Abstract
The initiation of sporulation in Bacillus subtilis results primarily from phosphoryl group input into the phosphorelay by histidine kinases, the major kinase being kinase A. Kinase A is active as a homodimer, the protomer of which consists of an approximately 400-amino-acid N-terminal putative signal-sensing region and a 200-amino-acid C-terminal autokinase. On the basis of sequence similarity, the N-terminal region may be subdivided into three PAS domains: A, B, and C, located from the N- to the C-terminal end. Proteolysis experiments and two-hybrid analyses indicated that dimerization of the N-terminal region is accomplished through the PAS-B/PAS-C region of the molecule, whereas the most amino-proximal PAS-A domain is not dimerized. N-terminal deletions generated with maltose binding fusion proteins showed that an intact PAS-A domain is very important for enzymatic activity. Amino acid substitution mutations in PAS-A as well as PAS-C affected the in vivo activity of kinase A, suggesting that both PAS domains are required for signal sensing. The C-terminal autokinase, when produced without the N-terminal region, was a dimer, probably because of the dimerization required for formation of the four-helix-bundle phosphotransferase domain. The truncated autokinase was virtually inactive in autophosphorylation with ATP, whereas phosphorylation of the histidine of the phosphotransfer domain by back reactions from Spo0F∼P appeared normal. The phosphorylated autokinase lost the ability to transfer its phosphoryl group to ADP, however. The N-terminal region appears to be essential both for signal sensing and for maintaining the correct conformation of the autokinase component domains.
The initiation of sporulation is a complex process dependent on several regulatory pathways with unique signals and controls. A major signal transduction system involved in this process is the phosphorelay, which processes and integrates signals of many types to control the level of phosphorylation of the Spo0A transcription factor (2, 11). Activation of Spo0A by phosphorylation induces the synthesis of sporulation-specific sigma factors (17) and proteins regulating their activity (1), among others, and represses the synthesis of stationary-phase repressors such as AbrB (18). Since sporulation and stationary phase are whole-cell events, it should not be surprising that the regulation of the phosphorelay is very complex.
In its basic form the phosphorelay appears to be an expanded version of the familiar two-component system used to regulate a variety of pathways (6). Signals of some type activate a kinase to autophosphorylate on a histidine residue. The phosphoryl group is subsequently transferred to an aspartate of a response regulator, Spo0F, then to a second histidine of a phosphotransferase, Spo0B, and finally to an aspartate of the response regulator domain of Spo0A. This series of phosphoryl transfers may be interrupted and short circuited by specific phosphatases for Spo0F∼P or Spo0A∼P (16). Such phosphatases are regulated by cellular events for which the onset of sporulation is unfavorable (13). Thus, environmental and cellular signals promoting sporulation lead to phosphate input into the phosphorelay and opposing signals dephosphorylate the component proteins, making the entire pathway a signal integration circuit (11, 14).
If signal input is the province of the kinases of the phosphorelay, in order to understand how sporulation is induced it becomes important to uncover the identity of the signal ligands activating these kinases. Bioinformatic approaches to histidine kinases of the Bacillus subtilis genome identified five closely related kinases, KinA, KinB, KinC, KinD (YkvD), and KinE (YkrQ), that were likely to phosphorylate Spo0F (4). KinA was known to be the major source of phosphoryl groups for the phosphorelay (15), and KinB is an important secondary source. In a kinA kinB double mutant, sporulation is decreased by 5 to 6 orders of magnitude (22). Because of its major role in sporulation and because it is a cytoplasmic soluble enzyme, KinA has been the most studied of this group of kinases. In this communication, experiments to explore the secondary structure of KinA are presented.
MATERIALS AND METHODS
Construction of KinA mutants.
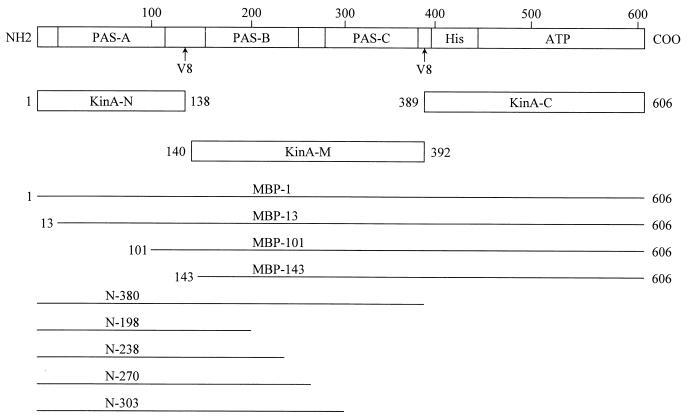
DNA fragments encoding KinA-N (Met1 to Glu139), KinA-M (Tyr140 to Lys392), and KinA-C (Lys389 to Lys606) were generated by PCR using appropriate primer pairs. The PCR products were subcloned into NcoI and BamHI sites of plasmid pET16b (Novagen) to generate expression vectors pET16b-KinA-N, pET16b-KinA-M, and pET16b-KinA-C. Mutants of KinA at His405, H405R, H405V, and H405S, were constructed using the MutaGene Phagemid Mutagenesis Kit and appropriate mutagenic oligonucleotides (Bio-Rad). The coding regions of these expression vectors were analyzed and confirmed by DNA sequencing.
Construction and purification of the MBP fusions.
