Abstract
The study of nonhuman primates (NHP) can provide significant insights into our understanding numerous infectious agents. The etiological agent of COVID-19, SARS-CoV-2 virus, first emerged in 2019 and has so far been responsible for the deaths of over 4 million people globally. In the frenzied search to understand its pathogenesis and immunology and to find measures for prevention and control of this pandemic disease, NHP, particularly macaques, are the preferred model because they manifest similar clinical signs and immunologic features as humans. However, possible latent, subclinical, and opportunistic infections not previously detected in animals participating in a study may obscure experimental results and confound data interpretations in testing treatments and vaccine studies for COVID-19. Certain pathophysiologic changes that occur with SARS-CoV-2 virus infection are similar to those of simian pathogens. The current review discusses numerous coinfections of COVID-19 with other diseases and describes possible outcomes and mechanisms in COVID-19 studies of NHP that have coinfections. Due to the urgency triggered by the pandemic, screening that is more rigorous than usual is necessary to limit background noise and maximize the reliability of data from NHP COVID-19 studies. Screening for influenza virus, selected respiratory bacteria, and regional endemic pathogens such as vector-borne agents, together with the animal’s individual exposure history, should be the main considerations in selecting a NHP for a COVID-19 study. In addition, because NHP are susceptible to the SARS-CoV-2 virus, management and surveillance measures should be established to prevent transmission to healthy animals from infected colony animals and husbandry staff. This review presents compiled data on the use of NHP in COVID-19 studies, emphasizing the need to create the most reliable NHP model for those studies by extensive screening for other pathogens.
Abbreviations: ABSL, animal biosafety level; ACE-2, angiotensin-converting enzyme; ARDS, acute respiratory distress syndrome; CNPRC, California National Primate Research Center; E, envelope; ESR, erythrocyte sedimentation rate; HAV, hepatitis A virus; HBV, hepatitis B virus; HGF hepatocyte growth factor; HTLV, human T-cell lymphotropic virus; IFN, interferon; IL, interleukin; IP, inducible protein; M, matrix; MCP, monocyte chemotactic proteins; MCSF, macrophage colony-stimulating factor; MIP, macrophage inflammatory protein; N, nucleocapsid; NSP, non-structural proteins; RdRp, RNA-dependent RNA polymerase; S, spike; SARS-CoV-2, Severe Acute Respiratory Syndrome-coronavirus-2; SFV, simian foamy virus; SOP, standard operating procedures; SRV/D, simian retrovirus type D; STLV, simian T-lymphotropic virus; TB, tuberculosis; TGF, transforming growth factor; TMPRSS2 transmembrane serine protease 2
Introduction
The coronavirus SARS-CoV-2 (Severe Acute Respiratory Syndrome-coronavirus-2) was first identified in November 2019 in China.41 Since that time, the human toll of the COVID-19 pandemic has heightened the need to perform studies using animals to develop a better understanding of the pathogenic and immunologic profile of coronaviruses.25
Studies in animals have historically provided invaluable information relevant to diseases that affect humans. Of the animals used to assess viral replication and the associated disease caused by SARS-CoV-2, the most promising results to date have been obtained from mice, hamsters, ferrets, and nonhuman primates (NHP). Other animals such as mink, cats, dogs, chickens, ducks, and fruit bats have shown less illness and lower susceptibility to infection even though they are thought to be directly related to the ecology and evolution of the current virus.46 NHP, due to their genetic, physiologic, and biochemical homology with humans, model clinical-pathologic events and immune responses associated with human pathogens more accurately by manifesting measurable in vivo symptoms, allowing the clinical assessment of the success or failure of vaccines.5,21 The scientific value of NHP research is fully recognized in various studies of infectious, degenerative, reproductive, and cardiovascular diseases.5,63 Vaccine trials with the SARS-Cov-2 virus in NHP have shown that NHP have significant immune responses and can produce neutralizing activities without pathologic change or any other type of side effect.14,17,32,52 Moreover, most authors state that rhesus monkeys (Macaca mulatta) are the most suitable NHP model for vaccine studies.12,17,38,66,76
To study COVID-19 in animals, either for a better understanding of the pathogenesis and immunologic mechanisms triggered by the virus or to establish the safety and efficacy of potential therapeutic and immunogenic compounds, numerous factors associated with possible latent, subclinical, and opportunistic infections must be considered. Such infections can interfere with experimental results related to clinical abnormalities, histopathologic lesions, physiologic parameter changes, and nonspecific immune responses.5,35 Certain microorganisms establish persistent infections with a broad spectrum of pathogenic parameters that depend directly on environmental factors or the host itself.35 Due to the immunocompromising effect of the SARS-CoV-2 virus, protocols for experimental infection with this virus may reactivate other previously unknown pathogens, leading to unexpected symptoms due to these unidentified pathogens. This scenario has highly negative effects on economics and science due to a possible loss of accurate data from the experiments.5,6
The selection of NHP for COVID-19 experiments must include a careful and detailed laboratory screening. Thus, before the transfer of the selected animals to facilities of animal biosafety level 3 or 4 (ABSL-3 or ABSL-4), individuals must undergo rigorous clinical and laboratory exams to document that they are free of SARS-CoV-2 virus75 and other potential pathogens that may alter the pathologic and immunologic responses characteristic of the virus.6 The risk of transmission of SARS-CoV-2 and other infectious agents by presymptomatic or asymptomatic carriers must be determined.6,23,74 Even before the pandemic, the Federation of European Laboratory Animal Science Associations (FELASA) provided guidance for health monitoring of Old and New World NHP by listing the main viral, bacterial and parasitic pathogens that should be tested based on to their respective zoonotic characteristics, degree of virulence, or ability to produce immunosuppression that could influence research results.6 The various experimental designs that use COVID-19 should incorporate preliminary screens to determine the most relevant agents for each project. Publications about simian models for COVID-19 studies show that more experimental infections are conducted on NHP originating from specific pathogen-free colonies (SPF).38,59,75 However, no regulation addresses the conduct of such research in non-SPF animals, which could be used if screening is comprehensive. In addition to precautions regarding screening in order to select study animals, the COVID-19 pandemic requires comprehensive health care and preventive measures in the NHP colony. Because NHP are susceptible to the SARS-CoV-2 virus, they are subject to cross contamination if such measures are not incorporated into a properly designed health surveillance program. This concern has been increasing due to constant viral mutations.9 The goal of these precautions is to protect institutions from this virus through the improvement of animal management and Standard Operating Procedures (SOP) for staff.57,75
Given the premise that research conducted with NHP can provide data to expand knowledge about the clinical and pathogenic characteristics of the SARS-CoV-2 virus, the present analysis aims to present information about the use of the NHP for COVID-19 studies beyond routine prophylactic and therapeutic strategies. Special emphasis is placed on detecting potential underlying infectious agents that may mask experimental results while also preventing transmission of the SARS-CoV-2 virus to healthy NHP and staff.
Pathogenesis and Immunology of the SARS-CoV-2 Virus in Humans and NHP
SARS-CoV-2 is a single-stranded RNA virus, characterized by two groups of proteins: i) Structural proteins, such as Spike (S) proteins, which target all coronaviruses and bind to receptors on the host cell; Nucleocapsid protein (N), which protects the genetic information of the virus, Matrix protein (M), and Envelope protein (E); and ii) Replicase Complex Encoding Non-Structural Proteins, such as proteases (nsp3 and nsp5) and RdRp (nsp12).79
The S protein present in the SARS-CoV-2 membrane is necessary for entry of the virus into the host cell by means of the angiotensin-converting enzyme 2 (ACE-2) receptor. After being released into the cytoplasm, the viral RNA genome is translated into two structural proteins and polyproteins, which assist in viral replication. After infection, the host cell activates the innate and adaptive immune response in an immediate and well-coordinated manner.51
The humoral immune response mediated by B cells is protective due to the production of neutralizing antibodies. Patients with COVID-19 have high levels of plasma chemokines and cytokines such as interleukins (IL- 1, IL-2, IL-4, IL-7, IL-10, IL-12, IL-13, and IL-17), interferon- γ inducible protein 10 (IP-10), macrophage colony-stimulating factor (MCSF), monocyte chemotactic proteins-1 (MCP-1), GCSF, hepatocyte growth factor (HGF), IFN-γ, macrophage inflammatory protein-1α (MIP-1α), and TNF-α, etc., in amounts that are associated with the severity of the disease.36
Lymphopenia and the ‘cytokine storm’ play a significant role in the pathogenesis of COVID-19. This ‘cytokine storm’ is responsible for the onset of viral sepsis, followed by inflammation-induced lung injury, which in turn is related to other complications such as acute respiratory distress syndrome (ARDS), pneumonia, respiratory failure, septic shock, organ failure, and potentially death.11,13,79
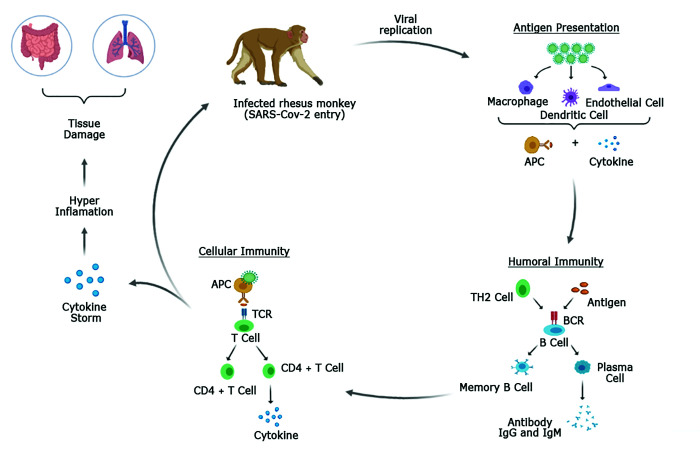
Likewise, in rhesus macaques, experimental infection by the SARS-CoV-2 virus promotes a rapid and transient change in innate immune responses and an increase in the number of auxiliary follicular CD4 T cells in peripheral blood.34 The pattern is similar to that of humans with regard to humoral and cellular immune responses, as assessed by the serologic profile and production of cytokines (MIP1β and IFNγ), respectively (Figure 1).12 Vaccine trials in rhesus monkeys showed the induction of high levels of neutralizing activity and Th1 responses with low to undetectable Th2 responses, as also occurs in humans.14
Figure 1.
