Abstract
American Indians (AI) experience disproportionately high prevalence of suicide and substance use disorders (SUD). However, accounting for risk burden (e.g. historical trauma and discrimination), the likelihood of mental health disorders or SUD is similar or decreased compared with the broader population. Such findings have spurred psychological research examining the protective factors, but no studies have investigated its potential neural mechanisms. Inhibitory control is one of the potential neurobehavioral construct with demonstrated protective effects, but has not been examined in neuroimaging studies with AI populations specifically. We examined the incidence of suicidal thoughts and behaviors (STB) and SUD among AI (n = 76) and propensity matched (sex, age, income, IQ proxy and trauma exposure) non-Hispanic White (NHW) participants (n = 76). Among the AI sample, functional magnetic resonance imaging (fMRI) data recorded during the stop-signal task (SST) was examined in relation to STB and SUDs. AIs relative to NHW subjects displayed lower incidence of STB. AIs with no reported STBs showed greater activity in executive control regions during the SST compared with AI who endorsed STB. AI without SUD demonstrated lower activity relative to those individual reporting SUD. Results are consistent with a growing body of literature demonstrating the high level of risk burden driving disparate prevalence of mental health concerns in AI. Furthermore, differential activation during inhibitory control processing in AI individuals without STB may represent a neural mechanism of protective effects against mental health problems in AI. Future research is needed to elucidate sociocultural factors contributing protection against mental health outcomes in AIs and further delineate neural mechanisms with respect to specific concerns (e.g. SUD vs STB).
Keywords: American Indians, suicide risk, inhibitory control, fMRI, substance use, protective factors
Background
Previous studies reported that American Indian (AI)1 populations suffer from disproportionately high prevalence of suicide and substance use disorders (SUD) (Beals, 2005; Brave Heart et al., 2016). Alarmingly, AI populations have demonstrated the highest increase in suicide rates of any ethnic group from 1999 to 2017, 139% for females and 71% for males (Curtin and Hedegaard, 2019). However, disparate prevalence has often been noted in the literature without consideration of factors, which likely drive higher levels of risk in these communities; specifically, it has been noted that systemic racism confers disproportionate health risks in particular racial categories (Jones, 2000). Among AI communities, many disproportionate risk factors, such as historical trauma, discrimination and trauma exposure contribute to increased risk for mental health concerns (Alcántara and Gone, 2007; Walls et al., 2021). Appropriate contextualization of research in health and mental health among marginalized populations is a critical consideration in this field due to the potential for harm to communities from unintended biases, misinterpretations and promulgation of stereotypes and prejudice (Beals et al., 2009; Jordan et al., 2021).
When researchers contextualize mental health concerns with respect to risk factors (e.g. trauma exposure and socioeconomic status), disparate prevalence has been demonstrated to vanish (Beals et al., 2002). In some work, AI individuals actually show lower prevalence of certain conditions (e.g. depression, anxiety; Beals, 2005). For example, the prevalence of post-traumatic stress disorder (PTSD) among AIs may be inflated due to higher prevalence of trauma exposure (Robin et al., 1997; Beals et al., 2002; Manson et al., 2005). Thus, mental health disparities among AIs are likely driven by increased risk burden (Beals, 2005; Evans-Campbell et al., 2006; King et al., 2009; Kading et al., 2015). However, contextualizing mental health concerns with respect to particular risk factors is an underdeveloped area of research particularly in the context of SUD.
There is a burgeoning body of previous and ongoing work, which has focused on mental health research and the development of community programs to mitigate risk burden and promote well-being among AI populations (Les Whitbeck et al., 2004; Blue Bird Jernigan et al., 2015; Walters et al., 2019). Furthermore, recent research in AI mental health has focused on strength-based approaches for understanding the protective and resilience factors (Stumblingbear-Riddle and Romans, 2012; Wexler, 2014; Elm et al., 2016; Oré et al., 2016; Teufel-Shone et al., 2018), which is a significant gap in quantitative research in AI populations. Specific calls related to strengths-based research and protective factors in AIs highlight the need for understanding the mechanisms of protective effects, multi-level interaction and consideration of unintended impacts among other recommendations (Allen et al., 2022).
Clinical neuroscience techniques hold significant promise for extending the level of analysis in mental health research in AI and characterizing mechanisms of risk and protective factors. Notably, broader calls for clinical neuroscience work aim at integrating knowledge of brain and nervous system function from basic science research into an understanding of etiology and maintenance of mental disorders and substance use with an ultimate goal of informing intervention and prevention efforts (e.g. Insel and Quirion, 2005; NIDA, 2010; NIMH, 2021). Unfortunately, very little research has investigated psychological and neural mechanisms underlying the risk for protection against mental health problems within AI populations. In fact, AI populations are largely absent in mental health neuroscience research in general (e.g. neuroimaging and genetics research). This is problematic as advances in clinical neuroscience identified in the general population may not be generalized to AI communities; furthermore, there may be unique aspects of AI populations that confer protection against mental health difficulties that neuroscience could be helpful in further delineating. This underscores the need to have clinical neuroscience research within AI populations to inform our understanding of potential common and unique protective and risk factors. Furthermore, such work represents an intersection of the cutting edge of both AI mental health research, which calls for understanding the mechanisms and multi-level interactions (Allen et al., 2022) and clinical neuroscience priorities in increasing diversity and understanding the environmental influences on risk for mental health conditions (NIMH, 2021).
The dearth of neuroscience research among AIs is a critical gap in the overarching goal of reducing mental health disparities among AI and there are numerous potential functional domains that may be relevant for the study of neuroscientific indicators of adaptive functioning in AI populations. A prudent first step is to examine well-established adaptive neurocognitive functions among AI samples to determine the generalizability of findings within this population and establish associations with mental health concerns. Inhibitory control is a cognitive function that serves to prevent and regulate impulsive behaviors (Logan et al., 1997) and prepotent behavioral responses during both neutral and emotional situations (Diamond, 2013). Higher levels of inhibitory control are associated with lower levels of multiple forms of psychopathology across behavioral and functional brain studies (Aupperle et al., 2012; McTeague et al., 2016; White and Grant, 2017). Neural correlates of adaptive inhibitory control have also been associated with decreased risk for SUD (Holmes et al., 2016) and increased ability to regulate craving for substances (Kober et al., 2010). Furthermore, deficits in inhibitory control are associated with increased risk of suicidal behavior (Nock et al., 2010; Jollant et al., 2011). Notably, specific brain regions associated with inhibitory control are sensitive to functional context and measurement strategy (e.g. task design). The dorsolateral prefrontal cortex (dlPFC), in particular, has been implicated in inhibitory control in emotional contexts (Goldstein et al., 2007). Additionally, a well-established empirical paradigm for indexing inhibitory control (e.g. SST; Matthews et al., 2005) indicates blood-oxygenation level dependent (BOLD) signal increases in the dlPFC and inferior frontal gyrus (IFG) under inhibitory control demands. Both the IFG and dlPFC have also been implicated in research examining response inhibition deficits associated with SUD (see Spechler et al., 2016). Thus, inhibitory control as indexed by BOLD signal response in IFG and dlPFC during SST is an ideal candidate for the initial exploration of a potential mechanism for adaptive cognitive function that may be inversely related to mental health concerns among AI populations.
