Abstract
The flagellated kinetoplastid protozoan and causative agent of human Chagas disease, Trypanosoma cruzi, inhabits both invertebrate and mammalian hosts over the course of its complex life cycle. In these disparate environments, T. cruzi uses its single flagellum to propel motile life stages and in some instances, to establish intimate contact with the host. Beyond its role in motility, the functional capabilities of the T. cruzi flagellum have not been defined. Moreover, the lack of proteomic information for this organelle, in any parasite life stage, has limited functional investigation. In this study, we employed a proximity-dependent biotinylation approach based on the differential targeting of the biotin ligase, TurboID, to the flagellum or cytosol in replicative stages of T. cruzi, to identify flagellar-enriched proteins by mass spectrometry. Proteomic analysis of the resulting biotinylated protein fractions yielded 218 candidate flagellar proteins in T. cruzi epimastigotes (insect stage) and 99 proteins in intracellular amastigotes (mammalian stage). Forty of these flagellar-enriched proteins were common to both parasite life stages and included orthologs of known flagellar proteins in other trypanosomatid species, proteins specific to the T. cruzi lineage and hypothetical proteins. With the validation of flagellar localization for several of the identified candidates, our results demonstrate that TurboID-based proximity proteomics is an effective tool for probing subcellular compartments in T. cruzi. The proteomic datasets generated in this work offer a valuable resource to facilitate functional investigation of the understudied T. cruzi flagellum.
Introduction
Trypanosoma cruzi is the uniflagellate protozoan parasite that causes Chagas disease, a chronic disease with severe outcomes including cardiomyopathies and gastrointestinal motility disorders 1,2. T. cruzi has a complex life cycle that involves both invertebrate and mammalian hosts, in which the parasite undergoes marked developmental changes and alternates between actively dividing (‘epimastigote’ or ‘amastigote’ forms in insect and mammalian hosts, respectively) and non-dividing ‘trypomastigote’ forms in both hosts (life cycle schematic; Supplementary Fig. 1). In mammals, infection is initiated by motile trypomastigotes that actively invade host cells before converting to the non-motile amastigote stage that replicates in the host cytoplasm. Intracellular T. cruzi amastigotes begin to replicate ~24 hours post-infection (hpi) and undergo several rounds of cell division before converting back to trypomastigotes that eventually rupture the host cell membrane (between ~90–120 hpi) to allow dissemination of the parasite and infection of new tissue sites. Once T. cruzi infection is established in mammalian hosts, parasites typically persist at low levels for the life of the host, giving rise to chronic infections that can trigger inflammation and pathology.
In both insect and mammalian hosts, T. cruzi can establish intimate contact with host structures using its single flagellum 3–5. In triatomine vectors, epimastigotes attach to the hindgut by forming a hemidesmosome-like structure between the distal part of the flagellum and host rectal epithelium 5. This attachment prevents the parasites from being flushed from the insect and is important for promoting differentiation to the infectious metacyclic stage 5. In mammalian host cells, cytosolically-localized T. cruzi amastigotes establish intermittent contact with host mitochondria using their short motile flagellum 3,6. Unlike the motile trypomastigote and epimastigote stages of T. cruzi, that have elongated flagella (up to 15 µm in length 7), replicative intracellular amastigotes have a truncated flagellum (~2.7 µm) that extends just beyond the opening of the flagellar pocket 6. Also, T. cruzi amastigotes retain a 9+2 axonemal structure found in motile trypanosomatid life stages 8, but lack a paraflagellar rod, a unique lattice-like structure that runs parallel to the axoneme in these organisms 9, and which is associated with several functions including flagellar motility and signal transduction 10. It has long been assumed that the minimal amastigote flagellum serves no function other than to provide a structural platform for flagellar outgrowth during differentiation to motile life stages 11. However, recent observations that the flagellum of intracellular T. cruzi amastigotes undergoes low frequency aperiodic ‘beating’ inside mammalian host cells 6 and makes physical contact with the host mitochondria 3,6, indicate that the amastigote flagellum has a functional role within the host cell. The interaction between the T. cruzi amastigote flagellum and host mitochondria is comparable to the intimate contact observed between the flagellum of intracellular Leishmania mexicana amastigotes and the host parasitophorous vacuole membrane 12. In the case of Leishmania, it has been postulated that the amastigote flagellum has a sensory role and/or functions in the delivery of parasite material to the infected host cell 12,13. It is therefore reasonable to predict that the T. cruzi amastigote flagellum may have similar role(s) in its interactions with the intracellular environment of the host cell.
In addition to critical roles in motility, eukaryotic flagella (i.e. cilia) and non-motile cilia have emerged as important sensory organelles that are equipped with signal transduction systems and second messengers such as cyclic AMP (cAMP) 14 and calcium 15,16 that coordinate cellular responses to external stimuli. Functions beyond cell locomotion have also been ascribed to the flagellum of motile trypanosomatid life stages 11,13,17–19, where the best understood example of sensory integration in these organisms is the role of flagellar receptor-type adenylate cyclases and cAMP-depending signaling in pH taxis and social motility in the insect stage of Trypanosoma brucei 20–23. In Leishmania, flagellar aquaporin has been implicated in osmotaxis 24 in the insect stage, and the flagellar membrane may be a critical site for glucose 25,26 and arginine 27 sensing in these parasites. Indeed, in both T. brucei and Leishmania, near comprehensive flagellar proteomes have been generated using shot-gun proteomics of isolated flagella 28–30 or of detergent and high salt extracted fractions of the parasite, yielding axonemal and paraflagellar rod proteins 31. Further, in T. brucei, specific domains of the flagellum have been partially mapped using proximity-dependent biotinylation including flagellar attachment zone proteins 32 and the flagellar tip 33, a specialized signaling domain.
By comparison, we have little knowledge of the molecular composition of the T. cruzi flagellum. Beyond a core axonemal proteome that is predicted based on conservation across trypanosomatid species and life stages 34, few flagellar proteins that have the potential to serve as a functional interface with the host environment have been identified in any T. cruzi life stage 9,35,36. The best characterized is the flagellar calcium-binding protein (FCaBP), a dual-acylated, 24 kDa Ca2+-sensing protein that tethers to the inner leaflet of the flagellar membrane 37. FCaBP is expressed in all T. cruzi life stages and is conserved across other trypanosomatid species, but its precise role in the biology of these organisms is unknown beyond its role as a calcium binding protein 36,38,39. Additionally, we have recently localized small myristoylated protein 1–1 (TcSMP1–1) to the flagellum in amastigotes 6, but the overall proteomic landscape of the T. cruzi flagellum remains largely uncharacterized.
In this study, we pursued a targeted, proximity-dependent biotinylation (BioID) approach to identify flagellar membrane and membrane-proximal flagellar proteins in the replicative stages of T. cruzi. We report the identification of 218 and 99 candidate flagellar proteins in T. cruzi epimastigotes and intracellular amastigote stages, respectively, many of which are conserved in other trypanosomatid species with evidence of flagellar localization. Approximately 20% of the candidate flagellar proteins were found to be restricted to the T. cruzi lineage, including a hypothetical protein that we confirmed localizes to the flagellar tip in T. cruzi epimastigotes and intracellular amastigotes. The novel BioID dataset identified here provides a critical foundation for investigation of the T. cruzi flagellum and its role in mediating interactions with diverse host environments.
Results
Flagellar and cytosolically targeted TurboID retains activity in T. cruzi
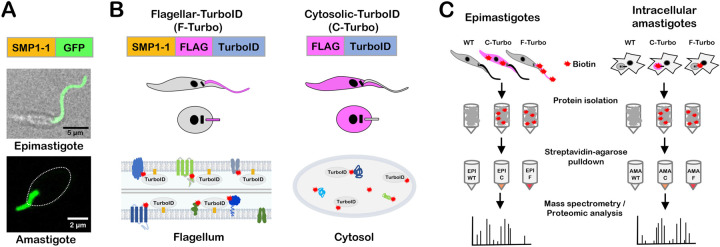
To facilitate the identification of flagellar proteins in T. cruzi using a proximity-dependent biotinylation approach, we generated transgenic parasites that express the biotin ligase, TurboID 40 in the parasite flagellum, as an in-frame fusion with C-terminal FLAG-tagged T. cruzi small myristoylated protein 1–1 (TcSMP1–1) (Fig. 1A,B; SMP1-1-FLAG-TurboID; ‘F-Turbo’). TcSMP1–1 was chosen as the endogenous ‘bait’ protein for flagellar localization of TurboID given its near exclusive localization in the flagellum in both replicative stages of T. cruzi, epimastigotes and amastigotes 6 (Fig. 1A), and because TcSMP1–1 contains the N-myristoylation sequence motif (MGXXXS/T) required for localization and tethering to the inner flagellar membrane, as demonstrated in Leishmania 41. The strategy of targeting TurboID to the flagellum using TcSMP1–1 is expected to increase the likelihood of identifying flagellar membrane and associated proteins while minimizing capture of axonemal proteins. To control for non-flagellar TurboID expression in the F-Turbo parasites, we generated an independent transgenic line that expresses FLAG-TurboID in the cytoplasm (Fig. 1B; ‘C-Turbo’). The parallel processing of F-Turbo and C-Turbo parasites, along with parental (WT) parasites that lack TurboID expression (Fig. 1C), will allow for background subtraction and identification of flagellar-enriched proteins in F-Turbo versus C-Turbo within the same parasite life cycle stage.
