Abstract
Background and aims
The pain of labour is very severe. Most women prefer painless labour to routine labour if they are aware of the methods of analgesia. The aim of this study was to evaluate the effect of dexmedetomidine intravenous infusion on labour pain management in primipara term pregnant women.
Methods
In this nonrandomised clinical trial with control group, all primipara term pregnant women from August 2019 to March 2020 were included. In the intervention group, after the active phase of labour, dexmedetomidine was given according to the protocol and continued until phase 2 of labour. The control group received no intervention to reduce pain. Patients in both groups were evaluated for fetal heart rate, Apgar scores, vital signs, pain intensity, and sedation score.
Results
There were no significant differences in primary fetal heart rate, primary maternal hemodynamics, and mean Apgar scores at 1 and 5 minutes between the two groups (p > .05). There was no significant difference in the mean fetal heart rate in different stages between the two groups. Intragroup analysis in the intervention group showed that mean systolic and diastolic blood pressures were significantly decreased after drug administration but were in the normal range. The active phase of labour in the intervention group was significantly shorter than in the control group (p = 0.002). The mean Visual Analogue Scale (VAS) score after dexmedetomidine administration decreased significantly from 9.25 at baseline to 4.61 after drug administration, 3.88 during labour, and 1.88 after placental expulsion. The mean Ramsay Sedation Scale score after dexmedetomidine administration increased significantly from 1.00 at baseline to 2.05 after drug administration, 2.22 during labour, and 2.05 after placental expulsion.
Conclusion
Based on the study's results, the administration of dexmedetomidine to manage labour pain with careful monitoring of mother and fetus is recommended.
Keywords: dexmedetomidine, intravenous analgesia, painless labour
Introduction
Labour pain generally is very severe. Even the use of distraction techniques, such as music and video games, does not delay the time to seek analgesia [1]. Failure to communicate between a gynaecologist and the patient leads to obstacles in the patient's knowledge of the painless delivery method and therefore the tendency of pregnant women to have caesarean section [2]. Based on a previous investigation, it has been reported that most women prefer these methods instead of routine labour if they are aware of analgesic alternatives [3]. Using analgesia for delivery reduces the incidence of elective caesarean section [4]. Most painless delivery methods accelerate labour by reducing the duration of active phase [5,6,7,8]. In addition, pain relief is associated with a decreased prevalence of postpartum depression [9, 10].
Although most studies on painless delivery focus on the epidural method, which has been recognized as a safe and uncomplicated procedure for the mother and infant [11,12,13,14,15], this procedure may sometimes fail, or it may be contraindicated, or the mother may refuse to use the neuroaxial method [16, 17].
Dexmedetomidine is a specific α2 agonist, with sedative and analgesic effects and the least probable respiratory depression [18]. It has been used successfully to control shivering due to spinal caesarean section and has had no significant hemodynamic complications [19].
Because there are limited studies on this issue and previous case reports, intravenous (IV) dexmedetomidine was used in labour and no complication was observed in the mother and infant [16, 17, 20,21,22,23], we aimed to evaluate the effect of dexmedetomidine IV infusion on labour pain management in primipara term pregnant women who are contraindicated or refused for an epidural procedure.
Methods
In this nonrandomised clinical trial with control group, all primipara term pregnant women, American Society of Anesthesiologists (ASA) I and II, who were referred to Baqiyatallah Hospital in Tehran, Iran, from August 2019 to March 2020 were included. In this study a convenience sampling method for data gathering was used. Patients with fetal distress, latent phase of labour, prolonged premature rupture of membranes, liver failure, renal failure, 2- or 3-degree heart block, neuropsychiatric diseases, and drug abusers were excluded. Mothers who were candidates for pain relief through IV drugs, after obtaining written consent, were included in the intervention group, and mothers who were not candidates for pain relief and who decided to experience labour pain entered the control group after accepting the project.
In the active phase of labour (diagnosed by a gynaecologist), the patients were monitored for systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse rate (PR), respiratory rate (RR), and oxygen saturation (SpO2), and their vital signs were recorded in the questionnaire every 5 to 15 minutes after placental expulsion. Fetal heart rate (FHR) monitoring was performed continuously and recorded every 5 minutes. FHR1, FHR2, and FHR3 were recorded at baseline, during administration of a bolus dose of dexmedetomidine, and during labour, respectively. Patients were instructed on how to assess pain. In the intervention group, after recording the initial effacement and dilatation, dexmedetomidine was infused intravenously for 10 minutes at a dose of 1.00 μg/kg (0.4% solution), followed by infusion of 0.2 to 1.00 μg/kg/h. The dose of continuous infusion was based on the rate of analgesia by the Visual Analogue Scale score (VAS ≤ 3) or sedation by the Ramsay Sedation Scale score (RSS < 5). The infusion continued until stage 2 of labour, and this period was recorded as stage 1. In the event of hemodynamic complications (SBP < 100 or PR < 60 or FHR < 100), the drug was temporarily discontinued, and IV atropine at a dose of 0.01 mg/kg and IV ephedrine at an initial dose of 10 mg were administered.
