EM, Koralnik IJ. 2021. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann Clin Transl Neurol 8:1073–1085. 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halfmann PJ, Iida S, Iwatsuki-Horimoto K, Maemura T, Kiso M, Scheaffer SM, Darling TL, Joshi A, Loeber S, Singh G, Foster SL, Ying B, Case JB, Chong Z, Whitener B, Moliva J, Floyd K, Ujie M, Nakajima N, Ito M, Wright R, Uraki R, Warang P, Gagne M, Li R, Sakai-Tagawa Y, Liu Y, Larson D, Osorio JE, Hernandez-Ortiz JP, Henry AR, Ciuoderis K, Florek KR, Patel M, Odle A, Wong L-YR, Bateman AC, Wang Z, Edara V-V, Chong Z, Franks J, Jeevan T, Fabrizio T, DeBeauchamp J, Kercher L, Seiler P, Gonzalez-Reiche AS, Sordillo EM, Chang LA, van Bakel H, Simon V, Alburquerque B, Alshammary H, Amoako AA, Aslam S, Banu R, Cognigni C, Espinoza-Moraga M, Farrugia K, van de Guchte A, Khalil Z, Laporte M, Mena I, Paniz-Mondolfi AE, Polanco J, Rooker A, Sominsky LA, Douek DC, Sullivan NJ, Thackray LB, Ueki H, Yamayoshi S, Imai M, Perlman S, Webby RJ, Seder RA, Suthar MS, García-Sastre A, Schotsaert M, Suzuki T, Boon ACM, Diamond MS, Kawaoka Y. 2022. SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters. Nature 603:687–692. 10.1038/s41586-022-04441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartman AL, Nambulli S, McMillen CM, White AG, Tilston-Lunel NL, Albe JR, Cottle E, Dunn MD, Frye LJ, Gilliland TH, Olsen EL, O’Malley KJ, Schwarz MM, Tomko JA, Walker RC, Xia M, Hartman MS, Klein E, Scanga CA, Flynn JL, Klimstra WB, McElroy AK, Reed DS, Duprex WP. 2020. SARS-CoV-2 infection of African green monkeys results in mild respiratory disease discernible by PET/CT imaging and shedding of infectious virus from both respiratory and gastrointestinal tracts. PLoS Pathog 16:e1008903. 10.1371/journal.ppat.1008903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassan AO, Case JB, Winkler ES, Thackray LB, Kafai NM, Bailey AL, McCune BT, Fox JM, Chen RE, Alsoussi WB, Turner JS, Schmitz AJ, Lei T, Shrihari S, Keeler SP, Fremont DH, Greco S, McCray PB, Jr, Perlman S, Holtzman MJ, Ellebedy AH, Diamond MS. 2020. A SARS-CoV-2 infection model in mice demonstrates protection by neutralizing antibodies. Cell 182:744–753. 10.1016/j.cell.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M, Anheim M, Meziani F. 2020. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med 382:2268–2270. 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imai M, Iwatsuki-Horimoto K, Hatta M, Loeber S, Halfmann PJ, Nakajima N, Watanabe T, Ujie M, Takahashi K, Ito M, Yamada S, Fan S, Chiba S, Kuroda M, Guan L, Takada K, Armbrust T, Balogh A, Furusawa Y, Okuda M, Ueki H, Yasuhara A, Sakai-Tagawa Y, Lopes TJS, Kiso M, Yamayoshi S, Kinoshita N, Ohmagari N, Hattori SI, Takeda M, Mitsuya H, Krammer F, Suzuki T, Kawaoka Y. 2020. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc Natl Acad Sci USA 117:16587–16595. 10.1073/pnas.2009799117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarius S, Pache F, Körtvelyessy P, Jelčić I, Stettner M, Franciotta D, Keller E, Neumann B, Ringelstein M, Senel M, Regeniter A, Kalantzis R, Willms JF, Berthele A, Busch M, Capobianco M, Eisele A, Reichen I, Dersch R, Rauer S, Sandner K, Ayzenberg I, Gross CC, Hegen H, Khalil M, Kleiter I, Lenhard T, Haas J, Aktas O, Angstwurm K, Kleinschnitz C, Lewerenz J, Tumani H, Paul F, Stangel M, Ruprecht K, Wildemann B. 2022. Cerebrospinal fluid findings in COVID-19: A multicenter study of 150 lumbar punctures in 127 patients. J Neuroinflammation 19:19. 10.1186/s12974-021-02339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang RD, Liu MQ, Chen Y, Shan C, Zhou YW, Shen XR, Li Q, Zhang L, Zhu Y, Si HR, Wang Q, Min J, Wang X, Zhang W, Li B, Zhang HJ, Baric RS, Zhou P, Yang XL, Shi ZL. 2020. Pathogenesis of SARS-CoV-2 in transgenic mice expressing human angiotensin-converting enzyme 2. Cell 182:50–58. 