Figure 1. T. cruzi life cycle and schematic of TurboID-expressing lines generated for proximity-dependent biotinylation experiments.
(A) Live confocal images of SMP1-1-GFP localized to the flagellum of T. cruzi epimastigotes and an intracellular amastigote; white oval denotes position of amastigote body. (B) Strategy for generating stable T. cruzi lines expressing TurboID in the flagellum using SMP1–1 as the endogenous bait protein or in the cytoplasm of epimastigotes and amastigotes, where addition of exogenous biotin will mediate biotinylation (red star) of proteins in close proximity to TurboID in both settings. FLAG-epitope is included to facilitate TurboID localization in transfectants. (C) Flow chart outlining the experimental protocol used for identification of biotinylated proteins in epimastigotes (left) and intracellular amastigotes (right). For both life stages, wild-type (‘WT’), cytoplasmic-TurboID (‘C’) and flagellar-TurboID (‘F’) parasites (from left to right in the illustration) were exposed to biotin and the biotinylated protein fraction in protein lysates captured on streptavidin-agarose beads and subjected to mass spectrometry for identification and subsequent proteomic analysis.
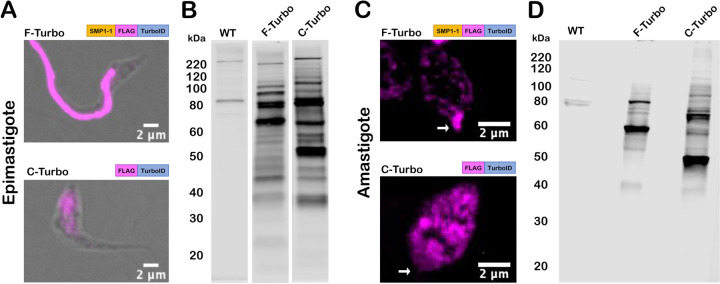
TurboID expression in transgenic T. cruzi parasites was confirmed by indirect immunofluorescence microscopy of fixed parasites stained with an antibody to the FLAG tag epitope, located immediately upstream of TurboID (Fig. 2). The flagella of F-Turbo parasites were brightly stained (Fig. 2A,C; F-Turbo) indicating that trafficking of TurboID to the flagellum occurred in both T. cruzi epimastigote (Fig. 2A; F-Turbo) and amastigote (Fig. 2C; F-Turbo) life stages. While most of the FLAG signal was localized to the flagellum in epimastigotes (Fig. 2A; F-Turbo), signal was detected in the body of intracellular amastigotes in addition to the brightly stained flagellum (Fig. 2C; F-Turbo), which may be due to overexpression of the SMP1-1-FLAG-TurboID fusion protein. C-Turbo parasites, generated as a proteomic control for non-flagellar TurboID-dependent biotinylation (Fig. 1C), were confirmed to express cytosolic FLAG in both parasite life stages (Fig. 2A,C; C-Turbo). To determine if TurboID is active in T. cruzi, total protein lysates were prepared from WT and Turbo-expressing parasites, following brief exposure to exogenous biotin, were probed with streptavidin-DyLight™ 800 to detect biotinylated proteins (Fig. 2B,D). As expected, multiple biotinylated proteins were revealed in lysates derived from F-TurboID and C-TurboID epimastigotes (Fig. 2B) and amastigotes (Fig. 2D) whereas few biotinylated proteins were detected in the parental (WT) controls. Differences in the biotinylated protein profiles observed when comparing F-Turbo to C-Turbo within a single life stage (Fig. 2B,D) likely reflect the differential localization patterns for TurboID in these parasite lines. Combined, these results confirm the expression of active TurboID in the flagellum (F-Turbo) or cytosol (C-Turbo) in both replicative stages of T. cruzi.
Figure 2. TurboID localization and activity in T. cruzi.
(A,C) Fluorescence microscopy images of fixed T. cruzi epimastigotes (A) or amastigotes (C) expressing SMP1-1-FLAG-TurboID (F-Turbo) (top) or FLAG-TurboID (C-Turbo) (bottom) stained for FLAG epitope (anti-FLAG)(pink). In (C), white arrows indicate the position of the amastigote flagellum. (B,D) Biotinylated proteins in lysates of WT, F-Turbo, and C-Turbo T. cruzi epimastigote (B) and amastigotes (D) detected with streptavidin-Dylight800.
Proteomic identification of candidate flagellar proteins in T. cruzi
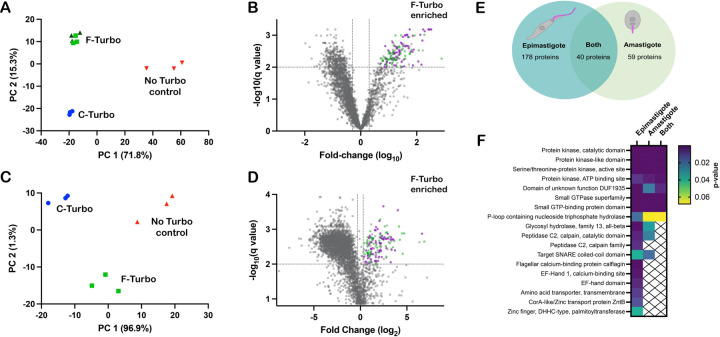
Biotinylated proteins in lysates generated from WT, F-Turbo and C-Turbo T. cruzi epimastigotes or intracellular amastigotes, were captured on immobilized streptavidin beads and identified using high performance liquid chromatography combined with mass spectrometry (Fig. 1C). Three independent biological replicates were analyzed for each parasite line, with the exception of F-Turbo epimastigotes, for which triplicate samples from two independent transfections were included. Peptide identification and relative intensity data obtained for replicate samples from each parasite line are represented in Supplementary Table 1. Principal component analysis (PCA) identified overall trends in the proteomic data obtained for T. cruzi epimastigotes (Fig. 3A) and amastigotes (Fig. 3C), revealing that biological replicates from individual parasite lines (WT, F-Turbo, C-Turbo) formed discrete clusters that were well separated from each other. As the replicates from independent F-Turbo epimastigote lineages were indistinguishable, these samples were pooled for subsequent analyses. Prior to data filtering and analysis, protein intensity scores were averaged across biological replicates within individual experimental groups (Supplementary Table 1).
Figure 3. Proximity proteome analysis identifies flagellar-enriched proteins in T. cruzi.
Principal component analysis (PCA) of biotinylome data plotted for WT (no TurboID control), flagellar-TurboID (F-Turbo) and cytosolic-TurboID (C-Turbo) for T. cruzi epimastigotes (A) and intracellular amastigotes (C). The two independent F-Turbo groups in the epimastigote PCA plot are represented in green (triangles and squares). Volcano plots (B,D) with fold-change (F-Turbo vs C-Turbo; x-axis) and adjusted p-value (q-value; y-axis) for T. cruzi epimastigote (B) and amastigote (D) proteomic data. Horizontal lines represent a q-value of 0.01 and the two vertical lines indicate the cut-offs for fold change (2-fold). The top right quadrants in each plot (B,D) contain proteins that are significantly enriched in F-Turbo proteomes (q≤0.01, ≥2-fold change). Known trypanosomatid flagellar proteins (purple circles) and hypothetical proteins (green circles) are shown for the F-Turbo enriched proteins. (E) Venn diagram depicting the number of proteins identified as enriched in the F-Turbo samples of T. cruzi epimastigote and amastigote stages. (F) Interpro domains assigned by DAVID that are significantly enriched in F-Turbo samples (i.e. proteins found in the upper right quadrant of each volcano plot) in epimastigotes, amastigotes and those common to both life stages; p-value is a modified Fisher exact, for protein enrichment analysis.
Streptavidin-bound proteins identified by mass spectrometry in WT parasites (which lack TurboID) represent the ‘background’ signal of endogenously biotinylated proteins and proteins that bound non-specifically to immobilized streptavidin. Thus, proteins represented at less than 100-fold enriched over the WT samples in F-Turbo or C-Turbo epimastigote samples were removed before subsequent analysis. Also, any proteins identified in less than 4/6 of the F-Turbo samples were removed (Supplementary Table 2). Volcano plots revealed the protein subsets significantly enriched in F-Turbo and C-Turbo samples in epimastigotes (Fig. 3B) and amastigotes (Fig. 3D). Proteins found to be significantly enriched in F-Turbo over C-Turbo (fold-change > 2; q-value ≤ 0.01) as well as proteins identified uniquely in F-Turbo samples (i.e., not present in C-Turbo samples from the same parasite life stage) are listed in Supplementary Table 3. From this analysis, 218 proteins were identified as significantly enriched in F-Turbo samples from T. cruzi epimastigotes and 99 proteins in amastigotes (Supplementary Table 3).
The T. cruzi SMP1–1 proximity proteome includes known trypanosomatid flagellar proteins.
