Highlights
-
•
We developed a laboratory paradigm to assess impaired control over alcohol use.
-
•
Most heavy drinkers were unable to resist alcohol consumption after an initial exposure.
-
•
Alcohol craving was significantly associated with rate of lapse in our paradigm.
-
•
Craving may therefore be important factor in impaired control over drinking.
Keywords: Alcohol self-administration, Alcoholism, Alcohol abstinence, Craving, Impaired control
Abstract
Background
Roughly half of patients with alcohol use disorder prefer non-abstinence based approaches to treatment. However, only individuals who can limit their alcohol use after low-risk consumption are most likely to benefit from these approaches. This pilot study developed a laboratory-based intravenous alcohol self-administration paradigm to determine the characteristics of individuals who could successfully resist consuming alcohol after an initial exposure.
Methods
Seventeen non-treatment seeking heavy drinkers completed two versions of an intravenous alcohol self-administration paradigm designed to assess impaired control over alcohol use. In the paradigm, participants received a priming dose of alcohol and then entered a 120-min resist phase, in which they received monetary rewards if they resisted self-administering alcohol. We used Cox proportional hazards regression to determine the impact of craving and Impaired Control Scale scores on rate of lapse.
Results
64.7% of participants across both versions of the paradigm were unable to resist alcohol for the duration of the session. Craving at baseline (HR = 1.07, 95% CI 1.01-1.13, p = 0.02) and following priming (HR = 1.08, 95% CI 1.02-1.15, p = 0.01) were associated with rate of lapse. Individuals who lapsed endorsed greater attempts to control their drinking over the prior six months compared to individuals who resisted.
Conclusions
This study provides preliminary evidence that craving may be predictive of risk of lapse in individuals who are trying to limit alcohol intake after consuming a small initial amount of alcohol. Future studies should test this paradigm in a larger and more diverse sample.
1. Introduction
It is estimated that 6–14% of American adults meet the diagnostic threshold for alcohol use disorder in a given year (Grant et al., 2015; SAMHSA, 2019). Of these individuals, only an estimated 7.6% receive some form of treatment (SAMHSA, 2019). Certain clinics and treatment programs, including 12-step facilitation programs, promote an abstinence-based treatment approach which defines recovery as total abstinence from alcohol (Nash, 2020). Other programs endorse harm reduction or non-abstinence-based approaches, in which heavy drinkers are supported in their efforts to reduce their drinking to safer levels (Charlet and Heinz, 2017). Around 45% of treatment-seeking drinkers prefer a non-abstinence-based approach over an abstinence-based approach (Heather et al., 2010; Hodgins et al., 1997). However, only individuals who can prevent themselves from consuming additional alcohol after low-risk consumption are likely to achieve maximal benefits from non-abstinence-based approaches.
Ability to resist alcohol is directly linked to the psychological construct of impaired control. Impaired control is considered to be a core feature of addiction and is defined as an inability to limit drug use despite the intention to do so (Leeman et al., 2012). One of the most common self-report measures used to assess impaired control over alcohol use is the Impaired Control Scale (Heather et al., 1993). However, individuals may not display good insight into their level of impaired control, especially since poor insight is known to be a central feature of addiction (Goldstein et al., 2009), which may in turn impact the reliability of self-report measures. Human laboratory models can be used to overcome this limitation as they assess alcohol consumption behaviors directly. We sought to develop a novel laboratory approach which would provide both continuous and categorical data related to impaired control. A novel laboratory paradigm could potentially be used to improve understanding of individual differences in ability to resist alcohol and prove helpful in testing novel pharmacotherapies.
In this pilot study, we developed a human laboratory paradigm to assess impaired control over alcohol consumption based on a well-validated smoking lapse paradigm (McKee, 2009), which has been used to investigate whether certain psychological factors and pharmacological treatments impact an individual's ability to resist smoking (McKee et al., 2012; Roche et al., 2014; McKee et al., 2011; Leeman et al., 2010; Verplaetse et al., 2017; Roberts et al., 2018; Verplaetse et al., 2018). In the current study, individuals completed a session in which they were given a priming dose of intravenous (IV) alcohol and then entered a 120-min resist phase, in which they were paid to resist infusing additional alcohol. The model was hypothesized to provide a laboratory measure of impaired control as participants were provided with an incentive to limit their alcohol consumption following a priming dose; failure to resist was hypothesized to represent a breakdown of intention to limit consumption. We tested two different reward schedules: a fixed-reward schedule, in which individuals received a consistent monetary reward for each minute they resisted and a de-escalating reward schedule, in which the monetary reward per minute of resistance gradually diminished over the course of the session. We examined whether each paradigm met target model behavior, that is, whether the average time to lapse was at roughly the halfway point of the paradigm (McKee, 2009). A paradigm that demonstrates target model behavior has advantages for interventional studies, as it allows experimenters to examine whether a given intervention increases or decreases ability to resist. By contrast, it would be difficult to study whether an intervention affects ability to resist in a paradigm where the average participant lapses very early on (floor effect) or in the last minute of the session (ceiling effect). We also examined the characteristics that influenced rate of lapse. Specifically, we hypothesized that individuals who endorsed higher levels of craving and greater degree of failed attempts to control their drinking over the past 6 months would exhibit the highest rate of lapse.