The intention of the current study was to explore potential adaptive functioning mechanisms and provide a foundation for a strength-based neuroscientific perspective of mental health among AI populations. There is precedent and potential for stigma and inadvertent harm that can come from research on mental health and substance use conditions affecting Indigenous communities (e.g. Beals et al., 2009; Drabiak-Syed, 2010). Thus, aim 1 of the current study examines the incidence of suicidal thoughts and behaviors (STB) reported during clinical interview and SUD across the lifespan among a sample of AI individuals in comparison to a non-Hispanic white (NHW) sample, to demonstrate that sociodemographic and risk factors (i.e. trauma-exposure) likely account for potential disparities present in this sample consistent with previous research (e.g. Beals et al., 2002). Aim 2 of the current study is to delineate difference in neural markers of inhibitory control for AI individuals with and without STB and for those with vs without SUD. Notably, aim 2 is conducted only within the AI group as the intention of the study is to establish potential neural mechanisms of adaptive cognitive function relative to mental health concern in this population. Furthermore, comparisons across racial groups are inconsistent with the goals of the current work as race is not appropriately conceptualized as a causal (Holland, 2003) or biological (Templeton, 2013) variable and may be better conceptualized as epi-phenomenological considering the differential exposure to modifiable factors (e.g. income, access to healthcare, education and discrimination) across racial categories. Regarding the first aim, we hypothesized that when propensity matching for sociodemographic and risk factors AIs would demonstrate equivalent and or lower incidence of STB and SUD than NHW, consistent with previous research. With respect to aim 2, we hypothesized AI individuals with either no STB or without SUD would exhibit higher levels of activation in inhibitory control networks (Spechler et al., 2016) (i.e. dlPFC and IFG) during response inhibition in the SST compared with AI individuals with STB and/or SUD.
Methods and materials
Participants
Participants for the current study were drawn from the first 500 participants in the baseline assessment of the Tulsa-1000 (T1000) study (Victor et al., 2018). The total T1000 sample is divided into two halves (n = 500/sample), the first half for exploratory studies and the second half with controlled access is for confirmatory, replication and preregistered studies. The current investigation falls in the former category. The T1000 study recruited treatment seeking adults (age 18–55) from the Tulsa community and local treatment facilities with mood, anxiety, substance use and eating disorders, as well as healthy comparison participants, in order to identify neural, behavioral, self-report, physiological and biological variables derived from blood-based analytes to identify factors with potential clinical utility. Screening for anxiety, mood, substance use and eating disorders was based on Patient Health Questionnaire-9 score ≥ 10, Overall Anxiety Sensitivity and Impairment Scale ≥ 8; Drug Abuse Screening Test-10 > 2 Sick, Control, One, Fat, Food Questionnaire score ≥ 2. Full screening procedures and inclusion/exclusion criteria are reported in the open access T1000 protocol paper (Victor et al., 2018). This study was reviewed and approved by the Western Institutional Review Board. All participants provided written informed consent prior to participation, in accordance with the Declaration of Helsinki, and were compensated for participation (ClinicalTrials.gov identifier: #NCT02450240).
Participants in the current analyses self-identified their race and ethnicity. Those who self-identified as AI to any degree were included in the AI group (n = 89) and those who identified as NHW were included in the comparison group (n = 348) for a total initial sample of (n = 437). Participants were administered the MINI International Neuropsychiatric Interview (6.0 or 7.0) (Sheehan and Lecrubier, 2010; Sheehan et al., 2015) by clinical study staff to assess lifetime mental disorders, defined by the Diagnostic and Statistical Manual of Mental Disorders (4th or 5th Edition; American Psychiatric Association, 2000, 2013). Demographic information and screening measure scores are described in Table 1. Thirteen individuals in the AI group were excluded from analyses due to missing data on variables of interest resulting in a sample of (n = 76) for analysis of incidence rates. Propensity matching procedures (see Analytic Strategy) generated a matched sample of NHW participants (n = 76) from the broader pool of participants. For subsequent fMRI BOLD signal analysis among the AI subsample eight subjects were excluded for poor quality data (see Neuroimaging Data Processing section below) resulting in a final sample size of n = 68.
Table 1.
Demographic and screening data in the full sample for American Indian (AI) and non-Hispanic White (NHW) groups
| AI (n = 89) | NHW (n = 387) | ||||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | t | df | p | |
| Age (years) | 34.65 | 9.61 | 34.59 | 11.07 | 0.05 | 156 | 0.96 |
| Income ($) | 28 238.38 | 32 874.05 | 54 634.89 | 79 409.82 | −4.57 | 313 | <0.001 |
| PHQ | 8.27 | 5.59 | 9.52 | 6.68 | −1.82 | 162 | 0.07 |
| DAST | 4.20 | 3.85 | 2.46 | 3.54 | 3.89 | 130 | <0.001 |
| OASIS | 7.17 | 5.01 | 7.57 | 4.60 | −0.69 | 130 | 0.49 |
| SCOFF | 0.63 | 1.02 | 0.97 | 1.27 | −2.67 | 168 | 0.008 |
| Frequency | Frequency | ||||||
| Education (categories) | Χ 2 = 10.66, p = 0.01 | ||||||
| Less than high school | 15 (16.67%) | 26 (7.51%) | |||||
| High school or GED | 24 (26.67%) | 69 (19.94%) | |||||
| Some college | 24 (26.67%) | 127 (36.71%) | |||||
| College degree or higher | 27 (30%) | 124 (35.83%) | |||||
| Sex (female) | 52 (57.78%) | 229 (65.81%) | Χ 2 = 2.00, p = 0.16 | ||||
Note: PHQ = Patient Health Questionnaire-9; DAST = Drug Abuse Screening Test; OASIS = Overall Anxiety Severity and Impairment Scale; SCOFF = ‘SCOFF’ questionnaire screening tool for eating disorders.
Procedure
Overall procedures included clinical interview and assessment sessions and a neuroimaging session completed within a 2-week period. Only details relevant to the current analyses are presented here, but the full protocols of the parent project are available (Victor et al., 2018).
Clinical interview and measures
The MINI clinical interview was administered by trained study staff and during this session participants provided self-reported information regarding demographics (i.e. age, income, race and ethnicity), trauma exposure and completed the Wide Range Achievement Test (Wilkinson and Robertson, 2006). Trauma exposure was assessed using Traumatic Events Questionnaire (Vrana and Lauterbach, 1994) and the Childhood Trauma Questionnaire (Bernstein et al., 2003). STB was assessed by the MINI interview as indicated on the suicidality module with questions regarding suicidal ideation, intent, plan and previous suicidal behaviors within the past month. Specifically, individuals who met criteria for any level of STB (i.e. low, moderate and high) were included in the STB group and those who did not were included in the no-STB group. The presence of SUD was established based on any substance use disorder diagnosis within the year prior to participation integrating reporting information from the MINI and the Tulsa Life Chart (Aupperle et al., 2020), with life time substance use verified by the Customary Drinking and Drug Use Record (Brown et al., 1998). See supplementary information for frequency of various current substances of choice present in the matched samples (Table S1), number of individuals with SUD diagnosed by the MINI or Tulsa Life Chart or both and those without SUD (Table S2).
