Abstract
We aimed to describe practice patterns and outcomes in patients with extracorporeal membrane oxygenation (ECMO) support throughout the coronavirus 2019 (COVID-19) pandemic, with the hypothesis that mortality would improve as we accumulated knowledge and experience. We included 48 patients supported on veno-venous ECMO (VV-ECMO) at a single institution between April 2020 and December 2021. Patients were categorized into three waves based on cannulation date, corresponding to the wild-type (wave 1), alpha (wave 2), and delta (wave 3) variants. One hundred percent of patients in waves 2 and 3 received glucocorticoids, compared with 29% in wave 1 (p < 0.01), and the majority received remdesivir (84% and 92% in waves 2 and 3, vs. 35% in wave 1; p < 0.01). Duration of pre-ECMO noninvasive ventilation was longer in waves 2 and 3 (mean 8.8 days and 3.9 days, vs. 0.7 days in wave 1; p < 0.01), as was time to cannulation (mean 17.2 and 14.6 days vs. 8.8 days in wave 1; p < 0.01) and ECMO duration (mean 55.7 days and 43.0 days vs. 28.4 days in wave 1; p = 0.02). Mortality in wave 1 was 35%, compared with 63% and 75% in waves 2 and 3 (p = 0.05). These results suggest an increased prevalence of medically refractory disease and rising mortality in later variants of COVID-19.
Keywords: ECMO, COVID-19, mortality, outcomes, trends
For the past 2 years, intensive care units across the globe have seen the overwhelming impact of coronavirus 2019 (COVID-19) with an increased number of patients presenting with acute respiratory distress syndrome (ARDS),1,2 the most critically ill of whom are managed with veno-venous extracorporeal membrane oxygenation (VV-ECMO).3 Early observational studies of ECMO use in patients with medically refractory COVID-19 demonstrated mortality rates as high as 90%4; significantly above the standard mortality rates of 30–50% seen in patients with ARDS from earlier trials.5–9 For this reason, ECMO was not widely adopted as a management strategy for COVID-19–related ARDS in the early months of the pandemic, and guidelines for ECMO utilization in these patients were largely absent. Nevertheless, many institutions with established programs elected to offer ECMO as salvage therapy in patients with COVID pneumonia that was refractory to conventional interventions, and studies performed later in the pandemic demonstrated outcomes similar to those in ECMO for ARDS,10,11 encouraging progressive uptake of ECMO in appropriate individuals in the COVID-19 population.
As the pandemic progressed, management guidelines shifted considerably,10 altering the profile of patients cannulated for ECMO and complicating our understanding of patient outcomes. Furthermore, availability of COVID-19 vaccinations and introduction of new viral variants has added additional complexity. Although research on ECMO outcomes based on viral variant has been limited, recent retrospective studies have raised concern for inferior patient outcomes in the subsequent wave of COVID-19 patients managed with ECMO,12 with results extending through the end of 2020.
To date, no retrospective analysis of ECMO outcomes in COVID-19 patients has included the delta wave of COVID-19, a strain that proved deadlier and more virulent than those before it.13 Our objective was to characterize the shifts in practice patterns and mortality in ECMO patients across the course of the COVID-19 pandemic by analyzing three distinct waves of patients, representing the timing of the wild-type, alpha, and delta viral variants. We hypothesized that individuals on ECMO during the delta wave of COVID-19 would demonstrate improved survival relative to the first wave, given the optimization of management guidelines and increased provider experience since the onset of the pandemic.
Methods
Study Design and Population
We conducted a single institution, retrospective observational study in adult COVID-19 patients on VV-ECMO support between March 2020 and December 2021. Approval for the study was obtained from our institutional review board. Patients were divided into three categories based on time of ECMO initiation: patients were categorized as wave 1 if they were cannulated between April and August of 2020, wave 2 if they were cannulated between October 2020 and May 2021, and wave 3 if they were cannulated between August and December of 2021. These categories represent a natural separation in COVID-19 waves, in which there was greater than 1 month between cannulations, and correspond to timing of the wild-type, alpha variant, and delta variant spikes in COVID-19 cases observed in the state of Maryland.14
Inclusion and Exclusion Criteria
All patients over 18 years of age who were cannulated with VV-ECMO for COVID-19 ARDS were included in the study. Patients at our institution were considered for VV-ECMO if they had ARDS by the Berlin definition and their disease met the inclusion criteria of the ECMO to Rescue Lung Injury in Severe ARDS (EOLIA) trial.15,16 These criteria included PaO2:FiO2 ratio <80 for 6 hours, PaO2:FiO2 ratio <50 for 3 hours, or pH <7.25 with PCO2 >60 mmHg for 6 hours. In order to be considered for ECMO support, our cohort was also required to demonstrate a failure to substantially respond to additional conventional interventions such as prone positioning, neuromuscular blockade, and inhaled pulmonary vasodilators.
