Abstract
Plasmodium vivax uses Duffy binding protein (PvDBP1) to bind to the Duffy Antigen-Chemokine Receptor (DARC) to invade human erythrocytes. Individuals who lack DARC expression (Duffy-negative) are thought to be resistance to P. vivax. In recent years, P. vivax malaria is becoming more prevalent in Africa with a portion of these cases detected in Duffy-negatives. Apart from DBP1, members of the reticulocyte binding protein (RBP) and tryptophan-rich antigen (TRAg) families may also play a role in erythrocyte invasion. While the transcriptomes of the Southeast Asian and South American P. vivax are well documented, the gene expression profile of P. vivax in Africa and more specifically the expression level of several erythrocyte binding gene candidates as compared to DBP1 are largely unknown. This paper characterized the first P. vivax transcriptome in Africa and compared with those from the Southeast Asian and South American isolates. The expression of 4,404 gene transcripts belong to 12 functional groups including 43 specific erythrocyte binding gene candidates were examined. Overall, there were 10–26% differences in the gene expression profile amongst the geographical isolates, with the Ethiopian and Cambodian P. vivax being most similar. Majority of the gene transcripts involved in protein transportation, housekeeping, and host interaction were highly transcribed in the Ethiopian P. vivax. Erythrocyte binding genes including PvRBP2a and PvRBP3 expressed six-fold higher than PvDBP1and 60-fold higher than PvEBP/DBP2. Other genes including PvRBP1a, PvMSP3.8, PvMSP3.9, PvTRAG2, PvTRAG14, and PvTRAG22 also showed relatively high expression. Differential expression was observed among geographical isolates, e.g., PvDBP1 and PvEBP/DBP2 were highly expressed in the Cambodian but not the Brazilian and Ethiopian isolates, whereas PvRBP2a and PvRBP2b showed higher expression in the Ethiopian and Cambodian than the Brazilian isolates. Compared to Pvs25, the standard biomarker for detecting female gametocytes, PvAP2-G (PVP01_1440800), GAP (PVP01_1403000), and Pvs47 (PVP01_1208000) were highly expressed across geographical samples. These findings provide an important baseline for future comparisons of P. vivax transcriptomes from Duffy-negative infections and highlight potential biomarkers for improved gametocyte detection.
Keywords: Plasmodium vivax, transcriptomes, erythrocyte invasion ligands, gene expression profiles, gametocyte detection
1. Introduction
Plasmodium vivax Duffy binding protein (PvDBP1), which binds to the cysteine-rich region II of the human glycoprotein Duffy Antigen-Chemokine Receptor (DARC) (1–3), was previously thought to be the exclusive invasion mechanism for P. vivax (4). However, reports of P. vivax infections in Duffy-negative individuals (3) have raised important questions of how P. vivax invades erythrocytes that lack DARC expression. It was hypothesized that either mutations in PvDBP1 or a weakened expression of DARC allowed P. vivax to invade Duffy-negative erythrocytes (5, 6). Despite several mutational differences observed in PvDBP1 between Duffy-positive and Duffy-negative infections, these differences do not lead to binding of Duffy-negative erythrocytes (4) and suggested an alternative invasion pathway.
The P. vivax nuclear genome is ~29 megabases with 6,642 genes distributed amongst 14 chromosomes (7). Remarkably, across the P. vivax genome, approximately 77% of genes are orthologous to P. falciparum, P. knowlesi, and P. yoelii (8). Genes involved in key metabolic pathways, housekeeping functions, and membrane transporters are highly conserved between P. vivax and P. falciparum (8). However, at the genome level, P. vivax isolates from Africa, Southeast Asia, South America, and Pacific Oceania are significantly more polymorphic than the P. falciparum ones (9, 10), likely due to differences in distributional range, transmission intensity, frequency of gene flow via human movement, and host susceptibility (11).
In P. vivax, erythrocyte binding protein (PvEBP), reticulocyte binding protein (PvRBP), merozoite surface protein (PvMSP), apical membrane antigen 1 (PvAMA1), anchored micronemal antigen (PvGAMA), Rhoptry neck protein (PvRON), and tryptophan-rich antigen genes (PvTRAg) families have been suggested to play a role in erythrocyte invasion (9, 12). PvDBP1, PvMSP1, PvMSP7, and PvRBP2c were previously shown to be highly polymorphic (13–17). PvEBP, a paralog of PvDBP1, harbors the hallmarks of a Plasmodium red blood cell invasion protein and is similar to PcyM DBP2 sequences in P. cynomolgi that contains a Duffy-binding like domain (18). Binding assay of PvEBP region II (171–484) showed moderate binding to Duffy-negative erythrocytes (4). Both PvDBP1 and PvEBP (PvEBP/DBP2 hereafter) exhibit high genetic diversity and are common antibody binding targets associated with clinical protection (19, 20). Several members of PvRBP2 (PvRBP2a, PvRBP2b, PvRBP2c, PvRBP2d, PvRBP2e, PvRBP2p1, and PvRBP2p2) are orthologous to PfRh2a, PcyRBP2, and PfRh5, with PvRBP2a and PfRh5 share high structural similarity (21, 22). PvRBP2b and PvRBP2c are orthologous to PcyRBP2b and PcyRBP2c, respectively (23). The receptor for PvRBP2a was previously identified as CD98, a type II transmembrane protein that links to one of several L-type amino acid transporters (24); the receptor for PvRBP2b is transferrin receptor 1 (TfR1) (25). The PvRBP2b-TfR1 interaction plays a critical role in reticulocyte invasion in Duffy-positive infections (25). MSP1 also shows a strong binding affinity, with high-activity binding peptides (HABPs) clustered close to fragments at positions 280–719 and 1060–1599 (26), suggesting a critical role in erythrocyte invasion. Although the MSP7 gene family shows no binding potential, it forms a complex with PvTRAg36.6 and PvTRAg56.2 on the surface, likely for stabilization at the merozoite surface (27). A comparison of P. vivax transcriptomes between Aotus and Saimiri monkeys indicated that the expression of six PvTRAg genes in Saimiri P. vivax was 37-fold higher than in the Aotus monkey strains (28), five of which bind to human erythrocytes (27, 29). Although most PvTRAg receptors remain poorly characterized, the receptor of PvTRAg38 has been identified as Band 3 (30).
Recent progress in transcriptomic sequencing of P. vivax in non-human primates has provided an overview of stage-specific gene expression profile and structure, of which thousands of splices and unannotated untranslated regions were characterized (31, 32) The transcriptomes of Cambodian (33) and Brazilian (34) P. vivax field isolates showed high expression levels and large populational variation amongst host-interaction transcripts. Heterogeneity of gene expression has been documented amongst P falciparum-infected samples, implying that the parasites can modulate the gene transcription process through epigenetic regulation (35). However, the transcriptomic profile of African P. vivax remains unexplored, and it is unclear if there is heterogeneity among the geographical isolates. In addition, our previous study found that two CPW-WPC genes PVP01_0904300 and PVP01_1119500 expressed in the male gametocytes, and Pvs230 (PVP01_0415800) and ULG8 (PVP01_1452800) expressed in the female gametocytes were highly expressed relative to Pvs25 in the Ethiopian P. vivax (36). While these genes have a potential to be used for gametocyte detection, it remains unclear if such expressional patterns are similar in other geographical isolates.
In this study, we aimed to 1) examine the overall gene expression profile of 10 Ethiopian P. vivax with respect to different intraerythrocytic lifecycle stages; 2) determine the expression levels of previously characterized erythrocyte binding gene candidates (9); 3) compare gene expression profiles of the Ethiopian P. vivax with the Cambodian (33) and Brazilian (34) isolates from in vitro especially for the erythrocyte binding and male/female gametocyte gene candidates. These findings provide the first description of the P. vivax transcriptomes in Africa. A systematic comparison of gene expression profiles among the African, Southeast Asian, and South American isolates will deepen our understanding of P. vivax transcriptional machinery and invasion mechanisms.