Immunopathogenesis of SARS-CoV-2 virus in an experimentally infected rhesus monkey (Macaca mulatta). Adapted from Chatterjee and colleagues (2020).13 Scheme created by BioRender - http://BioRender.com.
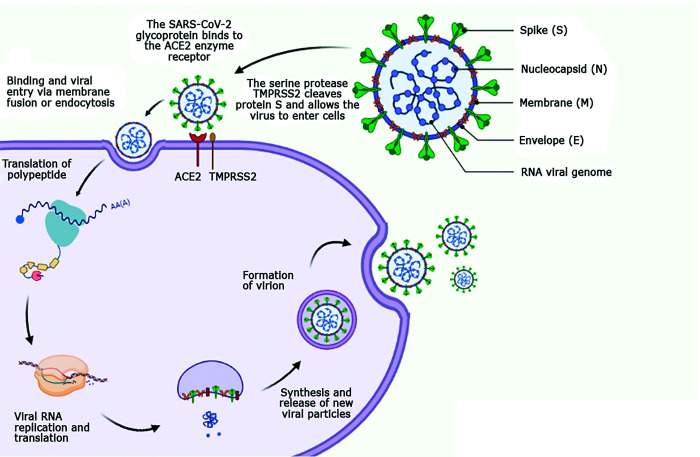
ACE-2 and TMPRSS2 Receptors in SARS-COV-2 Infection
Acute respiratory infections are responsible for the high rate of human and animal mortality and morbidity associated with viruses of the Coronaviridae family. The presence of the viral receptor in the host is a key determinant for infection by the virus.37 Since the 2002 emergence of the SARS-CoV-1 virus, numerous studies on the pathogenesis of acute respiratory syndrome (SARS) have revealed that infection with this coronavirus begins through the binding of protein S (spike) to the ACE-2 receptor (Figure 2). The ACE-2 receptor is a multifunctional peptide that facilitates the entry of the SARS-COV-2 virus into cell membranes and functions as a key receptor for infection.45
Figure 2.
Mechanism of action and life cycle of the SARS-CoV-2 virus, with emphasis on the entry site into the cell membrane through the ACE-2 and TMPRSS2 receptors. Adapted from Machhi and colleagues (2020)41 and Monteil and colleagues (2020).45 Scheme created by BioRender - http://BioRender.com.
The ACE-2 enzyme is widely found on the surface of the endothelium of several cell types in kidneys, intestines, heart, and lungs, with the latter being the most dominant site (type II alveolar cells).45 The expression of ACE-2 in the heart occurs through excessive activation of the renin-angiotensin system (RAS). Hypertension, congestive heart failure, and atherosclerosis are examples of pathologies that lead to stimulation of ACE-2.37 Less markedly, Serine Protease Transmembrane 2 (TMPRSS2) also has a role in infection with SARS-COV-2 virus. The TMPRSS2 (whose function is still unknown) is a cell surface protein expressed mainly by the endothelial cells of the respiratory (nasal and bronchial epithelia) and gastrointestinal tracts.58
Upon entering the body, SARS-CoV-2 binds through its S glycoprotein to its receptors (ACE-2 and TMPRSS2), which cleave protein S, allowing the virus to enter the cells. The cells then sequentially internalize the viral particle, fuse the membranes to release of the viral RNA, replicate and translate the viral RNA and, finally, synthesize and release new viral particles.58
For an animal to provide a model for a disease, the pathogen should be able to infect the animal using the same cellular receptor that it uses in humans and then successfully replicate inside the host. The infection should also induce clinical symptoms that are similar to those of humans; sometimes mild symptoms are also of interest by providing information about immune responses against the pathogen.16
Like humans, many animal species have the ACE-2 enzyme, which induces a cascade of enzymatic and protein activation, regulating the renin-angiotensin system in specific organs of the body, and providing a receptor for SARS-CoV-2.37 Analyzing the ACE-2 amino acid sequence in various animal species, including NHP, cats, dogs, ferrets, cattle, sheep, goats, pigs, horses, rodents, bats, etc., has verified that most species have similar sequences.37 With regard to NHP ACE-2, rhesus and cynomolgus (Macaca fascicularis) monkeys have the same amino acid sequence as humans, whereas this sequence differs in four specific amino acids in common marmosets (Calltithrix jacchus).38
Because the 12 key amino acid residues present in human ACE-2 are also present in ACE-2 of macaques,44 macaques are a close model system to humans, and therefore the choice of animal for vaccine testing against COVID-19. Studies with macaques have shown that they develop pulmonary infiltrates, which can be seen in radiographs, have high viral loads in swabs of nose and throat, and shed virus in the upper and lower respiratory tract.47,53 Taken together, the disease symptoms in rhesus macaques match with the milder symptoms of SARS-CoV-2 infection in humans. However, levels of the chemokines IL1ra, IL10, IL15, MCP-1, IL6, and TGFα are not significantly altered on days 1 and 3 after infection.47 SARS-CoV-2 affects older people more severely than young ones; similarly, older macaques show higher viral loads in the nose and throat and prolonged viral shedding in the upper respiratory tract.53 Thus, the rhesus monkeys are considered a suitable model for vaccine efficacy studies, as they have immunologic and clinical responses similar to those of humans, providing an opportunity to study how monkeys protect themselves from coronavirus.16
Most candidate vaccines for COVID-19 have been produced to induce antibody responses against protein S, facilitate the binding of the antigen to the ACE-2 receptor, and thus trigger virus-cell-to-cell-membrane fusion.14 Studies with different formulations of vaccines have already identified strong specific immune responses in NHP with high affinity for the ACE-2 receptor.14,32,66 In contrast, immunologic tests demonstrated that although elevated levels of the ACE-2 enzyme were found in the ciliated epithelium of the upper airways, marked expression of ACE-2 was not present in lungs infected with the SARS-CoV-2 virus either in humans or NHP. This unexpected finding requires further examination.60
Clinical Manifestations of COVID-19 and Potential Coinfections in Humans and NHP
Human patients commonly infected with COVID-19 present with fever, dry cough, tiredness, muscle pain, sore throat, headache, nasal congestion, conjunctivitis, loss of smell and taste, vomiting, diarrhea, and skin rashes. About 80% of confirmed SARS-CoV-2 infections have mild symptoms or are asymptomatic and most recover with no sequelae. About 15% of infections result in severe disease, requiring oxygen, and 5% are very serious infections that require assisted ventilation in a hospital environment. The most severe cases can progress to pneumonia, accompanied by severe respiratory failure, septicemia, organ failure, and progress to death. The signs of worsening of the disease can be sudden, appearing usually during the second week with shortness of breath, pain or pressure in the chest, blue tone in the fingers, and disturbances in speech and movement.70 Severe symptoms and mortality in humans are higher in people aged 60+ and thus has age-related pathology in humans. Older patients or those with comorbidities can develop severe, progressive disease and unfavorable outcomes.74
NHP infected experimentally by the SARS-CoV virus develop milder symptoms than those seen in humans.57 On the first day of infection, the animals exhibit changes in their breathing pattern, accompanied by a pulmonary infiltrate that remains for at least 1 wk. They may have mild or absent fever and weight loss,47,57 in addition to hematologic changes, such as leukocytosis and neutrophilia (like humans), with a return to normal values in a few days.57
To date, few natural COVID-19 infections have been reported in NHP. Three gorillas from the San Diego Zoo Safari Park, CA, USA, confirmed positive for SARS-CoV-2 in January 2021, were the first recorded cases of natural infection of the virus in NHP. After two of the gorillas in the social group developed a cough, COVID-19 infection was confirmed. Since then, the gorillas have been periodically monitored. Despite the careful protocols of the zoo, the transmission likely occurred through a staff member with an asymptomatic infection.64 The second report was at the Atlanta Zoo, GA, USA, where 13 gorillas tested positive for COVID-19, probably transmitted from a positive keeper with no clinical signs. In this case, the gorillas presented with runny nose, mild cough, and loss of appetite, but had no serious clinical consequences and recovered promptly.39 Thus, the main source of transmission to NHP has been from humans.57
During experimental infection, the genera Macaca and Cercopithecus, some species of Papio, and the neotropical species Callithrix jacchus showed signs of active infection, with variable clinical disease. Experimental studies on COVID-19 in NHP are still ongoing.24,75
Some infections can alter the host’s immune responses, and synergism with COVID-19 can contribute to a more severe clinical evolution.15 The coinfecting pathogens can be detected at variable intervals during the COVID-19 disease course and remain an important consideration in targeted treatment strategies for COVID-19 patients.33 In COVID-19 patients with Human Immunodeficiency Virus (HIV) coinfection, for instance, the lower level of CD4 + T lymphocyte counts caused by HIV infection can result in a relatively weak production of specific antibodies against SARS-CoV-2, which directly compromises the outcome of the COVID-19 disease.73 The immunologic impairment caused by simian retroviruses requires the elimination of simian retroviruses from cells or animals used in COVID-19 studies, as the results would be strongly confounded. However, much remains to be clarified about the immunologic interactions between HIV and SARS-CoV-2, and further studies are urgently required to resolve this lack of data.