10.1016/j.cell.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiao L, Yang Y, Yu W, Zhao Y, Long H, Gao J, Ding K, Ma C, Li J, Zhao S, Wang H, Li H, Yang M, Xu J, Wang J, Yang J, Kuang D, Luo F, Qian X, Xu L, Yin B, Liu W, Liu H, Lu S, Peng X. 2021. The olfactory route is a potential way for SARS-CoV-2 to invade the central nervous system of rhesus monkeys. Signal Transduct Target Ther 6:169. 10.1038/s41392-021-00591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kantonen J, Mahzabin S, Mäyränpää MI, Tynninen O, Paetau A, Andersson N, Sajantila A, Vapalahti O, Carpén O, Kekäläinen E, Kantele A, Myllykangas L. 2020. Neuropathologic features of four autopsied COVID-19 patients. Brain Pathol 30:1012–1016. 10.1111/bpa.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Käufer C, Schreiber CS, Hartke A-S, Denden I, Stanelle-Bertram S, Beck S, Kouassi NM, Beythien G, Becker K, Schreiner T, Schaumburg B, Beineke A, Baumgärtner W, Gabriel G, Richter F. 2022. Microgliosis and neuronal proteinopathy in brain persist beyond viral clearance in SARS-CoV-2 hamster model. EBioMedicine 79:103999. 10.1016/j.ebiom.2022.103999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim YI, Kim SG, Kim SM, Kim EH, Park SJ, Yu KM, Chang JH, Kim EJ, Lee S, Casel MAB, Um J, Song MS, Jeong HW, Lai VD, Kim Y, Chin BS, Park JS, Chung KH, Foo SS, Poo H, Mo IP, Lee OJ, Webby RJ, Jung JU, Choi YK. 2020. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe 27:704–709. 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knight AC, Montgomery SA, Fletcher CA, Baxter VK. 2021. Mouse models for the study of SARS-CoV-2 infection. Comp Med 71:383–397. 10.30802/AALAS-CM-21-000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumari P, Rothan HA, Natekar JP, Stone S, Pathak H, Strate PG, Arora K, Brinton MA, Kumar M. 2021. Neuroinvasion and encephalitis following intranasal inoculation of SARS-CoV-2 in K18-hACE2 mice. Viruses 13:132. 10.3390/v13010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lean FZX, Núñez A, Spiro S, Priestnall SL, Vreman S, Bailey D, James J, Wrigglesworth E, Suarez-Bonnet A, Conceicao C, Thakur N, Byrne AMP, Ackroyd S, Delahay RJ, van der Poel WHM, Brown IH, Fooks AR, Brookes SM. 2022. Differential susceptibility of SARS-CoV-2 in animals: Evidence of ACE2 host receptor distribution in companion animals, livestock and wildlife by immunohistochemical characterisation. Transbound Emerg Dis 69:2275–2286. 10.1111/tbed.14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, Chekkoury-Idrissi Y, Hans S, Delgado IL, Calvo-Henriquez C, Lavigne P, Falanga C, Barillari MR, Cammaroto G, Khalife M, Leich P, Souchay C, Rossi C, Journe F, Hsieh J, Edjlali M, Carlier R, Ris L, Lovato A, De Filippis C, Coppee F, Fakhry N, Ayad T, Saussez S. 2020. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur Arch Otorhinolaryngol 277:2251–2261. 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee MH, Perl DP, Nair G, Li W, Maric D, Murray H, Dodd SJ, Koretsky AP, Watts JA, Cheung V, Masliah E, Horkayne-Szakaly I, Jones R, Stram MN, Moncur J, Hefti M, Folkerth RD, Nath A. 2021. Microvascular injury in the brains of patients with Covid-19. N Engl J Med 384:481–483. 10.1056/NEJMc2033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu S, Zhao Y, Yu W, Yang Y, Gao J, Wang J, Kuang D, Yang M, Yang J, Ma C, Xu J, Qian X, Li H, Zhao S, Li J, Wang H, Long H, Zhou J, Luo F, Ding K, Wu D, Zhang Y, Dong Y, Liu Y, Zheng Y, Lin X, Jiao L, Zheng H, Dai Q, Sun Q, Hu Y, Ke C, Liu H, Peng X. 2020. Comparison of nonhuman primates identified the suitable model for COVID-19. Signal Transduct Target Ther 5:157. 