Abstract
Down syndrome regression disorder (DSRD) is a clinical symptom cluster consisting of neuropsychiatric regression without an identifiable cause. This study evaluated the clinical effectiveness of IVIg and evaluated clinical characteristics associated with relapse after therapy discontinuation. A prospective, multi-center, non-randomized, observational study was performed. Patients met criteria for DSRD and were treated with IVIg. All patients underwent a standardized wean off therapy after 9–12 months of treatment. Baseline, on therapy, and relapse scores of the Neuropsychiatric Inventory Total Score (NPITS), Clinical Global Impression-Severity (CGI-S), and the Bush-Francis Catatonia Rating Scale (BFCRS) were used to track clinical symptoms. Eighty-two individuals were enrolled in this study. Patients had lower BFCRS (MD: −6.68; 95% CI: −8.23, −5.14), CGI-S (MD: −1.27; 95% CI: −1.73, −0.81), and NPITS scores (MD: −6.50; 95% CI: −7.53, −5.47) while they were on therapy compared to baseline. Approximately 46% of the patients (n = 38) experienced neurologic relapse with wean of IVIg. Patients with neurologic relapse were more likely to have any abnormal neurodiagnostic study (χ2 = 11.82, p = 0.001), abnormal MRI (χ2 = 7.78, p = 0.005), and abnormal LP (χ2 = 5.45, p = 0.02), and a personal history of autoimmunity (OR: 6.11, p < 0.001) compared to patients without relapse. IVIg was highly effective in the treatment of DSRD. Individuals with a history of personal autoimmunity or neurodiagnostic abnormalities were more likely to relapse following weaning of immunotherapy, indicating the potential for, a chronic autoimmune etiology in some cases of DSRD.
Keywords: down syndrome, IVIg, regression, neuropsychiatric, immunotherapy, neuroinflammation
Introduction
Down syndrome (DS) is the most common cause of intellectual disability worldwide and occurs in 1 in 800 live births in the United States1. Neurologic and psychiatric diseases in this population are well described, although the last decade has seen an increasing frequency of reports of the onset of subacute developmental regression of unclear etiology in individuals considered too young to develop Alzheimer’s disease and too old to develop autism spectrum disorder. This condition has been referred to as Down Syndrome Regression Disorder (DSRD) and has primarily been reported in young persons with DS between ages 10 and 30 years2,3. Symptoms include a subacute loss of previously acquired developmental skills in the areas of language, communication, cognition, executive function, behavioral, and adaptive skills2,4−7. Other symptoms can include psychiatric manifestations, bradykinesia, catatonia, and rapid onset insomnia4,5,7,8. DSRD can be severe and significantly impact both the quality of life and autonomy of persons with DS.
Therapeutic interventions for this condition are broad and have ranged from antipsychotics to immunotherapy4,9. A minority of individuals with DSRD may have a neuroinflammatory etiology to the disease, confirmed by the presence of abnormal neurodiagnostic studies and dramatic immunotherapy responsiveness in some patients4,9,10. While immunotherapy provides a tool to rapidly reverse this clinical syndrome, guidance on dosing and duration of therapy remains unclear.
This study sought to examine changes in clinical measures of functionality, gait, catatonia, and neuropsychiatric symptoms among individuals with DSRD receiving IVIg, investigate possible demographic, lab, and clinical factors linked to responsiveness to immunotherapy with IVIg, and assess the likelihood of successful treatment tapering once improvement of symptoms has been achieved.
Materials And Methods
IRB and Data Availability
IRB approval was obtained for this study with waived assent authorized in patients not capable of providing assent. Consent was obtained from caregivers (if < 18 years) or legal guardians (if > 18 years) when assent could not be obtained. Anonymized data is available to qualified researchers upon request.
Participants and Study Design
All individuals evaluated in the DS neurology clinic at multiple institutions were evaluated for participation in this study. Inclusion criteria included age between 8 and 26 years at the time of symptom onset, diagnosis of either possible or probable DSRD per expert consensus guidelines3, and completion of clinical neurodiagnostic studies (EEG, MRI, and Lumbar Puncture (LP)). Confirmation of diagnosis was performed by an arbiter with no knowledge of the case (MK). Exclusion criteria which included, age < 8 or > 26 years at the time of symptom onset, active cardiac or pulmonary disease, frequent infection (defined as more than two infections requiring antibiotics or antivirals per year), a history of neoplasia or receipt of chemotherapy, structural brain malformation on neuroimaging, active or a history of epilepsy (excluding febrile seizure), current use of electroconvulsive therapy and use of any immunotherapy not related to DSRD. Previously published cases of individuals receiving immunotherapy were also excluded4,9. Patients were permitted to be on psychotropic medications (e.g., selective serotonin reuptake inhibitor, antipsychotics, etc.) although once started on immunotherapy, dosing was locked with the exception of weaning if indicated. Individuals with co-morbid diagnoses of ASD were not excluded although they were all required meeting consensus criteria for DSRD.
Demographic data, medical history, and results of clinical and diagnostic investigations were collected through clinical documentation. Radiographic data was reviewed independently by a board-certified neuroradiologist. This study did not involve a control population of children with DS without DSRD as there are no other established indications for IVIg in DS.
Visit Schedule
Prior to enrollment in the study, all patients were clinically evaluated and diagnosed with DSRD as per published guidelines (baseline)3. Patients were evaluated clinically at + 0 days (baseline), + 90 days, and + 180 days (+/− 7 days) after the initiation of IVIg. In addition to these scheduled visits, patients had the option for more frequent urgent visits when necessary. Behavioral and neuropsychiatric testing assessments were performed at baseline and at + 180 days. During the titration period, patients were evaluated at standardized time points of + 35 days, + 77 days, and + 119 days at the time of subsequent infusions (+/− 7 days). Behavioral and neuropsychiatric assessments were performed at the time of clinical relapse (urgent evaluation) or + 119 days, whichever came first.
Behavioral/Neuropsychiatric Assessment
Given that DSRD has a wide variety of presenting symptoms, we employed several validated study tools in tandem to capture differences in disease severity. Research coordinators administered the Clinical Global Impression-Severity (CGI-S) scale at all clinical visits. At the baseline visit, the severity scale was performed and during follow up visits the improvement scale versions of this 7-point scale were administered. Physician evaluators also completed the Neuropsychiatric Inventory Questionnaire (NPI-Q) and the Bush-Francis Catatonia Rating Scale (BFCRS) at all clinical visits. These tests were utilized to capture global functional improvement (CGI), neuropsychiatric symptoms (NPI-Q), and catatonia (BFCRS). To assess global motor impairment, patients also had a timed 25-foot walk (25FTW) completed as part of their clinical evaluations when able to participate and follow directions. Higher scores for each of these metrics (CGI-S, NPI-Q, BFCRS, and 25FTW) was indicative of increased severity of symptoms.
