Abstract
Physical pain may lead to aggressive behavior in a social context. However, it is unclear whether this is related to changes of social information processing. Thus, this study aimed to investigate the neural mechanisms underlying pain-induced aggression using functional magnetic resonance imaging. In the experiment, 59 healthy participants were recruited: 31 were treated with topical capsaicin cream (pain group) and 28 with hand cream (control group). Participants completed a social network aggression task, during which they underwent two phases: feedback processing and attack exerting. The results revealed that participants in the pain group exhibited more aggression than those in the control group. During the feedback-processing phase, physical pain reduced brain activation in the right insula, left orbitofrontal cortex and anterior cingulate cortex, which typically exhibited stronger activation in response to negative (and positive) vs neutral social feedback in the control group. However, during the attack-exerting phase, pain did not significantly alter the activation of the dorsolateral prefrontal cortex. These findings suggest that pain increased aggression, while before that, it suppressed brain activities of the salience network involved in the process of salient social information and the value system associated with the value representation of social events.
Keywords: pain, aggression, social information, salience network, fMRI
Introduction
As an aversive stimulus, pain inevitably leads to negative consequences among individuals. For example, individuals with pain are more likely to experience negative affects including anxiety, depression and anger (Gatchel et al., 2007). Patients with chronic pain were found to exhibit several cognitive deficits, such as disturbances in attention allocation, low performance in the episodic memory task and worse decision-making in economic games (Apkarian et al., 2004). Furthermore, in recent years, much attention has been devoted to the negative impact of pain on social behaviors, in particular, aggression. For example, acute pain induced by inserting a hand into ice-cold water increased the likelihood of hurting an available target (Berkowitz, 1993). It was also found that recalling an experience of social or physical pain produced more desire to aggress (Riva et al., 2011). These findings demonstrated an increased effect of pain on aggressive behavior and prompted us to further explore how physical pain increases aggression.
Aggression is defined as any behavior directed toward another individual with a proximate intent to cause harm (Anderson and Bushman, 2002). A social information processing (SIP) model was developed to explain individuals’ aggressive behaviors, which suggests that individuals with disruptive behavioral problems perceive, interpret and make decisions about social information in ways that increase their likelihood of engaging in aggression (Dodge and Crick, 1990). This indicates that processing social cues has a prominent impact on aggressive behavior. For example, a previous study reported that individuals who exhibited hostile attribution toward ambiguous social–emotional information were more likely to engage in aggression (Coccaro et al., 2016). However, several studies have reported the potential consequences of physical pain on SIP. For example, adolescents with chronic pain may interpret non-supportive social information from close friends as distressing (Forgeron et al., 2011). Similarly, participants exposed to painfully cold water (i.e. acute pain) reported more feelings of being ignored or excluded (Riva et al., 2011). Thus, we speculated that pain may affect aggression within a social situation by changing the processing of social information.
How might SIP be affected by physical pain and, thus, lead to increased aggression? One possible explanation is that pain exaggerates the experience of SIP. More specifically, individuals experiencing pain respond more negatively to negative information and more positively to positive information and, thus, exhibit stronger aggressive behavior specifically toward a target who provides negative feedback in a social context. Another possibility is that physical pain may cause a negative shift in SIP. This assumption is based on the pain overlap theory (Eisenberger and Lieberman, 2004), which posits that social and physical pain have similar psychological responses and shared neurological underpinnings (Eisenberger et al., 2010; Riva et al., 2011). Thus, we assume that individuals who experience physical pain implicitly carry a similar effect to social pain and, consequently, exert more aggression toward both negative and positive feedback. In fact, a recent study reported that valence ratings of pain-related, negative and positive words became more negative after a painful electrical prime was applied (Brodhun et al., 2021).
To better understand the underlying neural mechanisms by which physical pain affects SIP and subsequent aggressive behavior, we considered three brain areas. First, the salience network plays a central role in the detection of salient stimuli in the environment, thus contributing to the processing of negative social information. It mainly consists of the anterior insula and the anterior cingulate cortex (ACC) (Goulden et al., 2014). Second, the value representation system plays an important role in evaluating both positive and negative social information. The orbitofrontal cortex (OFC) is implicated in representing the value of nearly all reward types, such as food, money and social rewards (Levy and Glimcher, 2012), and is also involved in punishments such as money loss (O’Doherty et al., 2001; Elliott et al., 2010). Third, regarding the key brain regions associated with aggressive behavior, the dorsolateral prefrontal cortex (DLPFC) in the executive functioning network should be considered (Goulden et al., 2014). Many studies have reported increased DLPFC activity in association with a high inhibitory control ability and a low degree of aggression (Choy et al., 2018; Van de Groep et al., 2021). For example, Van de Groep et al. (2021) reported that higher activity in the DLPFC when receiving negative social feedback is likely to inhibit subsequent noise blast aggression.