Neuroimaging session
Participants completed 288 trials of the SST (Matthews et al., 2005) over approximately 8 min and 32 s during fMRI. In this task, participants were asked to respond to an ‘X’ and ‘O’ with either a right or left button press, but on 25% of the trials (n = 72), an auditory tone and color change (i.e. ‘stop-signal’) indicated they should not respond. Notably, this task was previously examined in a portion of the T1000 sample (n =121) with respect to anxiety, depression and cannabis use (Spechler et al., 2020). Trials were presented in six blocks of 48 trials with 12 s breaks between blocks. Difficulty of the trials was manipulated by delay of stop-signal following target stimuli calibrated to each individual during practice trials. Easy trials having longer delays (i.e. 500, 400 and 300 ms) and hard trials having shorter delays (i.e. 200, 100 and 0 ms) relative to participants mean reaction RT on practice trials. For the current study, response inhibition is indexed as the percent signal change (PSC) in BOLD response on hard compared with easy successful stop trials.
Functional magnetic resonance imaging (fMRI) was acquired during the SST with two identical GE MR750 3 T scanners consisting of contiguous echo-planar imaging (EPI) volumes (39 axial slices, TR/TE = 2000/27 ms, flip angle = 78°, sampling bandwidth = 250 kHz, FOV/slice thickness = 240/2.9 mm, 128 × 128 matrix producing 1.875 × 1.875 × 2.9 mm voxels, 256 volumes, R/L frequency encoding direction, SENSE acceleration factor = 2). Additionally, high-resolution structural images were obtained through a 3D axial T1-weighted magnetization-prepared rapid acquisition with gradient echo sequence (TR/TE = 5/2.0 12 ms, FOV/slice thickness = 240/0.9 mm, flip angle = 8°, sampling bandwidth = 31.25 kHz, 256 × 256 matrix producing 0.938 × 0.938 × 0.9 mm voxels, 186 axial slices, SENSE acceleration factor = 2).
Neuroimaging data processing
The current study included AI participants with quality fMRI data (n = 68). Participants were excluded if they had an average Euclidean norm of the derivatives of the six motion parameters (ENORM) greater than 0.3 across and had significant artifacts on visual inspection, or no SST data available. Neuroimaging data were processed and analyzed using Analysis of Functional Neuroimaging (AFNI; http://anfi.nimh.nih.gov) software (Cox, 2012). The three initial EPI volumes during the run were removed to allow time for signal stabilization and noise adaptation. Then data processing included despiking, correction for slice timing, co-registering to anatomical volumes, correction for motion via affine registration, smoothing (4 × 4 × 4 mm3 full width at half maximum) and normalizing to Montreal Neurological Institute standard space (resampled voxel size 2 × 2 × 2 mm3). General linear model analyses were employed to model task-related brain activation. Modeling included five task-related regressors: successful easy stops, successful hard stops, unsuccessful easy stops, unsuccessful hard stops and successful go trials. Additionally, nuisance regressors included six motion parameters (roll/pitch/yaw/x/y/z translation) and four baseline polynomials. Any TR with an ENORM greater than 0.3 or an outlier fraction greater than 0.1 (via 3dToutcount) was censored at the regression step. Model fits were estimated from single subject general linear models (i.e. beta coefficients for condition contrasts hard minus easy). Data were then extracted for regions of interest (ROIs) corresponding to brain regions activated in the SST previously (IFG and dlPFC) (Matthews et al., 2005) using the Brainnetome Atlas (BNA) (Fan et al., 2016); specific Brainnetome labels ROIs are reported in supplementary materials; Table S3). ROIs were constructed for the left and right hemisphere separately to account for potential laterality effects. Only voxels with a temporal signal to noise ratio of greater than 50 were included in ROI analyses. Task effect images from the parent sample (Figure S1 and Table S4) and exploratory whole brain analyses in the AI subsample can be found in supplementary material (Figures S2 and S3).
Analytic strategy
Incidence of STB and SUD
Propensity matching was employed to examine incidence of STB and SUD in the current sample, while properly accounting for sociodemographic factors and psychological risk factors. Propensity score matching is used in observational studies to reduce bias introduced by covariates by balancing them across two comparison groups (D’Agostino, 1998). Propensity matching was conducted using the MatchIt R program (Ho et al., 2011) following procedural recommendations from Randolph et al. (2014). Individuals who self-identified as AI in the T1000 study were matched with NHW individuals from the original sample on sociodemographic variables (i.e. sex, age, income, education and WRAT reading score), which have been shown to be associated with differential risk for STB and SUD, as well as differential fMRI metrics of executive function (Graham et al., 2010; Patrick et al., 2012; Page et al., 2014). Due to its role as a psychological risk factor, trauma exposure (i.e. CTQ and TES) was also included in the matching procedure. The resulting 1-to-1 subsample of participants was across two groups, AI and NHW. Propensity matching is employed to address confounding in the context of observational studies (Austin, 2011) often results in balance across comparison groups with respect to variables included in the matching; however, this result should be verified analytically (Ohlsson and Kendler, 2020). Thus, results of the procedures with respect to matching variables can be seen in Table 2. To evaluate the relative incidence of STB and SUD, chi-square analyses were conducted across the two groups on each variable separately.
Table 2.
Propensity matched verification in matched American Indian (AI) and non-Hispanic White (NHW) participants
| AI (n = 76) | NHW (n = 76) | ||||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | t | df | p | |
| Age (years) | 33.36 | 9.46 | 34.48 | 10 | −0.56 | 150 | 0.58 |
| WRAT | 60.25 | 6.24 | 60.66 | 6.43 | 0.40 | 150 | 0.69 |
| Income ($) | 25 221.45 | 26 942.43 | 22 968.00 | 27 369.13 | −0.51 | 150 | 0.61 |
| TES occurrence (total) | 3.88 | 3.25 | 4.13 | 3.62 | 0.45 | 148 | 0.65 |
| TES intensity (worst) | 10.38 | 7.69 | 10.87 | 6.89 | 0.41 | 148 | 0.68 |
| CTQ denial | 8.25 | 3.43 | 7.99 | 3.11 | −0.50 | 149 | 0.62 |
| CTQ emotional abuse | 10.45 | 5.17 | 10.99 | 5.06 | 0.65 | 150 | 0.52 |
| CTQ emotional neglect | 11.08 | 5.28 | 11.58 | 4.87 | 0.61 | 149 | 0.54 |
| CTQ physical abuse | 8.88 | 4.02 | 8.99 | 4.33 | 0.16 | 149 | 0.88 |
| CTQ physical neglect | 7.97 | 3.28 | 7.96 | 3.57 | −0.02 | 149 | 0.98 |
| Frequency | Frequency | ||||||
| Education (categories) | Χ 2 = 6.85, p = 0.08 | ||||||
| Less than high school | 11 (14.74%) | 6 (7.90%) | |||||
| High school or GED | 20 (26.32%) | 17 (22.37%) | |||||
| Some college | 21 (27.63%) | 36 (47.37%) | |||||
| College degree or higher | 24 (31.58%) | 17 (22.37%) | |||||
| Sex (female) | 46 (60.53%) | 46(60.53%) | n/a | ||||
Note: WRAT = Wide Ranging Achievement Test; TES = Traumatic Experiences Scale; CTQ = Childhood Trauma Questionnaire.
