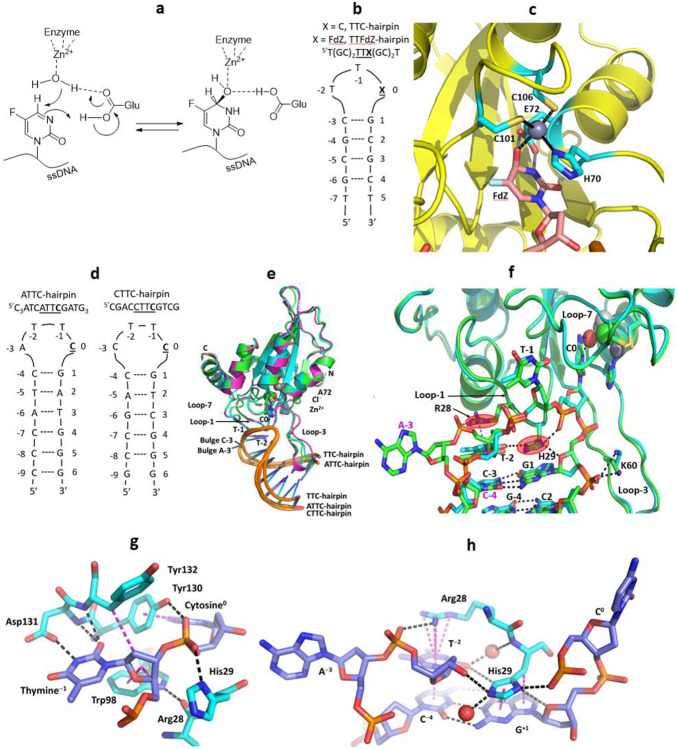
Fig. 1: Structures of substrates and inhibitor of A3A, and mechanism of inhibition.
a, Schematic of reaction of FdZ with water activated by Zn2+, analogous to that of cytidine/cytosine deaminases, showing critical role of general acid-base Glu72. b, TTFdZ- and TTC-hairpins. c, Structure of wildtype A3A with TTFdZ-hairpin inhibitor with FdZ hydrolyzed and coordinated to the active-site Zn2+. Glu72 and metal-binding ligands (His70, Cys106 and Cys106) are highlighted in cyan. d, ATTC- and CTTC-hairpin substrates of A3A with four-residue loops. e, Superposition of structures of A3A-E72A in complex with three-residue loop TTC-hairpin (cyan) and four-residue loop ATTC-hairpin (green) and CTTC-hairpin (magenta). f, Superposition of A3A-E72A in complex with TTC-hairpin (cyan) and four-residue loop ATTC-hairpin (green). The critical roles played by His29 and Arg28 in determining the conformation of the loop that makes these hairpins better substrates for wildtype A3A than corresponding linear ssDNA are highlighted. g, Interactions of Loop-7 and Loop-1 residues of A3A-E72A with the TC recognition motif. h, Interactions of Arg28 and His29 of Loop 1 of A3A-E72A with the ATTC-hairpin. Key hydrogen-bonding interactions are shown by black dashes. Cation-π interactions between Arg28 and T−2, π-π interactions between T−2 and G−4 and between His29 and G+1, and dispersion interactions are shown as magenta dashes. Zn2+ are shown as grey spheres and Cl− as green spheres. All structures share a similar crystal packing, whereby the base of the stem of one molecule stacks with that of the other molecule in the asymmetric unit (Extended Data Fig. S2).