The present study aimed to unveil the mechanisms by which pain influences SIP before affecting aggressive behavior by performing a functional magnetic resonance imaging (fMRI) experiment. In this experiment, capsaicin cream was used to induce physical pain in one-half of the participants. Next, participants completed a modified social network aggression task (SNAT) (Achterberg et al., 2016), in which they received social feedback (positive, neutral or negative) and responded to it by giving an electric shock as an index of aggression. The brain activities of social feedback and subsequent aggressive behaviors were recorded in both the pain and control groups. It was hypothesized that behaviorally, individuals in the pain group would exhibit more aggression. We further examined two possibilities for how SIP is affected at the neural level. First, if pain has an exaggeration effect, we would observe stronger brain activation of the salience network and the value representation system in the pain group when responding to social information of positive or negative valence. For the second possibility, if pain has a negative shift effect, we would observe decreased brain activation in the pain group in both the salience network and the value representation system when responding to all types of social information. These brain regions may have been predominantly involved in physical pain processing and, consequently, limited resources were allocated to SIP. Under painful conditions, individuals may devote more attention to their pain; as such, the ability to process social information is diminished. In parallel, we examined whether an increase in aggression due to pain would be associated with a decrease in inhibitory control function by reducing the activity of the DLPFC.
Methods
Participants
This study initially recruited 63 healthy Chinese students as paid volunteers. Then, a total of 59 participants were involved in data analysis (30 females, 29 males; age: M = 21.1 years, s.d. = 2.0), as two participants were excluded due to excessive head motion during the fMRI scanning and another two due to void data (i.e. they did not respond to any trial). The participants were divided into two groups [31 in pain condition (male: 16; female: 15) and 28 in control condition (male: 13; female: 15)] with age and gender being matched between groups (Table 1). The Buss–Perry Aggression Questionnaire was used to measure the participants’ aggression traits (Buss and Perry, 1992) prior to the task. No significant difference was observed between the two groups for the personality trait of aggression [t (57) = 0.16, P = 0.877, Table 1]. All participants self-reported no history of psychiatric illness or chronic pain disorders. This study was approved by the University Committee on Human Research Protection of East China Normal University. All participants signed their informed consent.
Table 1.
Demographics of the pain and control groups
| Item | Control group | Pain group |
|---|---|---|
| Gender | 13 male,15 female | 16 male, 15 female |
| Age | 21.0 ± 1.6 | 21.2 ± 2.3 |
| Aggression trait | 1.56 ± 0.41 | 1.54 ± 0.54 |
Pain induction and assessment
The procedure of pain induction was the same as what we reported in our previous studies (Wang et al., 2018, 2020). It was a safe and noninvasive paradigm based on the heat/capsaicin sensitization model (Modir and Wallace, 2010). In the painful treatment, 0.1 ml of Capzasin-HP cream (0.1% capsaicin) was applied to a 2 × 2 cm2 area on the volar side of the dominant forearm. In the non-painful control treatment, hand cream was administrated to the same area. The area was then covered with plastic film to ensure skin contact and accumulate body heat to produce heat allodynia. After applying the cream, we asked participants to wait for 25 min until the capsaicin produced stable moderate painful feelings.
Pain sensation was assessed using a subjective numerical pain rating with an 11-point visual analog scale (0 corresponds to ‘no pain at all’ and 10 corresponds to ‘worst imaginable pain’) (Carlsson, 1983; Huang et al., 2013). Participants were asked to rate the intensity of pain three times: right after the cream was applied, 25 min after pain induction and at the end of the task when they came out of the fMRI scanner. Meanwhile, the emotional states were also assessed 25 min after pain induction by the positive and negative affect schedule (Crawford and Henry, 2004). The pain group and the control group showed no differences in positive emotion [t (57) = 1.20, P = 0.236] and negative emotion [t (57) = 0.53, P = 0.599] after having capsaicin or hand cream on the forearm for 25 min, suggesting that the pain induction by capsaicin did not affect participants’ emotional states.
Stimuli and procedure
A modified SNAT was used to induce participants’ aggressive behavior. The SNAT consisted of two stages. The first stage was conducted at least 1 week before fMRI scanning. The participants were asked to fill out a resume and upload a photo of themselves. Then, participants were presented with resumes and photos of another 30 college students and evaluated their resumes with three types of feedback (positive: I like your resume; negative: I dislike your resume and neutral: neither like nor dislike). Meanwhile, they were informed that their resumes were also evaluated with the same criteria by those 30 individuals. Unbeknownst to participants, their resumes were not judged by others, and photos of 30 students were actually taken from an existing database (Taiwanese Facial Expression Image Database; Chen and Yen, 2007).