Maltose binding protein (MBP) fusions to the entire or NH2-truncated KinA were constructed by using the pMal-c2 vector (New England Biolabs). Restriction-tagged PCR primers were used to amplify the kinA gene from the chromosome of JH642. These PCR products were digested with EcoRI and BamHI and then cloned into the EcoRI and BamHI sites of pMal-c2 to generate in-frame fusions. Expression of the MBP fusion proteins was driven by the inducible tac promoter upstream of malE on pMal-c2. The constructs were sequenced to confirm that they encoded the correct in-frame product without PCR-generated mutations. The MBP fusion proteins were purified from DH5α by amylose affinity chromatography, according to the specifications of the manufacturer (New England Biolabs). Briefly, cells were disrupted by sonication (8 times for 15 s each time) in sonication buffer (SB) (25 mM Tris [pH 8.0], 1 mM EDTA, 1 mM dithiothreitol [DTT], 10 mM KCl, 5 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride [PMSF]), and then debris and membranes were removed by centrifugation. The supernatants were passed through a 1-ml amylose resin column (New England Biolabs), previously equilibrated with SB buffer. The column was washed with 10 column volumes of SB buffer to remove unbound proteins. The fusion proteins were subsequently eluted with SB buffer containing 10 mM maltose, and fractions containing the desired proteins (determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE]) were pooled, spun, and loaded (0.5 ml/min) onto a HiLoad 16/10 Q-Sepharose High Performance (HP) column (1.6 by 10 cm; Pharmacia). The column was washed with 100 mM KCl (2 ml/min), and the MBP fusion proteins were eluted by a 100-ml linear gradient of 100 to 450 mM KCl (2 ml/min). Fractions containing fusion proteins were pooled and dialyzed overnight at 4°C against KinA storage buffer (50 mM Tris [pH 8.0], 1 mM DTT, 40% glycerol).
Expression and purification of wild-type and mutant kinases.
Wild-type KinA and four His405 mutants (H405R, H405Y, H405V, and H405S) were expressed and purified by the methods described by Grimshaw et al. (5). KinA-N, KinA-M, and KinA-C were expressed in Escherichia coli BL21(DE3) with expression vectors pET16b-KinA-N, pET16b-KinA-M, and pET16b-KinA-C, respectively. E. coli cells harboring the expression plasmid were grown at 37°C with shaking in Luria-Bertani medium containing ampicillin (100 μg/ml). When the culture reached an optical density at 600 nm (OD600) of 0.6, the incubation temperature was reduced to 30°C and shaking was continued for 1 h. At this time, the cells were induced by adding isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 0.2 mM, followed by incubation for 3 to 5 h. The cells were harvested, and cell lysates were prepared by the method established for KinA (5). KinA-N, KinA-M, and KinA-C were purified from the cell lysates using a three-step purification scheme (ammonium sulfate precipitation, Q-Sepharose ion exchange, and Sephacryl-S100 gel filtration chromatography), with each step optimized for individual KinA fragments. Briefly, saturated ammonium sulfate (4.1 M at 25°C) was added dropwise to the cell lysates with stirring on ice. The final concentration of ammonium sulfate was 35% for KinA-M and KinA-C and 40% for KinA-N. After an additional 20 min of stirring, the suspensions were centrifuged at 23,400 × g for 1 h at 4°C. The pellets were resuspended in 10 ml of buffer A (25 mM Tris-HCl, pH 8.0, at 4°C, 10 mM KCl, and 1 mM EDTA). The suspensions were dialyzed against 2 liters of buffer A using 6,000- to 8,000-molecular-weight cutoff (6 to 8K, MWCO) Spectra/Por dialysis tubing (Spectrum) for 2 h, followed by a buffer change and overnight dialysis. NRII, a histidine kinase from E. coli, and its cognate response regulator, NRI, were purified as described previously (10).
The dialysates were centrifuged at 11,950 × g for 10 min at 4°C to remove any precipitated material, and the supernatant was loaded onto a Pharmacia HiLoad 16/10 Q-Sepharose HP column equilibrated with buffer A. For purification of KinA-N, the column was washed with 200 mM KCl in buffer A until the OD280 approached baseline values and was then eluted by a 250-ml linear gradient of 10 to 300 mM KCl in buffer A. For KinA-C, the column was washed with 170 mM KCl in buffer A and eluted by a 250-ml linear gradient of 170 to 265 mM KCl in buffer A. Fractions containing the desired KinA fragments as determined by SDS-PAGE were pooled and concentrated in a Centriprep 10 concentrator (Amicon) to ∼10 ml. The concentrated pool was then loaded onto a Pharmacia Sephacryl-S100 high-resolution (HR) column equilibrated with buffer A. Homogeneous KinA-N, KinA-C, or KinA-M was eluted and detected by SDS-PAGE. Routinely, an ATPase assay was performed on the fractions using the method of Grimshaw et al. (5). Fractions with low ATPase activity were pooled and concentrated in a Centriprep 10 as before and dialyzed into storage buffer (50 mM Tris-HCl [pH 8.0], 1 mM DTT, and 40% glycerol) at 4°C overnight. All the preparations were stored at −20°C.
Limited proteolysis of KinA.
KinA (0.5 mg/ml) was incubated with V8 protease (Sigma; type IVII-B) at a protease/KinA ratio of 1/50 or 1/25 (wt/wt) in buffer containing 0.5 N NH4HCO3, pH 8.0, at 37°C. At specific time intervals 5× SDS-PAGE sample buffer (250 mM Tris-HCl [pH 6.8], 10% [vol/vol] glycerol, 1% [wt/vol] SDS, 280 mM 2-mercaptoethanol, and 0.01% [wt/vol] bromophenol blue) was added to the reaction mixture to inactivate V8 protease. The digests were then analyzed by SDS–10% PAGE. To isolate proteolytic fragments of KinA, the digest mixture was loaded onto a Pharmacia Superose-12 gel filtration column equilibrated with buffer B (25 mM Tris [pH 8.0], 10% glycerol, and 1 mM 2-mercaptoethanol). The column was eluted with the same buffer, and fractions (0.5 ml) were collected and analyzed by SDS-PAGE.
N-terminal sequence and MS analysis.
N-terminal amino acid sequence analysis was performed using an Applied Biosystems 470A protein sequencer equipped with an on-line phenylthiohydantoin analyzer (model 120A). Mass spectronomy analysis was performed using matrix-assisted laser desorption ionization mass spectroscopy (MALDI-MS on a Vestec 2000 MALDI-TOF-MS with a Lumonics NAG laser tuned at 355 nm). All the analyses were performed at the core facility at The Scripps Research Institute.