The searchable TrypTag database 42, which contains localization data for 7,487 T. brucei proteins, was used as a resource to identify orthologues in the T. cruzi flagellar-enriched protein dataset (Supplementary Table 3) that have demonstrated flagellar localization in T. brucei. Of the 218 flagellar-enriched proteins in T. cruzi epimastigotes, 145 have orthologs that are represented in the TrypTag database and of these, 75 proteins exhibit at least partial flagellar localization in T. brucei bloodstream forms 42. Similar results emerged from the T. cruzi amastigote data where orthologs of 75 of the 99 proteins found to be enriched in amastigote F-Turbo samples had orthologs in T. brucei and were endogenously tagged, 44 of these showed at least partial flagellar localization. A comparison of the flagellar-enriched proteins identified in both T. cruzi epimastigotes and amastigotes revealed 40 proteins common to both life stages (Fig. 3E), of which 29 have orthologs that are represented in the TrypTag database (Supplementary Table 3) and 20 proteins exhibited some flagellar localization in T. brucei. Examples of confirmed flagellar proteins in other trypanosomatids that are significantly enriched in the T. cruzi flagellar proximity proteome include: flagellar membrane 8 43, flabarin 44, flagellar attachment zone 14 32, casein kinase I 42, CARP3 20 and cysteine peptidase, Clan CA, family C2 (calpain 1.3) 42. Although a significant proportion of the flagellar candidates identified in T. cruzi epimastigotes and amastigotes fall into the ‘hypothetical’ category (i.e., no annotation), the datasets were found to be enriched in kinase domains, calpain domains, and small GTP binding protein / GTPase domains (Fig. 3F).
Selected flagellar candidates localize to the T. cruzi flagellum in epimastigotes and amastigotes.
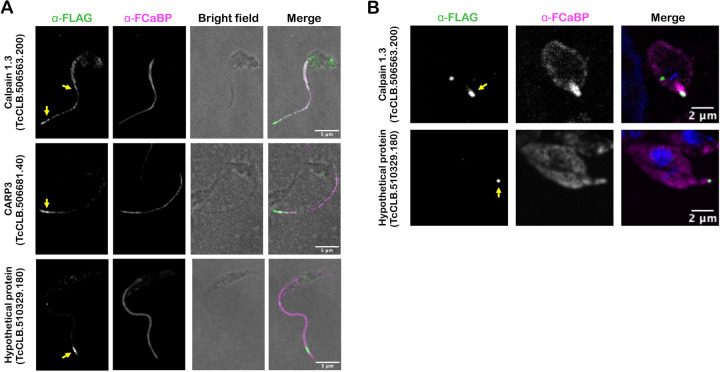
To localize candidate flagellar proteins in T. cruzi, we prioritized those that were significantly enriched in both the epimastigote and amastigote F-Turbo datasets and that included one or more of the following characteristics: (a) sequence motifs predicting membrane localization; (b) predicted role in signaling based on annotation or (c) were unique to the T. cruzi lineage (i.e., no obvious orthologues in other trypanosomatid species). Based on these criteria, six proteins were selected for endogenous FLAG-tagging and subsequent subcellular localization (Table 1) using primers and homology-directed repair templates shown in (Supplementary Fig. 4). Four of the 6 proteins were successfully tagged and three of which exhibited flagellar localization in T. cruzi epimastigotes: calpain 1.3 (TcCLB.506563.200), CARP3 (TcCLB.506681.40) and hypothetical protein (TcCLB.510329.180) (Fig. 4A; Supplementary Fig. 2). Another hypothetical protein (TcCLB.509965.20) was not verified as flagellar as the FLAG epitope signal localized to the parasite body (data not shown). Calpain 1.3-mRuby2-smFP FLAG exhibited a punctate pattern of labeling along the entire length of the T. cruzi epimastigote flagellum (Fig. 4A), whereas expression appeared to be restricted to the flagellar tip in the intracellular amastigote stage (Fig. 4B). Both CARP3 and the hypothetical protein (TcCLB.510329.180) localized to the distal region of the epimastigote flagellum (Fig. 4A) and hypothetical protein (TcCLB.510329.180) also localized to the flagellar tip in amastigotes (Fig. 4B). We were unable to determine CARP3 localization in amastigotes, due to undetectable signal for CARP3-mRuby2-smFP FLAG expression in this life stage, despite clear signal and flagellar localization in epimastigotes (Fig. 4A). Nonetheless, the successful identification of a subset of flagellar-localized proteins in intracellular T. cruzi amastigotes and epimastigotes provides initial validation of the proteomic datasets generated from proximity-dependent labeling.
Table 1:
Candidate flagellar proteins in T. cruzi selected for endogenous tagging.
| Name | TriTryp ID | Successfully Tagged? | Reason for selection |
|---|---|---|---|
| Calpain 1.3 | TcCLB.506563.200 | Yes | Potential signaling role |
| CARP3 | TcCLB.506681.40 | Yes | Potential signaling role |
| Hypothetical Protein | TcCLB.510329.180 | Yes | No known ortholog |
| Hypothetical Protein | TcCLB.509965.20 | Yes | No known T. brucei ortholog |
| ATPase | TcCLB.506925.410 | No | Potential transmembrane protein |
| Hypothetical Protein | TcCLB.509011.50 | No | Potential signaling role |
Candidates from the 40 proteins identified in both T. cruzi epimastigotes and amastigotes selected for endogenous epitope tagging and localization.
Figure 4. Flagellar localization of candidate flagellar proteins in T. cruzi.
Endogenous tagging reveals flagellar localization of candidate flagellar proteins: calpain 1.3-smFLAG (TcCLB.506563.200), CARP3-smFLAG (TcCLB.506681.40), or hypothetical protein-FLAG (TcCLB.510329.180) in T. cruzi epimastigotes (A) or intracellular amastigotes (B). In all cases, the FLAG tag was detected in fixed parasites using an anti-FLAG antibody and secondary antibody (green) and the flagellum was detected using anti-FCaBP and secondary antibody (magenta). The FLAG signal in the flagellum is indicated (yellow arrow).
Discussion
In the present work we demonstrate the successful use of a proximity labeling tool in the protozoan parasite, Trypanosoma cruzi. With the goal of identifying flagellar membrane and/or associated proteins in T. cruzi as candidates for mediating physical or functional interactions with insect or vertebrate hosts, the biotin ligase TurboID was targeted to the parasite flagellum as a fusion protein with the inner flagellar membrane protein, SMP1–1. Overexpression of the fusion protein was well-tolerated in the parasite, with no interference in the ability to transition between axenic epimastigotes, the T. cruzi life stage in which DNA transfection and selection is performed, and the intracellular stages in mammalian cells. This offered the opportunity to perform a comparative analysis of the two main replicative stages of T. cruzi, one that is motile with an elongated flagellum (epimastigote) and the other that is non-motile with a short flagellum (intracellular amastigote). Furthermore, inclusion of cytosolic-TurboID expressing parasites in the analysis aided in the differential identification of flagellar-enriched biotinylated proteins derived from SMP1-1-FLAG-TurboID parasites. This approach yielded 218 flagellar candidates in epimastigotes and 99 proteins in amastigotes, where 40 proteins were common to both T. cruzi life stages. Flagellar localization was confirmed for a subset of proteins in this dataset, based on endogenous epitope-tagging, and many more were predicted based on demonstrated localization in the related trypanosomatids, T. brucei or Leishmania spp.
The functional capabilities of the T. cruzi flagellum are broadly uncharacterized, beyond its role in propelling motile life stages and anchoring epimastigotes to the rectal mucosa in the insect vector 4,45,46. However, the recent recognition that the flagellum of cytosolically-localized intracellular amastigotes is capable of beating and establishes physical contact with host mitochondria 3,6, points to a potential role for the amastigote flagellum in host environmental sensing. Although little is known regarding the sensory capabilities of trypanosomatids in general, significant progress has been made toward a molecular understanding of pH taxis and social motility in the insect stages of T. brucei 21,23. The sensory system involves regulation of cyclic AMP levels, modulated by flagellar receptor-type adenylate cyclases and cyclic AMP phosphodiesterases22 and the involvement of a cyclic AMP responsive protein (CARP3), which is thought to act in a complex with adenylate cyclases 20. Our discovery, that CARP3 is expressed in the replicative stages of T. cruzi, including intracellular amastigotes (despite the inability to localize the tagged protein in this life stage), is quite exciting given the established role of CARP3 in T. brucei 20. In T. brucei CARP3 is known to co-localize with calpain 1.3 at the distal region of the flagellum, where the two proteins may physically interact 20. We show that the calpain 1.3 ortholog is also expressed in the flagellum of T. cruzi, where it localizes exclusively to the flagellar tip in intracellular amastigotes, a recognized signaling domain in trypanosomatids 33(p2),47. Calpain 1.3 belongs to a sub-family of cysteine peptidases that are predicted to be catalytically inactive as they lack one or more of the active site amino acid residues 48. Proteins with these features are thought to play a role in calcium homeostasis / signaling, including the calcium-based regulation of adenylate cyclase complexes 20,49. Despite the lack of social motility in T. cruzi, the expression of flagellar CARP3 and calpain 1.3 in this species is a strong indicator that the T. cruzi flagellum is equipped to sense and integrate signals from the environment. Dissection of the functional roles of CARP3 and calpain 1.3 in T. cruzi is expected to be instrumental in establishing the existence of flagellum-based environmental sensing in this parasite. In addition, a functional investigation of the hypothetical protein (TcCLB.509965.20) that also localizes to the distal end of the T. cruzi flagellum but lacks an obvious ortholog in T. brucei or Leishmania, has the potential to reveal novel biological or mechanistic insights into the role of the T. cruzi flagellum in different life stages.
