Abstract
Pulmonary hypertension (PH) is a prevalent disease of the pulmonary vasculature that is characterised by considerable morbidity and mortality. Substantial efforts have been made in recent years to improve disease recognition, diagnosis and management, and this is reflected in current guidelines. The haemodynamic definition of PH has been revised and a definition for exercise PH has been provided. Risk stratification has been refined and the importance of comorbidities and phenotyping have been highlighted. These changes provide an opportunity to potentially identify pulmonary vascular disease at an earlier stage and to enhance patient-centred, goal-orientated treatment decisions. A promising fourth treatment pathway for pulmonary arterial hypertension and potential targeted therapies for group 3 PH are on the horizon, concepts which seemed inconceivable only a few years ago. Beyond medication, there is a greater appreciation for the importance of supervised training in stable PH and the possible role of interventional therapies in select cases. The landscape of PH is changing and it is characterised by progress, innovation and opportunities. In this article, we highlight some of the new trends in PH, with a specific focus on the revised European Society of Cardiology/European Respiratory Society 2022 guidelines for the diagnosis and management of PH.
Short abstract
In this article we highlight some of the new trends in pulmonary hypertension, with a specific focus on the revised ESC/ERS 2022 guidelines for the diagnosis and management of PH. https://bit.ly/3Waum3Q
Definitions and diagnosis
The global burden of pulmonary hypertension (PH) is substantial, with an estimated prevalence of approximately 1% of the world's population. Our understanding of the epidemiology, pathology and pathobiology of PH has evolved, and this has been paralleled by advances in disease management and treatment. The revised European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines for the diagnosis and treatment of PH offers a comprehensive overview of progress in the field [1].
The haemodynamic definition of PH was re-examined at the Sixth World Symposium on PH and subsequently revised (table 1). A mean pulmonary artery pressure (mPAP) threshold of >20 mmHg is now used to define PH [1]. This reflects a greater appreciation for normal values (14±3 mmHg) and their prognostic relevance [1]. Similarly, the pulmonary vascular resistance (PVR) threshold has been reduced and a lower value of >2 Wood units (WU) now defines a pre-capillary pattern of PH. This revised threshold was chosen in light of recent evidence that shows that PVR values greater than 2.2 WU are associated with increased mortality and that normal values are closer to ∼0.9±0.4 WU [1, 2]. Furthermore, a definition of exercise PH has also been provided, which may facilitate earlier identification of pulmonary vascular disease. Dedicated studies are required to clarify the treatment implications of the new guidelines for patients with an mPAP between 21 and 24mmHg, a PVR between 2 and 3 WU and for those who meet the criteria for exercise PH.
TABLE 1.
Overview of the haemodynamic definitions of pulmonary hypertension (PH)
| Haemodynamic definitions | mPAP | PVR | PAWP |
| PH | >20 mmHg | ||
| Pre-capillary PH | >20 mmHg | >2 WU | ≤15 mmHg |
| Isolated post-capillary PH | >20 mmHg | ≤2 WU | >15 mmHg |
| Combined post- and pre-capillary PH | >20 mmHg | >2 WU | >15 mmHg |
| Unclassified PH # | >20 mmHg | ≤2 WU | ≤15 mmHg |
| Exercise PH | mPAP/CO slope >3 mmHg·min·L−1 between rest and exercise | ||
CO: cardiac output; mPAP: mean pulmonary artery pressure; PAWP: pulmonary artery wedge pressure; PVR: pulmonary vascular resistance. #: these patients are frequently characterised by elevated pulmonary blood flow and, although they have PH, they do not fulfil the criteria of pre- or post-capillary PH.
Echocardiography remains an important tool to estimate the probability of PH. The peak tricuspid regurgitation velocity and additional echocardiographic signs of PH can stratify patients into low, intermediate or high probability of PH. The TAPSE/sPAP (tricuspid annular plane systolic excursion/systolic pulmonary artery pressure) ratio is a new addition to the echocardiographic probability assessment. It is a noninvasive assessment of right ventricular (RV) and pulmonary artery coupling and a ratio <0.55 mm·mmHg−1 is suggestive of PH [1].
The importance of accurate and regular risk assessment in PH cannot be overstated. Multiparametric risk assessment is recommended at each consultation to estimate the 1-year mortality risk and to inform management decisions. Risk can be stratified into low (<5%), intermediate (5–20%) and high (>20%) using the three-strata model from the recent ESC/ERS risk stratification table [1]. A sizable proportion of patients with pulmonary arterial hypertension (PAH) are categorised into the intermediate risk stratum and, therefore, considerable efforts have been made to further substratify this cohort. The intermediate stratum can be further divided into intermediate-low and intermediate-high risk groups, creating a four-strata model. While the three-strata model is still recommended at the initial assessment, the four-strata model can be employed at interval follow-ups using three noninvasive parameters, as follows: World Health Organization (WHO) functional class (FC), 6 min walk distance (6MWD) and N-terminal pro B-type natriuretic peptide (NT-proBNP). Risk assessment should be integrated with individual patient characteristics in order to guide patient-centred treatment decisions.
Group 1 PAH
Group 1 PAH is a progressive vasculopathy of the pulmonary circulation. It has an estimated incidence of six cases per million adults and can affect all age groups. The demographics of PAH are changing and the clinical phenotype has evolved. PAH is now commonly identified in older patients, with concomitant comorbidities. Therefore, detailed clinical phenotyping is critical to ensure that the correct diagnosis is made.