Gel electrophoresis and fast protein liquid chromatography (FPLC) size exclusion chromatography.
SDS-PAGE was performed according to the work of Laemmli (8). Nondenaturing PAGE was performed essentially the same as SDS-PAGE except that SDS was omitted. The gels were stained with Coomassie Brilliant Blue R-250. Molecular weights of KinA and its fragments were determined on nondenaturing gels using the nondenatured protein molecular weight marker kit as standards by following the manufacturer's instructions (Sigma).
Gel filtration was performed on a Pharmacia Sephacryl-S200 HR column equilibrated with buffer containing 25 mM Tris-HCl, pH 8.0, at 4°C, 100 mM KCl, and 20 mM EDTA. To determine the molecular weight of KinA and its fragments, the column was calibrated using the gel filtration molecular weight markers as standards by following the manufacturer's instructions (Sigma).
Phosphorylation assays and purification of phosphorylated proteins.
Phosphorylation reactions (total volume, 40 μl) were carried out in reaction buffer (50 mM K-Epps buffer [pH 8.5], 0.1 mM EDTA, 20 mM MgCl2, 5% [vol/vol] glycerol) containing 5 μCi of purified [γ-32P]ATP (3,000 mCi/mmol; Du Pont-NEN) mixed with cold ATP to give a final ATP concentration of 400 μM. Kinase (KinA, KinA mutants, KinA-N, KinA-M, KinA-C, or NRII) alone or in combination with either other kinases, Spo0F, or NRI, was included at the indicated concentrations. The reaction was initiated by addition of ATP. After incubation at 20°C for the designated period, the reaction was terminated by addition of 0.2 volume of 5× SDS-PAGE sample buffer, and the sample was immediately frozen on dry ice and thawed just prior to analysis by SDS-PAGE. Samples were loaded onto a 15% gel and electrophoresed at a constant voltage (150 V) for 2.5 h or until the dye front had migrated at least 75% of the gel length. The lower portion of the gel containing the dye front was removed to reduce background radiation due to unincorporated [γ-32P]ATP. The gel was dried at 80°C under a vacuum and exposed to Kodak X-Omat AR film for 1 h at room temperature. Sometimes the gel was exposed to a PhosphorImager screen for 0.5 h at room temperature and the bands of interest were quantitated using a PhosphorImager with ImageQuant software (Molecular Dynamics).
To obtain large amounts of phosphorylated protein, the phosphorylation reaction was carried out in reaction buffer in a total volume of 1 ml containing 80 μCi of purified [γ-32P]ATP (3,000 mCi/mmol; Du Pont-NEN) mixed with cold ATP to give a final ATP concentration of 400 μM. KinA (1.2 μM) or KinA-C (5 μM) was included with or without Spo0F (20 μM). After incubation at room temperature for 30 min (KinA with or without Spo0F) or 1 h (KinA-C with or without Spo0F), the reaction was loaded onto a Pharmacia Sephacryl-S100, HR column equilibriated with the reaction buffer. The column was eluted with the reaction buffer, and 0.5-ml fractions were collected. Fractions under discrete peaks as monitored by OD280 were pooled. The pools were analyzed by liquid scintillation counting, SDS-PAGE, and thin-layer chromatography (TLC) followed by autoradiography. Purified phosphoproteins were stored at −20°C.
Phosphotransfer and dephosphorylation assays.
Phosphotransfer and dephosphorylation assays were performed in the reaction buffer containing one of the purified 32P-labeled phosphorylated proteins (KinA∼P, KinA-C∼P, NRII∼P, or Spo0F∼P), either alone or in combination with one or two of the following as indicated: Spo0F (10 μM), KinA (1 μM), KinA-C (1 μM), KinA-N (1 μM), KinA-M (1 μM), and ADP (400 μM). After incubation at room temperature for 3 min, the reaction mixture was subjected either to SDS-PAGE or to TLC. For TLC analysis aliquots were removed from the reaction mixture and spotted onto a polyethyleneimine (PEI)-cellulose TLC plate. The TLC plate was developed with 2 M acetic acid–3.2 M LiCl, 1:1 (vol/vol). After development the plate was air dried and subjected to autoradiography overnight. For SDS-PAGE analysis the reaction was terminated by addition of 0.2 volume of 5× SDS-PAGE sample buffer, and the sample was analyzed by SDS-PAGE followed by either autoradiography or PhosphorImager analysis as described above.
Direct photoaffinity labeling of the KinA carboxyl-terminal domain with [γ-32P]ATP and [α-32P]ATP.
The carboxyl-terminal domain of KinA was photoaffinity labeled with [γ-32P]ATP or [α-32P]ATP using a method based on those described previously (3, 21). Each reaction mixture (50 μl) contained 5 μM KinA-C and 80 μCi of label at a final concentration of 2 μM total ATP. Reaction mixtures were incubated for 30 min on ice in the dark, after which 25 μl was removed and exposed to UV light for 60 min on ice. The remainder of the reaction mixtures were kept on ice in the dark. UV light was generated by two 15-W General Electric G15T8 UV lamps at a distance of 3 cm from the sample. Samples were then subjected to SDS-PAGE with subsequent autoradiography.
Yeast two-hybrid analyses.
The KinA N-terminal region was amplified by PCR from chromosomal DNA using oligonucleotide primers that placed an EcoRI site at the first codon and a PstI site at codon 380. This fragment was cloned in both pGAD424 and pGBT9 (Clontech). Fragments of this cloned DNA were generated by cleavage with EcoRI and the naturally occurring restriction sites EcoRV (codons 1 to 198), AccI (codons 1 to 238), PvuII (codons 1 to 270), and NdeI (codons 1 to 303). These fragments were cloned in pGAD424 and also pGAD9 and tested against the corresponding opposite full-length plasmids. Transformation in yeast strains and complementation growth were carried out according to the Clontech yeast protocols handbook.