The proteomic datasets generated here offer new opportunities to pursue the localization and functional analyses of many uncharacterized proteins, some of which are unique to the T. cruzi lineage. While some of the annotated proteins are not predicted to localize to the flagellum (based on annotation and TrypTag localization), including proteins involved in protein trafficking which may have encountered SMP1-1-FLAG-TurboID en route to the flagellum, there are a number of proteins with transmembrane domains or N-myristoylation consensus sequences that predict membrane-association. SMP-1 contains the N-myristoylation sequence motif (MGXXXS/T) known to direct flagellar localization and tethering to the inner flagellar membrane in Leishmania, where it associates tightly with detergent-resistant membranes (lipid rafts) 41 and forms homodimers 41. As such, SMP1–1 may preferentially interact with other membrane proteins associated with lipid rafts in the flagellar membrane. It is notable that a number of proteins that were among the strongest ‘hits’ in the flagellar candidate pool, such as calpain 1.3 and CARP3, also have MGXXXS/T motifs. While this motif is insufficient to direct a protein to the flagellum 41, proteins with lipid anchoring motifs or transmembrane domains could be prioritized for future studies of the T. cruzi flagellum. Notably, we did not identify adenylate cyclases in our data, even though CARP3 and calpain 1.3 were identified in a proximity-labeling study in T. brucei designed to identify flagellar tip proteins that interact with adenylate cyclase 1 33. While the proximity-dependent labeling approach used in this study enabled the discovery of a subset of flagellar proteins in T. cruzi amastigotes (where physical isolation of the short amastigote flagellum may not be feasible), it is understood that the resulting proteomes derived for the two parasite life stages are not comprehensive and many T. cruzi flagellar membrane proteins remain to be identified. With the discovery of additional flagellar proteins in T. cruzi, opportunities will be presented to use one or more of these proteins as alternative bait proteins for proximity labeling with a view to expanding the flagellar proteome in this understudied parasite.
Overall, we have presented the first use of proximity-dependent biotinylation in T. cruzi for the identification of more than 200 candidate flagellar proteins across two parasite life stages, thereby creating an important resource for the research community. As more information becomes available for the T. cruzi amastigote flagellum, it is expected to provide some context for the biological role of the flagellum in infection and these interactions may be the key for specific targeting of parasite function and viability within the mammalian host. Future investigation focused on identifying the function of potential sensory flagellar candidates in T. cruzi epimastigote and amastigote flagella, may aid in the discovery of these currently unknown host-parasite interaction mechanisms.
Methods and Materials
Reagents
Compounds were purchased and diluted to stock concentrations: Biotin, 100 mM in DMSO (Sigma Aldrich, St. Louis, Missouri, USA). Phenylmethylsulfonyl fluoride (PMSF), 10 mM in isopropanol (Sigma Aldrich, St. Louis, Missouri, USA). Tosyl-L-lysyl-chloromethane hydrochloride (TLCK), 5 mM in DMSO (Abcam, Cambridge, United Kingdom).
Mammalian cell culture
Normal Human Neonatal Dermal Fibroblasts (NHDF; Lonza, Basel, Switzerland) and monkey kidney epithelial cells (LLC-MK2; American Type Culture Collection) were maintained in Dulbecco’s modified Eagle medium (DMEM; HyClone, Logan, Utah, USA) supplemented with 10% heat-inactivated FBS (Gibco, Waltham, Massachusetts, USA), 25 mM glucose, 2 mM L-glutamine, and 100 U/mL penicillin-streptomycin (DMEM-10) at 37°C and 5% CO2.
Growth and maintenance of T. cruzi
Trypanosoma cruzi Tulahuén LacZ clone C4 was obtained from the American Type Culture Collection (ATCC, PRA-330; ATCC, Manassas, Virginia, USA). The epimastigote stage was propagated at 28°C in liver infusion tryptose (LIT) medium (4 g/L NaCl, 0.4 g/L KCl, 8 g/L Na2HPO4, 2 g/L dextrose, 3 g/L liver infusion broth, 5 g/L tryptose, with 25 mg/L hemin and 10% heat-inactivated FBS). The mammalian cell infection cycle was initiated with metacyclic trypomastigotes arising within stationary phase epimastigote cultures that were shifted from LIT to DMEM + 2% FBS (DMEM-2) for 5 days at 28°C. Metacyclic-enriched cultures were washed in DMEM-2 and incubated with confluent LLC-MK2 monolayers at 37°C, 5% CO2 to allow invasion. Mammalian stage trypomastigotes that emerged from infected LLC-MK2 cells (within 5–10 days) were harvested from culture supernatants and used to infect fresh LLC-MK2 monolayers. This cycle was continued on weekly basis to maintain the mammalian-infective stages of T. cruzi in culture. For experimental infections, trypomastigotes collected from LLC-MK2 maintenance cultures were pelleted at 2060 x g for 10 minutes and pellets were incubated at 37°C, 5% CO2 for 2–4 hours to allow motile trypomastigotes to swim up into the supernatant. Purified trypomastigotes in the supernatant were collected, washed once in DMEM-2 and utilized to infect sub-confluent monolayers of NHDF as indicated.
Generation of stable T. cruzi transfectants
T. cruzi strains expressing TcSMP1–1GFP were previously generated 6. A plasmid containing the TurboID sequence was a kind gift of Jeffrey Dvorin (Harvard Medical School). Each TurboID construct was used to replace the GFP-P2A-puro cassette in a modified pTREX plasmid 50,51 containing either SMP1-1-GFP or GFP alone. The inserts in the pTREX backbone to generate the F-Turbo plasmids were SMP1-1-TurboID-P2A-puro (F-Turbo-P) or SMP-1-1-TurboID-T2A-puro (F-Turbo-T). The inserts and backbone were assembled using the NEB HiFi DNA assembly kit (New England Biolabs, Ipswich, Massachusetts, USA), resulting in the final plasmids. For the cytosolic control, TurboID-P2A-puro was amplified using PCR and then inserted into the pTREX-GFP backbone, replacing GFP between the SpeI and XmaI cut sites using or through restriction enzyme cloning. To generate TurboID-expressing parasites T. cruzi epimastigotes were transfected with 15 μg of the respective DNA. Prior to transfection log-phase T. cruzi epimastigotes, were pelleted at 2060 x g for 10 minutes, resuspended in 100 μL of Tb BSF buffer 52 (4×107 parasites) and placed into a sterile 2 mm gap cuvette with the appropriate DNA and transfected using an Amaxa Nucleofector II (Lonza, Basel, Switzerland; U-33 program). Parasites were immediately transferred to LIT medium for 24 hours before adding 10 μg/mL puromycin (Invivogen, San Diego, California, USA) or 50 μg/mL blasticidin (Invivogen, San Diego, California, USA) for selection.
CRISPR/Cas9-facilitated epitope tagging of genomic loci in T. cruzi was performed as described 53. Briefly, each gene of interest was PCR-amplified from genomic DNA (T. cruzi Tulahuen strain) and PCR products sequenced. Two gRNA binding sites near the 3’ region of each gene of interest were identified using EuPaGDT 54. Editing of the previously modified pTREX-n-Cas9 plasmid 51 (Addgene plasmid 68708), performed to exchange the previous gRNA sequence was achieved using a Q5 mutagenesis kit (New England Biolabs, Ipswich, Massachusetts, USA). gRNA sequences were inserted into pTREX-n-Cas9 using primers specific to the gene of interest in Supplementary Table 4, such that the previous gRNA sequence was replaced. The template for generating homology-directed repair DNA for gene tagging was constructed by inserting a P2A viral skip peptide in frame with a downstream blasticidin-S deaminase (BSD) or puromycin N-acetyl-transferase (puro) and TOPO cloned into a pCR4 backbone (Thermo Fisher, Waltham, MA, United States of America) 51. Homology template was amplified from this template using ultramer pairs (Supplementary Table 4) that provided 100 bp of homology for the gene of interest, the FLAG tag and 20 bp of homology to template. Parasite were transfected as above with 25 μg of each gRNA-specific pTREX-n-Cas9 plasmid to the gene of interest and 50 μg of homology repair template. Correct integration of the endogenous tag and drug cassette was established via PCR (Supplementary Fig. 2).