10.1038/s41392-020-00269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luis MB, Liguori NF, López PA, Alonso R. 2021. SARS-CoV-2 RNA detection in cerebrospinal fluid: Presentation of two cases and review of literature. Brain Behav Immun Health 15:100282. 10.1016/j.bbih.2021.100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. 2020. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 77:683–690. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, Mushumba H, Fitzek A, Allweiss L, Dandri M, Dottermusch M, Heinemann A, Pfefferle S, Schwabenland M, Sumner Magruder D, Bonn S, Prinz M, Gerloff C, Püschel K, Krasemann S, Aepfelbacher M, Glatzel M. 2020. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol 19:919–929. 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCray PB, Jr, Pewe L, Wohlford-Lenane C, Hickey M, Manzel L, Shi L, Netland J, Jia HP, Halabi C, Sigmund CD, Meyerholz DK, Kirby P, Look DC, Perlman S. 2007. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol 81:813–821. 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meekins DA, Gaudreault NN, Richt JA. 2021. Natural and experimental SARS-CoV-2 infection in domestic and wild animals. Viruses 13:1993. 10.3390/v13101993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, Laue M, Schneider J, Brünink S, Greuel S, Lehmann M, Hassan O, Aschman T, Schumann E, Chua RL, Conrad C, Eils R, Stenzel W, Windgassen M, Rößler L, Goebel HH, Gelderblom HR, Martin H, Nitsche A, Schulz-Schaeffer WJ, Hakroush S, Winkler MS, Tampe B, Scheibe F, Körtvélyessy P, Reinhold D, Siegmund B, Kühl AA, Elezkurtaj S, Horst D, Oesterhelweg L, Tsokos M, Ingold-Heppner B, Stadelmann C, Drosten C, Corman VM, Radbruch H, Heppner FL. 2021. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci 24:168–175. 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 56.Monchatre-Leroy E, Lesellier S, Wasniewski M, Picard-Meyer E, Richomme C, Boué F, Lacôte S, Murri S, Pulido C, Vulin J, Salguero FJ, Gouilh MA, Servat A, Marianneau P. 2021. Hamster and ferret experimental infection with intranasal low dose of a single strain of SARS-CoV-2. J Gen Virol 102:001567. 10.1099/jgv.0.001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mukerji SS, Solomon IH. 2021. What can we learn from brain autopsies in COVID-19? Neurosci Lett 742:135528. 10.1016/j.neulet.2020.135528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munster VJ, Feldmann F, Williamson BN, van Doremalen N, Pérez-Pérez L, Schulz J, Meade-White K, Okumura A, Callison J, Brumbaugh B, Avanzato VA, Rosenke R, Hanley PW, Saturday G, Scott D, Fischer ER, de Wit E. 2020. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature 585:268–272. 10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oladunni FS, Park JG, Pino PA, Gonzalez O, Akhter A, Allué-Guardia A, Olmo-Fontánez A, Gautam S, Garcia-Vilanova A, Ye C, Chiem K, Headley C, Dwivedi V, Parodi LM, Alfson KJ, Staples HM, Schami A, Garcia JI, Whigham A, Platt RN, 2nd, Gazi M, Martinez J, Chuba C, Earley S, Rodriguez OH, Mdaki SD, Kavelish KN, Escalona R, Hallam CRA, Christie C, Patterson JL, Anderson TJC, Carrion R, Jr, Dick EJ, Jr, Hall-Ursone S, Schlesinger LS, Alvarez X, Kaushal D, Giavedoni LD, Turner J, Martinez-Sobrido L, Torrelles JB. 2020. Lethality of SARS-CoV-2 infection in K18 human angiotensin-converting enzyme 2 transgenic mice. Nat Commun 11:6122. 10.1038/s41467-020-19891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan T, Chen R, He X, Yuan Y, Deng X, Li R, Yan H, Yan S, Liu J, Zhang Y, Zhang X, Yu F, Zhou M, Ke C, Ma X, Zhang H. 2021. Infection of wild-type mice by SARS-CoV-2 B.1.351 variant indicates a possible novel cross-species transmission route. Signal Transduct Target Ther 6:420. 10.1038/s41392-021-00848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pelà G, Goldoni M, Solinas E, Cavalli C, Tagliaferri S, Ranzieri S, Frizzelli A, Marchi L, Mori PA, Majori M, Aiello M, Corradi M, Chetta A. 2022. Sex-related differences in long-COVID-19 syndrome. J Womens Health (Larchmt) 31:620–630. 10.1089/jwh.2021.0411. [DOI] [PubMed] [Google Scholar]
