Abstract
We present a case of a 60‐year‐old female with a history of liver cirrhosis, alcohol abuse, and chronic venous insufficiency who presented with maggot‐infested wounds on her legs, bilateral buttocks, and groin area. Two sets of blood cultures grew Wohlfahrtiimonas chitiniclastica. She underwent wound debridement and treatment with cefazolin.
Keywords: bacteremia, infectious diseases, maggots, myiasis
Since its recent discovery, the Gram‐negative bacillus Wohlfahrtiimonas chitiniclastica has remained unfamiliar to many clinicians. Further investigation of this organism is warranted given its documented, but rare, pathogenic potential.
1. INTRODUCTION
Myiasis is a parasitic infestation of animals with dipterous larvae. Myiasis is a term derived from the Greek word, myia, meaning fly. The parasitic fly Wohlfahrtia magnifica remains a serious pest of livestock of Eastern Europe, the Mediterranean and Middle Asia but has been seldom reported in humans in the United States, let alone the rest of the world. 1 Wohlfahrtiimonas chitiniclastica was first isolated from the third‐stage larvae of Wohlfahrtia magnifica fly in 2008. Wohlfahrtiimonas chitiniclastica is a strictly aerobic, non‐motile bacillus with strong chitinase activity, which is thought to play a role in the metamorphosis of the fly. In this paper, we report a case of a 60‐year‐old female resident of the midwestern United States with Wohlfahrtiimonas chitiniclastica monobacteremia secondary to maggot‐infested wounds of the lower extremities and sacrum.
2. CASE DESCRIPTION
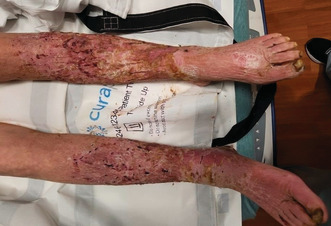
A 60‐year‐old female with a past medical history of liver cirrhosis, alcohol abuse, peripheral vascular disease, chronic venous insufficiency, and severe protein‐calorie malnutrition presented with bilateral leg pain and difficulty ambulating. She stated that she had frequent falls starting about 3 months ago. The patient reported losing her balance, however, she denied any loss of consciousness or dizziness. She was able to move around the house only using a wheelchair. The patient had decreased appetite for the past few months and ate only about one meal a day. Her last drink was after she was discharged from the hospital 6 months ago. The patient had a 40 pack‐year history of smoking. She denied fever, chills, chest pain, shortness of breath, abdominal pain, nausea, or vomiting. She was afebrile and tachycardic with a heart rate of 107 beats/minute on initial presentation. On her physical exam, a large black eschar approximately 12 cm × 10 cm was found on her sacrum. She also had multiple malodorous, maggot‐infested large open wounds on her legs, bilateral buttocks, and groin area. Her heels were erythematous and thickened with callous formation. The skin of her lower extremities was indurated, swollen, erythematous, and covered with multiple old wounds. There was bilateral lower extremity deep, scalloped ulcerations with visualization of dermal and subcutaneous layers. No drainage was noted but large patches of thick skin were visibly peeling (Figure 1).
FIGURE 1.
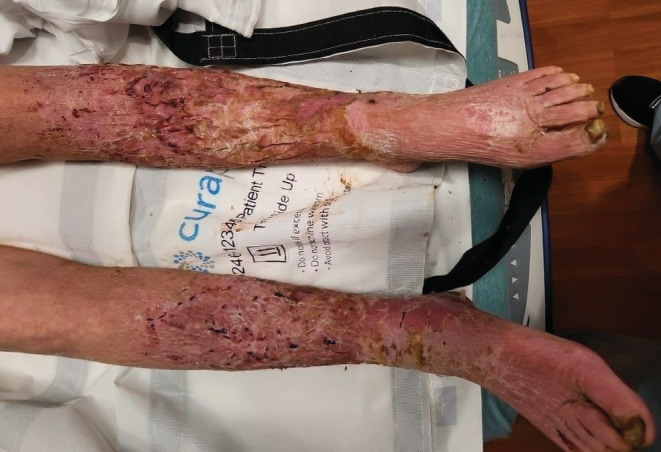
Initial clinical appearance of maggot‐infested lower extremities.