Detection of biotinylated protein fractions in T. cruzi
Epimastigotes:
1.5 × 108 epimastigotes were pelleted at 2060 x g for 10 minutes, resuspended in 1 ml of LIT and incubated with 50 µM biotin for 10 minutes at 37oC. Parasites were washed twice with ice-cold PBS then resuspended in 1 ml cell lysis buffer 55 (0.5% Nonidet P-40, 500 mM NaCl, 5 mM EDTA, 1 mM DTT, 50 mM Tris-Base, 0.4% SDS, pH 7.4) with Roche cOmplete™ Protease Inhibitor (Sigma-Aldrich, St. Louis, Missouri, USA), 100 μM PMSF and 10 μM TLCK. Lysates were sonicated using 3 pulses of 30 seconds at 100% amplitude (Q700 sonicator, QSONICA, Newton, Connecticut), with 15 sec breaks between to cool the tubes on ice. Samples were centrifuged at 16,000 x g for 20 minutes at 4°C and the supernatant was collected. Aliquots of clarified lysate (3 × 106 parasite equivalents) were resolved by SDS-PAGE (Mini-PROTEAN®TGX protein gel; Bio-Rad, Hercules, California, USA), transferred to PVDF membrane (Immobilon®-FL, MilliporeSigma, Burlington, Massachusetts, USA) and probed with Streptavidin DyLight™ 800 (Thermo Fisher Scientific, Waltham, Massachusetts, USA) to detect biotinylated proteins.
Intracellular amastigotes:
At 48 hpi, T. cruzi-infected NHDF monolayers in T-150 flasks were exposed to 100 µM biotin for 10 minutes at 37°C, 5% CO2. Monolayers were then rinsed three times with cold PBS and incubated with 2 mL of cell lysis buffer (as above). Flasks were agitated manually for 5 minutes then cells were scraped and transferred into a tube containing 0.5 μL of benzonase (Sigma-Aldrich, St. Louis, Missouri, USA). Tubes were placed on a rotative wheel for 15 minutes at room temperature, then sonicated as above. Amastigote loading volumes were normalized via Western blot as follows. Equal volumes of serially diluted protein lysates, generated for WT, F-Turbo and C-Turbo infected NHDF, were resolved by SDS-PAGE (Mini-PROTEAN®TGX gels), transferred to PVDF membrane and probed with a rabbit antibody to trypanosome BiP 56, followed by α-Rabbit Alexa Flour 647. A LiCor Odessy® CLx imager was used to measure BiP signal in each sample (Image Studio). Relative BiP densities were used to adjust the volumes of each amastigote lysates (confirmed by independent western blots) prior to loading on streptavidin beads.
Isolation of biotinylated proteins
Protein lysates were loaded onto Pierce™ High-Capacity Streptavidin Agarose (Thermo Fisher Scientific, Waltham, Massachusetts, USA). 100 μL and 150 μL packed bead volumes were used for epimastigote and amastigote samples, respectively, and incubated on a rotative wheel overnight at 4°C. For all following steps, washes consisted of adding 1 mL of the indicated buffer and placing the tube on the rotative wheel for 5 minutes, then spinning down the agarose beads for 1 minute at 500 x g, as previously described 55. Beads with bound protein were subjected to 5 washes with Buffer 1 (8 M urea, 200 mM NaCl, 100 mM Tris, pH 8.0) with 0.2% sodium dodecyl sulfate (SDS), 5 washes with Buffer 1 containing 2% SDS, and 5 washes with Buffer 1 with no SDS were completed at room temperature. Next, 2 washes with 200 mM NaCl, 100 mM Tris, at a pH of 7.0 and 2 washes with Tris, pH 8.0 were completed at 4°C. Washed beads were adjusted to pH 7.5 with 200 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) and bound proteins were reduced using 5 mM dithiothreitol (Sigma-Aldrich) at 37°C for 1 h, followed by alkylation of cysteine residues using 15 mM iodoacetamide (Sigma-Aldrich) in the dark at room temperature for 1 h. Excessive iodoacetamide was quenched using 10 mM dithiotheritol. Protein mixtures were diluted in 1:6 ratio (v/v) using ultrapure water prior to digestion using sequencing grade trypsin (Promega) at 37°C for 16 h. Digested peptides were subsequently desalted using self-packed C18 STAGE tips (3M EmporeTM) 57 for LC-MS/MS analysis.
Mass spectrometry
Epimastigotes:
Desalted peptides were resuspended in 0.1% (v/v) formic acid and loaded onto HPLC-MS/MS system for analysis on an Orbitrap Q-Exactive Exploris 480 (Thermo Fisher Scientific) mass spectrometer coupled to an Easy nanoLC 1000 (Thermo Fisher Scientific) with a flow rate of 250 nl/min. The stationary phase buffer was 0.5 % formic acid and mobile phase buffer was 0.5 % (v/v) formic acid in acetonitrile. Chromatography for peptide separation was performed using increasing organic proportion of acetonitrile (5 – 40 % (v/v)) over a 120 min gradient) on a self-packed analytical column using PicoTip™ emitter (New Objective, Woburn, MA) using Reprosil Gold 120 C-18, 1.9 μm particle size resin (Dr. Maisch, Ammerbuch-Entringen, Germany). The mass spectrometry analyzer operated in data dependent acquisition mode with a top ten method at a mass range of 300–2000 Da. Data were processed using MaxQuant software (version 1.5.2.8) 58 with the following setting: oxidized methionine residues and protein N-terminal acetylation as variable modification, cysteine carbamidomethylation as fixed modification, first search peptide tolerance 20 ppm, main search peptide tolerance 4.5 ppm. Protease specificity was set to trypsin with up to 2 missed cleavages allowed. Only peptides longer than five amino acids were analyzed, and the minimal ratio count to quantify a protein is 2 (proteome only). The false discovery rate (FDR) was set to 1% for peptide and protein identifications. Database searches were performed using the Andromeda search engine integrated into the MaxQuant environment 59 against the UniProt Trypanosoma cruzi strain CL Brener (352153) database containing 19,242 entries (March 2020). “Matching between runs” algorithm with a time window of 0.7 min was employed to transfer identifications between samples processed using the same nanospray conditions. Protein tables were filtered to eliminate identifications from the reverse database and common contaminants.
Amastigotes:
Desalted peptides were resolubilized in 0.1% (v/v) formic acid and loaded onto HPLC-MS/MS system for analysis on an Orbitrap Q-Exactive Exploris 480 (Thermo Fisher Scientific) mass spectrometer coupled to an FAIMS Pro Interface system and Easy nanoLC 1000 (Thermo Fisher Scientific) with a flow rate of 300 nl/min. The stationary phase buffer was 0.1 % formic acid, and mobile phase buffer was 0.1 % (v/v) formic acid in 80% (v/v) acetonitrile. Chromatography for peptide separation was performed using increasing organic proportion of acetonitrile (5 – 40 % (v/v)) over a 120 min gradient) on a self-packed analytical column using PicoTip™ emitter (New Objective, Woburn, MA) using Reprosil Gold 120 C-18, 1.9 μm particle size resin (Dr. Maisch, Ammerbuch-Entringen, Germany). High precision iRT calibration was used for samples processed using the same nanospray conditions 60. The mass spectrometry analyzer operated in data independent acquisition mode at a mass range of 300–2000 Da, compensation voltages of −50/−70 CVs with survey scan of 120,000 and 15,000 resolutions at MS1 and MS2 levels, respectively. Data were processed using Spectronaut™ software (version 15; Biognosys AG) 61 using directDIA™ analysis with default settings, including: oxidized methionine residues, biotinylation, protein N-terminal acetylation as variable modification, cysteine carbamidomethylation as fixed modification, initial mass tolerance of MS1 and MS2 of 15 ppm. Protease specificity was set to trypsin with up to 2 missed cleavages were allowed. Only peptides longer than seven amino acids were analyzed, and the minimal ratio count to quantify a protein is 2 (proteome only). The false discovery rate (FDR) was set to 1% for peptide and protein identifications. Database searches were performed against the UniProt Trypanosoma cruzi strain CL Brener (352153) database containing 19,242 entries (March 2020). Protein tables were filtered to eliminate identifications from the reverse database and common contaminants.
Principal component analysis
All protein intensity scores were uploaded to Metaboanalyst 5.0 62 to perform statistical analysis, with one factor. Data was entered as peak intensities and filtered using the interquartile range, then normalized by sum and log transformed. 2D PCA scores were plotted in Prism GraphPad.
Volcano plots
For epimastigote data, protein intensity scores for all proteins that were found to be 100-fold or higher enriched in the F-Turbo or C-Turbo samples over wild type were loaded into Prism GraphPad, and Log10 transformed. Multiple unpaired t-tests were run on the data with a false discovery rate of 1% and the results were reported as F-Turbo – C-Turbo, with -log10(q value) reported for the volcano plot. For amastigote data, Spectronaut™ software was used to generate the statistical analysis of the amastigote proteomics. Within the DIA analysis pipeline, the default settings were used, including a false discovery rate of 1% and a n unpaired Student’s t-test was performed. Fold changes were reported as an average Log10 (epimastigotes) or Log2 (amastigotes) ratio for the volcano plot. Volcano plot was created in Prism GraphPad. Post-analysis, six proteins were excluded from the amastigote flagellar enriched list, as they were not identified in 2 of 3 biological replicates. Additionally, all allelic duplicates were removed to ensure that the proteins listed in the final tables were represented by a single gene identifier corresponding to the CL Brener reference genome (TriTrypDB; https://tritrypdb.org/), but paralogs remain.