Coinfection with Simian Immunodeficiency Virus (SIV) and SARS-CoV-2 can be studied as a model for HIV and COVID-19 coinfections in humans. Experimental infections of COVID-19 in NHP with latent microorganisms that have tropism for immune cells or for the gastrointestinal and respiratory systems directly alter experimental results. Some studies suggest that HIV1 infection induces IFN-I, which may to some extent curtail the SARS-CoV-2 infection, thus leading to persistently undetectable RNA.43 Also, a possible influence of HIV1-induced immune dysfunction on immune responses to and clearance of SARS-CoV-2, although HIV was not related directly to the occurrence of COVID-19.43 In addition, retroviral drugs routinely used to control HIV infection may help to prevent the infection by COVID-19.7,43 Therefore, despite the lack of a clear relationship between HIV and COVID-19 in terms of susceptibility and disease evolution, and given the contradictions that still require resolution, screening for simian retroviruses is important to eliminate any possible confounding effects in NHP studies of COVID-19.
Simian retroviruses can interfere with vaccine responses to several viral pathogens in NHP.35 In general retroviruses infect cells that mediate the immune response, and a subset of these viruses impair immune function. Old World NHP are often infected with the Simian T-Lymphotropic Virus (STLV), a retrovirus closely related to human T-cell lymphotropic viruses (HTLV-1 and 2). Its cellular tropism included both lymphoid and nonlymphoid tissues. The infection is usually subclinical and long-lasting. Cases of generalized lymphadenopathy and splenomegaly are associated with this virus. Persistent lymphocytosis and atypical lymphocytes are observed in infected NHP, with cachexia, gastroenteritis and diarrhea secondary bacterial infections, weight loss, and episodes of anemia and lymphopenia.5,67 One report suggests that patients infected with HTLV are more vulnerable to signs and symptoms of COVID-19 and can develop the most severe form of the disease; this could occur because HTLV causes dysregulation of CD4 and CD8 T lymphocyte proliferation and an immune dysfunction that would predispose to severe cases of COVID-19 in association with HTLV.10
Simian Retrovirus type D (SRV/D) can cause morbidity and mortality in the genus Macaca, mainly affecting animals in a breeding group.35 Clinically, infection can result in a syndrome characterized by profound immunosuppression and death associated with opportunistic infections. Immunodeficiency is followed by generalized lymphadenopathy, splenomegaly, chronic diarrhea, progressive weight loss, fever, severe lymphocyte cell depletion, and hematologic abnormalities, such as anemia, leukopenia, and thrombocytopenia. The virus is also associated with neoplastic processes, such as cutaneous fibrosarcomas and retroperitoneal fibromatosis, which is an exacerbated proliferation of fibrous tissue in the abdominal cavity.30
Simian Immunodeficiency Virus (SIV) is common in African monkeys, including Chlorocebus aethiops and Papio spp., which are usually asymptomatic carriers of the virus. SIV is not naturally present in wild populations of Asian monkeys, but they can be experimentally infected with the virus. SIV therefore has a relatively low risk of natural infection, and continuous health screening for this pathogen is not necessary in rhesus macaques.6
Simian Foamy Virus (SFV) is a highly prevalent retrovirus in most species of NHP, approaching 100% prevalence in some populations. Although it is considered nonpathogenic, SFV is highly cytolytic and can interfere with studies that require the growth or maintenance of cell cultures.61 In this regard, SARS-CoV-2 research that uses simian cell lines49 may have compromised viral replication if cells are contaminated by SFV.
Concurrent coinfections in COVID-19 can also change the respiratory microbiome and thus stimulate immune cells to produce more severe inflammation.27 Among the most important respiratory viruses in NHP, measles stands out as a highly infectious paramyxovirus of the genus Morbillivirus. Infection spreads rapidly, notably with profound consequences that can be fatal in marmosets.69 The disease causes immunosuppression with transient dysfunction of both humoral and cellular immune responses. Transmission occurs through aerosols and direct contact with surfaces. Animals develop maculopapular rashes, conjunctivitis, blepharitis, and malaise.26 Coronavirus and Measles morbillivirus have an evolutionary connection, as they share core proteins; coinfections with SARS-CoV-2 may generate genetic exchange and recombinant viruses.48
Other airway viral infections, such as influenza virus,29 could also confound COVID-19 research findings. Influenza virus and SARS-CoV-2 infections have highly similar respiratory symptoms, including high fever, cough, headache, and even pneumonia. Influenza coinfection can provoke COVID-19 hyper-inflammatory states with higher incidence of acute cardiac injury caused by the overexpression of ACE-2 induced by the high production of IFN.27
Another relevant respiratory disease is tuberculosis (TB). NHP are highly sensitive to TB and their most frequent pathogen is Mycobacterium tuberculosis. TB is common in captive apes, with a chronic or subacute course and an incubation period of 1 to 3 mo. Transmission occurs through contaminated food or contact with infected apes or humans, and it is the most important bacterial disease in NHP due to its ability to spread quickly. Symptoms include cough, progressive weight loss, alopecia, fatigue, weakness, pneumonia, prostration, and cachexia.20 Because it is a highly communicable disease, all people who work directly with NHP must undergo periodic TB tests.3,31 During a TB coinfection with COVID-19, the cytokine storm produced by COVID-19 may reactivate latent TB or exacerbate active TB. Lung damage caused by TB may also intensify disease severity due to SARS-CoV-2.15 Other respiratory bacteria can also compromise studies of experimental COVID-19 infection in NHP; these bacteria include Streptococcus pneumoniae, Bordetella bronchiseptica, Klebsiella pneumoniae, and Burkholderia pseudomallei (formerly Pseudomonas). Such bacteria should be investigated in endemic areas,6 as the association of SARS-CoV-2 with one of these bacterial agents can trigger fatal sepsis.27
Pneumonyssus simicola mites, which cause pulmonary acariasis, are endemic in some regions of the world and common in macaques. Animals usually do not present clinical signs but may have episodes of cough. The diagnosis is usually made postmortem with the identification of cystic and granulomatous lung lesions that can be confused with lesions from other infections (for example, TB).4,28 The presence of this lung mite must be evaluated in experiments with COVID-19, as lung lesions caused by the mite complicate understanding of the actual lesions caused by the coronavirus.
NHP can harbor enteric infectious agents that may be latent6 and can alter gastrointestinal symptoms identified during the COVID-19 infection. A possible pathogenic reactivation and consequent digestive coinfection with such agents can similarly exacerbate symptoms that would be expected to occur only after SARS-Cov-2 infection. One report describes high gastrointestinal ACE-2 mRNA that interacts strongly with SARS-CoV-2 in the gastrointestinal system, which has high microbial diversity and possible chances of immunosuppression and coinfections.27
Among the main agents that affect the gastrointestinal tract of NHP is hepatitis virus, especially hepatitis A (HAV) and B (HBV) viruses, which affect both humans and great apes;6 transmission of natural HBV infection has been reported among wild populations of cynomolgus macaques.18 Hepatic complications associated with COVID-19 are particularly concerning for people with HBV coinfection and preexisting liver complications (for example, cirrhosis, liver failure, or hepatocellular carcinoma).27 One study suggests that the SARS-CoV-2 virus could lead to liver injury mainly by binding to the ACE-2 receptors on hepatocytes, causing an immune-mediated hepatic injury through activation of cytokine storm,2 and increasing alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyl transferase (GGT) levels, with moderately elevated prothrombin time (PT). Therefore, extensive screening for hepatitis B infections in critical COVID-19 patients can be useful for disease progression analysis and effective treatment planning in patients with diagnostic indications of severely abnormal liver function.27
Other common gastrointestinal bacteria include campylobacteriosis (Campylobacter jejuni), salmonellosis (Salmonella typhimurium/enteritidis), shigellosis (Shigella flexneri), and yersinioses (Yersinia enterocolitica/pseudotuberculosis). Infections are characterized by enterocolitis, fever, malaise, myalgia, abdominal pain, and fetid and watery diarrhea. Transmission occurs via the orofecal route, and animals can be asymptomatic carriers.6,56
Intestinal protozoa such as Entamoeba histolytica, Balantidium coli, and Giardia sp. can cause intermittent or severe diarrhea when present in high numbers, particularly in immunocompromised individuals.56,78 Intestinal helminths affecting various primate species include Strongyloides and Trichuris, which are easily detected and treated and cause no major health problems.6 Coinfection with a parasite appears to be associated with reduced severity of COVID-19, suggesting that immunomodulatory responses caused by the parasite may silence the hyperinflammation associated with severe COVID-19 disease.68
Given this information, we emphasize the need to consider all agents that can cause signs in NPH that are similar to those of the SARS-Cov-2 virus, as such coinfections could confound data and its interpretation. On the other hand, such coinfections can be studied as a model for similar coinfections in humans.
