Abstract
The concept that different systems control episodic and surge secretion of gonadotropin-releasing hormone (GnRH) was well established by the time that GnRH was identified and formed the framework for studies of the physiological roles of GnRH, and later kisspeptin. Here, we focus on recent studies identifying the neural mechanisms underlying these two modes of secretion, with an emphasis on their core components. There is now compelling data that kisspeptin neurons in the arcuate nucleus that also contain neurokinin B (NKB) and dynorphin (i.e., KNDy cells) and their projections to GnRH dendrons constitute the GnRH pulse generator in mice and rats. There is also strong evidence for a similar role for KNDy neurons in sheep and goats, and weaker data in monkeys and humans. However, whether KNDy neurons act on GnRH dendrons and/or GnRH soma and dendrites that are found in the mediobasal hypothalamus (MBH) of these species remains unclear. The core components of the GnRH/luteinising hormone surge consist of an endocrine signal that initiates the process and a neural trigger that drives GnRH secretion during the surge. In all spontaneous ovulators, the core endocrine signal is a rise in estradiol secretion from the maturing follicle(s), with the site of estrogen positive feedback being the rostral periventricular kisspeptin neurons in rodents and neurons in the MBH of sheep and primates. There is considerable species variations in the neural trigger, with three major classes. First, in reflex ovulators, this trigger is initiated by coitus and carried to the hypothalamus by neural or vascular pathways. Second, in rodents, there is a time of day signal that originates in the suprachiasmatic nucleus and activates rostral periventricular kisspeptin neurons and GnRH soma and dendrites. Finally, in sheep nitric oxide-producing neurons in the ventromedial nucleus, KNDy neurons and rostral kisspeptin neurons all appear to participate in driving GnRH release during the surge.
Keywords: GnRH pulses, GnRH surge, kisspeptin, KNDy neurons, steroid feedback
1 |. INTRODUCTION
The concept that tonic luteinising hormone (LH) secretion and the LH surge are controlled by largely separate mechanisms was first proposed by Everett and Sawyer in 19491 and received convincing support from the pioneering work of Halasz and Gorski in the 1960s.2 This model was generally accepted when gonadotropin-releasing hormone (GnRH) was identified as the hypothalamic hormone controlling gonadotropin secretion, and so it is not surprising that it established important parameters for studies of the GnRH neural network. For example, the issue of which GnRH neurons were required for episodic and surge secretion received considerable attention. More recently, it led directly to the hypothesis that different populations of kisspeptin neurons mediate these two modes of GnRH secretion,3 a hypothesis that underlies many of the studies of the role of kisspeptin in reproductive neuroendocrinology. Here, we review recent evidence on the neural mechanisms underlying these two modes of secretion, with an emphasis on the core components of each.
2 |. EPISODIC GnRH/LH SECRETION
The pulsatile nature of LH release was first recognized by the Knobil laboratory in 19704 and, a decade after the identification of GnRH in 1971,5,6 this led to the concept of the “GnRH pulse generator”7,8 as a neural ensemble generating episodic GnRH secretion responsible for pulsatile LH release. This section aims to summarize the progress that has been made in unravelling the genesis of pulsatile secretion and highlights on-going issues and questions.
2.1 |. Characteristics of pulsatile LH secretion
The pulsatile release of LH has been documented in a range of species, with most investigations being undertaken in humans, monkeys, sheep, rats and mice. Across these species, the pattern and frequency of pulsatile LH secretion remains remarkably consistent, with one pulse occurring every 45–60 min in the follicular phase and pulse frequency slowing considerably during the luteal/estrous phase.9 In seasonally breeding species such as the sheep, the frequency of pulsatile LH secretion can be even slower than in the luteal phase during the non-breeding season.10 Adult males exhibit a marked sex difference in pulsatile LH release, with pulses typically occurring once every 3 h but in a relatively disorderly manner.9
It is very likely that the pulse generator is operational before birth with investigations demonstrating a slow pulsatile pattern of LH secretion at mid-gestation in sheep11 and evidence for LH pulses in the early infantile period in humans and monkeys.12–14 As detailed elsewhere,15–17 LH pulse frequency is very much reduced, or even stopped, during the neonatal period only to re-emerge in early adolescence to initiate puberty. Despite its key importance,18,19 the mechanisms (e.g., afferent input or epigenetic changes) responsible for the re-activation of the pulse generator at puberty remain undecided.20
Many studies have examined pulsatile LH secretion in gonadectomized animals and in human females after the menopause, when gonadal steroid negative feedback actions are minimal and the “free-running” state of the pulse generator is apparent. Although there is a uniform increase in LH pulse amplitude across all species, the effects on pulse frequency are more variable. The number of LH pulses increases substantially following gonadectomy in males of all species and in rodent females, whereas there can be little change in LH pulse frequency in female primates and sheep compared with their follicular phase.9 To highlight just how different this can be, the gonadectomy of a male mouse moves the pulse generator from operating, on average, once every 170 min to once every 15 min,21 whereas the gonadectomy of a female monkey leaves LH pulse frequency at approximately one pulse every 60 min, unchanged from the follicular phase rate.4,22 The reasons for these little appreciated sex and species differences in gonadal steroid negative feedback on the pulse generator are not known.
3 |. RELATIONSHIP OF PULSATILE GnRH AND LH SECRETION
The pulsatile profile of GnRH secretion was demonstrated using the “gold-standard” portal bleeding approach of Caraty et al.23 and Clarke et al.,24 as well as with “push–pull” perfusion techniques measuring GnRH levels in and around the median eminence.25–28 Within the technical limitations of sampling frequency and hormone assays, these investigations have demonstrated that pulsatile LH secretion is highly correlated with pulsatile GnRH secretion. Importantly, high resolution sampling of portal blood in ovariectomized (OVX) ewes revealed that GnRH pulses resemble a “square-wave” episode of secretion with an abrupt increase in GnRH secretion occurring over 2 min followed by a 4–5 min plateau phase of variable secretion and an abrupt decline over 3 min.29,30
4 |. NON-LINEAR DYNAMICS OF GnRH AND LH SECRETION AND INTER-PULSE SECRETION
Although the abrupt episodic release of GnRH into the portal system reliably accounts for the regular pulsatile profile of LH secretion, it is important to recognize the existence of inter-pulse secretion and also that there is a dynamic range within which the pulse generator can operate effectively.
The half-life of human and rat LH in circulation varies from 13–40 min31,32 and, accordingly, this equates to a LH pulse duration of approximately 30–60 min in intact animals.33–36 However, outside of the normal physiological state, different pulse generator frequencies can distort and even collapse pulsatile LH secretion. For example, pulse generator frequencies faster than the LH half-life result in each LH pulse beginning before the preceding LH pulse has dissipated. This is encountered in gonadectomized rodents and monkeys where frequent LH pulses occur on a high “baseline” level of LH. Driving the pulse generator at even faster frequencies can result in desensitization within the signaling cascade and the collapse of pulsatile hormone secretion.37,38 This effect of rapid frequencies generates a non-linear, inverted U-shaped relationship between GnRH pulse generator frequency and pulsatile LH secretion that, although not likely to be of physiological relevance, is important when considering experimental manipulations of the system.38
It is apparent from high-temporal resolution measurements that there is a low basal amount of fluctuating secretion of GnRH and LH between pulses.29,33 It is unknown whether this originates from small fluxes in the activity of GnRH neurons themselves or results from the arcuate nucleus (ARN) kisspeptin neuron pulse generator. Interestingly, in sheep, this basal fluctuation increases as the surge onset approaches39; whether this represents desynchronization of pulse generator activity or the initial stages of the GnRH surge generator remains to be determined. However, the physiological role, if any, of inter-pulse fluctuations in GnRH release into the portal vasculature remains unknown.
5 |. NEURAL ENSEMBLE COMPRISING THE PULSE GENERATOR
Multiple models have been proposed to explain the episodic nature of GnRH secretion over the last 20 years.40–42 Although it was always clear that the GnRH neurons were a central component of the pulse generator, controversy existed as to whether the episodic nature of GnRH release was an intrinsic property of GnRH neurons or whether it was driven by an extrinsic mechanism. The impressive ARN multi-unit recording correlates of pulsatile LH secretion43–45 evidenced that an episode generator existed, although attempts to unambiguously identify the cells giving rise to this signal were inconclusive.46,47 Following the discovery of the critical role of kisspeptin in the regulation of GnRH secretion and the realization that ARN kisspeptin neurons may be interconnected, multiple laboratories began questioning their potential role as the GnRH pulse generator.9,48,49 Kisspeptin neuron-selective live cell imaging and optogenetic strategies were eventually able to provide evidence that the ARN kisspeptin neurons were indeed the GnRH pulse generator in mice.50 The remarkable similarity between ARN multi-unit recordings obtained from rats, goats and monkeys and ARN kisspeptin neuron synchronization events in the mouse (see below) indicates that these cells are very likely to be the mammalian GnRH pulse generator.9
In mice and rats, the ARN kisspeptin neuron pulse generator regulates the distal projections of the GnRH neurons near the median eminence (termed “dendrons” because they have characteristics of both axons and dendrites), and not the GnRH cell bodies (Figure 1), to drive pulsatile GnRH secretion.51,52 It appears that electrical activity in the GnRH neuron cell bodies and proximal dendrites may have little role in generating pulsatile GnRH secretion with the dendron operating as an autonomous regulatory zone driven by the ARN kisspeptin neurons.51,53 Although surprising, this is compatible with the ability of brain slices without GnRH cell bodies to maintain pulsatile GnRH secretion.54 It is also consistent with the lack of evidence for electrical synchronization between GnRH neuron cell bodies in adults, although this was identified in cell culture preparations.55,56 Earlier studies had tested the possibility that kisspeptin acts at GnRH terminals in the median eminence of rats, although no kisspeptin-GnRH synapses were found.57 Instead, the ARN kisspeptin neurons use volume transmission to control activity in the distal dendron in the ARN.52 It is important to note that the dendron also receives many direct synaptic inputs from other afferents in mice,58 which may be the conduit for modulatory inputs to GnRH release.
FIGURE 1.
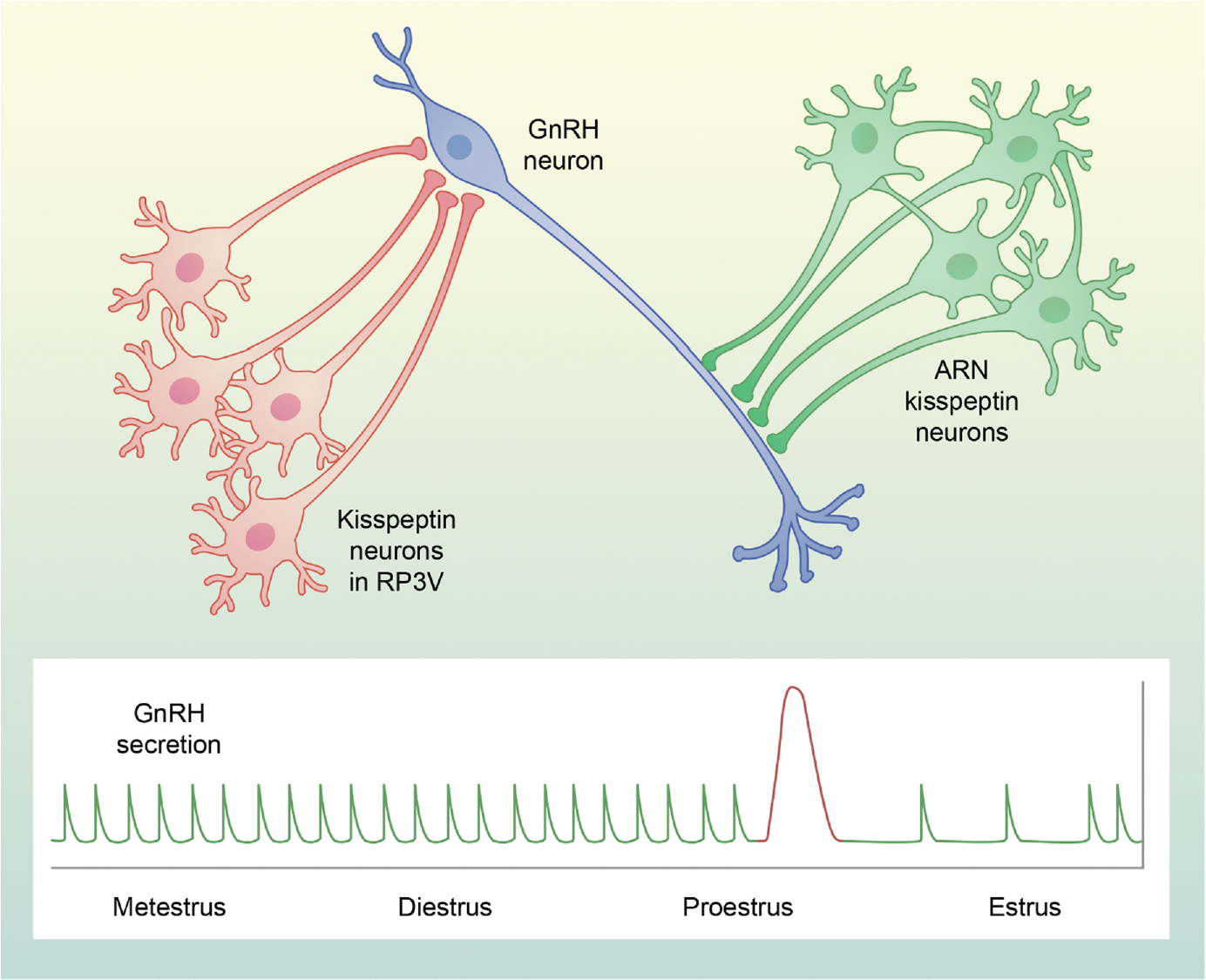
Schematic illustrating the role of kisspeptin neurons in the control of pulsatile and surge secretion of gonadotropin-releasing hormone (GnRH) in mice and rats. Arcuate nucleus (ARN) kisspeptin (KNDy) neurons (green) are an interconnected network that fires synchronously to release kisspeptin on to GnRH (blue) dendrons and drive GnRH and luteinising hormone (LH) pulses (green at bottom) at a constant frequency during most of the estrous cycle. Rostral periventricular area of the third ventricle (RP3V) kisspeptin neurons (red) are stimulated by rising titers of estradiol on proestrus and triggered by a signal from the suprachiasmatic nucleus (not shown) to stimulate GnRH cells bodies to release GnRH during the LH surge (red at bottom) on the afternoon of proestrus. Note that, although KNDy neurons are considered to be the GnRH pulse generator in other species, they may act not solely on GnRH dendrons, but rather on cell bodies in the mediobasal hypothalamus (for details, see text)
A key question is to what extent the rodent organization of the pulse generator is applicable to other species. In species such as sheep and humans, many GnRH neuron cell bodies reside within the mediobasal hypothalamus (MBH) and are known to be direct targets for ARN kisspeptin neurons.59,60 Moreover, there is strong evidence that GnRH cell bodies in the MBH of sheep are the source of GnRH released during a pulse. Specifically, pharmacological or pheromonal stimulation of episodic LH secretion increased Fos expression in GnRH neurons in the MBH, but not the preoptic area (POA)61 and, based on internalization of kappa opioid receptors, dynorphin is released onto GnRH cells in the MBH, but not those in the POA, at the termination of a pulse.62 KNDy neurons (i.e., cells containing kisspeptin, NKB and dynorphin in the ARN) do project to the median eminence in sheep63 and goats,64 but likely do not synapse of GnRH neurons there based on data from the latter65; whether the dendron exists in species outside the rodent is an unresolved question.51
It is important to emphasize that, although the ARN kisspeptin neurons represent the core machinery generating an episodic signal, their sex- and state-dependent frequency of operation is almost certainly dictated or at least modulated by extrinsic inputs to these cells (see below). In this sense, the GnRH pulse generator comprises a core population of kisspeptin neurons modulated by a widely dispersed network of afferent neurons.66,67 Although optogenetic “re-setting” strategies have indicated that episode generation is an inherent property of the ARN kisspeptin neuron population,50 the degree to which different afferent inputs modulate the frequency of episode generation is unknown. As noted above, the distal dendron itself receives many synaptic inputs within the zone of kisspeptin volume transmission providing a locus through which afferent inputs could modulate episodic GnRH release independent of kisspeptin.
