Abstract
Background
Incidence of postoperative nausea and vomiting (PONV) in susceptible patients can be unacceptably high (70–80% reported incidence). This study was designed to evaluate the effect of palonosetron and ondansetron in preventing PONV in high-risk patients undergoing gynaecological laparoscopic surgery.
Methodology
In this randomised, controlled, double-blind trial, nonsmoking females 18–70 years and weighing 40–90 kg, scheduled for elective laparoscopic gynaecological surgeries, were enrolled into the ondansetron (Group A, n=65) or palonosetron (Group B, n=65) group. Palonosetron (1 mcg/kg 4) or ondansetron (0.1 mg/kg 4) were administered just before induction. Postoperatively, incidence of nausea, vomiting, PONV (scored on a scale of 0–3), need for rescue antiemetic, complete response, patient satisfaction, and adverse effects were evaluated for up to 48 h following surgery.
Results
The overall PONV scores and postoperative nausea score during 0–2 h and 24–48 h were comparable, but PONV scores (P=0.023) and postoperative nausea scores (P=0.010) during 2–24 h were significantly lesser in Group B compared to Group A. There was no statistically significant difference in the postoperative vomiting score or retching during 0–48 h. The amount of first-line rescue antiemetic used during 2–24 h was significantly higher in Group A (56%) than in Group B (31%) (P=0.012; P<0.05). Complete response to the drug during 2–24 h was significantly higher (P=0.023) in Group B (63%) compared to Group A (40%), whereas response was comparable during 0–2 h and 24–48 h. Both groups had comparable incidences of adverse effects and patient satisfaction scores.
Conclusion
Palonosetron has superior antinausea effect, less need of rescue antiemetics, and lesser incidence of total PONV in comparison to ondansetron during 2–24 h and comparable effect to ondansetron during the 0–2 h and 24–48 h postoperative periods in high-risk patients undergoing gynaecological laparoscopic surgery.
Keywords: Palonosetron, ondansetron, postoperative nausea and vomiting, laparoscopy, gynaecological surgery, general anaesthesia
Introduction
Anaesthesia practice has improved significantly in the last few decades owing to the advancement in drug therapy. However, postoperative nausea and vomiting (PONV) remains a distressing symptom second only to pain [1]. PONV can lengthen hospital stay and cause delayed recovery. In cases with prolonged vomiting, morbidities including pulmonary aspiration, bleeding, wound dehiscence, and dehydration can occur, leading to adverse consequences.
There is multifactorial etiology and pathophysiology of PONV that involves multiple receptor pathways. Risk factors identified using the Apfel simplified risk scoring system increase likelihood of PONV by 18–22% per risk factor, emphasizing the significance of prevention and control by anaesthetists [2]. Laparoscopic surgeries are now emerging as a preferred technique for diagnostic and/or therapeutic gynaecological procedures. However, the incidence of PONV is high with such procedures (40–75%) [3].
Traditional antiemetics such as phenothiazines, antihistamines, metoclopramide, and droperidol have been replaced by newer 5-hydroxytryptamine type-3 receptor antagonists (5-HT3RA) owing to their higher efficacy, longer/sustained activity, and favourable side effect profile [4,5]. Among these, ondansetron is the most frequently used drug. Recently, second generation 5-HT3RA palonosetron has been reported to have better receptor binding affinity and a very long plasma half-life of 40 hours, allowing extension of the anti-PONV effect to the second and third postoperative day [6,7,8].
Although recent literature supports the use of either ondansetron or palonosetron, certain studies support the use of one over the other. For patients with high-risk factors, anti-emetic efficacy and potency of palonosetron prophylaxis remains debatable in the late postoperative period [9,10]. Also, some studies comparing the effectiveness of palonosetron with ondansetron in PONV prophylaxis following laparoscopic surgery have shown controversial results; further research is needed to provide better clinical evidence [8,11]. So we undertook this randomized controlled trial (RCT) to gather more data to evaluate and compare the efficacy of palonosetron with ondansetron for PONV prophylaxis for 48 hours in high-risk patients undergoing laparoscopic gynaecological surgery.