NHP as a Model for Studying COVID-19
Among several animal models with different biologic characteristics that have been used to study the SARS-CoV-2 virus for its pathology, transmissibility, host responses to the virus, and tests for the efficacy of drugs and vaccines,46 NHP most closely resemble humans due to their phylogenetic similarities.21 In addition, M. mulatta currently appears to be the most appropriate simian model for COVID-19 vaccine studies. The search for simian models is extremely important for elucidation of the pathogenesis of the virus and the development of the most effective intervention strategies against infection.23
Some species, such as rhesus monkeys, cynomolgus, green monkeys (Chlorocebus aethiops), and common marmosets have already shown susceptibility to the virus through experimental infections.25 These species experimentally infected by three strains of SARS-associated coronavirus (Urbani, PUMC-01, and HKU39849) and developed viral replication with symptoms including pneumonia in different degrees of severity depending on the physiologic characteristics of the infected species.22,24,33,42,54
Among the primate species that have shown susceptibility to infection, the best simian model may not yet have been identified because all species studied had viral replication in the lungs, virus shedding in respiratory secretions, and histopathologic changes similar to those seen in human infections.52 However, some studies indicate that responses to the viral infection that most resemble those of humans are most consistent in rhesus monkeys, suggesting that this is the most suitable of all NHP species tested to date.12,17,38,66,76
Marmosets (Callithrix jacchus) have potential as a model for investigating the pathogenesis of the SARS-CoV virus. One study24 reported that the infection caused pneumonia consisting of mononuclear multifocal interstitial cells, accompanied by multinucleated cells, edema, and bronchiolitis. At necropsy, viral RNA was detected in tracheobronchial and myocardial lymph nodes, and inflammatory changes were observed in the liver, with marked multifocal lymphocytic hepatitis and necrosis of individual hepatocytes.24
Acute COVID-19 infection has been characterized in two Old World monkeys, M. mulatta and M. fascicularis, and in a New World species, C. jacchus. The infected NHP showed several clinical features of human COVID-19 (fever, weight loss, and pneumonia).38 Radiographic imaging revealed lung abnormalities in the form of nodules, masses, and interstitial patterns. Gross lesions of the lungs at necropsy matched the abnormal spots seen on chest radiographs taken right before necropsy. Severe histopathologic changes showed thickening of the pulmonary septum and the infiltration of inflammatory cells, diffuse hemorrhage and necrosis in the lungs, and inflammatory infiltration in the trachea and bronchus, especially in M. mulatta. Virus was detected in the lung tissues of the two macaque species but not in C. jacchus.38
Despite different susceptibilities in the Old and New World monkeys to SARS-CoV-2, M. mulatta species showed a more severe response to the virus than did the others, including higher levels of inflammatory cytokines and more pathologic changes in lung tissues. Its homology with humans in terms of the amino acid fragments of ACE-2 may give this species a greater degree of susceptibility to SARS-CoV-2.38
Three NHP, young rhesus, cynomolgus, and green monkeys analyzed in different laboratories, had high levels of viral replication in both upper and lower respiratory tracts between 7 and 14 d after infections, with pathologic features of viral pneumonia and variable induction of mild clinical disease.12,47,53 All research on experimental infection in NHP has produced only mild clinical disease, in contrast to the situation in humans.46 Like humans, older rhesus monkeys (> 15 y old) develop more severe disease than do younger ones (3 to 5 y old), with more active viral replication for 14 d after SARS-CoV-2 challenge. Old rhesus macaques exhibited diffuse severe interstitial thickened alveolar septum caused by inflammation and edema.76 Another study reported that viral shedding in the upper respiratory tract was more prolonged in aged cynomolgus macaques (15 to 20 y of age) than in younger animals (4 to 5 y old).53 Elderly rhesus and cynomolgus monkeys secrete the virus through the nose and throat for longer periods than do younger individuals.52,76 Higher viral loads were also seen in the lung tissue of elderly rhesus, and advanced age in rhesus monkeys was also associated with a higher incidence of radiologic and histopathologic changes.46 Such findings highlight the importance of including age as a factor in the selection criteria for experimental animals for therapeutic testing of severe stages of disease.
The imaging diagnosis of NHP infected by intrabronchial and aerosol exposure revealed radiographic chest abnormalities within 2 d after infection; these tended to resolve within 11 to 15 d after infection. Evidence of the spread of live virus was found in the respiratory and gastrointestinal tracts. Hematologic changes were activation of T cells, mild lymphopenia, and neutrophilia.46
To date, the similarities that the NHP share with humans related to SARS-CoV-2 infection are as follows: i) viral replication in the upper and lower respiratory tract; ii) pneumonia with bilateral pulmonary involvement, focal edema, and inflammation of the alveoli; iii) immunologic aspects linked to the presence of seroconversion, neutralizing antibody titers, marked proliferation of T cells and proinflammatory cytokines; and iv) a demographic profile characterized by a more severe clinical disease in older individuals.46
One study reported an expressive cellular response in NHP and robust protection against repeat exposure to the virus.SARS-CoV-2 infection and reinfection in rhesus monkeys present promising data, showing a natural induction of protective immunity.46 Such results support immunologic approaches for prevention and treatment of the infection.12,46 In rhesus macaques vaccinated with Adenovirus-vector-based vaccine (ChAdOx1 nCoV-19), viral load was significantly reduced in bronchoalveolar lavage fluid and lower respiratory tract tissue as compared with control animals, and vaccinated animals did not develop pneumonia.66 These data corroborate the statement that clinical signs or severity of histopathologic changes improved with vaccination.65
The vaccines tested in NHP so far induced production of neutralizing antibodies, especially in rhesus monkeys, suggest that such immunogens promote protection.32 Inactivated and live virus vaccines (produced from ChAndeno virus S ptn, Ad 26 virus S ptn, DNA S ptn, mRNA S ptn, Recombinat S ptn, and S ptn-like particles) have already been tested in rhesus monkeys.32 Vaccination resulted in reductions in viral replication in the lower respiratory tract and, to a lesser extent, in the upper tract after challenge with SARS-CoV-2, indicating that these vaccines may more effectively block disease at the level of the lower respiratory tract.
Health Monitoring and Surveillance for COVID-19 in NHPs
Because possible endemic agents may interfere with the interpretation of research results in NHP, health monitoring programs are essential tools for outlining strategies and procedures for screening for microorganisms.6,35 A multitude of infectious agents (including viruses, bacteria, parasites, and fungi) can interact with the SARS-CoV-2 virus to alter molecular pathogenesis. This pathogenic interaction underlies the molecular mechanisms that lead to the severe disease of critically ill patients with COVID-19. The interactions of multiple pathogens (especially lung organisms), SARS-CoV-2, and the host are important factors that complicate the accurate diagnosis, treatment, and prognosis of COVID-19, and even leads to increased mortality. Simultaneously, coinfecting microbiota may use new strategies to escape host defense mechanisms by altering both innate and adaptive immune responses to further aggravate SARS-CoV-2 pathogenesis.27
In this regard, based on our experience with the use of NHP for biomedical research, we propose that when selecting NHP for research, the animals should be removed from their respective family groups and transferred to an isolation area while still in the breeding unit for adaptation to individual cages and performing the necessary clinical and laboratory tests before the start of the study. Once animals are ready for the study, they are transferred to different animal rooms for acclimation and modified handling. Although they continue to receive all necessary care, they inevitably become stressed in face of the new environment. The initial confinement stress can trigger changes in an individual’s homeostasis, resulting in abnormal clinical conditions due to immunodepression.5 After adaptation to the new housing conditions (usually between 30 to 45 d), biologic parameters tend to normalize and the proposed study can begin.5 However, if the animal has a latent infection that was not detected during study screening, a pathogenic reactivation may occur during the period of immunodepression.35
To develop any biomedical study on infectious diseases, the study director must confirm the health status of the selected animals, with health certifications validated by the responsible veterinarian and laboratory reports. Five classifications of undesirable agents to the scientific protocols must be researched and animals excluded from the experiment upon their detection: A) pathogenic agent: infections that cause clinical disease and mortality in immunocompetent animals; B) opportunistic agent: present in the environment or as part of the normal commensal flora with no clinical disease, but becomes pathogenic under certain conditions, acting synergistically with other infections; C) interfering agent: ability to interfere in outcomes or interpretation of scientific protocols; D) zoonotic agent: naturally transmitted between vertebrate animals and humans; E) anthroponotic agent: can be transmitted from humans to animals.6
Analysis of the health conditions must be based on these categories of agents, including the simian pathogens recommended by FELASA.6 Screening these pathogens should be part of a program of periodic health monitoring of an animal population, as well as a component of animals being considered for experimental infection with SARS-Cov-2. Thus, in addition to SARS-CoV-2 virus,6 a detailed screening of other relevant infectious agents must be conducted and repeated when necessary, depending on the time and course of the experiment for an appropriate follow-up of the results. Because the topic of this manuscript is possible pathophysiologic changes resulting from coinfection by the SARS-CoV-2 virus and other microorganisms, we included the main agents whose clinical, hematologic, biochemical, and immunologic changes, when present, could exacerbate the course of the coronavirus infection and cause misinterpretation of clinical diagnoses.