Definitions of Abnormal Neurodiagnostics
Electroencephalogram (EEG)
Focal or generalized slowing, focal epileptiform discharges out of any cortex, or seizure were considered abnormal. Generalized discharges were considered abnormal although inconsistent with the diagnosis of DSRD. All patients had to have at least one prior EEG that did not demonstrate these results previously.
MRI
All MRIs had to be performed on a 3T scanner with and without contrast administration. Any abnormality beyond a structural malformation (e.g., Chiari malformation) was considered abnormal. Patients did not require a prior “normal” MRI.
Lumbar Puncture (LP):
Abnormalities were defined as having any of the following: WBC count > 5 cells/mm3, total protein > 60 mg/dL, presence of oligoclonal bands, an IgG index of > 0.66, and/or an elevated neopterin (> 33 nmol/mL). Samples with over 1,000 RBC were excluded from analysis. Patients did not require a prior “normal” LP
Therapeutic Interventions
A high concentration formulation of IVIg was utilized in all patients (10%, 100 mg/dL) at each dosing period. Patients were administered either Gammagard, Privagen, or Octagam formulations of IVIg depending on local infusion policies and regional restrictions on use. Once a patient was started on a particular formulation of IVIg, they had to continue on that same formulation unless an infusion reaction occurred. IVIg was dosed at 2 g/kg (administered over two days) for the induction dose followed by 1 g/kg (administered over one day) for maintenance dosing as per prior dosing regimens in pediatric inflammatory neurologic disease11,12. The timeframe between maintenance doses was 28 days +/−3 days and infusion protocols are presented in Appendix A. Steroids were not co-administered for any infusion unless as a treatment for medication reaction. Infusions could be administered at an outpatient infusion center or at home. In all situations, infusions were administered by a registered nurse.
Therapeutic Wean
All patients were weaned off IVIg using a standard protocol. After 9 months of IVIg therapy, the frequency of infusions was reduced from every four to every five weeks, then to every six weeks, then to every seven. Completed wean off therapy would take 18 total weeks. If there was no clinical return of symptoms (e.g., catatonia, mutism etc.), IVIg therapy was then discontinued; if there was recurrence of symptoms, the patient was placed back on an every four week infusion schedule. A relapse was defined as any sustained worsening (≥ 3 days) in any of the symptoms listed on the international DSRD criteria checklist and was determined by the evaluating clinician3.
Safety Assessments
Patients were asked standardized screening questions to report any adverse events (AE) on therapy at two time points (+ 90 days and + 180 days (+/− 7 days)) during the study period; they were also asked about intercurrent use of antibiotics or antivirals, urgent or emergent medical care evaluations, hospitalizations, or febrile illnesses. All potential AEs were reported.
During infusions, a nurse was available at bedside for rapid triage of reactions to IVIg administration. All infusion reactions were evaluated and escalated to the treating physician when appropriate. At the first sign of an infusion reaction, the infusion was paused, and only resumed after medical clearance by the supervising physician.
Statistical Analysis
The primary outcomes of interest were the 25FTW, BFCRS, CGI-S, NPI-Q Total Scores (NPITS) collected at multiple time points (i.e., baseline, on-therapy, and after-therapy). These scores were analyzed using mixed-effects regression models with an unstructured covariance model for the “within subject repeated measures” and “indicators for time post-baseline to capture change in outcome mean scores from baseline”. The models further allowed for fixed effects for demographics and disease biometrics and their interactions with time, with effects added individually.
Additional mixed-effects models with similar covariance structure were used including the fixed effects for those with relapse relative to those without, categorical indicators for time post-baseline, and the interaction between relapse and time. The models further allowed for interaction effects between demographics and disease biometrics and relapse and time.
Demographics, disease biometrics, and baseline clinical features were compared between patients with and without neurologic relapse using chi-square (χ2) or Fisher’s exact tests for categorical variables, and t-test for continuous variables. Univariate logistic regression was used to model the association between relapse and individual demographics, disease biometrics, and baseline clinical features. Factors which differed significantly by relapse were entered into a separate multivariable logistic regression model. All analyses were conducted using Stata/MP version-17.0 (StataCorp. 2021. College Station, TX: StataCorp LLC.).
Results
Demographics and Clinical Features
Ninety-three patients were identified for potential review, of which 82 (88%) met all inclusion criteria. The most common reasons for exclusions were age > 26 years at symptom onset (n = 8, 73%), prior receipt of immunotherapy unrelated to DSRD (n = 2, 18%), and history of epilepsy (n = 1, 9%). Demographics and clinical features of cases are reported in Table 1.