Materials and Methods
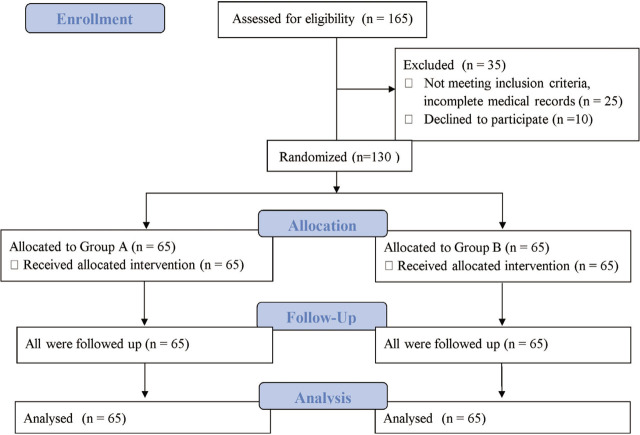
We conducted this study over one year after obtaining approval from the hospital's Institutional Ethics Committee. Female patients between 18–70 years, of ASA grade I–II planned for laparoscopic gynaecological surgeries, nonsmoking, and weighing 40–90 kgs, were enrolled in the study. Written informed consent was obtained. Exclusion criteria included weight >90 kg; history of PONV and motion sickness; known hypersensitivity to study drugs; evidence of major organ dysfunction; pregnancy; lactation; and existing gastrointestinal disease. Patients already on anti-emetics, steroids, or psychomimetic drugs preoperatively, on chemotherapeutic agents in the last few weeks, those unable to cooperate and unwilling to participate in the study, were also excluded (Figure 1).
Figure 1.
Consort flow diagram.
This is a prospective randomised controlled double-blind study. We used simple randomisation (chit in box system) to divide the patients in two groups of 65 each. Group A received ondansetron 0.1 mg/kg (maximum 8 mg intravenously [IV]) and Group B received palonosetron 1 mcg/kg (maximum 75 mcg IV) [12]. The syringes (labelled ‘antiemetic’, diluted with normal saline, total volume 5 ml) containing the study drug were prepared by anaesthesiologists not involved in the study. All the anaesthesiologists and the patients involved in the study were blinded to group allotment. All patients had more than three risk factors (female, nonsmoker, postoperative opioids use, and laparoscopic gynaecological surgery under general anaesthesia) and hence came under the high-risk category for PONV [2,13].
The preanaesthetic regimen and anaesthesia procedure were standardized for all. After recording baseline vitals, patients received the respective antiemetic drugs just before induction. Induction of anaesthesia was achieved with fentanyl 2mcg/kg IV and propofol (1%) 1.5–2mg/kg IV, and endotracheal intubation facilitated by atracurium (0.5 mg/kg) IV. Intraoperative monitoring involved electrocardiography, noninvasive blood pressure, pulse oximetry, capnography (EtCO2). Anaesthesia was maintained with controlled mechanical ventilation (EtCO2 between 30–40mmHg) and anaesthetic gases (sevoflurane in 50% oxygen and air). At completion of surgery, residual neuromuscular blockade was reversed with neostigmine 0.05mg/kg IV and glycopyrrolate 0.01mg/kg IV and trachea was extubated. Multimodal analgesia was instituted with morphine 1mg IV (SOS basis in post-anaesthesia care unit [PACU]), paracetamol 1gm IV six hourly and diclofenac 1mg/kg IV eight hourly in ward.
An episode of PONV was defined as either a spell of nausea (unpleasant sensation with an urge to vomit), retching (involuntary, laboured, spasmodic contractions of the respiratory muscles without expulsion of stomach contents), or vomiting (forceful expulsion of stomach contents from mouth), and scored on a scale of 0–3 as per scoring system (Table 1) [14,15]. All data was collected for 0–2 h in PACU and from 2–48 h (2–24 h and 24–48 h) in postoperative ward. Complete response was specified as: no need to administer rescue antiemetics and absence of PONV. ‘Treatment failure’ implied patients who experienced PONV despite receiving antiemetics. First line rescue antiemetic drug in both groups (ondansetron 4 mg IV) was given for PONV and repeated after 30 min if symptoms persisted, followed by second line or ultimate rescue antiemetic drug (dexamethasone 4 mg IV). Ondansetron was used as first line rescue antiemetic due to slow onset of action of dexamethasone. Drug-related adverse effects (headache, dizziness, drowsiness, constipation, and ECG changes) were recorded. Rating for overall satisfaction after surgery (satisfied, neutral, dissatisfied) was enquired from the patients.
Table 1.
Scoring system used for assessing postoperative nausea, vomiting, and PONV.
| Score | Postoperative nausea score | Postoperative vomiting score | PONV score |
|---|---|---|---|
| 0 | None | None | No nausea /vomiting /retching /no rescue antiemetic required |
| 1 | Mild, intermittent nausea | One vomit only | Nausea |
| 2 | Constant, moderate nausea | Several vomits | Retching |
| 3 | Severe nausea | Repeated retching/vomiting | Vomiting |
PONV score: Postoperative nausea and vomiting score
Primary outcome measured in our study was incidence of overall PONV, postoperative nausea, and vomiting in the first 48 h following surgery. Secondary outcomes were requirement of rescue antiemetic (total amount administered), complete response to study drugs, patient satisfaction score, and incidence of adverse effects.