2. Material and methods
2.1. Participants
Twenty-one participants were recruited for this study. Of these, seventeen completed the experimental laboratory session measuring ability to resist alcohol. Participants between 21 and 60 years of age were included in this study. Only individuals who were non-treatment seeking heavy drinkers were included in this study, with heavy drinking defined as consuming on average > 15 standard drinks per week for women and > 20 standard drinks per week for men over the past 90 days as measured using the Timeline Followback interview. Participants also needed to endorse at least one binge drinking day per week over the past 90 days, defined as 4+ drinks for females or 5+ drinks for males in a single day (NIAAA, 2004). All but two of our participants met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria for current alcohol use disorder (Table 1), although this was not one of our inclusion criteria. Individuals who met current criteria for other substance use disorders and were not in remission based on the Structured Clinical Interview for DSM-5, Research Version (SCID-5) (First et al., 2015) or who were actively using substances other than tobacco based on self-report or urine toxicology screen (an immunoassay which tested for cannabis, cocaine, amphetamines, opioids, and benzodiazepines) were excluded from the study. Individuals with any other current DSM-5 diagnoses requiring intervention (e.g. major depressive disorder, bipolar disorder, posttraumatic stress disorder) or lifetime history of serious medical illness were also excluded. Individuals with elevated transaminase levels (greater than 3 times the upper limit of normal), positive hepatitis or HIV tests, or clinically significant abnormal electrocardiogram (ECG) findings were also excluded. Those who were pregnant (based on urine beta-hCG test), breastfeeding, or intended to become pregnant or breastfeed were not permitted to enroll. Individuals taking prescription or over-the-counter medication known to interact with alcohol or affect alcohol metabolism in the 2–4-week period prior to the study were also excluded. Individuals with a current or prior history of alcohol-induced flushing reactions were not permitted to enroll. Participants needed to be able and willing to abstain from alcohol for 24 h prior to each alcohol self-administration session. Individuals with a history of clinically significant withdrawal symptoms or a Clinical Institute Withdrawal Assessment, revised (CIWA-Ar) score (Sullivan et al., 1989) of greater than 8 at the screening visit were not permitted to enroll. Individuals who were currently seeking treatment for AUD or who had undergone inpatient or outpatient detoxification or treatment for alcohol problems in the past 6 months were not permitted to enroll.
Table 1.
Group characteristics.
|
Group Characteristics | |||||
|---|---|---|---|---|---|
|
Participants Who Resisted (N = 6) |
Participants Who Lapsed (N = 11) |
||||
| N | % | N | % | Chi-square value | |
| Sex (Male/Female) | 6/0 | 100%/0.0% | 9/2 | 81.8%/18.2% | 1.2 |
| Alcohol Use Disorder Diagnosisa | 5 | 83.3% | 10 | 90.9% | 0.2 |
| Paradigm Version (Fixed/De-Escalating) | 3/3 | 50.0%/50.0% | 4/7 | 36.4%/63.6% | 0.3 |
| Household Income (≤39,999/>40,000) | 1/5 | 16.7%/83.3% | 7/4 | 63.6%/36.4% | 3.4 |
| Mean | SD | Mean | SD | t- value | |
| Age (years) | 40.2 | 12.0 | 35.0 | 10.8 | 0.9 |
| Full Scale IQb | 109.2 | 10.3 | 111.5 | 12.7 | -0.3 |
| AUDIT score | 16.7 | 5.9 | 14.9 | 4.6 | 0.7 |
| Total Lifetime Drinksc | 23016.0 | 15550.3 | 15135.0 | 13268.5 | 1.1 |
| Total Drinks, Last 90 Daysd | 376.3 | 104.2 | 334.7 | 130.1 | 0.7 |
| Binge Drinking Days, Last 90 Daysd | 40.3 | 22.3 | 31.6 | 20.5 | 0.8 |
AUDIT: Alcohol Use Disorder Identification Test.
Diagnosis of alcohol use disorder was assessed by the SCID-IV or SCID-5.
Full Scale IQ was derived from the Wechsler Abbreviated Scale of Intelligence-II (WASI-II). WASI-II data was missing from one participant who resisted.
Total lifetime drinks was estimated using the Lifetime Drinking History interview.
Assessed by the 90-day Timeline Followback interview.