The control group received no intervention to reduce pain. Routine care was performed in these patients.
The beginning of stage 2 until the birth of the infant was recorded as stage 2, and stage 3 was indicated from the birth of the infant until the placental expulsion.
Patients in both groups were evaluated for pain intensity by a VAS score and sedation by an RSS score up to 15 minutes after placental expulsion. After birth neonatal Apgar scores at 1 and 5 minutes were recorded. Fifteen minutes after placental expulsion, satisfaction of midwives was assessed in the intervention group using the 5-point Likert index (1 = highly dissatisfied, 2 = dissatisfied, 3 = no comment, 4 = satisfied, 5 = strongly satisfied).
Ethical Considerations
This research project was approved by the Ethics Committee of Baqiyatallah University of Medical Sciences (IR.BMSU. REC.1397.250; date: 2019-02-15), and it was registered in the Iranian registry of clinical trials (IRCT20161022030421N5 date: 2019-07-20). Consent letters were obtained from participants.
Data analysis
The data were analyzed by SPSS-22 software. Quantitative data were analyzed using descriptive software and displayed as mean ± standard deviation (SD). Chi-square tests were used to compare percentages or frequencies. The normality of quantitative data was assessed by the Kolmogorov-Smirnov test. An independent sample t-test was used to compare parametric data between the two groups. A paired t-test was used to compare quantitative parameters before and after the intervention. In this study a probability value less than .05 was considered statistically significant.
Results
A total of 46 patients were studied, including 20 patients in the intervention group and 26 patients in the control group. No patient was excluded in the two groups. Two patients in control group and three in intervention group underwent cesarean section for different reasons. There was no significant difference in mean age (p = .79), height (p = .94), and weight (p = .2) between the intervention and control groups. Comparison of mean cervical dilatation and effacement between the two groups showed no significant difference in mean dilatation (p = .89) and effacement (p = .87). The comparison of demographic characteristics between the two groups is shown in (Table 1).
Table 1.
Comparison of demographic characteristics between the two groups
| groups | N | Minimum | Maximum | Mean | Std. Deviation | p-value | |
|---|---|---|---|---|---|---|---|
| Age (year) | intervention | 20 | 22 | 38 | 27.9000 | 4.51 | 0.79 |
| control | 26 | 18 | 36 | 26.5385 | 4.76 | ||
|
| |||||||
| Height (cm) | intervention | 20 | 157 | 170 | 162.7500 | 4.35 | 0.94 |
| control | 26 | 147 | 173 | 162.6154 | 7.40 | ||
|
| |||||||
| Weight (kg) | intervention | 20 | 59 | 88 | 77.0000 | 13.79 | 0.2 |
| control | 26 | 51 | 98 | 72.1154 | 11.77 | ||
|
| |||||||
| Dilatation (cm) | intervention | 20 | 4 | 8 | 5.42 | 5.42 | 0.89 |
| control | 26 | 4 | 7 | 5.38 | 5.38 | ||
|
| |||||||
| Effacement (%) | intervention | 20 | 30 | 90 | 53.15 | 53.15 | 0.87 |
| control | 26 | 30 | 80 | 53.84 | 53.84 | ||
The mean duration of the first stage of labour in the two intervention and control groups was 81.23 ± 75.83 minutes and 192.75 ± 120.76 minutes, respectively (p = .002). There was no significant difference in the mean duration of the second (p = .95) and third (p = .47) stages of delivery between the groups (Table 2).
Table 2.
Comparing mean duration of labor stages (minutes) between the two groups
| groups | N | Minimum | Maximum | Mean | Std. Deviation | p-value | |
|---|---|---|---|---|---|---|---|
| Stage I | intervention | 17 | 10 | 300 | 81.23 | 75.83 | 0.002 |
| control | 24 | 33 | 402 | 192.75 | 120.76 | ||
|
| |||||||
| Stage II | intervention | 17 | 10 | 90 | 42.47 | 27.58 | 0.95 |
| control | 24 | 10 | 93 | 41.95 | 23.87 | ||
|
| |||||||
| Stage III | intervention | 17 | 4 | 10 | 5.94 | 1.71 | 0.47 |
| control | 24 | 4 | 50 | 7.58 | 9.16 | ||
Two patients (8.33%) in the intervention group and no patients in the control group needed assisted delivery device, and no significant difference was noted between the groups (p = .22). The need for oxytocin in the control and intervention groups was 10 out of 26 patients (38.46%) and 8 out of 20 patients (40%), respectively. There was no significant difference in the frequency of patients who were in need o oxytocin administration between the two groups (p = .91).