Table 1.
Demographics and clinical data (N = 82)
| No Relapse (n = 44; 53.7%) | Relapse (n = 38; 46.3%) | All (n = 82) | ||||
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Sex | ||||||
| Female | 26 | 59.1% | 18 | 47.4% | 44 | 53.7% |
| Male | 18 | 40.9% | 20 | 52.6% | 38 | 46.3% |
| Race | ||||||
| Caucasian | 34 | 77.3% | 31 | 81.6% | 65 | 79.3% |
| Black | 6 | 13.6% | 4 | 10.5% | 10 | 12.2% |
| Asian | 4 | 9.1% | 3 | 7.9% | 7 | 8.5% |
| Ethnicity | ||||||
| Non-Hispanic | 13 | 29.5% | 13 | 34.2% | 26 | 31.7% |
| Hispanic | 31 | 70.5% | 25 | 65.8% | 56 | 68.3% |
| Age at symptom onset | 14.1 | (3.5) | 15.6 | (4.6) | 14.8 | (4.1) |
| Age at diagnosis | 16.5 | (4.1) | 17.9 | (5.1) | 17.2 | (4.6) |
| Age at Therapy | 16.8 | (4.1) | 18.4 | (4.9) | 17.5 | (4.5) |
| Δ Age at Symptom Onset and Age at Diagnosis | 2.4 | (1.9) | 2.3 | (1.8) | 2.4 | (1.8) |
| Δ Age at Therapy and Age at Diagnosis* | 0.3 | (0.5) | 0.5 | (0.6) | 0.4 | (0.6) |
| Δ Age at Therapy and Age at Symptom Onset | 2.7 | (2.1) | 2.8 | (1.7) | 2.8 | (1.9) |
| Time (months) to Symptom Peak | 3.7 | (2.5) | 3.3 | (2.5) | 3.5 | (2.5) |
| Trigger Present | 20 | 45.5% | 20 | 52.6% | 40 | 48.8% |
| Type of Trigger | ||||||
| Infection | 9 | 20.5% | 7 | 18.4% | 16 | 19.5% |
| Change of School/Work/Home Environment | 6 | 13.6% | 5 | 13.2% | 11 | 13.4% |
| Loss of Family/Caregiver/Friend | 2 | 4.5% | 2 | 5.3% | 4 | 4.9% |
| Death | 1 | 2.3% | 2 | 5.3% | 3 | 3.7% |
| Change in Residence | 0 | 0.0% | 2 | 5.3% | 2 | 2.4% |
| Abuse | 1 | 2.3% | 1 | 2.6% | 2 | 2.4% |
| Medical Change | 1 | 2.3% | 1 | 2.6% | 2 | 2.4% |
| Disease Biometrics | ||||||
| Probable DSRD Criteria | 17 | 38.6% | 13 | 34.2% | 30 | 36.6% |
| History of Personal Autoimmune Disease | 18 | 45.0% | 15 | 46.9% | 33 | 45.8% |
| Serum Cytokines | 7 | 26.9% | 13 | 54.2% | 20 | 40.0% |
| Catatonia | 30 | 68.2% | 30 | 78.9% | 60 | 73.2% |
| EEG Abnormal* | 7 | 17.5% | 12 | 37.5% | 19 | 26.4% |
| MRI Abnormal** | 4 | 10.0% | 12 | 37.5% | 16 | 22.2% |
| Lumbar Puncture Abnormal** | 3 | 7.5% | 9 | 28.1% | 12 | 16.7% |
| Any Neurodiagnostic Study Abnormal** | 9 | 22.5% | 20 | 62.5% | 29 | 40.3% |
| Psychotropic Medications at Baseline | 35 | 79.5% | 30 | 78.9% | 65 | 79.3% |
| Benzodiazepines | 22 | 50% | 17 | 44.7% | 39 | 47.6% |
| SSRI/SNRIs | 17 | 38.6% | 10 | 26.3% | 27 | 32.9% |
| Antipsychotics | 8 | 18.2% | 7 | 18.4% | 17 | 20.7% |
| Anticonvulsants | 2 | 4.5% | 5 | 13.1% | 7 | 8.5% |
| Mood Stabilizers | 3 | 6.8% | 0 | 0% | 3 | 3.6% |
| Prior Immunotherapy | 3 | 6.8% | 1 | 2.6% | 4 | 4.9% |
| IVIg Brand | ||||||
| Gammaguard | 27 | 61.4% | 28 | 73.7% | 55 | 67.1% |
| Octagam | 11 | 25.0% | 7 | 18.4% | 18 | 22.0% |
| Privagen | 6 | 13.6% | 3 | 7.9% | 9 | 11.0% |
| IVIg Duration | 7.5 | (2.4) | 7.8 | (2.1) | 7.6 | (2.3) |
| Baseline Clinical Features | ||||||
| 25 Foot Walk** | 9.4 | (4.5) | 12.3 | (6.4) | 10.7 | (5.6) |
| Bush Francis Severity Score | 16.6 | (9.9) | 19.5 | (10.9) | 17.9 | (10.4) |
| CGI Severity of Illness** | 3.3 | (1.3) | 4.1 | (1.5) | 3.6 | (1.4) |
| NPI Total Score** | 18.7 | (5.7) | 21.8 | (5.9) | 20.1 | (6.0) |
| NPI Delusions | 3.8 | (1.9) | 3.6 | (1.6) | 3.7 | (1.8) |
| NPI Hallucinations* | 1.2 | (1.4) | 1.8 | (1.7) | 1.5 | (1.5) |
| NPI Agitation | 2.1 | (1.4) | 1.9 | (1.6) | 2.0 | (1.5) |
| NPI Anxiety | 0.8 | (0.9) | 0.6 | (0.7) | 0.7 | (0.8) |
| NPI Apathy* | 2.5 | (1.5) | 3.1 | (1.6) | 2.8 | (1.5) |
| NPI Irritability** | 1.7 | (1.2) | 2.5 | (1.5) | 2.1 | (1.4) |
| NPI Euphoria | 0.5 | (0.8) | 0.6 | (0.8) | 0.5 | (0.8) |
| NPI Disinhibition | 0.5 | (0.9) | 0.7 | (1.4) | 0.5 | (1.2) |
| NPI Aberrant Motor | 2.6 | (1.7) | 3.3 | (1.9) | 2.9 | (1.8) |
| NPI Night Time | 2.3 | (1.7) | 2.7 | (1.5) | 2.5 | (1.6) |
| NPI Appetite/Eating | 0.7 | (1.0) | 1.0 | (1.5) | 0.9 | (1.2) |
p < 0.1 (italic font);
p < 0.05 (bold font).
Data are mean (SD) or frequency and percentage %. The frequency and percentages of incomplete variables are as follows: History of Personal Autoimmune Disease (n = 10; 12.2%), Serum Cytokines (n = 32; 39%), EEG Abnormal (n = 10; 12.2%), MRI Abnormal (n = 10; 12.2%), Lumbar Puncture Abnormal (n = 10; 12.2%), and Any Neurodiagnostic Study Abnormal (n = 10; 12.2%). Δ = difference between. Multiple responses allotted for types of trigger and psychotropic medications at baseline.
Therapeutics and Safety
Gammagard was the most common IVIg formulation administered (67%). In total, only two patients (2.4%) had AEs reported during the study period. One patient had an infusion reaction (rash) during the third infusion and one developed wheezing two hours into her fourth infusion. Infusions were temporarily paused but completed after therapeutic intervention. Both patients continued to receive infusions with no further AEs. No participant developed deep venous thrombosis or clotting, headache, aseptic meningitis, or other known side effects of IVIg. Nearly 20% (n = 16) of patient’s caregivers reported subjective improvements in skin conditions such as hidradenitis suppurativa, eczema, and psoriasis. This was not systematically asked by clinicians but was information volunteered by families in some circumstances.