Idiopathic PAH (IPAH) is typically the most common subgroup within group 1 PAH. Historically, IPAH was a disease of young female patients and plexiform lesions were considered the histological hallmark of the disease. However, there is mounting evidence to demonstrate that distinct phenotypes exist. Age, comorbidities and the diffusion capacity for carbon monoxide (DLCO) are useful parameters to phenotype subjects with PAH. DLCO assessment is a noninvasive means to assess the integrity of the alveolar-capillary membrane and is a valuable biomarker in pulmonary vascular disease [3]. Trip et al. [3] demonstrated that individuals with IPAH and marked reductions in DLCO (<45%) are typically older, male, report greater tobacco exposure and frequently have coronary heart disease. Despite similar haemodynamic profiles to subjects with higher DLCO, these individuals are more symptomatic and have worse outcomes. Cluster analysis of the COMPERA registry identified a similar cohort, which was comprised of older, predominantly male patients, who had frequent comorbidities and a history of smoking [4]. These patients also had a low DLCO and worse survival, further emphasising the prognostic role of DLCO. This is not restricted to group 1 PAH, as similar findings have also been observed in subjects with group 2 [5], 3 [6] and 4 PH.
The importance of phenotyping in PAH can also be extended to histopathology. In a study of 50 pulmonary specimens from patients with IPAH, detailed histopathological analysis revealed two distinct vascular phenotypes [7]. Plexiform vasculopathy was observed in 52% and the remaining 48% demonstrated a nonplexiform vasculopathy. The latter was characterised by vascular rarefaction and distinct microvascular remodelling. Interestingly, subjects with a nonplexiform vasculopathy were more commonly male and had lower DLCO. This new research further underscores the importance of characterising the spectrum of IPAH disease and the potential role of DLCO to enhance the clinical picture. Indeed, as our understanding of genetics increases, it can help us refine our clinical phenotyping. Recently, KDR mutations are shown to be associated with a particular form of familial PAH characterised by low DLCO and radiological evidence of parenchymal lung disease, including interstitial lung disease (ILD) and emphysema [8].
Group 2 PH
Group 2 PH associated with left heart disease (PH-LHD) represents the most prevalent form of PH [1]. In PH-LHD, retrograde transmission of increased left-sided pressures into the pulmonary circulation results in PH. This can be accompanied by endothelial dysfunction and pulmonary vascular remodelling and a pre-capillary component if left untreated. The haemodynamic patterns of PH-LHD include isolated post-capillary PH or combined post- and pre-capillary PH (CpcPH) [1]. The presence of PH in patients with left heart disease has been associated with increased morbidity and mortality, especially if the PVR is elevated >5 WU [1].
Establishing the diagnosis of PH-LHD can be challenging, particularly in cases of PH with heart failure with preserved ejection fraction (PH-HFpEF) which can be difficult to differentiate from PAH at times, especially when the pulmonary arterial wedge pressure (PAWP) is borderline. Careful clinical assessment, probability scores and fluid challenge at right heart catheterisation (RHC) can help differentiate these conditions [9]. Patient characteristics that might suggest PH-LHD include older age, the presence of metabolic syndrome, structural left heart disease, left atrial dilatation and left ventricular hypertrophy [1]. Validated probability scores such as the Heart Failure Association-PEFF [10] and the H2FPEF scores can assess for the presence of HFpEF, and composite echocardiography scores [11] have been developed to help discriminate pre- and post-capillary PH. Fluid challenge during RHC can unmask left heart disease. This typically comprises a 500 ml bolus of saline, delivered over 5–10 min with careful monitoring of pulmonary haemodynamics, though further validation of this method is required [1].
Optimisation of LHD is the priority in the treatment of group 2 PH. Drugs approved for the treatment of PAH are generally not recommended in PH-LHD. PAH-specific therapy vasodilates the pulmonary vasculature and reduces RV afterload. This in turn leads to improved RV output and increased blood flow to the left heart. In subjects with PH-LHD, this may result in pulmonary oedema as a noncompliant left heart may not tolerate this increase blood flow. However, PAH-specific therapy may be trialled in specific instances, particularly in CpcPH when the PVR is >5 WU and the PAWP is <15 mmHg after diuresis. These cases should be managed in specialised PH units and monitored closely for potential side-effects [1]. Phosphodiesterase type-5 (PD5) inhibitors have been prescribed in patients with HFpEF and CpcPH with evidence of clinical and haemodynamic benefits [1, 12].
Emerging therapies for group 2 PH include sodium–glucose cotransporter 2 (SGLT2) inhibitors, glucagon-like peptide-1 agonists and angiotensin receptor neprilysin inhibitors (ARNIs). The SGLT2 inhibitor empagliflozin and the ARNI sacubitril/valsartan have shown some promise in subjects with group 2 PH undergoing invasive monitoring with pulmonary artery sensor devices [13, 14]. Patients with heart failure treated with empagliflozin (n=33) in the EMBRACE-HF (empagliflozin evaluation by measuring impact on haemodynamics in patients with heart failure) trial had significant reductions in pulmonary artery diastolic pressure [13]. Similarly, patients with heart failure transitioned to sacubitril/valsartan had significant improvements in pulmonary artery pressures in a retrospective case series of 18 subjects. These results will require further exploration in larger PH-LHD cohorts.
Group 3 PH
Group 3 PH associated with lung disease and/or hypoxia includes obstructive lung diseases or emphysema, restrictive lung diseases and hypoventilation syndromes [1]. Alterations to the clinical classification of group 3 PH and revised treatment recommendations have been made in recent guidelines [1]. The term sleep-disordered breathing is no longer used and has been replaced by “hypoventilation syndromes”, reflecting that isolated obstructive sleep apnoea rarely results in significant PH. Additionally, lymphangioleiomyomatosis (LAM) has been relocated to group 3 PH, given that PH in LAM is generally mild and associated with underlying parenchymal lung disease [15]. A PVR threshold of >5 WU has been chosen to differentiate severe from nonsevere PH in group 3 PH.