Laboratory analysis revealed a white blood cell count of 6.3 (reference range 3.8–10.4) × 103/microL, hemoglobin of 10.1 (reference range 13.8–17.2) g/dL, platelet count of 369 (reference range 150–350) × 103/microL, hypocalcemia, creatine kinase levels of 702 (reference range 30–150) μ/L, elevated aspartate aminotransferase and alanine aminotransferase to 100 U/L. On the computed tomography scan of the pelvis, mild fat stranding and edema of the bilateral thighs were noted, likely related to cellulitis. There were no signs of fluid collection to suggest an abscess. Her chest x‐ray showed no acute process. Duplex ultrasonography of the lower extremities showed no evidence of deep venous thrombosis.
Infectious Diseases was consulted with recommendations of starting vancomycin and cefepime for broad‐spectrum microbial coverage. Acetaminophen, hydromorphone, and tramadol were used for pain control. Bedside surgical wound debridement of ulcers with removal of maggots and wet‐to‐dry dressings were performed. The urine culture was negative. After preliminary blood culture identification of a Gram‐negative organism, her empiric coverage was de‐escalated. Once Wohlfahrtiimonas chitiniclastica was identified in blood cultures, she was subsequently transitioned to a 3‐day course of cefazolin. Vector identification was not performed in our patient. Repeat blood cultures were negative. On the date of discharge, the patient reported significant improvement, was hemodynamically stable, and was medically optimized for a transition to outpatient care.
3. DISCUSSION
Wohlfahrtiimonas chitiniclastica is a strictly aerobic, oxidase‐positive, catalase‐positive, non‐spore‐forming, non‐motile, and mesophilic Gram‐negative bacillus first isolated from the obligate ectoparasitic fly Wohlfahrtia magnifica. 1 Other potential vectors for the distribution of infection include Chrysomya megacephalea, Lucilia sericata, and Musca domestica. 2 Female flies deposit eggs in wounds and mucosal surfaces where they feed on the tissue creating a nidus for infection. To date, there have been fewer than 20 published cases of Wohlfahrtiimonas chitiniclastica infection in humans reported worldwide. Based on previous reports, the majority of infected patients had poor personal hygiene, substandard living conditions, alcohol dependence, tobacco use, chronic wounds, and documented myiasis. Sites of isolation have ranged from the skin, bone, soft tissue, and blood. Clinical manifestations have included cellulitis, wound infections, osteomyelitis, and bacteremia. 3 , 4 Many cases documented larval infestation of wounds, which is a highly plausible mechanism of transmission and pathogenesis in our patient's case. Wohlfahrtiimonas chitiniclastica bacteremia is typically polymicrobial, commonly concurrently isolated with Escherichia coli, Morganella morganii, Proteus mirabilis, Providencia rettgeri, and Staphylococcus aureus. 5 Blood cultures should not be routinely obtained due to their generally low yield. In this patient's case, the surface area and depth of the wounds increased our suspicion for hematogenous seeding and resultant bacteremia. In addition, the patient's immunocompromised status due to liver cirrhosis raised suspicion of other atypical organisms in an immunocompromised host. It is important to note that the exclusive isolation of Wohlfahrtiimonas chitiniclastica in our patient supports the idea that this novel organism was the sole cause of the patient's bacteremia. Given that growth was reported in all samples supports true bacteremia as opposed to contamination.
Reported clinical isolates of Wohlfahrtiimonas chitiniclastica are broadly susceptible to antibiotics used for Gram‐negative infections, including beta‐lactams, carbapenems, and fluoroquinolones. Resistance to fosfomycin, amikacin, and tobramycin has been described. 4 , 6 Antibiotic sensitivity testing conducted in our laboratory indicated that Wohlfahrtiimonas chitiniclastica was pan‐sensitive (Table 1).
TABLE 1.