2.2. Study procedures
All participants provided consent for and completed an initial screening and evaluation protocol at the National Institutes of Health (NIH), in which they underwent a medical assessment, blood tests (including liver function tests, hepatitis serology, and HIV serology), a urine drug screen, a 90-day Timeline Followback interview (Sobell and Sobell, 1992), a SCID-IV or SCID-5 interview (First et al., 2015), the Wechsler Abbreviated Scale of Intelligence-II (Wechsler, 2011), the Lifetime Drinking History interview (Skinner and Sheu, 1982), and a comprehensive battery of self-report assessments including the Alcohol Use Disorders Identification Test (Saunders et al., 1993), the Alcohol Flushing Questionnaire (Johnson et al., 1984), and the Impaired Control Scale (Heather et al., 1993). Following this, individuals who were deemed eligible completed a baseline assessment visit in which they provided written informed consent for our study. Participants then completed a battery of psychological tasks and were given an ecological momentary assessment (EMA) device to assess drinking behaviors outside of the laboratory (results to be reported separately). Participants were subsequently scheduled for a “free-access” laboratory session in which they could self-administer intravenous alcohol in an ad-libitum fashion (Stangl et al., 2017). The purpose of the free-access session was to acclimate participants to the intravenous alcohol self-administration procedure. Participants were then scheduled for a laboratory visit in which they completed the novel “resist” paradigm. Alcohol self-administration sessions were conducted in a room at the day hospital unit in the NIH Clinical Center.
2.3. Intravenous alcohol administration paradigms
Under controlled experimental conditions designed to achieve uniformity across participants, individuals consuming oral doses of alcohol standardized to their total body water at fixed intervals show a 3-4 fold range in blood alcohol concentration (BAC) vs. time curves due to inter-individual differences in ethanol absorption and metabolism (Ramchandani et al., 2009). Intravenous alcohol administration largely eliminates this issue by bypassing the gastrointestinal tract and first-pass metabolism. In our IV alcohol self-administration paradigm, the participant presses a button when they wish to consume alcohol and our infusion software delivers an IV infusion of alcohol that is designed to raise the individual's breath alcohol concentration (BrAC) by 7.5mg% over 2.5 min. With this approach, it is possible to achieve nearly identical increases in BrAC over a fixed time frame, which is ideal for experiments such as our resist paradigm in which we aim for each individual to receive identical exposures of alcohol. This is important as differential exposure to alcohol between participants during the priming phase could influence induction of craving and therefore affect ability to resist during the resist phase of our paradigm.
2.4. Free-access alcohol self-administration session
All participants were scheduled for a free-access alcohol self-administration session prior to the resist session (Gowin et al., 2017; Sloan et al., 2020; Sloan et al., 2018; Stangl et al., 2017). Participants first completed a 25-min priming phase, in which they were instructed to press the button four times (once every 2.5 min) over the first 10 min of the session. Participants were then given 15 min to experience the effects of the priming dose of alcohol. Following this, participants completed a 125-min free-access phase in which they could infuse as much alcohol as they wanted up to a safety ceiling BrAC of 120mg%.
2.5. Resist session
In the resist session, participants were given a priming dose of IV alcohol and then paid to resist additional alcohol consumption. We employed two different reward schedules in our paradigm (see Fig. S1). In the fixed-reward schedule, individuals received $0.10 for each minute they resisted infusing alcohol. In the de-escalating reward schedule, participants initially received $0.20 per minute of resistance, but the amount of compensation decreased by $0.01 every six minutes such that individuals received $0.01 per minute of resistance in the last six minutes of the paradigm. Participants therefore earned a total of $12.00 if they resisted for the whole session using the fixed-reward schedule and $12.60 if they resisted for the whole session using the de-escalating reward schedule. There were two other important differences in the procedures used after switching over to the de-escalating reward schedule. Firstly, individuals who were tested with the fixed-reward schedule received a lower priming dose of alcohol (4 button presses, targeted at 25 mg%) than those who were tested with the de-escalating reward schedule (6 button presses, targeted at 40mg%). Following both primes, we provided a sufficient delay for the participants to achieve close to a zero BrAC (i.e. < 5 mg%) before they started the resist phase of the session. Secondly, when we switched over to the de-escalating reward schedule, we required participants to stay at the hospital overnight following the session. This change was enacted as there was some concern that individuals might choose not to infuse alcohol in order to leave the hospital earlier (participants were not permitted to leave after the study session until their breath alcohol concentration fell to 2mg% due to safety considerations). 7 participants completed the paradigm using the fixed-reward schedule and the next 10 participants completed the paradigm using the de-escalating reward schedule.
The resist session was structured as follows. Upon arrival, individuals completed a breathalyzer test and urine drug screen, both of which needed to be negative in order to proceed with the session. Females also needed to provide a negative urine pregnancy test in order to proceed. Participants needed to abstain from alcohol for at least 24 h prior to the session. Participants were assessed with the CIWA-Ar to ensure that they were not experiencing significant symptoms of alcohol withdrawal (a CIWA-Ar score of < 8 was needed to proceed with the session). Individuals were given a standardized 350 kilocalorie meal. An IV catheter was then inserted into the cubital fossa of their non-dominant arm. Participants first completed the priming phase of the session, which was 30 min for the fixed-reward schedule and 45 min for the de-escalating reward schedule. Craving was measured at baseline and during the priming phase using the Alcohol Urge Questionnaire (Bohn et al., 1995). Following the priming phase, participants completed the 120-min resist phase in which they were paid for each minute they were able to resist consuming additional alcohol. Individuals could stop resisting at any time throughout this period. The following questionnaires were administered every 15 min during the resist period: the Drug Effects Questionnaire (Morean et al., 2013), the Alcohol Urge Questionnaire (Bohn et al., 1995), the brief version of the Biphasic Alcohol Effects Scale (Rueger and King, 2013), the Subjective Units of Distress Scale, and five questions related to mood. During the resist session participants were not allowed to use their devices (e.g. mobile phones) or watch television. When an individual signaled that they wished to quit resisting, the time was recorded and the individual was able to begin self-administering alcohol until the end of the 120-min resist interval (the participant could infuse alcohol ad libitum up to a breath alcohol concentration of 120mg%).