Similar to group 2 PH, optimisation of the underlying disease is the priority in the management of group 3 PH. Former guidelines recommended against the use of PAH-specific therapy in this group due to concerns regarding worsening ventilation/perfusion (V′/Q′) mismatch and a lack of evidence demonstrating significant benefits [16]. However, following the results of the phase 3 INCREASE trial, inhaled treprostinil can now be considered in subjects with ILD and PH [17]. This randomised controlled trial explored the role of inhaled treprostinil versus placebo in 326 patients with ILD. At 16 weeks there was a significant improvement in the 6MWD in the treatment arm (+31 m), with associated improvements in NT-proBNP and reduced clinical worsening events [17]. These results were met with considerable excitement, as this is the first time that a PH therapy has been recommended for patients with group 3 PH. The delivery route of this drug may be the key to its success, as the drug is preferentially delivered to a well-ventilated lung. Furthermore, pre-clinical studies suggest that treprostinil may have anti-fibrotic effects, which will be explored further in the TETON study (NCT04708782). Moreover, in patients with severe PH associated with ILD, the revised ESC/ERS guidelines propose that PD5 inhibitors may be considered on a case-by-case basis in expert centres [1].
There are currently no approved PH-targeted therapies for subjects with PH associated with COPD. Some of these patients can have severe PH that is out of proportion to the underlying lung disease. This group is often defined as a “pulmonary vascular phenotype” and can be difficult to differentiate from IPAH with comorbid COPD. The PERFECT study (NCT03496623) is a phase 3 randomized controlled trial (RCT) of inhaled treprostinil in patients with PH associated with COPD and a PVR >4 WU that will hopefully address this pertinent question. In patients with severe group 3 PH, it is preferable to take an individualised approach to treatment decisions. Referral for lung transplant assessment should be considered in eligible subjects.
Group 4 PH
Considerable advances have been made in the diagnosis and management of chronic thromboembolic PH (CTEPH), which is the predominant subgroup within group 4 PH. CTEPH has a distinct treatment pathway and differs from the other PH groups in that it is potentially curable. It is characterised by fibrotic obstructions within the pulmonary arterial tree and a secondary microvasculopathy. The incidence of CTEPH probably ranges from two to six cases per million adults [1]. Disease identification has been advanced by the development of CTEPH prediction scores, a greater appreciation of potential risk factors and new imaging modalities. While V′/Q′ scintigraphy is an effective tool to screen for CTEPH, new imaging modalities such as magnetic resonance imaging perfusion, dual-energy computed tomography (CT) angiography and iodine subtraction mapping are currently being explored.
Substantial progress has also been made in disease management. Risk stratification tools such as the ESC/ERS risk stratification table and the REVEAL scores have been validated in CTEPH. Lifelong anticoagulation is indicated in all cases of CTEPH and there is growing evidence to demonstrate that direct oral anticoagulants are a safe alternative to warfarin [18]. However, warfarin is still indicated in cases of antiphospholipid syndrome, which may affect up to 10% of individuals with CTEPH. Medical therapies such as riociguat [19] and treprostinil [20] have demonstrated efficacy in CTEPH and additional therapies such as macitentan at higher doses are under investigation (NCT04271475). The treatment algorithm for CTEPH has evolved and now includes multimodal therapy with PH-specific therapy, balloon pulmonary angioplasty (BPA) and pulmonary endarterectomy (PEA). In the recently published RACE trial, PVR reduction at week 26 was more pronounced with BPA than with riociguat, but treatment-related serious adverse events (SAEs) were more common with BPA. However, there were fewer BPA-related SAEs among patients pre-treated with riociguat in the follow-up study. The RACE study points to the potential benefits of a multimodality approach in patients with inoperable CTEPH [21, 42].
Group 5 PH
Group 5 PH comprises a heterogeneous mix of disorders that may be complicated by PH. A common theme within this group is that the precise mechanisms driving PH are often poorly understood and frequently multifactorial in nature. PH-tailored treatments have therefore been limited; however, there has been some progress in the field of sarcoidosis-associated PH (SAPH).
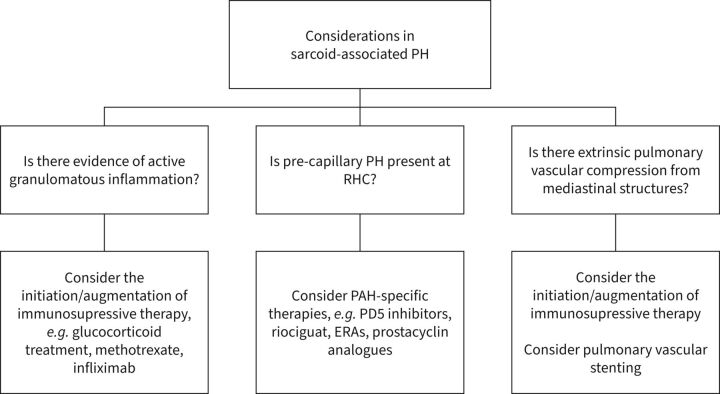
SAPH is a prevalent condition that is estimated to effect 6–20% of patients with sarcoidosis [22]. The nature of PH in SAPH is varied and therefore treatment is tailored to the underlying aetiology. For example, patients with SAPH and a group 1 phenotype may benefit from PAH-specific therapy, while those with active fibrotic lung disease may respond to immunosuppressive therapies. Additionally, those with extrinsic pulmonary artery compression from fibrosing mediastinitis may improve with pulmonary artery stenting.
In a large cohort of patients with severe pre-capillary SAPH, an improvement in short-term haemodynamic profiles was observed in patients treated with PAH-targeted therapies [23]. Interestingly, this was not accompanied by an improvement in 6MWD. Further studies have also suggested a role of PAH-specific therapies such as riociguat in select SAPH cases, which will require confirmation in larger cohorts [24]. Immunosuppressive therapies may also benefit patients with SAPH and active granulomatous disease. 18F-labelled fluorodeoxyglucose positron emission tomography combined with CT is a valuable tool to identify metabolically active sarcoidosis and patients who might benefit from this strategy. However, the associated radiation exposure should be considered, particularly in younger patients. Unfortunately, the prognosis of severe SAPH is poor. Patients who do not respond to the above interventions should have the treatment plan revisited and consideration of timely referral for lung transplantation and/or palliative care input (figure 1) [22, 23, 25].
FIGURE 1.
