Abstract
High-affinity iron uptake in gram-negative bacteria depends upon TonB, a protein which couples the proton motive force in the cytoplasmic membrane to iron chelate receptors in the outer membrane. To advance studies on TonB structure and function, we expressed a recombinant form of Escherichia coli TonB lacking the N-terminal cytoplasmic membrane anchor. This protein (H6-′TonB; Mr, 24,880) was isolated in a soluble fraction of lysed cells and was purified by virtue of a hexahistidine tag located at its N terminus. Sedimentation experiments indicated that the H6-′TonB preparation was almost monodisperse and the protein was essentially monomeric. The value found for the Stokes radius (3.8 nm) is in good agreement with the value calculated by size exclusion chromatography. The frictional ratio (2.0) suggested that H6-′TonB adopts a highly asymmetrical form with an axial ratio of 15. H6-′TonB captured both the ferrichrome-iron receptor FhuA and the ferric enterobactin receptor FepA from detergent-solubilized outer membranes in vitro. Capture was enhanced by preincubation of the receptors with their cognate ligands. Cross-linking assays with the purified proteins in vitro demonstrated that there was preferential interaction between TonB and ligand-loaded FhuA. Purified H6-′TonB was found to be stable and thus shows promise for high-resolution structural studies.
Bacteria seeking to colonize aerobic environments at physiological pH are faced with the practical insolubility of an essential nutrient, iron. Further limiting the availability of iron in vertebrate hosts are iron-chelating proteins, such as lactoferrin and transferrin. Gram-negative bacteria respond to iron deficiency by derepressing expression of a series of high-affinity iron transport systems (7, 8, 52). Such systems bind iron chelates (siderophores and host iron-binding compounds) by means of receptors located in the outer membrane. A parallel system for uptake of vitamin B12 is induced when cobalamins are scarce in the environment. Bound ligands are then transported into the periplasm for internalization by ATP-binding cassette transporters located in the cytoplasmic membrane. The outer membrane transport step depends upon the electrochemical proton gradient across the cytoplasmic membrane (5, 20, 49) and upon the cytoplasmic membrane-anchored proteins TonB, ExbB, and ExbD to transduce this energy across the periplasm (23, 32, 35, 40, 45). To date, more than 20 outer membrane proteins whose functions depend upon TonB have been identified (57).
Recently, significant advances in understanding high-affinity iron uptake in bacteria came from determinations of the three-dimensional crystal structures at atomic resolution of the ferrichrome-iron receptor FhuA (17, 37) and the ferric-enterobactin receptor FepA (11) of Escherichia coli. Perhaps the most surprising part of the receptor structures was the N-terminal 160-amino-acid cork or plug that is organized into a globular domain and is held in the 22-strand β-barrel by an extensive network of hydrogen bonds and salt bridges. An elegant structural comparison of FhuA with and without bound ferrichrome-iron revealed uncoiling of the switch helix (residues 24 to 29) from the cork and striking displacement of certain residues (for example, Glu19 by more than 1.7 nm) upon ligand binding (17, 37). What is the outcome of transduction of this signal across the N-terminal cork domain? An earlier study identified conformational changes in an N-terminal periplasmic region of FhuA in response to binding of ferricrocin, a ferrichrome analogue (42). Such ligand-dependent alterations, which may be closely related to those defined by X-ray crystallography, are likely to promote the association between TonB and TonB-dependent receptors, as has been demonstrated for FhuA (43) and for FepA (32), and thereby promote ligand translocation through the receptor. Such a mechanism ensures efficient expenditure of energy since TonB interacts preferentially with those receptors to which ligand has bound (24, 40). However, the molecular mechanism of energy transduction remains unclear. Kadner and colleagues (12, 13) recently identified interacting pairs of residues in the vitamin B12 receptor BtuB and TonB; certain cysteine substitution mutations toward the N terminus of BtuB (the TonB box) were effectively disulfide cross-linked in vivo to phenotypically compensatory cysteine substitutions around amino acid 160 of TonB. A recent spectroscopy study (39) monitored the dynamics of spin-labeled BtuB variants within isolated outer membranes. Addition of substrate resulted in rapid unfolding of the N-terminal part of the protein in the vicinity of the spin labels. Combined, the results that have been obtained demonstrate that the TonB box of BtuB cycles between constrained and accessible conformations and that the latter may allow TonB to bind to the TonB box of BtuB (13, 39). Complete removal of the TonB box, however, does not eliminate all TonB-dependent receptor activity. Indeed, a FhuA variant carrying a deletion of most of the cork domain (FhuAΔ5-160) and thus devoid of its TonB box still transported ferrichrome in a TonB- and energy-dependent manner, albeit at a rate that was significantly lower than the wild-type FhuA rate (6).
TonB is essentially a periplasmic protein with an uncleaved hydrophobic N terminus (47) anchored in the cytoplasmic membrane both in Salmonella enterica serovar Typhimurium (21) and in E. coli (50). How might TonB maintain its connection to the cytoplasmic membrane and at the same time modulate the activity of receptors in the outer membrane? The proline-rich regions between residues 70 and 102 of TonB from E. coli ([EP]4X13[KP]6) (46) and between residues 66 and 106 of TonB from S. enterica serovar Typhimurium ([EP]5X13[KP]7) (21) adopt an extended structure (16, 21, 58) that may contribute to the interaction between the C-terminal 60 residues of TonB (2, 31, 35) and outer membrane-localized receptors under certain osmotic conditions (28).
The N-terminal anchor also plays a critical role in the function of TonB (22, 25). Although certain TonB variants with altered N termini retain the ability to bind to outer membrane receptors (22, 29, 35), point mutations affecting this predicted α-helical transmembrane domain disrupt interaction with the cytoplasmic membrane protein ExbB and abolish ferric siderophore transport and killing by TonB-dependent phages and protein antibiotics termed colicins (29, 32). Mutations in ExbB or ExbD or overexpression of TonB without concomitant overexpression of ExbB or ExbD increased the rate of degradation of TonB and reduced TonB-dependent receptor activity (1, 18, 53). Such susceptibility to endogenous proteolysis has impeded isolation of TonB from E. coli. To overcome these difficulties, we expressed a soluble derivative of E. coli TonB lacking the N-terminal cytoplasmic membrane anchor. Here we describe purification of this hexahistidine-tagged TonB variant (H6-′TonB), show that it retains affinity for the TonB-dependent receptors FhuA and FepA, and demonstrate that it responds to the ligand occupation status of these receptors. The stability of H6-′TonB and its ability to interact with TonB-dependent receptors imply that the protein is amenable to further structure-function studies.
MATERIALS AND METHODS
Bacterial strains and reagents.
E. coli XL-1 Blue [recA1 endA1 gyrA96 thi hsdR17(rK− mK+) supE44 relA1 lac (F′ proAB+ lacIqZΔM15::Tn10); Stratagene, La Jolla, Calif.], DH5α [φ80dlacZΔM15 recA1 endA1 gyrA96 thi-1 hsdR17 (rK− mK+) phoA λ− supE44 relA1 deoR Δ(lacZYA-argF)U169; Life Technologies, Cergy Pontoise, France], and ER2566 [F− λ− fhuA2 (lon) ompT lacZ::T7 gene1 gal sulA11 Δ(mcrC-mrr)114::IS10 R(mcr-73::mini-Tn10)2 R(zgb-210::Tn10) endA1 (dcm); New England Biolabs, Beverly, Mass.] were used for chromosomal DNA isolation, routine subcloning, and protein expression, respectively. Strains KP1060 [GM1 Δ(ompT-fepA-entF)] (54), KP1120 [KP1060 Δ(trp-tonB-opp-ana)467] (22) KP1060fhuA (43), and KP1120fhuA (43) were used to evaluate the specificity and titer of polyclonal anti-TonB chicken serum. QIAprep spin miniprep columns, a QIAquick PCR purification kit, and Ni2+-nitrilotriacetate (NTA) agarose resin were purchased from Qiagen (Courtaboeuf, France); plasmid pET-28 was obtained from Novagen (Madison, Wis.). Restriction enzymes, Pwo thermostable proofreading DNA polymerase, and T4 DNA ligase were purchased from Eurogentec (Seraing, Belgium). Antihistidine monoclonal antibody, bromochloroindolyl phosphate, nitroblue tetrazolium, phenylmethylsulfonyl fluoride, and formaldehyde (37% solution in water) were obtained from Sigma-Aldrich (Saint Quentin Fallavier, France). Protease inhibitor cocktail (Complete) tablets and 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride (Pefabloc SC) were purchased from Roche Diagnostics (Meylan, France) and were used as recommended by the manufacturer.
Cloning of TonB.