- 62.Philippens IHCHM, Böszörményi KP, Wubben JAM, Fagrouch ZC, van Driel N, Mayenburg AQ, Lozovagia D, Roos E, Schurink B, Bugiani M, Bontrop RE, Middeldorp J, Bogers WM, de Geus-Oei L-F, Langermans JAM, Verschoor EJ, Stammes MA, Verstrepen BE. 2022. Brain inflammation and intracellular α–synuclein aggregates in macaques after SARS-CoV-2 infection. Viruses 14:776. 10.3390/v14040776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Port JR, Yinda CK, Owusu IO, Holbrook M, Fischer R, Bushmaker T, Avanzato VA, Schulz JE, Martens C, van Doremalen N, Clancy CS, Munster VJ. 2021. SARS-CoV-2 disease severity and transmission efficiency is increased for airborne compared to fomite exposure in Syrian hamsters. Nat Commun 12:4985. 10.1038/s41467-021-25156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qureshi AI, Abd-Allah F, Al-Senani F, Aytac E, Borhani-Haghighi A, Ciccone A, Gomez CR, Gurkas E, Hsu CY, Jani V, Jiao L, Kobayashi A, Lee J, Liaqat J, Mazighi M, Parthasarathy R, Steiner T, Suri MFK, Toyoda K, Ribo M, Gongora-Rivera F, Oliveira-Filho J, Uzun G, Wang Y. 2020. Management of acute ischemic stroke in patients with COVID-19 infection: Report of an international panel. Int J Stroke 15:540–554. 10.1177/1747493020923234. [DOI] [PubMed] [Google Scholar]
- 65.Rathnasinghe R, Strohmeier S, Amanat F, Gillespie VL, Krammer F, García-Sastre A, Coughlan L, Schotsaert M, Uccellini MB. 2020. Comparison of transgenic and adenovirus hACE2 mouse models for SARS-CoV-2 infection. Emerg Microbes Infect 9:2433–2445. 10.1080/22221751.2020.1838955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. 2020. Neuropathology of COVID-19: A spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol 140:1–6. 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roberts A, Lamirande EW, Vogel L, Jackson JP, Paddock CD, Guarner J, Zaki SR, Sheahan T, Baric R, Subbarao K. 2008. Animal models and vaccines for SARS-CoV infection. Virus Res 133:20–32. 10.1016/j.virusres.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rockx B, Kuiken T, Herfst S, Bestebroer T, Lamers MM, Oude Munnink BB, de Meulder D, van Amerongen G, van den Brand J, Okba NMA, Schipper D, van Run P, Leijten L, Sikkema R, Verschoor E, Verstrepen B, Bogers W, Langermans J, Drosten C, Fentener van Vlissingen M, Fouchier R, de Swart R, Koopmans M, Haagmans BL. 2020. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 368:1012–1015. 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rogers JP, Watson CJ, Badenoch J, Cross B, Butler M, Song J, Hafeez D, Morrin H, Rengasamy ER, Thomas L, Ralovska S, Smakowski A, Sundaram RD, Hunt CK, Lim MF, Aniwattanapong D, Singh V, Hussain Z, Chakraborty S, Burchill E, Jansen K, Holling H, Walton D, Pollak TA, Ellul M, Koychev I, Solomon T, Michael BD, Nicholson TR, Rooney AG. 2021. Neurology and neuropsychiatry of COVID-19: A systematic review and meta-analysis of the early literature reveals frequent CNS manifestations and key emerging narratives. J Neurol Neurosurg Psychiatry 92:932. [DOI] [PubMed] [Google Scholar]