2. Materials and Methods
2.1. Ethics statement and data availability
Scientific and ethical clearance was obtained from the institutional scientific and ethical review boards of Jimma University, Ethiopia and University of North Carolina, Charlotte, USA. Written informed consent/assent for study participation was obtained from all consenting heads of households, parents/guardians (for minors under 18 years old), and individuals who were willing to participate in the study. Sequences for the 10 Ethiopian transcriptomes are available on the National Center for Biotechnology Information Short Read Archive under BioProject: PRJNA784582. All code is available on GitHub at https://github.com/colbyford/vivax_transcriptome_comparisons.
2.2. Sample preparation
Ten microscopy-confirmed P. vivax samples were collected from Duffy positive patients at hospitals in Jimma, Ethiopia. These patients had 4,000 parasites/µL parasitemia and had not received prior antimalarial treatment. A total of 10mL whole blood was preserved in sodium heparin tubes at the time of collection. Red blood cell pellets were isolated and cryo-preserved with two times glycerolyte 57 and stored in liquid nitrogen. Prior to culture, samples were thawed by adding 0.2V of 12% NaCl solution drop-by-drop followed by a 5-minute room temperature incubation. Ten-times volume of 1.6% NaCl solution was then added drop-by-drop to the mixture and the samples were centrifuged at 1000 rcf for 10 minutes to isolate the red blood cell pellet. This process was repeated with a 10x volume of 0.9% NaCl. Following centrifugation, the supernatant was removed via aspiration, and 18mL of sterile IMDM (also containing 2.5% human AB plasma, 2.5% HEPES buffer, 2% hypoxanthine, 0.25% albumax, and 0.2% gentamycin) per 1mL cryo mixture was added to each sample for a final hematocrit of 2%. 10% Giemsa thick microscopy slides were made to determine the majority parasite stage and duration of incubation required, averaging 20–22 hours for the majority trophozoites and 40–44 hours for the majority ring. Samples were incubated at 37°C in a 5% O2, 5% CO2 atmosphere to allow growth to the schizont stage. In vitro maturation was validated through microscopic smears 20–40 hours after the initial starting time, dependent on the majority stage (Supplementary Figure 1A). To minimize oxidative stress, each culture was checked more than two times and returned to a 5% oxygen environment immediately after checking.
Cultured pellets were isolated via centrifugation and placed in 10x volume trizol for RNA extraction. RNA extraction was performed using direct-zol RNA prep kit according to the manufacturer’s protocol, followed by two rounds of DNA digestion using the DNA-free kit (Zymo). Samples were analyzed with a nanodrop 2000 and RNA Qubit to ensure sample concentrations were above 150 ng total for library construction. For samples with no significant amount of DNA or protein contaminants, RNA libraries were constructed using Illumina rRNA depletion library kits according to the manufacturer’s protocol. Completed libraries were quality checked using a bioanalyzer to ensure adequate cDNA was produced before sequencing. Sample reads were obtained using Illumina HiSeq 2×150bp configuration to obtain at least 35 million reads per sample. Sequence reads were aligned with HISAT2 (37), using the Rhisat2 R package (38) to the P01 P. vivax reference genome and all human reads were filtered out using SAMtools (39) (implemented in the R package (40)). The alignment was mapped to the P01 reference annotation using the Rsubread package (41).
2.3. Data analyses
To further confirm samples were majority schizont stage, sequence reads of each sample were deconvoluted in CIBERSORTx (42) based on P. berghei homologs (43). We used the published matrix to determine the frequency of expression for each gene calculated for rings, trophozoites, and schizonts, respectively. Transcripts that were expressed 30% or more were sorted into their respective stages (Supplementary Figure 1B). All reads were annotated using the Rsubread package and classified into 12 different categories by function. We then examined the top 30 transcribed genes using the counts per million (CPM) metric.
Our previously published whole genome sequence data identified several mutations and structural polymorphisms in genes from the PvEBP, PvRBP, PvMSP, and PvTRAg gene families that are likely to involve in erythrocyte invasion (9). Specific binding regions in some of the genes such as PvDBP1, PvEBP/DBP2, PvRBP2b, and PvMSP3 have been identified (44). To further explore the putative function, we compared relative expression levels of 43 erythrocyte binding gene candidates (Supplementary Table 1) in the 10 Ethiopian P. vivax samples with other geographical isolates that were of majority schizont stage. We used the CPM and TPM (transcripts per million) metrics in R package edgeR (45). The CPM metric was used to obtain the top 30 transcripts overall and does not consider gene length, while TPM considers gene length for normalization and allows an unbiased conclusion to be made relative between and to other transcriptomes (34). We then transformed the data using log(2)TPM+1 to illustrate relative expression levels via a heat map with an average abundance. We also selected 25 gametocyte gene candidates, 15 of which were shown to correlate to female gametocyte development and nine to male gametocytes (36, 46), to assess their expression levels relative to the standard Pvs25 in the samples. In addition, we examined the expression of AP2-G that is a critical transcription factor for both male and female gametocyte development (47).
2.4. Comparison of datasets
RNA-seq data of four in vitro Cambodian (33) and two in vitro Brazilian (34) P. vivax samples were downloaded from the GitHub repository and analyzed with the same bioinformatic methods described above to minimize potential batch effects. Samples were deconvoluted using the same matrix. The Ethiopian P. vivax samples were cultured and sequenced using similar protocol as the Cambodian (33) and Brazilian (34) ones with slight modifications in media and library preparation. We obtained the average expression and standard deviation in TPM for each gene target and determined potential difference in transcription levels by conducting pairwise differential expression (DE) analysis among the Cambodian, Brazilian, and Ethiopian samples. The expression level of 6,829 genes were examined for DE by edgeR dream (45, 48) and variancePartition (49), with adjusted p-value<1.0e-6 for DE gene concordance. A linear mixed effects models was used to ensure accuracy in triplicated Brazilian samples, and the Kenward-Roger method was used to estimate the effective degree of freedom for hypothesis testing due to small sample sizes.
3. Results
3.1. Overview of the Ethiopian P. vivax transcriptomes
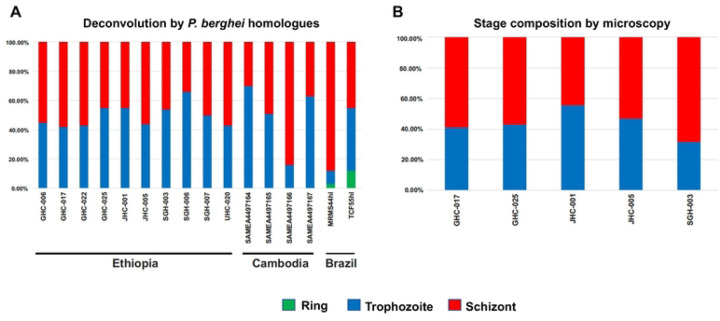
All 10 Ethiopian P. vivax samples originated from Duffy-positive patients. Based on deconvolution, all 10 Ethiopian P. vivax samples had similar proportions of trophozoite and schizont stage (Figure 1A). Only less than 1% of the sequence reads belong to the ring stage. Microscopic results corroborated the deconvolution analyses showing similar proportion of parasite stages in a subset of samples (Figure 1B). The deconvolution of P. vivax sequence reads from the Cambodian and Brazilian samples also showed no significant difference in the proportions of trophozoites or schizonts (P>0.05; Figure 1A).
Figure 1.
(A) CIBERSORTx deconvolution of the 10 Ethiopian, four Cambodian, and two Brazilian P. vivax transcriptomes using a P. berghei homologue matrix. No significant difference was observed in the proportion of trophozoites and schizonts amongst the isolates (p>0.05). (B) Parasite stage based on microscopic analysis of five Ethiopian P. vivax samples. No significant difference was observed between microscopy and computational deconvolution for these samples (p>0.05).