2.6. Statistical analysis
To assess participant behavior during experimental laboratory sessions, we report the percentage of individuals who lapsed and the mean time to lapse (+/- standard deviation) for both the fixed-reward and the de-escalating versions of the paradigm. We assigned individuals who resisted throughout the entire session a mean time to lapse of 120 min. We used Fisher's exact test to compare the percentage of individuals who lapsed between different versions of the paradigm and an Independent Samples t-test to compare mean resist time between versions. We used one sample t-tests to determine whether each model demonstrated target model behavior, that is, whether the mean time to lapse in each model was significantly different than 60 min (the half-way point of the laboratory session) (McKee, 2009). To compare whether the rate of lapse differed between paradigm versions, we constructed Kaplan-Meier curves and used a log-rank test to compare the two curves.
In order to assess the effect of craving at baseline and following the priming dose of alcohol on rate of lapse, we used Cox proportional hazards models controlling for paradigm version (i.e. fixed-reward versus de-escalating). We split the sample into high- and low- craving (using a cut-off of 20 for the Alcohol Urge Questionnaire) for graphical representation with accompanying log-rank tests; figures depicting the effect of craving on rate of lapse by paradigm version can be found in the supplement (Figs. S2 and S3). Additional Cox proportional hazards models were created controlling for other potentially relevant covariates including age, sex, baseline drinking (AUDIT score, total drinks in the past 90 days, and binge drinking days over the past 90 days), household income (coded as a binary variable), total lifetime drinks, and full scale IQ; these additional models are available in the supplement (Tables S1 and S2).
We conducted Cox proportional hazards models testing whether ICS scores predicted rate of lapse. We also examined the difference between Impaired Control Scale scores between individuals who lapsed and individuals who resisted. The ICS consists of three parts, an Attempted Control scale, a Failed Control scale, and a Perceived Control Scale. We used Independent Samples t-tests to compare each score between groups, using a Bonferroni-corrected alpha to control for multiple comparisons (alpha = 0.05/3 = 0.017; uncorrected p-values are reported). Depictions of the mean score of each group split by paradigm version can be found in the supplement (Figs. S4–S6). To control for confounding variables, we conducted separate linear regression models controlling for the covariates listed above; these additional models are also available in the supplement (Table S3).
3. Results
3.1. Model behavior for fixed-reward and de-escalating reward schedules
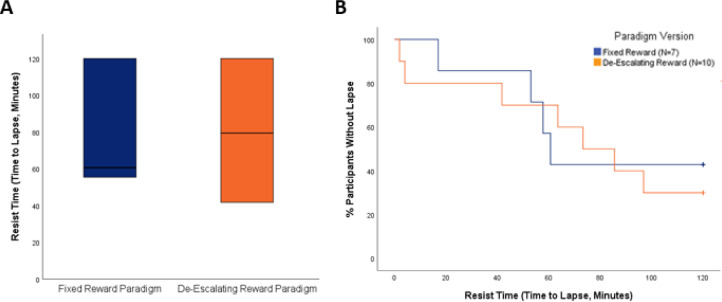
Four out of 7 participants (57.1%) lapsed in the fixed-reward version of the paradigm whereas 7 out of 10 participants (70.0%) lapsed in the de-escalating reward version of the paradigm. The mean time to lapse was 78.34 min (SD = 41.54 min) using the fixed reward-schedule and 72.68 min (SD = 45.01 min) using the de-escalating reward schedule. There were no significant differences in mean time to lapse (t(15) = 0.26, p = 0.80, Fig. 1(a)), the proportion of individuals who lapsed (Fisher's Exact Test p = 0.64), or the rate of lapse (log-rank Χ2 = 0.07, p = 0.79, Fig. 1(b)) between the two versions of the paradigm. A major goal of paradigm development was determining whether each version of the paradigm met target model behavior, that is, whether the reward schedules produced a mean time to lapse at roughly the halfway point of the paradigm (60 min). The time to lapse for both the fixed-reward (t(6) = 1.17, p = 0.29) and de-escalating reward (t(9) = 0.89, p = 0.40) versions of the paradigm did not differ significantly from 60 min.
Fig. 1.
(A) Box plots depicting time to lapse in the fixed reward and de-escalating version of the paradigm. The horizontal line in the middle of each box represents the median value, whereas the bottom and top borders represent the 25th and 75th percentile values respectively. There were no significant differences in mean time to lapse (t(15) = 0.26, p = 0.80) or in the proportion of individuals who lapsed (Fisher's Exact Test p = 0.64) between paradigm versions. (B) Kaplan-Meier curves demonstrating that the rate of lapse was not significantly different by paradigm version (log-rank Χ2 = 0.07, p = 0.79).