Exclusion criteria included age over 60 years, body mass index (BMI) over 45 kg/m2 (over 50 if age <45), mechanical ventilation for greater than 7 days (10 days if age <45), irreversible neurologic injury, severe comorbidities (cirrhosis, advanced chronic obstructive pulmonary disease, congestive heart failure, or chronic kidney disease stage 3 or 4), immunocompromised state, malignancy with life expectancy less than 5 years, or contraindication to anticoagulation.
Patient Management
All patients were managed according to institutional VV-ECMO guidelines, which were unchanged during the course of the study period. These guidelines provide detailed criterion for patient selection, initial ECMO settings, management by organ system, troubleshooting, and weaning (Supplemental Document 1, Supplemental Digital Content 1, http://links.lww.com/ASAIO/A879). Weaning protocols were initiated for patients who demonstrated evidence of lung recovery, including larger tidal volumes on pressure control or airway pressure release ventilation (APRV) or improved compliance on volume control ventilation. Patients were considered appropriate for decannulation once able to maintain adequate gas exchange with ECMO sweep gas at zero liters per minute for 16–24 hours.
Data Collection and Definitions
Pre-ECMO characteristics were collected on all patients, including patient demographics, preexisting comorbidities, vaccination status, baseline inflammatory markers, Sequential Organ Failure Assessment (SOFA) score, and APACHE II score. Patient management strategies before cannulation were recorded, including ventilation time and noninvasive ventilation time before cannulation, time between COVID-19 diagnosis and cannulation, need for vasopressors, administration of COVID-19 therapies (glucocorticoids, hydroxychloroquine, remdesivir, and anti-IL-6 monoclonal antibodies), and use of rescue therapies such as APRV, neuromuscular blockade, inhaled nitric oxide, or proning.
Outcomes
The primary outcome of interest was inhospital mortality. Secondary outcomes included duration of ECMO run, length of stay, acute kidney injury, bleeding sequelae, major vascular thrombosis, secondary infection, and cause of death. Bleeding sequelae were defined as any significant bleeding that required a blood transfusion, and included oropharyngeal, nasopharyngeal, tracheal, gastrointestinal, intracranial, pulmonary, and cannulation site bleeding. Major vascular thrombosis included deep vein thrombosis or pulmonary emboli.
Statistical Analysis
Categorical variables for each wave of COVID-19 were created based on the timing of cannulation, as previously described (wave 1, wave 2, and wave 3). Demographics, management, and outcomes of our population were presented as proportions for categorical variables and median and interquartile range or mean and standard deviation for continuous variables. Comparisons between groups were performed using Analysis of Variance (ANOVA) or Kruskal-Wallis testing for continuous variables and χ2 or Fischer’s exact tests for categorical variables, as appropriate. For variables with significant differences on the omnibus test, we then performed pairwise comparisons using χ2 and Fisher’s exact tests for categorical variables and Student’s t-test or Dunn’s test for continuous variables. Finally, we performed an exploratory data analysis comparing survivors and nonsurvivors and evaluated the impact of the aforementioned variables on patient outcomes.
Based on our initial results, a post hoc analysis was performed to further investigate the relationship between inhospital mortality and time since the onset of the pandemic. In order to capture the natural progression of management strategies and introduction of viral variants, we created a continuous variable representing time since the onset of the pandemic. We then performed bivariate logistic regression evaluating the relationship between mortality and time since the onset of the pandemic. Statistical analysis was performed using Stata/IC 16 (StataCorp LLC, College Station, TX, software). Statistical significance was set to a p-value ≤ 0.05 for all tests.