- 70.Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, Sánchez-Larsen Á, Layos-Romero A, García-García J, González E, Redondo-Peñas I, Perona-Moratalla AB, Del Valle-Pérez JA, Gracia-Gil J, Rojas-Bartolomé L, Feria-Vilar I, Monteagudo M, Palao M, Palazón-García E, Alcahut-Rodríguez C, Sopelana-Garay D, Moreno Y, Ahmad J, Segura T. 2020. Neurologic manifestations in hospitalized patients with COVID-19. Neurology 95:e1060–e1070. 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rutkai I, Mayer MG, Hellmers LM, Ning B, Huang Z, Monjure CJ, Coyne C, Silvestri R, Golden N, Hensley K, Chandler K, Lehmicke G, Bix GJ, Maness NJ, Russell-Lodrigue K, Hu TY, Roy CJ, Blair RV, Bohm R, Doyle-Meyers LA, Rappaport J, Fischer T. 2022. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat Commun 13:1745. 10.1038/s41467-022-29440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ryan KA, Bewley KR, Fotheringham SA, Slack GS, Brown P, Hall Y, Wand NI, Marriott AC, Cavell BE, Tree JA, Allen L, Aram MJ, Bean TJ, Brunt E, Buttigieg KR, Carter DP, Cobb R, Coombes NS, Findlay-Wilson SJ, Godwin KJ, Gooch KE, Gouriet J, Halkerston R, Harris DJ, Hender TH, Humphries HE, Hunter L, Ho CMK, Kennard CL, Leung S, Longet S, Ngabo D, Osman KL, Paterson J, Penn EJ, Pullan ST, Rayner E, Skinner O, Steeds K, Taylor I, Tipton T, Thomas S, Turner C, Watson RJ, Wiblin NR, Charlton S, Hallis B, Hiscox JA, Funnell S, Dennis MJ, Whittaker CJ, Catton MG, Druce J, Salguero FJ, Carroll MW. 2021. Dose-dependent response to infection with SARS-CoV-2 in the ferret model and evidence of protective immunity. Nat Commun 12:81. 10.1038/s41467-020-20439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salguero FJ, White AD, Slack GS, Fotheringham SA, Bewley KR, Gooch KE, Longet S, Humphries HE, Watson RJ, Hunter L, Ryan KA, Hall Y, Sibley L, Sarfas C, Allen L, Aram M, Brunt E, Brown P, Buttigieg KR, Cavell BE, Cobb R, Coombes NS, Darby A, Daykin-Pont O, Elmore MJ, Garcia-Dorival I, Gkolfinos K, Godwin KJ, Gouriet J, Halkerston R, Harris DJ, Hender T, Ho CMK, Kennard CL, Knott D, Leung S, Lucas V, Mabbutt A, Morrison AL, Nelson C, Ngabo D, Paterson J, Penn EJ, Pullan S, Taylor I, Tipton T, Thomas S, Tree JA, Turner C, Vamos E, Wand N, Wiblin NR, Charlton S, Dong X, Hallis B, Pearson G, Rayner EL, Nicholson AG, Funnell SG, Hiscox JA, Dennis MJ, Gleeson FV, Sharpe S, Carroll MW. 2021. Comparison of rhesus and cynomolgus macaques as an infection model for COVID-19. Nat Commun 12:1260. 10.1038/s41467-021-21389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schlottau K, Rissmann M, Graaf A, Schön J, Sehl J, Wylezich C, Höper D, Mettenleiter TC, Balkema-Buschmann A, Harder T, Grund C, Hoffmann D, Breithaupt A, Beer M. 2020. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: an experimental transmission study. Lancet Microbe 1:e218–e225. 10.1016/S2666-5247(20)30089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schurink B, Roos E, Radonic T, Barbe E, Bouman CSC, de Boer HH, de Bree GJ, Bulle EB, Aronica EM, Florquin S, Fronczek J, Heunks LMA, de Jong MD, Guo L, du Long R, Lutter R, Molenaar PCG, Neefjes-Borst EA, Niessen HWM, van Noesel CJM, Roelofs J, Snijder EJ, Soer EC, Verheij J, Vlaar APJ, Vos W, van der Wel NN, van der Wal AC, van der Valk P, Bugiani M. 2020. Viral presence and immunopathology in patients with lethal COVID-19: A prospective autopsy cohort study. Lancet Microbe 1:e290–e299. 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, Liu R, He X, Shuai L, Sun Z, Zhao Y, Liu P, Liang L, Cui P, Wang J, Zhang X, Guan Y, Tan W, Wu G, Chen H, Bu Z. 2020. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 368:1016–1020. 