Therapeutic Effects
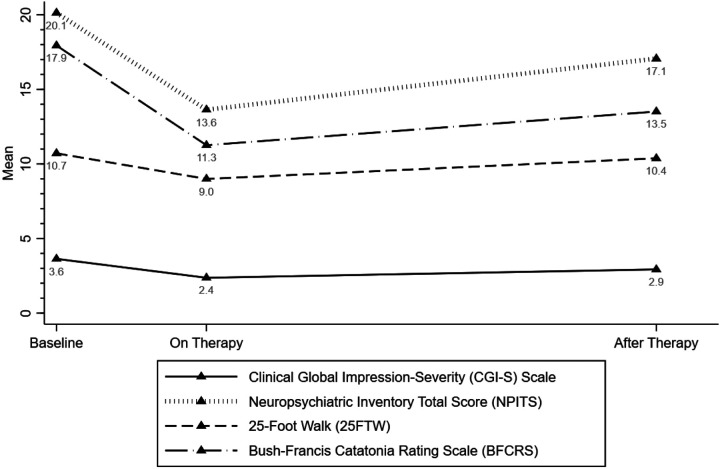
Changes in primary clinical outcome measures are displayed in Fig. 1 and Table 2. While on therapy, in comparison to baseline, patients had lower scores for 25FTW (mean difference (MD): −1.72; 95% confidence interval (CI): −2.42, −1.01), BFCRS (MD: −6.68; 95% CI: −8.23, −5.14), CGI-S (MD: −1.27; 95% CI: −1.73, −0.81), and NPITS (MD: −6.50; 95% CI: −7.53, −5.47). Furthermore, after therapy, lower mean scores were observed for BFCRS (MD: −4.43; 95% CI: −5.89, −2.97), CGI-S (MD: −0.71; 95% CI: −0.95, −0.47), and NPITS (MD: −3.07; 95% CI: −3.91, −2.23) but not 25FTW (MD: −0.34; 95% CI: −0.91, 0.24), compared to baseline.
Figure 1.
Clinical features including behavioral and neuropsychiatric assessments over the study period.
Table 2.
Estimated regression coefficient with 95% confidence interval [CI] from repeated measures analyses for all patients.
| Coef. | SE | 95% CI | p-value | |
|---|---|---|---|---|
| 25-Foot walk | ||||
| Prior to Therapy | 0.00 | 0.00 | ||
| On-Therapy** | −1.72 | 0.36 | [−2.42, −1.01] | 0.0000 |
| After-Therapy | −0.34 | 0.29 | [−0.91, 0.24] | 0.2509 |
| Bush-Francis Score | ||||
| Prior to Therapy | 0.00 | 0.00 | ||
| On-Therapy** | −6.68 | 0.79 | [−8.23, −5.14] | 0.0000 |
| After-Therapy** | −4.43 | 0.75 | [−5.89, −2.97] | 0.0000 |
| CGI-Severity Score | ||||
| Prior to Therapy | 0.00 | 0.00 | ||
| On-Therapy** | −1.27 | 0.23 | [−1.73, −0.81] | 0.0000 |
| After-Therapy** | −0.71 | 0.12 | [−0.95, −0.47] | 0.0000 |
| Total NPI Score | ||||
| Prior to Therapy | 0.00 | 0.00 | ||
| On-Therapy** | −6.50 | 0.53 | [−7.53, −5.47] | 0.0000 |
| After-Therapy** | −3.07 | 0.43 | [−3.91, −2.23] | 0.0000 |
p < 0.1 (italic font);
p < 0.05 (bold font).
The repeated measure analyses were carried out using longitudinal mixed-effects regression models with restricted maximum likelihood estimation and Kenward-Roger method for small-sample adjustment
Clinical Response Variables:
There was evidence that changes in clinical responses differed by disease-related factors such as the presence of catatonia and treatment with prior immunotherapy as well as neurodiagnostic study abnormalities. Clinical responses were more profound in individuals with catatonia, those who had received prior immunotherapy for the treatment of DSRD and those with any neurodiagnostic study abnormality (Table 3).
Table 3.
Estimated means with 95% confidence interval [CI] for clinical outcomes differed by potential disease biometrics
| On-Therapy | After-Therapy | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean change from baseline | SE | 95% CI | p-value | Mean change from baseline | SE | 95% CI | p-value | |
| 25-Foot walk | ||||||||
| With catatonia | −2.39 | 0.40 | [−3.17, −1.62] | 0.0000 | −0.75 | 0.33 | [−1.40, −0.09] | 0.0254 |
| Without catatonia | 0.13 | 0.65 | [−1.15, 1.41] | 0.8457 | 0.78 | 0.55 | [−0.30, 1.86] | 0.1580 |
| With any neurodiagnostic abnormalities | −3.14 | 0.60 | [−4.32, −1.96] | 0.0000 | 0.31 | 0.551 | [−0.68, 1.30] | 0.5397 |
| Without any neurodiagnostic abnormalities | −1.00 | 0.50 | [−1.97, −0.02] | 0.0447 | −0.86 | 0.42 | [−1.68, −0.05] | 0.0379 |
| Prior immunotherapy | −4.05 | 1.61 | [−7.21, −0.89] | 0.0120 | −4.07 | 1.26 | [−6.55, −1.60] | 0.0013 |
| No prior immunotherapy | −1.60 | 0.37 | [−2.31, −0.88] | 0.0000 | −0.14 | 0.29 | [−0.71, 0.42] | 0.6130 |
| Bush-Francis Score | ||||||||
| With catatonia | −8.45 | 0.84 | [−10.10, −6.80] | 0.0000 | −5.45 | 0.85 | [−7.11, −3.79] | 0.0000 |
| Without catatonia | −1.86 | 1.39 | [−4.59, 0.87] | 0.1811 | −1.64 | 1.40 | [−4.38, 1.11] | 0.2432 |
| With any neurodiagnostic abnormalities | −9.86 | 1.30 | [−12.41, −7.32] | 0.0000 | −3.45 | 1.29 | [−5.98, −0.92] | 0.0075 |
| Without any neurodiagnostic abnormalities | −5.28 | 1.07 | [−7.37, −3.19] | 0.0000 | −5.56 | 1.06 | [−7.63, −3.48] | 0.0000 |
| EEG abnormal | −10.42 | 1.62 | [−13.60, −7.24] | 0.0000 | −6.00 | 1.60 | [−9.14, −2.86] | 0.0002 |
| EEG normal | −5.94 | 0.97 | [−7.85, −4.04] | 0.0000 | −4.25 | 0.96 | [−6.12, −2.37] | 0.0000 |
| MRI abnormal | −8.44 | 1.83 | [−12.02, −4.85] | 0.0000 | −1.56 | 1.70 | [−4.90, 1.78] | 0.3590 |
| MRI normal | −6.75 | 0.98 | [−8.67, −4.83] | 0.0000 | −5.61 | 0.91 | [−7.39, −3.82] | 0.0000 |
| LP abnormal | −9.42 | 1.41 | [−12.17, −6.66] | 0.0000 | −1.67 | 1.15 | [−3.92, 0.59] | 0.1470 |
| LP normal | −6.25 | 0.63 | [−7.48, −5.02] | 0.0000 | −3.55 | 0.51 | [−4.56, −2.54] | 0.0000 |
| Prior immunotherapy | −9.00 | 3.58 | [−16.01, −1.99] | 0.0119 | −11.25 | 3.31 | [−17.73, −4.77] | 0.0007 |
| No prior immunotherapy | −6.56 | 0.81 | [−8.15, −4.98] | 0.0000 | −4.08 | 0.75 | [−5.54, −2.61] | 0.0000 |
| CGI-Severity Score | ||||||||
| With catatonia | −1.58 | 0.27 | [−2.10, −1.06] | 0.0000 | −0.63 | 0.14 | [−0.92, −0.35] | 0.0000 |