Results
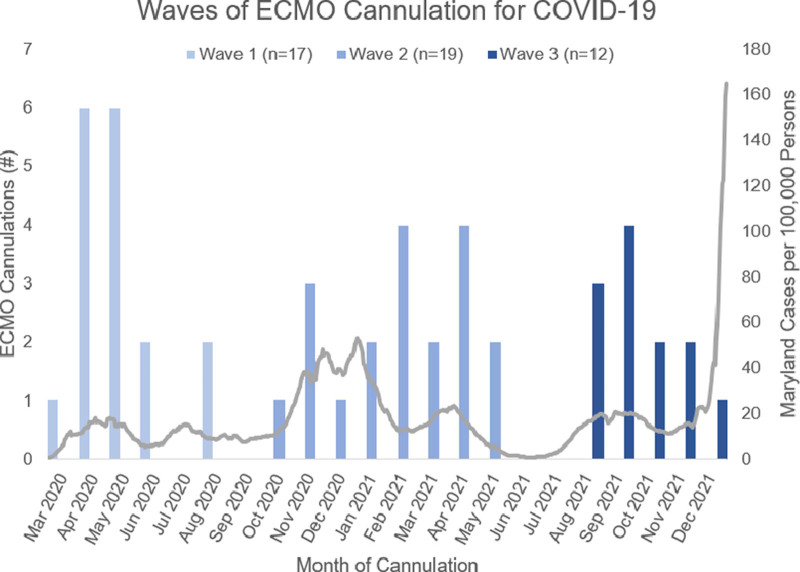
A total of 50 ECMO cannulations were performed in patients with severe COVID-19 ARDS. We excluded two VA ECMO cannulations from our analysis. Of the 48 patients included, there were 17 patients (35%) included in wave 1, 19 patients (40%) included in wave 2, and 12 patients (25%) included in wave 3 (Figure 1).
Figure 1.
Histogram representing frequency of cannulations between April 1, 2020 and December 31, 2021. Wave 1 patients were cannulated between April and August 2020. Wave 2 (alpha wave) patients were cannulated between October 2020 and May 2021. Wave 3 (delta wave) patients were cannulated between August and December 2021.
The majority of patients (73%) were male. Common comorbidities included obesity, hypertension, diabetes mellitus, and hyperlipidemia (Table 1). Patients in all three cohorts were similar in age, BMI, and comorbidities, but there was a higher predominance of White patients in wave 3 compared with waves 1 and 2 (92% in wave 3 compared with 21% in wave 2 and 0% in wave 1; p < 0.01). No patients in wave 1 were vaccinated. Two patients in wave 2 were partially vaccinated, and two patients in wave 3 were fully vaccinated (p = 0.06). Inflammatory markers were elevated in all waves, but the extent of elevation was similar. Patients in wave 3 had a higher APACHE II score compared with those in waves 1 and 2 (mean 22.3 in wave 3 compared with 14.6 in wave 2 and 15.9 in wave 1; p < 0.01).
Table 1.
Patient Characteristics Among Three Waves of COVID-19 Patients Receiving Extracorporeal Membrane Oxygenation
| Patient Characteristics | Wave 1 (n = 17) | Wave 2 (n = 19) | Wave 3 (n = 12) | p |
|---|---|---|---|---|
| Age, years | 51 (38–56) | 53 (44–55) | 46 (34–49.5) | 0.12 |
| Body mass index, kg/m2 | 31.5 (29.2–34.1) | 34.1 (29.3–39.4) | 35.2 (31.9–39.3) | 0.41 |
| Sex | ||||
| Male | 13 (76%) | 12 (63%) | 10 (83%) | 0.50 |
| Female | 4 (24%) | 7 (37%) | 2 (17%) | |
| Race | ||||
| African American†,‡ | 6 (35%) | 9 (47%) | 0 (0%) | <0.01* |
| Asian | 1 (6%) | 1 (5%) | 0 (0%) | |
| Hispanic† | 10 (59%) | 5 (26%) | 1 (8%) | |
| White†,‡ | 0 (0%) | 4 (21%) | 11 (92%) | |
| Preexisting comorbidities | ||||
| Hypertension | 7 (41%) | 6 (32%) | 4 (33%) | 0.87 |
| Hyperlipidemia | 4 (24%) | 4 (21%) | 2 (17%) | 1.00 |
| Diabetes | 6 (35%) | 7 (37%) | 0 (0%) | 0.21 |
| Coronary artery disease | 1 (6%) | 1 (5%) | 1 (8%) | 1.00 |
| Tobacco use | 1 (6%) | 0 (0%) | 0 (0%) | 0.60 |
| Vaccination | ||||
| None | 17 (100%) | 17 (89%) | 10 (83%) | 0.06 |
| Partial | 0 (0%) | 2 (11%) | 0 (0%) | |
| Full | 0 (0%) | 0 (0%) | 2 (17%) | |
| Baseline inflammatory markers | ||||
| IL-6 | 290 (167–710) | 438 (19–3080) | 648 (213–3399) | 0.72 |
| CRP | 16.6 (9.9–34.2) | 5.6 (2.4–18.6) | 16.8 (5.2–23) | 0.16 |
| Ferritin‡ | 1012 (705–1900) | 705 (531–1114) | 1587 (1379–2986) | 0.03* |
| SOFA score†,‡ | 7.8 ± 2.0 | 7.2 ± 2.1 | 10.1 ± 2.4 | <0.01* |
| APACHE II score†,‡ | 15.9 ± 6.0 | 14.6 ± 5.8 | 22.3 ± 5.6 | <0.01* |
Continuous variables are presented as mean ± SD or median (IQR). Categorical variables are presented as n (%).