RESULTS
Proteolytic cleavage of KinA.
KinA is a soluble, non-membrane-associated kinase with a molecular weight of 69,107 and consisting of 606 residues (Fig. 1). Homology studies have shown that the N-terminal 380 residues may contain three PAS domains of about 100 residues each (19). In order to gain some appreciation of the secondary structure of this N-terminal domain, proteolysis experiments with protease V8 were undertaken to identify linker and compactly folded sections of this region. Short-term proteolysis at low protease V8 concentrations released a semistable 56K fragment and a 30K fragment resistant to further proteolysis. Amino-terminal sequencing revealed that both fragments contained the sequence STTYI located just C-terminal to Glu136. The 30K fragment was determined to have a second cleavage at Glu391. Longer times of proteolysis with higher levels of V8 protease did not further cleave the 30K fragment, suggesting that this portion of the N-terminal region is tightly associated and that potential protease V8 cleavage sites are not available to the enzyme. The primary cleavage site at Glu136 is located between two potential PAS domains in a very hydrophilic and potentially disordered linker region. The second cleavage site at Glu391 is C-terminal to the last PAS domain and precedes the beginning of the catalytic region of kinase (Fig. 1).
FIG. 1.
Domain structure of kinase A and extent of fragments used to probe kinase A activities. The top diagram shows the location and extent of the PAS domains (PAS-A, PAS-B, and PAS-C), the histidine phosphotransfer domain (His), and the ATP-binding domain in the primary sequence of kinase A. All numbers indicate the relevant amino acids where fragments start or stop. The KinA-N, KinA-M, and KinA-C fragments delineate the extent of the constructs expressed as proteins that were originally defined by the protease V8 cleavage sites of intact kinase A. MBP fragment numbers indicate the N-terminal amino acid of kinase A fused in frame to the MBP. N fragment numbers indicate the C-terminal amino acid of the kinase A fragment used in the yeast two-hybrid system.
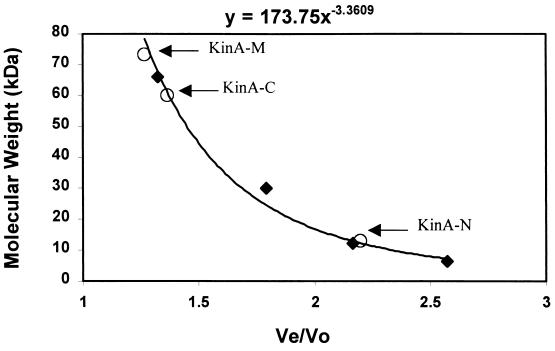
In order to study the properties of the potential domains generated by protease V8, expression vectors for each fragment were constructed and the expressed proteins were purified to homogeneity. The proteins generated were KinA-N (Met1 to Glu136), KinA-M (Tyr140 to Lys392), and KinA-C (Lys389 to Lys606). The amino-terminal sequence of each purified protein was determined to confirm its identity. Molecular weight determinations were carried out by gel filtration on a Sephacryl S-100 FPLC column (Fig. 2) and by electrophoresis on nondenaturing PAGE. Identical results were obtained with the two systems. Native KinA ran as a dimer in the PAGE system and on the S-100 column (data not shown). The protease-resistant middle section of KinA, KinA-M, ran as a dimer and very close to the expected dimeric molecular weight (56K) in the PAGE system (data not shown). On the Sephacryl S-100 column it tends to run larger and may aggregate under these buffer conditions. The catalytic domain of KinA, KinA-C (dimer, 48K) was also a dimer by both methods of analyses. In contrast, the N-terminal PAS domain-containing fragment ran as a monomer by both methods.
FIG. 2.
Molecular weight determination of expressed KinA domains by gel filtration. The molecular weights of expressed KinA fragments were analyzed using a Sephacryl S-100 FPLC column. Standard molecular weight markers (⧫) were bovine serum albumin (66 kDa), carbonic anhydrase (29 kDa), cytochrome c (12.4 kDA), and aprotinin (6.5 kDa). The column's voided volume (V0) was determined by the migration of dextran blue (2,000 kDa). KinA-N, KinA-M, and KinA-C were eluted in a single peak at the elution volumes (Ve) of 66, 28, and 41 ml, respectively. Their molecular sizes were determined as 13, 60, and 73.3 kDa.
Two-hybrid analysis of the N-terminal region of KinA.
To further define those regions of the KinA N-terminal region responsible for dimerization, a series of fragments (Fig. 1) were expressed in the yeast two-hybrid system and assayed for growth by genetic complementation (data not shown). The various fragments cloned in pGAD were assayed against the entire N-terminal region (N-380) cloned in pGBT9. No complementation was observed for pGADN-198 or pGADN-238, which contain the PAS-A domain and portions of PAS-B. Complementation was observed with pGADN-270 and pGADN-303, both of which contain intact PAS-A and PAS-B domains. The same results were obtained regardless of whether the fragments were cloned in pGAD424 or pGBT9 and tested for complementation against the full-length N-terminal region in the opposite plasmid. These results are consistent with the physical studies indicating that dimerization was a property of that portion of the N-terminal region containing the PAS-B and PAS-C domains and that the PAS-A-containing region did not appear to form a dimer, at least by itself.
MBP fusions to KinA.