| Without catatonia | −0.41 | 0.44 | [−1.27, 0.45] | 0.3519 | −0.91 | 0.24 | [−1.38, −0.44] | 0.0001 |
| With any neurodiagnostic abnormalities | −2.21 | 0.38 | [−2.96, −1.46] | 0.0000 | −0.45 | 0.21 | [−0.86, −0.04] | 0.0324 |
| Without any neurodiagnostic abnormalities | −0.81 | 0.31 | [−1.43, −0.20] | 0.0096 | −1.00 | 0.17 | [−1.34, −0.66] | 0.0000 |
| MRI abnormal | −2.31 | 0.53 | [−3.35, −1.28] | 0.0000 | −0.19 | 0.28 | [−0.73, 0.36] | 0.5018 |
| MRI normal | −1.11 | 0.28 | [−1.66, −0.55] | 0.0001 | −0.95 | 0.15 | [−1.24, −0.65] | 0.0000 |
| LP abnormal | −2.58 | 0.61 | [−3.77, −1.39] | 0.0000 | −0.33 | 0.33 | [−0.98, 0.31] | 0.3128 |
| LP normal | −1.13 | 0.27 | [−1.67, −0.60] | 0.0000 | −0.87 | 0.15 | [−1.16, −0.58] | 0.0000 |
| Total NPI Score | ||||||||
| With catatonia | −7.40 | 0.59 | [−8.55, −6.25] | 0.0000 | −3.22 | 0.50 | [−4.20, −2.23] | 0.0000 |
| Without catatonia | −4.05 | 0.97 | [−5.95, −2.14] | 0.0000 | −2.68 | 0.83 | [−4.31, −1.05] | 0.0012 |
| With any neurodiagnostic abnormalities | −9.55 | 0.83 | [−11.17, −7.93] | 0.0000 | −2.03 | 0.73 | [−3.46, −0.61] | 0.0052 |
| Without any neurodiagnostic abnormalities | −4.91 | 0.68 | [−6.24, −3.58] | 0.0000 | −4.05 | 0.60 | [−5.22, −2.88] | 0.0000 |
| EEG abnormal | −10.00 | 1.06 | [−12.08, −7.92] | 0.0000 | −2.11 | 0.91 | [−3.90, −0.31] | 0.0213 |
| EEG normal | −5.62 | 0.63 | [−6.87, −4.38] | 0.0000 | −3.64 | 0.55 | [−4.71, −2.57] | 0.0000 |
| MRI abnormal | −10.13 | 1.17 | [−12.42, −7.83] | 0.0000 | −1.81 | 0.99 | [−3.76, 0.13] | 0.0678 |
| MRI normal | −5.82 | 0.62 | [−7.05, −4.60] | 0.0000 | −3.64 | 0.53 | [−4.68, −2.60] | 0.0000 |
| LP abnormal | −9.42 | 1.41 | [−12.17, −6.66] | 0.0000 | −1.67 | 1.15 | [−3.92, 0.59] | 0.1470 |
| LP normal | −6.25 | 0.63 | [−7.48, −5.02] | 0.0000 | −3.55 | 0.51 | [−4.56, −2.54] | 0.0000 |
p < 0.1 (italic font); p < 0.05 (bold font).
In total, there were 12 individuals who did not respond to therapy. Amongst non-responders, 11/12 (93%) had no neurodiagnostic study abnormalities, 11/12 (93%) had no altered mental status, 11/12 (93%) had no developmental regression (7%), and 10/12 (23%) had no catatonia. Additionally, 8/12 (67%) had neither altered mental status, developmental regression or catatonia.
Neurodiagnostic Abnormalities and Disease Severity
Patients with any neurodiagnostic abnormalities had higher means at baseline for 25FTW (MD: 3.50; 95% CI: 0.84, 6.15) and Total NPI (MD: 3.16; 95% CI: 0.42, 5.90) compared to those without any abnormalities. Lower mean of CGI-S (MD: −0.81; 95% CI: −1.26, −0.37) was observed for patients with any neurodiagnostic abnormalities while on-therapy compared to baseline. Moreover, patients with any neurodiagnostic abnormalities had higher mean scores of 25FTW (MD: 4.67; 95% CI: 2.70, 7.07), BFCRS (MD: 6.69; 95% CI:2.40, 10.98), CGI-S (MD:1.13; 95% CI: 0.43, 1.84), and Total NPI (MD: 5.17; 95% CI: 2.51, 7.83) after-therapy compared to those without any abnormalities. A borderline difference was observed between patients with and without neurodiagnostic abnormalities while on-therapy (MD: 1.35; 95% CI: −0.002, 2.71).
With regards to specific neurodiagnostics, patients with EEG abnormality had lower mean for BFCRS and NPITS while on-therapy and after-therapy relative to baseline. Similar pattern was observed for patients without EEG abnormality while on-therapy and after-therapy compared to baseline. Patients with abnormal EEG had lower mean NPITS while on-therapy (MD: −3.54; 95% CI: −6.04, −1.05) compared to those without EEG abnormality. Patients with abnormal neuroimaging had lower means for BFCRS, CGI-S, and NPITS while on-therapy compared to baseline. Similarly, patients without neuroimaging abnormalities had lower BFCRS, CGI-S, and NPITS while on-therapy and also after-therapy compared to baseline. While patients with abnormal neuroimaging had lower mean for CGI-S while on-therapy (MD: −0.69; 95% CI: −1.24, −0.14) compared to those with normal neuroimaging, they had higher means of CGI-S (MD: 1.28; 95% CI: 0.44, 2.12) and NPITS (MD: 5.00; 95% CI: 1.76, 8.24) after-therapy compared to patients with normal neuroimaging.
Patients with LP abnormalities had lower means of BFCRS, CGI-S, and NPITS while-on therapy compared to baseline. Those with a normal LP had lower mean levels of BFCRS, CGI-S, and NPITS while on-therapy and after-therapy relative to baseline. Patients with abnormal LP had higher mean levels at baseline for CGI-S (MD: 0.93; 95% CI: 0.04, 1.83) and NPITS (MD: 3.88; 95% CI: 0.26, 7.51) compared with patients without such abnormality. Moreover, higher mean levels for CGI-S (MD: 1.47; 95% CI: 0.53, 2.40) and NPITS (MD: 5.77; 95% CI: 2.17, 9.36) were observed for patients with LP abnormality after-therapy compared to those without abnormality.