We calculated the sample size based on observed incidence of PONV during 24 h. Using an alpha value (0.05) and power 80%, 65 patients per study group were found to be sufficient to detect a significant difference of 25% in incidence of PONV between the palonosetron and ondansetron groups [12,16]. We performed statistical testing with SPSS [Version 17.0, Chicago: SPSS Inc.]. Continuous variables were expressed as mean ± SD, and categorical variables as absolute numbers and percentages. Normally distributed continuous variables were compared using Student's t-test. Chi-square test or Fisher's exact test were used to compare nominal categorical data as deemed appropriate. P-value <0.05 was observed as statistically significant.
Results
The study enrolled 130 patients with no dropouts. There were no statistically significant differences between the study groups in patient characteristics and anaesthesia time (Table 2). Preoperative, intraoperative, and postoperative vitals recorded were comparable between the study groups. There was no difference in postoperative morphine requirements between both the groups and none of the patients received more than one dose of morphine (Table 2).
Table 2.
Patient characteristics and duration of anaesthesia.
| Group A [n=65] | Group B [n=65] | P-value | |
|---|---|---|---|
| Age [years] | 37.40 ± 9.59 | 39.51 ± 8.67 | 0.191 |
| Weight [kg] | 64.90 ± 11.10 | 65.10 ± 8.53 | 0.909 |
| ASA grade [I/II] | 32/33 [49.2%/50.8%] | 39/26 [60%/40%] | 0.218 |
| Duration of anaesthesia [min] | 150.85 ± 57.42 | 145.08 ± 36.23 | 0.495 |
| Morphine requirement in PACU [Number of patient/%] | 20 [30.76] | 16 [24.61] | 0.435 |
Data are mean ± SD or numbers of patients [%]; ASA grade [American Society of Anesthesiologists grade]
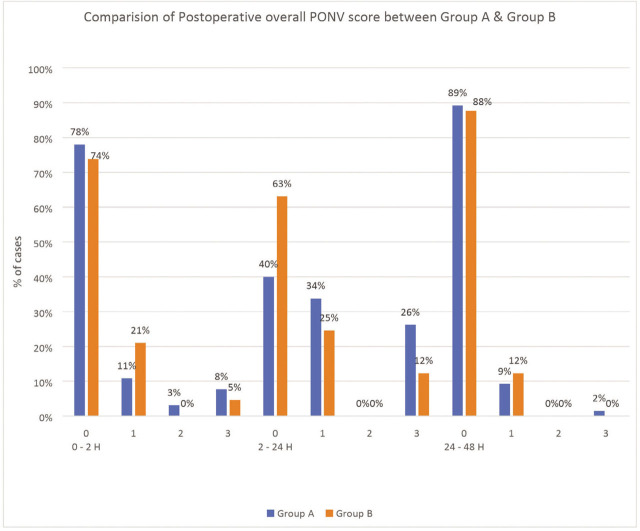
The overall PONV scores and postoperative nausea score during 0–2 and 24–48 h were comparable between the two groups. However, there was significantly lower PONV score (p=0.023) and postoperative nausea score (p=0.010) during 2–24 h in Group B (palonosetron) compared to Group A (ondansetron). During the 2–24 h postoperative period, 63% were free from PONV in Group B compared to 40% in Group A (Figure 2) and 66% patients in Group B were free from nausea compared to only 40% in Group A (Table 3 and Table 4; P <0.05).
Figure 2.
Comparison of postoperative overall PONV score between group A [Ondansetron] and group B [Palonosetron]; 0–3 on x-axis denotes PONV scoring system used at various time intervals in postoperative period [Table 1].
Table 3.
Postoperative nausea and vomiting (PONV) score.
| Overall PONV score | Group A [n=65] Frequency [%] | Group B [n=65] Frequency [%] | P-value | |
|---|---|---|---|---|
| 0–2 h | 0 | 51 [78] | 48 [74] | 0.218 |
| 1 | 7 [11] | 14 [21] | ||
| 2 | 2 [3] | 0 [0] | ||
| 3 | 5 [8] | 3 [5] | ||
| 2–24 h | 0 | 26 [40] | 41 [63] | 0.023* |
| 1 | 22 [34] | 16 [25] | ||
| 2 | 0 [0] | 0 [0] | ||
| 3 | 17 [26] | 8 [12] | ||
| 24–48 h | 0 | 58 [89] | 57 [88] | 0.177 |
| 1 | 6 [9] | 8 [12] | ||
| 2 | 0 [0] | 0 [0] | ||
| 3 | 1 [2] | 0 [0] | ||
Values are number of patients [%];
P< 0.05 for the Group B compared with Group A
Table 4.