Furthermore, there was no significant difference in FHR1 between the groups (p = .98). Mean FHR1 was 132.55 ± 9.11 in the intervention group and 132.50 ± 9.88 in the control group. The mean FHR1, FHR2, and FHR3 in the intervention group were 132.55 ± 9.11, 134.38 ± 8.40, and 133.44 ± 8.02, respectively. There was no significant difference in the mean of FHR1 and FHR2 (p = .31), mean of FHR1 and FHR3 (p = .42), and mean of FHR2 and FHR3 (p = .51) in the intervention group. Mean FHR1 and FHR3 in the control group were 132.50 ± 9.88 and 135.08 ± 8.84, respectively. There was no significant difference in the mean of FHR1 and FHR3 in the control group (p = .51).
Mean baseline DBP in the intervention and control groups was 81.88 ± 6.39 and 81.00 ± 8.82, respectively. There was no significant difference in mean baseline DBP between the two groups (p = 0.72), indicating the homogeneity of samples before the study.
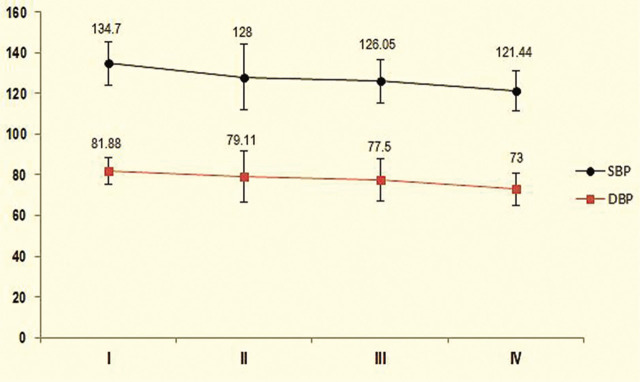
The mean of the first, second, third, and fourth DBP in the intervention group was 81.88 ± 6.39, 79.11 ± 12.8, 77.50 ± 10.47, and 73.0 ± 8.01, respectively (Figure 1).
Figure 1.
Comparison of mean systolic and diastolic blood pressure (mmHg) in the intervention group during the study.
I = Primary SBP and DBP; II = during bolus dose of dexmedetomidine; III = during labour; IV = after placental expulsion.
There was no significant difference in the mean of the first and second DBP (p = .16). However, there was a significant difference between the mean of the first and third DBP (p = 0.039) and the mean of the first and fourth DBP (p = .023). There was no significant difference in the mean of the second and third DBP (p = .31), second and fourth DBP (p = .11), and third and fourth DBP (p = .09; Figure 1).
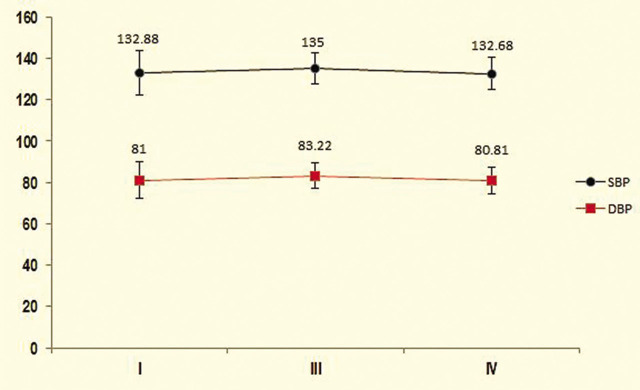
The mean of the first, third, and fourth DBP in the control group was 81.00 ± 8.82, 83.22 ± 6.07, and 80.81 ± 6.27, respectively. There was no significant difference between the mean of the first to third DBP in the control group (p = .25; Figure 2).
Figure 2.
Comparison of mean systolic and diastolic blood pressure (mmHg) in the control group during the study.
I = Primary SBP and DBP; III = during labour; IV = after placental expulsion.
Mean baseline SBP in the intervention group was 134.70 ± 10.68 and in the control group, 132.88 ± 10.77. There was no significant difference in mean baseline SBP between the two groups (p = .34), indicating that the samples were homogeneous before drug intervention.
Mean baseline in the second, third, and fourth SBP in the intervention group was 134.70 ± 10.68, 128.0 ± 16.32, 126.05 ± 10.66, and 121.44 ± 9.90 mmHg, respectively. Significant differences were observed in the mean of the first and second SBP (p = .034), first and third SBP (p = .028) and first and fourth SBP (p = .019). There was no significant difference in the mean of the second and third SBP (p = .42), but there was a significant difference in the mean of the second and fourth SBP (p = .043) and third and fourth SBP (p = .047; Figure 1). The mean of the first, third, and fourth SBP in the control group was 132.88 ± 10.77, 135.00 ± 7.63, and 132.68 ± 7.42, respectively. There was no significant difference between the mean of first and third SBP (p = .14; Figure 2).