Change in Clinical Features Incorporating Neurologic Relapse
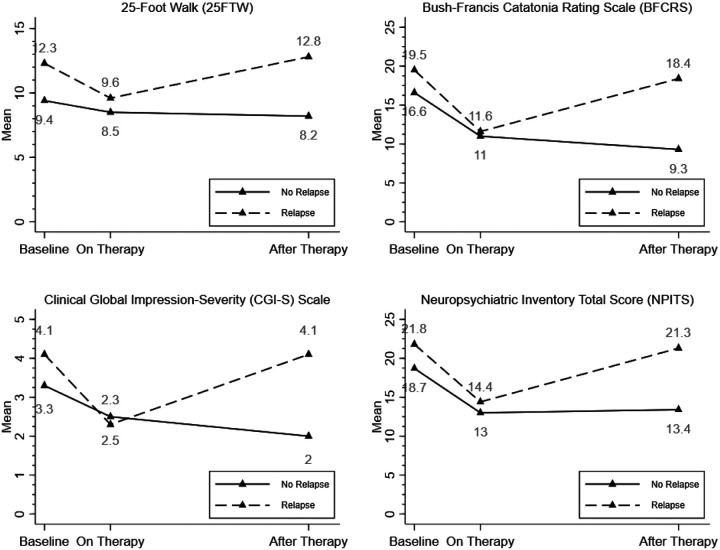
Therapeutic response across all clinical measures was sustained in individuals who did not relapse although those that did relapse had scores return to baseline levels (Fig. 2). We observed a significant reduction in mean scores of all clinical outcomes while on-therapy compared to baseline for patients with relapse (Appendix B). For patients without relapse, there was also evidence of reduction in means of all the outcomes while on-therapy compared to baseline, except for 25FTW. Additionally, significant reduction in scores were observed among these patients without relapse when comparing after-therapy with baseline. Patients with relapse had higher baseline means for all the clinical outcomes, except for BFCRS and higher mean scores for all the clinical outcomes after-therapy compared to patients without relapse (Appendix C)
Figure 2.
Clinical features including behavioral and neuropsychiatric assessments over the study period for patients with and without neurologic relapse.
Clinical Features and Relapse
There was evidence that the association between clinical responses and sex, catatonia, any neurodiagnostic abnormalities, and treatment with prior immunotherapy differed by neurologic relapse. Of note, individuals with any neurodiagnostic abnormality who did relapse had lower mean scores on the 25FTW while on-therapy and higher mean after-therapy relative to baseline. Sub analysis of the impact of non-IVIg medications (e.g., anti-depressants) on relapse was not possible due to highly heterogenous treatments, yielding only 12 patients with identical regimens.
Risk of Relapse and Neurodiagnostic Abnormalities
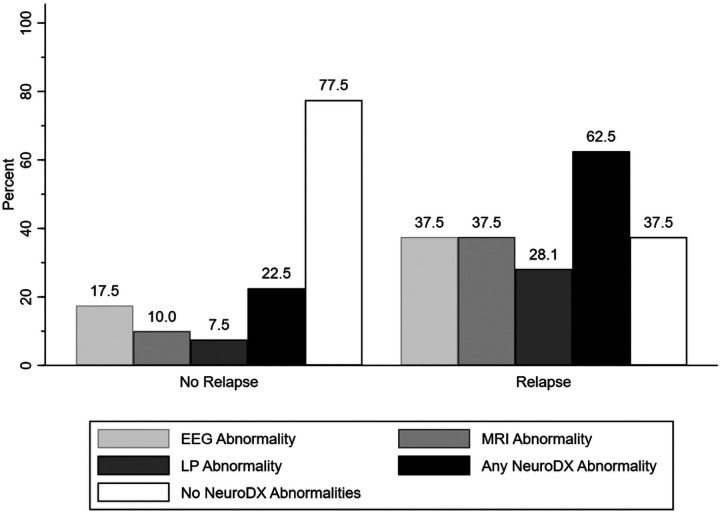
Approximately 46% of the patients (n = 38) experienced a relapse of symptoms. Patients who relapsed were more likely to have any abnormal neurodiagnostic study (χ2 = 11.82; p = 0.001), abnormal MRI (χ2 = 7.78; p = 0.005), and abnormal LP (χ2 = 5.45; p = 0.02) compared to patients without relapse (Fig. 3). Individuals with a history of personal autoimmunity were six times more likely to relapse than those without (OR: 6.11, p < 0.001, 95%CI: 2.69–12.13). Additionally, patients with relapse had higher baseline 25FTW (p = 0.0222), CGI Severity of illness Score (p = 0.0152), NPI Irritability Score (p = 0.005), and NPITS (p = 0.0171) than those without relapse (Table 1).
Figure 3.
Neurodiagnostic abnormality presence in patients with (n = 44) and without (n = 38) neurologic relapse.
Predictors of Relapse
Unadjusted analysis
There was no evidence of significant association between individual demographic characteristics and neurologic relapse after therapy, except a borderline association with the difference between age at therapy and age at diagnosis (OR: 2.20, 95% CI: 0.98, 4.93). Higher odds of relapse were associated with MRI abnormality (OR: 5.40, 95% CI: 1.54, 18.97), LP abnormality (OR: 4.83, 95% CI: 1.18, 19.70), and any neurodiagnostic abnormality (OR: 5.74, 95% CI: 2.05, 16.10).
Relapse was associated with a number of baseline clinical features, where the odds of relapse increased by one unit for each increase in 25FTW (OR: 1.11, 95% CI: 1.01, 1.21), CGI-S (OR: 1.49, 95% CI: 1.07, 2.07), NPI irritability (OR: 1.65, 95% CI: 1.14, 2.39), and NPITS (OR: 1.10, 95% CI: 1.01, 1.19). In addition, borderline associations were observed between relapse and baseline NPI Hallucinations (OR: 1.31, 95% CI: 0.97, 1.77) and NPI Apathy (OR: 1.32, 95% CI: 0.98, 1.77) scores.
Adjusted analysis
The disease biometrics and baseline clinical features that differed significantly by relapse were included in a model adjusting for sex, ethnicity, and difference between age at therapy and age at diagnosis. Analysis revealed a greater risk of relapse after therapy was associated with any neurodiagnostic abnormality (aOR: 4.34, 95% CI: 1.39, 13.53). No evidence of significant association was observed between relapse and other baseline covariates was present.
Discussion
Individuals with DSRD were responsive to immunotherapy on a variety of clinical measures, consistent with previously published data4,9,10. Patients demonstrated improvements in functional status (CGI), gait (25FTW), catatonia (BFCRS and 25FTW), and neuropsychiatric symptoms (NPITS). Further, treatment of these patients for a period of 9–12 months yielded sustained improvement in 47% of individuals after IVIg immunotherapy was weaned off. Those with a history of personal autoimmunity or baseline neurodiagnostic abnormalities were more likely to experience a clinical relapse upon wean of immunotherapy. These findings advance our understanding of the DSRD phenotype having immunologic origins in a subset of individuals.