Inhibitory control
The second aim of the study is to determine if within the AI sub-sample, there is an association between neural indicators of inhibitory control and STB or SUD. Welch’s two sample t-tests, which are robust to the assumption of homogeneity of variance (Zimmerman, 2004; Field et al., 2012), were used to examine mean PSC differences within a priori ROIs (i.e. dlPFC [3 bilateral ROIs], IFG [2 bilateral ROIs]) using BNA defined ROIs (see Table S3, Figure S4) to compare individuals with and without current STB and SUD. Exploratory whole brain analyses were also conducted (see supplementary material).
Results
Incidence comparisons
The groups did not differ on matching variables following the propensity matching procedures (see Table 2). With respect to STB, individuals in the AI group displayed a lower incidence when compared with the matched sample of NHW (Χ2 = 6.81, P = 0.009, OR = 0.42; Figure 1A). Regarding SUD incidence, there were no differences between the AI group and NHW group (Χ2 = 0.028, P = 0.87; Figure 1B). Chi-squared analysis in the unmatched samples can be found in supplementary material.
Fig. 1.
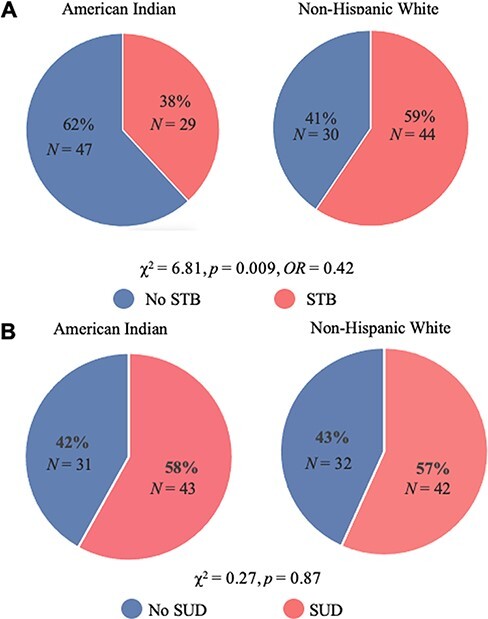
Pie charts displaying incidence rates comparison between AI and NHW in the matched sample.
Note: STB = suicidal thoughts and behaviors; SUD = substance use disorder.
Neuroimaging
Among the AI sample, individuals without STB, relative to individuals with STB, demonstrated increased BOLD signal activation in the left dlPFC (BNA-ROI-19: t(49.06) = 2.44, P = 0.02 Hedge’s g = 0.60 CI95 = [0.09, 1.11]; BNA-ROI-21: t(57.71) = 2.15, P = 0.04, Hedge’s g = 0.50 CI95 = [−0.01, 1.01]); right dlPFC (BNA-ROI-20: t(51.9) = 2.06, P = 0.04, Hedge’s g = 0.50 CI95 = [−0.01, 1.01]) and left IFG (BNA-ROI-31: t(45.393) = 2.22, P = 0.03, Hedge’s g = 0.56 CI95 = [0.05, 1.07]) during hard vs easy contrast of the SST. No other BNA ROIs were significantly different between STB groups (P’s = 0.06–0.22).
Interestingly, with respect to SUD, individuals without SUD demonstrated significantly lower BOLD signal response in the left IFG (BNA-ROI-31: t(57.36) = −2.00, P = 0.05 Hedge’s g = −1.581 CI95= [−1.96, −1.20]) and left dlPFC (BNA-ROI-19: t(57.12) = −2.56, P = 0.01 Hedge’s g = −1.582 CI95= [−1.96, −1.20]; BNA-ROI-21: t(58.53) = −2.1, P = 0.03 Hedge’s g = −1.583 CI95= [−1.96, −1.20]); no other BNA ROIs showed significant differences between SUD groups (P’s = 0.06–0.49). See supplement for exploratory whole-brain linear mixed effect models. Of note, the dlPFC group differences based on STB were also significant in whole-brain analyses (voxel-wise threshold of P < 0.005; cluster corrected at P < 0.05 using a threshold of 83 voxels; Figure S2).
Discussion
This study addressed two questions: (i) to determine the incidence of STB and SUD in AI individuals, after accounting for sociodemographic variables and trauma exposure and (ii) to examine possible neural mechanisms by examining associations of STB and SUD with neural markers of inhibitory control in AI participants. There were three main results. First, after accounting for sociodemographic variables as well as trauma exposure as a risk factor, AI demonstrated lower incidence of STB and equivalent rates of SUD relative to a matched NHW sample. Second, AI participants with no STB relative to those with reported STB showed greater activity in executive control regions during the SST. Third, AI individuals with a history of SUD showed higher activation in executive control regions than those with no history. These findings are an important addition to this literature as AIs have displayed the largest increase in suicide rates of any ethnic group in the recent decade (Curtin and Hedegaard, 2019) and have displayed high prevalence of SUD (Beals, 2005; Brave Heart et al., 2016). These data extend previous findings that indicate the high level of risk (i.e. trauma exposure) accounting for inflated prevalence of PTSD in a Southwestern AI tribe (Robin et al., 1997). Taken together, this body of evidence supports two critical implications: (i) there is an extremely high risk burden in AI communities (Les Whitbeck et al., 2004; Alcántara and Gone, 2007) and (ii) when accounting for risk level and socioeconomic variables, AI individuals may have important protective factors against poor mental health (i.e. STB and SUD). This is consistent with previous research demonstrating high incidence of positive mental health among two AI communities despite disproportionately high stressors (Kading et al., 2015).
The current investigation also explored the potential for neural factors that may be protective among the AI sub-sample. Results from the fMRI BOLD signal data indicate that individuals with no STB demonstrated greater activation in brain regions associated with executive control (i.e. dlPFC and IFG; Matthews et al., 2005) during a response inhibition task than those at risk. Extant literature supports the beneficial role of inhibitory control on emotion regulation (Diamond, 2013) and documents low levels of inhibitory control across multiple psychopathologies (McTeague et al., 2016) and suicidality (Jollant et al., 2011). Thus, current results extend this literature by demonstrating that inhibitory control is associated with lower STB among AIs. Although, the potential adaptive effects of inhibitory control are not unique to AIs, this study provides initial evidence that inhibitory control is relevant for clinical neuroscience research among AIs and future work in this area should aim to identify factors that support optimal inhibitory control among AIs specifically.