6 |. CHARACTERISTICS OF THE ARN KISSPEPTIN NEURON PULSE GENERATOR
The in vivo monitoring of ARN kisspeptin neurons in the mouse with GCaMP fiber photometry has provided detailed information on the episodic behavior of this population.21,50,68 In intact mice, abrupt episodes of synchronized activity last for 1–2 min and occur 5–6 min before the peak of every LH pulse measured from blood taken from the tip of the tail. This is mirrored by optogenetic studies where the artificial activation of kisspeptin neurons for 1 min at 10 Hz generates LH pulses identical to endogenous LH pulses.50 Marked sex differences exist, most notably in the patterning of synchronization episodes. Male mice exhibit synchronization events on average every 3 h throughout the day and night but there is no regular distribution of inter-event intervals indicating that timing is a near-stochastic process.21 By contrast, female mice exhibit much more regular synchronization episodes that occur approximately every 45 min in metestrus, diestrus and proestrus but then slow to occur approximately once every 180 min in estrus.68 These characteristics of the pulse generator mirror the known profiles of pulsatile LH secretion in intact mice33,36 but the photometry approach provide a much higher temporal resolution of pulse generator activity (in the order of seconds) and can be measured continuously for over 24 h.69
Marked changes in kisspeptin neuron pulse generator activity occur after gonadectomy with time-dependent increases in the frequency, duration and amplitude of the synchronization events.21 Most strikingly, the duration and profile of synchronizations is changed so that an individual event can now comprise multiple quickly occurring individual “calcium spikes” that can go on without reaching baseline for up to 10 min. Studies in the monkey looking at ARN multi-unit activity have observed a similar phenomenon (see below) and GnRH pulses in OVX sheep last for approximately 8 min.29 Although the increase in synchronization event frequency drives the increased LH pulse frequency in gonadectomized rodents, the significance of each event being prolonged is unclear.
There is a remarkable concordance between synchronization events recorded from ARN kisspeptin neurons in mice and the multi-unit electrical recordings made from the ARN of rats, goats and monkeys with abrupt changes in activity preceding each LH pulse. In all species, event duration in intact animals is approximately 1–3 min and extended to 10–20 min after gonadectomy.43,45,70–72 Interestingly, it may be that it is only the first minute or two of synchronized activity that determines LH pulse dynamics; the duration of multi-unit events was not found to be associated with differences in LH pulse dynamics in the monkey70 and, in ovariectomized sheep where long duration synchronization events would be expected, the ascending phase of the GnRH pulse only lasts approximately 2 min.29 Although the identity of cells generating multi-unit activity is not yet conclusively established, it appears very likely that they are kisspeptin neurons.47
7 |. OPERATION OF THE ARN KISSPEPTIN (KNDy) NEURON PULSE GENERATOR
An important feature of the arcuate kisspeptin neurons is that they co-express many different neurotransmitters with glutamate, NKB and dynorphin A receiving most attention although several other neuropeptides are expressed in a species-specific manner.73–75 The robust expression of NKB and dynorphin A in almost all arcuate kisspeptin neurons in laboratory animals has led to the useful moniker of “KNDy neurons” for this population.48 Furthermore, the realization that KNDy neurons expressed transcripts for neurokinin 3 receptor (NK3R, encoded by Tacr3) and kappa opioid receptors (KORs, encoded by Oprk1)76 and the corresponding peptides77–79 resulted in the popular KNDy hypothesis of pulse generation.76 This posits that NKB and dynorphin act autosynaptically through recurrent collateral projections amongst KNDy neurons to synchronize their activity. At first, NKB plays the dual role of stimulator of kisspeptin release and synchronizer of large groups of KNDy neurons and, with a short time delay, dynorphin A and KOR undergo an upregulation process and the inhibitory action of KOR activation then decreases NKB and kisspeptin release, which ends each kisspeptin and GnRH pulse.
The basic premise for the KNDy hypothesis has been supported by electrophysiological studies revealing that essentially all KNDy neurons in mice are excited or inhibited by NKB and dynorphin A, respectively80,81 and optogenetic experiments showing that KNDy neurons communicate with each other using NKB and dynorphin A.82 It is likely that KNDy signaling also exists in other species, with goats, sheep and rats all exhibiting somewhat similar anatomical relationships.76,79,83,84 Remarkably, however, there is presently little evidence for dynorphin expression by KNDy neurons in humans,73 although there is support for its transcript being present in ARN (infundibular), and possibly in KNDy, neurons in pre- and postmenopausal women.85
Although attractive, many aspects of the KNDy hypothesis remain untested; in particular, how dynorphin signaling may be delayed relative to NKB release, what mechanisms underlie when a synchronization event is initiated and how a synchronization event can terminate over such a wide range of times (2–15 min). Recent data obtained in rodents indicate that combinatorial KNDy signaling is not occurring at the GnRH neuron itself as only kisspeptin (not glutamate, NKB or dynorphin A) modulates the activity of the dendron.52 In sheep, there is evidence that dynorphin is released onto MBH GnRH cell bodies at the termination of a pulse,62 although the functional significance of this has yet to be tested. One recent suggestion has been that nitric oxide-expressing cells within the ARN are involved in helping to provide a termination signal.86 A major hurdle to investigating these key issues has been the inability to observe spontaneous synchronized activity patterns amongst KNDy neurons in acute brain slice preparations.87
8 |. ESTROGEN FEEDBACK AT THE ARN KISSPEPTIN (KNDy) NEURON PULSE GENERATOR
The GnRH pulse generator is the key target for negative feedback regulation of sex steroids in both sexes.88–90 As estradiol (E2) and androgen levels decrease, GnRH pulsatility quickly ramps up to increase sex steroid synthesis in the gonads through the pulsatile release of LH from the pituitary. However, there is little evidence supporting the presence of estrogen receptor α (ERα) or androgen receptor (required for the central inhibitory action of sex steroids upon GnRH secretion) in GnRH neurons. By contrast, KNDy neurons express ERα and are considered to be a likely target for the estrogen feedback suppression of the pulse generator.91,92 There is substantial evidence in mice for the ERα-dependent modulation of the key genes that encode the three KNDy ligands, kisspeptin, NKB and dynorphin A, as well as their receptors NK3R and KOR in the ARN.76,93,94 Once the circulating levels of sex steroids reach a homeostatic set point, all of these genes become inhibited to potentially reduce signaling amongst the KNDy neurons and thereby suppress pulsatile LH secretion. These inhibitory effects in the ARN have been observed in a large number of studies and several species,95,96 although sometimes discrepancies exist between mRNA and protein levels.97 Whether these discrepancies reflect the effects of estrogen on translation, rapid transport of peptides to nerve terminals or uncoupling of synthesis and secretion is difficult to determine. As noted above, major changes in frequency, duration and amplitude of KNDy neuron synchronization events occur following gonadectomy. Intriguingly, however, estrogen negative feedback of LH secretion remains present when ERα is deleted from KNDy neurons93,94 or even when KNDy neurons are ablated in rats.98 Similarly, the acute knockdown of ERα in KNDy neurons was found to have no effect on LH secretion.99 This indicates that the robust estrogen-regulated gene program in KNDy neurons noted above is not essential for the altered functional output of the kisspeptin pulse generator. There has similarly been difficulty observing any marked differences in the electrical activity of KNDy neurons in the brain slice following gonadectomy100,101 and, if anything, estradiol augments channels predicted to increase the excitability of KNDy neurons.102–104 Thus, the involvement and contribution of KNDy neurons in the estrogen negative feedback mechanism in rodents is unclear and remains a key unanswered question in the field.
9 |. AFFERENT REGULATION OF THE KISSPEPTIN NEURON PULSE GENERATOR
The successful perpetuation of a species relies on the ability of their reproductive system to adapt to changing environmental conditions, from circadian and seasonal changes to food availability and social interactions. This translates into the need for a plethora of upstream neuronal circuits that fine-tune kisspeptin pulse generator frequency to regulate fertility. Kisspeptin-selective viral mapping studies in mice indicate that there are at least 24 different brain regions that provide direct inputs to KNDy neurons.66,67 We focus here on two of that have received considerable attention recently.
9.1 |. Tac1 neuron afferents to the pulse generator
As part of the negative feedback of sex steroids, additional hypothalamic neurons might contribute to the regulation of kisspeptin pulses. Neurons expressing the Tac1 gene in rodents (i.e., Tac1 neurons), encoding substance P (SP) and neurokinin A (NKA), are scattered though the ARN and are abundant in the adjacent ventromedial nucleus (VMN).105 These Tac1 neurons respond to decreasing levels of E2 with a surge in Tac1 expression, suggesting an increase in presynaptic output of SP and NKA onto KNDy neurons, which express the SP receptor (NK1R) but not the NKA receptor (NK2R).105 Direct evidence from electrophysiological recordings of Kiss1 neurons demonstrate that the activation of NK1R leads to action potentials in Kiss1 neurons,80,105 which translates into an increase in circulating LH levels.84 Moreover, congenital deletion of Tac1 in mice (Tac1KO) leads to changes in the frequency and amplitude of LH pulses.106,107 Altogether, there is sufficient evidence supporting a role for Tac1 neurons as a source of afferent regulation of kisspeptin pulses.
9.2 |. Afferents carrying metabolic information to the pulse generator
Energetic availability is arguably the most potent external regulator of the reproductive axis. During periods of famine, the ability to reproduce is dramatically reduced or completely blocked to conserve vital energy.108,109 This translates into a significant decrease in LH pulses.110 Despite the acute stress response of fasting, which can inhibit LH pulsatility through cortisol, prolactin and other stress-induced factors,111 a myriad of metabolic factors also contribute to the short and long term regulation of kisspeptin pulses. One of these is glucose, with hypoglycemia acting via noradrenergic neurons in the brain stem and paraventricular dynorphin neurons to inhibit KNDy cells.109,112 KNDy neurons express receptors for a number of other metabolic factors, including leptin (Lepr),113 insulin (IR)114 and ghrelin (GHSR),115 which are critical peripheral factors that transmit the immediate energetic state to the hypothalamus. However, the selective deletion of these receptors from KNDy neurons does not induce the level of suppression of the reproductive axis observed during fasting,116–118 suggesting that the metabolic regulation of reproduction happens mostly at additional, upstream levels. In this regard, agouti-related peptide (AgRP)/neuropeptide Y (NPY)/GABA neurons, activated by hunger, inhibit KNDy neurons directly using all three transmitters.119,120 By contrast, although POMC neurons likely innervate KNDy neurons and αMSH stimulates LH release in a kisspeptin-dependent manner,121 electrophysiological studies in prepubertal and adult females in diestrus do not show a direct action of αMSH on KNDy neurons.120,121 Although no experimental evidence exists for the direct action of AgRP or POMC neurons in the control of GnRH pulses, based on their direct synaptic appositions with KNDy neurons, it is likely that these energy sensing neurons modulate KNDy neurons to affect pulse generation.
10 |. GENERATION OF GnRH PULSES BEYOND KNDy NEURONS
As would be expected, considerable redundancy exists within the neural circuitry controlling fertility. As few as 10% of GnRH neurons are necessary for normal fertility in male mice122 and, equally, only 20% of ARN kisspeptin neurons are sufficient for normal pulsatile LH secretion to occur in rats.19 However, reflecting their positioning in the cascade of information through the neural network, deletions of Gnrh1123 and Kiss1r124–127 result in complete, irreversible infertility. This contrasts with the deletion of many other genes where only small delays or reversible deficits are found in specific aspects of fertility. Intriguingly, however, the embryonic (but not adult) deletion of kisspeptin-expressing cells is compatible with fertility128 indicating that, under extreme circumstances, the network can compensate and develop in an as yet unknown manner to generate sufficient patterned GnRH secretion. This highlights the plasticity of the GnRH neuronal network as a whole and its ability to compensate for abnormalities during embryonic development. As such, it is difficult to determine the minimal components of the pulse generator with genetic methodologies or spontaneous mutations that delete specific molecules during embryonic development.