Beneficial clinical responses to immunotherapy (IVIg) were high in this cohort at roughly 85% (70/82), consistent with prior studies4,9,10. These improvements were most notable in individuals with catatonia or neurodiagnostic study abnormalities, which is consistent with previously published data4. Non-responders to therapy were less likely to have neurodiagnostic study abnormalities (7%), altered mental status (7%), developmental regression (7%), and catatonia (17%). Although these findings were observed in a limited cohort (n = 12), it does indicate that some clinical features could be more predictive of non-immunotherapy responsive disease. Conversely, this study also expands on the concept that even in patients without definitive neurodiagnostic abnormalities, there may be a role for immune-based interventions as well, highlighting need to prioritize identification of sensitive and specific biomarkers in DSRD.
Duration of immunotherapy has remained an important question in individuals with DSRD since the first cohorts of immunotherapy-responsive patients were published.10 This study used a slow therapeutic wean, consistent with previously published literature, in order to avoid rebound after abrupt discontinuation13,14. Ultimately, the 53% of individuals that did relapse upon wean of IVIg may represent a cohort of individuals where the etiology of their DSRD-related symptoms is potentially inflammatory in nature. Etiologies to DSRD remain poorly elucidated although emerging evidence for a neuroinflammatory component to the disease in a minority of individuals has gained traction3,9. Thus, the data presented in this report not only serves to highlight the therapeutic effect of IVIg in DSRD, but also provides proof of concept that the immunomodulatory effects of this therapeutic may be treating a potential, albeit unknown, inflammatory target. This is supported the observed 4.34 times greater risk of relapse in individuals with neurodiagnostic study abnormalities (including early/accelerated mineralization and CSF abnormalities) which are indicative of potentially interferon driven immune dysregulation, a concept more well established in systemic disease presentations in persons with DS15–18. It has been established that individuals with DS are at great risk for a variety of autoimmune disorders19–25, and thus it would be reasonable to consider the brain as another potential target of immune dysregulation.
This study is not without limitations. Selection and severity bias is present in the exclusion of patients who did not undergo neurodiagnostic work up. Investigators involved in this study were early adopters of immunotherapy in persons with DSRD and thus both a referral and selection bias in favor of use of IVIg may be present. Severity bias could also decrease the likelihood of response to any therapy, but the authors do contemplate if more severe cases are associated with inflammatory etiologies which is still undetermined. This same severity bias could have explained the responsiveness to IVIg and the high rates of neurologic relapse in this data set. Sub analysis of non-immunotherapy was not possible in this study due to the low numbers of patients on the same regimen. Clinical treatment heterogeneity, utilization of weight-based dosing and lack of algorithmic therapeutic interventions in DSRD are major limiting factors in this disease and yielded only 12 patients who were on exactly the same treatments at the time of immunotherapy initiation. In addition, patients were on a variety of different psychotropic medications at baseline and while these were not changed during this study, add an additional layer of complexity to interpreting and generalizing the results. With regards to metrics, the use of CGI is subjective measure prone to recall bias, although this was mitigated by the use of two objective, physician-based metrics. This study did not assess family response to treatments (e.g., functional improvements in homelife for family) although the authors acknowledge this would be an important variable to assess in future study. Importantly, this study was not randomized nor controlled which should temper interpretation. Standardized immunotherapy regimens and wean schedules mitigated some of this although further randomized, controlled, trials are desperately needed in this space. Different formulations of IVIg had to be used although no significant differences between formulations was identified. Additionally in our demographic and clinic response variable analysis, we observed statistical (and lack thereof) differences in study tools; this may reflect differences in disease severity between those comparison groups or more likely reflect the study’s low power. Finally, the authors note that given the rare nature of DSRD, low study populations limit the generalizability of these findings and the authors caution clinicians to evaluate each case individually.
In summary, amongst a cohort of individuals with DSRD, immunotherapy was effective in the treatment of the clinical symptom cluster. Individuals with neurodiagnostic abnormalities of any type were significantly less likely to be able to wean off of immunotherapy, indicating the potential for, a chronic immune etiology in some cases of DSRD. These results must be tempered by multiple study limitations although provide a basis for further investigations into randomized controlled, double-blinded, biomarker and therapeutic trials in this emerging disease.
Acknowledgements:
This work was supported by the Biostatistics Core at The Saban Research Institute, Children’s Hospital Los Angeles and by grants UL1TR001855 and UL1TR000130 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health.
Funding and Support:
The authors report no funding for this study, therapeutics were obtained and administered on a clinical basis.
Conflicts of Interest:
Dr. Santoro reports receiving federal funding from the NHLBI and NICHD for the study of autoimmune disease in persons with Down syndrome and the role of immunotherapy in persons with Down syndrome regression disorder. Drs. Patel and Kammery also receive federal funding from the NICHD for the study of autoimmune disease in persons with Down syndrome and the role of immunotherapy in persons with Down syndrome regression disorder.
Abbreviations
- 25FTW
Timed 25-foot walk
- 95% CI
95% Confidence Interval
- BFCRS
Bush-Francis Catatonia Rating Scale
- CGI-S
Clinical Global Impression-Severity Score
- CSF
Cerebrospinal Fluid
- DS
Down Syndrome
- DSRD
Down Syndrome Regression Disorder
- EEG
Electroencephalogram
- IVIg
Intravenous Immunoglobulin
- LP
Lumbar puncture
- MD
Mean Difference
- MRI
Magnetic Resonance Imaging
- NPITS
Neuropsychiatric Inventory Total Score
Footnotes
Supplementary Files
Contributor Information
Jonathan Santoro, Children’s Hospital Los Angeles.
Noemi Spinazzi, Kennedy-Krieger Institute and Johns Hopkins University.
Robyn Filipink, Kennedy-Krieger Institute and Johns Hopkins University.
Panteha Hayati-Rezvan, Kennedy-Krieger Institute and Johns Hopkins University.
Ryan Kammeyer, Kennedy-Krieger Institute and Johns Hopkins University.
Lina Patel, Kennedy-Krieger Institute and Johns Hopkins University.
Elise Sannar, Kennedy-Krieger Institute and Johns Hopkins University.
Luke Dwyer, Kennedy-Krieger Institute and Johns Hopkins University.
Abhik Banerjee, Kennedy-Krieger Institute and Johns Hopkins University.
Mellad Khoshnood, Kennedy-Krieger Institute and Johns Hopkins University.
Sabaj Jafarpour, Kennedy-Krieger Institute and Johns Hopkins University.
Natalie Boyd, Kennedy-Krieger Institute and Johns Hopkins University.