Interpro domain enrichment analysis
DAVID 63 enrichment was used to assign Interpro domains to all of the proteins found to be significantly enriched in either of the F-Turbo samples. No weighting of the data was completed. Interpro domains with a p-value ≤ 0.05 were considered significantly enriched.
Indirect immunofluorescence microscopy
Epimastigotes were fixed directly in growth medium with the addition of paraformaldehyde (1% final concentration in PBS) for 10-minute at 4°C. Fixed parasites were pelleted by centrifuging for 10 minutes at 4000 x g and resuspended in PBS. 10 μL of the parasite solution was dropped onto poly-L-lysine coated slides and allowed to dry completely prior to staining. For immunostaining of intracellular amastigotes, T. cruzi-infected NHDF on round cover glass (12 mm, #1.5; Electron Microscopy Sciences, Hatfield, Pennsylvania, USA) were fixed at 48 hours post-infection with 1% (v/v) paraformaldehyde/PBS. All steps of the immunostaining protocol were preceded by three washes with PBS and carried out at room temperature. Parasites were permeabilized with a 0.1% Triton-X 100 solution (JT Baker, Phillipsburg, New Jersey, USA) for 10 minutes and blocked with 3% BSA (Sigma-Aldrich, St. Louis, Missouri, USA) in PBS for 1 hour. The primary antibody solution containing 1:400 mouse α-FLAG (clone M2, Sigma-Aldrich, St. Louis, Missouri, USA) and/or 1:1,500 rabbit α-FCaBP 64 in 1% BSA in PBS was added for 1 hour, followed by a 1:1000 α-Mouse Alexa Flour 594 and/or α-Rabbit Alexa Flour 647 solution in 1% BSA in PBS for 1 hour. DAPI (0.2 μg/mL; Thermo Fisher Scientific, Waltham, Massachusetts, USA) in PBS was added for 5 minutes, and following washes, coverslips were placed onto slides with Prolong® Diamond mountant (Thermo Fisher Scientific, Waltham, Massachusetts, USA). After setting for 24 hours parasites were imaged using a Yokogawa CSU-X1 spinning disk confocal system paired with a Nikon Ti-E inverted microscope and an iXon Ultra 888 EMCCD camera (100X objective). Image processing, analysis, and display were performed using ImageJ Fiji software 65.
Supplementary Material
Supplementary Table 1: Raw proteomics data for epimastigotes and intracellular amastigotes TurboID experiment. Each sheet contains the protein identity and intensity score for all samples in either epimastigotes or amastigotes.
Supplementary Table 2: Filtered proteomics data for epimastigotes and intracellular amastigotes TurboID experiment. Each sheet contains the protein identity and intensity score for all samples in either epimastigotes or amastigotes that was used for statistical analysis.
Supplementary Table 3: Proteins enriched in the flagellar TurboID expressing samples for epimastigotes and intracellular amastigotes. Each sheet contains information about the proteins enriched in either epimastigotes, amastigotes, or both epimastigotes and amastigotes.
Supplementary Table 4: Primers for all PCR and endogenous tagging. Primer pairs are listed for all experiments described.
Supplementary Figure 1. Model of the T. cruzi life cycle. Schematic of the T. cruzi life cycle highlighting the insect stage ‘epimastigote’ that is propagated axenically in liquid culture and gives rise to the infectious ‘metacyclic trypomastigote’. Trypomastigotes, whether derived from epimastigotes or as the end product of a single lytic cycle in a mammalian cell are motile, non-dividing forms of the parasite that actively invade a mammalian host cell. Inside a host cell, the ‘trypomastigote’ transforms into the replicative intracellular ‘amastigote’ stage by 18 hours post-infection (hpi). Amastigotes undergo several rounds of proliferation, dividing by binary fission (between ~24–90 hpi), before they stop dividing and differentiate into trypomastigotes, that eventually lyse the infected host cell and disseminate infection. Stable transfection and drug selection is performed in the epimastigote stage (lightning bolt symbolizes electroporation). Once stable genomic changes are confirmed in epimastigotes, these parasites are used to establish the mammalian infection cycle starting with metacyclic trypomastigotes as outlined above.
Supplementary Figure 2: PCR confirmation of endogenous tags for candidate proteins. A. Schematic showing the region of amplification and DNA gel with corresponding bands for A. calpain 1.3-smFLAG (TcCLB.506563.200), B. CARP3-smFLAG (TcCLB.506681.40), and C. hypothetical protein-FLAG (TcCLB.510329.180). Ladder run on all DNA gels is Thermo Scientific GeneRuler DNA Ladder.
Importance.
Trypanosoma cruzi is a protozoan parasite that causes Chagas disease, which contributes substantial morbidity and mortality in South and Central America. Throughout its life cycle, T. cruzi interacts with insect and mammalian hosts via its single flagellum, establishing intimate contact with host membranes. Currently, few flagellar proteins have been identified in T. cruzi that could provide insight into the mechanisms involved in mediating physical and biochemical interactions with the host. Here, we set out to identify flagellar proteins in the main replicative stages of T. cruzi using a proximity-labeling approach coupled with mass spectrometry. The >200 candidate flagellar proteins identified represent the first large scale identification of candidate flagellar proteins in T. cruzi with preliminary validation. These data offer new avenues to investigate the biology of T. cruzi - host interactions, a promising area for development of new strategies aimed at the control of this pathogen.
Acknowledgments
We thank the Sabri Ülker Center’s Advanced Imaging Lab at the Harvard T.H. Chan School of Public Health for microscopy training and access. Additionally, we would like to thank Zon Weng Lai from the Harvard Chan Advanced Multi-omics Platform at the Harvard T.H. Chan School of Public Health for his assistance in interpreting the mass spectrometry data. We thank Jeffrey Dvorin (Boston Children’s Hospital) for providing the TurboID plasmid, David Engman (Cedars-Sinai Medical Center) for the gift of the anti-FCaBP antibody and Jay Bangs (The State University of New York at Buffalo) for anti-BiP antibody. We also thank Lucas Pagura for his thoughtful discussion. This work was supported by NIH grant R21AI135520 awarded to BAB and NIH training grant 5T32AI049928.
Footnotes
Competing interests
No competing interests declared.
References
- 1.Bern C. Chagas’ Disease. N Engl J Med. 2015;373(5):456–466. doi: 10.1056/NEJMra1410150 [DOI] [PubMed] [Google Scholar]
- 2.Stanaway JD, Roth G. The burden of Chagas disease: estimates and challenges. Glob Heart. 2015;10(3):139–144. doi: 10.1016/j.gheart.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 3.Lentini G, Dos Santos Pacheco N, Burleigh BA. Targeting host mitochondria: A role for the Trypanosoma cruzi amastigote flagellum. Cell Microbiol. 2018;20(2). doi: 10.1111/cmi.12807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nogueira NFS, Gonzalez MS, Gomes JE, de Souza W, Garcia ES, Azambuja P, Nohara LL, Almeida IC, Zingales B, Colli W. Trypanosoma cruzi: involvement of glycoinositolphospholipids in the attachment to the luminal midgut surface of Rhodnius prolixus. Exp Parasitol. 2007;116(2):120–128. doi: 10.1016/j.exppara.2006.12.014 [DOI] [PubMed] [Google Scholar]
- 5.Schmidt J, Kleffmann T, Schaub GA. Hydrophobic attachment of Trypanosoma cruzi to a superficial layer of the rectal cuticle in the bug Triatoma infestans. Parasitol Res. 1998;84(7):527–536. doi: 10.1007/s004360050443 [DOI] [PubMed] [Google Scholar]
- 6.Won MM, Krüger T, Engstler M, Burleigh BA. The intracellular amastigote of Trypanosoma cruzi maintains an actively beating flagellum. mBio. In Press. [DOI] [PMC free article] [PubMed]
- 7.Gonçalves CS, Ávila AR, de Souza W, Motta MCM, Cavalcanti DP. Revisiting the Trypanosoma cruzi metacyclogenesis: morphological and ultrastructural analyses during cell differentiation. Parasit Vectors. 2018;11:83. doi: 10.1186/s13071-018-2664-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gadelha C, Wickstead B, McKean PG, Gull K. Basal body and flagellum mutants reveal a rotational constraint of the central pair microtubules in the axonemes of trypanosomes. J Cell Sci. 2006;119(Pt 12):2405–2413. doi: 10.1242/jcs.02969 [DOI] [PubMed] [Google Scholar]
- 9.Gardiner PJ. Pellicle-associated structures in the amastigote stage of Trypanosoma cruzi and Leishmania species. Ann Trop Med Parasitol. 1974;68(2):167–176. doi: 10.1080/00034983.1974.11686935 [DOI] [PubMed] [Google Scholar]