p-value ≤ 0.05.
Comparison of wave 1 and wave 3 (p < 0.05).
Comparison of wave 2 and wave 3 (p < 0.05).
COVID-19, coronavirus 2019; IL-6, Interleukin 6; CRP, C-Reactive Protein; SOFA, Sequential Organ Failure Assessment; APACHE II, Acute Physiology and Chronic Health Evaluation; IQR, interquartile range.
Differences in management paradigms between the three waves can be seen in Table 2. Compared with the first wave, patients in subsequent waves were more likely to be managed with steroids (100% in waves 2 and 3 compared with 29% in wave 1; p < 0.01) and remdesivir (84% and 92% in waves 2 and 3 compared with 35% in wave 1; p < 0.01). Patients in waves 2 and 3 had a longer duration of time on noninvasive oxygenation compared with wave 1 (mean 8.8 days in wave 2 and 3.9 days in wave 3 compared with 0.7 days in wave 1; p < 0.01) and a similar duration of mechanical ventilation before cannulation (mean 3.7 days in wave 2 and 4.2 days in wave 3 compared with 5.5 days in wave 1; p = 0.19). Patients in waves 2 and 3 had a longer duration of time between initial COVID-19 diagnosis and ECMO cannulation than patients in wave 1 (median 17.2 days in wave 2 and 14.6 days in wave 3 compared with 8.8 days in wave 1; p < 0.01).
Table 2.
Management Strategies Among Three Waves of COVID-19 Patients Receiving ECMO
| Management | Wave 1 (n = 17) | Wave 2 (n = 19) | Wave 3 (n = 12) | p |
|---|---|---|---|---|
| Noninvasive ventilation time, days †,‡,§ | 0.71 ± 0.77 | 8.8 ± 4.4 | 3.9 ± 3.6 | <0.01* |
| Pre-ECMO ventilation time, days | 5.5 ± 2.9 | 3.7 ± 2.5 | 4.2 ± 3.7 | 0.19 |
| Diagnosis to cannulation time, days †,§ | 8.8 ± 6.3 | 17.2 ± 3.4 | 14.6 ± 3.7 | <0.01* |
| APRV | 9 (53%) | 12 (63%) | 3 (25%) | 0.11 |
| Inhaled nitric oxide | 15 (88%) | 19 (100%) | 9 (75%) | 0.06 |
| Prone positioning | 17 (100%) | 18 (95%) | 12 (100%) | 1.00 |
| Neuromuscular blockade | 18 (100%) | 20 (100%) | 12 (100%) | 1.00 |
| Vasopressors | 13 (76%) | 15 (79%) | 12 (100%) | 0.21 |
| COVID-19 therapies | ||||
| Glucocorticoids †,‡,§ | 5 (29%) | 19 (100%) | 12 (100%) | <0.01* |
| Hydroxychloroquine | 2 (12%) | 1 (5%) | 0 (0%) | 0.61 |
| Remdesivir †,‡,§ | 6 (35%) | 16 (84%) | 11 (92%) | <0.01* |
| Anti-IL-6 | 10 (59%) | 5 (26%) | 7 (58%) | 0.14 |
Data are presented as mean ± SD or n (%).