Mean baseline PR in the intervention group was 98.05 ± 20.12 minutes and in the control group, 92.53 ± 17.41 minutes. There was no significant difference in the mean baseline PR between the two groups (p = .32), indicating that the samples were homogeneous before drug use.
Mean first, second, third, and fourth PR in the intervention group was 98.05 ± 20.12, 93.44 ± 19.43, 92.66 ± 16.74, and 93.11 ± 13.86, respectively. There was no significant difference in the mean PR during the different stages. Mean first, third, and fourth PR in the control group was 92.53 ± 17.41, 93.33 ± 12.82, and 92.70 ± 12.16, respectively. There was no significant difference in the mean PR at different stages (p = .45).
No patients in either group needed treatment with atropine or ephedrine.
Mean baseline RR was 14.55 ± 1.23 in the intervention group and 14.34 ± 1.29 in the control group. There was no significant difference in the mean baseline RR between the two groups (p = .59), indicating that the samples were homogeneous prior to drug intervention.
Mean baseline, second, third, and fourth RR in the intervention group was 14.55 ± 1.23, 13.88 ± 1.18, 13.44 ± 1.09, and 13.27 ± 0.89, respectively. Mean first, third, and fourth RR in the control group was 14.34 ± 1.29, 13.75 ± 0 3.02, and 13.58 ± 3.02, respectively. There was no significant difference between the mean of the first to third RR (p = .21). Also, no patient in the two groups had SpO2 < 94.
Mean baseline VAS score was 9.25 ± 1.37 in the intervention group and 9.46 ± 1.14 in the control group. There was no significant difference in the mean baseline VAS score between the two groups (p = .57), indicating that the samples were homogeneous before drug intervention.
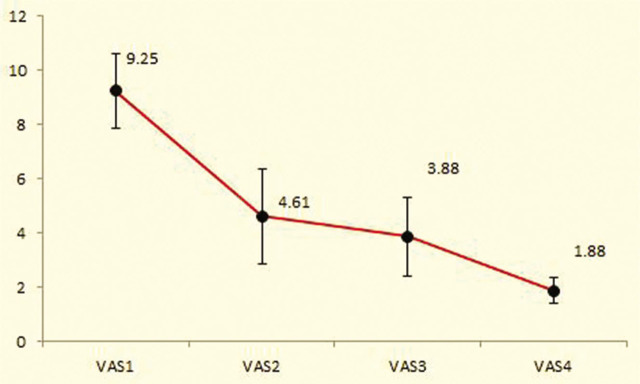
Mean baseline, second, third, and fourth VAS scores in the intervention group were 9.25 ± 1.37, 4.61 ± 1.75, 3.88 ± 1.45, and 1.88 ± 0.47, respectively. There were significant differences in the mean of the first and second VAS (p < .001), first and third VAS (p < .001), first and fourth VAS (p < .001), second and fourth VAS (p < .001), and third and fourth VAS scores (P<0.001), but the mean of the second and third VAS scores was not significant (p = .38; Figure 3).
Figure 3.
Comparison of the mean VAS score in the intervention group during the study.
VAS1 = primary VAS; VAS2 = after bolus dose of dexmedetomidine; VAS3 = during labour; VAS4 = after placental expulsion.
Mean first and third VAS scores in the control group were 9.46 ±1.14 and 9.45 ± 1.17, respectively. However, the fourth VAS score was 3.12 ± 2.25 significantly lower than the first and third VAS score (p < .001).
Mean baseline RSS score was 1.00 ± 0.00 in intervention group and 1.11 ± 0.32 in the control group. There was no significant difference in the mean baseline RSS score between the two groups (p = .12), indicating that the samples were homogeneous before drug intervention.
Mean baseline, second, third, and fourth RSS scores in the intervention group were 1.0 ± 0.00, 2.05 ± 0.63, 2.22 ± 0.54, and 2.05 ± 0.23, respectively. There was a significant difference between the mean of the first and second RSS scores (p < .001), first and third RSS scores (p < .001), and first and fourth RSS scores (p < .001), but there was no significant difference in the mean scores of the second and third RSS, and second and fourth RSS, and third and fourth RSS (p = .36).
The mean of the first, third, and fourth RSS scores in the control group were 1.11 ± 0.32, 1.12 ± 0.33, and 1.12 ± 0.33, respectively. There was no significant difference in the mean of the first, third, and fourth RSS scores (p = .16).