The issue of unknown compensation is indeed problematic when considering the necessity of NKB and dynorphin A in pulse generator functioning. Patients with inactivating mutations in the NKB/NK3R system often reverse from their hypogonadal state129,130 and NKB or NK3R knockout mice are fertile.131,132 A body of evidence suggests that the tachykinin family possesses a large degree of cross-reactivity.133,134 Indeed, it is only when all three tachykinin receptors are blocked that kisspeptin neurons are unable to be activated by NKB in brain slices80 or when gonadectomized rats significantly show a decrease in LH levels.135 These effects clearly speak to the ability of tachykinins to compensate in the absence of one of the individual ligand/receptor systems. To investigate the question of cross-reactivity in vivo, a complete tachykinin deficient mouse was generated (Tac1/Tac2KO). Interestingly, males were fertile, and females only had the positive feedback of sex steroids (i.e., preovulatory LH surge) disrupted. Although LH pulses were less frequent and irregular in these mice, they were still able to activate the HPG axis.106 It remains conceivable that compensatory pathways could have arisen in these knockout models during development. The possibility of developmental compensation can be addressed with pharmacological approaches, and these point to species differences in the degree of redundancy in tachykinin signaling. Thus, although specific NK3R antagonists do not block LH pulses in OVX rats,135 they readily do so in ruminants and primates.95 Similarly, much higher doses of agonists to neurokinin A and substance P than NKB are needed to increase the frequency of LH pulses in sheep136 and this differential increases dramatically when specific agonists are used in goats.137
In terms of dynorphin A, both Pdyn138 and Oprk1139 knockout mice are fertile and naloxone does not prevent LH pulses in humans or mice,130 suggesting that other inhibitory mechanisms may intervene to end every kisspeptin pulse. On the other hand, naloxone increases the frequency and amplitude of LH pulses in women and monkeys in the luteal phase and in NKB deficient humans and mice.130,140 Moreover, naloxone acutely increases the amplitude and duration of GnRH pulses in sheep30 and kappa opioid receptor antagonists increase GnRH pulse frequency in sheep141 and multi-unit ARN activity in goats.84 These actions of kappa opioid antagonists support a role of opioid receptors in the initiation of pulse generator synchronization events as well as their dynamics. Of note, the role of dynorphin A as a fundamental component of the pulse generator is also challenged by the relative absence of dynorphin A in human KNDy neurons.73 The notion of additional components in the termination of each kisspeptin pulse would reconcile these species differences. As is the case for the tachykinins, clarification of the importance of dynorphin A in pulse generation will likely require acute KNDy neuron-selective manipulations in vivo.
A curious observation is the induction of small, highly variable fluctuations in LH secretion by continuous i.v. kisspeptin administration in humans with hypogonadal hypogonadism resulting from NKB/NK3R mutations.142 Similarly, the partial suppression of LH pulses with NKB antagonism was fully restored by continuous i.v. infusion of kisspeptin in OVX sheep.143 Although the deficits and mechanisms may be different in these two scenarios, it is unclear how continuous i.v. kisspeptin is able to stimulate episodic LH secretion. Nevertheless, this is reminiscent of optogenetic experiments in which continuous low frequency (1 Hz) activation of KNDy neurons in mice facilitates repetitive LH pulses despite this level of stimulation being insufficient to initiate an LH pulse directly.144,145 Thus, it is possible that sufficient continuous activation of KNDy neurons can move them above their threshold for intrinsic episodic synchronization or, at least, generate irregular patterns of activity that feed through to the GnRH neuron dendron. Given that KNDy neurons themselves are unresponsive to kisspeptin,80 it is unclear how i.v. kisspeptin achieves this. These observations serve to reinforce the importance of continuing work to attain a full understanding of how the kisspeptin neuron pulse generator operates.
11 |. GnRH/LH SURGE
Our understanding of the neural mechanisms controlling the generation of GnRH/LH pulses has been advanced by the concept of a core component, consisting of KNDy and GnRH neurons, and a variety of external inputs to this core. In this section, we attempt to apply this concept to the neural systems controlling the preovulatory GnRH and LH surges. In its most basic form, the core components of the surge consist of an endocrine signal that initiates the process and a neural trigger that drives GnRH secretion during the surge. One important consideration to keep in mind at the outset is that there are significant species differences in the neural mechanisms responsible for the LH surge, ranging from reflex ovulators through rodents, sheep and primates, all of which have GnRH surges to humans, who may not have any neural component.146,147 This approach, as well as space limitations, precludes a discussion of many neural systems that modulate the preovulatory LH surge, but do not appear to be core components; information on these can be found in several recent reviews.35,147–150
12 |. ENDOCRINE COMPONENT
12.1 |. Estradiol
There is very little species variation in the primary ovarian signal that initiates the LH surge: in almost all mammalian species studied to date, the key endocrine requirement for the preovulatory LH surge is an elevated circulating concentration of estradiol that lasts for several hours.147,148 From a physiological perspective, this is an ideal signal by which the ovary can indicate that its follicles now contain mature ova ready to be released. The minimal duration of this signal varies somewhat across species with estimates of approximately 24 h in rats151 and 36 h in monkeys152; in sheep, it varies from 4 to 8 h as the breeding season progresses.153 Although an increment in estradiol levels is a key component of the signal, relatively modest increases are sufficient in rats,154 mice,155 sheep156 and monkeys.152 The one exception to this general pattern of estrogen positive feedback is the horse, in which follicular ablation to disrupt estradiol secretion does not affect the LH surge.157 It should be noted in this regard that preovulatory LH secretion is prolonged (about 5 days) and of low amplitude compared to the LH surge in other species.157
There is more species variation with respect to the sites where estradiol acts to initiate the LH surge, although this specifically applies to neural sites of action. Based on the LH increment induced by exogenous GnRH, estradiol has clear stimulatory effects on the pituitary in spontaneous ovulators (except for the pig147), but not reflex ovulators.158 This effect most likely reflects an increase in the number of GnRH receptors in the pituitary159 and is evident after relatively prolonged (8–36 h, depending on the species) exposure to estradiol.147 There is considerably less information on the neural sites of estrogen positive feedback, with the most definitive data in rodents and sheep. In the former, there is compelling evidence that estradiol acts in the rostral periventricular area of the third ventricle (RP3V),160 most likely in kisspeptin neurons in this region.150 In sheep, local administration of estradiol identified the VMN and ARN as sites of estrogen positive feedback161 and more recent data have pointed to neurons containing somatostatin (SST) and neuronal nitric oxide (nNOS)162 in the VMN, and KNDy neurons163 in the ARN. It should be pointed out that these results do not exclude other neural systems that are also important to the surge; these will be discussed later in this section. Knife cut studies have demonstrated that the core components of the surge system in monkeys are located in the MBH-pituitary unit,164 and estradiol acts in the ARN (infundibular nucleus), but not the VMN, to induce an LH surge.165
12.2 |. Progesterone
The effects of progesterone varies temporally: acute increases are usually stimulatory, whereas chronic increases are almost always inhibitory. The acute stimulatory actions of progesterone have been observed in rodents, primates and a number of other species,148 although they may not be physiologically important in all of them. For example, in monkeys, estrogen treatment alone can induce normal LH surges and, in women, the stimulatory effects of progesterone are modest compared to those of estradiol.166 In rodents, however, progesterone produces a dramatic increase in amplitude of the LH surge. There is a large increase in progesterone concentrations on proestrus that parallels the LH surge167 and, based on replacement studies, this is essential for the amplitude of the normal preovulatory LH surge.147,148,167 Interestingly, progesterone receptors (PR) may be even more important than the native steroid because PR antagonists suppress the LH surge in rats168 and knockout of PR in mice prevents estrogen positive feedback.169 This raises the possibility that ligand-independent activation of PR, or local action by neurosteroids, may play an important role in the estrogen-induced surge in these species.170 In sheep, no acute stimulatory effects of progesterone have been observed, but the increase in progesterone levels during the luteal phase that precedes the follicular phase results in a significantly greater secretion of GnRH (but not LH) during the surge.171
By contrast to the species differences in the acute stimulatory actions of progesterone, chronic exposure to progesterone blocks the estrogen-induced surge in all spontaneous ovulators. This effect of progesterone has been known for 85 years,147 and has likely been conserved to prevent inappropriate LH surges during the luteal phase, pseudopregnancy (in rodents), and pregnancy.172 Interestingly, an analogous inhibition is seen in response to the proestrous progesterone surge in rodents: this prevents the daily LH surges that are seen in estrogen-treated OVX rats,151 on subsequent days of the estrous cycle.173 These inhibitory effects of progesterone are difficult to investigate in reflex ovulators because progesterone inhibits estrous behavior in most of these species.158 However, a recent study found no effect of progesterone on the LH surge in llamas.174 One simple explanation for this species difference is that inappropriate LH surges do not occur in reflex ovulators when progesterone is elevated because estrous behavior and hence coitus does not occur under these circumstances. There is indirect evidence that the stimulatory actions of progesterone in rats increases the GnRH surge175 and direct evidence in sheep that chronic progesterone blocks the GnRH surge.176 The exact sites of progesterone action are largely unexplored, although there is evidence that progesterone acts on kisspeptin neurons in the RP3V to stimulate GnRH secretion in rodents177 and on estrogen-responsive cells in the ARN to block the surge in sheep.178
In summary, an increase in estradiol is the key endocrine signal that induces the preovulatory LH surge, whereas progesterone can have both stimulatory and inhibitory effects depending on the species and timing of its increased secretion. We thus propose that estradiol is the core endocrine component of the preovulatory surge mechanism, with the effects of progesterone being less important and more analogous to an external input to the core components.
13 |. NEURAL TRIGGER
By contrast to the core endocrine component, which is an increase in estradiol secretion in all species, there are marked species differences in the trigger that initiates GnRH secretion during the surge.147 In general, these triggers can be grouped into three major classes: (1) reflex ovulators; (2) rodents in which the surge can only occur at a specific time of day; and (3) other spontaneous ovulators.
13.1 |. Reflex ovulators
The most obvious example of a neural trigger occurs in reflex ovulators because coitus is required for the LH surge. This category includes a large number of mammalian species,158,179 but the neural components of this trigger have only been studied in a few of these, with most work being carried out in rabbits. A detailed consideration of this system is beyond the scope of this review but, in general, fairly diffuse neural inputs from the vaginal and cervix converge on noradrenergic neurons in the brain stem, which then pass the neural signal on to key hypothalamic neural populations (Figure 2). With the exception of GnRH neurons, the important hypothalamic neurons remain to be established. There is one study reporting that mating increases Fos expression in POA, but not ARN, kisspeptin neurons in musk shrews,180 but the role of kisspeptin neurons has not been studied in other reflex ovulators. An interesting subset of reflex ovulators (that emphasizes the variability in these triggers) are camelids, including alpacas and llamas. In these species, coitus is required for ovulation but the signal that coitus has occurred is carried via the circulatory, not the nervous, system. Specifically, there is compelling evidence that nerve growth factor (NGF) in semen is absorbed into the uterine circulation, travels to the hypothalamus and induces the preovulatory surge in GnRH and LH.181–184 Interestingly, in llamas, the stimulatory effects of NGF are dramatically increased by estradiol185 but, as noted above, they are not blocked by progesterone.174 It is not clear whether NGF plays any role in ovulation in other reflex ovulators but, based on the ability of chemical lesions of noradrenergic neurons to block ovulation in rabbits,186 the neural pathway is more critical.
FIGURE 2.
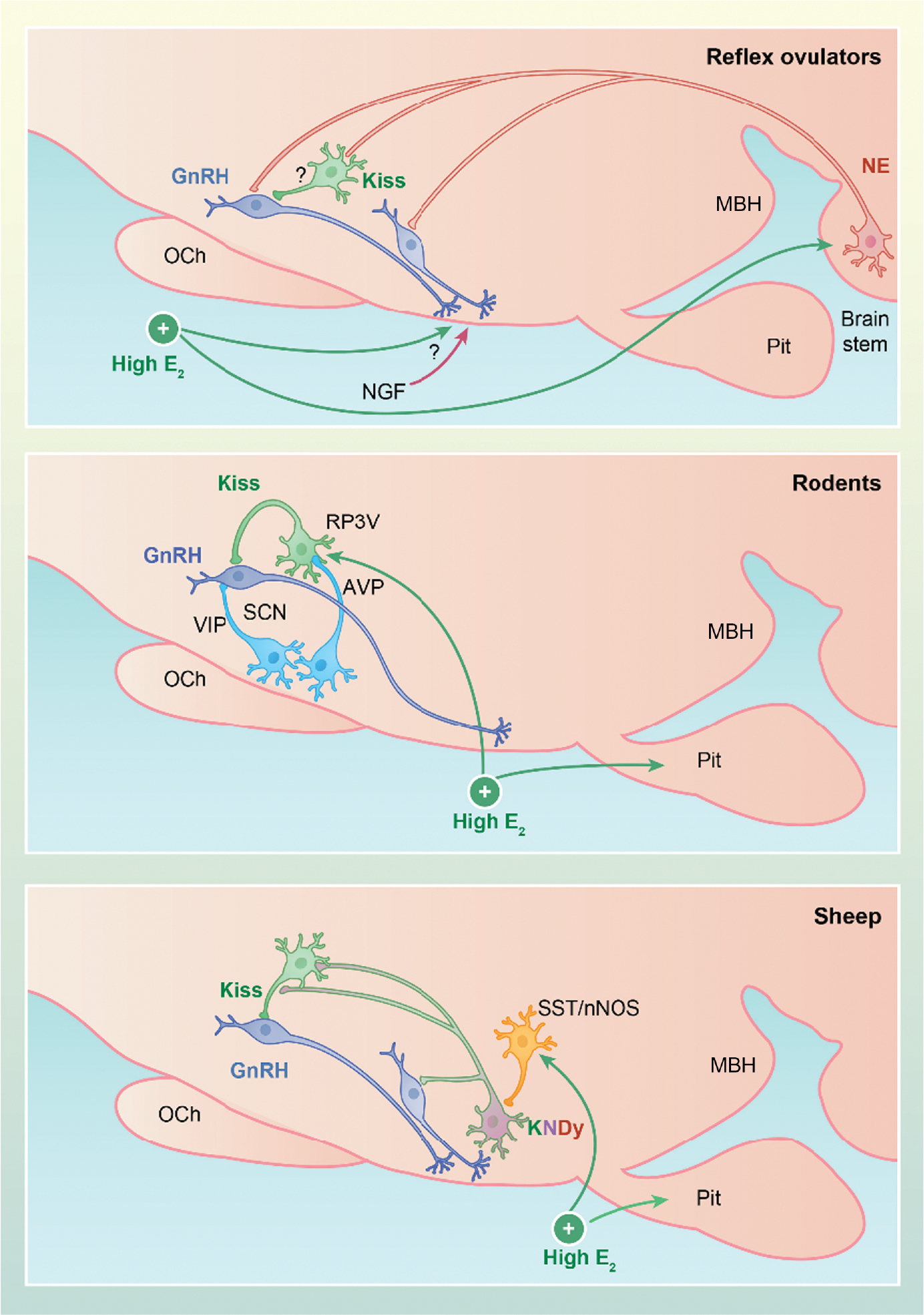
Schematic representation of core components of the gonadotropin-releasing hormone (GnRH) surge generator in reflex ovulators (top), rats and mice (middle), and sheep (bottom). In many reflex ovulators, GnRH release is triggered by a coitus, which acts via noradrenergic (NE) neurons (red) in the brain stem to stimulate rostral kisspeptin (green) neurons (musk shrew) and/or GnRH (blue) neurons in the preoptic area (POA) (rabbits) or mediobasal hypothalamus (MBH) (ferrets). Note that, although Fos expression increases in rostral kisspeptin neurons in the musk shrew, there is no direct evidence that this reflects noradrenergic input as indicated by the question mark on this pathway. Ovarian estradiol facilitates this pathway by acting on NE neurons and likely other neurons in the hypothalamus. In other reflex ovulators, nerve growth factor (NGF) in the semen is carried via the blood stream to stimulate GnRH secretion by mechanisms that remain to be determined. In rodents (middle), estradiol acts on kisspeptin neurons in the rostral periventricular area of the third ventricle (RP3V), which transmits a daily signal from the suprachiasmatic nucleus (SCN) to GnRH cells bodies to drive the preovulatory GnRH surge. Vasoactive intestinal peptide (VIP)-containing neurons from the suprachiasmatic nucleus (SCN) to GnRH neurons may also facilitate this process. In sheep (bottom), estradiol acts on ventromedial nucleus (VMN) neurons that produce nitric oxide and somatostatin (orange), immediately activating KNDy cells (i.e., cells containing kisspeptin, neurokinin B and dynorphin) (purple). This sets in motion unknown processes that, approximately 20 h later, activate these two neural populations and rostral kisspeptin neurons to drive the GnRH surge. Note that the somatostatin (SST)/neuronal nitric oxide (nNOS) neurons likely project to POA GnRH neurons, although this connection has been omitted for simplicity and because these studies used SST, not SST/nNOS, to identify these close contacts. AVP, arginine vasopressin; E2, estradiol; OCh, optic chiasm; Pit, pituitary
13.2 |. Rodents
The neural trigger for the LH surge in rats and mice has been extensively studied and is thus the most-well characterized of the different neural systems responsible for the generation of the GnRH/LH surge. The concept that ovulation in rats is driven by a neural signal occurring only once a day was first proposed in 1950 by Everett and Sawyer.187 Subsequent studies from many groups interested in circadian rhythms, in general, and their role in reproduction, specifically, have demonstrated that this daily signal originates in the suprachiasmatic nucleus (SCN).148,150,167 This signal appears to be carried in part by arginine vasopressin (AVP)-positive afferents from the SCN to the ERα-expressing kisspeptin cells in the RP3V and by SCN projections that contain vasoactive intestinal peptide (VIP) to GnRH cells bodies in the POA.150 Thus, the core neural elements of the GnRH surge generator mechanism in rats and mice consists of VIP and AVP neurons in the SCN, estrogen-responsive RP3V kisspeptin neurons, and POA GnRH soma and processes that project to the median eminence (Figure 2).