Global test for differences between waves (p-value ≤0.05).
Comparison of wave 1 and wave 3 (p < 0.05).
Comparison of wave 2 and wave 3 (p < 0.05).
Comparison of wave 1 and wave 2 (p < 0.05).
APRV, airway pressure release ventilation; COVID-19, coronavirus 2019; ECMO, extracorporeal membrane oxygenation; IL-6, Interleukin 6.
Overall, the mortality rate was 43% during the study period of interest. Inhospital mortality was highest in wave 3 and lowest in wave 1 (75% vs. 35%; p = 0.05). Most common cause of death was refractory pulmonary disease (37%), followed by sepsis with multiorgan failure (22%), intracranial hemorrhage (ICH) (19%), and hemorrhagic shock (11%). Despite standardized anticoagulation algorithms throughout the study period of interest, there was a 25% prevalence of fatal ICH in wave 3, compared with 0% in wave 1 (p = 0.03). Complications included bleeding, secondary infection, major vascular thrombosis, and acute kidney injury requiring renal replacement therapy (Table 3). There were no significant differences in prevalence of complications between the three waves. The duration of ECMO support was longer in the second wave than in the first wave (mean 55.7 days in wave 2 compared with 28.4 days in wave 1; p < 0.01). Length of stay was also longer in wave 2 compared with wave 1 (mean 75.4 days in wave 2 vs. 47.9 days in wave 1; p = 0.01).
Table 3.
Complications and Outcomes Among Three Waves of COVID-19 Patients Receiving ECMO
| Outcomes | Wave 1 (n = 17) | Wave 2 (n = 19) | Wave 3 (n = 12) | p |
|---|---|---|---|---|
| Complications | ||||
| Acute kidney injury | 7 (41%) | 5 (26%) | 5 (42%) | 0.59 |
| Bleeding sequelae | 13 (77%) | 16 (80%) | 10 (83%) | 0.90 |
| Thrombosis | 7 (41%) | 4 (21%) | 3 (25%) | 0.41 |
| Secondary infection | 15 (88%) | 13 (65%) | 11 (92%) | 0.25 |
| ECMO time, days † | 28.4 ± 19.0 | 55.7 ± 36.1 | 43.0 ± 26.4 | 0.02* |
| Length of stay † | 47.9 ± 23.6 | 75.4 ± 37.8 | 52.8 ± 30.4 | 0.03* |
| Inhospital death § | 6 (35%) | 12 (63%) | 9 (75%) | 0.05* |
| Intracranial hemorrhage | 0 (0%) | 2 (17%) | 3 (33%) | |
| Sepsis with multiorgan failure | 1 (17%) | 2 (17%) | 3 (33%) | |
| Hemorrhagic shock | 1 (17%) | 2 (17%) | 0 (0%) | |
| Refractory pulmonary disease | 2 (33%) | 5 (42%) | 3 (33%) | |
| Other | 2 (33%) | 1 (8%) | 0 (0%) | |
Data are presented as mean ± SD or n (%).
Global test for differences between waves (p-value ≤ 0.05).
Comparison of wave 1 and wave 2 (p < 0.05).
Comparison of wave 1 and wave 3 (p < 0.05).
COVID-19, coronavirus 2019; ECMO, extracorporeal membrane oxygenation.