The tonB gene (46) was amplified by PCR from 20 ng of chromosomal DNA of E. coli XL-1 Blue. The reaction mixture included 1.5 U of the proofreading DNA polymerase Pwo and 50 pmol (each) of primers that were designed to incorporate 5′ NdeI and 3′ XhoI sites for cloning the amplified product into pET-28. The sequences of the PCR primers were as follows: forward primer, 5′-TCAGTCCATATGCATCAGGTTATTGAACTA (the NdeI site is underlined; the CAT codon in bold face type encodes His33 of the TonB sequence [46]); and reverse primer, 5′-TCGATCCTCGAGTTACTGAATTTCGGTGGT (the XhoI site is underlined; the TTA in boldface type is reverse and complementary to the stop codon of the chromosomal tonB sequence and is preceded by the codon for the C-terminal Gln239 of the TonB sequence). PCR products were trimmed with NdeI and XhoI and ligated to pET-28 that had been restricted with the same enzymes.
Expression and purification of soluble histidine-tagged TonB.
The plasmid encoding H6-′TonB was transformed into E. coli ER2566, and 500-ml cultures in Luria broth plus kanamycin (30 μg/ml) were induced with 0.5 mM isopropyl β-d-thiogalactopyranoside (IPTG). Cells were collected by centrifugation and suspended in 50 ml of 100 mM sodium phosphate, pH 7.9. Protease inhibitor cocktail tablets and phenylmethylsulfonyl fluoride were added, and all steps were carried out at 4°C or on ice. Cells were lysed by sonication, and the cleared supernatant was mixed with 5 ml of equilibrated Ni2+-NTA agarose resin in batch for 30 min. Protein was eluted with an imidazole step gradient and was dialyzed extensively against 100 mM sodium phosphate, pH 7.9.
Anti-TonB antibodies.
White Leghorn hens (Genaxis, Montigny le Bretonneux, France) were immunized subcutaneously at 3-week intervals with 100 μg of purified H6-′TonB in Freund's complete adjuvant. Immune serum drawn 2 weeks after the first boost revealed anti-TonB titers of 1:15,000 relative to the preimmune control serum in an enzyme-linked immunosorbent assay (ELISA) with purified H6-′TonB. The specificity of the anti-TonB immune serum was confirmed by blotting against whole-cell extracts of E. coli KP1060 (TonB+) and KP1120 (tonB).
Characterization of H6-′TonB and of the H6-′TonB–FhuA.H6 complex by size exclusion chromatography.
Proteins were applied to a Superose 12 HR 30/10 column (Pharmacia) equilibrated either in 100 mM sodium phosphate buffer (pH 7.9) or in 20 mM Tris-HCl (pH 8.0)–150 mM NaCl–0.1% lauryldimethylamine oxide (TLN buffer). The flow rate was kept at 0.25 ml/min. To calibrate the Superose 12 column, we obtained experimental values for the elution volumes (Ve) of the following standard globular proteins with known Stokes radii (Rs): thyroglobulin (Rs = 8.6 nm; Ve = 8.8 ml), ferritin (Rs = 6.3 nm; Ve = 10.7 ml), catalase (Rs = 5.2 nm; Ve = 11.7 ml), aldolase (Rs = 4.6 nm; Ve = 12.0 ml), bovine serum albumin (Rs = 3.5 nm; Ve = 12.5 ml), ovalbumin (Rs = 2.8 nm; Ve = 13.4 ml), chymotrypsinogen A (Rs = 2.1 nm; Ve = 14.9 ml), and RNase A (Rs = 1.75 nm; Ve = 15.5 ml). The partition coefficient (KD) was calculated with the equation KD = (Ve − V0)/(Vt − V0), where V0 is the void volume of the column (7.3 ml) and Vt is the total volume of the column (21.0 ml). Dextran blue 2000 was used as a marker of the void volume. The total volume was measured by using NaNO3 (80 mM) (3, 34). The calibration curve Rs as a function of KD(data not shown) was the fit obtained with a polynomial of degree 3 (correlation coefficient = 0.989).
For size exclusion chromatography of the FhuA–H6-′TonB complex, hexahistidine-tagged FhuA protein (FhuA.H6) (42) was purified from TLN buffer-solubilized outer membrane preparations of E. coli HO830fhuA(pHX405) as described previously (27). For experiments with ferricrocin-iron, the iron-loaded, FhuA-specific siderophore was preincubated with samples at a twofold molar excess relative to FhuA.H6 prior to chromatography. Under these conditions, in the presence of detergent, ferricrocin-iron was efficiently bound by FhuA.H6, as shown by the reduction in the area of the ferricrocin-iron (Mr, 740) peak (data not shown on chromatograms). Size exclusion chromatography was performed at room temperature.
Analytical ultracentrifugation.
Sedimentation velocity and sedimentation equilibrium measurements were obtained by using an AN 60-Ti rotor in a Beckman XL-A analytical ultracentrifuge equipped with an optical absorbance system (Laboratoire d'Enzymologie, Centre National de la Recherche Scientifique, Gif-sur-Yvette, France). All measurements were obtained at 20°C and in 100 mM sodium phosphate, pH 7.9. The concentration of H6-′TonB was 0.8 mg/ml. For velocity centrifugation, a rotor speed of 12,000 rpm was used. Sedimentation equilibrium runs were performed at 12,000 and 18,000 rpm. After the final set of equilibrium scans was recorded, the rotor speed was increased to 30,000 rpm to clear the meniscus of residual protein in order to calculate the baseline absorbance and thus the corrected concentration at each radial increment. XL-A data analysis software (Beckman) was used to calculate the protein distributions at equilibrium. Various fitting models for single data sets were used to obtain best fits in which the residuals were distributed evenly around the mean, meeting normality assumptions. The partial specific volume of the protein (ν̄) was calculated on the basis of the amino acid composition and was taken to be 0.7376 cm3/g. The buffer density (ρ) and viscosity (η) were taken to be 1.007 g/ml and 1.04, respectively. The Rs of the protein was calculated from the sedimentation coefficient (S20, w) by using the following equation:
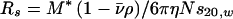 |
1 |
where M* is the molecular mass of the anhydrous protein and N is Avogadro's number. The frictional ratio (Rs/Rmin) was calculated as described by Tanford et al. (56) by using the relationship:
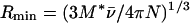 |
2 |
Interaction assays.
For small-scale solution assays we used wild-type FhuA protein that was purified by ion-exchange chromatography and chromatofocusing (3) and H6-′TonB that was purified as described above. All incubations were performed at room temperature. Purified FhuA (10 μg; 120 pmol) was incubated either with a 20-fold molar excess of ferricrocin-iron or without the siderophore in TLN buffer in a total volume of 100 μl for 5 min. Purified H6-′TonB (2.4 μg; 100 pmol) was added, and incubation was continued for 1 h. To each tube 5 μl of packed Ni2+-NTA agarose resin that previously had been equilibrated in TLN buffer was added. After incubation for 30 min, the resin was pelleted by centrifugation and washed extensively with TLN buffer. Bound protein was eluted with 20 μl of 100 mM EDTA in TLN buffer, mixed with an equal volume of denaturing electrophoresis sample buffer, boiled for 2 min, resolved on 10% polyacrylamide gels, and visualized by Coomassie blue staining. Immunoblotting of duplicate gels (data not shown) using either an antihistidine monoclonal antibody or anti-TonB polyclonal chicken antibodies and anti-FhuA monoclonal antibody Fhu8.3 (41) confirmed the identity and integrity of each protein.
ELISA.
Microtiter plates (Nunc ImmunoSorp Delta; Life Technologies, Cergy Pontoise, France) were coated with (per well) 1 μg (40 pmol) of H6-′TonB in 50 mM Tris-HCl (pH 8.0)–150 mM NaCl (TBS) containing 0.1% Triton X-100 overnight at 4°C. The wells were blocked with TBS containing 0.1% Triton X-100, 0.1% bovine serum albumin, and 1% nonfat milk (blocking buffer). An overlay of 1 μg (12 pmol) of purified FhuA.H6 in blocking buffer, either preincubated or not preincubated with a 20-fold molar excess of ferricrocin-iron, was added to each well, and each plate was incubated for 1 h at 37°C. The wells were washed with blocking buffer, and anti-FhuA monoclonal antibodies (Fhu4.1 or Fhu8.1 as hybridoma culture supernatants diluted 10-fold in blocking buffer) were added. After 1 h of incubation at 37°C followed by extensive washing, primary antibodies were detected with alkaline phosphatase-conjugated anti-mouse antibodies and para-nitrophenylphosphate. Mean background A405 values were subtracted, and the antibody reactivities (A405 values) for eight replicates were analyzed by using the Student t test. Other controls, to which no H6-′TonB was added before blocking and subsequent addition of FhuA.H6 or ferricrocin-loaded FhuA.H6, showed background levels of antibody binding.
Formaldehyde cross-linking.
Purified H6-′TonB (4 μg) in 100 mM sodium phosphate (pH 7.9) was incubated with purified FhuA.H6 (4 μg) in a buffer consisting of 100 mM sodium phosphate (pH 7.9), 100 mM NaCl, and 0.9% octylglucoside for 30 min at the ambient temperature. In some experiments, NaCl was included at a final concentration of 1 M. Formaldehyde was added to a concentration of 0.1% (vol/vol), and the cross-linking reaction was allowed to proceed for either 5 or 20 min at the ambient temperature. Samples were mixed with electrophoresis sample buffer, heated at 60°C for 5 min, and applied to sodium dodecyl sulfate (SDS)–7.5% polyacrylamide gels. Proteins were visualized by immunoblotting by using anti-FhuA monoclonal antibodies or anti-TonB polyclonal chicken antibodies and alkaline phosphatase-conjugated secondary antibodies with bromochloroindolyl phosphate and nitroblue tetrazolium. Alternatively, antihistidine monoclonal antibodies were used to reveal both H6-′TonB and FhuA.H6 on the same blot. Formaldehyde targets lysine, cysteine, and tyrosine and, to a lesser extent, tryptophan, histidine, aspartate, and arginine for cross-linking (10).