This activation pattern was in the opposite direction for individuals with SUD relative to no history of SUD. Thus, inhibitory control-related BOLD signal was greater in AI individuals with relative to those without SUD. Notably, inhibitory control disruptions are a key risk factor for SUD (Heitzeg et al., 2015). However findings regarding fMRI activation are mixed with respect to dlPFC and IFG BOLD signal differences in substance use disorder (Zilverstand et al., 2018; Hildebrandt et al., 2021; Le et al., 2021). Direction of activation differences (hypo/hyperactivation) may be dependent on substance type and current use vs abstinence (see: Le et al., 2021); furthermore, a recent systematic review indicates that degree of substance use and substance related difficulties may play a key role in differential neural activity associated with response inhibition (Hildebrandt et al., 2021). Importantly, current data include substance use disorder diagnosis within the past year and participants report a range of substance preferences (see Table S1). Our data suggest that AI individuals with substance use disorder show greater activation in prefrontal cortical regions during inhibition. However, more work is needed to delineate the nuance in the relationship of substance type and use vs abstinence and neural markers of inhibition broadly and especially among AI populations.
These findings point to the intriguing possibility of culturally based resilience to mental health problems among AI individuals that may be evidenced by adaptive neurocognitive functioning. Recent research has identified specific cultural influences among AIs (i.e. enculturation, spirituality and traditional healing) and differences in social support (i.e. extended family networks and tribal connection) that may be protective against poor mental health (i.e. SUD and suicidality; Fleming and Ledogar, 2008) and predictive of well-being (LaFromboise et al., 2006; Kirmayer et al., 2011). There are multiple factors of particular salience to AI individuals (e.g. enculturation and spirituality) and communities (e.g. social connectedness; LaFromboise et al., 2006; Fleming and Ledogar, 2008; Kirmayer et al., 2011) that may enhance inhibitory control (Tsethlikai, 2015). Theoretical work indicates that sociocultural connections convey the resilience against major life stressors (Doane et al., 2012) and play an important role in the development of the ability to cognitively manipulate information (Dunbar, 2003). Specifically, rooted in evolutionary theory, the social brain hypothesis posits that social group complexity in humans is closely evolutionarily interconnected with cognitive control processes, perhaps given the use of such processes for tracking and navigating the structure of their social networks and relationships therein (Dunbar, 2003; Stout, 2010). Future work is needed to establish the potential relationships between cultural factors and adaptive neurocognitive function among AI populations.
Current findings should be contextualized within notable limitations to the data. The cross-sectional nature of these data precludes the ability to make causal or directional inferences regarding identified relationships. Due to the novelty of the current approach, we chose to focus analyses on mental health difficulties noted as particularly relevant for AI populations (Beals, 2005; Brave Heart et al., 2016; Curtin and Hedegaard, 2019) and physical and mental health comorbidities were not evaluated in the present analysis. Notably, suicidality was indexed based on the MINI clinical interview suicidality module rather than previous suicidal behaviors. However, this module has been shown to be predictive of suicidal behaviors (Roaldset et al., 2012). Although dichotomizing the data into risk and absence of reported risk eliminates some granularity in the measurement of suicide risk, it is a reasonable first approximation of suicidality in the current sample. Future investigations are needed to examine cognitive control suicide related ideation and behaviors among AI, as the current study was not designed or powered to examine suicide with a high degree of specificity. Furthermore, this examination comprises secondary data analysis that was not designed to address the particular risk and protective factors among AI. Thus, there was no data collected regarding specific risk factors (e.g. historical trauma and discrimination) and/or cultural protective factors (e.g. familial structures, social support and traditional culture engagement) that may be present within this sample. Notably in the unmatched samples, AI demonstrated lower STB and increased SUD incidence relative to NHW; however, this does not preclude the examination of neural mechanisms of protective effects in the current sample. Importantly, there is substantial heterogeneity among AI peoples with over 573 federally recognized tribes in the USA and variability in whether individuals live on or off reservations and in urban or rural settings (Weaver, 2012); the current analyses cannot account for the influence of heterogeneity among the AI sample. Although the current investigation focused on sociodemographic factors and trauma exposure as risk factors for STB, it is important to note that other factors also play a role in risk for STB (e.g. depression and other psychopathology symptoms). Furthermore, mechanistic research in STB among AI populations is very limited; thus, the field would benefit from future investigations examining mechanisms of the relationship between symptoms of psychopathology and STB among AIs. Additionally, inhibitory control only represents one potential mechanism of protective factors against STB and SUD. Future research is needed to examine culturally specific variables that may convey protective effects as well as a broader range of potential neural mechanisms.
Conclusions
The current findings indicate that, when accounting for sociodemographic and psychological risk factors (i.e. trauma exposure), STB are lower and SUD is not different among a sample of AI compared with NHWs recruited from the general community. Furthermore, examination of fMRI BOLD signal activation during SST revealed that AI individuals without STB displayed greater activation in prefrontal cortical regions associated with executive control (e.g. dlPFC and IFG) than AI with STB. In sum, contrary to conclusions drawn from raw prevalence statistics (Curtin and Hedegaard, 2019), contextualization of prevalence of STB indicate there may be protective factors against STB among AI. Furthermore, inhibitory control may represent a potential mechanistic aspect of adaptive neural functioning relevant for AI mental health research, thus adding to a burgeoning literature on cultural factors that promote mental health among AIs (Kading et al., 2015).
Supplementary Material
Acknowledgements
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
It is important to note that terms such as American Indian/Alaska Native (AIAN), Native, Indigenous, First Peoples, have often been used interchangeably and it is most respectful to use terminology individuals and communities prefer. For the current study, we use the term AI to refer to individuals from heterogenous Native tribes represented in northeastern Oklahoma.
Contributor Information
Evan J White, Laureate Institute for Brain Research, Tulsa, OK 74136, USA; Oxley School of Community Medicine, University of Tulsa, Tulsa, OK 74119, USA.
Mara J Demuth, Laureate Institute for Brain Research, Tulsa, OK 74136, USA.
Mariah Nacke, Laureate Institute for Brain Research, Tulsa, OK 74136, USA.
Namik Kirlic, Laureate Institute for Brain Research, Tulsa, OK 74136, USA.
Rayus Kuplicki, Laureate Institute for Brain Research, Tulsa, OK 74136, USA.
Philip A Spechler, Laureate Institute for Brain Research, Tulsa, OK 74136, USA.
Timothy J McDermott, Laureate Institute for Brain Research, Tulsa, OK 74136, USA; Department of Psychology, University of Tulsa, Tulsa, OK 74104, USA.
Danielle C DeVille, Laureate Institute for Brain Research, Tulsa, OK 74136, USA; Department of Psychology, University of Tulsa, Tulsa, OK 74104, USA.