Rebecca Partridge, Kennedy-Krieger Institute and Johns Hopkins University.
Grace Gombolay, Kennedy-Krieger Institute and Johns Hopkins University.
Alison Christy, Kennedy-Krieger Institute and Johns Hopkins University.
Diego Real de Asua, Kennedy-Krieger Institute and Johns Hopkins University.
Maria del Carmen Ortega, Kennedy-Krieger Institute and Johns Hopkins University.
Melanie Manning, Kennedy-Krieger Institute and Johns Hopkins University.
Heather Van Mater, Kennedy-Krieger Institute and Johns Hopkins University.
Gordon Worley, Kennedy-Krieger Institute and Johns Hopkins University.
Cathy Franklin, Kennedy-Krieger Institute and Johns Hopkins University.
Maria Stanley, Kennedy-Krieger Institute and Johns Hopkins University.
Ruth Brown, Kennedy-Krieger Institute and Johns Hopkins University.
George Capone, Kennedy-Krieger Institute and Johns Hopkins University.
References
- 1.de Graaf G, Buckley F, Skotko BG. Estimates of the live births, natural losses, and elective terminations with Down syndrome in the United States. Am J Med Genet A. 2015;167a(4):756–767. [DOI] [PubMed] [Google Scholar]
- 2.Rosso M, Fremion E, Santoro SL, et al. Down Syndrome Disintegrative Disorder: A Clinical Regression Syndrome of Increasing Importance. Pediatrics. 2020;145(6). [DOI] [PubMed] [Google Scholar]
- 3.Santoro JD, Patel L, Kammeyer R, et al. Assessment and Diagnosis of Down Syndrome Regression Disorder: International Expert Consensus. Front Neurol. 2022;13:940175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santoro JD, Partridge R, Tanna R, et al. Evidence of neuroinflammation and immunotherapy responsiveness in individuals with down syndrome regression disorder. J Neurodev Disord. 2022;14(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santoro SL, Cannon S, Capone G, et al. Unexplained regression in Down syndrome: 35 cases from an international Down syndrome database. Genet Med. 2020;22(4):767–776. [DOI] [PubMed] [Google Scholar]
- 6.Walpert M, Zaman S, Holland A. A Systematic Review of Unexplained Early Regression in Adolescents and Adults with Down Syndrome. Brain Sci. 2021;11(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Worley G, Crissman BG, Cadogan E, Milleson C, Adkins DW, Kishnani PS. Down Syndrome Disintegrative Disorder: New-Onset Autistic Regression, Dementia, and Insomnia in Older Children and Adolescents With Down Syndrome. J Child Neurol. 2015;30(9):1147–1152. [DOI] [PubMed] [Google Scholar]
- 8.Mircher C, Cieuta-Walti C, Marey I, et al. Acute Regression in Young People with Down Syndrome. Brain Sci. 2017;7(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santoro SL, Baumer NT, Cornacchia M, et al. Unexplained regression in Down syndrome: Management of 51 patients in an international patient database. Am J Med Genet A. 2022. [DOI] [PubMed] [Google Scholar]
- 10.Cardinale KM, Bocharnikov A, Hart SJ, et al. Immunotherapy in selected patients with Down syndrome disintegrative disorder. Dev Med Child Neurol. 2019;61(7):847–851. [DOI] [PubMed] [Google Scholar]
- 11.Iro MA, Sadarangani M, Absoud M, et al. ImmunoglobuliN in the Treatment of Encephalitis (IgNiTE): protocol for a multicentre randomised controlled trial. BMJ Open. 2016;6(11):e012356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iro MA, Martin NG, Absoud M, Pollard AJ. Intravenous immunoglobulin for the treatment of childhood encephalitis. Cochrane Database Syst Rev. 2017;10(10):Cd011367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.N’Kaoua E, Attarian S, Delmont E, et al. Immunoglobulin shortage: Practice modifications and clinical outcomes in a reference centre. Rev Neurol (Paris). 2022;178(6):616–623. [DOI] [PubMed] [Google Scholar]
- 14.Adrichem ME, Lucke IM, Vrancken A, et al. Withdrawal of intravenous immunoglobulin in chronic inflammatory demyelinating polyradiculoneuropathy. Brain. 2022;145(5):1641–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flores-Aguilar L, Iulita MF, Kovecses O, et al. Evolution of neuroinflammation across the lifespan of individuals with Down syndrome. Brain. 2020;143(12):3653–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verstegen RHJ, Kusters MAA. Inborn Errors of Adaptive Immunity in Down Syndrome. J Clin Immunol. 2020;40(6):791–806. [DOI] [PubMed] [Google Scholar]
- 17.Waugh KA, Araya P, Pandey A, et al. Mass Cytometry Reveals Global Immune Remodeling with Multi-lineage Hypersensitivity to Type I Interferon in Down Syndrome. Cell Rep. 2019;29(7):1893–1908.e1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan KD, Evans D, Pandey A, et al. Trisomy 21 causes changes in the circulating proteome indicative of chronic autoinflammation. Sci Rep. 2017;7(1):14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santoro JD, Lee S, Wang AC, et al. Increased Autoimmunity in Individuals With Down Syndrome and Moyamoya Disease. Front Neurol. 2021;12:724969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aversa T, Valenzise M, Corrias A, et al. In children with autoimmune thyroid diseases the association with Down syndrome can modify the clustering of extra-thyroidal autoimmune disorders. J Pediatr Endocrinol Metab. 2016;29(9):1041–1046. [DOI] [PubMed] [Google Scholar]
- 21.Aversa T, Valenzise M, Salerno M, et al. Metamorphic thyroid autoimmunity in Down Syndrome: from Hashimoto’s thyroiditis to Graves’ disease and beyond. Ital J Pediatr. 2015;41:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostermaier KK, Weaver AL, Myers SM, Stoeckel RE, Katusic SK, Voigt RG. Incidence of Celiac Disease in Down Syndrome: A Longitudinal, Population-Based Birth Cohort Study. Clin Pediatr (Phila). 2020;59(12):1086–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludvigsson JF, Lebwohl B, Green PH, Chung WK, Mårild K. Celiac disease and Down syndrome mortality: a nationwide cohort study. BMC Pediatr. 2017;17(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rohrer TR, Hennes P, Thon A, et al. Down’s syndrome in diabetic patients aged < 20 years: an analysis of metabolic status, glycaemic control and autoimmunity in comparison with type 1 diabetes. Diabetologia. 2010;53(6):1070–1075. [DOI] [PubMed] [Google Scholar]
- 25.Juj H, Emery H. The arthropathy of Down syndrome: an underdiagnosed and under-recognized condition. J Pediatr. 2009;154(2):234–238. [DOI] [PubMed] [Google Scholar]