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shuffrey LC, Firestein MR, Kyle MH, Fields A, Alcántara C, Amso D, Austin J, Bain JM, Barbosa J, Bence M, Bianco C, Fernández CR, Goldman S, Gyamfi-Bannerman C, Hott V, Hu Y, Hussain M, Factor-Litvak P, Lucchini M, Mandel A, Marsh R, McBrian D, Mourad M, Muhle R, Noble KG, Penn AA, Rodriguez C, Sania A, Silver WG, O’Reilly KC, Stockwell M, Tottenham N, Welch MG, Zork N, Fifer WP, Monk C, Dumitriu D. 2022. Association of birth during the COVID-19 pandemic with neurodevelopmental status at 6 months in infants with and without in utero exposure to maternal SARS-CoV-2 infection. JAMA Pediatr 176:e215563. 10.1001/jamapediatrics.2021.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sia SF, Yan LM, Chin AWH, Fung K, Choy KT, Wong AYL, Kaewpreedee P, Perera R, Poon LLM, Nicholls JM, Peiris M, Yen HL. 2020. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 583:834–838. 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, Adams G, Hornick JL, Padera RF, Jr, Sabeti P. 2020. Neuropathological features of Covid-19. N Engl J Med 383:989–992. 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S, Lu P, Weizman OE, Liu F, Dai Y, Szigeti-Buck K, Yasumoto Y, Wang G, Castaldi C, Heltke J, Ng E, Wheeler J, Alfajaro MM, Levavasseur E, Fontes B, Ravindra NG, Van Dijk D, Mane S, Gunel M, Ring A, Kazmi SAJ, Zhang K, Wilen CB, Horvath TL, Plu I, Haik S, Thomas JL, Louvi A, Farhadian SF, Huttner A, Seilhean D, Renier N, Bilguvar K, Iwasaki A. 2021. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med. 218:e20202135. 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spinato G, Fabbris C, Polesel J, Cazzador D, Borsetto D, Hopkins C, Boscolo-Rizzo P. 2020. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA 323:2089–2090. 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stone S, Rothan HA, Natekar JP, Kumari P, Sharma S, Pathak H, Arora K, Auroni TT, Kumar M. 2021. SARS-CoV-2 variants of concern infect the respiratory tract and induce inflammatory response in wild-type laboratory mice. Viruses 14:27. 10.3390/v14010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stout AE, Guo Q, Millet JK, de Matos R, Whittaker GR. 2021. Coronaviruses associated with the superfamily Musteloidea. mBio 12:e02873-20. 10.1128/mbio.02873-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun SH, Chen Q, Gu HJ, Yang G, Wang YX, Huang XY, Liu SS, Zhang NN, Li XF, Xiong R, Guo Y, Deng YQ, Huang WJ, Liu Q, Liu QM, Shen YL, Zhou Y, Yang X, Zhao TY, Fan CF, Zhou YS, Qin CF, Wang YC. 2020. A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe 28:124–133. 10.1016/j.chom.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Szabo MP, Iba M, Nath A, Masliah E, Kim C. 2022. Does SARS-CoV-2 affect neurodegenerative disorders? TLR2, a potential receptor for SARS-CoV-2 in the CNS. Exp Mol Med 54:447–454. 10.1038/s12276-022-00755-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang X, Zheng F. 2022. A review of ischemic stroke in COVID-19: Currently known pathophysiological mechanisms. Neurol Sci 43:67–79. 10.1007/s10072-021-05679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tostanoski LH, Wegmann F, Martinot AJ, Loos C, McMahan K, Mercado NB, Yu J, Chan CN, Bondoc S, Starke CE, Nekorchuk M, Busman-Sahay K, Piedra-Mora C, Wrijil LM, Ducat S, Custers J, Atyeo C, Fischinger S, Burke JS, Feldman J, Hauser BM, Caradonna TM, Bondzie EA, Dagotto G, Gebre MS, Jacob-Dolan C, Lin Z, Mahrokhian SH, Nampanya F, Nityanandam R, Pessaint L, Porto M, Ali V, Benetiene D, Tevi K, Andersen H, Lewis MG, Schmidt AG, Lauffenburger DA, Alter G, Estes JD, Schuitemaker H, Zahn R, Barouch DH. 2020. Ad26 vaccine protects against SARS-CoV-2 severe clinical disease in hamsters. Nat Med 26:1694–1700. 