Comparing the mean Apgar scores of the two groups showed no significant difference at 1 minute (p = .09) and 5 minutes (p = .34). Mean Apgar scores at 1 and 5 minutes in the intervention group were 9.06 ± 0.25 and 9.87 ± 0.34, respectively. Mean Apgar scores at 1 and 5 minutes in the control group were 8.84 ± 0.47 and 9.32 ± 0.68, respectively. The mean score of midwifery satisfaction of the patients in the intervention group was 4.00 ± 0.91 based on the 5-point Likert scale.
Discussion
In this study we assessed the effect of IV dexmedetomidine infusion on pain score and neonatal Apgar score in primipara term pregnant women in two groups, intervention and control. Intragroup analysis in the intervention group showed that mean SBP and DBP were significantly decreased after drug administration but were in the normal range, and no patient needed treatment. The first stage of labour in the intervention group was significantly shorter than in the control group. There was a significant difference in mean duration of the first stage of labour between the intervention and control groups, but there was no significant difference between the mean duration of the second and third stages of labour between the two groups. There was no significant difference in the mean baseline VAS score between the two groups, but intragroup studies showed that the mean pain score decreased significantly after dexmedetomidine administration, during labour, and after placental expulsion. There was no change in the mean pain score in the control group until delivery. The Ramsay Sedation Scale score increased significantly after drug administration, during labour, and after placental expulsion.
The administration of dexmedetomidine, without any complications in mother and neonate, significantly reduced labour pain and accelerated the first stage of labour.
In a review of related articles, there were no clinical studies regarding the use of dexmedetomidine to manage labour pain, and there were only a few case studies that reported the use of dexmedetomidine in pregnant women for a variety of reasons (16, 17, 20–23). In these case studies, dexmedetomidine was proven safe for the fetus and newborn. This study was consistent with studies by Palanisami (16), Mendoza (17), Souza (20), Abu-Halaweh (21), El Tahan (22), and Newman (23) and showed that maternal dexmedetomidine infusion had no complication on the fetus or neonate.
In a review of articles on labour pain management, there are many findings related to different epidural methods that consider these methods of analgesi suitable for labour pain management. In a study by Deshmukh et al on pregnant women, investigators compared the effect of epidural analgesia with a control group and concluded that epidural analgesia had no effect on labour duration, but with excellent analgesic effect, along with an improved neonatal Apgar score. They suggested that the epidural method was suitable for painless delivery [24]. Karn et al also considered the epidural as the most effective method of analgesia for labour [2].
However, this procedure had some complications, as Uemura et al reported prolonged sensory dysfunction in a perineal area following epidural analgesia in a 36-year-old pregnant mother, due to ropivacaine neurotoxicity [25]. In recent studies simpler methods comparable to the epidural method of pain control have been recommended. Faied et al in 2019 conducted a study comparing intradermal injection of distilled water with epidural bupivacaine in the first phase of labour. The analgesia in both the epidural and sterile water groups was good compared to normal saline. The analgesia was comparable in the epidural and sterile water groups. Complications were more common in the epidural group than in normal saline and distilled water. The authors suggested that distilled water injection was a safe and effective method for reducing first-stage labour pain similar to that of bupivacaine [26]. Even the epidural method for controlling postoperative pain in painful surgeries such as knee arthroplasty and sternotomy has been questioned due to the complications of this method; researchers prefer to use simpler regional methods and even local anesthetic infiltration [27,28,29,30].
Another factor, considered more important than pain reduction, was patient satisfaction. In this regard, Richardson et al performed a study on pregnant women who underwent epidural analgesia, nitrous oxide, or nitrous oxide initiation followed by epidural analgesia. While 92% of the neuroaxial group reported a good analgesic effect with this method, only 52% of the nitrous oxide group reported a good analgesic effect. Overall satisfaction of the nitrous oxide group was 93%; in the neuroaxial group, 97%. Despite the great difference in the analgesic rate of the nitrous oxide method, the rate of satisfaction was close to that of the neuroaxial method. The authors stated that factors other than the extent of pain relief, such as maintaining a sense of childbirth, mobility, and force, seem to influence mothers' satisfaction with analgesia [3]1).
In a study by Sia et al, the efficacy of clonidine and dexmedetomidine on human pregnant myometrial stripes was obtained from 6 patients during elective caesarean section. Their results showed that dexmedetomidine increased uterine contractility at plasma concentrations of 1 × 109 g/mL [32]. Another study by Sia and Sng found that if dexmedetomidine was used correctly during labour, it could cause hemodynamic relaxation and stability with minimal risk of respiratory depression in the pregnant woman [33]. Like both Sia studies, the current study showed that administering dexmedetomidine led to a significant change in the first stage of labour, and hemodynamic changes were in the normal range; no maternal respiratory depression was observed.