After certifying that the preselected animals are COVID-19 negative, they should undergo two selection phases. The first is the clinical evaluation phase in which a medical physical is performed, including weighing, body palpation, temperature measurement, heart and respiratory rate, oral evaluation, clinical examination in general, and deworming. The clinical analysis should include general clinical parameters (complete hemogram and blood plasma or serum biochemistry) and assess factors that are compromised by the SARS-CoV-2 virus. Animals with changes that are consistent with the SARS-CoV-2 virus infection, such as leukocytosis, lymphopenia, high erythrocyte sedimentation rate (ESR), thrombocytopenia, D dimer elevation, decreased albumin, increased bilirubin, increased creatinine, high C reactive protein, and high lactate dehydrogenase (LDH),41 must be excluded from the study. Imaging, including radiology and ultrasound, and if possible, tomography, can provide a diagnostic assessment of possible changes that may be mistaken for COVID-19. This first phase of clinical evaluation lasts 1 mo, which is the time needed for the animal to adapt to the first isolation area after leaving the social group. The second screening phase is examination for pathogens (viruses, bacteria, and parasites), including those that commonly affect the animals (recommended by FELASA)6 and those considered endemic in the evaluated region.3,6
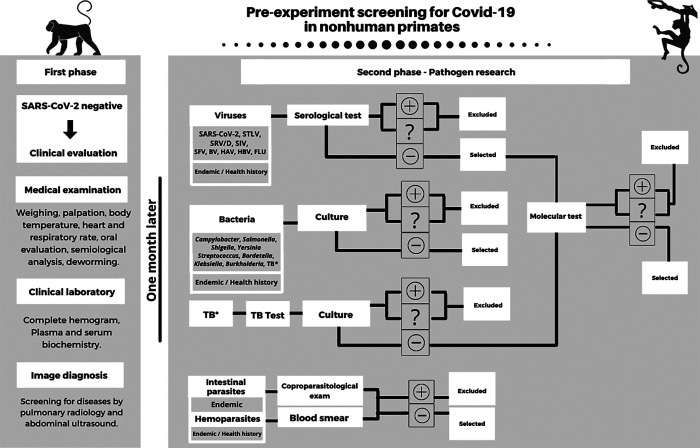
The health history of individual animals should also be considered, based on the annual or biannual health monitoring program. Historical data are of paramount importance in choosing additional pathogens to be investigated during screening. Two different techniques should be used to confirm the diagnosis, usually serologic and molecular in the case of viruses and culture for enteric and respiratory bacteria. In the case of TB, both a tuberculin test and a molecular test should be done. An examination for intestinal parasites and of a blood smear for hematozoa should also be performed. Animals with positive or indeterminate findings should be excluded from the study, and negative animals selected to continue (Figure 3).3,5,6
Figure 3.
Pre-experiment screening recommendations for COVID-19 studies in nonhuman primates. STLV: Simian T-Lymphotropic Virus; SRV/D: Simian Retrovirus type D; SIV: Simian Immunodeficiency Virus; SFV: Simian Foamy Virus; BV: Herpesvirus B; HAV: Hepatitis A virus; HBV: Hepatitis B Virus; FLU: Influenza virus. TB: Tuberculosis. Selection of animals is divided into the clinical and pathogen research phases. Animals with positive (+) and indeterminate (?) results are excluded from the study, while negative are selected. Viral panel includes serology and confirmation with molecular tests. Tuberculosis (TB) history in the animal husbandry requires association of tests. Pathogens were highlighted according to the FELASA recommendations,6 alerting endemics in the region and pathogens included in the health history of the scientific animal creation. Other clinical tests and pathogens based on each experimental protocol should be considered. Scheme created by BioRender - http://BioRender.com.
In addition to the possible confounding coinfections already discussed throughout this text, macaques are susceptible to changes caused by Cercopithecine herpesvirus 1 (Herpes B virus). Herpes B virus is the zoonotic virus of greatest concern for those working with macaques, as the virus can be fatal in humans.62 Infection is transmitted via bites, scratches, sexual contact, or contact between saliva and other body fluids of an infected animal. Viremia is intermittent and does not always coincide with symptoms. The most prevalent clinical signs in macaques are vesicular lesions in the oral cavity. Like other herpesviruses, B virus exhibits latency, particularly in the trigeminal ganglion, which leads to lifelong infection and quiescence for extended periods. During these periods, antibody titers may decrease below the detection limits of serologic assays. Titers may rise again periodically, especially under ‘stress’ or immunosuppression.6 Immunocompetent human patients may undergo reactivation of the herpes zoster virus due to the pathogenic process associated with SARS-CoV-2 virus. The interaction of herpes zoster virus and SARS-CoV-2 could result in trigeminal neuropathy.19
Opportunistic infections that normally arise from other unexpectedly reactivated agents1 should also be screened, including cytomegalovirus, Epstein-Barr virus, rotavirus, SV40, candidiasis, cryptosporidiosis, histoplasmosis, toxoplasmosis, and aspergillosis.5,6 Personnel should be aware of studies on immunosuppressive therapy against severe cases of COVID-19, as these agents increase the risk of opportunistic infections.1
Finally, infectious diseases carried by vectors are prevented through a program to isolate NHP from wild animals, especially rodents, insects, and birds.3,6 Stainless steel mosquito net screens are an effective method to prevent the entry of arboviruses (for example, dengue, yellow fever, zika virus, and chikungunya).3 A coinfection of arboviruses with SARS-Cov-2 could lead to greater symptom severity,77 especially due to lower leukocyte, neutrophil, lymphocyte, and platelet counts, which could contribute to higher mortality.55
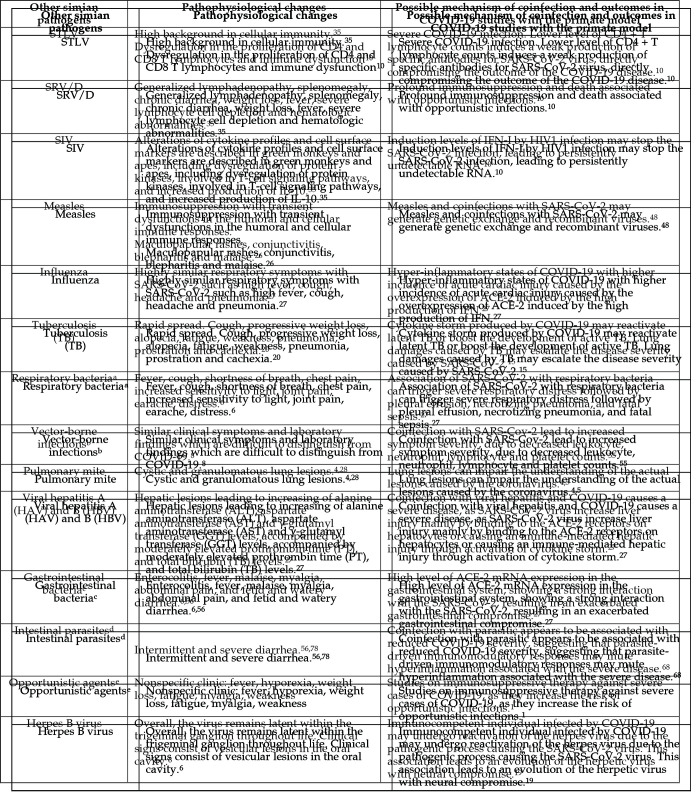
The pathophysiologic changes that occur with SARS-CoV-2 and coinfection with other simian pathogens are summarized in Figure 4. Coinfection may require a differential diagnosis and alter outcomes in COVID-19 studies in NHP. All the coinfections discussed above are based on reported histories of interference with results from research studying pathogens other than COVID-19. The concerns and hypothetical specifications raised relate to particular pathogens due to their clinical and immunologic similarities to SARS-Cov-2 virus, their high virulence and destructive features, or their zoonotic potential. Respiratory viruses and bacterial infections warrant particular consideration due to their SARS-Cov-2-like transmission characteristics and clinical manifestations which can cause more serious disease than any single agent.40,49 For example, STLV-infected NHP have high background levels in cellular immunity assays as compared with other simian pathogens,35 which can invalidate the entire experimental design of a study. Equally important are coinfections with vector-borne infections, especially Flaviviridae, because they have clinical symptoms and laboratory findings that are difficult to distinguish from COVID-19.8
Figure 4.
Pathophysiological changes caused by the SARS-CoV-2 virus and coinfections with other simian pathogens, possible mechanisms of coinfections and outcomes in COVID-19 studies with the primate model. aRespiratory bacteria: Streptococcus pneumoniae, Bordetella bronchiseptica, Klebsiella pneumoniae and Burkholderia pseudomallei; bVector-borne infections: arboviruses, such as dengue, yellow fever, zika virus and chikungunya; cGastrointestinal bacteria: Campylobacter, Salmonella, Shigella, Yersinia; dIntestinal parasites: Entamoeba histolytica, Balantidium coli, Giardia sp., Strongyloides, Trichuris; eOpportunistic agents: herpesviruses (that is cytomegalovirus, epstein-barr virus), rotavirus, SV40, candidiasis, cryptosporidiosis, histoplasmosis, toxoplasmosis, candidiasis, aspergillosis, gastrointestinal agents, respiratory agents.
We strongly propose to expand the screening protocol for COVID-19 studies in NHP from the SARS-CoV-2 virus test and pathogens recommended by FELASA6 to include agents that occur in endemic areas (especially the vector-borne infections), pathogens identified in the health history of individual animals, and tests for respiratory agents such as influenza virus,28 Streptococcus pneumoniae, and Bordetella bronchiseptica (Figure 3). The final experimental design should include both test frequency and the number of NHP in the study based on the specifics of the protocol and approval or licensing by relevant laws or policies related to ethics in the use of animals in research.