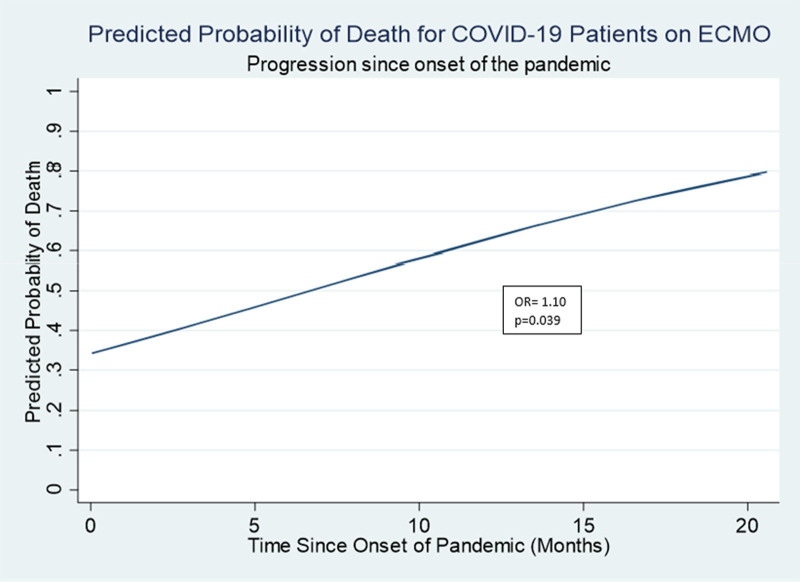
In an exploratory analysis, there were no significant differences in patients’ baseline characteristics between survivors and nonsurvivors (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/ASAIO/A880). Nonsurvivors were more commonly male, White, and had a higher proportion of comorbidities such as hypertension, hyperlipidemia, coronary artery disease, and malnutrition. Of the nonsurvivors, 30% had baseline malnutrition, whereas only 5% of the survivors were malnourished (p = 0.05). There were no major differences in complications between survivors and nonsurvivors. Survivors had shorter ECMO runs (mean 30.3 days compared with 52.6; p < 0.01) and had less time between diagnosis and cannulation (mean 11.1 days compared with 15.4; p = 0.02). There was no significant difference in days of noninvasive ventilation between survivors and nonsurvivors (median 2 days in survivors compared with 5 days in nonsurvivors; p = 0.16). Survivors were cannulated an average of 216 days from the onset of the pandemic, compared with an average of 340 days from the onset of the pandemic in nonsurvivors (p = 0.03). Similarly, a post hoc analysis demonstrated an increased probability of death over time during the study period of interest (Figure 2). Using a bivariate logistic regression model, we observed a 10% increased odds of mortality for each additional month since the onset of the pandemic (p = 0.04).
Figure 2.
Predicted probabilities of death calculated from a univariate logistic regression model assessing the relationship between time since the onset of the pandemic and probability of death. ECMO, extracorporeal membrane oxygenation.
Discussion
Our objective was to characterize the patient presentation, management paradigms, and outcomes of patients supported with VV-ECMO during the COVID-19 pandemic. There were no major differences in baseline demographics between the first two waves, but the notable difference was the number of days patients were receiving supplemental oxygen before their intubation. The number of days of PPV or oxygen from high flow nasal cannula (HFNC) was significantly longer in the second and third waves compared with the first. Furthermore, nearly all the patients were treated with steroids and a great majority were also treated with remdesivir after the first wave. ECMO cannulation was occurring later in patients’ disease courses and the sickest ARDS cases were those refractory to standard treatment. The mortality during the first wave was comparable to patients with non-COVID ARDS15 at approximately 35%, but this rose dramatically to 60% during the second wave, and 75% during the third wave.
The observed trends in patient management reflect an improved understanding of COVID-19 and its management that has occurred over the course of the pandemic. For example, while early management of COVID-19 was largely supportive with an emphasis on early mechanical ventilation, the standard of care now favors prolonged trials of high-flow nasal cannula oxygen or noninvasive positive pressure ventilation in the absence of other indications for mechanical ventilation.17 Other shifts in patient management include the widespread adoption of pharmaceutical support with dexamethasone, remdesivir, and monoclonal antibody therapies for patients with escalating supplemental oxygen requirements.18,19 We hypothesized that this expanding knowledge of COVID-19 would result in improved patient outcomes, but our results suggest otherwise.
Given the optimization of medical therapy seen later on in the pandemic, we believe the higher mortality observed in the latest cohort reflects selection bias of patients with medically refractory disease rather than inferior patient management. Patients who were cannulated early on in the pandemic may not have required ECMO cannulation (and would therefore not have been included in our study sample), had they benefited from the same medical optimization as our most recent cohort. Conversely, patients who required ECMO cannulation in the later waves of the pandemic were those refractory to treatments that we now consider to be standard of care and, therefore, represented a sicker patient population at baseline. This hypothesis is further supported by the higher APACHE II scores seen in the most recent cohort. The pattern of rising mortality over time may, therefore, represent a higher proportion of treatment-refractory disease in the later waves, and phenotypes of COVID-19 with worse overall prognosis. Similarly, although nonsurvivors tended to have longer durations of time between initial diagnosis and ECMO cannulation, we believe this to be an association and not the cause of mortality. Consultation for ECMO cannulation was occurring later on in the disease course of patients in the second and third waves of the pandemic, which may reflect the efficacy of newer treatment modalities in preventing rapid deterioration, as well as the current emphasis on delayed intubation.