Capture of FepA and FhuA by H6-′TonB.
E. coli ER2566 (fepA+ fhuA) and KP1060 (fhuA+ fepA) were grown to saturation in 500 ml of L broth with 100 μM 2,2-dipyridyl, harvested by centrifugation, and suspended in 40 ml of TBS. Cells were lysed by sonication on ice, and the lysate was centrifuged at 15,000 × g for 20 min at 4°C to pellet membranes and unlysed cells. The pellet was resuspended in 10 ml of TBS containing 5 mM EDTA and 1% lauryldimethylamine oxide. After stirring for 30 min at the ambient temperature, the samples were centrifuged, and the resulting supernatants were dialyzed against TLN buffer to remove the EDTA. Total protein concentrations were determined by the detergent-compatible bicinchoninic acid assay (55) and adjusted to 2 mg/ml with TLN buffer. For a typical capture assay, 5 μg of purified H6-′TonB was incubated for 1 h at the ambient temperature with 200 μg of total TLN buffer-soluble membrane extract containing either FepA (extract of strain ER2566) or FhuA (extract of strain KP1060) that in some experiments was preincubated for 10 min with either 2 μg of purified colicin B (an FepA-specific protein antibiotic) or 10 μg of ferricrocin-iron (an FhuA-specific iron chelate). The equivalent of 10 μl of packed Ni2+-NTA agarose resin was added to each tube, and the samples were incubated for 30 min at the ambient temperature. The resin was pelleted, washed extensively with TLN buffer, and incubated with 20 μl of 100 mM EDTA in TLN buffer to elute the bound protein. Eluted protein samples were each mixed with an equal volume of electrophoresis sample buffer and boiled, and 8-μl portions were applied to SDS–7.5% polyacrylamide gels. After transfer to nitrocellulose membranes, proteins were revealed by immunoblotting with monoclonal antibodies directed against either FepA (a gift from M. A. McIntosh) or FhuA (antibody Fhu8.3) (41). For control experiments the same protocol was used except that H6-′TonB was omitted.
RESULTS
Expression and purification of hexahistidine-tagged TonB.
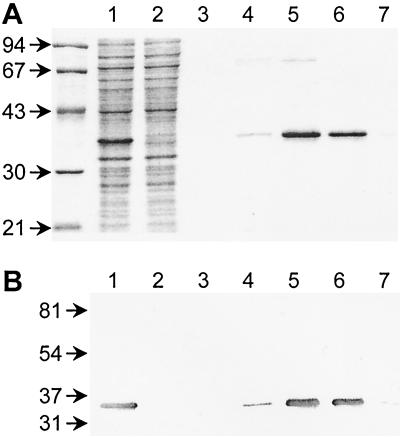
The tonB gene was amplified from the chromosome of E. coli by using PCR primers designed to eliminate codons for its N-terminal 32-amino-acid hydrophobic predicted transmembrane anchor (50). Vector-derived sequences appended a 20-amino-acid N-terminal extension, including a stretch of six histidine residues, to the truncated TonB to facilitate purification. Cell fractionation indicated that the majority of the membrane anchorless histidine-tagged TonB protein was present in the cytoplasm. In Ni2+-chelate chromatography, a major H6-′TonB peak was recovered with 100 and 150 mM imidazole steps (Fig. 1A). Interestingly, H6-′TonB (Mr, 24,880) migrated with a relative mobility of ca. 35 kDa in SDS-polyacrylamide gel electrophoresis (PAGE) gels, like wild-type TonB (Mr, 26,100; relative mobility, 36 kDa [32, 47]). The identity of H6-′TonB was confirmed by immunoblotting duplicate gels with a monoclonal antibody specific for the hexahistidine tag (Fig. 1B) and with polyclonal chicken antibodies specific for TonB. Despite the presence of minor contaminating proteins that migrated with a relative mobility of ca. 75 kDa, H6-′TonB represented 90 to 95% of the eluted protein, as judged from Coomassie blue- or silver-stained gels. The yield of H6-′TonB protein was 10 mg from 500 ml of induced culture, and the protein was stable for several days at room temperature and for several weeks at 4°C.
FIG. 1.
Purification of histidine-tagged TonB. Expression of H6-′TonB in E. coli ER2566 was induced with IPTG. H6-′TonB was purified from the soluble cell extract by Ni2+-chelate chromatography. (A) Coomassie blue-stained gel. (B) Immunoblot of a duplicate gel probed with antihistidine monoclonal antibodies. The masses of marker proteins (in kilodaltons) are indicated on the left. Lane 1, clarified supernatant after cell lysis; lane 2, flowthrough after binding to Ni2+-NTA agarose; lanes 3 to 7, elution profiles with 50, 100, 150, 200, and 250 mM imidazole respectively.
Characterization of H6-′TonB by sedimentation equilibrium and sedimentation velocity.
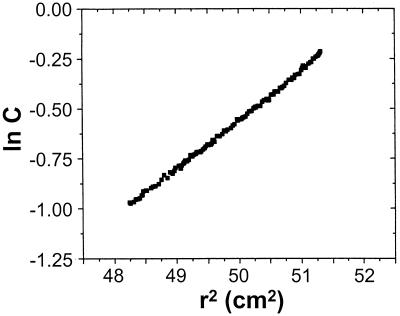
The state of aggregation of H6-′TonB and its molecular mass were assessed from a sedimentation equilibrium. A plot of the natural logarithm of the corrected protein concentration versus the square of the radial distance (Fig. 2) revealed a slight deviation from linearity, an indication of minor heterogeneity in the sample. The slope of this plot yields a quantity that is formally equal to M∗(1 − ν̄ρ), where M∗ is the molecular mass of the anhydrous protein, ν̄ is the partial specific volume of the protein, and ρ is the buffer density. A molecular mass of 30 kDa was deduced from values obtained at both 12,000 and 18,000 rpm. The deviation from the theoretical molecular mass of H6-′TonB (24.9 kDa) led us to search for contributions from a protein(s) having a higher molecular mass. Two further fittings were performed with data collected at 18,000 rpm (data not shown). Both the ideal, two-independent-population model and the self-association model were consistent with 85 to 90% of the protein being monomeric and the remainder being dimeric.
FIG. 2.
Sedimentation equilibrium of H6-′TonB. Solutions of H6-′TonB (0.8 mg/ml in 100 mM sodium phosphate, pH 7.9) were centrifuged overnight at 18,000 rpm at 20°C. The corrected protein concentration, (C) was calculated from the A280 and was plotted as a function of distance from the center of rotation (r). The molecular mass of H6-′TonB was estimated by fitting the concentration gradients to a single-ideal-component model.
Sedimentation velocity experiments allowed determination of the sedimentation coefficient. From the value found (1.4 S) we calculated (equation 1) that the Rs for the H6-′TonB protein was 3.8 nm. A value of 1.9 nm for Rmin was calculated with equation 2. The value found for the frictional ratio (Rs/Rmin), 2.0, suggests that H6-′TonB is rather elongated. Similar calculations were done assuming a hydration level of 0.3 g of H2O/g of protein (45), and these calculations led to a value for Rmin of 2.1 nm and a value for the frictional ratio of 1.8. Data were best fit to a hydrated cylinder model with a length of 24 nm and a diameter of 1.6 nm, corresponding to an axial ratio (a/b) of 15.
Size exclusion chromatography of H6-′TonB.