During the second stage, participants came to the laboratory and underwent an fMRI scanning. We modified the SNAT by asking participants to give an electric shock instead of a noise blast. It would overcome the shortcoming of a noise blast being intervened by the fMRI machine noise. We also believed that the electric shock seemed more deterrent and aggressive. During the experiment, participants were first given a mild electric shock and asked to rate its intensity and likability. The purpose was to make participants vividly feel the intensity of the electric shock that they would exert on others during the fMRI scanning. After that, either capsaicin cream or hand cream was applied to them. Participants were then asked to fill out the demographic questionnaire. Thirty minutes after pain treatment, participants entered into the fMRI scanner. The fMRI scanning consisted of four runs of 15 randomly ordered trials. The total 60 trials were made up of 20 trials for each type of social feedback (positive, neutral and negative) from the 30 students who had pretended to judge the participants’ resumes. Figure 1 displays an overview of one modified SNAT trial. Each trial started with a fixation screen of 500 ms, followed by the social feedback for 2500 ms (feedback-processing phase). After a fixation screen jittered between 3000 and 5000 ms, a bar with the intensity of the electric shock appeared on the screen, which was presented for a total of 5000 ms (attack-exerting phase). During this period, participants could exert an electric shock ranging from 0 (no shock) to 10 (the highest imaginable intensity of shock) by pressing two buttons (turn up and turn down). The intensity of the electric shock was initialized as 0. It would go higher if the participants pressed the button ‘right arrow’, and it would go down by pressing the ‘left arrow’. According to their reaction, we calculated that the minimum of validness reached 75.93% (see the response of each participant in Supplementary Table S1). After that, another fixation screen jittered between 0 and 11 550 ms was presented. Thus, each run took 280 s, and the total scanning (including T1 image acquisition time) of each participant was ∼25 min.
Fig. 1.

A trail of SNAT. Three types of social feedback were shown on the board. The initial stage of the bar indicating an electric shock was set at 0.
fMRI acquisition and preprocessing
Image acquisition was performed on a 3T Siemens scanner at the Shanghai Key Laboratory of Magnetic Resonance of East China Normal University. Functional images were acquired by using a T2-weighted, echo-planar imaging sequence [repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle (FA) = 90°, slice thickness = 4 mm, field of view (FOV) = 224 × 224 mm2 and voxel size = 3.5 × 3.5 × 4 mm3]. High-resolution anatomical images were acquired by using a T1-weighted sequence (TR = 440 ms, TE = 2.51 ms, FA = 90°, slice thickness = 3 mm, FOV = 220 × 220 mm2 and voxel size = 0.69 × 0.69 × 3.75 mm3).
Functional image preprocessing was carried out using, Statistical Parametric Mapping 12 (SPM12; https://www.fil.ion.ucl.ac.uk/spm) a toolbox run on the software MATLAB. The first eight images of each run of the scan were excluded to account for T1-stabilization effects. For each participant, the functional images were slice-timing corrected and realigned to the first image, followed by normalization to the Montreal Neurological Institute (MNI) template, and then spatially smoothed by a Gaussian kernel with an isotropic of 6 mm full width at half maximum.
During preprocessing, artifact time points were identified as time points for which there was (i) 2 mm or more composite motion relative to the previous time point or (ii) a fluctuation in the global signal that exceeded a threshold of three standard deviations from the mean global signal. It was conducted via the Artifact Detection Tools (ART toolbox; https://www.nitrc.org/projects/artifact_detect/). Participants were dropped if 10% or more of the time points collected were identified as artifact time points. This criterion resulted in dropping two participants from the sample.
fMRI data analysis
Confirmatory region of interest analyses
Region of interest (ROI) analysis was conducted to examine the effect of pain on the neural activity of SIP and subsequent aggressive behavior within the social context. For the feedback-processing phase, five ROIs were predefined, as they were selected from the previous study (Achterberg et al., 2016) which adopted a similar experimental task. It included the left insula (−33, 23, −2), right insula (36, 17, −11), ACC (6, 35, 16), left OFC (−33, 20, −14) and right OFC (45, 29, −2). For the attack-exerting phase, two ROIs were predefined and selected similarly (Seeley et al., 2007). They were the left DLPFC (−32, 44, 16) and the right DLPFC (44, 36, 20). These ROIs were defined as spheres with a radius of 9 mm centered at those MNI coordinates using the MarsBaR toolbox (http://marsbar.sourceforge.net). Beta values of different conditions were extracted, respectively, for the two phases. These values were then subjected to repeated-measure Analysis of variance (ANOVA) with Feedback (positive, neutral and negative) and treatment (pain and control) using the SPSS 23 (IBM Corporation, Armonk, NY, USA).
Exploratory whole-brain analyses
Whole-brain statistical analyses were performed on participants’ fMRI data using a general linear model. At the first level, the fMRI time series were modeled and convolved with the hemodynamic response function. We established two models separately for the two phases. For the feedback-processing phase, the event of social feedback was identified with the onset and a duration of 2500 ms and then modeled with three regressors as the neutral, positive and negative feedback. Similarly, for the attack-exerting phase, the event of aggression was identified with the onset and a duration of 5000 ms and then modeled with separate regressors for attack after receiving neutral, positive and negative feedback. For the second-level analysis, the first-level contrast images were subjected to an ANOVA of Feedback (neutral, negative or positive) and Treatment (pain and control). After that, t-tests with respect to the contrast of negative vs neutral and positive vs neutral feedback were conducted in the pain group and the control group, respectively. Significant brain activations were determined by a voxel threshold of P < 0.001 and a spatial extent threshold of k > 20.