Our findings of rising mortality in the population of COVID-19 patients supported on ECMO are consistent with those reported by Barbaro et al.10 in a retrospective review of the Extracorporeal Life Support Organization registry. In evaluating outcomes of COVID-19 patients supported on ECMO during 2020, the authors report increased mortality in those who started ECMO after May 2020, despite a higher proportion of patients being treated with remdesivir and steroids. Our data expand on these results by offering a perspective on patient outcomes in 2021. Although our patients in later waves have similar proportions of preexisting comorbidities and the same inclusion criteria for ECMO consideration as the first wave, our mortality has continued to rise.
In addition to the broad trends, we have observed in management strategies and patient outcomes, our institutional experience highlights several other interesting patterns that have emerged over time. One notable example exists in the relationship between preexisting malnutrition and mortality. Interestingly, when comparing survivors and nonsurvivors, we found that only 5% of survivors had malnutrition, compared with 30% of nonsurvivors. Similarly, in a meta-analysis of 4,187 COVID-19 patients not supported on ECMO, Abate et al. 20 found a 10-fold increase in mortality among hospitalized patients with COVID-19 and poor nutrition. These findings collectively emphasize the importance of optimizing nutritional status in COVID-19 patients.
It is also worth noting the high incidence of fatal ICH in the delta wave of ECMO patients at our institution. An astounding 25% of patients in the delta wave demonstrated severe ICH with herniation and brain death, compared with a combined 6% in the first two waves, despite all cohorts being maintained with a standardized and equivalent anticoagulation regimen. Although it is beyond the scope of this paper to pose a causal relationship between COVID-19 and ICH, this association has been described previously. In a case series published by Abbas et al.,21 the authors describe the phenomenon of ICH in COVID-19 patients and specifically comment on the unexpected features of this population, including an extraordinarily young age and unusually high mortality. This association, if present, would only be magnified in the ECMO population, in whom anticoagulation is the standard of care. Furthermore, when considering anticoagulation, the risk of ICH must be balanced with the risk of thrombosis; these patients are faced both with the risk of thrombosis related to the thrombogenic properties of the extracorporeal circuit as well as the prothrombotic characteristics of the coronavirus itself.22 At our institution, given the high prevalence of ICH in our latest wave, we have since decreased our partial thromboplastin time (PTT) goal to 40–50 in hopes of avoiding future intracranial catastrophes. Further research would be of value in investigating the potential relationship between COVID-19 infection and ICH and the optimal anticoagulation regimen for this patient population.
To our knowledge, this is the only report to date of outcomes in COVID-19 patients supported on ECMO that compares data extending through the occurrence of the delta variant. The primary limitation of our study is the small sample size, which limited the statistical power of our analysis and its corresponding scientific accuracy. Further, our data is from a single institution and may not be generalizable to all institutions with COVID-19 patients supported on ECMO. Subsequent analysis with a large, multi-institutional database would be of value in verifying external validity. Finally, there are elements of the COVID-19 pandemic that are unable to be quantified in a study such as this, such as the potential impact of facility and resource limitations on clinical decision-making. Although our institution always had an adequate supply of ECMO circuits, the pandemic placed strain on our hospital personnel and intensive care unit capacity in an unprecedented manner.
In summary, mortality for COVID-19 patients supported on ECMO has increased at our institution over the course of the pandemic. While our initial wave of patients demonstrated mortality comparable to non-COVID patients, we observed 75% mortality in our latest wave despite optimization of medical management and prolonged ECMO support. Although we believe this rising mortality reflects a shift in the baseline presentation of patients considered for ECMO cannulation rather than inferior patient management, it does pose concern regarding patient selection for ECMO cannulation. As we strive to optimize resource allocation, this study emphasizes the need for reassessment of cannulation criteria in a population that has significantly changed since the onset of the pandemic.
Acknowledgment
HERALD (Hopkins Exploration, Research, and Advancement in Life support Devices) Investigators are as follows: Matthew Acton MD, Diane Alejo, Kate Calligy RN, Scott Anderson, Benjamin Shou BS, Shrey Kapoor BS, Marc Sussman MD, Christopher Wilcox DO MS, Patricia Brown RD-AP CNSC, Anna Peeler RN.