Overview of important considerations in patients with sarcoidosis-associated pulmonary hypertension (SAPH) [22, 23, 25]. ERA: endothelin receptor antagonists; PAH: pulmonary arterial hypertension; PD5: phosphodiesterase type-5 inhibitor; PH: pulmonary hypertension; RHC: right heart catheterisation.
Novel pathways in PAH
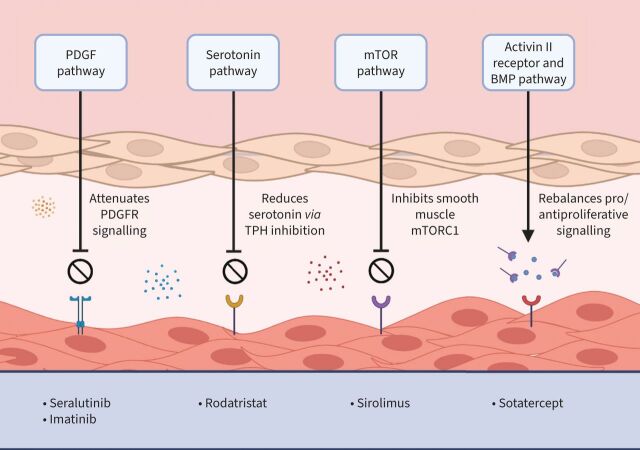
Current therapeutic targets in PAH include the endothelin pathway, the nitric oxide–soluble guanylate cyclase–cyclic guanosine monophosphate pathway and the prostacyclin pathway. These three pathways have been the cornerstone of PAH therapy for over a decade. However, there are a number of emerging therapies for PAH in clinical development that may revolutionise the field (figure 2). Some of these agents are outlined below.
FIGURE 2.
Emerging therapies for pulmonary arterial hypertension. BMP: bone morphogenetic protein; mTOR: mechanistic target of rapamycin; mTORC1: mTOR complex 1; PDGF: platelet-derived growth factor; PDGFR: platelet-derived growth factor receptor; TPH: tryptophan hydroxylase.
Sotatercept
Sotatercept is a novel, first-in-class fusion protein that binds and sequesters select transforming growth factor-β superfamily ligands. This rebalances the pro- and anti-proliferative signalling in PAH and augments the bone morphogenetic protein receptor (BMPR) II pathway [26]. In the phase 2 PULSAR study, treatment with sotatercept for 24 weeks resulted in significant reductions in PVR in patients with PAH. These patients had FC II or III symptoms at baseline and were established on background double or triple combination therapy. Improvements in 6MWD and NT-proBNP were also observed [26]. In the open-label extension, common side-effects included headache, diarrhoea and nasopharyngitis. Haematological side-effects such as thrombocytopaenia occurred in 17.3% of cases and telangiectasia occurred in 10.6%, following an average duration of therapy of 1.5 years [27].
Sirolimus
The mTOR (mechanistic target of rapamycin) signalling pathway is an important regulator of cellular homeostasis. Overactivation of this pathway has been implicated in a number of disease states including PAH. mTOR acts via two distinct complexes, mTOR complex 1 (mTORC1) and mTORC2 [28]. The activity of smooth muscle mTORC1 is enhanced in PAH and this is associated with pulmonary vascular proliferation and remodelling. Therefore, the inhibition of mTORC1 may provide a novel therapeutic pathway in PAH. Current evidence suggests that approved prostacyclin agents such as treprostinil may also have activity via this pathway and may attenuate pulmonary artery smooth muscle cell proliferation and vascular remodelling [29]. Phase 1/2 studies of an albumin-bound nanoparticle form of sirolimus called rapamycin (ABI-009) are currently underway (NCT02587325) [30]. This drug has a high accumulation within the lung and is currently being studied in patients with severe PAH and FC III symptoms despite treatment with two or more PAH-targeted therapies [30].
Seralutinib
The platelet-derived growth factor receptor (PDGFR), colony stimulating factor 1 receptor (CSF1R) and stem cell factor receptor (c-KIT) pathways have been implicated in the pathobiology of PAH. Targeting these pathways could attenuate pulmonary vascular inflammation and proliferation in PAH. Seralutinib is a PDGFR, CSF1R and c-KIT kinase inhibitor that upregulates BMPRII protein expression. Pre-clinical studies have demonstrated efficacy of inhaled seralutinib in animal models of PAH [31]. The TORREY study is a phase 2 study of inhaled seralutinib in patients with PAH and FC II and III symptoms [32]. The primary end-point will evaluate changes in PVR at 24 weeks.
Imatinib
Imatinib also has inhibitory effects on PDGFR and c-KIT and was investigated in patients with PAH in the IMPRES study [33]. This 24-week phase 3 RCT explored the impact of oral imatinib on the 6MWD in symptomatic subjects with PAH. While treatment with imatinib resulted in improved exercise capacity and haemodynamic profiles in patients with PAH, SAEs and side-effects were common and limited its use. Subdural haematomas occurred in eight patients who were also prescribed anticoagulation [33]. Interest in imatinib was not deterred by these results, and the potential role of inhaled imatinib is currently under investigation in the IMPAHCT study (NCT05036135). The inhaled route of drug delivery offers the benefit of delivering the drug directly to the target organ, while minimising potential systemic side-effects.
Rodatristat ethyl
Rodatristat ethyl is a tryptophan hydroxylase inhibitor designed to reduce the body's peripheral production of serotonin. ELEVATE 2 is a proof-of-concept phase 2 study of rodatristat ethyl in patients with PAH (ClinicalTrials.gov Identifier: NCT04712669).
Beyond medication for PH
Nonpharmacological interventions for subjects with PH is an area of expanding knowledge and experience. Our understanding of the benefits and safety of exercise training in chronic stable PH has increased [34]. Interventional procedures such as BPA for inoperable CTEPH are now incorporated into formal guidelines. Pulmonary artery stenting and atrial septostomy, while not currently featured in the treatment algorithm in the guidelines, are valuable interventions when used in specific scenarios and pulmonary artery denervation (PADN) may ultimately have a role as an adjuvant to medical therapy in select cases. This section outlines some of the nonpharmacological interventions for PH and recent advances in the field.