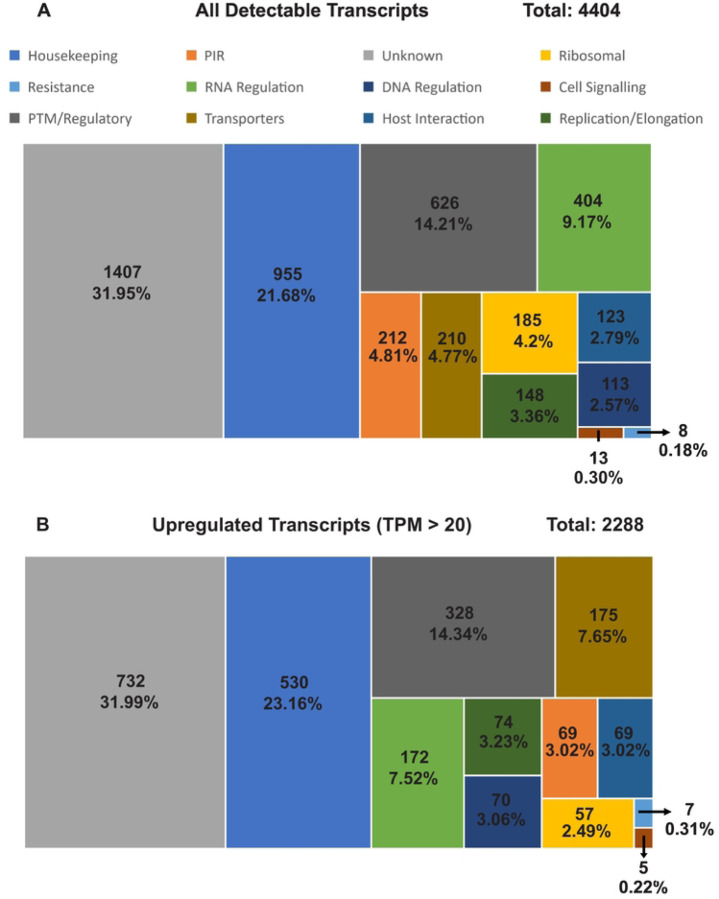
Overall, about 64% (4,404 out of 6,830) of the genes were detected with transcription in the Ethiopian P. vivax (Supplementary Table 2). Of the 4,404 genes, 69% (2,997) were annotated with known functions and 31% (1,407 genes) remain uncharacterized (Figure 2A). We normalized each sample expression profile to TPM to remove technical bias in the sequences and ensure gene expressions were directly comparable within and between samples (Supplementary Table 2). Of the 2,997 genes with known function, 21.7% are responsible for housekeeping, and 14.2% genes for post-translation modifications (PTMs) and regulation. The PIR proteins account for 4.8% (212) of all the identified genes and ~2.8% of the genes are involved in host-pathogen interactions. Nearly 52% of all detectable transcripts (2,288 genes) were expressed at a threshold of 20 TPM or above, which were considered as highly transcribed (Figure 2B). These highly transcribed transcripts showed similar proportions of gene categories including unknown, PTM/regulatory, DNA regulation, replication/elongation, host interactions, cell signaling, and resistance. Only transcripts involved in transport and housekeeping showed a slight increase of 2.9% and 1.48%, respectively, indicating a higher activity relative to the other categories. By contrast, transcripts involved in RNA regulation, PIR, and ribosomal activity showed a slight decrease of 2.19%, 1.79%, and 1.71%, indicating an overall lower activity compared to other categories (Figure 2B).
Figure 2.
Categorization of (A) all detectable transcripts and (B) upregulated (TPM > 20) transcripts for the Ethiopian P. vivax by gene function. The numbers shown represent the number of transcripts along with the overall percentage compared to all detected transcripts. Transcripts that were not detected were removed from the analysis. Only transcripts involved in transport and housekeeping showed a slight increase of 2.9% and 1.48%, respectively in the number of upregulated transcripts, indicating a higher activity relative to the other categories. By contrast, transcripts involved in RNA regulation, PIR, and ribosomal activity showed a slight decrease of 2.19%, 1.79%, and 1.71%, indicating an overall lower activity compared to other categories.
3.2. Top 30 highly expressed transcripts of Ethiopian P. vivax
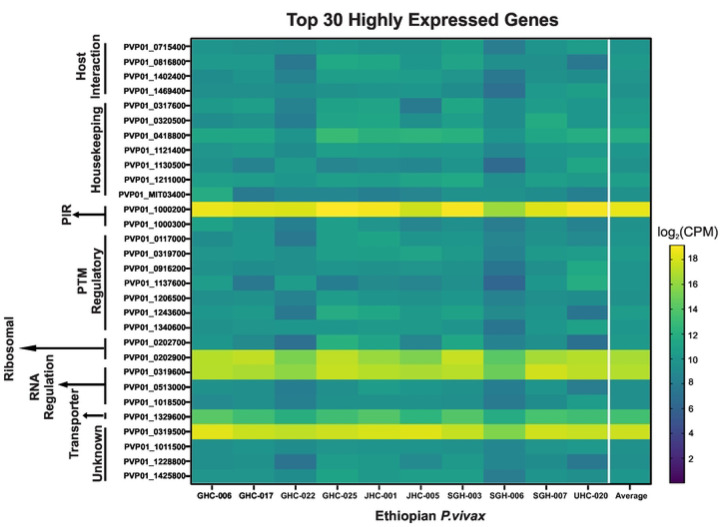
For the 10 Ethiopian P. vivax transcriptomes, four genes including PVP01_1000200 (PIR protein), PVP01_0202900 (18s rRNA), PVP01_0319600 (RNA-binding protein), and PVP01_0319500 (unknown function) were the most highly expressed among the others (Figure 3). Transcripts involved in housekeeping and PTM regulation each account for 23.3% of the top 30 highly expressed genes. Among genes involved in host-interactions, PVP01_0715400 (merozoite organizing protein), PVP01_0816800 (protein RIPR), PVP01_1402400 (reticulocyte binding protein 2a), and PVP01_1469400 (reticulocyte binding protein 3) are highly expressed. Five gene transcripts including PVP01_1000200 from the PIR family, PVP01_0319500 of unknown function, PVP01_0202900 a 18S rRNA, PVP01_1329600 a putative glutathione S-transferase, and PVP01_0418800 a putative pentafunctional AROM polypeptide showed most variable expression levels among the 10 samples, with a standard deviation of 20,000 and higher CPM (Figure 3). Three other genes including PVP01_0202700 (28S ribosomal RNA), PVP01_1137600 (basal complex transmembrane protein 1), PVP01_1243600 (replication factor C subunit 3) showed moderate variation ranging from 1,397 to 1,033 CPM. All other genes such as PVP01_1206500 (elongation factor Tu) and PVP01_1011500 (an unclassified protein) showed consistent expression level with variation under 1,000 CPM among samples (Figure 3).
Figure 3.
Heat map showing the top 30 highly transcribed genes based on log(2)CPM+1. Genes are arranged by different functions as indicated on the y-axis. Overall, four genes including PVP01_1000200 (PIR protein), PVP01_0202900 (18s rRNA), PVP01_0319600 (RNA-binding protein), and PVP01_0319500 (unknown function) from four different functional groups were shown to be most highly expressed among the others. Of interest, PVP01_0715400 (merozoite organizing protein), PVP01_0816800 (protein RIPR), PVP01_1402400 (reticulocyte binding protein 2a), and PVP01_1469400 (reticulocyte binding protein 3) were among the top 30 highly expressed genes involved in host interactions.