Jennifer L Stewart, Laureate Institute for Brain Research, Tulsa, OK 74136, USA; Oxley School of Community Medicine, University of Tulsa, Tulsa, OK 74119, USA.
John Lowe, School of Nursing, University of Texas at Austin, Austin, TX 78712, USA.
Martin P Paulus, Laureate Institute for Brain Research, Tulsa, OK 74136, USA; Oxley School of Community Medicine, University of Tulsa, Tulsa, OK 74119, USA.
Robin L Aupperle, Laureate Institute for Brain Research, Tulsa, OK 74136, USA; Oxley School of Community Medicine, University of Tulsa, Tulsa, OK 74119, USA.
Funding
Evan J. White Ph.D. receives funding support from the National Institute on Minority Health and Health Disparities (NIMHD) under Award No. K99MD015736. Rayus Kuplicki, Ph.D. Jennifer L. Stewart, Ph.D., Namik Kirlic, Ph.D., Jerzy Bodurka, Ph.D., Robin Aupperle Ph.D. and Martin Paulus M.D. receive funding from the National Institute of General Medical Sciences (NIGMS) Center Grant No. P20GM121312; Jennifer L. Stewart, Ph.D., has additional grant funding from the National Institute on Drug Abuse (R01DA050677); Robin Aupperle Ph.D. has additional grant funding from The National Institute of Mental Health (K23MH108707 and R01MH123691); and Martin Paulus, M.D. has additional grant funding from the National Institute of Drug Abuse (U01DA041089). Timothy McDermott, M.A., received support from the National Institute of Mental Health under Award No. F31MH122090. This work has been supported in part by The William K. Warren Foundation.
Conflict of interest
Evan J. White, Ph.D. Mara J. Demuth, M.A., Mariah Nacke, B.S., Namik Kirlic, Ph.D., Rayus Kuplicki, Ph.D., Phillip A. Spechler, Ph.D., Timothy J. McDermott, M.A., Danielle C. DeVille, M.A., Sahib Khalsa, M.D., Johnathan Savits Ph.D., Salvador Guinjoan M.D., Teresa Victor, Ph.D., Jennifer L. Stewart, Ph.D., John Lowe, R.N., Ph.D. and Robin L. Aupperle, Ph.D., have no financial conflicts of interests to disclose. Martin P. Paulus, M.D. is an advisor to Spring Care, Inc., a behavioral health startup, he has received royalties for an article about methamphetamine in UpToDate.
Supplementary data
Supplementary data are available at SCAN online.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Alcántara C., Gone J.P. (2007). Reviewing suicide in native American communities: situating risk and protective factors within a transactional–ecological framework. Death Studies, 31(5), 457–77.doi: 10.1080/07481180701244587. [DOI] [PubMed] [Google Scholar]
- Allen J., Wexler L., Rasmus S. (2022). Protective factors as a unifying framework for strength-based intervention and culturally responsive American Indian and Alaska native suicide prevention. Prevention Science, 23(1), 59–72.doi: 10.1007/s11121-021-01265-0. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . (2000). Diagnostic and Statistical Manual of Mental Disorders, 4th edn, Washington, DC: Text Revision, American Psychiatric Association. [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th edn, Washington, DC: American Psychiatric Association. [Google Scholar]
- Aupperle R.L., Melrose A.J., Stein M.B., Paulus M.P. (2012). Executive function and PTSD: disengaging from trauma. Neuropharmacology, 62(2), 686–94.doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle R.L., Paulus M.P., Kuplicki R., et al. (2020). Web-based graphic representation of the life course of mental health: cross-sectional study across the spectrum of mood, anxiety, eating, and substance use disorders. JMIR Mental Health, 7(1), e16919.doi: 10.2196/16919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin P.C. (2011). An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behavioral Research, 46(3), 399–424.doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beals J., Manson S.M., Shore J.H., et al. (2002). The prevalence of posttraumatic stress disorder among American Indian Vietnam veterans: disparities and context. Journal of Traumatic Stress, 15(2), 89–97.doi: 10.1023/A:1014894506325. [DOI] [PubMed] [Google Scholar]
- Beals J. (2005). Prevalence of DSM-IV disorders and attendant help-seeking in 2 American Indian reservation populations. Archives of General Psychiatry, 62(1), 99.doi: 10.1001/archpsyc.62.1.99. [DOI] [PubMed] [Google Scholar]
- Beals J., Belcourt-Dittloff A., Freedenthal S., et al. (2009). Reflections on a proposed theory of reservation-dwelling American Indian alcohol use: comment on Spillane and Smith (2007). Psychological Bulletin, 135(2), 339–43.doi: 10.1037/a0014819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D.P., Stein J.A., Newcomb M.D., et al. (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect, 27(2), 169–90.doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Blue Bird Jernigan V., Peercy M., Branam D., et al. (2015). Beyond health equity: achieving wellness within American Indian and Alaska Native communities. American Journal of Public Health, 105(Suppl 3), S376–9.doi: 10.2105/AJPH.2014.302447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brave Heart M.Y.H., Lewis-Fernández R., Beals J., et al. (2016). Psychiatric disorders and mental health treatment in American Indians and Alaska Natives: results of the National Epidemiologic Survey on alcohol and related conditions. Social Psychiatry and Psychiatric Epidemiology, 51(7), 1033–46.doi: 10.1007/s00127-016-1225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.A., Myers M.G., Lippke L., Tapert S.F., Stewart D.G., Vik P.W. (1998). Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol, 59(4), 427–38.doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Cox R.W. (2012). AFNI: what a long strange trip it’s been. NeuroImage, 62(2), 743–7.doi: 10.1016/j.neuroimage.2011.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin S.C., Hedegaard H. (2019). Suicide rates for females and males by race and ethnicity: United States, 1999 and 2017. 6.