Postoperative nausea score
| Overall nausea score | Group A [n=65] Frequency [%] | Group B [n=65] Frequency [%] | P-value | |
|---|---|---|---|---|
| 0–2 h | 0 | 51 [78.4] | 50 [77] | 0.101 |
| 1 | 7 [10.8] | 13 [20] | ||
| 2 | 0 [0] | 0 [0] | ||
| 3 | 7 [10.8] | 2 [3] | ||
| 2–24 h | 0 | 26 [40] | 43 [66] | 0.010* |
| 1 | 24 [37] | 15 [23] | ||
| 2 | 0 [0] | 0 [0] | ||
| 3 | 15 [23] | 7 [11] | ||
| 24–48 h | 0 | 58 [89] | 56 [86] | 0.593 |
| 1 | 7 [11] | 9 [14] | ||
| 2 | 0 [0] | 0 [0] | ||
| 3 | 0 [0] | 0 [0] | ||
Values are number of patients [%];
P < 0.05 for the Group B compared with Group A
Postoperative vomiting score or retching during 0–2 h, 2–24 h, and 24–48 h between the two groups were comparable (Table 5).
Table 5.
Postoperative vomiting score.
| Overall vomiting score | Group A [n=65] Frequency [%] | Group B [n=65] Frequency [%] | P-value | |
|---|---|---|---|---|
| 0–2 h | 0 | 59 [91] | 61 [95] | 0.061 |
| 1 | 6 [9] | 1 [2] | ||
| 2 | 0 [0] | 0 [0] | ||
| 3 | 0 [0] | 2 [3] | ||
| 2–24 h | 0 | 48 [74] | 56 [86] | 0.173 |
| 1 | 11 [17] | 7 [11] | ||
| 2 | 0 [0] | 0 [0] | ||
| 3 | 6 [9] | 2 [3] | ||
| 24–48 h | 0 | 64 [98.5] | 65 [100] | 1.000 |
| 1 | 1 [1.5] | 0 [0] | ||
| 2 | 0 [0] | 0 [0] | ||
| 3 | 0 [0] | 0 [0] | ||
Values are number of patients [%].
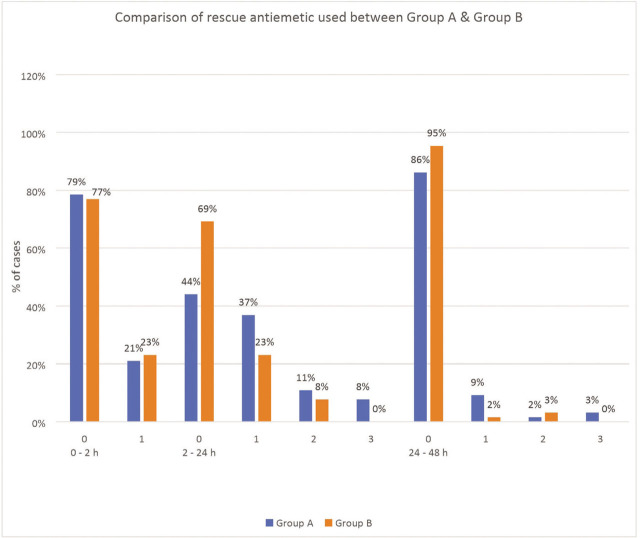
The amount of first line rescue antiemetic (ondansetron) used (Table 6, Figure 3) during 2–24 h was significantly higher in Group A than in Group B [P=0.012] whereas the amount of dexamethasone (second line or ultimate rescue antiemetic) used was similar in both the groups. Complete response to the drug in either group was comparable during 0–2 and 24–48 h, whereas during the 2–24 h period, complete response with palonosetron was significantly higher compared to ondansetron (63% vs. 40% for PONV and 69% vs. 44% for rescue antiemetic used, respectively; P<0.05; Tables 3 and 6; Figures 2 and 3),
Table 6.
Amount of ondansetron [first line rescue antiemetic drug] used.
| Amount of ondansetron [no. of doses] | Group A [n=65] Frequency [%] | Group B [n=65] Frequency [%] | P-value | |
|---|---|---|---|---|
| 0–2 h | 0 | 51 [79] | 50 [77] | 0.833 |
| 1 | 14 [21] | 15 [23] | ||
| 2–24 h | 0 | 29 [44] | 45 [69] | 0.012 |
| 1 | 24 [37] | 15 [23] | ||
| 2 | 7 [11] | 5 [8] | ||
| 3 | 5 [8] | 0 [0] | ||
| 24–48 h | 0 | 56 [86] | 62 [95] | 0.102 |
| 1 | 6 [9] | 1 [2] | ||
| 2 | 1 [2] | 2 [3] | ||
| 3 | 2 [3] | 0 [0] |
Values are number of patients [%].
Figure 3.
Comparison of amount of ondansetron [first line rescue antiemetic drug] used between group A [ondansetron] and group B [palonosetron] [0,1,2,3 on x-axis denotes the number of times rescue antiemetic drug administered during a time interval in post-operativ.