3.3. Differentially expressed genes among geographical P. vivax
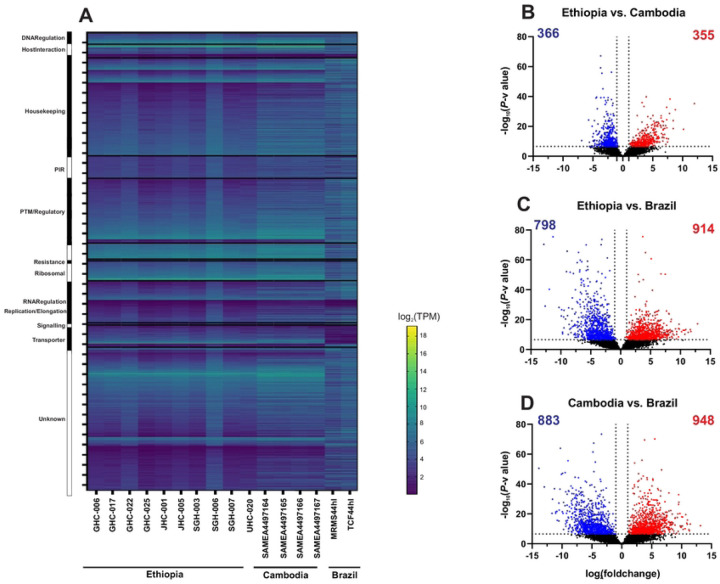
The overall gene expression profile was similar between the Ethiopian and Cambodian P. vivax, but different from the Brazilian ones (Figure 4A; Supplementary Table 3). Several genes involved in DNA regulation, host-interactions, replication, ribosomal, and transportation were upregulated in the Ethiopian and Cambodian isolates but showed considerable downregulation in Brazilian ones. Based on the Kenward-Roger DE analyses, a total of 1,831 differentially expressed genes were detected between the Cambodian and Brazilian isolates (CvB), 1,716 between the Ethiopian and Brazilian (EvB), and 721 between the Ethiopian and Cambodian (EvC) isolates (Figure 4B-D). The EvC analysis showed the lowest differentiation with only 10.6% of the entire transcriptome (Figure 4B), while EvB and CvB showed a greater differentiation of 25.1% and 26.8%, respectively (Figures 4C & D). For the 721 genes that were differentially expressed between the Cambodian and Ethiopian P. vivax, nearly half of them were significantly upregulated in Ethiopia compared to Cambodia (Figure 4B). Four genes including PVP01_0208700 (V-type proton ATPase subunit C), PVP01_0102800 (chitinase), PVP01_0404000 (PIR protein), and PVP01_0808300 (zinc finger (CCCH type protein) showed low levels of transcription (log10P-value>50; Figure 4B) compared to other DE genes. By contrast, two genes including PVP01_1329600 (glutathione S-transferase) and PVP01_MIT03400 (cytochrome b) were highly transcribed (log2fold change>10). For the 1,716 genes that were differentially expressed between the Ethiopian and Brazilian P. vivax, 914 of them were highly transcribed (Figure 3C). Of these, three genes including PVP01_1412800 (M1-family alanyl aminopeptidase), PVP01_0723900 (protein phosphatase-beta), and PVP01_0504500 (28S ribosomal RNA) showed a log10P-value greater then 75, indicating substantial expressional differences. For the 1,831 genes that were differentially expressed between the Cambodian and Brazilian P. vivax, 948 of them were highly transcribed (Figure 4D). Four genes including PVP01_1005900 (ATP-dependent RNA helicase DDX41), PVP01_0318700 (tRNAHis guanylyltransferase), PVP01_1334600 (60S ribosomal protein L10), and PVP01_1125300 (SURP domain-containing protein) showed substantial expressional differences with log10P-value greater than 75. Two genes, PVP01_0010550 (28S ribosomal RNA) and PVP01_0422600 (early transcribed membrane protein), were shown with low expression (log10fold change<−12), while one gene PVP01_0901000 (PIR protein) with substantial expression (log10fold change>12). These comparisons further demonstrated the differences in transcriptional patterns between geographical isolates.
Figure 4.
(A) Comparisons of the entire transcriptomes with genes sorted by functionality among the Ethiopian, Cambodian, and Brazilian P. vivax. The overall gene expression profile was nearly identical between the Ethiopian and Cambodian P. vivax, but different from the Brazilian isolates. Several genes involved in DNA regulation, host-interactions, replication, ribosomal, and transportation were upregulated in the Ethiopian and Cambodian isolates but showed considerable downregulation in Brazilian ones. (B-D) Volcano plots based on the Kenward-Roger DE analyses comparing differentially expressed genes between the (B) Ethiopian and Cambodian; (C) Ethiopian and Brazilian; (D) Cambodian and Brazilian isolates. Blue dots represent single genes that are downregulated in the comparison while red dots represent upregulated genes by comparison. About 10% of the detectable transcripts were differentially expressed between the Ethiopian and Cambodian P. vivax, but about 25% and 27% variations were detected between the Ethiopian and Brazilian as well as the Cambodian and Brazilian P. vivax, respectively. Overall, the Brazilian isolates had more genes that were upregulated compared to the Ethiopian and Cambodian ones.
3.4. Expression of genes related to erythrocyte invasion
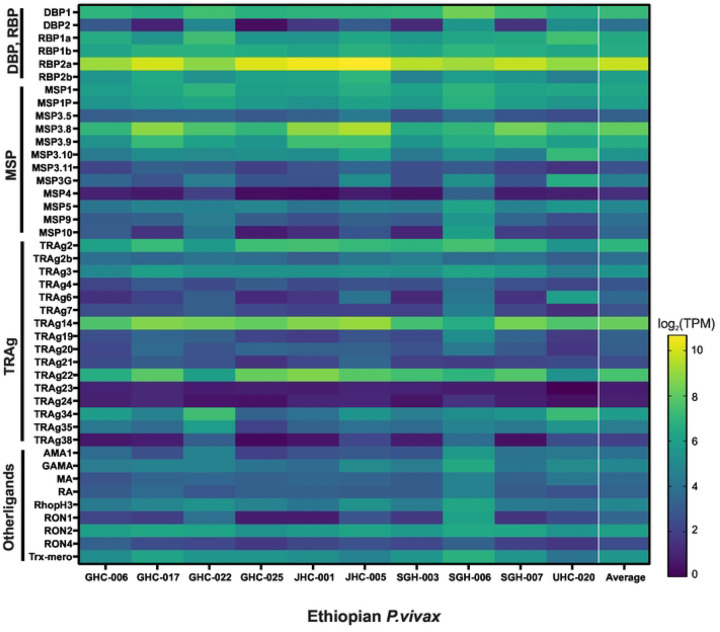
Of the 43 candidate genes associated with erythrocyte binding function, PvDBP1 on average showed about 10-fold higher expression than PvEBP/DBP2, which showed very low expression in four of the Ethiopian P. vivax samples (Figure 5). PvRBP2b showed four-fold higher expression than PvEBP/DBP2, but 50% less than PvDBP1. PvRBP2a showed consistently the highest expression across all samples, with about 6-fold, 67-fold, and 15-fold higher expression than PvDBP1, PvEBP/DBP2, and PvRBP2b, respectively. Other genes including PvMSP3.8, PvTRAg14, and PvTRAg22 also showed higher expression than PvDBP1. Of the 15 PvTRAg genes, only PvTRAg14 and PvTRAg22 showed expression higher than PvDBP1; PvTRAg23 and PvTRAg24 showed the lowest expression. Other putatively functional ligands including PvRA and PvRON4 showed 7–10 times lower expression compared to PvDBP1, though PvGAMA, PvRhopH3, PvAMA1, and PvRON2 were expressed higher than PvEBP/DBP2.
Figure 5.
Heatmap showing 43 genes associated with erythrocyte binding function in the Ethiopian P. vivax based on log(2)TPM+1 values. PvRBP2b showed four-fold higher expression on average than PvEBP/DBP2, but 50% less than PvDBP1. PvRBP2a showed consistently the highest expression across all samples, with about 6-fold, 67-fold, and 15-fold higher expression than PvDBP1, PvEBP/DBP2, and PvRBP2b, respectively. Other genes including PvMSP3.8, PvTRAg14, and PvTRAg22 also showed higher expression than PvDBP1.