- D’Agostino R.B. (1998). Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statistics in Medicine, 17(19), 2265–81.doi: . [DOI] [PubMed] [Google Scholar]
- Diamond A. (2013). Executive functions. Annual Review of Psychology, 64, 135–68.doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane L.S., Schumm J.A., Hobfoll S.E. (2012). The positive, sustaining, and protective power of resources: insights from conservation of resources theory. In: Törnblom, K., Kazemi, A., editors Handbook of Social Resource Theory: Theoretical Extensions, Empirical Insights, and Social Applications. New york, NY: Springer, 301–10.doi: 10.1007/978-1-4614-4175-5_19. [DOI] [Google Scholar]
- Drabiak-Syed K. (2010). Lessons from Havasupai Tribe v. Arizona State University Board of regents: recognizing group, cultural, and dignity harms as legitimate risks warranting integration into research practice. Journal of Health and Biomedical Law, 6, 175. [Google Scholar]
- Dunbar R. (2003). The social brain: mind, language, and society in evolutionary perspective. Annual Review of Anthropology, 32(1), 163–81.doi: 10.1146/ANNUREV.ANTHRO.32.061002.093158. [DOI] [Google Scholar]
- Elm J.H.L., Lewis J.P., Walters K.L., Self J.M. (2016). “I’m in this World for a Reason”: resilience and recovery among American Indian and Alaska Native Two Spirit Women. Journal of Lesbian Studies, 20(3–4), 352–71.doi: 10.1080/10894160.2016.1152813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans-Campbell T., Lindhorst T., Huang B., Walters K.L. (2006). Interpersonal violence in the lives of urban American Indian and Alaska Native women: implications for health, mental health, and help-seeking. American Journal of Public Health, 96(8), 1416–22.doi: 10.2105/AJPH.2004.054213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., Li H., Zhuo J., et al. (2016). The human Brainnetome Atlas: a new brain Atlas based on connectional architecture. Cerebral Cortex (New York, N.Y.: 1991), 26(8), 3508–26.doi: 10.1093/cercor/bhw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A.P., Miles J., Field Z. (2012). Discovering Statistics Using R. Los Angeles, CA: Sage. [Google Scholar]
- Fleming J., Ledogar R.J. (2008). Resilience and indigenous spirituality: a literature review. Pimatisiwin, 6(2), 47–64. [PMC free article] [PubMed] [Google Scholar]
- Goldstein M., Brendel G., Tuescher O., et al. (2007). Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: an emotional linguistic go/no-go fMRI study. NeuroImage, 36(3), 1026–40.doi: 10.1016/j.neuroimage.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Graham S., Jiang J., Manning V., et al. (2010). IQ-related fMRI differences during cognitive set shifting. Cerebral Cortex, 20(3), 641–9.doi: 10.1093/cercor/bhp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg M.M., Cope L.M., Martz M.E., Hardee J.E. (2015). Neuroimaging risk markers for substance abuse: recent findings on inhibitory control and reward system functioning. Current Addiction Reports, 2(2), 91–103.doi: 10.1007/s40429-015-0048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt M.K., Dieterich R., Endrass T. (2021). Neural correlates of inhibitory control in relation to the degree of substance use and substance-related problems – a systematic review and perspective. Neuroscience and Biobehavioral Reviews, 128, 1–11.doi: 10.1016/j.neubiorev.2021.06.011. [DOI] [PubMed] [Google Scholar]
- Ho D.E., Imai K., King G., Stuart E.A. (2011). MatchIt: nonparametric preprocessing for parametric causal inference. Journal of Statistical Software, 42(8), 1–28.doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]
- Holland P.W. (2003). CAUSATION AND RACE. ETS Research Report Series, 2003(1), i–21.doi: 10.1002/j.2333-8504.2003.tb01895.x. [DOI] [Google Scholar]
- Holmes A.J., Hollinshead M.O., Roffman J.L., Smoller J.W., Buckner R.L. (2016). Individual differences in cognitive control circuit anatomy link sensation seeking, impulsivity, and substance use. The Journal of Neuroscience, 36(14), 4038–49.doi: 10.1523/JNEUROSCI.3206-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T.R., Quirion R. (2005). Psychiatry as a clinical neuroscience discipline. JAMA?: The Journal of the American Medical Association, 294(17), 2221–4.doi: 10.1001/jama.294.17.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollant F., Lawrence N.L., Olié E., Guillaume S., Courtet P. (2011). The suicidal mind and brain: a review of neuropsychological and neuroimaging studies. The World Journal of Biological Psychiatry, 12(5), 319–39.doi: 10.3109/15622975.2011.556200. [DOI] [PubMed] [Google Scholar]
- Jones C.P. (2000). Levels of racism: a theoretic framework and a gardener’s tale. American Journal of Public Health, 90(8), 1212–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan A., Nich C., Babuscio T., Quainoo S., Carroll K. (2021). To the editor: our response to “substance use improvement depends on race/ethnicity: outpatient treatment disparities observed in a large US national sample”. Drug and Alcohol Dependence, 223, 108670.doi: 10.1016/j.drugalcdep.2021.108670. [DOI] [PubMed] [Google Scholar]
- Kading M.L., Hautala D.S., Palombi L.C., Aronson B.D., Smith R.C., Walls M.L. (2015). Flourishing: American Indian Positive Mental Health. Society and Mental Health, 5(3), 203–17.doi: 10.1177/2156869315570480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M., Smith A., Gracey M. (2009). Indigenous health part 2: the underlying causes of the health gap. The Lancet, 374(9683), 76–85.doi: 10.1016/S0140-6736(09)60827-8. [DOI] [PubMed] [Google Scholar]
- Kirmayer L.J., Dandeneau S., Marshall E., Phillips M.K., Williamson K.J. (2011). Rethinking resilience from indigenous perspectives. The Canadian Journal of Psychiatry, 56(2), 84–91.doi: 10.1177/070674371105600203. [DOI] [PubMed] [Google Scholar]
- Kober H., Mende-Siedlecki P., Kross E.F., et al. (2010). Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences, 107(33), 14811–6.doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFromboise T.D., Hoyt D.R., Oliver L., Whitbeck L.B. (2006). Family, community, and school influences on resilience among American Indian adolescents in the upper midwest. Journal of Community Psychology, 34(2), 193–209.doi: 10.1002/jcop.20090. [DOI] [Google Scholar]
- Le T.M., Potvin S., Zhornitsky S., Li C.-S.R. (2021). Distinct patterns of prefrontal cortical disengagement during inhibitory control in addiction: a meta-analysis based on population characteristics. Neuroscience and Biobehavioral Reviews, 127, 255–69.doi: 10.1016/j.neubiorev.2021.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Les Whitbeck B., Chen X., Hoyt D.R., Adams G.W. (2004). Discrimination, historical loss and enculturation: culturally specific risk and resiliency factors for alcohol abuse among American Indians. Journal of Studies on Alcohol, 65(4), 409–18.doi: 10.15288/jsa.2004.65.409. [DOI] [PubMed] [Google Scholar]
- Logan G.D., Schachar R.J., Tannock R. (1997). Impulsivity and inhibitory control. Psychological Science, 8(1), 60–4.doi: 10.1111/j.1467-9280.1997.tb00545.x. [DOI] [Google Scholar]
- Manson S.M., Beals J., Klein S.A., Croy C.D., The Ai-superpfp Team (2005). Social epidemiology of trauma among 2 American Indian Reservation populations. American Journal of Public Health, 95(5), 851–9.doi: 10.2105/AJPH.2004.054171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews S.C., Simmons A.N., Arce E., Paulus M.P. (2005). Dissociation of inhibition from error processing using a parametric inhibitory task during functional magnetic resonance imaging. NeuroReport, 16(7), 755–60.doi: 10.1097/00001756-200505120-00020. [DOI] [PubMed] [Google Scholar]
- McTeague L.M., Goodkind M.S., Etkin A. (2016). Transdiagnostic impairment of cognitive control in mental illness. Journal of Psychiatric Research, 83, 37–46.doi: 10.1016/j.jpsychires.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIDA . (2010). Strategic plan national institute on drug abuse. Available: https://nida.nih.gov/sites/default/files/stratplan.pdf [2021, June 12].