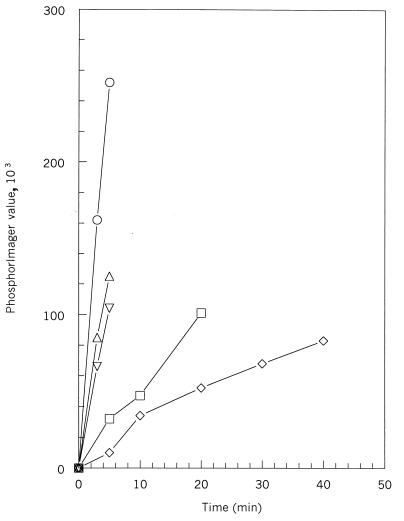
To investigate the functional role of the PAS-A domain, MBP fusions with deletions of sections of the PAS-A domain were accomplished with PCR fragments of KinA designed to make in-frame fusions when fused to MBP (Fig. 1). The inserts were sequenced to confirm the constructions, and the proteins were expressed and purified on amylose columns. The purified proteins ran as the expected molecular weights on SDS-PAGE gels. Assay of the four MBP fusions for autophosphorylation with ATP revealed that the full-length fusion, MBP-1, had about one-half of the native specific activity for this reaction (Fig. 3). MBP-13, with the first 12 amino acids deleted, gave an initial reaction rate 20% less than that of MBP-1. However, the initial reaction rates for MBP-101 and MBP-143 were decreased by 80 and 92%, respectively. These fusions delete most (MBP-101) or all (MBP-143) of the PAS-A domain and point out the importance of this domain in the in vitro activity of KinA.
FIG. 3.
Initial rates of autophosphorylation of MBP-kinase A fusion proteins. Autophosphorylation from [γ-32P]ATP was analyzed as a function of time. Samples were separated on SDS-PAGE and labeled KinA was quantitated by phosphorimaging. Symbols: ○, kinase A; ▵, MBP-1 fusion; ▿, MBP-13 fusion; □, MBP-101 fusion; ◊, MBP-143 fusion.
Functions of the isolated catalytic domain.
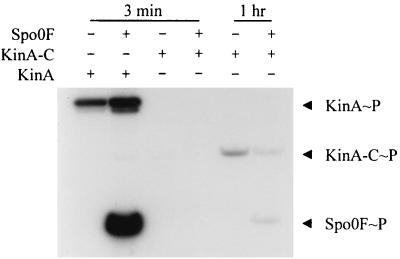
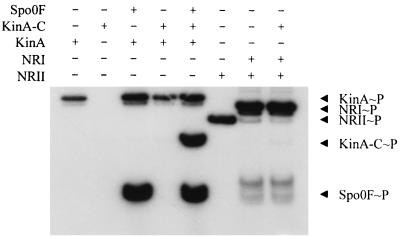
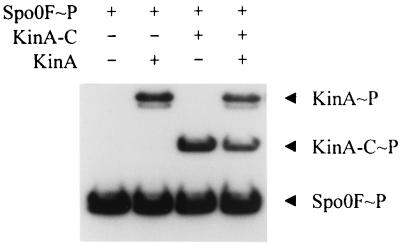
The purified expressed catalytic domain (KinA-C) was assayed for its ability to autophosphorylate and transfer phosphate to Spo0F. The autophosphorylation reaction was severely affected by the deletion of the N-terminal domain in KinA-C, but a discernible signal could be found after prolonged incubation (Fig. 4). There was no detectable autophosphorylation of KinA-C in the normal 3-min reaction, and the presence of Spo0F did not stimulate this reaction. However, incubation for 60 min resulted in a low level of phosphorylation of KinA-C, and transfer of the phosphoryl group to Spo0F could be detected. If KinA-C was incubated with KinA and Spo0F for the 3-min reaction time, KinA-C was heavily phosphorylated (Fig. 5). Spo0F is essential for this effect, since in its absence KinA-C is not phosphorylated in the presence of KinA alone. This suggested that KinA phosphorylated Spo0F and the labeling of KinA-C occurred via the back reaction from Spo0F∼P. Thus KinA-C appears to interact normally with Spo0F and to have an intact phosphotransferase function but lacks the ability to autophosphorylate. Furthermore, specificity was maintained in the back reaction, as no transfer from NRI∼P to KinA-C was detected (Fig. 5).
FIG. 4.
Phosphorylation activity of KinA and KinA-C. Various combinations of KinA (0.5 μM), KinA-C (5 μM), and Spo0F (10 μM), as indicated, were incubated with [γ-32P]ATP at 25°C for either 3 min or 1 h, as indicated. The phosphorylated proteins were subjected to SDS-PAGE and autoradiography as described in Materials and Methods.
FIG. 5.
Phosphotransfer reactions of KinA, KinA-C, and NRII. Various combinations of KinA (0.5 μM), KinA-C (5 μM), Spo0F (10 μM), NRII (0.5 μM), and NRI (5 μM) were incubated with [γ-32P]ATP at 25°C for 3 min. Individual reaction contents are indicated above the gel.
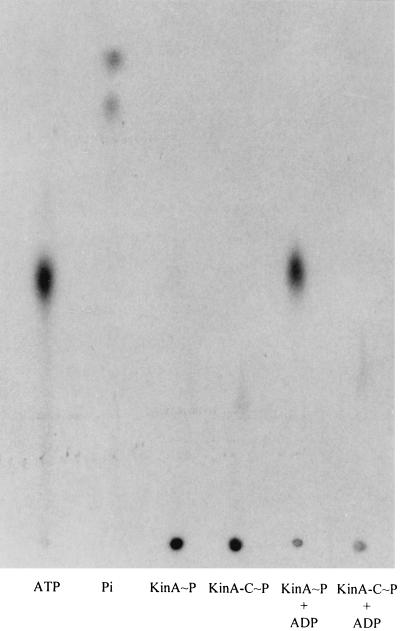
In order to eliminate possible artifacts in these results, direct transfer from labeled isolated components was tested. Purified Spo0F∼P transferred label to KinA-C as well as it transferred label to KinA (Fig. 6). Because the autophosphorylation reaction of KinA-C was severely decreased, it was of interest to see if the reverse reaction from labeled KinA-C∼P to ADP was similarly affected. Both purified KinA∼P and KinA-C∼P were incubated with 400 mM ADP to assay the back reaction of the kinase. KinA∼P plus ADP generated ATP, but no ATP was detected with KinA-C∼P and ADP (Fig. 7). Similar studies using Spo0F∼P and KinA or KinA-C and ADP showed that only KinA was capable of forming ATP (data not shown). Thus, Spo0F did not help to restore the missing back reaction of KinA-C. The inability of KinA-C to carry out either the autophosphorylation reaction or its reverse reaction suggested that the deletion of the N-terminal portion of KinA resulted in some conformational abnormality that disturbed the spatial arrangement of the ATP-binding domain and the active site histidine.