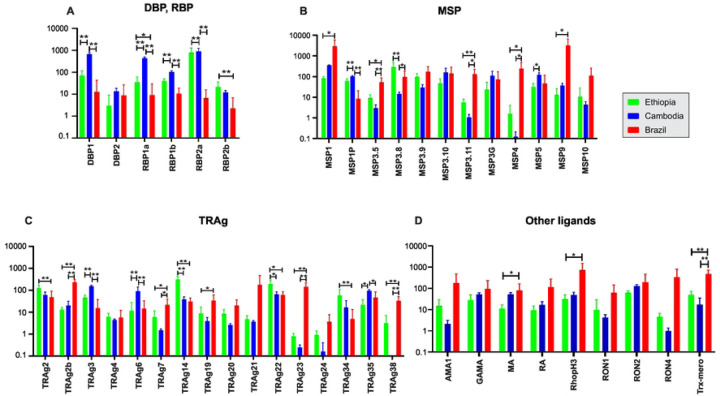
We further compared the expressional pattern of these 43 genes in the Ethiopian P. vivax with the Cambodian and Brazilian isolates (Figure 6). Members of the PvDBP and PvRBP gene family showed generally higher expression in the Cambodian P. vivax than the other isolates (Figure 6A). For instance, the expression of PvDBP1, PvRBP1a, and PvRBP1b were significantly higher in the Cambodian than the other isolates (P<0.01), whereas PvRBP2a and PvRBP2b showed higher expression in the Ethiopian P. vivax than the others. Compared to the PvDBP and PvRBP gene families, the expression patterns of PvMSP were different (Figure 6B). Most of the MSP gene members including PvMSP3.5, PvMSP3.11, and PvMSP4 showed substantially higher expression in the Brazilian P. vivax than the other isolates (P<0.01). Only PvMSP3.8 of the 12 PvMSP genes was expressed significantly higher in the Ethiopian than the others (P<0.01; Figure 6B). Of the 16 PvTRAg genes, PvTRAg14 and PvTRAg22 showed significantly higher expression in the Ethiopian isolates compared to the others (P<0.05; Figure 6C). Eight other members including PvTRAg2b, PvTRAg7, PvTRAg19, PvTRAg20, PvTRAg21, PvTRAg23, PvTRAg24, and PvTRAg38 showed significantly higher expression in the Brazilian isolates than the others (P<0.05; Figure 6C). The remaining nine putatively functional ligands showed relatively similar expression levels, except for PvMA, PvRhopH3, and PvTrx-mero that were highly expressed in the Brazilian isolates (P<0.05; Figure 6D).
Figure 6.
Comparisons of 43 genes associated with erythrocyte binding function based on log(2)TPM+1 values across the Ethiopian, Cambodian, and Brazilian P. vivax for (A) PvDBP1, PvEBP, and PvRBP genes; (B) PvMSP genes; (C) PvTRAg genes; (D) other putatively functional ligands. * denotes P-value < 0.05; ** denote P-value <0.01.
3.5. Expression of female and male gametocyte genes
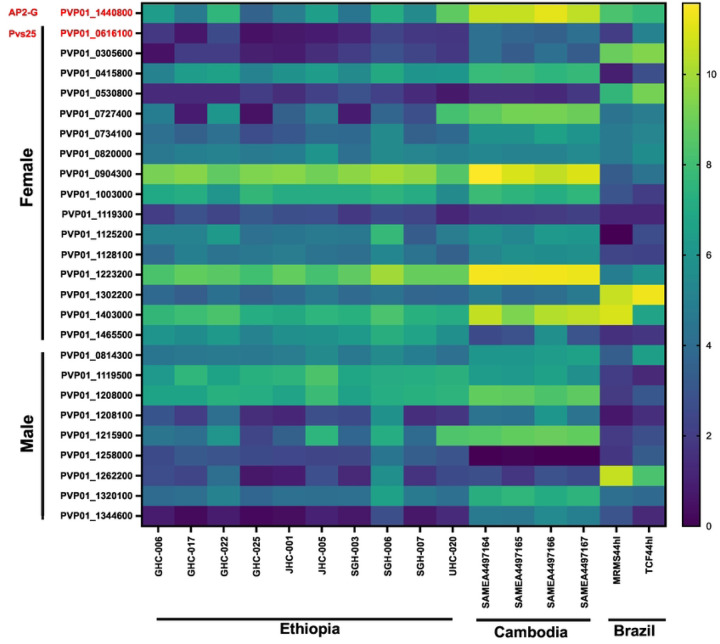
Based on the expression level of Pvs25 (PvP01_0616100), all 10 Ethiopian P. vivax samples contained submicroscopic gametocytes, in addition to the four samples from Cambodia and two samples from Brazil (Figure 7). Amongst the 26 gametocyte-related genes, PvAP2-G (PVP01_1440800) as well as the gametocyte associated protein, GAP (PVP01_1403000) and Pvs47 (PVP01_1208000) from female and male gametocytes, respectively, showed the highest expression across the Ethiopian, Cambodian, and Brazilian isolates, and were consistently higher than Pvs25 (Figure 7). This expression pattern suggests the potential utility of these three genes as better gametocyte biomarkers across geographical isolates. Other genes indicated differential expression patterns among isolates, e.g., the female gametocyte gene PVP01_0904300 (CPW-WPC family protein) showed consistently high levels of expression in both the Ethiopian and Cambodian isolates, though much lower in the Brazilian ones. On the other hand, PVP01_1302200 (high mobility group protein B1) and PVP01_1262200 (fructose 1,6-bisphosphate aldolase) from the female and male gametocytes showed the highest expression levels in Brazilian P. vivax but not the Ethiopian and Cambodian ones.
Figure 7.
Heatmap comparing 26 P. vivax gametocyte biomarker candidates across the Ethiopian, Cambodian, and Brazilian P. vivax. Based on the expression level of Pvs25, all 10 in vitro P. vivax samples from Ethiopia, four samples from Cambodia, and two samples from Brazil contained gametocytes. Three genes, PVP01_1440800 (PvAP2-G), PVP01_1403000 (gametocyte associated protein, GAP), and PVP01_1208000 (Pvs47) from female and male gametocytes, respectively, showed the highest expression across all geographical isolates, and were consistently higher than Pvs25.
4. Discussion
This study is the first to examine the transcriptomic profile of P. vivax from Africa and compare gene expression among geographical isolates. Approximately 32% of the detected transcripts are of unknown function, some of which such as PVP01_0319500, PVP01_1011500, and PVP01_1228800 were among the highest expressed and could play critical function. It is not surprising that 23% of the highly expressed transcripts belong to housekeeping function, such as several zinc fingers and ATP-synthase proteins. Besides, there is a large number of highly expressed protein regulators and PTMs that have not been thoroughly examined. For example, PVP01_1444000, a ubiquitin-activating enzyme, was among the highest expressed transcripts but with unclear function. Several other protein kinases, lysophospholipases, and chaperones were also highly expressed but their role in intercellular signaling pathways is unclear. It is worth noting that a great proportion of transcripts responsible for ribosomal protein production were also highly expressed. These ribosomal proteins support intraerythrocytic development of the parasites from one stage to another.
Members of the RBP family including PvRBP1a, PvRBP2a, PvRBP2b, and PvRBP3 were consistently highly expressed across the Ethiopian and Cambodian but not the Brazilian isolates, suggestive of potential differences in their role of erythrocyte invasion. Recent studies showed that the binding regions of PvRBP1a and PvRBP1b are homologous to that of PfRh4, and the amino acids at site ~339–599 were confirmed to interact with human reticulocytes (50). Though the host receptors of both PvRBP1a and PvRBP1b proteins are unclear, their receptors are neuraminidase resistant (22). The PvRBP2b-TfR1 interaction plays a critical role in reticulocyte invasion in Duffy-positive infections (25). PvRBP2d, PvRBP2e, and PvRBP3 are pseudogenes that share homology with other PvRBPs but encode for nonfunctional proteins (51). The extent to which of these PvRBP genes involve, if any, in erythrocyte invasion remains unclear and requires functional assays in broad samples. The high expression of PvRBP genes in Ethiopia could be related to a greater proportion of individuals with weak DARC expression (i.e., Duffy-negatives) (3); whereas in Cambodia and the inland regions of Brazil, populations are predominantly Duffy-positive (3). Given that P. falciparum can modulate gene expression in response to their hosts through epigenetic regulation (35, 52, 53), higher PvRBP expression in the Ethiopian P. vivax may allow the parasites to infect and adapt to both Duffy-positive and Duffy-negative populations (54). Further investigation on the expression and binding affinity of these PvRBP genes in different Duffy groups is necessary.