Antimicrobial susceptibilities for clinical isolates of Wohlfahrtiimonas chitiniclastica isolated from patient.
| Antibiotic | Minimum inhibition concentration (μg/mL) | Interpretation |
|---|---|---|
| Cefepime | <2.0 | Susceptible |
| Ceftazidime | <1.0 | Susceptible |
| Ciprofloxacin | <1.0 | Susceptible |
| Gentamicin | <1.0 | Susceptible |
| Piperacillin/Tazobactam | <4.0 | Susceptible |
| Tobramycin | <1.0 | Susceptible |
Most patients have significant clinical improvement with antibiotic treatment; however, monomicrobial bacteremia with Wohlfahrtiimonas chitiniclastica causing fatal sepsis has been described in the past. 7 Proper identification of Wohlfahrtiimonas chitiniclastica is crucial for prompt diagnosis and management. Previous studies indicate that VITEK 2 system has been occasionally mistaking Wohlfahrtiimonas chitiniclastica for Acinetobacter iwoffii, Comamonas testosteroni or Rhizobium radiobacter. Matrix‐assisted laser desorption ionization–time of flight mass spectrometry (MALDI‐TOF MS) and 16 S rRNA gene sequencing are recommended to be used as identification methods. 8
To the best of our knowledge, our case is one of the five cases of documented monomicrobial bacteremia caused by Wohlfahrtiimonas chitiniclastica in the United States. 6 Due to the rarity of infection, fast identification paired with antimicrobial susceptibility data is crucial for optimal patient outcomes.
4. CONCLUSION
This study highlights an emerging organism with pathogenic potential associated with flies, which often goes hand in hand with patients living in poor hygienic conditions. We would encourage any clinician taking care of a patient with similar clinical presentation to consider reporting new cases for further investigation of the ways of transmission of the pathogen, prevention, and treatment.
AUTHOR CONTRIBUTIONS
Marko Kozyk and Jordan Fisher wrote the manuscript. Jordan Fisher contributed to the management of the patient. Kateryna Strubchevska reviewed and edited the manuscript. All authors gave written consent for publication.
FUNDING INFORMATION
None for all authors.
CONFLICT OF INTEREST STATEMENT
None.
ETHICAL STATEMENT
The present study conforms to the ethical standards and guidelines of the journal.
INFORMED CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
None.
Kozyk M, Strubchevska K, Fisher J. Wohlfahrtiimonas chitiniclastica: A rare infection reported in an adult with liver cirrhosis. Clin Case Rep. 2023;11:e6972. doi: 10.1002/ccr3.6972
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Toth EM, Schumann P, Borsodi AK, Keki Z, Kovacs AL, Marialigeti K. Wohlfahrtiimonas chitiniclastica gen. Nov., sp. nov., a new gammaproteobacterium isolated from Wohlfahrtia magnifica (Diptera: Sarcophagidae). Int J Syst Evol Microbiol. 2008;58:976‐981. [DOI] [PubMed] [Google Scholar]
- 2. Dovjak P, Kroißenbrunner M, Iglseder B. Myiasis absent Wohlfahrtiimonas chitiniclastica bacteremia in a lung cancer patient: a case report. Eur J Med Res. 2021;26:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Connelly KL, Freeman E, Lin B, et al. Wohlfahrtiimonas chitiniclastica bloodstream infection due to a maggot‐infested wound in a 54‐year‐old male J glob. Infect Dis. 2019;11(3):125‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kopf A, Bunk B, Coldewey SM, Gunzer F, Riedel T, Schröttner P. Identification and antibiotic profiling of Wohlfahrtiimonas chitiniclastica, an underestimated human pathogen. Front Microbiol. 2021;12:712775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Connelly KL, Freeman E, Smibert OC, Lin B. Wohlfahrtiimonas chitiniclastica bloodstream infection due to a maggot‐infested wound in a 54‐year‐old male. J Glob Infect. 2019;11(3):125‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schröttner P, Rudolph WW, Damme U, Lotz C, Jacobs E, et al. Wohlfahrtiimonas chitiniclastica: current insights into an emerging human pathogen. Epidemiol Infect. 2017;145:1292‐1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Almuzara MN, Palombarani S, Tuduri A, et al. First case of fulminant sepsis due to Wohlfahrtiimonas chitiniclastica. J Clin Microbiol. 2011;49(6):2333‐2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hladík M, Lipovy B, Kaloudova Y, et al. Human infections by Wohlfahrtiimonas chitiniclastica: a mini‐review and the first report of a burn wound infection after accidental Myiasis in Central Europe. Microorganisms. 2021;9(9):1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