The incidence of adverse effects was similar between the two groups (Table 7). In terms of patient satisfaction score, higher satisfaction was observed with palonosetron compared to ondansetron (89% vs. 77%), 3% in each group were dissatisfied and the rest were neutral. However, this observation was not statistically significant.
Table 7.
Incidence of adverse events.
| Adverse effects | Group A Frequency [%] | Group B Frequency [%] | P-value |
|---|---|---|---|
| Headache | 10 [15] | 8 [12] | 0.800 |
| Dizziness | 4 [6] | 8 [12] | 0.364 |
| Drowsiness | 14 [22] | 17 [26] | 0.537 |
| Constipation | 4 [6] | 14 [22] | 0.020 |
| Allergic reaction | 0 [0] | 0 [0] | - |
| ECG changes | 0 [0] | 0 [0] |
Discussion
PONV is observed after general, regional, and local anaesthesia in a substantial proportion of patients even though antiemetic prophylaxis is used widely in modern anaesthesia practice. The etiology/pathophysiology of PONV is multifactorial and includes patient-related factors (young age, female sex, anxiety, history of PONV/motion sickness, genetic predisposition gastroparesis), surgical factors (laparoscopy, middle ear surgery) and mode of anaesthesia (TIVA or inhalational) [13,17,18].The simplified scoring system by Apfel and colleagues established four predisposing factors which increase the probability of PONV by 18–22% per risk factor [2]. These factors were well adjusted in our study and more than three risk factors as per this scoring system were present in both groups. Other factors like laparoscopic surgery (40–70% reported incidence),[3] surgery duration, and use of volatile anaesthetics also contributed to PONV[13] increasing the risk for developing PONV. Thus, it was not ethically feasible for us to include a control/placebo group, and preventing PONV was given priority similar to treating postoperative pain.
Several limitations of studies in literature include quality and design, variable inclusion criteria, and nonuniform dosages and measurement times which lead to clinical heterogeneity among the studies. One such meta-analysis has mentioned that more high-quality RCTs are needed to impart superior clinical evidence for rational clinical decisions regarding precise and effective choices for PONV prophylaxis in patients undergoing laparoscopic surgery [11]. Parameters such as patient demographics, anaesthesia regimen, postoperative analgesics, and duration and type of surgery were comparable and well controlled in our study. Hence, any variation in response is attributable to the characteristics and effects of study drugs.
Stimulation of 5-HT3 receptors is the main event involved in vomiting reflex initiation. Multifactorial agents and inputs arising from diverse areas are involved which initiate this reflex centrally by stimulating the 5-HT3 receptors located on the chemoreceptive trigger zone (CTZ) in the medulla; also, serotonin is released from small intestinal enterochromaffin cells which stimulates 5-HT3 receptors on vagal afferent fibres [15]. The 5-HT3RA are used commonly as they are more efficacious in treatment and prevention of PONV compared to other antiemetics and have an enviable safety profile with most side effects being mild and transient [4,5]. Palonosetron, a potent 5-HT3RA, has unique pharmacology, structure, and clinical effects, with longer half-life and stronger affinity for receptor binding than older 5-HT3RAs. Based on receptor binding studies, palonosetron interacts with 5-HT3 receptors in a manner different from ondansetron and granisetron by binding in an allosteric, positively cooperative manner at different sites [19]. It blocks substance-P associated response, has negative cooperativity with neurokinin-1 receptors by crosstalk, and prolonged effects with regard to receptor-ligand binding and responsiveness to serotonin [20]. In adults, elimination half-life of palonosetron is 40 h and may extend up to 48 h, in contrast to 3–6 h for ondansetron.
The consequences of PONV can vary from transient discomfort to serious complications, thus limiting the benefit of laparoscopy by delaying discharge or prolonging recovery. There is an increasing trend towards early discharge/enhanced recovery protocol (ERP) after surgery [13,21]. Therefore, a more potent and longer-acting drug will be more beneficial for such patients.
We undertook this study to gather more data to evaluate and compare the efficacy of palonosetron with ondansetron for PONV prophylaxis for 48 hours in high-risk patients undergoing laparoscopic gynaecological surgery. Our study showed significantly lower overall PONV and nausea scores in the palonosetron group compared to ondansetron group during 2–24 h (Tables 3 and 4; Figure 2). This could be explained by its better potency, longer half-life, and greater 5-HT3 receptor affinity [19,20]. The comparable PONV and nausea score observed during 24–48 h may be explained by lesser exposure to risk factors during this period (washout of inhalational agents, metabolism of opioids used in PACU, no surgical stimuli, and use of non-emetogenic drugs for pain control). Park et al. compared ondansetron with palonosetron in laparoscopic gynaecological surgery and reported incidence of PONV and nausea (not vomiting) was significantly lower with palonosetron compared to ondansetron during 0–24 h, which is in consensus with our study [16]. Similarly, Moon et al. studied PONV following thyroidectomies during the postoperative period (up to 24 h) and reported higher incidence of PONV with ondansetron (62%) than palonosetron (42%) [22].