13.3 |. Other spontaneous ovulators
It is much more difficult to identify the neural triggers in species in which the GnRH surge is not directly coupled to coitus or a time of day signal from the SCN. This too encompasses a large number of species, although only a few of these have been studied sufficiently to reach any detailed conclusions. It is clear that the LH surge in monkeys,152 sheep,188 and goats189 is not coupled to the time of day, but rather to when the increment in estradiol concentrations that leads to the LH surge occurs,152,156 and this is likely also true of a number of domestic animals.96,190 Thus, one way to view the trigger mechanism in these species is as a clock that is set in motion by the initiation of the estrogen signal. Interestingly, in sheep, this internal timer is independent of the initial estrogen signal. As noted above, the minimal duration of this signal is 4–8 h but, once this has been given, the timing of the LH surge is set; with the same dose of estradiol, it occurs approximately 20 h later regardless of whether the signal lasts for 4, 7 or 14 h.153,191,192 By contrast, estradiol concentrations need to be elevated until the initiation of the LH surge in monkeys.152 However, the duration of this signal is not immutable because the time between the estrogen signal and the LH surge is shorter with higher doses of estradiol in both sheep and monkeys.156,193 In sheep, which have a short 3-day follicular phase, the elevated progesterone from the previous luteal phase also slows the progression of this timer, so that the time between the estrogen signal and the LH surge is prolonged from 16 to 23 h.194 It should be noted that this effect of progesterone to prolong the duration of the GnRH surge generator is distinct from its action to time the occurrence of the follicular phase during the estrous cycle.195 The latter reflects the increase in episodic LH secretion caused by the fall in progesterone at luteolysis that drives the preovulatory rise in estradiol concentrations.196 The mechanisms underlying this timer are unknown but, given its duration, these could include induction of specific genes, synthesis of critical neurotransmitters and/or changes in synaptic inputs.
There is strong correlational data that kisspeptin neurons are involved in estrogen positive feedback in domestic animals96,190 and monkeys because estradiol increases kisspeptin expression in sheep,163,197 goats,198 pigs,199 cattle200,201 and monkeys.202,203 In three of these (sheep,163,197 goats189 and Japanese monkeys203), kisspeptin neurons are also more active, based on Fos expression, just before or during the LH surge. However, only in sheep is there sufficient information to develop a working model for the neural circuitry for the critical systems. Based on neuroanatomical and Fos data, one can propose that the core neural components of the trigger that induces the surge are: GnRH neurons in the POA and MBH, kisspeptin neurons in the ARN and POA, and SST neurons expressing nNOS in the VMN (Figure 2). The GnRH neurons are obviously an essential element of this core, and Fos increases are seen in them,176 in both populations of kisspeptin neurons197 and in SST-nNOS cells in the VMN during the surge,162,204 but not late in the follicular phase. Fos expression is a crude estimate of the role of a neural systems because it can obviously occur in systems that are not essential for the GnRH/LH surge.
Three other approaches can be used to assess the relative importance of systems showing Fos expression: (1) analyzing anatomical connections among these neural populations; (2) determining whether pharmacological manipulation alters the timing of the surge; and (3) identifying the systems that are absolutely essential for the GnRH/LH surge. There is little information on the latter two and it is somewhat contradictory: infusion of a Kiss1r antagonist into the lateral ventricle is the only pharmacological treatment that has been shown to delay the LH surge205 but this treatment only produces a 50% decrease in the amplitude of the LH surge in sheep.63,205 Interestingly, in rodents, a similar treatment completely blocks the LH surge.206 By contrast, infusion of the nitric oxide inhibitor, l-NG-nitro arginine methyl ester, into the lateral ventricle completely blocked the LH surge in ewes,162 although this study involved a limited number of animals and needs to be confirmed by other approaches. There is strong evidence that KNDy neurons in sheep project to GnRH cell bodies in the MBH and POA,60,141 GnRH fibers in the median eminence63 and the POA kisspeptin population,60 but not to SST-nNOS cells in the VMN,207 and these SST-nNOS neurons likely project to GnRH neurons,162,208 as well as both populations of kisspeptin cells.162,207,208 By contrast, the POA kisspeptin population projects to GnRH cells,60 but not KNDy60 or SST-nNOS207 neurons. Based on these connections and the increase in Fos expression reported in SST/nNOS (but not other SST neurons in the vlVMN) and KNDy cells shortly after a surge-inducing increment of estradiol, one current model for the interaction of these core components is that estradiol acts on the SST-nNOS cell population that quickly stimulates KNDy neurons and then both SST/nNOS and KNDy neurons stimulate POA kisspeptin neurons and GnRH neurons during the GnRH surge.162 It should be noted that this model may be overly simplistic and the core component may involve other systems, but it is consistent with the current data on this system. Moreover, as discussed below, other systems have major effects on the GnRH surge in ewes, but are likely modulatory rather than key components of the GnRH surge generator in this species.
14 |. CHARACTERISTICS OF THE HYPOTHALAMIC CORE COMPONENTS
14.1 |. GnRH neurons
Although it is obvious that GnRH neurons must be one of the key core elements in all species, the specific GnRH neurons responsible for the GnRH surge vary. To some extent, this reflects differences in the distribution of GnRH cell bodies. In rodents, Fos expression increases in GnRH neuron soma located primarily in the rostral POA and anterior hypothalamic area (AHA),209 where most GnRH cell bodies are found.148 In guinea pigs,210 rabbits211 and sheep,212 many GnRH neuron soma are found in the POA, but subsets are found in the AHA and MBH, whereas, in ferrets213 and monkeys,214 the majority of cell bodies are in the MBH. Not all of these GnRH subpopulations are necessarily active during the LH surge. In guinea pigs210 and rabbits,211 only those in the POA and AHA have an increase in Fos expression, whereas, in ferrets213 and sheep,176 there is a similar increase in Fos expression in POA, AHA and MBH GnRH neurons. By contrast, no Fos expression is seen in any GnRH neurons in monkeys during the LH surge,215 perhaps indicating a limitation of the use of Fos in this species as an activation marker.
Another unresolved issue is the minimum percentage of GnRH neurons needed for the surge. If one assumes that only those GnRH neurons expressing Fos are active, then approximately 40% of the GnRH soma are active during the surge in rats,209 sheep,176 guinea pigs210 and ferrets.213 It should be noted that this percentage is similar to, or slightly less than, the percentage of GnRH neurons projecting to the median eminence in rats148 and sheep,216 and so most neuroendocrine GnRH neurons may participate in the GnRH/LH surge in these species. However, there are data in mice and sheep suggesting that a much lower percentage is needed for the LH surge and ovulation. Hemizygous mice with a mutation that disrupted migration of GnRH neurons into the brain had a reduction in GnRH neuron cell number to 34% of that in wild-type (WT) mice, but showed normal estrous cycles and fertility.122 By contrast, their homozygous sisters with only 12% of the normal number of GnRH cells had either prolonged or absent estrous cycles and very low fertility. Thus, 34% of the normal GnRH cell numbers is sufficient for normal reproductive function. However, these mice had a much lower amplitude steroid-induced LH surge (1.6 ± 0.9 ng mL−1 vs. 7.4 ± 3.0 ng mL−1 for WT mice). One can conclude from these data that one-third of the normal GnRH cell number is needed for fertility, but a higher percentage is needed for a normal LH surge, indicating that a relatively small percentage of the LH surge is sufficient to induce ovulation. In sheep, there is no direct estimate of the minimal number of GnRH neurons required, but a comparison of GnRH concentrations in the hypophyseal portal blood during an estrogen-induced LH surge with those in which normal LH surges were induced with GnRH infusions indicates that only a small percentage of GnRH secretion during the surge is needed.217 Some of the excess GnRH secretion occurs after the end of the LH surge when GnRH release continues for several hours.218 However, if one limits this comparison to just the period of LH secretion, only approximately 18% of the normal amount of GnRH released is sufficient to produce a normal LH surge.217 Thus, the minimal percentage of GnRH neurons needed for the LH surge may be similar in sheep and rodents.
14.2 |. Kisspeptin neurons
Based on the ability of estradiol to stimulate kisspeptin expression in cells found in the RP3V,91 and earlier data implicating this area in estrogen positive feedback,160 it was proposed that this sub-population of kisspeptin neurons, but not those in the ARN, was critical for the GnRH surge in rodents3 (Figure 1). Because most,123,124,148,219,220 but not all,221 studies found Fos expression during the LH surge in this rostral population of kisspeptin neurons, but not in KNDy cells, it is now generally accepted they are core components of the GnRH surge system. Based on Fos expression, this model appears to apply to one reflex ovulator, the musk shrew,180 but kisspeptin neurons have not been examined in other reflex ovulators. By contrast, data on Fos and kisspeptin expression in sheep support a role for both the POA kisspeptin neurons and KNDy cells in the estrogen-induced LH surge.163,197 Approximately 30%–60% of kisspeptin neurons in rodents219,220 and sheep express Fos during the LH surge,197 whereas only 20%–40% of those in the musk shrew are active following coitus.180
At this time, there are only limited data from other domestic species on the role of kisspeptin subpopulations in the GnRH surge. In OVX goats,189 infusion of a high dose of estradiol that induced an LH surge increased Fos expression in POA, but not ARN, kisspeptin neurons. However, the estrogen infusion did not increase kisspeptin cell numbers in the POA, perhaps because they were higher than expected in controls, based on data from OVX animals in other species. Two reports compared kisspeptin expression between the luteal and follicular phases in cattle, with one reporting an increase in POA kisspeptin expression in the follicular phase,200 and the other reporting only an increase in the ARN.201 One simple explanation for this discrepancy is that samples were taken 1 day after luteolysis, before the estradiol concentration increased, in the latter, and 2 days after in the former, when estradiol concentrations were higher than those in the luteal phase. Thus, taken together, these two reports support the hypothesis that the follicular phase increase in estradiol stimulates POA kisspeptin expression in cattle. It is unclear whether kisspeptin increased in the ARN on day 2 after luteolysis because only two representative images were presented; in one, it appeared to increase, whereas in the other, it did not.200 In pigs, estradiol benzoate induced an increase in kisspeptin mRNA in the rostral population at onset of the LH surge, whereas it had no effect on levels of this mRNA in most regions of the ARN.199 Finally, the effects of estrogen in the guinea pig are complex because of discrepancies in message and protein levels.222 Thus, mRNA levels were elevated in the rostral, and suppressed in the ARN, population during both negative feedback (24 h after estrogen) and positive feedback (42 h after estrogen). By contrast, protein levels were decreased at both time points in the rostral populations, but decreased during negative feedback and increased during positive feedback in the ARN.