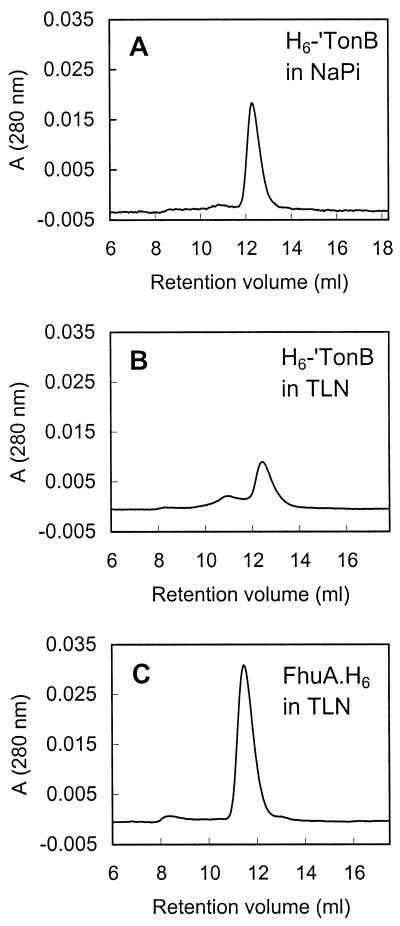
Gel filtration analysis of purified H6-′TonB in phosphate buffer revealed a single well-defined A280 peak that eluted at 12.1 ml (Fig. 3A). The gel filtration elution profile of purified H6-′TonB in the detergent-containing TLN buffer, which was used later in interaction studies with FhuA, was markedly different (Fig. 3B); H6-′TonB eluted as a major A280 peak (80% of the total peak area) at 12.5 ml and as a minor broad peak (15% of the total peak area) centered at 10.8 ml. The column was calibrated with proteins having known Rs, and the retention volumes of these proteins were not significantly different whether the column had been equilibrated in phosphate buffer or in TLN buffer. The Rs of H6-′TonB was estimated from the calibration curve of Rs versus KD; the value in phosphate buffer was 4.3 nm, whereas the major A280 peak in TLN buffer corresponded to an Rs of 4.1 nm. From the known sedimentation coefficient value (1.4 S), we estimated the molecular mass of H6-′TonB using equation 1 (see Materials and Methods). The values found (28 kDa in phosphate buffer, 26 kDa in TLN buffer) are in reasonable agreement with the molecular masses of H6-′TonB calculated from its sequence (24.9 kDa) and determined by sedimentation equilibrium (30 kDa). Determination of the Rs and molecular mass of the species corresponding to the minor peak that eluted in TLN buffer suffered from uncertainty due to the broadness of the peak.
FIG. 3.
Gel filtration chromatograms of purified H6-′TonB and FhuA.H6. Elution of protein from a Superose 12 column was recorded by tracing the A280. (A) H6-′TonB (270 μg) in 100 mM sodium phosphate (NaPi), pH 7.9. (B) H6-′TonB (250 μg) in detergent-containing TLN buffer. (C) FhuA.H6 (200 μg) in TLN buffer.
Purified H6-′TonB and wild-type FhuA interact in solution.
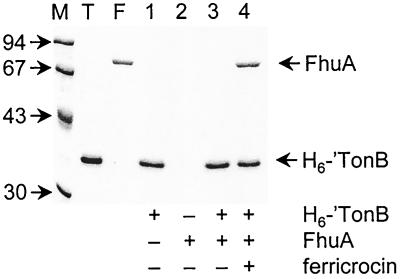
A small-scale solution assay was developed to investigate whether purified H6-′TonB could interact with FhuA, a prototypic TonB-dependent receptor of E. coli. A previous study demonstrated that purified histidine-tagged FhuA (FhuA.H6) (42) bound wild-type TonB from detergent-solubilized cell extracts and that the resulting complex could be captured from solution by using Ni2+-NTA agarose resin (43). Similar assays performed with H6-′TonB, therefore, necessitated the use of purified wild-type FhuA (3) so that only one of the two partners possessed the hexahistidine tag. By a number of criteria, including antibody reactivity, ligand binding in vitro and in vivo, and three-dimensional structure (17, 37, 42), wild-type FhuA and FhuA.H6 are structurally and functionally equivalent. After incubation of FhuA and H6-′TonB in vitro, complexes were captured with Ni2+-NTA agarose and examined by SDS-PAGE. While capture of FhuA by H6-′TonB apparently required prior binding of ferricrocin-iron to FhuA (Fig. 4, compare lanes 3 and 4), overloading of the gels (data not shown) allowed visualization of small amounts of FhuA that were recovered without addition of the siderophore. The FhuA-ferricrocin-iron complex that was incubated under the same conditions but in the absence of H6-′TonB showed no affinity for the resin (Fig. 4, lane 2), demonstrating the specificity of the capture. Since colicin B, an FepA-specific protein antibiotic, was unable to promote the FhuA–H6-′TonB interaction (data not shown), the small-scale assay therefore established that purified H6-′TonB formed a specific complex with FhuA since complex formation was greatly augmented by prior binding of ferricrocin-iron to the receptor.
FIG. 4.
Capture of purified wild-type FhuA by H6-′TonB is promoted by ferricrocin. H6-′TonB was incubated with FhuA or with ferricrocin-loaded FhuA, and complexes were captured with Ni2+-NTA agarose. Proteins were eluted from the resin, resolved by SDS-PAGE, and stained with Coomassie blue. The masses of marker proteins (lane M) (in kilodaltons) are indicated on the left. Lane T, 400 ng of H6-′TonB; lane F, 200 ng of wild-type FhuA; lane 1, control eluate from incubation of H6-′TonB without FhuA; lane 2, control eluate from incubation of FhuA-ferricrocin without H6-′TonB; lane 3, eluate from incubation of H6-′TonB with FhuA; lane 4, eluate from incubation of H6-′TonB with FhuA-ferricrocin.
Quantitation of ligand-induced formation of the H6-′TonB–FhuA.H6 complex.
An ELISA was developed to better quantify the interaction between FhuA and TonB. H6-′TonB was adsorbed to wells of a microtiter plate, the remaining protein binding sites were blocked, and a second FhuA.H6 or FhuA.H6-ferricrocin-iron antigen layer was added. After washing, bound FhuA.H6 was detected with anti-FhuA monoclonal antibodies Fhu4.1 and Fhu8.1 (41), each of which recognizes a different surface-exposed epitope of FhuA. The absorbance values were at least threefold higher for the FhuA.H6-ferricrocin-iron complex than they were for FhuA.H6 alone; with antibody Fhu4.1, the A405 without ferricrocin-iron was 0.65 ± 0.18 (mean ± standard deviation; n = 8), whereas with the ligand present the A405 increased by more than 200% to 2.04 ± 0.11. Similarly, with antibody Fhu8.1 the A405 without ferricrocin-iron was 0.42 ± 0.07, while in the presence of the ligand the A405 was 1.75 ± 0.17. As assessed by Student's t test, the differences in the mean absorbance values for the eight replicates were highly significant (P < 10−5). Since the reactivities of anti-FhuA monoclonal antibodies Fhu4.1 and Fhu8.1 are independent of the presence of ferricrocin-iron bound to the receptor (42), we concluded that ferricrocin-iron substantially increased the association between FhuA and TonB. A second ELISA, in which FhuA.H6 (either with or without pretreatment with ferricrocin-iron) was used to coat the microtiter plate, H6-′TonB was the second layer, and bound H6-′TonB was detected by anti-TonB polyclonal chicken antibodies, produced similar results.
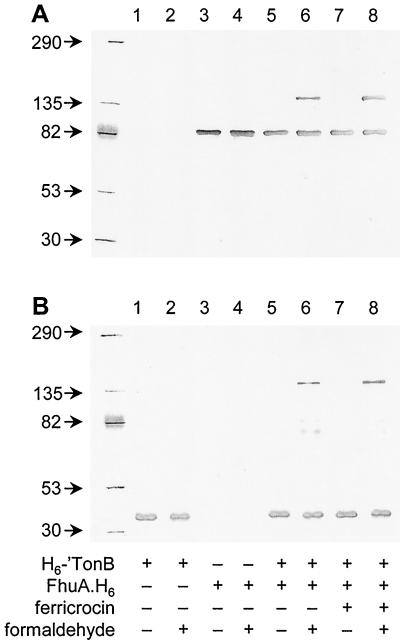
Purified H6-′TonB and FhuA.H6 proteins form a formaldehyde-cross-linked complex in vitro.
Previous studies identified complexes between TonB and FepA (54) and between TonB and FhuA (43) that were formed in vivo and were stabilized by formaldehyde cross-linking. We performed a formaldehyde-cross-linking experiment after allowing H6-′TonB and FhuA.H6 to interact in solution in an effort to estimate the molecular mass of the complex. FhuA.H6 remained exclusively monomeric in the presence of 0.1% formaldehyde (Fig. 5A). While purified H6-′TonB remained monomeric following incubation with formaldehyde (Fig. 5B, compare lanes 1 and 2), the protein showed a slight tendency to oligomerize into complexes with apparent mobilities between 70 and 80 kDa as visualized by immunoblotting of overloaded gels (data not shown). Incubation of the purified proteins together using an approximately threefold molar excess of H6-′TonB over FhuA led to recruitment of a fraction of each protein into a complex with a molecular mass of ca. 150 kDa (Fig. 5A and B, lane 6) as revealed by immunoblotting with FhuA- and TonB-specific antibodies. This finding can be considered in agreement with the relative mobility of the complex as determined after formaldehyde cross-linking in vivo (43), allowing for a certain degree of error given (i) the imprecision of relating relative mobility to molecular mass for unboiled cross-linked samples and (ii) the demonstrated aberrant migration of TonB and H6-′TonB in polyacrylamide gels. Preincubation of FhuA with ferricrocin-iron increased slightly the abundance of the TonB-FhuA complex without altering its apparent molecular mass (Fig. 5A and B, lane 8).
FIG. 5.