3.2. Associations between craving and rate of lapse
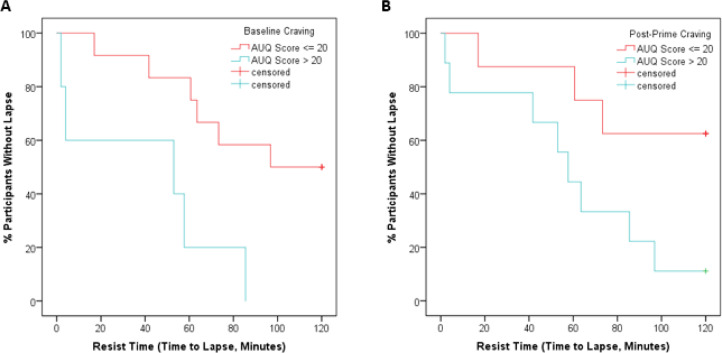
Both baseline craving and post-prime craving predicted rate of lapse, with participants who endorsed greater levels of craving displaying higher rates of lapse (HR = 1.07, 95% CI 1.01 to 1.13, p = 0.02 for baseline craving, HR = 1.08, 95% CI 1.02 to 1.15, p = 0.01 for post-prime craving; Figs. 2, S2, and S3). Paradigm version was not significantly associated with time to lapse in the above models. Additional models controlling for age, sex, baseline drinking, household income, total lifetime drinks, and full scale IQ did not have a major impact on our results (Tables S1 and S2).
Fig. 2.
The above Kaplan-Meier curves illustrate that during the laboratory paradigm, rates of lapse were higher in individuals with higher levels of craving (Alcohol Urge Questionnaire score > 20) at (a) baseline (log-rank Χ2 = 7.3, p < 0.01) and (b) following the priming dose of alcohol (log-rank Χ2 = 4.3, p < 0.05).
3.3. Self-reported impaired control and lapse behavior
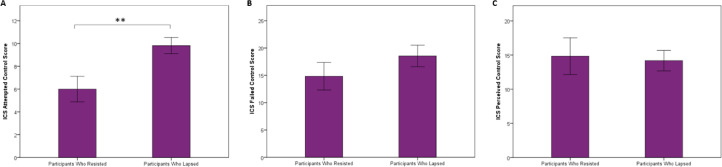
There was a trend level association between ICS Attempted Control score and rate of lapse using a Cox proportional hazards model controlling for paradigm version (HR = 1.18, 95% CI 0.98 to 1.42, p = 0.085). Cox proportional hazards models did not demonstrate any significant effect of ICS Failed Control score or ICS Perceived Control score on rate of lapse. Individuals who lapsed had significantly higher ICS Attempted Control scores than individuals who resisted (mean score: 9.82 versus 6.00, t(15) = -3.01, p < 0.01, Cohen's d = 1.49, Figs. 3(a) and S4), indicating that they had engaged in greater attempts to suppress their drinking over the preceding 6-month period. By contrast, there were no significant differences in ICS Failed Control scores or ICS Perceived Control scores between the lapse group versus the resist group (mean score: 18.55 vs. 14.83, t(15) = -1.14, p = 0.27, Cohen's d = 0.58 for ICS Failed Control score; mean score: 14.18 versus 14.83, t(15) = 0.23, p = 0.82, Cohen's d = 0.11 for ICS Perceived Control score, Figs. 3(b), (c), S5, and S6). We constructed linear regression models with ICS Attempted Control score as the dependent variable and whether an individual lapsed as a categorical independent variable controlling for age, sex, paradigm, baseline drinking, household income, total lifetime drinks, and full scale IQ. These models demonstrated that the association between whether an individual lapsed and ICS Attempted Control score remained significant when controlling for each of these covariates (see Table S3 for all models).
Fig. 3.
Individuals who lapsed during the session (N = 11) endorsed greater attempts to control their drinking over the last 6 months compared to individuals who resisted during the entire session (N = 6) (t(15) = -3.01, p < 0.01). Bars represent mean scores on each part of the Impaired Control Scale +/- standard error, **indicates p < 0.01. Panel (a) depicts ICS Attempted Control score, Panel (b) depicts Failed Control score, and Panel (c) depicts Perceived Control score.
4. Discussion
This pilot study evaluated two versions of a novel human laboratory paradigm designed to assess impaired control over alcohol use. In our paradigm, heavy drinkers were given a priming dose of IV alcohol and received monetary rewards for resisting further alcohol consumption. We evaluated two different reward schedules. In the first, participants received a fixed amount of money for each minute they resisted consuming additional alcohol and in the second, they received a de-escalating reward schedule such that the magnitude of the monetary reward per minute of resistance gradually diminished over the session. We found that both paradigms demonstrated target model behavior and produced similar rates of lapse. Across paradigms, individuals who endorsed greater levels of craving during the session displayed a diminished ability to resist further alcohol consumption. Individuals who lapsed also reported greater attempts to control their drinking over the preceding 6 months compared to those who resisted. This study provides initial evidence that higher levels of craving and a stronger history of attempting to control drinking behavior may be related to an impaired ability to resist alcohol consumption. Individuals with persistent high levels of craving or greater self-reported attempts to control their alcohol intake may therefore be less suitable for a non-abstinence based approach to treatment, which presumes the ability to abstain from additional alcohol after low levels of consumption.