To protect the health of NHP, breeding animals should be guarded against any contact with viral agents. Although some data support claims that NHP are susceptible to the SARS-CoV-2 virus, their role in maintaining the virus in the environment is unknown.57 The virus has undergone several mutations since it was first identified in late 2019, becoming increasingly virulent or contagious and able to adapt to new hosts. Research shows that initially, the virus had been mutating at a rate of about 1 to 2 mutations per month. However, some newer variants have accumulated significantly more mutations in shorter intervals, causing worldwide concern.9
Professionals working with NHP, whether for research or species preservation, including national parks, zoos, and other places that receive visitors, must restrict access during critical pandemic periods57 due to the high number of individuals with asymptomatic infections.23,57 To protect animals from this vulnerability, the California National Primate Research Center (CNPRC), CA initiated a health surveillance program to determine the SARS-CoV-2 viral status of its NHP population. The program involved policies such as: i) reduction in access to the CNPRC; ii) reduced operations, limiting interindividual contact (human to animal, human to human, animal to animal); iii) adequate use of personal protective equipment; iv) distancing between people; and v) establishing a surveillance program for humans who deal directly with animals. People who tested positive were barred from access. At the same time, biologic samples (nasal and oropharyngeal swabs, 1 g of feces, and 5 mL of whole blood without anticoagulant) were collected weekly from 10% of the population of rhesus monkeys for validated detection tests of the SARS-CoV-2 virus by RT-PCR and ELISA. Special barrier rooms were used to house animals that were removed from both the SPF and the conventional non-SPF colonies to identify them as uninfected and appropriate SARS-CoV-2 inoculation studies in biosafety level 3 conditions (BSL-3).75
Other primate centers worldwide have also been initiating efforts to protect NHP from possible unexpected exposure, establish techniques for collection, storage, and transport of biologic samples, and develop laboratory tests and biosafety protocols in accordance with the technical guidelines of the World Health Organization and pertinent legislation of each country.71,72 For conduct of technical activities with animals, different institutions adopt recommended prophylactic measures by rotating employees, adjusting routines according to the needs of each job, and always avoiding crowded groups. Furthermore, periodic examinations of staff must be required to detect new infections.72
References
- 1.Abdoli A, Falahi S, Kenarkoohi A. 2021. COVID-19-associated opportunistic infections: A snapshot on the current reports. Clin Exp Med. 10.1007/s10238-021-00751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alqahtani SA, Schattenberg JM. 2020. Liver injury in COVID-19: The current evidence. United European Gastroenterol J 8:509–519. 10.1177/2050640620924157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrade A, Andrade MCR, Marinho AM, Ferreira-Filho J. 2010. Biologia, manejo e medicina de primatas não humanos na pesquisa biomédica. [Biology, management and medicine of non-human primates in biomedical research]. Rio de Janeiro: Fiocruz. 472 p. [Google Scholar]
- 4.Andrade MCR, Marchevsky RS. 2007. Histopathologic finding of pulmonary acariasis in a rhesus monkeys breeding unit. Rev Bras Parasitol Vet 16:229–234. 10.1590/S1984-29612007000400009. [DOI] [PubMed] [Google Scholar]
- 5.Andrade MCR. 2017. Primatas não humanos para estudos biomédicos: Manejo, agentes infecciosos e monitoramento sanitário. [Non-human primates for biomedical studies: management, infectious agents, and health monitoring] Rev Soc Bras Ci Anim Lab 5:64–75 [Article in Portugese]. [Google Scholar]
- 6.Balansard I, Cleverley L, Cutler KL, Spangberg MG, Thibault-Duprey K, Langermans J. 2019. Revised recommendations for health monitoring of non-human primate colonies (2018). Lab Anim 53:429–446. 10.1177/0023677219844541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhaskaran K, Rentsch CT, MacKenna B, Schultze A, Mehrkar A, Bates CJ, Eddo RM, Morton CE, Bacon SCJ, Inglesby P, Douglas IJ, Walker AJ, McDonald HI. 2021. HIV infection and COVID-19 death: A population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV 8:e24–e32. 10.1016/S2352-3018(20)30305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bicudo N, Bicudo E, Costa JD, Castro JALP, Barra GB. 2020. Co-infection of SARS-CoV-2 and dengue virus: A clinical challenge. Braz J Infect Dis 24:452–454. 10.1016/j.bjid.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callaway E. 2020. Making sense of coronavirus mutations. Nature 585:174–177. 10.1038/d41586-020-02544-6. [DOI] [PubMed] [Google Scholar]
- 10.Carvalho LG, Rocha MEG, Chagas VM, Junior VRS, Aroucha AQMS, Correia MCB, Costa MFH. 2020. HTLV como fator de risco e gravidade ao COVID-19 - relato de dois casos. [HTLV as a risk factor and severity for COVID-19 - report of two cases]. Hematol Transfus Cell Ther 42: 536–537. [Article in Portuguese]. 10.1016/j.htct.2020.10.906. [DOI] [Google Scholar]
- 11.Catanzaro M, Fagiani F, Racchi M, Corsini E, Govini S, Lanni C. 2020. Immune response in COVID-19: Addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Target Ther 5:84. 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandrashekar A, Liu J, Martinot AJ, McMahan K, Mercado NB, Peter L, Tostanoski LH, Yu J, Maliga Z, Nekorchuk M, Busman-Sahay K, Terry M, Wrijil LM, Ducat S, Martinez DR, Atyeo C, Fischinger S, Burke JS, Slein MD, Pessaint L, Van Ry A, Greenhouse J, Taylor T, Blade K, Cook A, Finneyfrock B, Brown R, Teow E, Velasco J, Zahn R, Wegmann F, Abbink P, Bondzie EA, Dagotto G, Gebre MS, He X, Jacob-Dolan C, Kordana N, Li Z, Lifton MA, Mahrokhian SH, Maxfield LF, Nityanandam R, Nkolola JP, Schmidt AG, Miller AD, Baric RS, Alter G, Sorger PK, Estes JD, Andersen H, Lewis MG, Barouch DH. 2020. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 369:812–817. 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatterjee SK, Snigdha S, Munoz MN. 2020. Molecular pathogenesis, immunopathogenesis and novel therapeutic strategy against COVID-19. Front Mol Biosci 7:1–11. 10.3389/fmolb.2020.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbett KS, Flynn B, Foulds KE, Francica JR, Boyoglu-Barnum S, Werner AP, Flach B, O’Connell S, Bock KW, Minai M, Nagata BM, Andersen H, Martinez DR, Noe AT, Douek N, Donaldson MM, Nji NN, Alvarado GS, Edwards DK, Flebbe DR, Lamb E, Doria-Rose NA, Lin BC, Louder MK, O’Dell S, Schmidt SD, Phung E, Chang LA, Yap C, Todd J-PM, Pessaint L, Van Ry A, Browne S, Greenhouse J, Putman-Taylor T, Strasbaugh A, Campbell T-A, Cook A, Dodson A, Steingrebe K, Shi W, Zhang Y, Abiona OM, Wang L, Pegu A, Yang ES, Leung K, Zhou T, Teng I-T, Widge A, Gordon I, Novik L, Gillespie RA, Loomis RJ, Moliva JI, Stewart-Jones G, Himansu S, Kong W-P, Nason MC, Morabito KM, Ruckwardt TJ, Ledgerwood JE, Gaudinski MR, Kwong PD, Mascola JR, Carfi A, Lewis MG, Baric RS, McDermott A, Moore IN, Sullivan NJ, Roederer M, Seder RA, Graham BS. 2020. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med 383:1544–1555. 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crisan-Dabija R, Grigorescu C, Pavel CA, Artene B, Popa IV, Cernomaz A, Burlacu A. 2020. Tuberculosis and COVID-19: Lessons from the past viral outbreaks and possible future outcomes. Can Respir J 2020:1–10. 10.1155/2020/1401053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deb B, Shah H, Goel S. 2020. Current global vaccine and drug efforts against COVID-19: Pros and cons of bypassing animal trials. J Biosci 45:82. 10.1007/s12038-020-00053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng W, Bao L, Gao H, Xiang Z, Qu Y, Song Z, Gong S, Liu J, Yu P, Qi F, Xu Y, Li F, Xiao C, Lv Q, Xue J, Wei Q, Liu M, Wang G, Wang S, Yu H, Chen T, Liu X, Zhao W, Han Y, Qin C. 2020. Occular conjunctival inoculation of SARS-CoV-2 can cause mild COVID-19 in rhesus macaques. Nat Commun 11:4400. 10.1038/s41467-020-18149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupinay T, Gheit T, Roques P, Cova L, Chevallier-Queyron P, Tasahsu S, Le Grand R, Simon F, Cordier G, Wakrim L, Benjelloun S, Trépo C, Chemin I. 2013. Discovery of naturally occurring transmissible chronic hepatitis B virus infection among Macaca fascicularis from Mauritius Island. Hepatology 58:1610–1620. 10.1002/hep.26428. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira ACAF, Romão TT, Macedo YS, Pupe C, Nascimento OJM. 2020. COVID-19 and herpes zoster co-infection presenting with trigeminal neuropathy. Eur J Neurol 27:1748–1750. 10.1111/ene.14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flynn JL, Gideon HP, Mattila JT, Lin P. 2015. Immunology studies in non-human primate models of tuberculosis. Immunol Rev 264:60–73. 10.1111/imr.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fortman JD, Hewett TA, Halliday LC. 2018. The laboratory nonhuman primates. 2nd ed. Boca Raton (FL): CRC Press. [Google Scholar]