In monkeys, two groups202,203 reported that estrogen increased kisspeptin expression in the POA, and one of these203 also reported an increase in Fos expression in this kisspeptin population. The ARN was not examined in one of these, and estrogen failed to increase kisspeptin and Fos expression in the ARN in the other203 compared to untreated OVX monkeys; however, as noted above, a different conclusion might have been reached if estrogen-treated OVX monkeys had been used as controls. A third group223 observed an increase in kisspeptin expression in both the POA and ARN in the late follicular phase compared to the luteal and early follicular phase. Thus, although it is clear that estrogen stimulates kisspeptin expression in POA neurons in monkeys, its effects in the ARN remain to be determined. It should also be kept in mind, that the physiological significance of POA kisspeptin neurons must be evaluated in light of the early work that this area is not needed for the estrogen-induced LH surge in monkeys.164
It remains unclear how many kisspeptin neurons may be required for the surge. One recent study found that mice, in which only 28% of RP3V kisspeptin expressed ERα, did not exhibit the proestrous LH surge although, curiously, the mice continued to exhibit normal estrous cycles.99 Another study used genetic manipulations to decrease kisspeptin neuron number expression by about 50% (heterozygous) and 83% (homozygous) of that in WT female mice.224 There were few reproductive deficits in the heterozygous females, and the homozygous females had relatively normal estrous cycles, with occasional periods of prolonged estrus. However, only 30% of them were fertile, likely as result of a defect in ovulation because their ovaries contained antral preovulatory follicles, but few, or no, corpora lutea. Thus, somewhere between 17% and 50% of the RP3V kisspeptin neurons may be needed for the LH surge in mice.
15 |. UNRESOLVED ISSUES
There is now strong evidence on the core components of the neural trigger responsible for the GnRH surge in rodents and sufficient evidence to propose testable hypotheses in reflex ovulators and sheep. In the latter two species, clearly more work is needed to test the current models (Figure 2). For example, in sheep, there is only one study directly demonstrating a key role for NO in the surge162 and data pointing to a role for KNDy neurons163,197 is largely correlative in nature. In addition, most studies on the projections of these the VMN SST/nNOS neurons only examined SST staining, and so whether they specifically reflect the SST/nNOS neurons remains to be determined. Finally, there are several other nNOS-containing neurons in the hypothalamus that might be involved, although it is interesting to note that only those in the vlVMN appear to be estrogen-sensitive.225 There is even less information available in reflex ovulators with the only data on kisspeptin neurons coming from a study in musk shrews,180 not rabbits or ferrets, species in which much more information is available on the core components activated by copulation. In rodents, the relative importance of AVP and VIP projections to RP3V kisspeptin neurons and GnRH cells, respectively, is unresolved150 and there are no reports of endogenous activity of RP3V neurons during the LH surge similar to those of KNDy neural activity during episodic LH secretion. In addition, a possible role for KNDy neurons in the mechanisms generating the LH surge in rodents has not been completely excluded. For example, disrupting tachykinin signaling by knocking out both Tac1 and Tac2 or with antagonists to NK1 or NK3 blocked the LH surge in mice.106 These data indicate that tachykinins contribute to the LH surge, although the source of this input and whether it is part of the core component or one of many modulatory influences remains to be determined. More direct support for KNDy neurons comes from studies in rats in which many of these neurons were lesioned with saporin conjugated to a NK3R agonist.98,226 Surprisingly, these lesions increased the amplitude of the LH surge, and it has been proposed that these lesions disrupted inhibitory dynorphin inputs to RP3V kisspeptin cells.227 The physiological significance of this inhibitory input is unknown, but it would be interesting to determine whether it plays a role in the blockade of the LH surge on estrous that is induced by the progesterone increase on proestrus.173
In all species, the physiological role of afferent input to the core components of the GnRH surge has not been clearly identified for any of these inputs. Not surprisingly, functional studies in rats have identified more potential afferents than in any other species. These generally fall into stimulatory (including glutamate, norepinephrine and nitric oxide) and inhibitory (including the endogenous opioid peptides, β-endorphin and dynorphin, and RFRP3), although some can have both stimulatory or inhibitory effects.228 In reflex ovulators, early work in rabbits identified acetylcholine and norepinephrine as being essential to ovulation,229 although these likely reflect afferent pathways carrying the coital stimulus to hypothalamic neurons and are thus part of the core components. A few other neurotransmitters have been examined, but only neuropeptide Y and endogenous opioid peptides have been extensively studied. NPY appears to have stimulatory effects on GnRH, acting via norepinephrine, but its role in ovulation remains unclear,179 whereas it is generally agreed that opioids play no role in the LH surge in reflex ovulators.158 In sheep, as in these other species, norepinephrine inputs from the brain stem are stimulatory35,230 and endogenous opioid peptides are inhibitory.35 NKB-responsive cells in the retrochiasmatic area also stimulate GnRH secretion (via kisspeptin), but the phenotype of these cells has not been identified.205,231 Interestingly disrupting these stimulatory systems in sheep only decreases the amplitude of the LH surge in ewes,162,232 whereas it usually completely blocks it in rats.228 This difference raises the possibility that these afferent inputs play a more significant role in rodents than in sheep. Hopefully, future work will reconcile the data from these studies before the discovery of kisspeptin with the current model for the core components of the GnRH surge in these species.
16 |. SUMMARY AND CONCLUSIONS
The discovery of two different modes of LH and GnRH secretion, pulses and surge, which are controlled by distinct neural systems, was central to our understanding of the mechanisms underlying ovarian cyclicity and provided a relatively simple explanation for the ability of estradiol to both inhibit and stimulate LH and GnRH release. Subsequent work has led to the identification of neural elements that are core components of each. Studies in the rodent suggest that the GnRH pulse generator, represented by the ARN KNDy neurons, and the surge generator located in the RP3V operate relatively independently, with net GnRH release being the sum of the two generators operating at any one time.233 In essence, the pulse generator continues throughout the surge but is obscured by the massive release of GnRH.68 The pulse and surge generator appear to regulate different compartments of the GnRH neuron with the RP3V input to the cell bodies and ARN KNDy afferents directed at the dendron near the median eminence (Figure 1).53 Based on the constant frequency of LH pulses36 and KNDy neuron synchronization events68 during the murine estrous cycle, the distinct roles of rodent RP3V and ARN KNDy neurons, and the evidence that these two populations act on GnRH soma/dendrites and dendrons,233 respectively, it appears that these two systems function largely independently in rodents. By contrast, the characteristics of episodic GnRH secretion change dramatically leading up to the GnRH surge in ewes: both an increase in frequency and alteration in the shape of GnRH pulses occur followed by a progressive increase in GnRH secretion between pulses.234 Because the changes in frequency and pulse shape are induced by estrogen positive feedback,39,235 it is likely that the GnRH pulse generator in sheep participates in the generation of the GnRH surge. This hypothesis is consistent with evidence discussed above that KNDy neural activity increases during the LH surge in this species.163,197
Although we have focused on species differences, the most basic neural elements are similar across species that have been studied. Thus, KNDy neurons appear to be the critical neural populations essential for the synchronization of GnRH secretion during episodic secretion. Similarly, the rostral kisspeptin neural system appears to be a major player in the mechanisms responsible for the GnRH surge in most of them.
ACKNOWLEDGEMENTS
We thank the many students, post-doctoral fellows and colleagues who contributed to the development of these models. Supported by NIH R01 HD033916 (MNL; RLG), NIH R01 HD082135 (RLG; MNL), the Wellcome Trust (Grant # 212242/Z/18/Z), and NIH R01 HD090151 (VNM) and R01 HD099084 (VNM).
Funding information
NIH, Grant/Award Number: R01 HD033916, R01 HD082135, R01 HD090151 and R01 HD099084; Wellcome Trust, Grant/Award Number: 212242/Z/18/Z
Footnotes
CONFLICT OF INTERESTS
The authors declare that they have no conflicts of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/jne.13094.
DATA AVAILABILITY
Data sharing is not applicable to this review because no new data were created or analyzed.
REFERENCES
- 1.Everett JW, Sawyer CH, Markee JE. A neurogenic timing factor in control of the ovulatory discharge of luteinizing hormone in the cyclic rat. Endocrinology. 1949;44(3):234–250. [DOI] [PubMed] [Google Scholar]
- 2.Halasz B, Gorski RA. Gonadotrophic hormone secretion in female rats after partial or total interruption of neural afferents to the medial basal hypothalamus. Endocrinology. 1967;80(4):608–622. [DOI] [PubMed] [Google Scholar]
- 3.Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147(3):1154–1158. [DOI] [PubMed] [Google Scholar]
- 4.Dierschke DJ, Bhattacharya AN, Atkinson LE, Knobil E. Circhoral oscillations of plasma LH levels in the ovariectomized rhesus monkey. Endocrinology. 1970;87(5):850–853. [DOI] [PubMed] [Google Scholar]
- 5.Schally AV, Arimura A, Baba Y, et al. Isolation and properties of the FSH and LH-releasing hormone. Biochem Biophys Res Comm. 1971;43(2):393–399. [DOI] [PubMed] [Google Scholar]
- 6.Amoss M, Burgus R, Blackwell R, Vale W, Fellows R, Guillemin R. Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing factor (LRF) of ovine origin. Biochem Biophys Res Comm. 1971;44(1):205–210. [DOI] [PubMed] [Google Scholar]
- 7.Goodman RL, Karsch FJ. The hypothalmic pulse generator: A key determinant of reproductive cycles in sheep. In: Follet BK, Follet DE, eds. Biological Clocks in Seasonal Reproductive Cycles. John Wright and Sons; 1981: 223–236. [Google Scholar]
- 8.Pohl CR, Knobil E. The role of the central nervous system in the control of ovarian function in higher primates. Annu Rev Physiol. 1982;44:583–593. [DOI] [PubMed] [Google Scholar]
- 9.Herbison AE. The gonadotropin-releasing hormone pulse generator. Endocrinology. 2018;159(11):3723–3736. [DOI] [PubMed] [Google Scholar]
- 10.Goodman RL, Bittman EL, Foster DL, Karsch FJ. Alterations in the control of luteinizing hormone pulse frequency underlie the seasonal variation in estradiol negative feedback in the ewe. Biol Reprod. 1982;27(3):580–589. [DOI] [PubMed] [Google Scholar]
- 11.Mesiano S, Hart CS, Heyer BW, Kaplan SL, Grumbach MM. Hormone ontogeny in the ovine fetus. XXVI. A sex difference in the effect of castration on the hypothalamic-pituitary gonadotropin unit in the ovine fetus. Endocrinology. 1991;129(6):3073–3079. [DOI] [PubMed] [Google Scholar]
- 12.Foster DL, Mickelson IH, Ryan KD, Coon GA, Drongowski RA, Holt JA. Ontogeny of pulsatile luteinizing hormone and testosterone secretion in male lambs. Endocrinology. 1978;102(4):1137–1146. [DOI] [PubMed] [Google Scholar]
- 13.Plant TM. A striking sex difference in the gonadotropin response to gonadectomy during infantile development in the rhesus monkey (Macaca mulatta). Endocrinology. 1986;119(2):539–545. [DOI] [PubMed] [Google Scholar]
- 14.de Zegher F, Devlieger H, Veldhuis JD. Pulsatile and sexually dimorphic secretion of luteinizing hormone in the human infant on the day of birth. Pediatr Res. 1992;32(5):605–607. [DOI] [PubMed] [Google Scholar]
- 15.Foster DL, Hileman SM. Puberty in the sheep. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction, 4th ed. Elsevier; 2015: 1441–1485. [Google Scholar]
- 16.Plant TM, Witchel SF, Terasawa E. Puberty in nonhuman primates and humans. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction, 4th ed. Elsevier; 2015: 1487–1536. [Google Scholar]
- 17.Prevot V Puberty in the rat and mouse. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction, 4th ed. Elsevier; 2015: 1395–1439. [Google Scholar]
- 18.Uenoyama Y, Inoue N, Nakamura S, Tsukamura H. Central mechanism controlling pubertal onset in mammals: a triggering role of kisspeptin. Front Endocrinol. 2019;10:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagae M, Uenoyama Y, Okamoto S, et al. Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc Natl Acad Sci USA. 2021;118(5):e2009156118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naulé L, Maione L, Kaiser UB. A sensitive window of hypothalamic development and plasticity. Endocrinology. 2021;162(1):bqaa209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han SY, Kane G, Cheong I, Herbison AE. Characterization of GnRH pulse generator activity in male mice using GCaMP fiber photometry. Endocrinology. 2019;160(3):557–567. [DOI] [PubMed] [Google Scholar]
- 22.Norman RL, Lindstrom SA, Bangsberg D, Ellinwood WE, Gliessman P, Spies HG. Pulsatile secretion of luteinizing hormone during the menstrual cycle of rhesus macaques. Endocrinology. 1984;115(1):261–266. [DOI] [PubMed] [Google Scholar]
- 23.Caraty A, Orgeur P, Thiery JC. Demonstration of the pulsatile secretion of LH-RH into hypophysial portal blood of ewes using an original technic for multiple samples. Comptes rendus des seances de l’Academie des sciences. Serie III, Sciences de la vie. 1982;295(2):103–106. [PubMed] [Google Scholar]
- 24.Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111(5):1737–1739. [DOI] [PubMed] [Google Scholar]
- 25.Levine JE, Pau KY, Ramirez VD, Jackson GL. Simultaneous measurement of luteinizing hormone-releasing hormone and luteinizing hormone release in unanesthetized, ovariectomized sheep. Endocrinology. 1982;111(5):1449–1455. [DOI] [PubMed] [Google Scholar]