Impaired control over alcohol use has often been linked theoretically to impulsivity and executive function deficits (Leeman et al., 2012; Fillmore, 2003). However, it is possible that impaired control over drinking may be more closely linked to incentive salience. For example, in another small laboratory study assessing impaired control over alcohol in heavy drinking youth, participants were offered incentives to maintain their breath alcohol concentration below 80mg%. It was found that the impaired control group, defined as those who were not able to sustain their consumption below this threshold despite the presence of incentives, demonstrated higher post-priming levels of craving (Wardell et al., 2018). Our findings are also in accordance with smoking lapse paradigms, where craving has been found to be associated with time to lapse (Stevenson et al., 2017; Roche et al., 2014) and pharmacotherapy that reduced craving was found to increase ability to resist smoking (Verplaetse et al., 2017). Craving has also been associated with rate of achieving a binge-level alcohol exposure in ad-libitum IV alcohol self-administration paradigms (Sloan et al., 2020), demonstrating the overall importance of craving in alcohol self-administration behaviors. In clinical settings, craving has been associated with relapse to drinking following outpatient treatment (Bottlender and Soyka, 2004), residential treatment (Stohs et al., 2019), and detoxification (Ledda et al., 2019). Future studies should examine the relative strength of associations between measures of executive function, incentive salience, and impaired control in both the laboratory and real-world settings to better clarify the relationship between these constructs.
In our study, we compared impaired control over alcohol use in the laboratory with self-reported impaired control scale scores. We found that individuals who lapsed during the paradigm had numerically higher ICS Attempted Control and Failed Control scores versus individuals who resisted, although contrary to our hypothesis, only the former comparison was statistically significant. There was no difference in ICS Perceived Control scores, indicating that current perceptions of control over drinking may not match behavior in the laboratory. Our findings differed from those of the laboratory study assessing impaired control in heavy drinking youth, which did not find differences in ICS scores between the impaired control positive and negative group, but did find that ICS Failed Control scores were related to some laboratory indices of impaired control (Wardell et al., 2018).
This study had multiple limitations. Firstly, our sample size was small as we terminated the study early due to the COVID-19 pandemic; we were therefore likely underpowered for some of our statistical comparisons. A small sample size reduces power to detect true differences between groups, and use of small sizes also increases the probability that statistically significant findings are false positives (Button et al., 2013). It is therefore important to regard any findings from this pilot study as preliminary; future studies should attempt to replicate these findings in a larger and more diverse sample of heavy drinkers. This is especially true for comparisons between the two versions of the paradigm, as the sample size for each version (7 for the fixed-reward paradigm and 10 for the de-escalating reward paradigm) was small. Secondly, our sample was overwhelmingly male, so it is unclear if these findings extend to female heavy drinkers. Future studies should attempt to stratify recruitment by sex to determine whether there are any sex differences in lapse behavior and predictors of lapse. Thirdly, due to our small sample size, we were not able to create models simultaneously controlling for all covariates. Larger follow up studies should explore the effects of potential confounding variables on ability to resist alcohol, especially household income, which may impact the relative value of monetary rewards between individuals (Gowin et al., 2019). Also, we only tested two types of reward schedules in this study, but other reward schedules may have yielded different results. For example, a recent study testing a similar paradigm using oral alcohol found that individuals took longer to initiate drinking with a de-escalating reward schedule as compared to no reward schedule (McKee and Verplaetse, 2022). Other paradigm variations, such as adding stressors (McKee and Verplaetse, 2022), changing the priming dose of alcohol, or changing the time between the priming dose of alcohol and the start of the resist phase, could have also affected the results. Although IV alcohol self-administration has advantages such as providing very precise levels of alcohol exposure, IV administration is not a typical route of alcohol administration in real-world settings and eliminates certain important cues such as smell and taste. It will therefore be important to determine whether these findings extend to similar laboratory paradigms that utilize oral alcohol administration. Furthermore, for ethical reasons, our sample consisted only of non-treatment seeking heavy drinkers, which could limit generalizability to clinical populations.
5. Conclusions
This study evaluated an innovative paradigm designed to evaluate impaired control over alcohol use. Across the two reward schedules examined, after receiving a priming dose of alcohol, the majority of heavy drinkers were not able to resist additional alcohol consumption despite being paid to do so. Individuals who endorsed greater levels of craving during the session displayed higher rates of lapse. These results suggest that impaired control over alcohol intake can be modeled in the laboratory and that higher levels of craving in the presence of alcohol may reduce the ability to abstain from further consumption. Future studies should determine whether these findings reflect real-world patterns of drinking and can be extended to clinical samples. Furthermore, additional studies should evaluate whether this paradigm can be developed as a screening platform for novel pharmacotherapies. If so, we may be able to eventually use findings from these paradigms to improve our understanding of which patients are more likely to succeed with non-abstinence-based treatment approaches and develop novel interventions for alcohol use disorder.