Supplementary Material
Footnotes
Disclosure: The authors have no conflicts of interest to report.
S.M.C. was funded by NHLBI 1K23HL157610.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML and PDF versions of this article on the journal’s Web site (www.asaiojournal.com).
References
- 1.Shekar K, Badulak J, Peek G, et al. ; ELSO Guideline Working Group: Extracorporeal life support organization coronavirus disease 2019 interim guidelines: A consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. ASAIO J 66: 707–721, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G, Pesenti A, Cecconi M: Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: Early experience and forecast during an emergency response. JAMA 323: 1545–1546, 2020. [DOI] [PubMed] [Google Scholar]
- 3.Giani M, Redaelli S, Siragusa A, Fumagalli B, Rona R, Foti G: Extracorporeal gas exchange for acute respiratory distress syndrome: Open questions, controversies, and future directions. Membranes (Basel) 11: 172, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry BM, Lippi G: Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): Pooled analysis of early reports. J Crit Care 58: 27–28, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JJ, Hwang SM, Ko JH, et al. : Efficacy of veno-venous extracorporeal membrane oxygenation in severe acute respiratory failure. Yonsei Med J 56: 212–219, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camboni D, Philipp A, Lubnow M, et al. : Support time-dependent outcome analysis for veno-venous extracorporeal membrane oxygenation. Eur J Cardiothorac Surg 40: 1341–1346; discussion 1346, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Schmid C, Philipp A, Hilker M, et al. : Venovenous extracorporeal membrane oxygenation for acute lung failure in adults. J Heart Lung Transplant 31: 9–15, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Gray BW, Haft JW, Hirsch JC, Annich GM, Hirschl RB, Bartlett RH: Extracorporeal life support: Experience with 2000 patients. ASAIO J 61: 2–7, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badulak J, Antonini MV, Stead CM, et al. ; ELSO COVID-19 Working Group Members: Extracorporeal membrane oxygenation for COVID-19: Updated 2021 guidelines from the extracorporeal life support organization. ASAIO J 67: 485–495, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbaro RP, MacLaren G, Boonstra PS, et al. : Extracorporeal membrane oxygenation for COVID-19: Evolving outcomes from the international extracorporeal life support organization registry. Lancet 398: 1230–1238, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt M, Hajage D, Lebreton G, et al. : Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Resp Med 8: 1121–1131, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broman LM, Eksborg S, Lo Coco V, De Piero ME, Belohlavek J, Lorusso R: Extracorporeal membrane oxygenation for COVID-19 during first and second waves. Lancet Resp Med 9: e80–e81, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raman R, Patel KJ, Ranjan K: COVID-19: Unmasking emerging SARS-CoV-2 variants, vaccines and therapeutic strategies. Biomolecules 11: 993e1–99e30, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coronavirus - Maryland Department of Health: 2022. Available at: https://coronavirus.maryland.gov/. Accessed February 22, 2022.
- 15.Combes A, Hajage D, Capellier G, et al. : Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. NEJM 378: 1965–1975, 2018. [DOI] [PubMed] [Google Scholar]
- 16.Bernard GR, Artigas A, Brigham KL, et al. : The American-European consensus conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149: 818–824, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Oxygenation and Ventilation: 2021. Available at: https://www.covid19treatmentguidelines.nih.gov/management/critical-care/oxygenation-and-ventilation/. Accessed February 22, 2022.
- 18.Horby P, Lim WS, Emberson JR, et al. : Dexamethasone in hospitalized patients with Covid-19. NEJM 384: 693–704, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beigel JH, Tomashek KM, Dodd LE, et al. : Remdesivir for the treatment of Covid-19 - final report. NEJM 383: 1813–1826, 2020. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8294594/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abate SM, Chekole YA, Estifanos MB, Abate KH, Kabthymer RH: Prevalence and outcomes of malnutrition among hospitalized COVID-19 patients: A systematic review and meta-analysis. Clin Nutr ESPEN 43: 174–183, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbas R, El Naamani K, Sweid A, et al. : Intracranial hemorrhage in patients with coronavirus disease 2019 (COVID-19): A case series. World Neurosurg 154: e473–e480, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasuff H, Zochios V, Brodie D: Thrombosis and coagulopathy in COVID-19 patients requiring extracorporeal membrane oxygenation. ASAIO 66: 844–846, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.