Another invasion protein, RIPR, was also among the highly expressed transcripts in P. vivax. RIPR is currently known as a vaccine target in P. falciparum (55), where RIPR (PfRH5) binds to the erythrocyte receptor basigin (56, 57). The PfRh5 complex is composed of PfRh5, Ripr, CyRPA, and Pf113, which collectively promote successful merozoite invasion of erythrocytes by binding to basigin (BSG, CD147) (57, 58). A BSG variant on erythrocytes, known as Oka-, has been shown to reduce merozoite binding affinities and invasion efficiencies (56), though this has only been reported in individuals of Japanese ancestry (59). Despite the clear role of RIPR in P. falciparum, P. vivax RIPR does not seem to bind to BSG (60) and the exact role of RIPR and its binding target(s) remains unclear.
The KR-DE analysis showed 10–26% variation among the transcriptomes of the three countries, with the Ethiopian and Cambodian P. vivax being most similar whereas the Cambodian and Brazilian P. vivax most different. Genes that showed the highest levels of differentiation were those involved in housekeeping, PIR, and ribosomal functions. The exact reason for such differences amongst the geographical P. vivax isolates remains unclear. Previous whole genome sequencing analyses indicated that the Ethiopian, Cambodian, and Brazilian P. vivax are independent subpopulations, with isolates from Southeast Asia and East Africa share the most common ancestry (61). This genetic relationship may explain variations in the expression profiles. The high expression observed for some PIR proteins, such as PVP01_1000200, in the Cambodian and Ethiopian P vivax may suggest the prominent role of VIR antigens in epigenetic regulation associated with host exposure and immune responses (35, 52, 53), and such immune responses could vary in diverse geographical settings (62–64). Varying expression of ribosomal proteins, such as PVP01_0827400 (60S ribosomal protein L26) and PVP01_1013900 (40S ribosomal protein S9, putative) may be attributed to host nutrition, which is directly proportional to the speed of replication in P. berghei (65). In P. falciparum, host nutrition has been shown to significantly alter gene expression related to housekeeping, metabolism, replication, and invasion/transmission (65). Malnourishment has a protective effect to P. vivax infections in people from the western Brazilian Amazon (66). In zebra fish, sex determination can cause significant expressional differences in the housekeeping genes (67), suggesting that sexual development factors may alter expression profiles. The marked differences observed in the Brazilian isolates may also be attributed to the presence of ring stage parasites or oxidative stress related to different in vitro environments. Future studies should expand geographical samples of P. vivax and examine further host factors associated with gene expression.
In this study, the deconvolution of stage-specific transcripts was based on the P. berghei orthologues rather than the single-cell RNA-seq data of P. vivax because the latter showed little expression from the ring stage. To date, P. berghei remains the most comprehensively characterized single-cell data for both sexual and asexual blood stages of Plasmodium (68, 69), and their orthologues have been shown to be reliable for determining stage-specific transcripts (46). In primates, most P vivax genes have been shown to transcribe during a short period in the intraerythrocytic cycle (31) with a high proportion of late-schizont transcripts expressed as early as the trophozoite stage. In P. berghei, the process of gametocyte development and genes involve in sequestration are transcribed much earlier during the trophozoite-schizont transition stage. Male gametocyte development precursors are expressed in the asexual stages prior to the onset of gametocyte development (70, 71). For example, the transcription factor AP2-G in P. vivax expresses early in the asexual stage for parasites that are committed to sexual development (47). These factors hinder deconvolution efforts, making it challenging to identify precisely which genes are transcribed in each stage. Future studies should consider combining in vivo (rich in ring and trophozoites) and in vitro (rich in trophozoites and schizonts) RNA-seq data to provide a more comprehensive and reliable stage-specific model for deconvolution.
Low density P. vivax gametocytes in asymptomatic carriers can significantly contribute to transmission (72, 73). In areas with low transmission, submicroscopic infections are hidden reservoirs for parasites with high proportions of infectious gametocytes (74). The current gametocyte biomarkers Pvs25 (PVP01_0616100) and Pvs16 (PVP01_0305600) account only for female gametocytes (75), and grossly underestimate the total gametocyte densities. We previously described two alternative female (PVP01_0415800 and PVP01_0904300) and one male (PVP01_1119500) gametocyte genes that show higher expression than Pvs25 in the Ethiopian isolates (36). Nevertheless, these genes showed relatively low expression in the Cambodian and Brazilian isolates. By contrast, PvAP2-G (PVP01_1440800), GAP (PVP01_1403000), and Pvs47 (PVP01_1208000) were moderately expressed across all geographical isolates and at a level higher than Pvs25. These genes warrant further investigations on their potential utility as gametocyte biomarkers in low-density infections, as well as their exact role in gametocyte development.
5. Conclusion
This paper characterized the first P. vivax transcriptome from Africa and identified several host-interaction gene transcripts, including PvRBP2a, PvMSP3.8, PvTRAg14, and PvTRAg22 that were highly expressed compared to PvDBP1 in Duffy-positive individuals. These transcripts may play prominent roles in erythrocyte invasion and merit further investigations on their binding affinity and function. We further demonstrated 10–26% differences in the gene expression profile amongst the geographical isolates, with the Ethiopian and Cambodian P. vivax being most similar. These findings provide an important baseline for future comparisons of P. vivax transcriptomes from Duffy-negative infections. Furthermore, PvAP2-G (PVP01_1440800), GAP (PVP01_1403000), and Pvs47 (PVP01_1208000) of both female and male gametocytes showed higher expression than the standard Pvs25 in all geographical P. vivax. These gene may provide better gametocyte detection for low-density infections.
Acknowledgements.
We thank the field team from Jimma University for their technical assistance; the communities and hospitals for their support and willingness to participate in this research; and undergraduate students at UNC Charlotte for assistance with the experiments.
Financial support.
This research was funded by National Institutes of Health R01AI162947.
Footnotes
Potential conflicts of interest. The authors declare no conflict of interest.
Supplementary Files
Supplementary Table 1. Name and gene ID of 43 candidate invasion genes.
Supplementary Table 2. Raw reads of 10 Ethiopian P. vivax transcriptomes in CPM and TPM metrics, Raw reads of four Cambodian P. vivax transcriptomes in TPM metric, and Raw reads of four Brazilian P. vivax transcriptomes in TPM metric.
Supplementary Table 3. Kenward-Roger DE analyses comparing the differentially expressed genes between (a) Ethiopian and Cambodian, (b) Ethiopian and Brazilian, and (c) Cambodian and Brazilian P. vivax.