- 22.Fouchier RA, Kuiken T, Schutten M, van Amerongen G, van Doornum GJJ, van den Hoogen BG, Peiris M, Lim W, Stöhr K, Osterhaus ADME. 2003. Aetiology: Koch’s postulates fulfilled for SARS virus. Nature 423:240. 10.1038/423240a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furukawa NW, Brooks JT, Sobel J. 2020. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis 26:e1–e6. 10.3201/eid2607.201595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenough TC, Carville A, Coderre J, Somasundaran M, Sullivan JL, Luzuriaga K, Mansfied K. 2006. Pneumonitis and multi-organ system disease in common marmosets (Callithrix jacchus) infected with the severe acute respiratory syndrome-associated coronavirus. Am J Pathol 167:455–463. 10.1016/S0002-9440(10)62989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haagmans BL, Osterhaus ADME. 2006. Nonhuman primate models for SARS. PLoS Med 3:e194. 10.1371/journal.pmed.0030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall WC, Kovatch RM, Herman PH, Fox JG. 1971. Pathology of measles in rhesus monkeys. Vet Pathol 8:307–319. 10.1177/030098587100800403. [DOI] [PubMed] [Google Scholar]
- 27.Hoque MN, Akter S, Mishu ID, Islam MR, Rahman MS, Akhter M, Islam I, Hasan MM, Rahaman MM, Sultana M, Islam T, Hossain MA. 2021. Microbial co-infections in COVID-19: Associated microbiota and underlying mechanisms of pathogenesis. Microb Pathog 156:104941. 10.1016/j.micpath.2021.104941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson AL, Simonek GD, Keesler RI. 2017. Fatal pulmonary acariasis in an aged indoor rhesus macaque (Macaca mulatta). J Med Primatol 46:90–92. 10.1111/jmp.12260. [DOI] [PubMed] [Google Scholar]
- 29.Karlsson EA, Engel GA, Feeroz MM, San S, Rompis A, Lee BPY-H, Shaw E, Oh G, Schillaci MA, Grant R, Heidrich J, Schultz-Cherry S, Jones-Engle L. 2012. Influenza virus infection in nonhuman primates. Emerg Infect Dis 18:1672–1675. 10.3201/eid1810.120214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy RC, Shearer MH, Hildebrand W. 1997. Nonhuman primate models to evaluate vaccine safety and immunogenecity. Vaccine 15:903–908. 10.1016/S0264-410X(96)00277-0. [DOI] [PubMed] [Google Scholar]
- 31.Khan IH, Mendoza S, Yee J, Deane M, Venkateswaran K, Zhou SS, Barry PA, Lerche NW, Luciw PA. 2006. Simultaneous detection of antibodies to six nonhuman-primate viruses by multiplex microbead immunoassay. Clin Vaccine Immunol 13:45–52. 10.1128/CVI.13.1.45-52.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klasse PJ, Nixon DF, Moore JP. 2020. Immunogenicity of clinically relevant SARS-CoV-2 vaccines in nonhuman primates and humans. Sci Adv 7:eabe8065. 10.1126/sciadv.abe8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuiken T, Fouchier RA, Schutten M, Rimmelzwann GF, Amerongen G, Riel D, Laman JD, Jong T, Doornum G, Lim W, Ling AE, Chan PK, Tam JS, Zambin MC, Gopal R, Drosten C, Werf S, Escriou N, Osterhaus A. 2003. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet 362:263–270. 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lakshmanappa YS, Elizaldi SR, Roh JW, Schmidt BA, Carroll TD, Weaver KD, Smith JC, Verma A, Deere JD, Dutra J, Stone M, Franz S, Sammak RL, Olstad KJ, Reader JR, Ma Z, Nguyen NK, Watanabe J, Usachenko J, Immareddy R, Yee JL, Weiskopf D, Sette A, Hartigan-O’Connor D, McSorley SJ, Morrison JH, Tran NK, Simmons G, Busch MP, Kozlowski PA, Van Rompay KKA, Miller CJ, Iyer SS. 2021. SARS-CoV-2 induces robust germinal center CD4 T follicular helper cell responses in rhesus macaques. Nat Commun 12:541. 10.1038/s41467-020-20642-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lerche NW, Osborn KG. 2003. Simian retrovirus infections: Potential confounding variables in primate toxicology studies. Toxicol Pathol 31 1_suppl:103–110. 10.1080/01926230390174977. [DOI] [PubMed] [Google Scholar]
- 36.Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, Pan P, Wang W, Hu D, Liu X, Zhang Q, Wu J. 2020. Coronavirus infections and immune responses. J Med Virol 92:424–432. 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li R, Qiao S, Zhang G. 2020. Analysis of angiotensin-converting enzyme 2 (ACE2) from different species sheds some light on cross-species receptor usage of a novel coronavirus 2019-nCoV. J Infect 80:469–496. 10.1016/j.jinf.2020.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu S, Zhao Y, Wenhai Y, Yang Y, Gao J, Wang J, Kuang D, Yang M, Yang J, Ma C, Xu J, Qian X, Li H, Zhao S, Li J, Wang H, Long H, Zhou J, Luo F, Ding K, Wu D, Zhang Y, Dong Y, Liu Y, Zheng Y, Lin X, Jiao L, Zheng H, Dai Q, Sun Q, Hu Y, Ke C, Liu H, Peng X. 2020. Comparison of nonhuman primates identified the suitable model for COVID-19. Signal Transduct Target Ther 5:157. 10.1038/s41392-020-00269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luscombe R. [Internet]. 2021. Thirteen gorillas test positive for Covid at Atlanta zoo. [Cited: Sep 11th 2021]. Available at: https://www.theguardian.com/us-news/2021/sep/11/gorillas-coronavirus-covid-atlanta-zoo.
- 40.Ma L, Wang W, Le Grange JM, Wang X, Du S, Li C, Wei J. 2020. Coinfection of SARS-CoV-2 and other respiratory pathogens. Infect Drug Resist 13:3045–3053. 10.2147/IDR.S267238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Machhi J, Herskovitz J, Senan AM, Dutta D, Nath B, Oleynikov MD, Blomberg WR, Meigs DD, Hasan M, Patel M, Kline P, Chang RC-C, Chang L, Gendelman HE, Kevadiya BD. 2020. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J Neuroimmune Pharmacol 15:359–386. 10.1007/s11481-020-09944-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McAuliffe J, Vogel L, Roberts A, Fahle G, Fischer S, Shieh W-J, Butler E, Zaki S, St Claire M, Murphy B, Subbarao K. 2004. Replication of SARS coronavirus administered into the respiratory tract of African green, rhesus and cynomolgus monkeys. Virology 330:8–15. 10.1016/j.virol.2004.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medeiros KS, Silva LAS, Macêdo LTA, Sarmento AC, Costa APF, Eleutério J, Jr, Gonçalves AK. 2021. Potential impact of the COVID-19 in HIV-infected individuals: A systematic review. Rev Assoc Med Bras 67 Suppl 1:127–156. 10.1590/1806-9282.67.suppl1.20200754. [DOI] [PubMed] [Google Scholar]
- 44.Melin AD, Janiak MC, Marrone F, 3rd, Arora PS, Higham JP. 2020. Comparative ACE2 variation and primate COVID19 risk. Commun Biol 3:641. 10.1038/s42003-020-01370-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C, Prosper F, Romero JP, Wirnsberger G, Zhang H, Slutsky AS, Conder R, Montserrat N, Mirazimi A, Penninger JM. 2020. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 181:905–913.e7. 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muñoz-Fontela C, Dowling WE, Funnell SGP, Gsell PS, Riveros-Balta AX, Albrecht RA, Andersen H, Baric RS, Carroll MW, Cavaleri M, Qin C, Crozier I, Dallmeier K, de Waal L, de Wit E, Delang L, Dohm E, Duprex WP, Falzarano D, Finch CL, Frieman MB, Graham BS, Gralinski LE, Guilfoyle K, Haagmans BL, Hamilton GA, Hartman AL, Herfst S, Kaptein SJF, Klimstra WB, Knezevic I, Krause PR, Kuhn JH, Le Grand R, Lewis MG, Liu WC, Maisonnasse P, McElroy AK, Munster V, Oreshkova N, Rasmussen AL, Rocha-Pereira J, Rockx B, Rodríguez E, Rogers TF, Salguero FJ, Schotsaert M, Stittelaar KJ, Thibaut HJ, Tseng CT, Vergara-Alert J, Beer M, Brasel T, Chan JFW, García-Sastre A, Neyts J, Perlman S, Reed DS, Richt JA, Roy CJ, Segalés J, Vasan SS, Henao-Restrepo AM, Barouch DH. 2020. Animal models for COVID-19. Nature 586:509–515. 10.1038/s41586-020-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munster VJ, Feldmann F, Williamson BN, van Doremalen N, Pérez-Pérez L, Schulz J, Meade-White K, Okumura A, Callison J, Brumbaugh B, Avanzato VA, Rosenke R, Hanley PW, Saturday G, Scott D, Fischer ER, de Wit E. 2020. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature 585:268–272. 10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nascimento JSF, Castro RRT, Nascimento JKF, Knoploch BB, Duque PMCO, Neves MAO. 2020. Coinfection of SARS-CoV-2 and Measles morbillivirus in a front-line health worker in Rio de Janeiro, Brasil. Rev Assoc Med Bras 66:1027–1029. 10.1590/1806-9282.66.8.1027. [DOI] [PubMed] [Google Scholar]
- 49.Ogando NS, Dalebout TJ, Zevenhoven-Dobbe JC, Limpens RWAL, van der Meer Y, Caly L, Druce J, de Vries JJC, Kikkert M, Bárcena M, Sidorov I, Snijder EJ. 2020. SARS-coronavirus-2 replication in Vero E6 cells: Replication kinetics, rapid adaptation and cytopathology. J Gen Virol 101:925–940. 10.1099/jgv.0.001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ozaras R, Cirpin R, Duran A, Duman H, Arslan O, Bakcan Y, Kaya M, Mutlu H, Isayeva L, Kebanlı F, Deger BA, Bekeshev E, Kaya F, Bilir S. 2020. Influenza and COVID-19 coinfection: Report of 6 cases and review of the literature. J Med Virol 92:2657–2665. 10.1002/jmv.26125. [DOI] [PubMed] [Google Scholar]