Results
Behavioral results
A manipulation check was performed on whether capsaicin induced substantial pain intensity (Table 2). Subjective pain intensities showed no significant difference at the onset of capsaicin/hand cream application [0.26 vs 0.25; t (57) = 0.05, P = 0.963]. Then, the pain group reported a higher degree of pain intensity than the control group 25 min after the application [4.42 vs 0.21; t (57) = 8.92, P < 0.001] and at the end of the fMRI scanning [3.35 vs 0.21; t (57) = 6.53, P < 0.001]. The pain intensity ratings were not significantly different between the beginning and the end of fMRI scanning [t (30) = −1.95, P = 0.061] although it showed a slight reduction from 4.42 to 3.35. It demonstrated that the pain induction was successful as participants applied with capsaicin experienced sustained pain during the experiment.
Table 2.
Subjective pain assessment in the pain and control conditions
| Application | 0 min | 25 min | The end |
|---|---|---|---|
| Hand cream | 0.25 (0.80) | 0.21 (0.50) | 0.21 (0.50) |
| Capsaicin | 0.26 (0.51) | 4.42 (2.45) | 3.35 (2.50) |
To evaluate to what extent individuals’ aggressive behavior would be modulated by pain, a three-way mixed ANOVA was performed on the degree of aggression with the independent variables Feedback (within-subject factor: neutral, positive and negative), Treatment (between-subject factor: pain and control) and Sex (between-subject factor: male and female). The results showed a significant main effect of Feedback [F (2, 110) = 182.49, P <0.001], indicating that individuals exhibited more aggressive behavior after receiving negative social feedback compared with two other types of social feedback. The main effect of Treatment was significant [F (1, 55) = 4.94, P =0.030], as participants showed more aggressive behavior in the painful condition relative to the control condition (3.40 vs 2.47, Figure 2A). The main effect of Sex was also significant [F (1, 55) = 4.38, P = 0.041], showing that males had more aggression than females. However, the triple interaction was not significant [F (2, 110) = 1.14, P =0.325], nor did other two-way interactions (P > 0.05). These results indicated that pain would increase aggression, regardless of types of feedback and gender. In addition, Pearson correlation analysis showed a significantly positive correlation between the total attack value and the averaged pain intensity during scanning (r = 0.40, P = 0.002, Figure 2B). It suggested that the increased aggression was related to pain intensity.
Fig. 2.

Behavioral results of control and pain groups. (A) ANOVA of Feedback, Treatment and Gender on the attack value. (B) Correlation between subjective pain intensity and the total attack value.
Brain activities during feedback-processing phase
To examine the effect of pain on brain activities associated with SIP during the feedback-processing phase, ANOVAs of Feedback (positive, neutral and negative) and Treatment (pain and control) were performed on beta values of five predefined brain regions (the left insula, right insula, left OFC, right OFC and ACC; Figure 3). Significant main effects of Feedback were found at brain regions in the left insula [F (2, 114) = 10.94, P < 0.001], right insula [F (2, 114) = 9.25, P < 0.001], left OFC [F (2, 114)= 13.35, P < 0.001] and right OFC [F (2, 114) = 14.68, P < 0.001], except for the ACC [F (2, 114) = 0.62, P = 0.543]. It revealed that compared with neutral social feedback, negative and positive social feedback induced stronger brain activations. Significant main effects of Treatment were found in two regions: the right insula [F (1, 57) = 6.05, P = 0.017] and the ACC [F (1, 57) = 4.68, P = 0.035]. It indicated that pain generally decreased the activations of these two regions during the SIP phase.
Fig. 3.