- NIMH . (2021). NIMH strategic plan for research 2021 update. Available: https://www.nimh.nih.gov/sites/default/files/documents/about/strategic-planning-reports/NIMH-Strategic-Plan-for-Research-2021-Update.pdf [2021, June 12].
- Nock M.K., Hwang I., Sampson N.A., Kessler R.C. (2010). Mental disorders, comorbidity and suicidal behavior: results from the national comorbidity survey replication. Molecular Psychiatry, 15(8), 868–76.doi: 10.1038/mp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson H., Kendler K.S. (2020). Applying causal inference methods in psychiatric epidemiology: a review. JAMA Psychiatry, 77(6), 637–44.doi: 10.1001/jamapsychiatry.2019.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oré C.E., Teufel-Shone N.I., Chico-Jarillo T.M. (2016). American Indian and Alaska native resilience along the life course and across generations: a literature review. American Indian and Alaska Native Mental Health Research (Online), 23(3), 134–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page A., Morrell S., Hobbs C., et al. (2014). Suicide in young adults: psychiatric and socio-economic factors from a case–control study. BMC Psychiatry, 14(1), 68.doi: 10.1186/1471-244X-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick M.E., Wightman P., Schoeni R.F., Schulenberg J.E. (2012). Socioeconomic status and substance use among young adults: a comparison across constructs and drugs. Journal of Studies on Alcohol and Drugs, 73(5), 772–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph J.J., Falbe K., Manuel A.K., Balloun J.L. (2014). A step-by-step guide to propensity score matching in R. Practical Assessment, Research & Evaluation, 19(18), 1–6. [Google Scholar]
- Roaldset J.O., Linaker O.M., Bjørkly S. (2012). Predictive validity of the MINI suicidal scale for self-harm in acute psychiatry: a prospective study of the first year after discharge. Archives of Suicide Research: Official Journal of the International Academy for Suicide Research, 16(4), 287–302.doi: 10.1080/13811118.2013.722052. [DOI] [PubMed] [Google Scholar]
- Robin R.W., Chester B., Rasmussen J.K., Jaranson J.M., Goldman D. (1997). Prevalence and characteristics of trauma and posttraumatic stress disorder in a Southwestern American Indian Community. American Journal of Psychiatry, 154(11), 1582–8.doi: 10.1176/ajp.154.11.1582. [DOI] [PubMed] [Google Scholar]
- Sheehan D., Janavs J., Baker R., Sheehan K.H., Knapp E., Sheehan M. (2015). MINI international neuropsychiatric interview-version 7.0 ((MINI 7.0)). Medical Outcomes Systems Inc. [Google Scholar]
- Sheehan D., Lecrubier Y. (2010). The mini international neuropsychiatric interview version 6.0 (MINI 6.0). Medical Outcomes Systems Inc. [Google Scholar]
- Spechler P.A., Chaarani B., Hudson K.E., Potter A., Foxe J.J., Garavan H. (2016). Chapter 8 - Response inhibition and addiction medicine: from use to abstinence. In: Ekhtiari, H., Paulus, M. (editors) Progress in Brain Research. Vol. 223. Cambridge, MA: Elsevier, 143–64.doi: 10.1016/bs.pbr.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Spechler P.A., Stewart J.L., Kuplicki R., Paulus M.P. (2020). Parsing impulsivity in individuals with anxiety and depression who use cannabis. Drug and Alcohol Dependence, 217, 108289.doi: 10.1016/j.drugalcdep.2020.108289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout D. (2010). The evolution of cognitive control. Topics in Cognitive Science, 2(4), 614–30.doi: 10.1111/j.1756-8765.2009.01078.x. [DOI] [PubMed] [Google Scholar]
- Stumblingbear-Riddle G., Romans J.S.C. (2012). Resilience among urban American Indian adolescents: exploration into the role of culture, self-esteem, subjective well-being, and social support. American Indian and Alaska Native Mental Health Research (Online), 19(2), 1–19.doi: 10.5820/aian.1902.2012.1. [DOI] [PubMed] [Google Scholar]
- Templeton A.R. (2013). Biological races in humans. Studies in History and Philosophy of Biological and Biomedical Sciences, 44(3), 262–71.doi: 10.1016/j.shpsc.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufel-Shone N.I., Tippens J.A., McCrary H.C., Ehiri J.E., Sanderson P.R. (2018). Resilience in American Indian and Alaska native public health: an underexplored framework. American Journal of Health Promotion?: AJHP, 32(2), 274–81.doi: 10.1177/0890117116664708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsethlikai M. (2015). The cultural patterning of cognitive development. Human Development, 58(2), 97–102.doi: 10.1159/000381652. [DOI] [Google Scholar]
- Victor T.A., Khalsa S.S., Simmons W.K., et al. (2018). Tulsa 1000: a naturalistic study protocol for multilevel assessment and outcome prediction in a large psychiatric sample. BMJ Open, 8(1), e016620.doi: 10.1136/bmjopen-2017-016620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana S., Lauterbach D. (1994). Prevalence of traumatic events and post-traumatic psychological symptoms in a nonclinical sample of college students. Journal of Traumatic Stress, 7(2), 289–302.doi: 10.1007/BF02102949. [DOI] [PubMed] [Google Scholar]
- Walls M., Sittner K.J., Whitbeck L.B., et al. (2021). Prevalence of mental disorders from adolescence through early adulthood in American Indian and first nations communities. International Journal of Mental Health and Addiction, 19(6), 2116–30.doi: 10.1007/s11469-020-00304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters K.L., Walls M.L., Dillard D.A., Kaur J.S. (2019). Critical considerations for reviewing AIAN Research.
- Weaver H.N. (2012). Urban and indigenous: the challenges of being a Native American in the city. Journal of Community Practice, 20(4), 470–88.doi: 10.1080/10705422.2012.732001. [DOI] [Google Scholar]
- Wexler L. (2014). Looking across three generations of Alaska Natives to explore how culture fosters indigenous resilience. Transcultural Psychiatry, 51(1), 73–92.doi: 10.1177/1363461513497417. [DOI] [PubMed] [Google Scholar]
- White E.J., Grant D.M. (2017). Electrocortical consequences of image processing: the influence of working memory load and worry. Psychiatry Research: Neuroimaging, 261, 1–8.doi: 10.1016/j.pscychresns.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Wilkinson G., Robertson G. (2006). (WRAT4) Wide Range Achievement Test, 4th edn, Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Zilverstand A., Huang A.S., Alia-Klein N., Goldstein R.Z. (2018). Neuroimaging impaired response inhibition and salience attribution in human drug addiction: a systematic review. Neuron, 98(5), 886–903.doi: 10.1016/j.neuron.2018.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman D.W. (2004). A note on preliminary tests of equality of variances. The British Journal of Mathematical and Statistical Psychology, 57(Pt 1), 173–81.doi: 10.1348/000711004849222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