Formaldehyde cross-linking traps the FhuA-TonB complex. Samples of H6-′TonB were mixed with FhuA.H6, and formaldehyde was added to some samples as indicated at the bottom. Aliquots were resolved on duplicate SDS–7.5% polyacrylamide gels, and proteins were visualized by immunoblotting with either anti-FhuA monoclonal antibody Fhu4.1 (A) or anti-TonB polyclonal chicken antibodies (B). The masses of marker proteins (in kilodaltons) are indicated on the left. Lanes 1 and 2, H6-′TonB alone; lanes 3 and 4, FhuA.H6 alone; lanes 5 and 6, FhuA.H6 plus H6-′TonB; lanes 7 and 8, FhuA.H6-ferricrocin plus H6-′TonB. Lanes 1, 3, 5, and 7 contained untreated samples; the samples in lanes 2, 4, 6, and 8 were incubated with 0.1% formaldehyde for 20 min prior to electrophoresis.
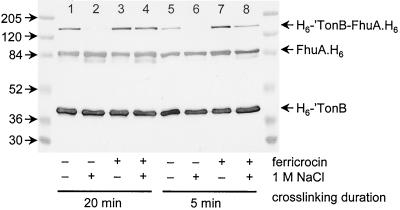
The cross-linking results demonstrated that FhuA formed a complex with TonB even in the absence of an FhuA-specific ligand. This result apparently conflicted with the results of the solution capture assay which demonstrated the need for ligand occupation of FhuA for formation of significant amounts of a complex with TonB. We addressed this apparent discrepancy by cross-linking in the presence of a high concentration of salt to increase the stringency of the interaction environment. While under stringent conditions in the absence of the ligand the efficiency of formaldehyde cross-linking was greatly reduced (Fig. 6, compare lanes 1 and 2 and lanes 5 and 6), the ferricrocin-loaded FhuA–H6-′TonB complex was stable in the presence of 1 M NaC1 (Fig. 6, lanes 4 and 8).
FIG. 6.
Ferricrocin stabilizes the FhuA-TonB complex against high salt concentrations. Samples of H6-′TonB and FhuA.H6 were mixed in the presence or absence of 1 M NaCl as indicated at the bottom, and then formaldehyde was added to all samples. Cross-linking was allowed to proceed for 20 min (lanes 1 to 4) or 5 min (lanes 5 to 8). Equal volumes of aliquots were subjected to SDS-PAGE, and H6-′TonB and FhuA.H6 proteins were visualized simultaneously by immunoblotting with antihistidine monoclonal antibodies. The masses of marker proteins (in kilodaltons) are indicated on the left. The samples in lanes 2, 4, 6, and 8 contained 1 M NaCl; the samples in lanes 3, 4, 7, and 8 were incubated in the presence of the FhuA-specific ligand ferricrocin prior to cross-linking.
Characterization of H6-′TonB–FhuA.H6 complexes by size exclusion chromatography.
Since results from the gel filtration experiments were in good agreement with the sedimentation data, size exclusion chromatography was used to further characterize the H6-′TonB–FhuA.H6 complex. Purified FhuA.H6 eluted in a major A280 peak at 11.6 ml (Fig. 3C). The value obtained for its Rs (4.8 nm) is close to that determined for wild-type FhuA in octylglucoside-containing buffer (4.5 nm) (3). It is therefore highly likely that the A280 peak corresponds to the monomeric form of FhuA.H6 plus its detergent belt (total Mr, 185,000). The presence of ferricrocin-iron had no effect on the elution profile of FhuA.H6 or H6-′TonB (data not shown). It should be noted that the A280 maximum of the H6-′TonB peak (Fig. 3A and B [270 and 250 μg of H6-′TonB applied, respectively]) was markedly lower than the A280 maximum of the FhuA.H6 peak (Fig. 3C [200 μg of FhuA.H6 applied]). Protein concentrations were determined carefully prior to chromatography (55) and were validated by estimation from both Coomassie blue- and silver-stained polyacrylamide gels with protein standards. The presence of a single tryptophan residue in H6-′TonB compared to nine tryptophan residues in FhuA.H6 may in part explain the relatively weak A280 of H6-′TonB.
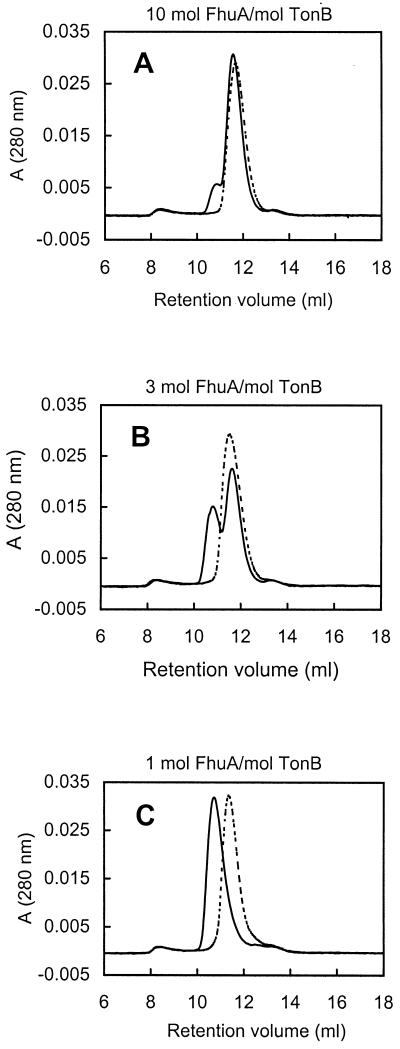
Addition of increasing amounts of H6-′TonB relative to FhuA.H6 in the absence of ferricrocin-iron did not significantly shift the position of the eluting peak (Fig. 7), which corresponded well to the pure FhuA.H6 peak (11.6 ml) (Fig. 3C). In marked contrast, addition of increasing amounts of H6-′TonB to ferricrocin-loaded FhuA.H6 progressively shifted the A280 peak to 10.7 ml (Fig. 7). At equimolar ratios of H6-′TonB and ferricrocin-loaded FhuA (Fig. 7C), the peak at 10.7 ml accounted for more than 96% of the total peak area. SDS-PAGE analysis (data not shown) confirmed the identities of FhuA.H6 and H6-′TonB in the eluates and their shift to earlier fractions when ferricrocin was present; even at the lowest molar ratio of H6-′TonB to ferricrocin-loaded FhuA (10-fold molar excess of FhuA) (Fig. 7A), H6-′TonB was found exclusively in fractions corresponding to the earliest elution volumes. These results are best explained by ferricrocin-induced formation of a complex between FhuA.H6 and H6-′TonB. The Rs of the complex, as deduced from the calibration curve relating Rs to KD, was 6.2 nm. Rmin was calculated by using equation 2 (see Materials and Methods); in this analysis the partial specific volume of FhuA was taken to be 0.776 cm3/g (3) and it was assumed that H6-′TonB bound to FhuA in its detergent-bound form (total Mr, 185,000) (3). An Rmin value was calculated by assuming that the H6-′TonB–FhuA.H6 molar stoichiometry was 1/1 with both proteins hydrated. With this value (Rmin = 4.4), we estimated that the frictional ratio (Rs/Rmin) was 1.4, a value significantly different from and between the frictional ratio values of H6-′TonB (1.8) and FhuA (1.2); the latter value is typical for a globular protein (3).
FIG. 7.
Analysis of H6-′TonB–FhuA.H6 interaction by gel filtration. Proteins were incubated together at various molar ratios and then applied to a Superose 12 column. Dotted lines, experiments conducted without ferricrocin; solid lines, ferricrocin added to the protein sample before it was applied to the column. In all experiments, the amount of FhuA.H6 applied to the column was 200 μg; the amount of H6-′TonB ranged from 5 μg (ca. 10-fold molar excess of FhuA) (A) to 18 μg (ca. 3-fold molar excess of FhuA) (B) to 60 μg (the concentrations of TonB and FhuA were approximately equal) (C).
Capture of FepA by histidine-tagged TonB.
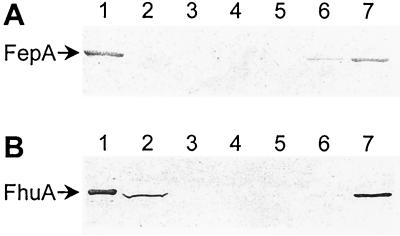
We hypothesized that H6-′TonB could bind not only to FhuA but also to other TonB-dependent receptors. The ferric enterobactin receptor FepA was chosen to test this hypothesis. E. coli cells expressing wild-type FepA from the chromosome were lysed, the membranes were solubilized in detergent-containing buffer, and portions (pretreated or not pretreated with the FepA-specific ligand colicin B [48]) were incubated with H6-′TonB. Complexes were captured with Ni2+-NTA agarose and identified by immunoblotting with an anti-FepA monoclonal antibody. FepA was detected as a single band at ca. 80 kDa in the eluate (Fig. 8A). Formation of the complex between FepA and H6-′TonB was specific since pretreatment with the FepA-specific ligand colicin B increased substantially the abundance of the FepA–H6-′TonB complex (Fig. 8A, compare lanes 6 and 7) and since FepA alone showed no affinity for the resin in the absence of H6-′TonB (Fig. 8A, lanes 2 and 3 [negative controls]). A control experiment confirmed that wild-type FhuA, also expressed from the chromosome, was bound by H6-′TonB; capture of wild-type FhuA was greatly enhanced by preincubation of wild-type FhuA with ferricrocin-iron (Fig. 8B, lanes 6 and 7) or with a crude cell extract containing the FhuA-specific protein antibiotic colicin M (data not shown).