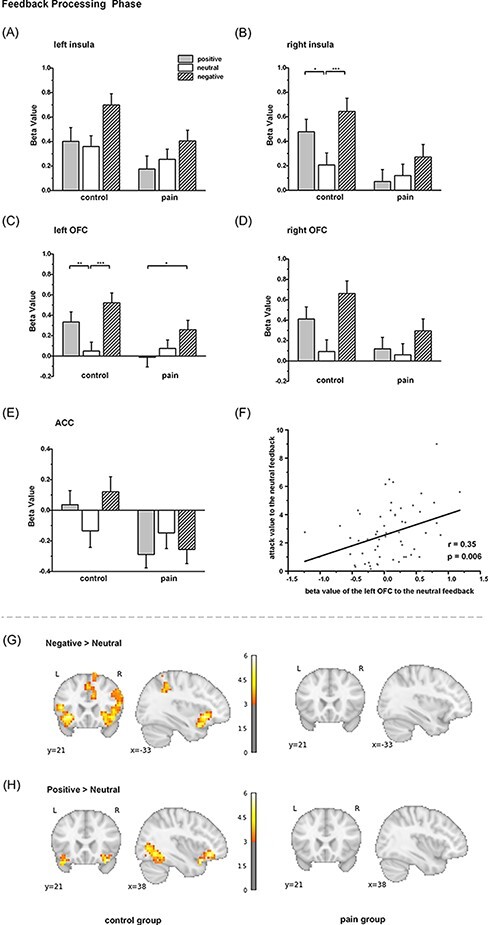
Pain effect of neural activation during the feedback-processing phase. Beta value of each condition for five ROIs: (A) left insula, (B) right insula, (C) left OFC, (D) right OFC and (E) ACC. (F) Correlation between the beta value of the left OFC to the neutral feedback in the feedback-processing phase and the attack value of the whole participants to the neutral feedback. Whole-brain analysis in the control and pain groups: (G) negative vs neutral contrast and (H) positive vs neutral contrast. *, ** and *** denote P < 0.05, P < 0.01 and P < 0.001, respectively. Color bar denotes the t-value of the contrast.
We found a significant interaction between Feedback and Treatment in three regions: the right insula [F (2, 114) = 3.22, p = 0.044], the ACC [F (2, 114) = 3.88, P = 0.023] and the left OFC [F (2, 114) = 4.52, P =0.013]. For the right insula, post hoc analysis (Sadik correction) indicated that in the control group, the beta values in the conditions of negative and positive feedback were significantly higher than those in the conditions of neutral feedback (negative vs neutral: 0.64 vs 0.21, P < 0.001; positive vs neutral: 0.48 vs 0.21, P =0.031), whereas these differences were not observed in the pain group (negative vs neutral: 0.27 vs 0.12, P = 0.344; positive vs neutral: 0.07 vs 0.12, P = 0.946). The left OFC showed a similar pattern, as dissociated brain activities were only observed in the control group (negative vs neutral: 0.52 vs 0.05, P < 0.001; positive vs neutral: 0.33 vs 0.05, P = 0.007) but not in the pain group (negative vs neutral: 0.26 vs 0.08, P = 0.148; positive vs neutral: −0.01 vs 0.08, P = 0.669). For the ACC, however, a marginally significant difference was observed in the control group (negative vs neutral: 0.12 vs −0.14, P = 0.058; positive vs neutral: 0.04 vs −0.14, P = 0.333) but not in the pain group (negative vs neutral: −0.26 vs −0.15, P = 0.644; positive vs neutral: −0.29 vs −0.15, P = 0.454). These findings implied that distinguished representations of different social feedback were eliminated by pain.
Correlation analysis was conducted to examine the relationship between brain activation of SIP and subsequent aggressive behavior. Interestingly, we found a significant positive correlation between the beta value of the left OFC and the subsequent attack value in the neutral feedback condition (r = 0.35, False Discovery Rate (FDR) P < 0.05; Supplementary Table S2; Figure 3F). The mediation analyses were also performed, but there were not any significant mediation effects on the brain activities in the feedback-processing phase (Supplementary Table S3).
Whole-brain analysis was further conducted to explore other potential brain areas that would carry the effects of pain. In the control group, the contrast (negative > neutral feedback) resulted in brain activations not only in the bilateral insula, ACC and OFC but also in the right cuneus, left inferior frontal gyrus, paracingulate cortex, occipital cortex and superior parietal lobule (Figure 3G; Table 3). The contrast (positive > neutral feedback) also resulted in brain activations of the OFC, lingual gyrus and occipital cortex (Figure 3H; Table 3). However, there were no significant brain activations in the pain group under these contrasts (Figure 3G and H). These findings revealed along with the ROI results that the painful situation may diminish the differentiated brain responses associated with different types of social feedback. But unfortunately, when we performed an analysis of the interaction effects of Feedback (either negative vs control or positive vs control) and Treatment, it resulted in no significant brain activations.
Table 3.
Whole-brain activation for Feedback ‘negative > neutral’ and ‘positive > neutral’ in the control group during the feedback-processing phase
| Brain region | t-value | Peak coordinates | Cluster | ||
|---|---|---|---|---|---|
| Negative > neutral | |||||
| Right cuneus | 6.05 | 13 | −91 | 20 | 88 |
| Left insula | 5.68 | −33 | 21 | −8 | 90 |
| Lateral occipital cortex | 5.46 | −50 | −70 | −8 | 69 |
| Inferior frontal gyrus | 5.16 | −47 | 21 | 8 | 22 |
| OFC | 5.13 | 38 | 28 | −8 | 201 |
| Right insula | 4.80 | 31 | 18 | −16 | |
| Middle occipital gyrus | 4.77 | 48 | −70 | −12 | 102 |
| Paracingulate cortex | 4.50 | −1 | 18 | 48 | 47 |
| Lateral occipital cortex | 4.34 | −8 | −67 | 64 | 24 |
| Superior parietal lobule | 4.32 | −47 | −42 | 56 | 24 |
| Intraparietal sulcus | 4.12 | −33 | −49 | 40 | 23 |
| Positive > neutral | |||||
| Lingual gyrus | 7.26 | 3 | −70 | 0 | 399 |
| Middle occipital lobe | 6.70 | 48 | −63 | −8 | 263 |
| OFC | 5.88 | 38 | 21 | −20 | 86 |
Note: Threshold: P < 0.001 (uncorrected) at the cluster level.