- 26.Levine JE, Ramirez VD. In vivo release of luteinizing hormone-releasing hormone estimated with push-pull cannulae from the mediobasal hypothalami of ovariectomized, steroid-primed rats. Endocrinology. 1980;107(6):1782–1790. [DOI] [PubMed] [Google Scholar]
- 27.Levine JE, Norman RL, Gliessman PM, Oyama TT, Bangsberg DR, Spies HG. In vivo gonadotropin-releasing hormone release and serum luteinizing hormone measurements in ovariectomized, estrogen-treated rhesus macaques. Endocrinology. 1985;117(2):711–721. [DOI] [PubMed] [Google Scholar]
- 28.Terasawa E, Krook C, Hei DL, Gearing M, Schultz NJ, Davis GA. Norepinephrine is a possible neurotransmitter stimulating pulsatile release of luteinizing hormone-releasing hormone in the rhesus monkey. Endocrinology. 1988;123(4):1808–1816. [DOI] [PubMed] [Google Scholar]
- 29.Moenter SM, Brand RM, Midgley AR, Karsch FJ. Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology. 1992;130(1):503–510. [DOI] [PubMed] [Google Scholar]
- 30.Goodman RL, Parfitt DB, Evans NP, Dahl GE, Karsch FJ. Endogenous opioid peptides control the amplitude and shape of gonadotropin-releasing hormone pulses in the ewe. Endocrinology. 1995;136(6):2412–2420. [DOI] [PubMed] [Google Scholar]
- 31.Hakola K, Haavisto AM, Pierroz DD, et al. Recombinant forms of rat and human luteinizing hormone and follicle-stimulating hormone; comparison of functions in vitro and in vivo. J Endocrinol. 1998;158(3):441–448. [DOI] [PubMed] [Google Scholar]
- 32.Burgon PG, Stanton PG, Robertson DM. In vivo bioactivities and clearance patterns of highly purified human luteinizing hormone isoforms. Endocrinology. 1996;137(11):4827–4836. [DOI] [PubMed] [Google Scholar]
- 33.Coquelin A, Desjardins C. Luteinizing hormone and testosterone secretion in young and old male mice. Am J Physiol. 1982;243(3):E2 57–E263. [DOI] [PubMed] [Google Scholar]
- 34.Fox SR, Smith MS. Changes in the pulsatile pattern of luteinizing hormone secretion during the rat estrous cycle. Endocrinology. 1985;116(4):1485–1492. [DOI] [PubMed] [Google Scholar]
- 35.Goodman RL, Inskeep EK. Control of the ovarian cycle of the sheep. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction, 4th ed. Elsevier; 2015: 1259–1305. [Google Scholar]
- 36.Czieselsky K, Prescott M, Porteous R, et al. Pulse and surge profiles of luteinizing hormone secretion in the mouse. Endocrinology. 2016;157(12):4794–4802. [DOI] [PubMed] [Google Scholar]
- 37.Wildt L, Hausler A, Marshall G, et al. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109(2):376–385. [DOI] [PubMed] [Google Scholar]
- 38.Han SY, Cheong I, McLennan T, Herbison AE. Neural determinants of pulsatile luteinizing hormone secretion in male mice. Endocrinology. 2020;161(2):bqz045. [DOI] [PubMed] [Google Scholar]
- 39.Evans NP, Dahl GE, Mauger D, Karsch FJ. Estradiol induces both qualitative and quantitative changes in the pattern of gonadotropin-releasing hormone secretion during the presurge period in the ewe. Endocrinology. 1995;136(4):1603–1609. [DOI] [PubMed] [Google Scholar]
- 40.Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19(3):302–330. [DOI] [PubMed] [Google Scholar]
- 41.Moenter SM, DeFazio AR, Pitts GR, Nunemaker CS. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front Neuroendocrinol. 2003;24(2):79–93. [DOI] [PubMed] [Google Scholar]
- 42.Terasawa E Luteinizing hormone-releasing hormone (LHRH) neurons: mechanism of pulsatile LHRH release. Vitam Horm. 2001;63:91–129. [DOI] [PubMed] [Google Scholar]
- 43.Kimura F, Nishihara M, Hiruma H, Funabashi T. Naloxone increases the frequency of the electrical activity of luteinizing hormone-releasing hormone pulse generator in long-term ovariectomized rats. Neuroendocrinology. 1991;53(1):97–102. [DOI] [PubMed] [Google Scholar]
- 44.O’Byrne KT, Knobill E. Electrophysiological approaches to gonadotrophin releasing hormone pulse generator activity in the rhesus monkey. Hum Reprod. 1993;8(Suppl 2):37–40. [DOI] [PubMed] [Google Scholar]
- 45.Mori Y, Tanaka T. Electrophysiological approach to the hypothalamic GnRH pulse generator. J Reprod Fertil Suppl. 1995;49:231–243. [PubMed] [Google Scholar]
- 46.Silverman AJ, Wilson R, Kesner JS, Knobil E. Hypothalamic localization of multiunit electrical activity associated with pulsatile LH release in the rhesus monkey. Neuroendocrinology. 1986;44(2):168–171. [DOI] [PubMed] [Google Scholar]
- 47.Ohkura S, Takase K, Matsuyama S, et al. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol. 2009;21(10):813–821. [DOI] [PubMed] [Google Scholar]
- 48.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maeda K, Ohkura S, Uenoyama Y, et al. Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brain Res. 2010;1364:103–115. [DOI] [PubMed] [Google Scholar]
- 50.Clarkson J, Han SY, Piet R, et al. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci USA. 2017;114(47):E10216–E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herbison AE. The dendron and episodic neuropeptide release. J Neuroendocrinol. 2021;33:e13024. [DOI] [PubMed] [Google Scholar]
- 52.Liu X, Yeo SH, McQuillan HJ, et al. Highly redundant neuropeptide volume co-transmission underlying episodic activation of the GnRH neuron dendron. eLife. 2021;10:e62455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L, Guo W, Shen X, et al. Different dendritic domains of the GnRH neuron underlie the pulse and surge modes of GnRH secretion in female mice. eLife. 2020;9:e53945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Purnelle G, Gerard A, Czajkowski V, Bourguignon JP. Pulsatile secretion of gonadotropin-releasing hormone by rat hypothalamic explants without cell bodies of GnRH neurons [corrected]. Neuroendocrinology. 1997;66(5):305–312. [DOI] [PubMed] [Google Scholar]
- 55.Herbison A Physiology of the adult gonadotropin-releasing hormone neuronal network. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction, 4th ed. Elsevier; 2015: 399–467. [Google Scholar]
- 56.Progress Constantin S. and challenges in the search for the mechanisms of pulsatile gonadotropin-releasing hormone secretion. Front Endocrinol. 2017;8:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uenoyama Y, Inoue N, Pheng V, et al. Ultrastructural evidence of kisspeptin-gonadotrophin-releasing hormone (GnRH) interaction in the median eminence of female rats: implication of axo-axonal regulation of GnRH release. J Neuroendocrinol. 2011;23(10):863–870. [DOI] [PubMed] [Google Scholar]
- 58.Moore AM, Prescott M, Czieselsky K, et al. Synaptic innervation of the GnRH neuron distal dendron in female mice. Endocrinology. 2018;159(9):3200–3208. [DOI] [PubMed] [Google Scholar]
- 59.Hrabovszky E, Ciofi P, Vida B, et al. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neuorsci. 2010;31(11):1984–1998. [DOI] [PubMed] [Google Scholar]
- 60.Merkley CM, Coolen LM, Goodman RL, Lehman MN. Evidence for changes in numbers of synaptic inputs onto KNDy and GnRH neurones during the preovulatory LH surge in the ewe. J Neuroendocrinol. 2015;27(7):624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boukhliq R, Goodman RL, Berriman SJ, Adrian B, Lehman MN. A subset of gonadotropin-releasing hormone neurons in the ovine medial basal hypothalamus is activated during increased pulsatile luteinizing hormone secretion. Endocrinology. 1999;140(12):5929–5936. [DOI] [PubMed] [Google Scholar]
- 62.Weems PW, Coolen LM, Hileman SM, et al. Evidence that dynorphin acts upon KNDy and GnRH neurons during GnRH pulse termination in the ewe. Endocrinology. 2018;159(9):3187–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith JT, Li Q, Yap KS, et al. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152(3):1001–1012. [DOI] [PubMed] [Google Scholar]
- 64.Wakabayashi Y, Yamamura T, Sakamoto K, Mori Y, Okamura H. Electrophysiological and morphological evidence for synchronized GnRH pulse generator activity among kisspeptin/neurokinin B/Dynorphin A (KNDy) neurons in goats. J Reprod Dev. 2013;59(1):40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsuyama S, Ohkura S, Mogi K, et al. Morphological evidence for direct interaction between kisspeptin and gonadotropin-releasing hormone neurons at the median eminence of the male goat: an immunoelectron microscopic study. Neuroendocrinology. 2011;94(4):323–332. [DOI] [PubMed] [Google Scholar]
- 66.Moore AM, Coolen LM, Lehman MN. Kisspeptin/Neurokinin B/Dynorphin (KNDy) cells as integrators of diverse internal and external cues: evidence from viral-based monosynaptic tract-tracing in mice. Sci Rep. 2019;9(1):14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yeo SH, Kyle V, Blouet C, Jones S, Colledge WH. Mapping neuronal inputs to Kiss1 neurons in the arcuate nucleus of the mouse. PLoS One. 2019;14(3):e0213927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McQuillan HJ, Han SY, Cheong I, Herbison AE. GnRH pulse generator activity across the estrous cycle of female mice. Endocrinology. 2019;160(6):1480–1491. [DOI] [PubMed] [Google Scholar]
- 69.Han SY, Clarkson J, Piet R, Herbison AE. Optical approaches for interrogating neural circuits controlling hormone secretion. Endocrinology. 2018;159(11):3822–3833. [DOI] [PubMed] [Google Scholar]
- 70.Williams CL, Thalabard JC, O’Byrne KT, et al. Duration of phasic electrical activity of the hypothalamic gonadotropin-releasing hormone pulse generator and dynamics of luteinizing hormone pulses in the rhesus monkey. Proc Natl Acad Sci USA. 1990;87(21):8580–8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilson RC, Kesner JS, Kaufman JM, Uemura T, Akema T, Knobil E. Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology. 1984;39(3):256–260. [DOI] [PubMed] [Google Scholar]
- 72.O’Byrne KT, Thalabard JC, Grosser PM, et al. Radiotelemetric monitoring of hypothalamic gonadotropin-releasing hormone pulse generator activity throughout the menstrual cycle of the rhesus monkey. Endocrinology. 1991;129(3):1207–1214. [DOI] [PubMed] [Google Scholar]
- 73.Skrapits K, Borsay BA, Herczeg L, Ciofi P, Liposits Z, Hrabovszky E. Neuropeptide co-expression in hypothalamic kisspeptin neurons of laboratory animals and the human. Front Neurosci. 2015;9:29. 10.3389/fnins.2015.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uenoyama Y, Nagae M, Tsuchida H, Inoue N, Tsukamura H. Role of KNDy neurons expressing kisspeptin, neurokinin B, and dynorphin A as a GnRH pulse generator controlling mammalian reproduction. Front Endocrinol. 2021;12:724632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ikegami K, Watanabe Y, Nakamura S, et al. Cellular and molecular mechanisms regulating the KNDy neuronal activities to generate and modulate GnRH pulse in mammals. Front Neuroendocrinol. 2021;64:100968. [DOI] [PubMed] [Google Scholar]
- 76.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489(3):372–386. [DOI] [PubMed] [Google Scholar]
- 78.Amstalden M, Coolen LM, Hemmerle AM, et al. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2010;22(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weems PW, Witty CF, Amstalden M, Coolen LM, Goodman RL, Lehman MN. kappa-Opioid receptor is colocalized in GnRH and KNDy cells in the female ovine and rat brain. Endocrinology. 2016;157(6):2367–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154:2750–2760. [DOI] [PubMed] [Google Scholar]
- 81.Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and kappa-opioid receptors in adult male mice. Endocrinology. 2013;154:2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qiu J, Nestor CC, Zhang C, et al. High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife. 2016;5:e16246. 162 10.17554/eLife.16246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498(5):712–726. [DOI] [PubMed] [Google Scholar]
- 84.Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rometo AM, Rance NE. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol. 2008;20(12):1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Constantin S, Reynolds D, Oh A, Pizano K, Wray S. Nitric oxide resets kisspeptin-excited GnRH neurons via PIP2 replenishment. Proc Natl Acad Sci USA. 2021;118(1):e2012339118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Piet R, de Croft S, Liu X, Herbison AE. Electrical properties of kisspeptin neurons and their regulation of GnRH neurons. Front Neuroendocrinol. 2015;36:15–27. [DOI] [PubMed] [Google Scholar]
- 88.Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202(4368):631–633. [DOI] [PubMed] [Google Scholar]
- 89.Plant TM. The neurobiological mechanism underlying hypothalamic GnRH pulse generation: the role of kisspeptin neurons in the arcuate nucleus. F1000Research. 2019;8:982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herbison AE. Physiology of the gonadotropin-releasing hormone neuronal network. In: Neiil JD, ed. Knobil and Neill’s Physiology of Reproduction, 3rd ed. Elsevier; 2006: 1415–1482. [Google Scholar]
- 91.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686–3692. [DOI] [PubMed] [Google Scholar]
- 92.Smith JT, Dungan HM, Stoll EA, et al. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146(7):2976–2984. [DOI] [PubMed] [Google Scholar]
- 93.Dubois SL, Acosta-Martinez M, DeJoseph MR, et al. Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor alpha in kisspeptin neurons. Endocrinology. 2015;156(3):1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Greenwald-Yarnell ML, Marsh C, Allison MB, et al. ERalpha in Tac2 neurons regulates puberty onset in female mice. Endocrinology. 2016;157(4):1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moore AM, Coolen LM, Porter DT, Goodman RL, Lehman MN. KNDy cells revisited. Endocrinology. 2018;159(9):3219–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Okamura H, Yamamura T, Wakabayashi Y. Kisspeptin as a master player in the central control of reproduction in mammals: an overview of kisspeptin research in domestic animals. Anim Sci J. 2013;84(5):369–381. [DOI] [PubMed] [Google Scholar]
- 97.Kanaya M, Iwata K, Ozawa H. Distinct dynorphin expression patterns with low- and high-dose estrogen treatment in the arcuate nucleus of female rats. Biol Reprod. 2017;97(5):709–718. [DOI] [PubMed] [Google Scholar]
- 98.Mittelman-Smith MA, Krajewski-Hall SJ, McMullen NT, Rance NE. Ablation of KNDy neurons results in hypogonadotropic hypogonadism and amplifies the steroid-induced LH surge in female rats. Endocrinology. 2016;157(5):2015–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang L, Vanacker C, Burger LL, et al. Genetic dissection of the different roles of hypothalamic kisspeptin neurons in regulating female reproduction. eLife. 2019;8:e43999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Croft S, Piet R, Mayer C, Mai O, Boehm U, Herbison AE. Spontaneous kisspeptin neuron firing in the adult mouse reveals marked sex and brain region differences but no support for a direct role in negative feedback. Endocrinology. 2012;153(11):5384–5393. [DOI] [PubMed] [Google Scholar]