FIG. 8.
H6-′TonB captures FepA and FhuA. Purified H6-′TonB was incubated with cell extracts containing either wild-type FepA or FhuA. Complexes were captured with Ni2+-NTA agarose, resolved by SDS-PAGE, and immunoblotted with a monoclonal antibody against FepA (A) or FhuA (B). (A) Lane 1, 15 μg of total soluble ER2566 extract; lane 2, control without added H6-′TonB; lane 3, control without added H6-′TonB but with colicin B added; lane 4, control eluate without added ER2566 cell extract; lane 5, control eluate without added ER2566 cell extract but with colicin B added; lane 6, eluate from incubation of H6-′TonB with ER2566 cell extract; lane 7, eluate from incubation of H6-′TonB with ER2566 cell extract preincubated with colicin B. (B) Lane 1, 100 ng of FhuA.H6 (migration standard); lane 2, 15 μg of total soluble KP1060 extract; lane 3, negative control capture experiment without added H6-′TonB; lane 4, control eluate without added KP1060 cell extract; lane 5, control eluate without added KP1060 cell extract but with ferricrocin-iron added; lane 6, eluate from incubation of H6-′TonB with KP1060 cell extract; lane 7, eluate from incubation of H6-′TonB with KP1060 cell extract preincubated with ferricrocin-iron.
The possibility that FepA and FhuA bound to H6-′TonB indirectly via colicin M and colicin B was discounted by a control experiment (Moeck, unpublished data) which demonstrated that colicin killing activity against susceptible E. coli strains in vivo was independent of prior incubation of the colicin solutions (colicin B purified protein, colicin M colicinogenic cell extract) with H6-′TonB and removal of any putative TonB-colicin complexes by precipitation with Ni2+-NTA agarose.
DISCUSSION
The work of Jaskula et al. (22) demonstrated that an anchorless form of TonB, although failing to energize TonB-dependent processes in vivo, remained able to form a complex with the ferric enterobactin receptor FepA. Experiments with the purified ferrichrome-iron receptor from E. coli indicated that wild-type TonB, solubilized from the cytoplasmic membrane, was capable of both binding the receptor and sensing its ligand occupation status (43). These results demonstrated that the interaction between receptors and both wild-type and N-terminally truncated TonB proteins could occur in the absence of the proton motive force and thus promoted the feasibility of in vitro reconstitution of high-affinity iron transport. To advance studies in this area, we isolated a TonB variant that lacked the N-terminal signal anchor. The favorable yield, purity, and stability of H6-′TonB make it a good candidate for crystallization and structure determination. Knowledge of the three-dimensional structure of TonB is essential for a complete understanding of bacterial iron nutrition and thus for designing iron transport inhibitors with potential therapeutic value against bacterial infections.
The homogeneity of the H6-′TonB preparation was ascertained by sedimentation equilibrium experiments. The data were consistent with 85 to 90% of the protein being monomeric and the remainder being dimeric. Size exclusion chromatography of H6-′TonB in phosphate buffer was indicative of a monodisperse and monomeric preparation, while 10 to 15% dimers were observed in another preparation of H6-′TonB (in detergent-containing TLN buffer). Cross-linking experiments indicated that H6-′TonB had a slight tendency to form oligomers with apparent mobilities between 70 and 80 kDa. These results are supported by the presence of H6-′TonB oligomers, as seen in mass spectrometry analysis (G. S. Moeck and B. Thatcher, unpublished data). A 77-kDa TonB-containing complex was observed in previous studies of in vivo cross-linking between TonB and other proteins of the cell envelopes of E. coli (54) and other gram-negative bacteria (30); however, the composition of the 77-kDa complex remains unknown. Taking into account the retarded migration of TonB in SDS-PAGE gels, it is likely that the 70- to 80-kDa complex which was observed in the present study represents dimers of H6-′TonB. In this regard, the native TonB amino acid sequence shows a weak propensity to form coiled coils (38) via interactions in a 14-amino-acid window (residues A199 to R212) near the C terminus. A β-strand–α-helix–β-strand motif involving this region has been proposed for TonB (26, 28).
Like wild-type TonB, H6-′TonB showed anomalous behavior in polyacrylamide gels. It migrated with a relative mobility of ca. 35 kDa, which is similar to the apparent molecular mass of wild-type TonB (35 to 36 kDa in SDS-PAGE gels [29, 47]) and is considerably different than would be predicted from its actual molecular mass of 25 kDa as deduced from its nucleotide sequence. It was concluded that the aberrant mobility of wild-type TonB stems from its characteristic proline-rich region since a TonB derivative carrying a deletion of this region (TonBΔ66-100) had a relative electrophoretic mobility in polyacrylamide gels that reflected its actual molecular mass (28). By inference, the aberrant migration of H6-′TonB indicates that its proline-rich region adopts a conformation which is similar to that of wild-type TonB.
H6-′TonB also behaved anomalously in gel filtration studies since its migration was delayed compared to that expected for a globular soluble protein with the equivalent molecular mass. Anomalous elution through gel filtration matrices has been described for elongated proteins, such as myosin and fibrinogen (44). The combination of size exclusion chromatography and sedimentation velocity measurements indeed indicated that H6-′TonB has an elongated form with an axial ratio of 15, so it may take the form of a rod that is 24 nm long and 1.6 nm in diameter.
These observations are reminiscent of recent data for the AcrA protein of the multidrug efflux complex of E. coli and for the TolA protein of the group A colicin import system of E. coli. These two proteins share features with TonB; they are anchored in or associated with the cytoplasmic membrane, and they are thought to extend into and across the periplasm. AcrA adopts a highly asymmetric form, as assessed by dynamic light scattering and analytical ultracentrifugation (59). This finding is consistent with the presence of a 75-amino-acid central region of AcrA that is predicted to be largely α-helical. This region may span a distance of 10 nm, thereby aiding in the formation of protein-protein contacts (60) across the cell envelope. Similarly, the central α-helical domain of TolA adopts a rigid form (14, 36), probably allowing the protein to span the periplasm to contact group A colicins, including colicins A and E1 (4, 15, 33). Based on these results and in agreement with a nuclear magnetic resonance study of a synthetic peptide corresponding to the central proline-rich region of TonB (9), we propose that the elongated form of H6-′TonB reflects the form adopted by wild-type TonB and may bridge the cytoplasmic and outer membranes, which are thought to be separated by 15 to 20 nm (19).
Size exclusion chromatography, ELISA, and in vitro capture experiments all showed that TonB-FhuA contact was greatly enhanced by prior binding of ferricrocin-iron to FhuA. Formaldehyde cross-linking stabilized a high-molecular-mass complex (relative mobility, ca. 150 kDa) that was detected by both anti-FhuA and anti-TonB antibodies. Preliminary results from mass spectrometry analysis (Moeck and Thatcher, unpublished) indicate that the stoichiometry of the complex corresponds to that of a H6-′TonB–FhuA.H6 heterodimer, equivalent to the stoichiometry observed for the site-directed cysteine-cross-linked BtuB-TonB heterodimer (relative mobility, 100 kDa) (12). By comparison, previous studies of in vivo cross-linking (30, 54) revealed a TonB-FepA complex with a relative mobility of ca. 195 kDa. Mass spectrometry and analytical ultracentrifugation using H6-′TonB may now be exploited to confirm the masses and stoichiometry of the various TonB-receptor complexes in a manner that is independent of their molecular forms. Furthermore, surface plasmon resonance techniques may now permit kinetic analyses of the interactions between TonB and purified TonB-dependent receptors, as demonstrated for the interactions between fragments of the E. coli TolA protein, a structural analogue of TonB, and the colicins which it translocates (14).
Two independent experiments indicated that H6-′TonB interacted in vitro with FhuA even in the absence of ferricrocin-iron. The H6-′TonB–FhuA complex was observed in Ni2+-NTA agarose capture experiments after the gel was overloaded and the H6-′TonB–FhuA.H6 complex was visualized after cross-linking of the two proteins. However, no evidence for formation of a complex in the absence of ferricrocin-iron was obtained by size exclusion chromatography. These results may reflect a weak affinity of TonB for its receptors in the absence of their cognate ligands, as previously noted (43, 54). Such weak TonB-receptor interactions are likely to be overrepresented by formaldehyde cross-linking since in the present study they were selectively disrupted with high concentrations of salt in the absence of an FhuA-specific ligand.