- 10.Portman N, Gull K. The paraflagellar rod of kinetoplastid parasites: From structure to components and function. Int J Parasitol. 2010;40(2):135. doi: 10.1016/j.ijpara.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly FD, Sanchez MA, Landfear SM. Touching the Surface: Diverse Roles for the Flagellar Membrane in Kinetoplastid Parasites. Microbiol Mol Biol Rev MMBR. 2020;84(2):e00079–19. doi: 10.1128/MMBR.00079-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gluenz E, Höög JL, Smith AE, Dawe HR, Shaw MK, Gull K. Beyond 9+0: noncanonical axoneme structures characterize sensory cilia from protists to humans. FASEB J Off Publ Fed Am Soc Exp Biol. 2010;24(9):3117–3121. doi: 10.1096/fj.09-151381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly FD, Yates PA, Landfear SM. Nutrient Sensing in Leishmania: Flagellum and Cytosol. Mol Microbiol. Published online October 28, 2020. doi: 10.1111/mmi.14635 [DOI] [PMC free article] [PubMed]
- 14.Saegusa Y, Yoshimura K. cAMP controls the balance of the propulsive forces generated by the two flagella of Chlamydomonas. Cytoskelet Hoboken NJ. 2015;72(8):412–421. doi: 10.1002/cm.21235 [DOI] [PubMed] [Google Scholar]
- 15.Bickerton P, Sello S, Brownlee C, Pittman JK, Wheeler GL. Spatial and temporal specificity of Ca2+ signalling in Chlamydomonas reinhardtii in response to osmotic stress. New Phytol. 2016;212(4):920–933. doi: 10.1111/nph.14128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christensen ST, Pedersen LB, Schneider L, Satir P. Sensory cilia and integration of signal transduction in human health and disease. Traffic. 2007;8(2):97–109. doi: 10.1111/j.1600-0854.2006.00516.x [DOI] [PubMed] [Google Scholar]
- 17.Camara M de los M, Bouvier LA, Miranda MR, Pereira CA. The flagellar adenylate kinases of Trypanosoma cruzi. FEMS Microbiol Lett. 2015;362(1):1–5. doi: 10.1093/femsle/fnu020 [DOI] [PubMed] [Google Scholar]
- 18.Langousis G, Hill KL. Motility and more: the flagellum of Trypanosoma brucei. Nat Rev Microbiol. 2014;12(7):505–518. doi: 10.1038/nrmicro3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peacock L, Bailey M, Carrington M, Gibson W. Meiosis and haploid gametes in the pathogen Trypanosoma brucei. Curr Biol CB. 2014;24(2):181–186. doi: 10.1016/j.cub.2013.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bachmaier S, Giacomelli G, Calvo-Alvarez E, Vieira LR, Abbeele JVD, Aristodemou A, Lorentzen E, Gould M, Brennand A, Dupuy JW, Forné I, imhof A, Bramkamp M, Salmon D, Rotureau B, Boshart M. A multi-adenylate cyclase regulator at the flagellar tip controls African trypanosome transmission. Published online 2022. doi: 10.21203/rs.3.rs-1216579/v1 [DOI] [PMC free article] [PubMed]
- 21.Shaw S, Knüsel S, Abbühl D, Naguleswaran A, Etzensperger R, Benninger M, Roditi I. Cyclic AMP signalling and glucose metabolism mediate pH taxis by African trypanosomes. Nat Commun. 2022;13(1):603. doi: 10.1038/s41467-022-28293-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberholzer M, Saada EA, Hill KL. Cyclic AMP Regulates Social Behavior in African Trypanosomes. mBio. 2015;6(3):e01954–14. doi: 10.1128/mBio.01954-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez MA, Saada EA, Hill KL. Insect stage-specific adenylate cyclases regulate social motility in African trypanosomes. Eukaryot Cell. 2015;14(1):104–112. doi: 10.1128/EC.00217-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figarella K, Uzcategui NL, Zhou Y, LeFurgey A, Ouellette M, Bhattacharjee H, Mukhopadhyay R. Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: possible role in volume regulation and osmotaxis. Mol Microbiol. 2007;65(4):1006–1017. doi: 10.1111/j.1365-2958.2007.05845.x [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Contreras D, Aslan H, Feng X, Tran K, Yates PA, Kamhawi S, Landfear SM. Regulation and biological function of a flagellar glucose transporter in Leishmania mexicana: a potential glucose sensor. FASEB J Off Publ Fed Am Soc Exp Biol. 2015;29(1):11–24. doi: 10.1096/fj.14-251991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piper RC, Xu X, Russell DG, Little BM, Landfear SM. Differential targeting of two glucose transporters from Leishmania enriettii is mediated by an NH2-terminal domain. J Cell Biol. 1995;128(4):499–508. doi: 10.1083/jcb.128.4.499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldman-Pinkovich A, Kannan S, Nitzan-Koren R, Puri M, Pawar H, Bar-Avraham Y, McDonald J, Sur A, Zhang WW, Matlashewski G, Madhubala R, Michaeli S, Myler PJ, Zilberstein D. Sensing Host Arginine Is Essential for Leishmania Parasites’ Intracellular Development. mBio. 2020;11(5). doi: 10.1128/mBio.02023-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oberholzer M, Langousis G, Nguyen HT, Saada EA, Shimogawa MM, Jonsson ZO, Nguyen SM, Wohlschlegel JA, Hill KL. Independent Analysis of the Flagellum Surface and Matrix Proteomes Provides Insight into Flagellum Signaling in Mammalian-infectious Trypanosoma brucei. Mol Cell Proteomics MCP. 2011;10(10):M111.010538. doi: 10.1074/mcp.M111.010538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beneke T, Demay F, Hookway E, Ashman N, Jeffery H, Smith J, Valli J, Becvar T, Myskova J, Lestinova T, Shafiq S, Sadlova J, Volf P, Wheeler RJ, Gluenz E. Genetic dissection of a Leishmania flagellar proteome demonstrates requirement for directional motility in sand fly infections. PLOS Pathog. 2019;15(6):e1007828. doi: 10.1371/journal.ppat.1007828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subota I, Julkowska D, Vincensini L, Reeg N, Buisson J, Blisnick T, Huet D, Perrot S, Santi-Rocca J, Duchateau M, Hourdel V, Rousselle JC, Cayet N, Namane A, Chamot-Rooke J, Bastin P. Proteomic analysis of intact flagella of procyclic Trypanosoma brucei cells identifies novel flagellar proteins with unique sub-localization and dynamics. Mol Cell Proteomics MCP. 2014;13(7):1769–1786. doi: 10.1074/mcp.M113.033357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broadhead R, Dawe HR, Farr H, Griffiths S, Hart SR, Portman N, Shaw MK, Ginger ML, Gaskell SJ, McKean PG, Gull K. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature. 2006;440(7081):224–227. doi: 10.1038/nature04541 [DOI] [PubMed] [Google Scholar]
- 32.Hu H, Zhou Q, Li Z. SAS-4 Protein in Trypanosoma brucei Controls Life Cycle Transitions by Modulating the Length of the Flagellum Attachment Zone Filament. J Biol Chem. 2015;290(51):30453–30463. doi: 10.1074/jbc.M115.694109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vélez-Ramírez DE, Shimogawa MM, Ray SS, Lopez A, Rayatpisheh S, Langousis G, Gallagher-Jones M, Dean S, Wohlschlegel JA, Hill KL. APEX2 Proximity Proteomics Resolves Flagellum Subdomains and Identifies Flagellum Tip-Specific Proteins in Trypanosoma brucei. mSphere. 2021;6(1):e01090–20. doi: 10.1128/mSphere.01090-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Branche C, Kohl L, Toutirais G, Buisson J, Cosson J, Bastin P. Conserved and specific functions of axoneme components in trypanosome motility. J Cell Sci. 2006;119(16):3443–3455. doi: 10.1242/jcs.03078 [DOI] [PubMed] [Google Scholar]
- 35.Jimenez V, Docampo R. Molecular and Electrophysiological Characterization of a Novel Cation Channel of Trypanosoma cruzi. PLOS Pathog. 2012;8(6):e1002750. doi: 10.1371/journal.ppat.1002750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engman DM, Krause KH, Blumin JH, Kim KS, Kirchhoff LV, Donelson JE. A novel flagellar Ca2+-binding protein in trypanosomes. J Biol Chem. 1989;264(31):18627–18631. [PubMed] [Google Scholar]
- 37.Maric D, McGwire BS, Buchanan KT, Olson CL, Emmer BT, Epting CL, Engman DM. Molecular Determinants of Ciliary Membrane Localization of Trypanosoma cruzi Flagellar Calcium-binding Protein. J Biol Chem. 2011;286(38):33109–33117. doi: 10.1074/jbc.M111.240895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buchanan KT, Ames JB, Asfaw SH, Wingard JN, Olson CL, Campana PT, Araújo APU, Engman DM. A flagellum-specific calcium sensor. J Biol Chem. 2005;280(48):40104–40111. doi: 10.1074/jbc.M505777200 [DOI] [PubMed] [Google Scholar]
- 39.Maldonado RA, Mirzoeva S, Godsel LM, Lukas TJ, Goldenberg S, Watterson DM, Engman DM. Identification of calcium binding sites in the trypanosome flagellar calcium-acyl switch protein. Mol Biochem Parasitol. 1999;101(1–2):61–70. doi: 10.1016/s0166-6851(99)00055-9 [DOI] [PubMed] [Google Scholar]
- 40.Branon TC, Bosch JA, Sanchez AD, Udeshi ND, Svinkina T, Carr SA, Feldman JL, Perrimon N, Ting AY. Efficient proximity labeling in living cells and organisms with TurboID. Nat Biotechnol. 2018;36(9):880–887. doi: 10.1038/nbt.4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tull D, Naderer T, Spurck T, Mertens HDT, Heng J, McFadden GI, Gooley PR, McConville MJ. Membrane protein SMP-1 is required for normal flagellum function in Leishmania. J Cell Sci. 2010;123(Pt 4):544–554. doi: 10.1242/jcs.059097 [DOI] [PubMed] [Google Scholar]