FIG. 6.
Phosphotransfer from purified Spo0F∼P to KinA and KinA-C. Purified γ-32P-labeled, phosphorylated Spo0F∼P (10 pmol) was incubated with various combinations of KinA-C (1 μM) and KinA (1 μM), as indicated, in reaction buffer at 25°C for 3 min. The samples were subjected to SDS-PAGE and autoradiography as described in Materials and Methods.
FIG. 7.
Phosphotransfer from KinA∼P and KinA-C∼P to ADP. Purified γ-32P-labeled KinA∼P (10 pmol) or KinA-C∼P (10 pmol) was incubated in the presence or absence of ADP (400 μM) as indicated in reaction buffer at 25°C for 3 min. The samples were subjected to TLC and autoradiography as described in Materials and Methods.
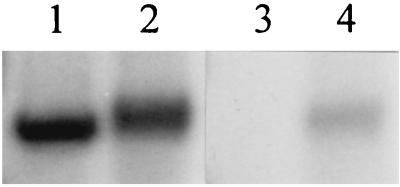
The loss of both the forward and reverse kinase reactions in KinA-C might be due to a large decrease in the ability to bind ATP or ADP or to a conformational abnormality that disturbs the active-site geometry. In the former case, a loss of ATP-binding activity might be detectable in vitro. To test this possibility, [γ-32P]ATP and [α-32P]ATP were incubated with KinA-C and a portion of each was cross-linked to the protein by UV irradiation (3). [γ-32P]ATP labeled KinA-C either by autophosphorylation or by UV irradiation (Fig. 8, lanes 1 and 2). In contrast, [α-32P] ATP labeled KinA-C only when cross-linked to it by UV irradiation (Fig. 8, lanes 3 and 4). Control experiments with intact KinA revealed that KinA-C bound about fivefold less ATP on a molar basis than KinA (data not shown). Although no kinetic experiments were undertaken, the data suggest that KinA-C is still capable of binding ATP at a reasonable level, and loss of affinity for ATP is unlikely to be solely responsible for the severe decreases observed for the kinase reactions.
FIG. 8.
UV cross-linking of [γ-32P]ATP and [α-32P]ATP to the KinA carboxy-terminal domain. KinA-C (5 μM) was incubated for 30 min on ice with 80 μCi of [γ-32P]ATP (lanes 1 and 2) or [α-32P]ATP (lanes 3 and 4) at a final concentration of 2 μM total ATP. Samples were exposed to UV light (lanes 2 and 4) or kept in the dark (lanes 1 and 3) for 60 min on ice and then subjected to SDS-PAGE and autoradiography.
Mutagenesis of KinA.
Mutagenesis experiments on KinA using PCR yielded a sporulation-deficient mutant in which phenylalanine residue 77 of the PAS-A domain was replaced by serine. This protein was expressed, purified, and assayed and found to have kinetic properties indistinguishable from those of the native KinA (data not shown). Residue F77 was deemed important for in vivo activity of KinA but not for its in vitro reactions. A second mutation, I280T, gave a sporulation-deficient phenotype. The affected residue is part of the putative PAS-C domain. This enzyme was not purified to test its activity in vitro.
kinA genes from Bacillus natto and from the B. subtilis “polish strain” were entirely sequenced and, aside from third-place substitutions in codons that did not change the coded amino acids, both strains had a tryptophan residue at position 187 instead of the serine found in B. subtilis 168 strains. The tryptophan substitution located in the putative PAS-B domain was transformed into the B. subtilis 168 strain, but no change in sporulation phenotype was observed.
The active-site histidine of the intact KinA was replaced by various amino acids to determine if phosphoryl acceptors other than histidine would function. Mutants H405Y, H405K, H405S, and H405V were produced by oligonucleotide mutagenesis, expressed, and purified. None of the mutants showed any ability to autophosphorylate, to transfer 32P from ATP to Spo0F, or to accept phosphate in a back reaction from labeled Spo0F∼P (no data shown). In contrast to many histidine kinases, KinA has little or no phosphatase activity on its product Spo0F∼P. None of the mutant proteins showed phosphatase activity on Spo0F∼P despite the fact that such mutations in other kinases retain or enhance phosphatase activity (7).
DISCUSSION
The initiation of sporulation is mainly controlled by the activity of KinA. The probable signal recognition and regulation region of KinA is within the N-terminal 400 amino acids. This region consists of three PAS domains identified by amino acid sequence homology to PAS domains of other proteins and designated PAS-A, PAS-B, and PAS-C. PAS domains have been implicated in dimerization as well as ligand sensing (19). Proteolysis experiments suggested that PAS-B and PAS-C form a compact protease-resistant structure with the same region of both protomers of the KinA dimer. Genetic complementation of N-terminal fragments in the dimer-detecting two-hybrid system showed that PAS-B could promote dimerization. There was no physical or genetic evidence for dimerization by the PAS-A domain. PAS-A appeared to be connected to the PAS-BC structure with a protease-sensitive linker. The C-terminal catalytic region of KinA, consisting of the active-site histidine-containing phosphotransferase domain and an ATP-binding domain, was a dimer both when proteolyzed from the native molecule and when produced alone. This region of KinA is likely to dimerize via the four-helix bundle of the phosphotransferase domain (20, 24). Thus, the active KinA dimer appears to be associated through PAS domain interaction in the signal input region and by two helices from each protomer making up the four-helix bundle in the catalytic domain. Both the entire N-terminal domain and the C-terminal catalytic domain complemented in a yeast two-hybrid system when tested against themselves but did not complement each other, i.e., no interaction between these two regions of the kinase was detected (data not shown).