In vitro experiments with H6-′TonB realistically may now be extended to further define the regions of contact between TonB and TonB-dependent receptors. The TonB box (51), a short stretch of sequence homology near the N termini of high-affinity outer membrane receptors, is one region of TonB-receptor contact. Site-directed disulfide cross-linking revealed close associations between residues in the TonB box of BtuB and residues around amino acid 160 of TonB (12, 13). Crystallographic analyses revealed dramatic ligand-induced changes on the periplasmic face of FhuA (17, 37), which occurred just C terminal to the TonB box of FhuA, which is located between amino acids 7 and 11. Similarly, the TonB box of BtuB underwent a striking rearrangement upon addition of Vitamin B12 to outer membrane preparations of E. coli; its helical conformation was converted into an extended, disordered structure that may have extended into the periplasm (39). The TonB box of FepA (residues 12 to 18) was sufficiently ordered in FepA crystals (11) to be modeled; however, its extended conformation and ambiguity about the ligand occupation status of the receptor in the crystal revealed little in terms of a distinct structure that might be sensed by TonB. The ligand-mediated conformational changes in FhuA and FepA that resulted in increased coupling with TonB, as manifested in the present work, may be closely related to those revealed by crystallography. Structural analyses of TonB-receptor cocrystals are necessary to define with precision the protein-protein contact surfaces. Purifying the H6-′TonB–FhuA.H6 complex by using preparative-scale size exclusion chromatography could render the complex amenable to such structural studies.
ACKNOWLEDGMENTS
We thank M. Bonhivers and P. Boulanger for stimulating discussions and for help with certain elements of this project. The assistance of R. Maranger in statistical analyses and in the preparation of figures is enthusiastically acknowledged. We thank L. Plançon and C. Khursigara for samples of FhuA, K. Postle for strains KP1060 and KP1120, J. W. Coulton for plasmids and anti-FhuA antibodies, and M. A. McIntosh for a sample of anti-FepA monoclonal antibody. G. Batelier (Laboratoire d'Enzymologie CNRS, Gif-sur-Yvette, France) and M. le Maire provided expertise for analytical ultracentrifugation experiments and analysis.
G.S.M. gratefully acknowledges postdoctoral fellowships from the Ministère des Affaires Etrangères, France, and from the Natural Sciences and Engineering Research Council, Canada.
REFERENCES
- 1.Ahmer B M M, Thomas M G, Larsen R A, Postle K. Characterization of the exbBD operon of Escherichia coli and the role of ExbB and ExbD in TonB function and stability. J Bacteriol. 1995;177:4742–4747. doi: 10.1128/jb.177.16.4742-4747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anton M, Heller K J. Functional analysis of a C-terminally altered TonB protein of Escherichia coli. Gene. 1991;105:23–29. doi: 10.1016/0378-1119(91)90509-a. [DOI] [PubMed] [Google Scholar]
- 3.Boulanger P, le Maire M, Bonhivers M, Dubois S, Desmadril M, Letellier L. Purification and structural and functional characterization of FhuA, a transporter of the Escherichia coli outer membrane. Biochemistry. 1996;35:14216–14224. doi: 10.1021/bi9608673. [DOI] [PubMed] [Google Scholar]
- 4.Bouveret E, Rigal A, Lazdunski C, Bénédetti H. Distinct regions of the colicin A translocation domain are involved in the interaction with TolA and TolB proteins upon import into Escherichia coli. Mol Microbiol. 1998;27:143–157. doi: 10.1046/j.1365-2958.1998.00667.x. [DOI] [PubMed] [Google Scholar]
- 5.Bradbeer C. The proton motive force drives the outer membrane transport of cobalamin in Escherichia coli. J Bacteriol. 1993;175:3146–3150. doi: 10.1128/jb.175.10.3146-3150.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun M, Killmann H, Braun V. The β-barrel domain of FhuAΔ5-160 is sufficient for TonB-dependent FhuA activities of Escherichia coli. Mol Microbiol. 1999;33:1037–1049. doi: 10.1046/j.1365-2958.1999.01546.x. [DOI] [PubMed] [Google Scholar]
- 7.Braun V, Killmann H. Bacterial solutions to the iron supply problem. Trends Biochem Sci. 1999;24:104–109. doi: 10.1016/s0968-0004(99)01359-6. [DOI] [PubMed] [Google Scholar]
- 8.Braun V, Hantke K, Köster W. Bacterial iron transport: mechanisms, genetics, and regulation. In: Sigel A, Sigel H, editors. Metal ions in biological systems. 35. Iron transport and storage in microorganisms, plants, and animals. New York, N.Y: Marcel Dekker; 1998. pp. 67–145. [PubMed] [Google Scholar]
- 9.Brewer S, Tolley M, Trayer I P, Barr G C, Dorman C J, Hannavy K, Higgins C F, Evans J S, Levine B A, Wormald M R. Structure and function of X-Pro dipeptide repeats in the TonB proteins of Salmonella typhimurium and Escherichia coli. J Mol Biol. 1990;216:883–895. doi: 10.1016/S0022-2836(99)80008-4. [DOI] [PubMed] [Google Scholar]
- 10.Brinkley M. A brief survey of methods for preparing protein conjugates with dyes, haptens, and cross-linking reagents. Bioconjug Chem. 1992;3:2–13. doi: 10.1021/bc00013a001. [DOI] [PubMed] [Google Scholar]
- 11.Buchanan S K, Smith B S, Venkatramani L, Xia D, Esser L, Palnitkar M, Chakraborty R, van der Helm D, Deisenhofer J. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat Struct Biol. 1999;6:56–63. doi: 10.1038/4931. [DOI] [PubMed] [Google Scholar]
- 12.Cadieux N, Bradbeer C, Kadner R J. Sequence changes in the Ton box region of BtuB affect its transport activities and interaction with TonB protein. J Bacteriol. 2000;182:5954–5961. doi: 10.1128/jb.182.21.5954-5961.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cadieux N, Kadner R J. Site-directed disulfide bonding reveals an interaction site between energy-coupling protein TonB and BtuB, the outer membrane cobalamin transporter. Proc Natl Acad Sci USA. 1999;96:10673–10678. doi: 10.1073/pnas.96.19.10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derouiche R, Lloubès R, Sasso S, Bouteille H, Oughideni R, Lazdunski C, Loret E. Circular dichroism and molecular modeling of the E. coli TolA periplasmic domains. Biospectroscopy. 1999;5:189–198. doi: 10.1002/(SICI)1520-6343(1999)5:3<189::AID-BSPY8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 15.Derouiche R, Zeder-Lutz G, Bénédetti H, Gavioli M, Rigal A, Lazdunski C, Lloubès R. Binding of colicins A and E1 to purified TolA domains. Microbiology. 1997;143:3185–3192. doi: 10.1099/00221287-143-10-3185. [DOI] [PubMed] [Google Scholar]
- 16.Evans J S, Levine B A, Trayer I P, Dorman C J, Higgins C F. Sequence imposed structural constraints in the TonB protein of Escherichia coli. FEBS Lett. 1986;208:211–216. doi: 10.1016/0014-5793(86)81020-1. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson A D, Hofmann E, Coulton J W, Diederichs K, Welte W. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science. 1998;282:2215–2220. doi: 10.1126/science.282.5397.2215. [DOI] [PubMed] [Google Scholar]
- 18.Fischer E, Günter K, Braun V. Involvement of ExbB and TonB in transport across the outer membrane of Escherichia coli: phenotypic complementation of exb mutants by overexpressed tonB and physical stabilization of TonB by ExbB. J Bacteriol. 1989;171:5127–5134. doi: 10.1128/jb.171.9.5127-5134.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham L L, Beveridge T J, Naninga N. Freeze-substitution of gram-negative eubacteria: general cell morphology and envelope profiles. J Bacteriol. 1991;173:1623–1633. doi: 10.1128/jb.173.5.1623-1633.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hancock R E W, Braun V. Nature of the energy requirement for the irreversible adsorption of bacteriophages T1 and φ80 to Escherichia coli. J Bacteriol. 1976;125:409–415. doi: 10.1128/jb.125.2.409-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hannavy K, Barr G C, Dorman C J, Adamson J, Mazengera L R, Gallagher M P, Evans J S, Levine B A, Trayer I P, Higgins C F. TonB protein of Salmonella typhimurium: a model for signal transduction between membranes. J Mol Biol. 1990;216:897–910. doi: 10.1016/S0022-2836(99)80009-6. [DOI] [PubMed] [Google Scholar]