- 42.Dean S, Sunter JD, Wheeler RJ. TrypTag.org: A Trypanosome Genome-wide Protein Localisation Resource. Trends Parasitol. 2017;33(2):80–82. doi: 10.1016/j.pt.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calvo‐Álvarez E, Bonnefoy S, Salles A, Benson FE, McKean PG, Bastin P, Rotureau B. Redistribution of FLAgellar Member 8 during the trypanosome life cycle: Consequences for cell fate prediction. Cell Microbiol. 2021;23(9):e13347. doi: 10.1111/cmi.13347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tetaud E, Lefebvre M, M’Bang-Benet DE, Crobu L, Blancard C, Sterkers Y, Pages M, Bastien P, Merlin G. TbFlabarin, a flagellar protein of Trypanosoma brucei, highlights differences between Leishmania and Trypanosoma flagellar-targeting signals. Exp Parasitol. 2016;166:97–107. doi: 10.1016/j.exppara.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 45.Kollien A, Schaub G. The Development of Trypanosoma cruzi in Triatominae. Parasitol Today. 2000;16(9):381–387. doi: 10.1016/S0169-4758(00)01724-5 [DOI] [PubMed] [Google Scholar]
- 46.Cámara M de los M, Balouz V, Centeno Cameán C, Cori CR, Kashiwagi GA, Gil SA, Macchiaverna NP, Cardinal MV, Guaimas F, Lobo MM, de Lederkremer RM, Gallo-Rodriguez C, Buscaglia CA. Trypanosoma cruzi surface mucins are involved in the attachment to the Triatoma infestans rectal ampoule. PLoS Negl Trop Dis. 2019;13(5):e0007418. doi: 10.1371/journal.pntd.0007418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varga V, Moreira-Leite F, Portman N, Gull K. Protein diversity in discrete structures at the distal tip of the trypanosome flagellum. Proc Natl Acad Sci U S A. 2017;114(32):E6546–E6555. doi: 10.1073/pnas.1703553114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rawlings ND, Barrett AJ. Families of cysteine peptidases. In: Methods in Enzymology. Vol 244. Proteolytic Enzymes: Serine and Cysteine Peptidases. Academic Press; 1994:461–486. doi: 10.1016/0076-6879(94)44034-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu W, Apagyi K, McLeavy L, Ersfeld K. Expression and cellular localisation of calpain-like proteins in Trypanosoma brucei. Mol Biochem Parasitol. 2010;169(1):20–26. doi: 10.1016/j.molbiopara.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 50.DaRocha WD, Silva RA, Bartholomeu DC, Pires SF, Freitas JM, Macedo AM, Vazquez MP, Levin MJ, Teixeira SMR. Expression of exogenous genes in Trypanosoma cruzi: improving vectors and electroporation protocols. Parasitol Res. 2004;92(2):113–120. doi: 10.1007/s00436-003-1004-5 [DOI] [PubMed] [Google Scholar]
- 51.Dumoulin PC, Vollrath J, Won MM, Wang JX, Burleigh BA. Endogenous Sterol Synthesis Is Dispensable for Trypanosoma cruzi Epimastigote Growth but Not Stress Tolerance. Front Microbiol. 2022;13:937910. doi: 10.3389/fmicb.2022.937910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schumann Burkard G, Jutzi P, Roditi I. Genome-wide RNAi screens in bloodstream form trypanosomes identify drug transporters. Mol Biochem Parasitol. 2011;175(1):91–94. doi: 10.1016/j.molbiopara.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 53.Lander N, Chiurillo MA, Vercesi AE, Docampo R. Endogenous C-terminal Tagging by CRISPR/Cas9 in Trypanosoma cruzi. Bio-Protoc. 2017;7(10). doi: 10.21769/BioProtoc.2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tarleton R, Peng D. EuPaGDT: a web tool tailored to design CRISPR guide RNAs for eukaryotic pathogens. Microb Genomics. 2015;1(4). doi: 10.1099/mgen.0.000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelly FD, Tran KD, Hatfield J, Schmidt K, Sanchez MA, Landfear SM. A Cytoskeletal Protein Complex is Essential for Division of Intracellular Amastigotes of Leishmania mexicana. bioRxiv. Published online April 30, 2020:2020.04.29.068445. doi: 10.1101/2020.04.29.068445 [DOI] [PMC free article] [PubMed]
- 56.Bangs JD, Uyetake L, Brickman MJ, Balber AE, Boothroyd JC. Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei. Divergent ER retention signals in a lower eukaryote. J Cell Sci. 1993;105 ( Pt 4):1101–1113. [DOI] [PubMed] [Google Scholar]
- 57.Rappsilber J, Ishihama Y, Mann M. Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal Chem. 2003;75(3):663–670. doi: 10.1021/ac026117i [DOI] [PubMed] [Google Scholar]
- 58.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26(12):1367–1372. doi: 10.1038/nbt.1511 [DOI] [PubMed] [Google Scholar]
- 59.Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M. Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res. 2011;10(4):1794–1805. doi: 10.1021/pr101065j [DOI] [PubMed] [Google Scholar]
- 60.Bruderer R, Bernhardt OM, Gandhi T, Reiter L. High-precision iRT prediction in the targeted analysis of data-independent acquisition and its impact on identification and quantitation. Proteomics. 2016;16(15–16):2246–2256. doi: 10.1002/pmic.201500488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruderer R, Bernhardt OM, Gandhi T, Miladinović SM, Cheng LY, Messner S, Ehrenberger T, Zanotelli V, Butscheid Y, Escher C, Vitek O, Rinner O, Reiter L. Extending the limits of quantitative proteome profiling with data-independent acquisition and application to acetaminophen-treated three-dimensional liver microtissues. Mol Cell Proteomics MCP. 2015;14(5):1400–1410. doi: 10.1074/mcp.M114.044305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pang Z, Zhou G, Ewald J, Chang L, Hacariz O, Basu N, Xia J. Using MetaboAnalyst 5.0 for LC-HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat Protoc. 2022;17(8):1735–1761. doi: 10.1038/s41596-022-00710-w [DOI] [PubMed] [Google Scholar]
- 63.Sherman BT, Hao M, Qiu J, Jiao X, Baseler MW, Lane HC, Imamichi T, Chang W. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. Published online March 23, 2022:gkac194. doi: 10.1093/nar/gkac194 [DOI] [PMC free article] [PubMed]
- 64.Maric D, Olson CL, Xu X, Ames JB, Engman DM. Calcium-dependent membrane association of a flagellar calcium sensor does not require calcium binding. Mol Biochem Parasitol. 2015;201(1):72–75. doi: 10.1016/j.molbiopara.2015.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Raw proteomics data for epimastigotes and intracellular amastigotes TurboID experiment. Each sheet contains the protein identity and intensity score for all samples in either epimastigotes or amastigotes.
Supplementary Table 2: Filtered proteomics data for epimastigotes and intracellular amastigotes TurboID experiment. Each sheet contains the protein identity and intensity score for all samples in either epimastigotes or amastigotes that was used for statistical analysis.
Supplementary Table 3: Proteins enriched in the flagellar TurboID expressing samples for epimastigotes and intracellular amastigotes. Each sheet contains information about the proteins enriched in either epimastigotes, amastigotes, or both epimastigotes and amastigotes.
Supplementary Table 4: Primers for all PCR and endogenous tagging. Primer pairs are listed for all experiments described.
Supplementary Figure 1. Model of the T. cruzi life cycle. Schematic of the T. cruzi life cycle highlighting the insect stage ‘epimastigote’ that is propagated axenically in liquid culture and gives rise to the infectious ‘metacyclic trypomastigote’. Trypomastigotes, whether derived from epimastigotes or as the end product of a single lytic cycle in a mammalian cell are motile, non-dividing forms of the parasite that actively invade a mammalian host cell. Inside a host cell, the ‘trypomastigote’ transforms into the replicative intracellular ‘amastigote’ stage by 18 hours post-infection (hpi). Amastigotes undergo several rounds of proliferation, dividing by binary fission (between ~24–90 hpi), before they stop dividing and differentiate into trypomastigotes, that eventually lyse the infected host cell and disseminate infection. Stable transfection and drug selection is performed in the epimastigote stage (lightning bolt symbolizes electroporation). Once stable genomic changes are confirmed in epimastigotes, these parasites are used to establish the mammalian infection cycle starting with metacyclic trypomastigotes as outlined above.
Supplementary Figure 2: PCR confirmation of endogenous tags for candidate proteins. A. Schematic showing the region of amplification and DNA gel with corresponding bands for A. calpain 1.3-smFLAG (TcCLB.506563.200), B. CARP3-smFLAG (TcCLB.506681.40), and C. hypothetical protein-FLAG (TcCLB.510329.180). Ladder run on all DNA gels is Thermo Scientific GeneRuler DNA Ladder.