References
- 1.Chitnis CE, Miller LH. Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte invasion. J Exp Med. 1994;180(2):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang XD, Kaslow DC, Adams JH, Miller LH. Cloning of the Plasmodium vivax Duffy receptor. Mol Biochem Parasitol. 1991;44(1):125–32. [DOI] [PubMed] [Google Scholar]
- 3.Howes RE, Patil AP, Piel FB, Nyangiri OA, Kabaria CW, Gething PW, et al. The global distribution of the Duffy blood group. Nat Commun. 2011;2:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunalan K, Lo E, Hostetler JB, Yewhalaw D, Mu J, Neafsey DE, et al. Role of Plasmodium vivax Duffy-binding protein 1 in invasion of Duffy-null Africans. Proc Natl Acad Sci U S A. 2016;113(22):6271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menard D, Barnadas C, Bouchier C, Henry-Halldin C, Gray LR, Ratsimbasoa A, et al. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc Natl Acad Sci U S A. 2010;107(13):5967–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menard D, Chan ER, Benedet C, Ratsimbasoa A, Kim S, Chim P, et al. Whole genome sequencing of field isolates reveals a common duplication of the Duffy binding protein gene in Malagasy Plasmodium vivax strains. PLoS Negl Trop Dis. 2013;7(11):e2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auburn S, Bohme U, Steinbiss S, Trimarsanto H, Hostetler J, Sanders M, et al. A new Plasmodium vivax reference sequence with improved assembly of the subtelomeres reveals an abundance of pir genes. Wellcome Open Res. 2016;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlton JM, Adams JH, Silva JC, Bidwell SL, Lorenzi H, Caler E, et al. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature. 2008;455(7214):757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford A, Kepple D, Abagero BR, Connors J, Pearson R, Auburn S, et al. Whole genome sequencing of Plasmodium vivax isolates reveals frequent sequence and structural polymorphisms in erythrocyte binding genes. PLoS Negl Trop Dis. 2020;14(10):e0008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White MT, Shirreff G, Karl S, Ghani AC, Mueller I. Variation in relapse frequency and the transmission potential of Plasmodium vivax malaria. Proc Biol Sci. 2016;283(1827):20160048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auburn S, Benavente ED, Miotto O, Pearson RD, Amato R, Grigg MJ, et al. Genomic analysis of a pre-elimination Malaysian Plasmodium vivax population reveals selective pressures and changing transmission dynamics. Nat Commun. 2018;9(1):2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roesch C, Popovici J, Bin S, Run V, Kim S, Ramboarina S, et al. Genetic diversity in two Plasmodium vivax protein ligands for reticulocyte invasion. PLoS Negl Trop Dis. 2018;12(10):e0006555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen SB, Wang Y, Kassegne K, Xu B, Shen HM, Chen JH. Whole-genome sequencing of a Plasmodium vivax clinical isolate exhibits geographical characteristics and high genetic variation in China-Myanmar border area. BMC Genomics. 2017;18(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornejo OE, Fisher D, Escalante AA. Genome-wide patterns of genetic polymorphism and signatures of selection in Plasmodium vivax. Genome Biol Evol. 2014;7(1):106–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diez Benavente E, Ward Z, Chan W, Mohareb FR, Sutherland CJ, Roper C, et al. Genomic variation in Plasmodium vivax malaria reveals regions under selective pressure. PLoS One. 2017;12(5):e0177134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearson RD, Amato R, Auburn S, Miotto O, Almagro-Garcia J, Amaratunga C, et al. Genomic analysis of local variation and recent evolution in Plasmodium vivax. Nat Genet. 2016;48(8):959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rice BL, Acosta MM, Pacheco MA, Carlton JM, Barnwell JW, Escalante AA. The origin and diversification of the merozoite surface protein 3 (msp3) multi-gene family in Plasmodium vivax and related parasites. Mol Phylogenet Evol. 2014;78:172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hester J, Chan ER, Menard D, Mercereau-Puijalon O, Barnwell J, Zimmerman PA, et al. De novo assembly of a field isolate genome reveals novel Plasmodium vivax erythrocyte invasion genes. PLoS Negl Trop Dis. 2013;7(12):e2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carias LL, Dechavanne S, Nicolete VC, Sreng S, Suon S, Amaratunga C, et al. Identification and Characterization of Functional Human Monoclonal Antibodies to Plasmodium vivax Duffy-Binding Protein. J Immunol. 2019;202(9):2648–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He WQ, Shakri AR, Bhardwaj R, Franca CT, Stanisic DI, Healer J, et al. Antibody responses to Plasmodium vivax Duffy binding and Erythrocyte binding proteins predict risk of infection and are associated with protection from clinical Malaria. PLoS Negl Trop Dis. 2019;13(2):e0006987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruszczyk J, Lim NT, Arnott A, He WQ, Nguitragool W, Roobsoong W, et al. Structurally conserved erythrocyte-binding domain in Plasmodium provides a versatile scaffold for alternate receptor engagement. Proc Natl Acad Sci U S A. 2016;113(2):E191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han JH, Lee SK, Wang B, Muh F, Nyunt MH, Na S, et al. Identification of a reticulocyte-specific binding domain of Plasmodium vivax reticulocyte-binding protein 1 that is homologous to the PfRh4 erythrocyte-binding domain. Sci Rep. 2016;6:26993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tachibana S, Sullivan SA, Kawai S, Nakamura S, Kim HR, Goto N, et al. Plasmodium cynomolgi genome sequences provide insight into Plasmodium vivax and the monkey malaria clade. Nat Genet. 2012;44(9):1051–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malleret B, El Sahili A, Tay MZ, Carissimo G, Ong ASM, Novera W, et al. Plasmodium vivax binds host CD98hc (SLC3A2) to enter immature red blood cells. Nat Microbiol. 2021;6(8):991–9. [DOI] [PubMed] [Google Scholar]
- 25.Gruszczyk J, Kanjee U, Chan LJ, Menant S, Malleret B, Lim NTY, et al. Transferrin receptor 1 is a reticulocyte-specific receptor for Plasmodium vivax. Science. 2018;359(6371):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez LE, Urquiza M, Ocampo M, Curtidor H, Suarez J, Garcia J, et al. Plasmodium vivax MSP-1 peptides have high specific binding activity to human reticulocytes. Vaccine. 2002;20(9–10):1331–9. [DOI] [PubMed] [Google Scholar]
- 27.Tyagi K, Hossain ME, Thakur V, Aggarwal P, Malhotra P, Mohmmed A, et al. Plasmodium vivax Tryptophan Rich Antigen PvTRAg36.6 Interacts with PvETRAMP and PvTRAg56.6 Interacts with PvMSP7 during Erythrocytic Stages of the Parasite. PLoS One. 2016;11(3):e0151065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunalan K, Sa JM, Moraes Barros RR, Anzick SL, Caleon RL, Mershon JP, et al. Transcriptome profiling of Plasmodium vivax in Saimiri monkeys identifies potential ligands for invasion. Proc Natl Acad Sci U S A. 2019;116(14):7053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyagi RK, Sharma YD. Erythrocyte Binding Activity Displayed by a Selective Group of Plasmodium vivax Tryptophan Rich Antigens Is Inhibited by Patients’ Antibodies. PLoS One. 2012;7(12):e50754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alam MS, Choudhary V, Zeeshan M, Tyagi RK, Rathore S, Sharma YD. Interaction of Plasmodium vivax Tryptophan-rich Antigen PvTRAg38 with Band 3 on Human Erythrocyte Surface Facilitates Parasite Growth. J Biol Chem. 2015;290(33):20257–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sa JM, Cannon MV, Caleon RL, Wellems TE, Serre D. Single-cell transcription analysis of Plasmodium vivax blood-stage parasites identifies stage- and species-specific profiles of expression. PLoS Biol. 2020;18(5):e3000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu L, Mok S, Imwong M, Jaidee A, Russell B, Nosten F, et al. New insights into the Plasmodium vivax transcriptome using RNA-Seq. Sci Rep. 2016;6:20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siegel SV, Chappell L, Hostetler JB, Amaratunga C, Suon S, Bohme U, et al. Analysis of Plasmodium vivax schizont transcriptomes from field isolates reveals heterogeneity of expression of genes involved in host-parasite interactions. Sci Rep. 2020;10(1):16667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rangel GW, Clark MA, Kanjee U, Goldberg JM, MacInnis B, Jose Menezes M, et al. Plasmodium vivax transcriptional profiling of low input cryopreserved isolates through the intraerythrocytic development cycle. PLoS Negl Trop Dis. 2020;14(3):e0008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cortes A, Crowley VM, Vaquero A, Voss TS. A view on the role of epigenetics in the biology of malaria parasites. PLoS Pathog. 2012;8(12):e1002943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ford A, Kepple D, Williams J, Kolesar G, Ford CT, Abebe A, et al. Gene Polymorphisms Among Plasmodium vivax Geographical Isolates and the Potential as New Biomarkers for Gametocyte Detection. Front Cell Infect Microbiol. 2021;11:789417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37(8):907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soneson C. hisat2: R Wrapper for HISAT2 Aligner. R package version 1120,. 2022.