- 22.Jaskula J C, Letain T E, Roof S K, Skare J T, Postle K. Role of the TonB amino terminus in energy transduction between membranes. J Bacteriol. 1994;176:2326–2338. doi: 10.1128/jb.176.8.2326-2338.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadner R J. Vitamin B12 transport in Escherichia coli: energy coupling between membranes. Mol Microbiol. 1990;4:2027–2033. doi: 10.1111/j.1365-2958.1990.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 24.Kadner R J, Heller K J. Mutual inhibition of cobalamin and siderophore uptake systems suggests their competition for TonB function. J Bacteriol. 1995;177:4829–4835. doi: 10.1128/jb.177.17.4829-4835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karlsson M, Hannavy K, Higgins C F. A sequence-specific function for the N-terminal signal-like sequence of the TonB protein. Mol Microbiol. 1993;8:379–388. doi: 10.1111/j.1365-2958.1993.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 26.Klebba P E, Rutz J M, Liu J, Murphy C K. Mechanisms of TonB-catalyzed iron transport through the enteric bacterial cell envelope. J Bioenerg Biomemb. 1993;25:603–611. doi: 10.1007/BF00770247. [DOI] [PubMed] [Google Scholar]
- 27.Lambert O, Moeck G S, Levy D, Plançon L, Letellier L, Rigaud J-L. An 8Å projected structure of FhuA, a ligand-gated channel of the Escherichia coli outer membrane. J Struct Biol. 1999;126:145–155. doi: 10.1006/jsbi.1999.4115. [DOI] [PubMed] [Google Scholar]
- 28.Larsen R A, Wood G E, Postle K. The conserved proline-rich motif is not essential for energy transduction by Escherichia coli TonB protein. Mol Microbiol. 1993;10:943–953. doi: 10.1111/j.1365-2958.1993.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 29.Larsen R A, Thomas M G, Postle K. Partial suppression of an Escherichia coli TonB transmembrane domain mutation (ΔV17) by a missense mutation in ExbB. Mol Microbiol. 1994;13:627–640. doi: 10.1111/j.1365-2958.1994.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 30.Larsen R A, Myers P S, Skare J T, Seachord C L, Darveau R P, Postle K. Identification of TonB homologs in the family Enterobacteriaceae and evidence for conservation of TonB-dependent energy transduction complexes. J Bacteriol. 1996;178:1363–1373. doi: 10.1128/jb.178.5.1363-1373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsen R A, Foster-Hartnett D, McIntosh M A, Postle K. Regions of Escherichia coli TonB and FepA proteins essential for in vivo physical interactions. J Bacteriol. 1997;179:3213–3221. doi: 10.1128/jb.179.10.3213-3221.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsen R A, Thomas M G, Postle K. Protonmotive force, ExbB and ligand-bound FepA drive conformational changes in TonB. Mol Microbiol. 1999;31:1809–1824. doi: 10.1046/j.1365-2958.1999.01317.x. [DOI] [PubMed] [Google Scholar]
- 33.Lazdunski C J, Bouveret E, Rigal A, Journet L, Lloubès R, Bénédetti H. Colicin import into Escherichia coli cells. J Bacteriol. 1998;180:4993–5002. doi: 10.1128/jb.180.19.4993-5002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Maire M, Aggerbeck L P, Monteilhet C, Andersen J P, Møller J V. The use of high-performance liquid chromatography for the determination of size and molecular weight of proteins: a caution and a list of membrane proteins suitable as standards. Anal Biochem. 1986;154:525–535. doi: 10.1016/0003-2697(86)90025-4. [DOI] [PubMed] [Google Scholar]
- 35.Letain T E, Postle K. TonB protein appears to transduce energy by shuttling between the cytoplasmic membrane and the outer membrane in Escherichia coli. Mol Microbiol. 1997;24:271–283. doi: 10.1046/j.1365-2958.1997.3331703.x. [DOI] [PubMed] [Google Scholar]
- 36.Levengood S K, Beyer W F J, Webster R E. TolA: a membrane protein involved in colicin uptake contains an extended helical region. Proc Natl Acad Sci USA. 1991;88:5939–5944. doi: 10.1073/pnas.88.14.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Locher K P, Rees B, Koebnik R, Mitschler A, Moulinier L, Rosenbusch J P, Moras D. Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell. 1998;95:771–778. doi: 10.1016/s0092-8674(00)81700-6. [DOI] [PubMed] [Google Scholar]
- 38.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 39.Merianos H J, Cadieux N, Lin C H, Kadner R J, Cafiso D S. Substrate-induced exposure of an energy-coupling motif of a membrane transporter. Nat Struct Biol. 2000;7:205–209. doi: 10.1038/73309. [DOI] [PubMed] [Google Scholar]
- 40.Moeck G S, Coulton J W. TonB-dependent iron acquisition: mechanisms of siderophore-mediated active transport. Mol Microbiol. 1998;28:675–681. doi: 10.1046/j.1365-2958.1998.00817.x. [DOI] [PubMed] [Google Scholar]
- 41.Moeck G S, Ratcliffe M J, Coulton J W. Topological analysis of the Escherichia coli ferrichrome-iron receptor by using monoclonal antibodies. J Bacteriol. 1995;177:6118–6125. doi: 10.1128/jb.177.21.6118-6125.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moeck G S, Tawa P, Xiang H, Turnbull J, Ismail A A, Coulton J W. Ligand-induced conformational change in the ferrichrome-iron receptor of Escherichia coli K-12. Mol Microbiol. 1996;22:459–471. doi: 10.1046/j.1365-2958.1996.00112.x. [DOI] [PubMed] [Google Scholar]
- 43.Moeck G S, Coulton J W, Postle K. Cell envelope signalling in Escherichia coli. Ligand binding to the ferrichrome-iron receptor FhuA promotes interaction with the energy-transducing protein TonB. J Biol Chem. 1997;272:28391–28397. doi: 10.1074/jbc.272.45.28391. [DOI] [PubMed] [Google Scholar]
- 44.Nozaki Y, Schechter N M, Reynolds J A, Tanford C. Use of gel chromatography for the determination of the Stokes radii of proteins in the presence and absence of detergents. A reexamination. Biochemistry. 1976;15:3884–3890. doi: 10.1021/bi00662a036. [DOI] [PubMed] [Google Scholar]
- 45.Postle K. TonB protein and energy transduction between membranes. J Bioenerg Biomemb. 1993;25:591–601. doi: 10.1007/BF00770246. [DOI] [PubMed] [Google Scholar]
- 46.Postle K, Good R F. DNA sequence of the Escherichia coli tonB gene. Proc Natl Acad Sci USA. 1983;80:5235–5239. doi: 10.1073/pnas.80.17.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Postle K, Skare J T. Escherichia coli TonB is exported from the cytoplasm without proteolytic cleavage of its amino terminus. J Biol Chem. 1988;263:11000–11007. [PubMed] [Google Scholar]
- 48.Pugsley A P, Reeves R. Characterization of group B colicin-resistant mutants of Escherichia coli K-12: colicin resistance and the role of enterochelin. J Bacteriol. 1976;127:218–228. doi: 10.1128/jb.127.1.218-228.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reynolds P R, Mottur G P, Bradbeer C. Transport of vitamin B12 in Escherichia coli K-12. Some observations on the roles of the gene products of btuC and tonB. J Biol Chem. 1980;255:4313–4319. [PubMed] [Google Scholar]
- 50.Roof S K, Allard J D, Bertrand K P, Postle K. Analysis of the Escherichia coli TonB membrane topology by use of PhoA fusions. J Bacteriol. 1991;173:5554–5557. doi: 10.1128/jb.173.17.5554-5557.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schram E, Mende J, Braun V, Kemp R M. Nucleotide sequence of the colicin B activity gene cba: consensus pentapeptide among TonB-dependent colicins and receptors. J Bacteriol. 1987;169:3350–3357. doi: 10.1128/jb.169.7.3350-3357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schryvers A B, Stojiljkovic I. Iron acquisition systems in the pathogenic Neisseria. Mol Microbiol. 1999;32:1117–1123. doi: 10.1046/j.1365-2958.1999.01411.x. [DOI] [PubMed] [Google Scholar]
- 53.Skare J T, Postle K. Evidence for a TonB-dependent energy transduction complex in Escherichia coli. Mol Microbiol. 1991;5:2883–2890. doi: 10.1111/j.1365-2958.1991.tb01848.x. [DOI] [PubMed] [Google Scholar]
- 54.Skare J T, Ahmer B M M, Seachord C L, Darveau R P, Postle K. Energy transduction between membranes. TonB, a cytoplasmic membrane protein, can be chemically cross-linked in vivo to the outer membrane receptor FepA. J Biol Chem. 1993;268:16302–16308. [PubMed] [Google Scholar]
- 55.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 56.Tanford C, Nozaki Y, Reynolds J A, Makino S. Molecular characterization of proteins in detergent solutions. Biochemistry. 1974;13:2369–2376. doi: 10.1021/bi00708a021. [DOI] [PubMed] [Google Scholar]
- 57.van der Helm D. The physical chemistry of bacterial outer membrane siderophore receptor proteins. In: Sigel A, Sigel H, editors. Metal ions in biological systems. 35. Iron transport and storage in microorganisms, plants, and animals. New York, N.Y: Marcel Dekker; 1998. pp. 355–401. [PubMed] [Google Scholar]
- 58.Williamson M P. The structure and function of proline-rich regions in proteins. Biochem J. 1994;297:249–260. doi: 10.1042/bj2970249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zgurskaya H I, Nikaido H. AcrA is a highly asymmetric protein capable of spanning the periplasm. J Mol Biol. 1999;285:409–420. doi: 10.1006/jmbi.1998.2313. [DOI] [PubMed] [Google Scholar]
- 60.Zgurskaya H I, Nikaido H. Cross-linked complex between oligomeric periplasmic lipoprotein AcrA and the inner-membrane-associated multidrug efflux pump AcrB from Escherichia coli. J Bacteriol. 2000;182:4264–4267. doi: 10.1128/jb.182.15.4264-4267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]