Role of funding source
Nothing declared.
Author disclosures
Nothing Declared.
CRediT authorship contribution statement
Matthew E. Sloan: Conceptualization, Methodology, Writing – original draft, Supervision. Joanna R. Sells: Writing – review & editing. Courtney L. Vaughan: Writing – review & editing. James K. Morris: Writing – review & editing. Nancy E. Ortega: Writing – review & editing. Sachin Sundar: Writing – review & editing. Soundarya Soundararajan: Writing – review & editing. Bethany L. Stangl: Writing – review & editing. Joshua Gowin: Writing – review & editing. Sumedha Chawla: Writing – review & editing. Nancy Diazgranados: Writing – review & editing. Sherry A. McKee: Methodology, Writing – review & editing. Andrew Waters: Conceptualization, Methodology, Writing – review & editing, Supervision. Vijay A. Ramchandani: Conceptualization, Methodology, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Acknowledgements
This study was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) Division of Intramural Clinical and Biological Research (Z1A AA000466 and Z1A AA000310). Development of the CAIS software used for the intravenous alcohol self-administration paradigm was supported by Sean O'Connor, MD, and Martin Plawecki, MD, PhD, at the Indiana Alcohol Research Center (NIH P60 AA007611). Interest in using the CAIS software for IV alcohol laboratory studies may be directed to Vijay Ramchandani (vijayr@mail.nih.gov) or Martin Plawecki (mplaweck@iupui.edu).
We would like to thank the medical staff who oversaw the safety of these participants, including Tonette Vinson, CRNP, and Yvonne Horneffer, CRNP. We could not have completed this study without the help of the research and clinical staff of the NIAAA intramural clinical program and the NIH Clinical Center 1-SE unit, 1-HALC alcohol clinic and 5SWS day hospital unit.
Footnotes
ClinicalTrials.gov Identifier: NCT00713492; https://clinicaltrials.gov/ct2/show/NCT00713492?term=vijay+ramchandani&draw=2&rank=5
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dadr.2022.100105.
Contributor Information
Matthew E. Sloan, Email: matthew.sloan@camh.ca.
Vijay A. Ramchandani, Email: vijayr@mail.nih.gov.
Appendix. Supplementary materials
References
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin. Exp. Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Bottlender M, Soyka M. Impact of craving on alcohol relapse during, and 12 months following, outpatient treatment. Alcohol Alcohol. 2004;39:357–361. doi: 10.1093/alcalc/agh073. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Charlet K, Heinz A. Harm reduction-a systematic review on effects of alcohol reduction on physical and mental symptoms. Addict. Biol. 2017;22:1119–1159. doi: 10.1111/adb.12414. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Drug abuse as a problem of impaired control: current approaches and findings. Behav. Cogn. Neurosci. Rev. 2003;2:179–197. doi: 10.1177/1534582303257007. [DOI] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, et al. American Psychiatric Association; Arlington, VA: 2015. Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV) pp. 1–94. [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, et al. The neurocircuitry of impaired insight in drug addiction. Trends Cogn. Sci. 2009;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin J, Sloan ME, Swan JE, et al. The relationship between delay discounting and alcohol dependence in individuals with and without comorbid psychopathology. Psychopharmacology (Berl.) 2019;236:775–785. doi: 10.1007/s00213-018-5113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for Alcohol Use Disorder and Rate of Alcohol Consumption. Am. J. Psychiatry. 2017;174:1094–1101. doi: 10.1176/appi.ajp.2017.16101180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heather N, Adamson SJ, Raistrick D, et al. Initial preference for drinking goal in the treatment of alcohol problems: I. Baseline differences between abstinence and non-abstinence groups. Alcohol Alcohol. 2010;45:128–135. doi: 10.1093/alcalc/agp096. [DOI] [PubMed] [Google Scholar]
- Heather N, Tebbutt JS, Mattick RP, et al. Development of a scale for measuring impaired control over alcohol consumption: a preliminary report. J. Stud. Alcohol. 1993;54:700–709. doi: 10.15288/jsa.1993.54.700. [DOI] [PubMed] [Google Scholar]
- Hodgins DC, Leigh G, Milne R, et al. Drinking goal selection in behavioral self-management treatment of chronic alcoholics. Addict. Behav. 1997;22:247–255. doi: 10.1016/s0306-4603(96)00013-5. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Nagoshi CT, Schwitters SY, et al. Further investigation of racial/ethnic differences and of familial resemblances in flushing in response to alcohol. Behav. Genet. 1984;14:171–178. doi: 10.1007/BF01065539. [DOI] [PubMed] [Google Scholar]
- Ledda R, Battagliese G, Attilia F, et al. Drop-out, relapse and abstinence in a cohort of alcoholic people under detoxification. Physiol. Behav. 2019;198:67–75. doi: 10.1016/j.physbeh.2018.10.009. [DOI] [PubMed] [Google Scholar]