In our study, the frequency of vomiting in the ondansetron group (26%) was greater than in the palonosetron group (14%) during 2–24 h follow-up; however, this was not statistically significant. The same holds true for 0–2 h and 24–48 h follow-up periods (Table 5). Kazemi-Kjelberg et al. suggested that 5-HT3RA are very efficacious in controlling vomiting rather than nausea [23], which corroborates our finding. Also, perhaps due to multifactorial pathophysiology of PONV and involvement of several receptors in the vomiting reflex (including serotonin 5-HT3, histamine H2, dopamine D2, alpha2 adrenergic, GABA, muscarinic cholinergic, and neurokinin1) [24], the difference in frequency of vomiting between groups could not attain statistical significance. More patients had retching in 2–24 h period in the ondansetron group (8% vs. none with palonosetron) but this was not statistically significant.
During the 2–24 h period, the number of patients who showed complete response was significantly greater with palonosetron than with ondansetron (Tables 3 and 6; Figures 2 and 3). A previous study by Park et al.[16] also reported similar findings, with more patients in the palonosetron group having a complete response than ondansetron group. This finding also explains why the amount of first line rescue antiemetic used was significantly more in ondansetron group compared to palonosetron group during the 2–24 h period (56% vs. 31%) (Table 6; Figure 3). The amount of dexamethasone (second line rescue antiemetic drug) required was comparable in both the groups.
The timing of ondansetron administration has been a topic of debate for a long time. Although administration before induction is recommended by the drug manufacturers, the relatively short half-life (3.5–6 h) may decrease antiemetic activity of ondansetron in procedures lasting over three hours. However, Joslyn et al.[25] in their study mentioned the rationale behind administration of ondansetron prior to the induction of anaesthesia was that a more accurate assessment of adverse events could be done (injection site reactions, dizziness or lightheadedness, as well as changes in haemodynamic parameters or ECG changes). Also, we believe it is more pertinent in high-risk patients that a prophylactic drug be administered prior to induction to antagonize the proposed mechanism of PONV, rather than at the end of surgery when the receptor pathway would have been already stimulated.
In our study, more patients in the palonosetron group were satisfied (89%) compared to the ondansetron group (77%). This was not statistically significant but probably reflects the better antiemetic profile of palonosetron. The incidence of adverse effects was similar in both study groups, suggesting similar safety profiles. Navari[26] found no clinically relevant differences among palonosetron, ondansetron, or dolasetron in laboratory, electrocardiographic, or vital sign changes, which agrees with our study. Findings by Park et al. corroborated with our findings related to patient satisfaction scores and incidence of adverse effects [16]. We didn’t find any ECG changes after drug administration, which correlates with the findings of Kim et al., who studied the effect of palonosetron on QTc interval in patients undergoing sevoflurane anaesthesia [27].
Consensus is emerging that antiemetic prophylaxis is not cost-effective in low-risk patients (10% or 20% expected risk) and is best accomplished in moderate, high-risk, or extremely high-risk patients with drug combinations. A single dose of palonosetron (longer half-life and better potency) seems more rational than multiple dosing with ondansetron, which might not be very desirable. Also, single dosing in the operation theatre can reduce the chance of drug interaction later in the postoperative period and can mitigate the higher cost of the newly developed drug. The decision/commitment to treat patients depends on several factors, including drug efficacy, baseline risk factors for PONV, adverse-effect profile, and the cost of acquiring the drug, which are non-identical among different settings [5,13].
In the late recovery period, the sustained antinausea effect of palonosetron compared to ondansetron assumes notable significance in ambulatory/day care and ERP settings. Up until now, palonosetron has been proven to prevent PONV through 24 h of the postoperative period, and efficacy beyond 24 h has not yet been demonstrated [16,22]. We extended the follow-up period through 48 h so we can suggest a cost-effective drug with coverage extending to the post-discharge period, leading to smoother recovery and decreased chances of readmission. In patients with medium to high risk for developing PONV, a combination therapy or multimodal approach with reliance on risk reduction strategy can better address this issue [28]. Also, recent guidelines reiterate and recommend multimodal prophylaxis when more than one risk factor is present [13,28]. There are some limitations in our study. First, even after following stringent exclusion criteria, we couldn’t exclude medications for comorbidities such as hypertension or diabetes mellitus that may influence the risk for PONV. Also, postoperative antibiotic regimens may differ in patients and can account for differences in PONV incidence. Second, we couldn’t evaluate the baseline incidence of PONV by inclusion of a placebo/control group, as it would have been unethical to withhold prophylaxis for patients at high risk for PONV. Third, we did our study based on optimal doses of ondansetron and palonosetron without knowledge of equipotent doses, and further studies are warranted to evaluate the equipotency of these drugs. Fourth, the administration time of ondansetron has been under debate for a long time. Manufacturers recommend administration before induction but considering its short half-life it is debatable that the difference in complete response in the late postoperative period may not be seen had it been given towards the end of surgery. Fifth, subjectivity in the assessment of patient satisfaction is unavoidable to some degree. These limitations need to be addressed and further multicentre studies with a large sample size may help provide data to overcome these shortcomings.