10.1038/s41591-020-1070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trimpert J, Vladimirova D, Dietert K, Abdelgawad A, Kunec D, Dökel S, Voss A, Gruber AD, Bertzbach LD, Osterrieder N. 2020. The Roborovski dwarf hamster is a highly susceptible model for a rapid and fatal course of SARS-CoV-2 infection. Cell Rep 33:108488. 10.1016/j.celrep.2020.108488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van de Ven K, van Dijken H, Wijsman L, Gomersbach A, Schouten T, Kool J, Lenz S, Roholl P, Meijer A, van Kasteren PB, de Jonge J. 2021. Pathology and immunity after SARS-CoV-2 infection in male ferrets is affected by age and inoculation route. Front Immunol 12:750229. 10.3389/fimmu.2021.750229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Veenhuis RT, Zeiss CJ. 2021. Animal Models of COVID-19 II. Comparative Immunology. ILAR J 62:17–34. 10.1093/ilar/ilab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vidal E, López-Figueroa C, Rodon J, Pérez M, Brustolin M, Cantero G, Guallar V, Izquierdo-Useros N, Carrillo J, Blanco J, Clotet B, Vergara-Alert J, Segalés J. 2022. Chronological brain lesions after SARS-CoV-2 infection in hACE2-transgenic mice. Vet Pathol 59:613–626. 10.1177/03009858211066841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.von Weyhern CH, Kaufmann I, Neff F, Kremer M. 2020. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet 395:e109. 10.1016/S0140-6736(20)31282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wan Y, Shang J, Graham R, Baric RS, Li F. 2020. Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. J Virol 94:e00127-20. 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wenzel J, Lampe J, Müller-Fielitz H, Schuster R, Zille M, Müller K, Krohn M, Körbelin J, Zhang L, Özorhan Ü, Neve V, Wagner JUG, Bojkova D, Shumliakivska M, Jiang Y, Fähnrich A, Ott F, Sencio V, Robil C, Pfefferle S, Sauve F, Coêlho CFF, Franz J, Spiecker F, Lembrich B, Binder S, Feller N, König P, Busch H, Collin L, Villaseñor R, Jöhren O, Altmeppen HC, Pasparakis M, Dimmeler S, Cinatl J, Püschel K, Zelic M, Ofengeim D, Stadelmann C, Trottein F, Nogueiras R, Hilgenfeld R, Glatzel M, Prevot V, Schwaninger M. 2021. The SARS-CoV-2 main protease M(pro) causes microvascular brain pathology by cleaving NEMO in brain endothelial cells. Nat Neurosci 24:1522–1533. 10.1038/s41593-021-00926-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Williams RK, Jiang GS, Holmes KV. 1991. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc Natl Acad Sci USA 88:5533–5536. 10.1073/pnas.88.13.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Winkler ES, Bailey AL, Kafai NM, Nair S, McCune BT, Yu J, Fox JM, Chen RE, Earnest JT, Keeler SP, Ritter JH, Kang LI, Dort S, Robichaud A, Head R, Holtzman MJ, Diamond MS. 2020. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol 21:1327–1335. 10.1038/s41590-020-0778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wood T, Moralejo D, Corry K, Fisher C, Snyder JM, Acuna V, Holden-Hunt A, Virk S, White O, Law J, Parikh P, Juul SE. 2019. A ferret model of inflammation-sensitized late preterm hypoxic-ischemic brain injury. J Vis Exp 153:e60131. 10.3791/60131. [DOI] [PubMed] [Google Scholar]