In 2018 Jia et al compared three groups of 40 women candidates for painless labour. Patient-controlled intravenous (PCIV) analgesia with remifentanil, patient-controlled epidural analgesia, and routine labour. The active phase was shorter in the two intervention groups than in the control group. Stages 2 and 3 did not differ in the three groups [5].
In 2019 Garg et al compared IV tramadol with IV paracetamol in labour analgesia. The paracetamol group showed better analgesia with less labour duration [6].
Zutshi et al studied 200 pregnant women in two groups to control labour pain. One hundred women received acetaminophen, and 100 women received normal saline. The duration of labour was shortened in both groups without any complications in mother and fetus [7]. Analgesia for labour, either epidural or IV (remifentanil, paracetamol, acetaminophen, or normal saline) was associated with a decrease in the active phase of labour, which was consistent with the current study.
Nguyen et al used PCIV analgesia with fentanyl in patients with a relative contraindication for epidural analgesia. In the control group, nalbuphine bolus was administered in alternate doses. The authors concluded that drug-related complications were very uncommon on infants and mothers, and IV fentanyl was a good option for women who have epidural contraindications. Patients' pain score was reduced by this method (reached about 8 at 2 and 3 hours) but returned to baseline after 3 hours [34]. In this method, the same as the current study, maternal and neonatal complications were uncommon, but decreased pain with fentanyl was not as good as in this study, and the pain score reached at least 8 but was not persistent. In the current study, the mean pain score was less than 5, and analgesia continued until delivery.
In a nationwide survey of obstetricians, anaesthesiologists, and midwives, Logtenberg et al investigated the incidence of serious complications associated with remifentanil infusion for painless delivery at 60 hospitals in the Netherlands over a 10-year period. Cases of insufficient pain control, maternal apnea, and even maternal cardiac arrest and neonatal respiratory depression were reported; however, all complications were treated. The authors therefore suggested that remifentanil should be used under the supervision of a trained professional [35]. In this study, none of the complications reported with remifentanil infusion were observed, as it was performed under the guidance of a trained anaesthesiologist, and the patients and fetuses were fully monitored.
Many patients in the medical center in this current study did not have consent to use neuroaxial pain management techniques; thus, in patients with refusal for epidural analgesia [21], spinal cord disorder [16], or contraindications to the regional analgesia [17], the use of dexmedetomidine which is an α2 agonist drug with appropriate sedative and analgesic effects, and no maternal or neonatal complications, were indicated. Dexmedetomidine accelerated the first stage of labour, and patients maintained a sense of childbirth, mobility, and force. Furthermore, dexmedetomidine is associated with fewer maternal and neonatal complications than other IV drugs used to manage labour pain. More extensive studies on the effect of dexmedetomidine on labour pain management and maternal and neonatal complications are needed.
Conclusion
Based on the results of this study, the administration of dexmedetomidine to manage labour pain with careful monitoring of mother and fetus is recommended. Further larger, multicentre studies are needed.
Acknowledgements
We would like to thank the Clinical Research Development Centre of Baqiyatallah Hospital for their kind cooperation.
Footnotes
Ethics approval and consent to participate:
This research project was approved by the Ethics Committee of Baqiyatallah University of Medical Sciences (IR.BMSU.REC.1397.250; date: 2019-02-15), and it was registered in the Iranian Registry of Clinical Trials (IRCT20161022030421N5; date: 2019-07-20). Consent letters were obtained from participants.
Consent for publication:
Not applicable.
Availability of data and materials:
The datasets used and/or analyzed during the current study will be available from the corresponding author on reasonable request.
Competing interests:
The authors declare that there are no competing interests.
Funding:
There is no funding in this study.
Authors' contributions:
Conceptual: AD and ML; data gathering: AD, AE, MD, and ML; data analyzing and drafting: AD, AE, MD, and ML. All authors read, revised, and approved the final version of the article.