Conclusion
Our study demonstrated that palonosetron produced a significantly lower incidence of overall PONV and postoperative nausea scores during the 2–24 h postoperative period compared with ondansetron in high-risk patients undergoing laparoscopic gynaecological surgery. The comparable PONV characteristics in study groups in the postoperative phase (0–2 h, 24–48 h) with a significant difference in response during 2–24 h (better anti-nausea effect, decrease in overall incidence of PONV, lesser need for rescue antiemetics postoperatively) emphasize higher efficacy and potency of palonosetron in long-term prophylaxis. This also merits use of palonosetron in ambulatory/day care surgery and ERP settings, and surgeries associated with high risk of PONV, thus ensuring smooth recovery and recuperation. A single-dose regimen of palonosetron can decrease the requirement for multiple administrations postoperatively as needed with ondansetron and thus this might prove to be cost-beneficial in the long term.
References
- [1].Halliday TA, Sundqvist J, Hultin M, Walldén J. Post-operative nausea and vomiting in bariatric surgery patients: an observational study. Acta Anaesthesiol Scand. 2017 May;61(5):471–9. doi: 10.1111/aas.12884. [DOI] [PubMed] [Google Scholar]
- [2].Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiol. 1999 Sep;91(3):693–700. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]
- [3].Apfel CC. Postoperative nausea and vomiting. Miller's Anesthesia. 2010:2729–55. [Google Scholar]
- [4].Chatterjee S, Rudra A, Sengupta S. Current concepts in the management of postoperative nausea and vomiting. Anesthesiol Res Pract. 2011;2011 doi: 10.1155/2011/748031. 748031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gan TJ, Meyer T, Apfel CC, Chung F, Davis PJ, Eubanks S. et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003 Jul;97(1):62–71. doi: 10.1213/01.ane.0000068580.00245.95. table of contents. [DOI] [PubMed] [Google Scholar]
- [6].Yang LPH, Scott LJ. Palonosetron: in the prevention of nausea and vomiting. Drugs. 2009 Nov 12;69(16):2257–78. doi: 10.2165/11200980-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [7].Kloth DD. New pharmacologic findings for the treatment of PONV and PDNV. Am J Health Syst Pharm. 2009 Jan 1;66(Suppl 1):S11–18. doi: 10.2146/ashp080462. [DOI] [PubMed] [Google Scholar]
- [8].Singh PM, Borle A, Gouda D, Makkar JK, Arora MK, Trikha A. et al. Efficacy of palonosetron in postoperative nausea and vomiting (PONV)-a meta-analysis. J Clin Anesth. 2016 Nov;34:459–82. doi: 10.1016/j.jclinane.2016.05.018. [DOI] [PubMed] [Google Scholar]
- [9].Kim SH, Hong JY, Kim WO, Kil HK, Karm MH, Hwang JH. Palonosetron has superior prophylactic antiemetic efficacy compared with ondansetron or ramosetron in high-risk patients undergoing laparoscopic surgery: A prospective, randomized, double-blinded study. Korean J Anesthesiol. 2013 Jun;64(6):517–23. doi: 10.4097/kjae.2013.64.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lee WS, Lee KB, Lim S, Chang YG. Comparison of palonosetron, granisetron, and ramosetron for the prevention of postoperative nausea and vomiting after laparoscopic gynecologic surgery: a prospective randomized trial. BMC Anesthesiol. 2015 Sep 3;15:121. doi: 10.1186/s12871-015-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu Q, Zhou C, Bao Z, Zhu Y. Effects of palonosetron and ondansetron on preventing nausea and vomiting after laparoscopic surgery. J Int Med Res. 2018 Jan;46(1):411–20. doi: 10.1177/0300060517715374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kovac AL, Eberhart L, Kotarski J, Clerici G, Apfel C. Palonosetron 04–07 Study Group. A randomized, double-blind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo in preventing postoperative nausea and vomiting over a 72-hour period. Anesth Analg. 2008 Aug;107(2):439–44. doi: 10.1213/ane.0b013e31817abcd3. [DOI] [PubMed] [Google Scholar]