- 98.Woolsey C, Borisevich V, Prasad AN, Agans KN, Deer DJ, Dobias NS, Heymann JC, Foster SL, Levine CB, Medina L, Melody K, Geisbert JB, Fenton KA, Geisbert TW, Cross RW. 2021. Establishment of an African green monkey model for COVID-19 and protection against re-infection. Nat Immunol 22:86–98. 10.1038/s41590-020-00835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang SJ, Wei TC, Hsu CH, Ho SN, Lai CY, Huang SF, Chen YY, Liu SJ, Yu GY, Dou HY. 2021. Characterization of virus replication, pathogenesis, and cytokine responses in Syrian hamsters inoculated with SARS-CoV-2. J Inflamm Res 14:3781–3795. 10.2147/JIR.S323026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ye Q, Zhou J, He Q, Li R-T, Yang G, Zhang Y, Wu S-J, Chen Q, Shi J-H, Zhang R-R, Zhu H-M, Qiu H-Y, Zhang T, Deng Y-Q, Li X-F, Liu J-F, Xu P, Yang X, Qin C-F. 2021. SARS-CoV-2 infection in the mouse olfactory system. Cell Discov 7:49. 10.1038/s41421-021-00290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yinda CK, Port JR, Bushmaker T, Offei Owusu I, Purushotham JN, Avanzato VA, Fischer RJ, Schulz JE, Holbrook MG, Hebner MJ, Rosenke R, Thomas T, Marzi A, Best SM, de Wit E, Shaia C, van Doremalen N, Munster VJ. 2021. K18-hACE2 mice develop respiratory disease resembling severe COVID-19. PLoS Pathog 17:e1009195. 10.1371/journal.ppat.1009195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zalpoor H, Akbari A, Samei A, Forghaniesfidvajani R, Kamali M, Afzalnia A, Manshouri S, Heidari F, Pornour M, Khoshmirsafa M, Aazami H, Seif F. 2022. The roles of Eph receptors, neuropilin-1, P2X7, and CD147 in COVID-19-associated neurodegenerative diseases: inflammasome and JaK inhibitors as potential promising therapies. Cell Mol Biol Lett 27:10. 10.1186/s11658-022-00311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zazhytska M, Kodra A, Hoagland DA, Frere J, Fullard JF, Shayya H, McArthur NG, Moeller R, Uhl S, Omer AD, Gottesman ME, Firestein S, Gong Q, Canoll PD, Goldman JE, Roussos P, tenOever BR, Jonathan BO, Lomvardas S. 2022. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell 185:1052–1064. 10.1016/j.cell.2022.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zeiss CJ, Compton S, Veenhuis RT. 2021. Animal Models of COVID-19. I. Comparative Virology and Disease Pathogenesis. Ilar j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhai C, Wang M, Chung HJ, Hassan M, Lee S, Kim HJ, Hong ST. 2021. Roborovski hamster (Phodopus roborovskii) strain SH101 as a systemic infection model of SARS-CoV-2. Virulence 12:2430–2442. 10.1080/21505594.2021.1972201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang AJ, Lee AC, Chu H, Chan JF, Fan Z, Li C, Liu F, Chen Y, Yuan S, Poon VK, Chan CC, Cai JP, Wu KL, Sridhar S, Chan YS, Yuen KY. 2021. Severe acute respiratory syndrome coronavirus 2 infects and damages the mature and immature olfactory sensory neurons of hamsters. Clin Infect Dis 73:e503–e512. 10.1093/cid/ciaa995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang L, Zhou L, Bao L, Liu J, Zhu H, Lv Q, Liu R, Chen W, Tong W, Wei Q, Xu Y, Deng W, Gao H, Xue J, Song Z, Yu P, Han Y, Zhang Y, Sun X, Yu X, Qin C. 2021. SARS-CoV-2 crosses the blood-brain barrier accompanied with basement membrane disruption without tight junctions alteration. Signal Transduct Target Ther 6:337. 10.1038/s41392-021-00719-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zheng J, Wong LR, Li K, Verma AK, Ortiz ME, Wohlford-Lenane C, Leidinger MR, Knudson CM, Meyerholz DK, McCray PB, Jr, Perlman S. 2021. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 589:603–607. 10.1038/s41586-020-2943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou B, Thao TTN, Hoffmann D, Taddeo A, Ebert N, Labroussaa F, Pohlmann A, King J, Steiner S, Kelly JN, Portmann J, Halwe NJ, Ulrich L, Trüeb BS, Fan X, Hoffmann B, Wang L, Thomann L, Lin X, Stalder H, Pozzi B, de Brot S, Jiang N, Cui D, Hossain J, Wilson MM, Keller MW, Stark TJ, Barnes JR, Dijkman R, Jores J, Benarafa C, Wentworth DE, Thiel V, Beer M. 2021. SARS-CoV-2 spike D614G change enhances replication and transmissibility. Nature 592:122–127. 10.1038/s41586-021-03361-1. [DOI] [PubMed] [Google Scholar]