- 51.Perlman S, Netland J. 2009. Coronaviruses post-SARS: Update on replication and Pathogenesis. Nat Rev Microbiol 7:439–450. 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roberts A, Lamirande EW, Vogel L, Jackson JP, Paddock CD, Guarner J, Zaki SR, Sheahan T, Baric R, Subbarao K. 2008. Animals models and vaccines for SARS-CoV infection. Virus Res 133:20–32. 10.1016/j.virusres.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rockx B, Kuiken T, Herfst S, Bestebroer T, Lamers MM, Oude Munnink BB, de Meulder D, van Amerongen G, van den Brand J, Okba NMA, Schipper D, van Run P, Leijten L, Sikkema R, Verschoor E, Verstrepen B, Bogers W, Langermans J, Drosten C, Fentener van Vlissingen M, Fouchier R, de Swart R, Koopmans M, Haagmans BL. 2020. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 368:1012–1015. 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rowe T, Gao G, Hogan RJ, Crystal RG, Voss TG, Grant RL, Bell P, Kobinger GP, Wivel NA, Wilson JM. 2004. Macaque model for severe acute respiratory syndrome. J Virol 78:11401–11404. 10.1128/JVI.78.20.11401-11404.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saddique A, Rana MS, Alam MM, Ikram A, Usman M, Salman M, Faryal R, Massab U, Bokhari H, Mian MS, Israr A. 2020. Emergence of co-infection of COVID-19 and dengue: A serious public health threat. J Infect 81:e16–e18. 10.1016/j.jinf.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saleh MN, Thomas JE, Heptinstall JR, Herbein JF, Wolf RF, Reichard MV, Zajac AM. 2017. Immunologic detection of Giardia duodenalis in a specific pathogen-free captive olive baboon (Papio cynocephalus anubis) colony. J Vet Diagn Invest 29:916–919. 10.1177/1040638717721580. [DOI] [PubMed] [Google Scholar]
- 57.Santos WJ, Guiraldi LM, Lucheis SB. 2020. Should we be concerned about COVID‐19 with nonhuman primates? Am J Primatol 82:e23158. 10.1002/ajp.23158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmitt CA, Bergey CM, Jasinska AJ, Ramensky V, Burt F, Svardal H, Jorgensen MJ, Freimer NB, Grobler JP, Turner TR. 2020. ACE2 and TMPRSS2 variation in savanna monkeys (Chlorocebus spp.): Potential risk for zoonotic/anthroponotic transmission of SARS-CoV-2 and a potential model for functional studies. PLoS One 15: e0235106. 10.1371/journal.pone.0235106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shan C, Yang-Feng Y, Yang XL, Zhou YW, Gao G, Peng Y, Yang L, Hu X, Xiong J, Jiang RD, Zhang HJ, Gao XX, Peng C, Min J, Chen Y, Si HR, Wu J, Zhou P, Wang YY, Wei HP, Pang W, Hu ZF, Lv LB, Zheng YT, Shi ZL, Yuan ZM. 2020. Infection with novel coronavirus (SARS-CoV-2) causes pneumonia in rhesus macaques. Cell Res 30:670–677. 10.1038/s41422-020-0364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soni S, Jiang Y, Tesfaigzi Y, Hornick JL, Çataltepe S. 2021. Comparative analysis of ACE2 protein expression in rodent, non-human primate, and human respiratory tract at baseline and after injury: A conundrum for COVID-19 pathogenesis. PLoS One 16:e0247510. 10.1371/journal.pone.0247510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swack NS, Hsiung GD. 1975. Pathogenesis of simian foamy virus infection in natural and experimental hosts. Infect Immun 12:470–474. 10.1128/iai.12.3.470-474.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tischer BK, Osterrieder N. 2010. Herpesviruses: A zoonotic threat? Vet Microbiol 140:266–270. 10.1016/j.vetmic.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Torres LB, Araujo BHS, Gomes de Castro PH, Romero Cabral F, Sarges Marruaz K, Silva Araujo M, Gomes da Silva S, Muniz JA, Cavalheiro EA. 2010. The use of New World primates for biomedical research: An overview of the last 4 decades. Am J Primatol 72:1055–1061. 10.1002/ajp.20864. [DOI] [PubMed] [Google Scholar]
- 64.United States Department of Agriculture. [Internet]. (2021). Confirmation of COVID-19 in gorillas at a California Zoo. [Cited: Jan 11th 2021]. Available at: https://www.aphis.usda.gov/aphis/newsroom/stakeholder-info/sa_by_date/sa-2021/sa-01/ca-gorillas-sars-cov-2
- 65.van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham JN, Port JR, Avanzato VA, Bushmaker T, Flaxman A, Ulaszewska M, Feldmann F, Allen ER, Sharpe H, Schulz J, Holbrook M, Okumura A, Meade-White K, Pérez-Pérez L, Edwards NJ, Wright D, Bissett C, Gilbride C, Williamson BN, Rosenke R, Long D, Ishwarbhai A, Kailath R, Rose L, Morris S, Powers C, Lovaglio J, Hanley PW, Scott D, Saturday G, de Wit E, Gilbert SC, Munster VJ. 2020. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 586:578–582. 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vogel AB, Kanevsky I, Che Y, Swanson KA, Muik A, Vormehr M, Kranz LM, Walzer KC, Hein S, Güler A, Loschko J, Maddur MS, Ota-Setlik A, Tompkins K, Cole J, Lui BG, Ziegenhals T, Plaschke A, Eisel D, Dany SC, Fesser S, Erbar S, Bates F, Schneider D, Jesionek B, Sänger B, Wallisch AK, Feuchter Y, Junginger H, Krumm SA, Heinen AP, Adams-Quack P, Schlereth J, Schille S, Kröner C, de la Caridad Güimil Garcia R, Hiller T, Fischer L, Sellers RS, Choudhary S, Gonzalez O, Vascotto F, Gutman MR, Fontenot JA, Hall-Ursone S, Brasky K, Griffor MC, Han S, Su AAH, Lees JA, Nedoma NL, Mashalidis EH, Sahasrabudhe PV, Tan CY, Pavliakova D, Singh G, Fontes-Garfias C, Pride M, Scully IL, Ciolino T, Obregon J, Gazi M, Carrion R, Jr, Alfson KJ, Kalina WV, Kaushal D, Shi PY, Klamp T, Rosenbaum C, Kuhn AN, Türeci Ö, Dormitzer PR, Jansen KU, Sahin U. 2021. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature 592:283–289. 10.1038/s41586-021-03275-y. [DOI] [PubMed] [Google Scholar]
- 67.Wachtman LM, Mansfield KG. 2008. Opportunistic infections in immunologically compromised nonhuman primates. ILAR J 49:191–208. 10.1093/ilar.49.2.191. [DOI] [PubMed] [Google Scholar]
- 68.White MPJ, McManus CM, Maizels RM. 2020. Regulatory T-cells in helminth infection: Induction, function and therapeutic potential. Immunology 160:248–260. 10.1111/imm.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Willy ME, Woodward RA, Thornton VB, Wolff AV, Flynn BM, Heath JL, Villamarzo YS, Smith S, Bellini WJ, Rota PA. 1999. Management of a measles outbreak among Old World nonhuman primates. Lab Anim Sci 49:42–48. [PubMed] [Google Scholar]
- 70.World Health Organization. [Internet]. 2020a. Coronavirus disease 2019 (COVID-19). [Cited: March 6th 2020]. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 71.World Health Organization. [Internet]. 2020b. Country & Technical Guidance - Coronavirus disease (COVID-19). [Cited: July 17th 2020]. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications.
- 72.World Health Organization. [Internet]. 2020c. Getting your workplace ready for COVID-19. [Cited: March 3rd, 2020]. Available at: https://www.who.int/docs/default-source/coronaviruse/advice-for-workplace-clean-19-03-2020.pdf.
- 73.Yang R, Gui X, Zhang Y, Xiong Y, Gao S, Ke H. 2020. Clinical characteristics of COVID-19 patients with HIV coinfection in Wuhan, China. Expert Rev Respir Med 15:403–409. 10.1080/17476348.2021.1836965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. 2020. Clinical course and outcomes of critically ill patients with SARS776 CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med 8:475–481. 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yee JL, Van Romapny KKA, Carpenter AB, Nham PB, Halley BM, Iyer SS, Hartigan-O’Connor DJ, Miller CJ, Roberts JA. 2020. SARS-CoV-2 surveillance for a non-human primate breeding research facility. J Med Primatol 49:322–331. 10.1111/jmp.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu P, Qi F, Xu Y, Li F, Liu P, Liu J, Bao L, Deng W, Gao H, Xiang Z, Xiao C, Lv Q, Gong S, Liu J, Song Z, Qu Y, Xue J, Wei Q, Liu M, Wang G, Wang S, Yu H, Liu X, Huang B, Wang W, Zhao L, Wang H, Ye F, Zhou W, Zhen W, Han J, Wu G, Jin Q, Wang J, Tan W, Qin C. 2020. Age-related rhesus macaque models of COVID-19. Animal Model Exp Med 3:93–97. 10.1002/ame2.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zahariadis G, Gooley TA, Ryall P, Hutchinson C, Latchford MI, Fearon MA, Jamieson FB, Richardson S, Kuschak T, Mederski B. 2006. Risk of ruling out severe acute respiratory syndrome by ruling in another diagnosis: Variable incidence of atypical bacteria coinfection based on diagnostic assays. Can Respir J 13:17–22. 10.1155/2006/862797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zanzani SA, Gazzonis AL, Epis S, Manfredi MT. 2016. Study of the gastrointestinal parasitic fauna of captive non-human primates (Macaca fascicularis). Parasitol Res 115:307–312. 10.1007/s00436-015-4748-9. [DOI] [PubMed] [Google Scholar]
- 79.Zhou Z, Ren L, Zhang L, Zhong J, Xiao Y, Jia Z, Guo L, Yang J, Wang C, Jiang S, Yang D, Zhang G, Li H, Chen F, Xu Y, Chen M, Gao Z, Yang J, Dong J, Liu B, Zhang X, Wang W, He K, Jin Q, Li M, Wang J. 2020. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe 27:883–890.e2. 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]