- 101.Vanacker C, Moya MR, DeFazio RA, Johnson ML, Moenter SM. Long-term recordings of arcuate nucleus kisspeptin neurons reveal patterned activity that is modulated by gonadal steroids in male mice. Endocrinology. 2017;158(10):3553–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ronnekleiv OK, Zhang C, Bosch MA, Kelly MJ. Kisspeptin and gonadotropin-releasing hormone neuronal excitability: molecular mechanisms driven by 17beta-estradiol. Neuroendocrinology. 2015;102(3):184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang C, Bosch MA, Qiu J, Ronnekleiv OK, Kelly MJ. 17beta-Estradiol increases persistent Na(+) current and excitability of AVPV/PeN Kiss1 neurons in female mice. Mol Endocrinol. 2015;29(4):518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qiu J, Rivera HM, Bosch MA, et al. Estrogenic-dependent glutamatergic neurotransmission from kisspeptin neurons governs feeding circuits in females. eLife. 2018;7:e35656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Navarro VM, Bosch MA, Leon S, et al. The integrated hypothalamic tachykinin-kisspeptin system as a central coordinator for reproduction. Endocrinology. 2015;156(2):627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.León S, Fergani C, Talbi R, et al. Tachykinin signaling is required for induction of the preovulatory luteinizing hormone surge and normal luteinizing hormone pulses. Neuroendocrinology. 2021;111(6):542–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Talbi R, Ferrari K, Choi JH, et al. Characterization of the action of tachykinin signaling on pulsatile LH secretion in male mice. Endocrinology. 2021;162(8):bqab074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Navarro VM. Metabolic regulation of kisspeptin - the link between energy balance and reproduction. Nat Rev Endocrinol. 2020;16(8):407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tsukamura H Kobayashi Award 2019: nThe neuroendocrine regulation of the mammalian reproduction. Gen Comp Endocrinol. 2022;315:113755. [DOI] [PubMed] [Google Scholar]
- 110.Kreisman MJ, Tadrousse KS, McCosh RB, Breen KM. Neuroendocrine basis for disrupted ovarian cyclicity in female mice during chronic undernutrition. Endocrinology. 2021;162(8):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McCosh RB, Breen KM, Kauffman AS. Neural and endocrine mechanisms underlying stress-induced suppression of pulsatile LH secretion. Mol Cell Endocrinol. 2019;498:110579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tsuchida H, Mostari P, Yamada K, et al. Paraventricular dynorphin A neurons mediate LH pulse suppression induced by hindbrain glucoprivation in female rats. Endocrinology. 2020;161(11):bqaa161. [DOI] [PubMed] [Google Scholar]
- 113.Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18(4):298–303. [DOI] [PubMed] [Google Scholar]
- 114.Campbell JN, Macosko EZ, Fenselau H, et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci. 2017;20(3):484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Frazao R, Dungan Lemko HM, da Silva RP, et al. Estradiol modulates Kiss1 neuronal response to ghrelin. Am J Physiol Endocrinol Metab. 2014;306(6):E606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol. 2003;23(22):7973–7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Donato J Jr, Cravo RM, Frazao R, et al. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Investig. 2011;121(1):355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qiu X, Dao H, Wang M, et al. Insulin and leptin signaling interact in the mouse kiss1 neuron during the peripubertal period. PLoS One. 2015;10(5):e0121974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Padilla SL, Qiu J, Nestor CC, et al. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc Natl Acad Sci USA. 2017;114(9):2413–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hessler S, Liu X, Herbison AE. Direct inhibition of arcuate kisspeptin neurones by neuropeptide Y in the male and female mouse. J Neuroendocrinol. 2020;32(5):e12849. [DOI] [PubMed] [Google Scholar]
- 121.Manfredi-Lozano M, Roa J, Ruiz-Pino F, et al. Defining a novel leptin-melanocortin-kisspeptin pathway involved in the metabolic control of puberty. Mol Metab. 2016;5(10):844–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology. 2008;149(2):597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Adachi S, Yamada S, Takatsu Y, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53(2):367–378. [DOI] [PubMed] [Google Scholar]
- 124.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100(19):10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. [DOI] [PubMed] [Google Scholar]
- 126.Uenoyama Y, Nakamura S, Hayakawa Y, et al. Lack of pulse and surge modes and glutamatergic stimulation of luteinising hormone release in Kiss1 knockout rats. J Neuroendocrinol. 2015;27(3):187–197. [DOI] [PubMed] [Google Scholar]
- 127.Topaloglu AK, Tello JA, Kotan LD, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366(7):629–635. [DOI] [PubMed] [Google Scholar]
- 128.Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci. 2011;14(6):704–710. [DOI] [PubMed] [Google Scholar]
- 129.Gianetti E, Tusset C, Noel SD, et al. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab. 2010;95(6):2857–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lippincott MF, Leon S, Chan YM, et al. Hypothalamic reproductive endocrine pulse generator activity independent of neurokinin B and dynorphin signaling. J Clin Endocrinol Metab. 2019;104(10):4304–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang JJ, Caligioni CS, Chan YM, Seminara SB. Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology. 2012;153(3):1498–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.True C, Nasrin Alam S, Cox K, Chan YM, Seminara SB. Neurokinin B is critical for normal timing of sexual maturation but dispensable for adult reproductive function in female mice. Endocrinology. 2015;156(4):1386–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Parnet P, Mitsuhashi M, Turck CW, Kerdelhue B, Payan DG. Tachykinin receptor cross-talk. Immunological cross-reactivity between the external domains of the substance K and substance P receptors. Brain Behav Immun. 1991;5(1):73–83. [DOI] [PubMed] [Google Scholar]
- 134.Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014;94(1):265–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Noritake K, Matsuoka T, Ohsawa T, et al. Involvement of neurokinin receptors in the control of pulsatile luteinizing hormone secretion in rats. J Reprod Dev. 2011;57(3):409–415. [DOI] [PubMed] [Google Scholar]
- 136.Fergani C, Mazzella L, Coolen LM, et al. Do substance P and neurokinin a play important roles in the control of LH secretion in ewes? Endocrinology. 2016;157(12):4829–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yamamura T, Wakabayashi Y, Ohkura S, Navarro VM, Okamura H. Effects of intravenous administration of neurokinin receptor subtype-selective agonists on gonadotropin-releasing hormone pulse generator activity and luteinizing hormone secretion in goats. J Reprod Dev. 2015;61(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sharifi N, Diehl N, Yaswen L, Brennan MB, Hochgeschwender U. Generation of dynorphin knockout mice. Brain Res Mol Brain Res. 2001;86(1–2):70–75. [DOI] [PubMed] [Google Scholar]
- 139.Kieffer BL. Opioids: first lessons from knockout mice. Trends Pharmacol Sci. 1999;20(1):19–26. [DOI] [PubMed] [Google Scholar]
- 140.Van Vugt DA, Bakst G, Dyrenfurth I, Ferin M. Naloxone stimulation of luteinizing hormone secretion in the female monkey: influence of endocrine and experimental conditions. Endocrinology. 1983;113(5):1858–1864. [DOI] [PubMed] [Google Scholar]
- 141.Goodman RL, Coolen LM, Anderson GM, et al. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959–2967. [DOI] [PubMed] [Google Scholar]
- 142.Young J, George JT, Tello JA, et al. Kisspeptin restores pulsatile LH secretion in patients with neurokinin B signaling deficiencies: physiological pathophysiological and therapeutic implications. Neuroendocrinology. 2013;97:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Clarke IJ, Li Q, Henry BA, Millar RP. Continuous kisspeptin restores luteinizing hormone pulsatility following cessation by a neurokinin B antagonist in female sheep. Endocrinology. 2018;159(2):639–646. [DOI] [PubMed] [Google Scholar]
- 144.Han SY, McLennan T, Czieselsky K, Herbison AE. Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc Natl Acad Sci USA. 2015;112(42):13109–13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Voliotis M, Li XF, De Burgh R, et al. The origin of GnRH pulse generation: an integrative mathematical-experimental approach. J Neurosci. 2019;39(49):9738–9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Plant TM. A comparison of the neuroendocrine mechanisms underlying the initiation of the preovulatory LH surge in the human, Old World monkey and rodent. Front Neuroendocrinol. 2012;33(2):160–168. [DOI] [PubMed] [Google Scholar]
- 147.Goodman RL. Neuroendocrine Control of Gonadotropin Secretion: Comparative Aspects. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction, 4th ed. Elsevier; 2015:1537–1574. [Google Scholar]
- 148.Herbison AE. Physiology of the Adult Gonadotropin-Releasing Hormone Neuronal Network. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction, 4th ed. Elsivier; 2015:388–467. [Google Scholar]
- 149.Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev. 2010;31(4):544–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Khan AR, Kauffman AS. The role of kisspeptin and RFamide-related peptide-3 neurones in the circadian-timed preovulatory luteinising hormone surge. J Neuroendocrinol. 2012;24(1):131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96(1):57–62. [DOI] [PubMed] [Google Scholar]
- 152.Karsch FJ, Weick RF, Butler WR, et al. Induced LH surges in the rhesus monkey: strength-duration characteristics of the estrogen stimulus. Endocrinology. 1973;92(6):1740–1747. [DOI] [PubMed] [Google Scholar]
- 153.Richter TA, Robinson JE, Evans NP. Progesterone blocks the estradiol-stimulated luteinizing hormone surge by disrupting activation in response to a stimulatory estradiol signal in the ewe. Biol Reprod. 2002;67(1):119–125. [DOI] [PubMed] [Google Scholar]
- 154.Goodman RL. A quantitative analysis of the physiological role of estradiol and progesterone in the control of tonic and surge secretion of luteinizing hormone in the rat. Endocrinology. 1978;102(1):142–150. [DOI] [PubMed] [Google Scholar]
- 155.Bronson FH. The regulation of luteinizing hormone secretion by estrogen: relationships among negative feedback, surge potential, and male stimulation in juvenile, peripubertal, and adult female mice. Endocrinology. 1981;108(2):506–516. [DOI] [PubMed] [Google Scholar]
- 156.Goodman RL, Legan SJ, Ryan KD, Foster DL, Karsch FJ. Importance of variations in behavioural and feedback actions of oestradiol to the control of seasonal breeding in the ewe. J Endocrinol. 1981;89(2):229–240. [DOI] [PubMed] [Google Scholar]
- 157.Ginther OJ, Gastal EL, Gastal MO, Beg MA. Regulation of circulating gonadotropins by the negative effects of ovarian hormones in mares. Biol Reprod. 2005;73(2):315–323. [DOI] [PubMed] [Google Scholar]
- 158.Bakker J, Baum MJ. Neuroendocrine regulation of GnRH release in induced ovulators. Front Neuroendocrinol. 2000;21(3):220–262. [DOI] [PubMed] [Google Scholar]
- 159.Turzillo AM, Nett TM. Regulation of GnRH receptor gene expression in sheep and cattle. J Reprod Fertil Suppl. 1999;54:75–86. [PubMed] [Google Scholar]
- 160.Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev. 2008;57(2):277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Caraty A, Fabre-Nys C, Delaleu B, et al. Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory gonadotropin releasing hormone surge in the ewe. Endocrinology. 1998;139(4):1752–1760. [DOI] [PubMed] [Google Scholar]
- 162.McCosh RB, Lopez JA, Szeligo BM, et al. Evidence that nitric oxide is critical for LH surge generation in female sheep. Endocrinology. 2020;161:bqaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Smith JT, Li Q, Pereira A, Clarke IJ. Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology. 2009;150(12):5530–5538. [DOI] [PubMed] [Google Scholar]
- 164.Krey LC, Butler WR, Knobil E. Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. I. Gonadotropin secretion. Endocrinology. 1975;96(5):1073–1087. [DOI] [PubMed] [Google Scholar]
- 165.Weick RF. Induction of the luteinizing hormone surge by intrahypothalamic application of estrogen in the rhesus monkey. Biol Reprod. 1981;24(2):415–422. [DOI] [PubMed] [Google Scholar]
- 166.Zeleznik AJ, Plant TM. Control of the menstrual cycle. In: Zeleznik AJ, Plant TM, eds. Knobil and Neill’s Physiology of Reproduction, 4th ed. Elsevier; 2015: 1307–1362. [Google Scholar]
- 167.Levine JE. Neuroendocrine control of the ovarain cycle of the rat. In: Zeleznik AJ, Plant TM, eds. Knobil and Neill’s Physiology of Reproduction, 4th ed. Elsevier; 2015: 1199–1257. [Google Scholar]
- 168.Ringstrom SJ, Szabo M, Kilen SM, Saberi S, Knox KL, Schwartz NB. The antiprogestins RU486 and ZK98299 affect follicle-stimulating hormone secretion differentially on estrus, but not on proestrus. Endocrinology. 1997;138(6):2286–2290. [DOI] [PubMed] [Google Scholar]
- 169.Chappell PE, Schneider JS, Kim P, et al. Absence of gonadotropin surges and gonadotropin-releasing hormone self-priming in ovariectomized (OVX), estrogen (E2)-treated, progesterone receptor knockout (PRKO) mice. Endocrinology. 1999;140(8):3653–3658. [DOI] [PubMed] [Google Scholar]
- 170.Sinchak K, Mohr MA, Micevych PE. Hypothalamic astrocyte development and physiology for neuroprogesterone induction of the luteinizing hormone surge. Front Endocrinol. 2020;11:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Caraty A, Skinner DC. Progesterone priming is essential for the full expression of the positive feedback effect of estradiol in inducing the preovulatory gonadotropin-releasing hormone surge in the ewe. Endocrinology. 1999;140(1):165–170. [DOI] [PubMed] [Google Scholar]
- 172.Evans NP, Richter TA, Skinner DC, Robinson JE. Neuroendocrine mechanisms underlying the effects of progesterone on the oestradiol-induced GnRH/LH surge. Reprod Suppl. 2002;59:57–66. [PubMed] [Google Scholar]