Exercise training and rehabilitation
Exercise training and rehabilitation is an important therapeutic intervention in PH. Historically, exercise was often discouraged in this population due to concerns regarding the risk of precipitating an arrhythmia, acute right heart failure, syncope and death. However, there is increasing evidence to demonstrate the safety and efficacy of exercise in patients with stable PH, with favourable effects on exercise capacity, quality of life and muscular function [34]. Furthermore, exercise training may also improve right ventricular function and pulmonary haemodynamics. An RCT of exercise training in 87 patients with severe PAH and inoperable CTEPH showed significant improvements in cardiac index (+9.3% versus −6.5%; p=0.002) and peak oxygen consumption during maximal exercise [35]. Recently, Grünig et al. [36] published the first multicentre RCT exploring the safety, feasibility and efficacy of exercise training as an adjunct to medical therapy in patients with PAH and CTEPH. 129 patients from 11 centres in 10 European countries were randomised and 116 patients completed the study. A significant improvement in 6MWD (30.7±57.9 m) was observed at 15 weeks, with additional improvements in quality-of-life scores, WHO FC and peak oxygen consumption [36]. An exercise prescription by a physiotherapist with a special interest in PH is preferable, specifying the training modalities, intensity and setting, as patients with PH can differ considerably in age, disease severity and comorbidities. Technological innovations and smart wearable devices enable remote monitoring of numerous parameters such as heart rate, rhythm and pulmonary artery pressures, which may further advance patient monitoring and safety [37].
BPA
CTEPH is characterised by organised fibrotic obstruction of pulmonary arteries and an associated microvasculopathy, with resultant PH. Percutaneous BPA is a staged, interventional procedure which dilates stenotic pulmonary artery segments using an endovascular balloon. BPA is an important therapeutic intervention for patients with CTEPH who are ineligible for PEA or have persistent PH following surgery.
BPA is a relatively new procedure for CTEPH. Technical advances in the field of paediatric cardiology and increased experience with balloon angioplasty in pulmonary artery stenoses led the way for the first BPA procedure in an adult patient with PH in 1988 [38]. Feinstein et al. [39] published the first BPA series of 18 patients with CTEPH in 2001. In this cohort, BPA resulted in significant improvements in FC, 6MWD and haemodynamic parameters at a median follow-up of 36 months; however, complications were not uncommon. The 30-day mortality was 5.5% and reperfusion pulmonary oedema occurred in 61% [39]. The procedure somewhat fell out of vogue until 2012, when Mizoguchi et al. [40] refined the procedure using intravascular ultrasound to determine the appropriate balloon size. The German [41] and French [42] national BPA series published in 2017 and 2019 contributed to the mounting evidence from Japanese centres regarding the role of BPA in inoperable CTEPH.
BPA is associated with greater improvements in PVR [21] and mPAP [43] in subjects with inoperable CTEPH when compared to medical therapy alone. Potential adverse events include vascular wall injury and lung injury such as reperfusion pulmonary oedema, haemoptysis and hypoxia. Pre-treatment with medical therapy may result in fewer BPA-related SAEs, especially in subjects with a PVR >4 WU [21]. Therefore, this recommendation has been cautiously incorporated into the revised ESC/ERS guidelines, while additional studies are pending [1].
Pulmonary artery stents and atrial septostomy
Endovascular pulmonary artery stenting or atrial septostomy are not applicable to the general PH population, although they can be valuable interventions when used in highly selected cases. Pulmonary artery stenting can ameliorate luminal patency, improve flow and provide symptomatic relief in subjects with extrinsic pulmonary artery compression due to fibrosing mediastinitis [44] or congenital heart disease [45, 46]. Furthermore, atrial septostomy can be a useful palliative intervention in patients with advanced PH and right heart failure. The first atrial septostomy in PPH (now IPAH) was published in 1983 by Rich et al. [47]. It involves the creation of a right to left atrial shunt to offload a failing right ventricle in advanced PAH. Contraindications to the procedure include the presence of moderate to severe hypoxia at baseline or severe elevations in right atrial pressure, which can make it difficult to accurately control the degree of shunting [48]. Atrial septostomy has been associated with an improvement in symptoms and haemodynamic parameters in subjects with PAH [49, 50] and should be considered on a case-by-case basis.
Pulmonary artery denervation
Cardiovascular homeostasis is modulated by a careful balance between the sympathetic nervous system (SNS) and the parasympathetic nervous system. Systemic and local changes in neurohormonal signalling, including enhanced SNS activity, have been documented in PAH [51]. This has been associated with disease progression and worse outcomes and provides a rationale for PADN as a means to address sympathetic activity and pulmonary vasoconstriction. PADN is an interventional procedure that obliterates sympathetic nerve fibres in the adventitia of the main pulmonary arteries.
In 2013, Chen et al. [52] published the first human experience of PADN in 13 subjects with IPAH. PADN resulted in significant reductions in the mPAP and significant improvements in 6MWD. This paved the way for further studies in this field, exploring the role of PADN in PAH [53, 54], combined pre- and post-capillary PH [55], and residual PH following PEA [56]. The Trophy-1 study investigated the role of therapeutic intravascular ultrasound PADN as an adjuvant therapy in PAH [54]. A percutaneous, noncontact catheter (TIVUSTM system) delivered a thermal ring to the main, right and left pulmonary arteries, to a depth of 10mm. 23 patients with PAH treated with dual oral or triple therapy underwent PADN, with no procedure-related SAEs. At 4–6 months follow-up, PADN was associated with significant reductions in PVR, increased 6MWD and improved daily activity in treated patients with PAH. However, heterogeneity in treatment response was observed and, therefore, further studies are required to specify the precise role of PADN in PAH.