References
- [1].Dixon CL, Monsivais L, Chamseddine P, Olson G, Pacheco LD, Saade GR. et al. The effect of distraction during labor induction on timing of analgesia request: a randomized clinical trial. Am J Perinatol. 2019;36(13):1351–6. doi: 10.1055/s-0038-1676974. [DOI] [PubMed] [Google Scholar]
- [2].Karn S, Yu H, Karna S, Chen L, Qiao D. Women's awareness and attitudes towards labor analgesia influencing practice between developed and developing countries. Adv Reproduc Sci. 2016;4(2):46–52. [Google Scholar]
- [3].Owa OO, Alao FO, Faturoti SO, Temenu BA, Lemadoro SA. Acceptability of painless labor among pregnant women in Owo, South-West Nigeria. Parity. 2015;1:2. [Google Scholar]
- [4].Sha X, Hu H, Yang J, Fang D, Li W, Zhang H. et al. Interventions to reduce the cesarean delivery rate in a tertiary hospital in China. J Matern Fetal Neonatal Med. 2019:1–9. doi: 10.1080/14767058.2019.1706475. [DOI] [PubMed] [Google Scholar]
- [5].Jia Z, Li Y, Jia H, Ren J, Xie N. Curative effect of remifentanil on labor analgesia in newborns. J Matern Fetal Neonatal Med. 2019:1–6. doi: 10.1080/14767058.2018.1533946. [DOI] [PubMed] [Google Scholar]
- [6].Garg N, Vanitha V. A randomized controlled trial of intravenous paracetamol and intravenous tramadol for labour analgesia. Obst Gynecol Res. 2019;2(2):3–13. [Google Scholar]
- [7].Zutshi V, Rani KU, Marwah S, Patel M. Efficacy of intravenous infusion of acetaminophen for intrapartum analgesia. J Clin Diagn Res. 2016;10(8):QC18. doi: 10.7860/JCDR/2016/19786.8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang N, Ou Y, Tang Y. Analgesic effects of continuous epidural anesthesia for painless labor in Chinese parturient women: a systematic review and meta-analysis. Int J Clin Exp Med. 2019;12(1):262–72. [Google Scholar]
- [9].Pourfathi H, Farzin H. Effect of painless labor on postpartum depression. J Obstet Gynecol Cancer Res. 2018;3(3):93–7. [Google Scholar]
- [10].Sun J, Xiao Y, Zou L, Liu D, Huang T, Zheng Z. et al. Epidural labor analgesia is associated with a decreased risk of the Edinburgh Postnatal Depression Scale in trial of labor after cesarean: a multicenter, prospective cohort study. BioMed Res Int. 2020;2020:1–16. doi: 10.1155/2020/2408063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ni J, Qin Y, Kang L, Wang L, Zhong Z, Yin S. Combined spinal-epidural anesthesia with sufentanil and ropivacaine for labor pain in women with pregnancy-induced hypertension. Int J Clin Exp Med. 2019;12(12):13902–7. [Google Scholar]
- [12].Mahmoodi F, Noroozi M, Mehr LA, Beigi M. Breastfeeding and its outcome in women receiving epidural analgesia for childbirth. Iran J Nurs Midwifery Res. 2019;24(5):355. doi: 10.4103/ijnmr.IJNMR_219_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cheng Q, Bi X, Zhang W, Lu Y, Tian H. Dexmedetomidine versus sufentanil with high- or low-concentration ropivacaine for labor epidural analgesia: a randomized trial. J Obst Gynaecol Res. 2019;45(11):2193–201. doi: 10.1111/jog.14104. [DOI] [PubMed] [Google Scholar]
- [14].Zhou X, Li J, Deng S, Xu Z, Liu Z. Ropivacaine at different concentrations on intrapartum fever, IL-6 and TNF-α in parturient with epidural labor analgesia. Exp Ther Med. 2019;17(3):1631–6. doi: 10.3892/etm.2018.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Layera S, Bravo D, Aliste J, Tran DQ. A systematic review of dural puncture epidural analgesia for labor. J Clin Anesth. 2019;53:5–10. doi: 10.1016/j.jclinane.2018.09.030. [DOI] [PubMed] [Google Scholar]
- [16].Palanisamy A, Klickovich R, Ramsay M, Ouyang D, Tsen L. Intravenous dexmedetomidine as an adjunct for labor analgesia and cesarean delivery anesthesia in a parturient with a tethered spinal cord. Int J Obstet Anesth. 2009;18(3):258–61. doi: 10.1016/j.ijoa.2008.10.002. [DOI] [PubMed] [Google Scholar]
- [17].Mendoza VJM. Dexmedetomidine as adjunct for analgesia in labor: a report of two cases. Colombian J Anesthesiol. 2012;40(1):79–81. [Google Scholar]
- [18].Weerink MA, Struys MM, Hannivoort LN, Barends CR, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clinical Pharmacokinetics. 2017;56(8):893–913. doi: 10.1007/s40262-017-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gambling D. Intravenous dexmedetomidine. Int J Obstet Anesth. 2019;39:148. doi: 10.1016/j.ijoa.2019.02.004. [DOI] [PubMed] [Google Scholar]