Brain activities during the attack-exerting phase
Similarly, ROI analysis was also conducted to examine the effect of pain on aggression in the attack-exerting phase. Beta values of the ROIs including the left DLPFC and the right DLPFC were, respectively, subjected to an ANOVA of Feedback (positive, neutral and negative) and Treatment (pain and control). The main effect of Feedback was significant in both the left DLPFC [F (2, 114) = 5.29, P = 0.006, Figure 4A] and the right DLPFC [F (2, 114) = 4.18, P = 0.018, Figure 4B]. However, the main effect of the Treatment was not significant [left DLPFC: F (1, 57) = 0.57, P = 0.452; right DLPFC: F (1, 57) = 0.46, P = 0.499], nor did the interactions of Feedback and Treatment [left DLPFC: F (2, 114) = 0.18, P = 0.836; right DLPFC: F (2, 114) = 0.06, P = 0.940].
Fig. 4.

Pain effect of neural activation during the attack-exerting phase. Beta value of each condition for two ROIs: (A) left DLPFC and (B) right DLPFC. Whole-brain analysis in the control and pain groups: (C) negative vs neutral contrast and (D) positive vs neutral contrast. Color bar denotes the t-value of the contrast.
Correlation analysis was also conducted to examine the relationship between these brain activations and aggressive behavior. We found significant positive correlations between the bilateral DLPFC and attack values to the positive feedback (left DLPFC: r = 0.608, FDR P < 0.05; right DLPFC: r = 0.405, FDR P < 0.05; Supplementary Table S2) for the whole sample. Meanwhile, both the pain and control groups showed the same pattern of these brain–behavior correlations (Supplementary Table S2). There was no significant mediation effect of the bilateral DLPFC within the effect of pain on aggression (Supplementary Table S3).
Furthermore, whole-brain analysis was also conducted on the contrasts for the attack-exerting phase. The contrast of negative vs neutral resulted in brain activations in the dorsomedial prefrontal cortex (DMPFC) and other prefrontal regions in the control group (Figure 4C, Table 4) but not in the pain group. Meanwhile, the contrast of positive vs neutral also resulted in the medial prefrontal cortex (MPFC) activations in the control group (Figure 4D, Table 4) but again, no significant difference of brain activations was found in the pain group. However, the interaction of Feedback (either negative vs control or positive vs control) and Treatment resulted in no significant brain activations. These results suggested that pain may not change brain activations associated with action performed during the attack-exerting phase.
Table 4.
Whole-brain activation for Feedback ‘negative > neutral’ and ‘positive > neutral’ in the control group during the attack-exerting phase
| Brain region | t-value | Peak coordinates | Cluster | ||
|---|---|---|---|---|---|
| Negative > neutral | |||||
| DMPFC | 6.00 | 6 | 49 | 40 | 217 |
| Medial prefrontal cortex | 5.47 | −12 | 21 | 60 | |
| Middle frontal gyrus | 4.78 | 41 | 25 | 48 | 63 |
| Superior frontal gyrus | 4.37 | 6 | 18 | 64 | 36 |
| Inferior frontal gyrus | 4.33 | 41 | 35 | −8 | 22 |
| Inferior parietal lobule | 4.17 | 55 | −46 | 52 | 30 |
| Positive > neutral | |||||
| Medial prefrontal cortex | 4.55 | −19 | 39 | 52 | 25 |
Note: Threshold: P < 0.001 (uncorrected) at the cluster level.
Discussion
The present study investigated how physical pain influenced aggressive behavior and attempted to reveal its underlying neural mechanisms by examining the prior brain activations associated with SIP. Behavioral results revealed that participants in the pain group exhibited more aggression than those in the control group. This was a general increase, regardless of the type of social feedback they received. The fMRI results revealed that, during the feedback-processing phase, physical pain reduced brain activation in the right insula, ACC and left OFC, which typically exhibited stronger activation in response to negative—and positive—vs neutral social feedback in a non-painful state. However, during the attack-exerting phase, the ROI analysis revealed that pain did not significantly alter activation in the DLPFC. These findings suggest that pain increases aggression after the suppression of brain activities related to SIP.