Conclusion
It is certainly an engaging time to work in the field of PH. New diagnostic criteria, refined risk stratification and nuanced phenotyping provide the opportunity to improve goal-orientated treatment decisions, which will hopefully translate to better clinical outcomes. New treatments on the horizon, including a potential fourth and perhaps fifth treatment pathway for PAH offer additional promise. Moving beyond medication, we have a greater appreciation for the importance of supervised exercise training in stable PH and the potential role of interventional therapies in select cases. The future holds much promise thanks to the hard work and collaboration of patients, scientists and physicians across the globe.
Acknowledgements
Figure 2 was created with biorender.com.
Provenance: Commissioned article, peer reviewed.
Previous articles in this series: No. 1: Simonneau G, Fadel E, Vonk Noordegraaf A, et al. Highlights from the International Chronic Thromboembolic Pulmonary Hypertension Congress 2021. Eur Respir Rev 2023; 32: 220132. No. 2: Buschulte K, Cottin V, Wijsenbeek M, et al. The world of rare interstitial lung diseases. Eur Respir Rev 2023; 32: 220161. No. 3: Dumoulin DW, Bironzo P, Passiglia F, et al. Rare thoracic cancers: a comprehensive overview of diagnosis and management of small cell lung cancer, malignant pleural mesothelioma and thymic epithelial tumours. Eur Respir Rev 2023; 32: 220174.
This article has an editorial commentary: https://doi.org/10.1183/16000617.0006-2023
Number 4 in the Series “The world of rare lung diseases” Edited by Michael Kreuter, Marc Humbert, Thomas Wagner and Marlies Wijsenbeek
Conflict of interest: S. Cullivan has nothing to disclose.
Conflict of interest: S. Gaine has received honoraria and speaker's fees from Actelion and Janssen Pharmaceuticals, outside the submitted work; received travel support from Actelion and Janssen Pharmaceuticals and ATAVANT, outside the submitted work; participated in a DSMB for Actelion and Janssen Pharmaceuticals, and is an advisory board member for ATAVANT, Bio Gossamer, and United Therapeutics, outside the submitted work.
Conflict of interest: O. Sitbon consults for and has received grants from Actelion, Bayer, and GlaxoSmithKline. He consults for MSD, Pfizer, and United Therapeutics, outside the submitted work.
References
- 1.Humbert M, Kovacs G, Hoeper MM, et al. . 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2023; 61: 2200879. doi: 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 2.Maron BA, Brittain EL, Hess E, et al. . Pulmonary vascular resistance and clinical outcomes in patients with pulmonary hypertension: a retrospective cohort study. Lancet Respir Med 2020; 8: 873–884. doi: 10.1016/S2213-2600(20)30317-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trip P, Nossent EJ, de Man FS, et al. . Severely reduced diffusion capacity in idiopathic pulmonary arterial hypertension: patient characteristics and treatment responses. Eur Respir J 2013; 42: 1575–1585. doi: 10.1183/09031936.00184412 [DOI] [PubMed] [Google Scholar]
- 4.Hoeper MM, Pausch C, Grünig E, et al. . Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J Heart Lung Transplant 2020; 39: 1435–1444. doi: 10.1016/j.healun.2020.09.011 [DOI] [PubMed] [Google Scholar]
- 5.Hoeper MM, Meyer K, Rademacher J, et al. . Diffusion capacity and mortality in patients with pulmonary hypertension due to heart failure with preserved ejection fraction. JACC Heart Fail 2016; 4: 441–449. doi: 10.1016/j.jchf.2015.12.016 [DOI] [PubMed] [Google Scholar]
- 6.Cottin V, Selman M, Inoue Y, et al. . Syndrome of combined pulmonary fibrosis and emphysema: an official ATS/ERS/JRS/ALAT research statement. Am J Respir Crit Care Med 2022; 206: e7–e41. doi: 10.1164/rccm.202206-1041ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nossent, EJ, Smits J, Seegers C, et al. , A new clinical phenotype with non-plexiform vasculopathy and distinctive microvessel remodeling among idiopathic pulmonary arterial hypertension patients. Am J Respir Crit Care Med 2022; 205: A5302. doi: 10.1164/ajrccm-conference.2022.205.1_MeetingAbstracts.A5302 [DOI] [Google Scholar]
- 8.Eyries M, Montani D, Girerd B, et al. . Familial pulmonary arterial hypertension by KDR heterozygous loss of function. Eur Respir J 2020; 55: 1902165. doi: 10.1183/13993003.02165-2019 [DOI] [PubMed] [Google Scholar]
- 9.Pieske B, Tschöpe C, de Boer RA, et al. . How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019; 40: 3297–3317. doi: 10.1093/eurheartj/ehz641 [DOI] [PubMed] [Google Scholar]
- 10.Pieske B, Tschöpe C, de Boer RA, et al. . How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019; 40: 3297–3317. doi: 10.1093/eurheartj/ehz641 [DOI] [PubMed] [Google Scholar]
- 11.D'Alto M, Romeo E, Argiento P, et al. . Echocardiographic prediction of pre- versus postcapillary pulmonary hypertension. J Am Soc Echocardiogr 2015; 28: 108–115. doi: 10.1016/j.echo.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 12.Guazzi M, Vicenzi M, Arena R, et al. . Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 2011; 124: 164–174. doi: 10.1161/CIRCULATIONAHA.110.983866 [DOI] [PubMed] [Google Scholar]
- 13.Nassif ME, Qintar M, Windsor SL, et al. . Empagliflozin effects on pulmonary artery pressure in patients with heart failure: results from the EMBRACE-HF trial. Circulation 2021; 143: 1673–1686. doi: 10.1161/CIRCULATIONAHA.120.052503 [DOI] [PubMed] [Google Scholar]