- 173.Freeman MC, Dupke KC, Croteau CM. Extinction of the estrogen-induced daily signal for LH release in the rat: a role for the proestrous surge of progesterone. Endocrinology. 1976;99(1):223–229. [DOI] [PubMed] [Google Scholar]
- 174.Carrasco RA, Leonardi CE, Pezo S, Adams GP. Hypothalamic involvement and the role of progesterone in the NGF-induced LH surge pathway. Reproduction. 2021;162(2):171–179. [DOI] [PubMed] [Google Scholar]
- 175.Lee WS, Smith MS, Hoffman GE. Progesterone enhances the surge of luteinizing hormone by increasing the activation of luteinizing hormone-releasing hormone neurons. Endocrinology. 1990;127(5):2604–2606. [DOI] [PubMed] [Google Scholar]
- 176.Moenter SM, Karsch FJ, Lehman MN. Fos expression during the estradiol-induced gonadotropin-releasing hormone (GnRH) surge of the ewe: induction in GnRH and other neurons. Endocrinology. 1993;133(2):896–903. [DOI] [PubMed] [Google Scholar]
- 177.Chappell PE, Levine JE. Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinology. 2000;141(4):1477–1485. [DOI] [PubMed] [Google Scholar]
- 178.Richter TA, Robinson JE, Lozano JM, Evans NP. Progesterone can block the preovulatory gonadotropin-releasing hormone/luteinising hormone surge in the ewe by a direct inhibitory action on oestradiol-responsive cells within the hypothalamus. J Neuroendocrinol. 2005;17(3):161–169. [DOI] [PubMed] [Google Scholar]
- 179.Kauffman ASR EF Neuroendocrine Control of Mating-Induced Ovulation. In: Neill JD, ed. Knobil and Neill’s Physiology of Reproduction, 3rd ed. Elsevier; 2006: 2283–2326. [Google Scholar]
- 180.Inoue N, Sasagawa K, Ikai K, et al. Kisspeptin neurons mediate reflex ovulation in the musk shrew (Suncus murinus). Proc Natl Acad Sci USA. 2011;108(42):17527–17532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.El Allali K, El Bousmaki N, Ainani H, Simonneaux V. Effect of the Camelid’s seminal plasma ovulation-inducing factor/beta-N GF: a kisspeptin target hypothesis. Front Vet Sci. 2017;4:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Adams GP, Ratto MH, Huanca W, Singh J. Ovulation-inducing factor in the seminal plasma of alpacas and llamas. Biol Reprod. 2005;73(3):452–457. [DOI] [PubMed] [Google Scholar]
- 183.Ratto MH, Leduc YA, Valderrama XP, et al. The nerve of ovulation-inducing factor in semen. Proc Natl Acad Sci USA. 2012;109(37):15042–15047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Silva ME, Smulders JP, Guerra M, et al. Cetrorelix suppresses the preovulatory LH surge and ovulation induced by ovulation-inducing factor (OIF) present in llama seminal plasma. Reprod Biol Endocrinol. 2011;9:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Silva ME, Recabarren MP, Recabarren SE, Adams GP, Ratto MH. Ovarian estradiol modulates the stimulatory effect of ovulation-inducing factor (OIF) on pituitary LH secretion in llamas. Theriogenology. 2012;77(9):1873–1882. [DOI] [PubMed] [Google Scholar]
- 186.Przekop F, Domanski E. Role of catecholamines in release of gonadotrophic hormones in the rabbit. Acta Physiol Pol. 1976;27(2):163–168. [PubMed] [Google Scholar]
- 187.Everett JW, Sawyer CH. A 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology. 1950;47(3):198–218. [DOI] [PubMed] [Google Scholar]
- 188.Jackson GL, Thurmon J, Nelson D. Estrogen-induced release of LH in the ovariectomized ewe: independence of time of day. Biol Reprod. 1975;13(3):358–362. [DOI] [PubMed] [Google Scholar]
- 189.Matsuda F, Nakatsukasa K, Suetomi Y, et al. The luteinising hormone surge-generating system is functional in male goats as in females: involvement of kisspeptin neurones in the medial preoptic area. J Neuroendocrinol. 2015;27(1):57–65. [DOI] [PubMed] [Google Scholar]
- 190.Scott CJ, Rose JL, Gunn AJ, McGrath BM. Kisspeptin and the regulation of the reproductive axis in domestic animals. J Endocrinol. 2019;240:R1–R16. [DOI] [PubMed] [Google Scholar]
- 191.Evans NP, Dahl GE, Padmanabhan V, Thrun LA, Karsch FJ. Estradiol requirements for induction and maintenance of the gonadotropin-releasing hormone surge: implications for neuroendocrine processing of the estradiol signal. Endocrinology. 1997;138(12):5408–5414. [DOI] [PubMed] [Google Scholar]
- 192.Harris TG, Dye S, Robinson JE, Skinner DC, Evans NP. Progesterone can block transmission of the estradiol-induced signal for luteinizing hormone surge generation during a specific period of time immediately after activation of the gonadotropin-releasing hormone surge-generating system. Endocrinology. 1999;140(2):827–834. [DOI] [PubMed] [Google Scholar]
- 193.Karsch FJ, Weick RF, Hotchkiss J, Dierschke DJ, Knobil E. An analysis of the negative feedback control of gonadotropin secretion utilizing chronic implantation of ovarian steroids in ovariectomized rhesus monkeys. Endocrinology. 1973;93(2):478–486. [DOI] [PubMed] [Google Scholar]
- 194.Skinner DC, Harris TG, Evans NP. Duration and amplitude of the luteal phase progesterone increment times the estradiol-induced luteinizing hormone surge in ewes. Biol Reprod. 2000;63(4):1135–1142. [DOI] [PubMed] [Google Scholar]
- 195.Hauger RL, Karsch FJ, Foster DL. A new concept for control of the estrous cycle of the ewe based on the temporal relationships between luteinizing hormone, estradiol and progesterone in peripheral serum and evidence that progesterone inhibits tonic LH secretion. Endocrinology. 1977;101(3):807–817. [DOI] [PubMed] [Google Scholar]
- 196.Karsch FJ, Foster DL, Legan SJ, Ryan KD, Peter GK. Control of the preovulatory endocrine events in the ewe: interrelationship of estradiol, progesterone, and luteinizing hormone. Endocrinology. 1979;105(2):421–426. [DOI] [PubMed] [Google Scholar]
- 197.Merkley CM, Porter KL, Coolen LM, et al. KNDy (Kisspeptin/Neurokinin B/Dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinology. 2012;153:5406–5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lehman MN, Merkley CM, Coolen LM, Goodman RL. Anatomy of the kisspeptin neural network in mammals. Brain Res. 2010;1364:90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tomikawa J, Homma T, Tajima S, et al. Molecular characterization and estrogen regulation of hypothalamic KISS1 gene in the pig. Biol Reprod. 2010;82(2):313–319. [DOI] [PubMed] [Google Scholar]
- 200.Hassaneen A, Naniwa Y, Suetomi Y, et al. Immunohistochemical characterization of the arcuate kisspeptin/neurokinin B/dynorphin (KNDy) and preoptic kisspeptin neuronal populations in the hypothalamus during the estrous cycle in heifers. J Reprod Dev. 2016;62(5):471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tanco VM, Whitlock BK, Jones MA, Wilborn RR, Brandebourg TD, Foradori CD. Distribution and regulation of gonadotropin-releasing hormone, kisspeptin, RF-amide related peptide-3, and dynorphin in the bovine hypothalamus. PeerJ. 2016;4:e1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vargas Trujillo M, Kalil B, Ramaswamy S, Plant TM. Estradiol upregulates kisspeptin expression in the preoptic area of both the male and female rhesus monkey (Macaca mulatta): implications for the hypothalamic control of ovulation in highly evolved primates. Neuroendocrinology. 2017;105(1):77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Watanabe Y, Uenoyama Y, Suzuki J, et al. Oestrogen-induced activation of preoptic kisspeptin neurones may be involved in the luteinising hormone surge in male and female Japanese monkeys. J Neuroendocrinol. 2014;26(12):909–917. [DOI] [PubMed] [Google Scholar]
- 204.Scanlan N, Dufourny L, Skinner DC. Somatostatin-14 neurons in the ovine hypothalamus: colocalization with estrogen receptor alpha and somatostatin-28(1–12) immunoreactivity, and activation in response to estradiol. Biol Reprod. 2003;69(4):1318–1324. [DOI] [PubMed] [Google Scholar]
- 205.Goodman RL, He W, Lopez JA, et al. Evidence that the LH surge in ewes involves both neurokinin B-dependent and -independent actions of kisspeptin. Endocrinology. 2019;160(12):2990–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pineda R, Garcia-Galiano D, Roseweir A, et al. Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology. 2010;151(2):722–730. [DOI] [PubMed] [Google Scholar]
- 207.Dufourny L, Lomet D. Crosstalks between kisspeptin neurons and somatostatin neurons are not photoperiod dependent in the ewe hypothalamus. Gen Comp Endocrinol. 2017;254:68–74. [DOI] [PubMed] [Google Scholar]
- 208.Goubillon ML, Caraty A, Herbison AE. Evidence in favour of a direct input from the ventromedial nucleus to gonadotropin-releasing hormone neurones in the ewe: an anterograde tracing study. J Neuroendocrinol. 2002;14(2):95–100. [DOI] [PubMed] [Google Scholar]
- 209.Lee WS, Smith MS, Hoffman GE. Luteinizing hormone-releasing hormone neurons express Fos protein during the proestrous surge of luteinizing hormone. Proc Natl Acad Sci USA. 1990;87(13):5163–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.King JC, Ronsheim P, Liu E, Powers L, Slonimski M, Rubin BS. Fos expression in luteinizing hormone-releasing hormone neurons of guinea pigs, with knife cuts separating the preoptic area and the hypothalamus, demonstrating luteinizing hormone surges. Biol Reprod. 1998;58(2):323–329. [DOI] [PubMed] [Google Scholar]
- 211.Caba M, Pau KY, Beyer C, Gonzalez A, Silver R, Spies HG. Coitus-induced activation of c-fos and gonadotropin-releasing hormone in hypothalamic neurons in female rabbits. Brain Res Mol Brain Res. 2000;78(1–2):69–79. [DOI] [PubMed] [Google Scholar]
- 212.Lehman MN, Robinson JE, Karsch FJ, Silverman AJ. Immunocytochemical localization of luteinizing hormone-releasing hormone (LHRH) pathways in the sheep brain during anestrus and the mid-luteal phase of the estrous cycle. J Comp Neurol. 1986;244(1):19–35. [DOI] [PubMed] [Google Scholar]
- 213.Bakker J, Kelliher KR, Baum MJ. Mating induces gonadotropin-releasing hormone neuronal activation in anosmic female ferrets. Biol Reprod. 2001;64(4):1100–1105. [DOI] [PubMed] [Google Scholar]
- 214.Silverman AJ, Antunes JL, Abrams GM, et al. The luteinizing hormone-releasing hormone pathways in rhesus (Macaca mulatta) and pigtailed (Macaca nemestrina) monkeys: new observations on thick, unembedded sections. J Comp Neurol. 1982;211(3):309–317. [DOI] [PubMed] [Google Scholar]
- 215.Sullivan KA, Witkin JW, Ferin M, Silverman AJ. Gonadotropin-releasing hormone neurons in the rhesus macaque are not immunoreactive for the estrogen receptor. Brain Res. 1995;685(1–2):198–200. [DOI] [PubMed] [Google Scholar]
- 216.Jansen HT, Hileman SM, Lubbers LS, Kuehl DE, Jackson GL, Lehman MN. Identification and distribution of neuroendocrine gonadotropin-releasing hormone neurons in the ewe. Biol Reprod. 1997;56(3):655–662. [DOI] [PubMed] [Google Scholar]
- 217.Karsch FJ, Bowen JM, Caraty A, Evans NP, Moenter SM. Gonadotropin-releasing hormone requirements for ovulation. Biol Reprod. 1997;56(2):303–309. [DOI] [PubMed] [Google Scholar]
- 218.Moenter SM, Caraty A, Karsch FJ. The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology. 1990;127(3):1375–1384. [DOI] [PubMed] [Google Scholar]
- 219.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26(25):6687–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28(35):8691–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kinoshita M, Tsukamura H, Adachi S, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146(10):4431–4436. [DOI] [PubMed] [Google Scholar]
- 222.Bosch MA, Xue C, Ronnekleiv OK. Kisspeptin expression in guinea pig hypothalamus: effects of 17beta-estradiol. J Comp Neurol. 2012;520(10):2143–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Smith JT, Shahab M, Pereira A, Pau KY, Clarke IJ. Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate. Biol Reprod. 2010;83(4):568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Popa SM, Moriyama RM, Caligioni CS, et al. Redundancy in Kiss1 expression safeguards reproduction in the mouse. Endocrinology. 2013;154(8):2784–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Dufourny L, Skinner DC. Influence of estradiol on NADPH diaphorase/neuronal nitric oxide synthase activity and colocalization with progesterone or type II glucocorticoid receptors in ovine hypothalamus. Biol Reprod. 2002;67(3):829–836. [DOI] [PubMed] [Google Scholar]
- 226.Helena CV, Toporikova N, Kalil B, et al. KNDy neurons modulate the magnitude of the steroid-induced luteinizing hormone surges in ovariectomized rats. Endocrinology. 2015;156(11):4200–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Clarke IJ, Sari IP, Qi Y, et al. Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology. 2008;149(11):5811–5821. [DOI] [PubMed] [Google Scholar]
- 228.Levine JE. Neuroendocrine control of the ovarian cycle of the rat. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction, 4th ed. Elsevier; 2015: 1199–1257. [Google Scholar]
- 229.Sawyer CH, Markee JE, Townsend BF. Cholinergic and adrenergic components in the neurohumoral control of the release of LH in the rabbit. Endocrinology. 1949;44(1):18–37. [DOI] [PubMed] [Google Scholar]
- 230.Rawson JA, Scott CJ, Pereira A, Jakubowska A, Clarke IJ. Noradrenergic projections from the A1 field to the preoptic area in the brain of the ewe and Fos responses to oestrogen in the A1 cells. J Neuroendocrinol. 2001;13(2):129–138. [DOI] [PubMed] [Google Scholar]
- 231.Porter KL, Hileman SM, Hardy SL, Nestor CC, Lehman MN, Goodman RL. Neurokinin-3 receptor activation in the retrochiasmatic area is essential for the full pre-ovulatory luteinising hormone surge in ewes. J Neuroendocrinol. 2014;26(11):776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Clarke IJ, Scott CJ, Pereira A, Pompolo S. The role of noradrenaline in the generation of the preovulatory LH surge in the ewe. Domest Anim Endocrinol. 2006;30(4):260–275. [DOI] [PubMed] [Google Scholar]
- 233.Herbison AE. A simple model of estrous cycle negative and positive feedback regulation of GnRH secretion. Front Neuroendocrinol. 2020;57:100837. [DOI] [PubMed] [Google Scholar]
- 234.Evans NP, Dahl GE, Mauger DT, Padmanabhan V, Thrun LA, Karsch FJ. Does estradiol induce the preovulatory gonadotropin-releasing hormone (GnRH) surge in the ewe by inducing a progressive change in the mode of operation of the GnRH neurosecretory system. Endocrinology. 1995;136(12):5511–5519. [DOI] [PubMed] [Google Scholar]
- 235.Evans NP, Dahl GE, Glover BH, Karsch FJ. Central regulation of pulsatile gonadotropin-releasing hormone (GnRH) secretion by estradiol during the period leading up to the preovulatory GnRH surge in the ewe. Endocrinology. 1994;134(4):1806–1811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this review because no new data were created or analyzed.