During the feedback-processing phase, we found a significant interaction between Feedback and Treatment in the right insula, ACC and left OFC. The right insula and the left OFC shared the same pattern, with higher activation when receiving both negative and positive feedback than when receiving neutral feedback in the control group. This was consistent with a previous study reporting enhanced insular activity in response to negative and positive feedback relative to neutral feedback (Achterberg et al., 2016). The insula is regarded to be one of the core nodes of the salience network. Its core function is postulated to detect salient stimuli (especially for negative events, but also to respond to positive events) and initiate appropriate control signals (Goulden et al., 2014; Menon and Uddin, 2010). Meanwhile, the OFC is regarded to be a core region of the valuation system because it was found to play a predominant role in encoding information values and guiding decisions (Northoff et al., 2000; Padoa-Schioppa and Cai, 2011; Groman et al., 2019). Interestingly, stronger activation in the right insula and the left OFC when processing negative and positive feedback was eliminated by physical pain. Moreover, OFC activities in response to neutral feedback in our study were positively correlated with subsequent attack values. Thus, the findings imply that pain affects SIP mainly by decreasing brain activities in the salience network and the value system, which may have an impact on subsequent attacking behavior.
The ACC was also influenced by pain, as evidenced by the main effect of treatment and a significant interaction effect. We found that ACC activity generally decreased when responding to all types of feedback in a painful state. The ACC has been regarded to be another key hub in the salience network but is specialized in processing negative affect, pain and cognitive control (Shackman et al., 2011). We speculated that the ACC may be occupied to process the unpleasantness of pain itself, which then results in a decreased representation of social information.
How would pain increase aggressive behavior while suppressing prior brain activities related to SIP? As painful events automatically capture an individual’s attention (Eccleston and Crombez, 1999), it is likely that those experiencing the pain condition cannot allocate sufficient cognitive resources to other salient stimuli in the environment, such as the positive and negative feedback in our study. As we have discussed, decreased ACC activity toward salient feedback occurred at the cost of processing physical pain, which may also be the case for the right insula and the left OFC. To this end, our results support the second hypothesis (i.e. negative shift effect of pain) but not the first (i.e. exaggeration effect of pain). More specifically, individuals experiencing physical pain implicitly carry a similar effect of social pain and, consequently, exert more aggression toward both negative and positive feedback. As Eisenberger et al. (2010) reported, participants exposed to endotoxins exhibited increased feelings of social disconnection (Eisenberger et al., 2010). Taken together, the results of the current study support the assertion that physical pain can aggravate negative social experiences.
During the attack-exerting phase, we found greater activation of the DLPFC and DMPFC in the contrast of negative vs neutral conditions, especially in the control group. The DLPFC is recognized as a key brain region for executive function, which plays a role in down-regulating aggressive behavior (Wagner et al., 2001). Previous studies have reported an association between DLPFC activity in response to negative feedback and subsequent aggressive behavior (Achterberg et al., 2016, 2020; Van de Groep et al., 2021). For example, it was found that increased DLPFC activity after receiving positive feedback leads to decreased aggression, indicating a potential role for the DLPFC in inhibiting control of aggression (Van de Groep et al., 2021). However, DLPFC activation in our study was probably unaffected by pain, which indicates that the increased aggression caused by pain was not likely related to a modulated function of the DLPFC (such as inhibition of control or other potential functions). On the contrary, the DMPFC has been identified as an important region engaged in cognitive processing to regulate emotions (Buhle et al., 2013; LeWinn et al., 2018). Thus, in our study, higher DMPFC activities responding to negative and positive social feedback compared to neutral feedback may help manage the emotions raised by social feedback. However, because we found no significant interaction effect of brain activation, the function of the DMPFC was also unlikely to be affected by pain. Therefore, we conclude that increased aggressive behavior is largely determined once the social information is processed, but not at the attack-exerting stage.
Meanwhile, the results of the present study suggest the potential roles of other brain networks in pain-induced social aggression. Previous studies have found that the mentalizing network (temporoparietal junction, cuneus and medial prefrontal cortex) plays an important role in this social-aggressive context (e.g. Beyer et al., 2014; Chester et al., 2018). We observed greater cuneus activity in the feedback-processing phase and greater DMPFC activity in the attack-exerting phase when responding to negative feedback in the control group. However, whole-brain results did not reach a significant threshold when comparing pain treatments. Moreover, other brain regions, such as the ventromedial prefrontal cortex (VMPFC), which plays a role in emotion regulation (Gilam et al., 2018), and the ventrolateral prefrontal cortex (VLPFC), which plays a role in self-regulatory processes (Chester et al., 2018), may also contribute to this process. However, these issues warrant further investigation.
The current study contributes to a better understanding of how pain affects aggression in two ways. First, it provides behavioral evidence from a social context to support the increased effect of pain on aggression (Berkowitz, 1993; Riva et al., 2011). The pain group exhibited higher aggression than the control group, and the total attack value was positively correlated with pain intensity. Second, this work highlights the role of SIP in pain-induced aggressive behavior, which supports the SIP model (Dodge and Crick, 1990). Decreased right insula, left OFC and ACC activities in the painful condition indicated that physical pain may decrease the detection and evaluation of salient social events, which in turn may contribute to subsequent aggressive behaviors.