- 14.Tran JS, Havakuk O, McLeod JM, et al. . Acute pulmonary pressure change after transition to sacubitril/valsartan in patients with heart failure reduced ejection fraction. ESC Heart Fail 2021; 8: 1706–1710. doi: 10.1002/ehf2.13225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freitas CSG, Baldi BG, Jardim C, et al. . Pulmonary hypertension in lymphangioleiomyomatosis: prevalence, severity and the role of carbon monoxide diffusion capacity as a screening method. Orphanet J Rare Dis 2017; 12: 74. doi: 10.1186/s13023-017-0626-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galiè N, Humbert M, Vachiery J-L, et al. . 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2015; 46: 903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 17.Waxman A, Restrepo-Jaramillo R, Thenappan T, et al. . Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med 2021; 384: 325–334. doi: 10.1056/NEJMoa2008470 [DOI] [PubMed] [Google Scholar]
- 18.Humbert M, Simonneau G, Pittrow D, et al. . Oral anticoagulants (NOAC and VKA) in chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2022; 41: 716–721. doi: 10.1016/j.healun.2022.02.002 [DOI] [PubMed] [Google Scholar]
- 19.Ghofrani HA, D'Armini AM, Grimminger F, et al. . Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369: 319–329. doi: 10.1056/NEJMoa1209657 [DOI] [PubMed] [Google Scholar]
- 20.Sadushi-Kolici R, Jansa P, Kopec G, et al. . Subcutaneous treprostinil for the treatment of severe non-operable chronic thromboembolic pulmonary hypertension (CTREPH): a double-blind, phase 3, randomised controlled trial. Lancet Respir Med 2019; 7: 239–248. doi: 10.1016/S2213-2600(18)30367-9 [DOI] [PubMed] [Google Scholar]
- 21.Jaïs X, Brenot P, Bouvaist H, et al. Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension (RACE): a multicentre, phase 3, open-label, randomised controlled trial and ancillary follow-up study. Lancet Respir Med 2022; 10: 961–971. 10.1016/S2213-2600(22)00214-4. [DOI] [PubMed] [Google Scholar]
- 22.Savale L, Huitema M, Shlobin O, et al. . WASOG statement on the diagnosis and management of sarcoidosis-associated pulmonary hypertension. Eur Respir Rev 2022; 31: 210165. doi: 10.1183/16000617.0165-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boucly A, Cottin V, Nunes H, et al. . Management and long-term outcomes of sarcoidosis-associated pulmonary hypertension. Eur Respir J 2017; 50: 1700465. doi: 10.1183/13993003.00465-2017 [DOI] [PubMed] [Google Scholar]
- 24.Baughman RP, Shlobin OA, Gupta R, et al. . Riociguat for sarcoidosis-associated pulmonary hypertension: results of a 1-year double-blind, placebo-controlled trial. Chest 2022; 161: 448–457. doi: 10.1016/j.chest.2021.07.2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baughman RP, Valeyre D, Korsten P, et al. . ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J 2021; 58: 2004079. doi: 10.1183/13993003.04079-2020 [DOI] [PubMed] [Google Scholar]
- 26.Humbert M, McLaughlin V, Gibbs JSR, et al. . Sotatercept for the treatment of pulmonary arterial hypertension. N Engl J Med 2021; 384: 1204–1215. doi: 10.1056/NEJMoa2024277 [DOI] [PubMed] [Google Scholar]
- 27.Humbert M, McLaughlin V, Gibbs JSR, et al. . Sotatercept for the treatment of pulmonary arterial hypertension: PULSAR open-label extension. Eur Respir J 2023; 61: 2201347. doi: 10.1183/13993003.01347-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goncharova EA, Simon MA, Yuan JX. mTORC1 in pulmonary arterial hypertension. At the crossroads between vasoconstriction and vascular remodeling? Am J Respir Crit Care Med 2020; 201: 1177–1179. doi: 10.1164/rccm.202001-0087ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He Y, Zuo C, Jia D, et al. . Loss of DP1 aggravates vascular remodeling in pulmonary arterial hypertension via mTORC1 signaling. Am J Respir Crit Care Med 2020; 201: 1263–1276. doi: 10.1164/rccm.201911-2137OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon M, Gomberg-Maitland M, Oudiz RJ, et al. . ABI-009, nab-sirolimus, an mTOR inhibitor with high lung accumulation in preclinical models is active in patients with severe pulmonary arterial hypertension. Am J Respir Crit Care Med 2019; 199: A4409. doi: 10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A4409 [DOI] [Google Scholar]
- 31.Galkin A, Sitapara R, Clemons B, et al. . Inhaled seralutinib exhibits potent efficacy in models of pulmonary arterial hypertension. Eur Respir J 2022; 60: 2102356. doi: 10.1183/13993003.02356-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frantz RP, Benza RL, Channick RN, et al. . TORREY, a phase 2 study to evaluate the efficacy and safety of inhaled seralutinib for the treatment of pulmonary arterial hypertension. Pulm Circ 2021; 11: 20458940211057071. doi: 10.1177/20458940211057071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoeper MM, Barst RJ, Bourge RC, et al. . Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the randomized IMPRES study. Circulation 2013; 127: 1128–1138. doi: 10.1161/CIRCULATIONAHA.112.000765 [DOI] [PubMed] [Google Scholar]
- 34.Grünig E, Eichstaedt C, Barberà J-A, et al. . ERS statement on exercise training and rehabilitation in patients with severe chronic pulmonary hypertension. Eur Respir J 2019; 53: 1800332. doi: 10.1183/13993003.00332-2018 [DOI] [PubMed] [Google Scholar]