- [20].Souza K, Anzoategui L, Pedroso W, Gemperli W. Dexmedetomidine in general anesthesia for surgical treatment of cerebral aneurysm in pregnant patient with specific hypertensive disease of pregnancy: case report. Rev Bras Anestesiol. 2005;55(2):212–6. doi: 10.1590/s0034-70942005000200008. [DOI] [PubMed] [Google Scholar]
- [21].Abu-Halaweh SA, Al Oweidi A-KS, Abu-Malooh H, Zabalawi M, Alkazaleh F, Abu-Ali H. et al. Intravenous dexmedetomidine infusion for labour analgesia in patient with preeclampsia. Eur J Anaesthesiol. 2009;26(1):86–7. doi: 10.1097/EJA.0b000e000000f3fb. [DOI] [PubMed] [Google Scholar]
- [22].El-Tahan M, Mowafi H, Al Sheikh I, Khidr A, Al-Juhaiman R. Efficacy of dexmedetomidine in suppressing cardiovascular and hormonal responses to general anaesthesia for caesarean delivery: a dose–response study. Int Journal Obstet Anesth. 2012;21(3):222–9. doi: 10.1016/j.ijoa.2012.04.006. [DOI] [PubMed] [Google Scholar]
- [23].Neumann M, Davio M, Macknet M, Applegate R II. Dexmedetomidine for awake fiberoptic intubation in a parturient with spinal muscular atrophy type III for cesarean delivery. Int J Obstet Anesth. 2009;18(4):403–7. doi: 10.1016/j.ijoa.2009.05.002. [DOI] [PubMed] [Google Scholar]
- [24].Deshmukh VL, Ghosh SS, Yelikar KA, Gadappa SN. Effects of epidural labour analgesia in mother and foetus. J Obstet Gynecol India. 2018;68(2):111–6. doi: 10.1007/s13224-017-1063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Uemura MT, Mezaki T, Shibasaki H, Takahashi R. Prolonged sensory impairment in the perineal region after painless delivery through lumbar epidural anesthesia. Neurol Clin Neurosci. 2019;7(1):43–4. [Google Scholar]
- [26].Faied SM, Abdalla AM, Youssef AYM. Intradermal sterile water injection versus epidural bupivacaine in painless first stage of labor. Egypt J Hosp Med. 2019;77(5):5589–96. [Google Scholar]
- [27].Cappiello G, Camarda L, Pulito G, Tarantino A, Di Martino D, Russi V. et al. Continuous femoral catheter for postoperative analgesia after total knee arthroplasty. Med Arch. 2020;74(1):54. doi: 10.5455/medarh.2020.74.54-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].El Shora HA, El Beleehy AA, Abdelwahab AA, Ali GA, Omran TE, Hassan EA. et al. Bilateral paravertebral block versus thoracic epidural analgesia for pain control post-cardiac surgery: a randomized controlled trial. Thorac Cardiovasc Surg. 2020;68(05):409–15. doi: 10.1055/s-0038-1668496. [DOI] [PubMed] [Google Scholar]
- [29].Liu X, Zhang H, Zhang H, Guo M, Gao Y, Du C. Local infiltration vs epidural analgesia for postoperative pain control after total knee or hip arthroplasty: a meta-analysis of randomized controlled trials. Medicine. 2020;99(44):1–10. doi: 10.1097/MD.0000000000022674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rawal N. Epidural analgesia for postoperative pain-improving outcome or adding risk? Best Pract Res Clin Anaesthesiol. 2021;35(1):53–65. doi: 10.1016/j.bpa.2020.12.001. [DOI] [PubMed] [Google Scholar]
- [31].Richardson MG, Lopez BM, Baysinger CL, Shotwell MS, Chestnut DH. Nitrous oxide during labor: maternal satisfaction does not depend exclusively on analgesic effectiveness. Anesth Analg. 2017;124(2):548–53. doi: 10.1213/ANE.0000000000001680. [DOI] [PubMed] [Google Scholar]
- [32].Sia A, Kwek K, Yeo G. The in vitro effects of clonidine and dexmedetomidine on human myometrium. Int J Obstet Anesth. 2005;14(2):104–7. doi: 10.1016/j.ijoa.2004.11.004. [DOI] [PubMed] [Google Scholar]
- [33].Sia AT, Sng BL. Intravenous dexmedetomidine for obstetric anaesthesia and analgesia: converting a challenge into an opportunity? Int J Obstet Anesth. 2009;18(3):204–6. doi: 10.1016/j.ijoa.2009.02.008. [DOI] [PubMed] [Google Scholar]
- [34].Nguyen T, Wang X, Wagner K, Izquierdo M, Bolden N. Labor analgesia when neuraxial anesthesia is relatively contraindicated: comparison of patient-controlled fentanyl and intermittent nalbuphine boluses. J Clin Anesth Manag. 2018;3(2) [Google Scholar]
- [35].Logtenberg S, Vink M, Godfried M, Beenakkers I, Schellevis F, Mol B. et al. Serious adverse events attributed to remifentanil patient-controlled analgesia during labour in the Netherlands. Int J Obstet Anesth. 2019;39:22–8. doi: 10.1016/j.ijoa.2018.10.013. [DOI] [PubMed] [Google Scholar]