The autophosphorylation activity of the C-terminal kinase domain was strongly dependent on the presence of the N-terminal domain. The C-terminal kinase domain showed no autophosphorylation activity with ATP in the usual 3-min assay but was capable of accepting a phosphoryl group from Spo0F∼P. Purified Spo0F∼P transferred its phosphoryl group equally well to the C-terminal kinase domain or to intact KinA, suggesting that interaction between Spo0F and the phosphotransfer domain was not affected in the truncated kinase. It is known from studies of the cocrystal of Spo0F and Spo0B (25), as well as from alanine-scanning mutagenesis of Spo0F (23), that the side chain interactions of Spo0F with phosphotransfer domains take place around the active-site aspartate. The apparent ability of Spo0F∼P to transfer its phosphoryl group equally to either intact or truncated KinA suggests that none of the important KinA residues of the phosphotransferase domain are perturbed in the truncated KinA. There was no evidence from alanine-scanning data that Spo0F made significant contacts with the ATP-binding domain.
The phosphorylated C-terminal kinase domain obtained by phosphoryl transfer from Spo0F∼P was incapable of generating ATP from ADP. This left open the possibility that the ATP binding site was perturbed or that the juxtaposition of the ATP-binding domain to the histidine of the phosphotransfer domain had been affected. None of the experiments allowed a distinction between these possibilities, except to point out the essential nature of the N-terminal region in the proper activity or structure of the kinase domain.
The N-terminal region is important for the activity of KinA by acting as a probable signal input domain as well as for the structural integrity of the C-terminal region. PAS-A appears to be very important for both of these activities. Deletion of the PAS-A domain in an MBP fusion protein causes more than 90% loss of the initial rate of autophosphorylation in the isolated protein. A mutation in PAS-A, F77S, destroys the in vivo activity of KinA. Based on homology to the structure of the PAS domain of photoactive yellow protein (PYP), F77 would correspond to PYP F92 (12). This residue is part of the β-scaffold making up the hydrophobic core of the PAS domain, and its replacement with a hydrophilic serine residue is likely to disturb the hydrophobic packing of the PAS domain. This has consequences in vivo that are not mimicked by the purified protein. It is tempting to speculate that the F77S mutation destroys the signal-ligand binding or activity of PAS-A. But in the absence of other evidence, it is also possible that the structural alteration predisposes the protein to rapid degradation in vivo. Regardless, the F77S KinA has native activity in vitro, suggesting that it is the presence of PAS-A, not its signal ligand activity, that accounts for maintaining C-terminal physical associations and enzymatic activities. The data also suggest that PAS-A may be in close physical contact with the C-terminal region, thereby allowing signal ligands to influence the autophosphorylation reaction of the ATP-binding domain. However, no interaction between the N-terminal and C-terminal domains was found by the two-hybrid system experiments or the mixing experiments to support this notion.
Amino acid substitutions were found in both PAS-B and PAS-C domains of KinA mutants. The S187W substitution found in other B. subtilis strains would be located in the core of PAS-B in a residue buried by the folding of the domain. We could not detect an alteration of KinA activity in vivo due to this residue change. The I280T mutation, on the other hand, gives a sporulation-deficient phenotype. Its putative equivalent amino acid in PYP is G29, and the side chain of its phenylalanine equivalent in ARNT, PER, and other PAS domain proteins is thought to project into the PAS core (19). The I280T mutation is a hydrophobic-to-hydrophilic substitution that would change the environment of the PAS core and likely disrupt its function, or it may affect the interaction of KinA with other as yet unidentified proteins. While this is speculation, it is clear that PAS-C is important for KinA function. A sporulation mutation originally designated gsiC82 is a KinA mutation that substitutes arginine for tryptophan at residue 288 (9). This substitution is located in the putative core of PAS-C and provides further evidence for its role in KinA function.
PAS domains are known to bind ligands such as FAD, FMN, or heme which act as oxygen or redox sensors to influence the activity of kinase catalytic domains (19). Purified KinA has no absorption spectrum for any of these ligands or for any other chromophore. There is no obvious homology between the ligand-binding region of any PAS domain and domains of known function. KinA is a sensor histidine kinase with multiple PAS domains in the signal input region. Whether these domains are synergistic or antagonize each other, allowing multiple signals to influence kinase activity, is open to speculation. What the PAS domains of KinA bind and how they regulate its activity remains unsolved.
ACKNOWLEDGMENTS
This research was supported, in part, by grants GM19416 and GM55594 from the National Institute of General Medical Sciences, National Institutes of Health, USPHS.
We thank Brian Rather and Corrado Fogher for isolation of mutants in KinA.
Footnotes
Publication 13630-MEM from the Department of Molecular and Experimental Medicine at The Scripps Research Institute.