- Leeman RF, O'Malley SS, White MA, et al. Nicotine and food deprivation decrease the ability to resist smoking. Psychopharmacology (Berl.) 2010;212:25–32. doi: 10.1007/s00213-010-1902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Patock-Peckham JA, Potenza MN. Impaired control over alcohol use: An under-addressed risk factor for problem drinking in young adults? Exp. Clin. Psychopharmacol. 2012;20:92–106. doi: 10.1037/a0026463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addict. Biol. 2009;14:99–107. doi: 10.1111/j.1369-1600.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, et al. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J. Psychopharmacol. 2011;25:490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Verplaetse TL. A novel human laboratory alcohol self-administration paradigm for medication screening: Modeling the ability to resist drinking and heavy drinking. Drug and Alcohol Dependence Reports. 2022;4 doi: 10.1016/j.dadr.2022.100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH, Shi J, et al. Developing and Validating a Human Laboratory Model to Screen Medications for Smoking Cessation. Nicotin Tob. Res. 2012;14:1362–1371. doi: 10.1093/ntr/nts090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, de Wit H, King AC, et al. The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology (Berl.) 2013;227:177–192. doi: 10.1007/s00213-012-2954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash AJ. The Twelve Steps and Adolescent Recovery: A Concise Review. Subst Abuse. 2020;14 doi: 10.1177/1178221820904397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA . 2004. NIAAA Council Approves Definition of Binge Drinking. [Google Scholar]
- Ramchandani VA, Plawecki M, Li TK, et al. Intravenous ethanol infusions can mimic the time course of breath alcohol concentrations following oral alcohol administration in healthy volunteers. Alcohol Clin. Exp. Res. 2009;33:938–944. doi: 10.1111/j.1530-0277.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- Roberts W, Shi JM, Tetrault JM, et al. Effects of varenicline alone and in combination with low-dose naltrexone on alcohol-primed smoking in heavy-drinking tobacco users: A preliminary laboratory study. Journal of addiction medicine. 2018;12:227. doi: 10.1097/ADM.0000000000000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DJ, Bujarski S, Moallem NR, et al. Predictors of smoking lapse in a human laboratory paradigm. Psychopharmacology (Berl.) 2014;231:2889–2897. doi: 10.1007/s00213-014-3465-x. [DOI] [PubMed] [Google Scholar]
- Rueger SY, King AC. Validation of the brief Biphasic Alcohol Effects Scale (B-BAES) Alcohol Clin. Exp. Res. 2013;37:470–476. doi: 10.1111/j.1530-0277.2012.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA . 2019. National Survey on Drug Use and Health: Detailed Tables. [Google Scholar]; Available at https://www.samhsa.gov/data/report/2019-nsduh-detailed-tables.
- Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J. Stud. Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Sloan ME, Gowin JL, Janakiraman R, et al. High-risk social drinkers and heavy drinkers display similar rates of alcohol consumption. Addict. Biol. 2020;25:e12734. doi: 10.1111/adb.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan ME, Klepp TD, Gowin JL, et al. The OPRM1 A118G polymorphism: converging evidence against associations with alcohol sensitivity and consumption. Neuropsychopharmacology. 2018;43:1530–1538. doi: 10.1038/s41386-017-0002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Measuring alcohol consumption: Psychosocial and biochemical methods. Humana Press; Totowa, NJ, US: 1992. Timeline follow-back: A technique for assessing self-reported alcohol consumption; pp. 41–72. [Google Scholar]
- Stangl BL, Vatsalya V, Zametkin MR, et al. Exposure-Response Relationships during Free-Access Intravenous Alcohol Self-Administration in Nondependent Drinkers: Influence of Alcohol Expectancies and Impulsivity. Int. J. Neuropsychopharmacol. 2017;20:31–39. doi: 10.1093/ijnp/pyw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JG, Oliver JA, Hallyburton MB, et al. Smoking environment cues reduce ability to resist smoking as measured by a delay to smoking task. Addict. Behav. 2017;67:49–52. doi: 10.1016/j.addbeh.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohs ME, Schneekloth TD, Geske JR, et al. Alcohol Craving Predicts Relapse After Residential Addiction Treatment. Alcohol Alcohol. 2019;54:167–172. doi: 10.1093/alcalc/agy093. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br. J. Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Verplaetse TL, Weinberger AH, Ashare RL, et al. Pilot investigation of the effect of carvedilol on stress-precipitated smoking-lapse behavior. J. Psychopharmacol. 2018;32:1003–1009. doi: 10.1177/0269881118767647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Weinberger AH, Oberleitner LM, et al. Effect of doxazosin on stress reactivity and the ability to resist smoking. J. Psychopharmacol. 2017;31:830–840. doi: 10.1177/0269881117699603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardell JD, Le Foll B, Hendershot CS. Preliminary evaluation of a human laboratory model of impaired control over alcohol using intravenous alcohol self-administration. J. Psychopharmacol. 2018;32:105–115. doi: 10.1177/0269881117723000. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Second Edition. Pearson; San Antonio, TX: 2011. Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.