- [13].Gan TJ, Belani KG, Bergese S, Chung F, Diemunsch P, Habib AS. et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2020;131(2):411–48. doi: 10.1213/ANE.0000000000004833. [DOI] [PubMed] [Google Scholar]
- [14].Dresner M, Dean S, Lumb A, Bellamy M. High-dose ondansetron regimen vs droperidol for morphine patient-controlled analgesia. Br J Anaesth. 1998 Sep;81(3):384–6. doi: 10.1093/bja/81.3.384. [DOI] [PubMed] [Google Scholar]
- [15].Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiol. 1992 Jul;77(1):162–84. doi: 10.1097/00000542-199207000-00023. [DOI] [PubMed] [Google Scholar]
- [16].Park SK, Cho EJ. A randomized, double-blind trial of palonosetron compared with ondansetron in preventing postoperative nausea and vomiting after gynaecological laparoscopic surgery. J Int Med Res. 2011;39(2):399–407. doi: 10.1177/147323001103900207. [DOI] [PubMed] [Google Scholar]
- [17].Gan TJ. Risk factors for postoperative nausea and vomiting. Anesth Analg. 2006 Jun;102(6):1884–98. doi: 10.1213/01.ANE.0000219597.16143.4D. [DOI] [PubMed] [Google Scholar]
- [18].Janicki PK, Vealey R, Liu J, Escajeda J, Postula M, Welker K. Genome-wide association study using pooled DNA to identify candidate markers mediating susceptibility to postoperative nausea and vomiting. Anesthesiol. 2011 Jul;115(1):54–64. doi: 10.1097/ALN.0b013e31821810c7. [DOI] [PubMed] [Google Scholar]
- [19].Rojas C, Stathis M, Thomas AG, Massuda EB, Massuda EB, Alt J. et al. Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg. 2008 Aug;107(2):469–78. doi: 10.1213/ane.0b013e318172fa74. [DOI] [PubMed] [Google Scholar]
- [20].Rojas C, Li Y, Zhang J, Stathis M, Alt J, Thomas AG. et al. The antiemetic 5-HT3 receptor antagonist palonosetron inhibits substance P-mediated responses in vitro and in vivo. J Pharmacol Exp Ther. 2010 Nov;335(2):362–8. doi: 10.1124/jpet.110.166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nelson G, Altman AD, Nick A, Meyer LA, Ramirez PT, Achtari C. et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations--Part I. Gynecol Oncol. 2016 Feb;140(2):313–22. doi: 10.1016/j.ygyno.2015.11.015. [DOI] [PubMed] [Google Scholar]
- [22].Moon YE, Joo J, Kim JE, Lee Y. Anti-emetic effect of ondansetron and palonosetron in thyroidectomy: a prospective, randomized, double-blind study. Br J Anaesth. 2012 Mar;108(3):417–22. doi: 10.1093/bja/aer423. [DOI] [PubMed] [Google Scholar]
- [23].Kazemi-Kjellberg F, Henzi I, Tramèr MR. Treatment of established postoperative nausea and vomiting: a quantitative systematic review. BMC Anesthesiol. 2001;1(1):2. doi: 10.1186/1471-2253-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gan TJ. Mechanisms underlying postoperative nausea and vomiting and neurotransmitter receptor antagonist-based pharmacotherapy. CNS Drugs. 2007;21(10):813–33. doi: 10.2165/00023210-200721100-00003. [DOI] [PubMed] [Google Scholar]
- [25].Joslyn AF. Ondansetron, clinical development for postoperative nausea and vomiting: current studies and future directions. Anesth. 1994 Jan;49(Suppl):34–7. doi: 10.1111/j.1365-2044.1994.tb03581.x. [DOI] [PubMed] [Google Scholar]
- [26].Navari RM. Palonosetron: A second-generation 5-hydroxytryptamine receptor antagonist. Future Oncol. 2006 Oct;2(5):591–602. doi: 10.2217/14796694.2.5.591. [DOI] [PubMed] [Google Scholar]
- [27].Kim HJ, Lee HC, Jung YS, Lee J, Min JJ, Hong DM. et al. Effect of palonosetron on the QTc interval in patients undergoing sevoflurane anesthesia. Br J Anaesth. 2014 Mar;112(3):460–8. doi: 10.1093/bja/aet335. [DOI] [PubMed] [Google Scholar]
- [28].Gan TJ, Belani KG, Bergese S. et al. Fourth Consensus Guidelines for the Management of Postoperative Nausea and Vomiting [published correction appears in Anesth Analg. 2020 Nov;131(5):e241] Anesth Analg. 2020;131(2):411–448. doi: 10.1213/ANE.0000000000004833. [DOI] [PubMed] [Google Scholar]