REFERENCES
- 1.Arigoni F, Duncan L, Alper S, Losick R, Stragier P. SpoIIE governs the phosphorylation state of a protein regulating transcription factor ςF during sporulating in Bacillus subtilis. Proc Natl Acad Sci USA. 1996;93:3238–3242. doi: 10.1073/pnas.93.8.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burbulys D, Trach K A, Hoch J A. The initiation of sporulation in Bacillus subtilis is controlled by a multicomponent phosphorelay. Cell. 1991;64:545–552. doi: 10.1016/0092-8674(91)90238-t. [DOI] [PubMed] [Google Scholar]
- 3.Doronin S, Dessauer C, Johnson R A. Direct photoaffinity labeling of individual cytosolic domains of adenylyl cyclase by [32P]2′-deoxy-3′-AMP and [α-32P]5′-ATP. J Biol Chem. 1998;273:32416–32420. doi: 10.1074/jbc.273.49.32416. [DOI] [PubMed] [Google Scholar]
- 4.Fabret C, Feher V A, Hoch J A. Two-component signal transduction in Bacillus subtilis: how one organism sees its world. J Bacteriol. 1999;181:1975–1983. doi: 10.1128/jb.181.7.1975-1983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grimshaw C E, Huang S, Hanstein C G, Strauch M A, Burbulys D, Wang L, Hoch J A, Whiteley J M. Synergistic kinetic interactions between components of the phosphorelay controlling sporulation in Bacillus subtilis. Biochemistry. 1998;37:1365–1375. doi: 10.1021/bi971917m. [DOI] [PubMed] [Google Scholar]
- 6.Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: American Society for Microbiology; 1995. [Google Scholar]
- 7.Hsing W, Russo F D, Bernd K K, Silhavy T J. Mutations that alter the kinase phosphatase activities of the two-component sensor EnvZ. J Bacteriol. 1998;180:4538–4546. doi: 10.1128/jb.180.17.4538-4546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laemmli U K. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 9.Mueller J P, Sonenshein A L. Role of the Bacillus subtilis gsiA gene in regulation of early sporulation gene expression. J Bacteriol. 1992;174:4374–4383. doi: 10.1128/jb.174.13.4374-4383.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ninfa A J, Ueno-Nishio S, Hunt T P, Robustell B, Magasanik B. Purification of nitrogen regulator II, the product of the glnL (ntrB) gene of Escherichia coli. J Bacteriol. 1986;168:1002–1004. doi: 10.1128/jb.168.2.1002-1004.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohlsen K L, Grimsley J K, Hoch J A. Deactivation of the sporulation transcription factor Spo0A by the Spo0E protein phosphatase. Proc Natl Acad Sci USA. 1994;91:1756–1760. doi: 10.1073/pnas.91.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pellequer J-L, Wager-Smith K A, Kay S A, Getzoff E D. Photoactive yellow protein: a structural prototype for the three-dimensional fold of the PAS domain superfamily. Proc Natl Acad Sci USA. 1998;95:5884–5890. doi: 10.1073/pnas.95.11.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perego M. A peptide export-import control circuit modulating bacterial development regulates protein phosphatases of the phosphorelay. Proc Natl Acad Sci USA. 1997;94:8612–8617. doi: 10.1073/pnas.94.16.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perego M. Kinase-phosphatase competition regulates Bacillus subtilis development. Trends Microbiol. 1998;6:366–370. doi: 10.1016/s0966-842x(98)01350-x. [DOI] [PubMed] [Google Scholar]
- 15.Perego M, Cole S P, Burbulys D, Trach K A, Hoch J A. Characterization of the gene for a protein kinase which phosphorylates the sporulation-regulatory proteins Spo0A and Spo0F of Bacillus subtilis. J Bacteriol. 1989;171:6187–6196. doi: 10.1128/jb.171.11.6187-6196.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perego M, Hoch J A. Protein aspartate phosphatases control the output of two-component signal transduction systems. Trends Genet. 1996;12:97–101. doi: 10.1016/0168-9525(96)81420-x. [DOI] [PubMed] [Google Scholar]
- 17.Stragier P, Losick R. Cascades of sigma factors revisited. Mol Microbiol. 1990;4:1801–1806. doi: 10.1111/j.1365-2958.1990.tb02028.x. [DOI] [PubMed] [Google Scholar]
- 18.Strauch M A. AbrB, a transition state regulator. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C.: American Society for Microbiology; 1993. pp. 757–764. [Google Scholar]
- 19.Taylor B L, Zhulin I B. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev. 1999;63:479–506. doi: 10.1128/mmbr.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomomori C, Tanaka T, Dutta R, Park H, Saha S K, Zhu Y, Ishima R, Liu D, Tong K I, Kurokawa H, Qian H, Inouye M, Ikura M. Solution structure of the homodimeric core domain of Escherichia coli histidine kinase EnvZ. Nat Struct Biol. 1999;6:729–734. doi: 10.1038/11495. [DOI] [PubMed] [Google Scholar]
- 21.Trach K A, Hoch J A. The Bacillus subtilis spo0B stage 0 sporulation operon encodes an essential GTP-binding protein. J Bacteriol. 1989;171:1362–1371. doi: 10.1128/jb.171.3.1362-1371.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trach K A, Hoch J A. Multisensory activation of the phosphorelay initiating sporulation in Bacillus subtilis: identification and sequence of the protein kinase of the alternate pathway. Mol Microbiol. 1993;8:69–79. doi: 10.1111/j.1365-2958.1993.tb01204.x. [DOI] [PubMed] [Google Scholar]
- 23.Tzeng Y-L, Hoch J A. Molecular recognition in signal transduction: the interaction surfaces of the Spo0F response regulator with its cognate phosphorelay proteins revealed by alanine scanning mutagenesis. J Mol Biol. 1997;272:200–212. doi: 10.1006/jmbi.1997.1226. [DOI] [PubMed] [Google Scholar]
- 24.Varughese K I, Madhusudan, Zhou X Z, Whiteley J M, Hoch J A. Formation of a novel four-helix bundle and molecular recognition sites by dimerization of a response regulator phosphotranserase. Mol Cell. 1998;2:485–493. doi: 10.1016/s1097-2765(00)80148-3. [DOI] [PubMed] [Google Scholar]
- 25.Zapf J W, Sen U, Madhusudan, Hoch J A, Varughese K I. A transient interaction between two phosphorelay proteins trapped in a crystal lattice reveals the mechanism of molecular recognition and phosphotransfer in signal transduction. Struct Fold Des. 2000;8:851–862. doi: 10.1016/s0969-2126(00)00174-x. [DOI] [PubMed] [Google Scholar]