- 35.Ehlken N, Lichtblau M, Klose H, et al. . Exercise training improves peak oxygen consumption and haemodynamics in patients with severe pulmonary arterial hypertension and inoperable chronic thrombo-embolic pulmonary hypertension: a prospective, randomized, controlled trial. Eur Heart J 2016; 37: 35–44. doi: 10.1093/eurheartj/ehv337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grünig E, MacKenzie A, Peacock AJ, et al. . Standardized exercise training is feasible, safe, and effective in pulmonary arterial and chronic thromboembolic pulmonary hypertension: results from a large European multicentre randomized controlled trial. Eur Heart J 2021; 42: 2284–2295. doi: 10.1093/eurheartj/ehaa696 [DOI] [PubMed] [Google Scholar]
- 37.McCormack C, Kehoe B, Hardcastle SJ, et al. . Pulmonary hypertension and home-based (PHAHB) exercise intervention: protocol for a feasibility study. BMJ Open 2021; 11: e045460. doi: 10.1136/bmjopen-2020-045460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voorburg JA, Cats VM, Buis B, et al. . Balloon angioplasty in the treatment of pulmonary hypertension caused by pulmonary embolism. Chest 1988; 94: 1249–1253. doi: 10.1378/chest.94.6.1249 [DOI] [PubMed] [Google Scholar]
- 39.Feinstein JA, Goldhaber SZ, Lock JE, et al. . Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 2001; 103: 10–13. doi: 10.1161/01.CIR.103.1.10 [DOI] [PubMed] [Google Scholar]
- 40.Mizoguchi H, Ogawa A, Munemasa M, et al. . Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 748–755. doi: 10.1161/CIRCINTERVENTIONS.112.971077 [DOI] [PubMed] [Google Scholar]
- 41.Olsson KM, Wiedenroth CB, Kamp JC, et al. . Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: the initial German experience. Eur Respir J 2017; 49: 1602409. doi: 10.1183/13993003.02409-2016 [DOI] [PubMed] [Google Scholar]
- 42.Brenot P, Jaïs X, Taniguchi Y, et al. . French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur Respir J 2019; 53: 1802095. doi: 10.1183/13993003.02095-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawakami T, Matsubara H, Shinke T, et al. . Balloon pulmonary angioplasty versus riociguat in inoperable chronic thromboembolic pulmonary hypertension (MR BPA): an open-label, randomised controlled trial. Lancet Respir Med 2022; 10: 949–960. doi: 10.1016/S2213-2600(22)00171-0 [DOI] [PubMed] [Google Scholar]
- 44.Welby JP, Fender EA, Peikert T, et al. . Evaluation of outcomes following pulmonary artery stenting in fibrosing mediastinitis. Cardiovasc Intervent Radiol 2021; 44: 384–391. doi: 10.1007/s00270-020-02714-z [DOI] [PubMed] [Google Scholar]
- 45.Reesink HJ, Henneman OD, van Delden OM, et al. . Pulmonary arterial stent implantation in an adult with Williams syndrome. Cardiovasc Intervent Radiol 2007; 30: 782–785. doi: 10.1007/s00270-007-9009-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis MJ, Kennedy KF, Ginns J, et al. . Procedural success and adverse events in pulmonary artery stenting: insights from the NCDR. J Am Coll Cardiol 2016; 67: 1327–1335. doi: 10.1016/j.jacc.2016.01.025 [DOI] [PubMed] [Google Scholar]
- 47.Rich S, Lam W. Atrial septostomy as palliative therapy for refractory primary pulmonary hypertension. Am J Cardiol 1983; 51: 1560–1561. doi: 10.1016/0002-9149(83)90678-1 [DOI] [PubMed] [Google Scholar]
- 48.Roy AK, Gaine SP, Walsh KP. Percutaneous atrial septostomy with modified butterfly stent and intracardiac echocardiographic guidance in a patient with syncope and refractory pulmonary arterial hypertension. Heart Lung Circ 2013; 22: 668–671. doi: 10.1016/j.hlc.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 49.Sandoval J, Gaspar J, Peïa H, et al. . Effect of atrial septostomy on the survival of patients with severe pulmonary arterial hypertension. Eur Respir J 2011; 38: 1343–1348. doi: 10.1183/09031936.00072210 [DOI] [PubMed] [Google Scholar]
- 50.Reichenberger F, Pepke-Zaba J, McNeil K, et al. . Atrial septostomy in the treatment of severe pulmonary arterial hypertension. Thorax 2003; 58: 797–800. doi: 10.1136/thorax.58.9.797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peters EL, Bogaard HJ, Vonk Noordegraaf A, et al. . Neurohormonal modulation in pulmonary arterial hypertension. Eur Respir J 2021; 58: 2004633. doi: 10.1183/13993003.04633-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen S-L, Zhang FF, Xu J, et al. . Pulmonary artery denervation to treat pulmonary arterial hypertension: the single-center, prospective, first-in-man PADN-1 study (first-in-man pulmonary artery denervation for treatment of pulmonary artery hypertension). J Am Coll Cardiol 2013; 62: 1092–1100. doi: 10.1016/j.jacc.2013.05.075 [DOI] [PubMed] [Google Scholar]
- 53.Chen J, Cui X, Qian Z, et al. . Multi-omics analysis reveals regulators of the response to PDGF-BB treatment in pulmonary artery smooth muscle cells. BMC Genomics 2016; 17: 781. doi: 10.1186/s12864-016-3122-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rothman AMK, Vachiery JL, Howard LS, et al. . Intravascular ultrasound pulmonary artery denervation to treat pulmonary arterial hypertension (TROPHY1): multicenter, early feasibility study. JACC Cardiovasc Interv 2020; 13: 989–999. doi: 10.1016/j.jcin.2019.12.027 [DOI] [PubMed] [Google Scholar]
- 55.Zhang H, Zhang J, Chen M, et al. . Pulmonary artery denervation significantly increases 6-min walk distance for patients with combined pre- and post-capillary pulmonary hypertension associated with left heart failure. JACC Cardiovasc Interv 2019; 12: 274–284. doi: 10.1016/j.jcin.2018.09.021 [DOI] [PubMed] [Google Scholar]
- 56.Romanov A, Cherniavskiy A, Novikova N, et al. . Pulmonary artery denervation for patients with residual pulmonary hypertension after pulmonary endarterectomy. J Am Coll Cardiol 2020; 76: 916–926. doi: 10.1016/j.jacc.2020.06.064 [DOI] [PubMed] [Google Scholar]