- 39.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25(16):2078–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan M PH, Obenchain V, Hayden N. Rsamtools: Binary alignment (BAM), FASTA, variant call (BCF), and tabix file import. R package version 2120. 2022.
- 41.Liao Y, Smyth GK, Shi W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 2019;47(8):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol. 2019;37(7):773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tebben K, Dia A, Serre D. Determination of the Stage Composition of Plasmodium Infections from Bulk Gene Expression Data. mSystems. 2022:e0025822. [DOI] [PMC free article] [PubMed]
- 44.Kepple D, Pestana K, Tomida J, Abebe A, Golassa L, Lo E. Alternative Invasion Mechanisms and Host Immune Response to Plasmodium vivax Malaria: Trends and Future Directions. Microorganisms. 2020;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim A, Popovici J, Menard D, Serre D. Plasmodium vivax transcriptomes reveal stage-specific chloroquine response and differential regulation of male and female gametocytes. Nat Commun. 2019;10(1):371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mancio-Silva L, Gural N, Real E, Wadsworth MH 2nd, Butty VL, March S, et al. A single-cell liver atlas of Plasmodium vivax infection. Cell Host Microbe. 2022;30(7):1048–60 e5. [DOI] [PubMed] [Google Scholar]
- 48.Hoffman GE, Roussos P. Dream: powerful differential expression analysis for repeated measures designs. Bioinformatics. 2021;37(2):192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffman GE, Schadt EE. variancePartition: interpreting drivers of variation in complex gene expression studies. BMC Bioinformatics. 2016;17(1):483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta S, Singh S, Popovici J, Roesch C, Shakri AR, Guillotte-Blisnick M, et al. Targeting a Reticulocyte Binding Protein and Duffy Binding Protein to Inhibit Reticulocyte Invasion by Plasmodium vivax. Sci Rep. 2018;8(1):10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hietanen J, Chim-Ong A, Chiramanewong T, Gruszczyk J, Roobsoong W, Tham WH, et al. Gene Models, Expression Repertoire, and Immune Response of Plasmodium vivax Reticulocyte Binding Proteins. Infect Immun. 2015;84(3):677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cui L, Miao J. Chromatin-mediated epigenetic regulation in the malaria parasite Plasmodium falciparum. Eukaryot Cell. 2010;9(8):1138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta AP, Chin WH, Zhu L, Mok S, Luah YH, Lim EH, et al. Dynamic epigenetic regulation of gene expression during the life cycle of malaria parasite Plasmodium falciparum. PLoS Pathog. 2013;9(2):e1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kepple D, Hubbard A, Ali MM, Abargero BR, Lopez K, Pestana K, et al. Plasmodium vivax From Duffy-Negative and Duffy-Positive Individuals Share Similar Gene Pools in East Africa. J Infect Dis. 2021;224(8):1422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ragotte RJ, Higgins MK, Draper SJ. The RH5-CyRPA-Ripr Complex as a Malaria Vaccine Target. Trends Parasitol. 2020;36(6):545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crosnier C, Bustamante LY, Bartholdson SJ, Bei AK, Theron M, Uchikawa M, et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480(7378):534–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Volz JC, Yap A, Sisquella X, Thompson JK, Lim NT, Whitehead LW, et al. Essential Role of the PfRh5/PfRipr/CyRPA Complex during Plasmodium falciparum Invasion of Erythrocytes. Cell Host Microbe. 2016;20(1):60–71. [DOI] [PubMed] [Google Scholar]
- 58.Partey FD, Castberg FC, Sarbah EW, Silk SE, Awandare GA, Draper SJ, et al. Correction: Kinetics of antibody responses to PfRH5-complex antigens in Ghanaian children with Plasmodium falciparum malaria. PLoS One. 2018;13(9):e0204452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sierra AY, Barrero CA, Rodriguez R, Silva Y, Moncada C, Vanegas M, et al. Splenectomised and spleen intact Aotus monkeys’ immune response to Plasmodium vivax MSP-1 protein fragments and their high activity binding peptides. Vaccine. 2003;21(27–30):4133–44. [DOI] [PubMed] [Google Scholar]
- 60.Rougeron V, Elguero E, Arnathau C, Acuna Hidalgo B, Durand P, Houze S, et al. Human Plasmodium vivax diversity, population structure and evolutionary origin. PLoS Negl Trop Dis. 2020;14(3):e0008072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benavente ED, Manko E, Phelan J, Campos M, Nolder D, Fernandez D, et al. Distinctive genetic structure and selection patterns in Plasmodium vivax from South Asia and East Africa. Nat Commun. 2021;12(1):3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bernabeu M, Lopez FJ, Ferrer M, Martin-Jaular L, Razaname A, Corradin G, et al. Functional analysis of Plasmodium vivax VIR proteins reveals different subcellular localizations and cytoadherence to the ICAM-1 endothelial receptor. Cell Microbiol. 2012;14(3):386–400. [DOI] [PubMed] [Google Scholar]
- 63.Requena P, Rui E, Padilla N, Martinez-Espinosa FE, Castellanos ME, Botto-Menezes C, et al. Plasmodium vivax VIR Proteins Are Targets of Naturally-Acquired Antibody and T Cell Immune Responses to Malaria in Pregnant Women. PLoS Negl Trop Dis. 2016;10(10):e0005009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cook J, Speybroeck N, Sochanta T, Somony H, Sokny M, Claes F, et al. Sero-epidemiological evaluation of changes in Plasmodium falciparum and Plasmodium vivax transmission patterns over the rainy season in Cambodia. Malar J. 2012;11:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar M, Skillman K, Duraisingh MT. Linking nutrient sensing and gene expression in Plasmodium falciparum blood-stage parasites. Mol Microbiol. 2021;115(5):891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alexandre MA, Benzecry SG, Siqueira AM, Vitor-Silva S, Melo GC, Monteiro WM, et al. The association between nutritional status and malaria in children from a rural community in the Amazonian region: a longitudinal study. PLoS Negl Trop Dis. 2015;9(4):e0003743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCurley AT, Callard GV. Characterization of housekeeping genes in zebrafish: male-female differences and effects of tissue type, developmental stage and chemical treatment. BMC Mol Biol. 2008;9:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hollin T, Le Roch KG. From Genes to Transcripts, a Tightly Regulated Journey in Plasmodium. Front Cell Infect Microbiol. 2020;10:618454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoo R, Zhu L, Amaladoss A, Mok S, Natalang O, Lapp SA, et al. Integrated analysis of the Plasmodium species transcriptome. EBioMedicine. 2016;7:255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu F, Yang F, Wang Y, Hong M, Zheng W, Min H, et al. A conserved malaria parasite antigen Pb22 plays a critical role in male gametogenesis in Plasmodium berghei. Cell Microbiol. 2021;23(3):e13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuda M, Kaneko I, Murata Y, Iwanaga S, Nishi T. Mechanisms of triggering malaria gametocytogenesis by AP2-G. Parasitol Int. 2021;84:102403. [DOI] [PubMed] [Google Scholar]
- 72.Abdelraheem MH, Bansal D, Idris MA, Mukhtar MM, Hamid MMA, Imam ZS, et al. Genetic diversity and transmissibility of imported Plasmodium vivax in Qatar and three countries of origin. Sci Rep. 2018;8(1):8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol. 2014;12(12):833–40. [DOI] [PubMed] [Google Scholar]
- 74.Hofmann NE, Gruenberg M, Nate E, Ura A, Rodriguez-Rodriguez D, Salib M, et al. Assessment of ultra-sensitive malaria diagnosis versus standard molecular diagnostics for malaria elimination: an in-depth molecular community cross-sectional study. Lancet Infect Dis. 2018;18(10):1108–16. [DOI] [PubMed] [Google Scholar]
- 75.Kosasih A, Koepfli C, Dahlan MS, Hawley WA, Baird JK, Mueller I, et al. Gametocyte carriage of Plasmodium falciparum (pfs25) and Plasmodium vivax (pvs25) during mass screening and treatment in West Timor, Indonesia: a longitudinal prospective study. Malar J. 2021;20(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]