The current work extends the social effects of pain. In recent years, some researchers have proposed that pain is complex, comprising sensory, emotional, cognitive and social components (Williams and Craig, 2016). In accordance with this novel insight, a growing research effort in psychology and neuroscience has focused on the impact of pain on social behaviors. For example, physical pain has been found to promote prosocial behaviors, such as cooperation and trust, implicating the evolutionarily adaptive significance of pain (Bastian et al., 2014; Ferris et al., 2016; Wang et al., 2018, 2019). The present study further extended the pain effect from prosocial behaviors to one type of negative social behavior (i.e. aggressive behavior). This may help explain why individuals with chronic pain exhibited low quality of social life and impaired social relationships (Forgeron et al., 2010; Hadi et al., 2019).
This study had some limitations. First, we only examined physical pain induced by capsaicin in the experimental settings. Whether the findings can be replicated among other types of pain (e.g. mechanical pain, laser heat and cold) should be verified. For chronic pain in particular (e.g. low back pain and postherpetic pain), the effect could even be altered because the motivation of patients with chronic pain is relatively decreased instead of being enhanced among those with acute pain (Navratilova and Porreca, 2014). Moreover, it would be intriguing to compare the effects of physical and social pain (e.g. social rejection, exclusion and loss). These speculations warrant further investigation, particularly in real-life situations. Second, we focused only on aggressive behaviors toward strangers. Previous research has found that social closeness is an important determinant of the impact of social feedback on aggression (Sip et al., 2015). Therefore, future research could take group membership into account by telling the participants that social feedback is from their friends or strangers. Third, we failed to find any mediation effect of the brain activities of SIP on the effect of pain on subsequent aggressive behavior. Thus, whether increased aggression is causally related to modulated brain activity requires further investigation.
In summary, our research demonstrates an increased effect of pain on aggression. Before that, pain suppresses brain activities of the salience network that is involved in the process of salient social information and the value system that is associated with the value representation of social events. This study provides novel insights for understanding the impact of physical pain on SIP, which may lead to subsequent negative social behavior. These findings support the negative shift hypothesis, in which physical pain has an effect similar to that of social pain.
Supplementary Material
Contributor Information
Yanfang Wang, Shanghai Key Laboratory of Mental Health and Psychological Crisis Intervention, Affiliated Mental Health Center (ECNU), School of Psychology and Cognitive Science, East China Normal University, Shanghai 200062, China; Institute of Brain and Education Innovation, East China Normal University, Shanghai 200062, China.
Lu Li, Shanghai Key Laboratory of Mental Health and Psychological Crisis Intervention, Affiliated Mental Health Center (ECNU), School of Psychology and Cognitive Science, East China Normal University, Shanghai 200062, China.
Junhao Cai, Department of Psychology, Vanderbilt University, Nashville, TN 37240, USA.
Huaifang Li, Department of Obstetrics and Gynecology, Tongji Hospital of Tongji University, Tongji University School of Medicine, Shanghai 200065, China.
Chenbo Wang, Shanghai Key Laboratory of Mental Health and Psychological Crisis Intervention, Affiliated Mental Health Center (ECNU), School of Psychology and Cognitive Science, East China Normal University, Shanghai 200062, China; Institute of Brain and Education Innovation, East China Normal University, Shanghai 200062, China; Shanghai Changning Mental Health Center, Shanghai 200335, China.
Funding
This work was supported by the Basic Research Project of the Shanghai Science and Technology Commission (20dz2260300; 19JC1410101) and the Fundamental Research Funds for the Central Universities (2017ECNU-HWFW023). It was also supported by grant from the National Natural Science Foundation of China (81873827).
Conflict of interest
The authors declared that they had no conflict of interest with respect to their authorship or the publication of this article.
Supplementary data
Supplementary data are available at SCAN online.
Author contributions
C.W., L.L. and H.L. designed the study. L.L. and J.C. performed the data collection. L.L. and Y.W. analyzed the data. C.W., Y.W. and H.L. wrote the manuscript. All authors read and provided the final approval of the paper.
Data availability
Datasets are available on request. The raw data and generated data during analyses supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
References
- Achterberg M., van Duijvenvoorde A.C., Bakermans-Kranenburg M.J., Crone E.A. (2016). Control your anger! The neural basis of aggression regulation in response to negative social feedback. Social Cognitive and Affective Neuroscience, 11(5), 712–20.doi: 10.1093/scan/nsv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achterberg M., van Duijvenvoorde A.C.K., van Ijzendoorn M.H., Bakermans-Kranenburg M.J., Crone E.A. (2020). Longitudinal changes in DLPFC activation during childhood are related to decreased aggression following social rejection. Proceedings of the National Academy of Sciences, 117(15), 8602–10.doi: 10.1073/pnas.1915124117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C.A., Bushman B.J. (2002). Human aggression. Annual Review of Psychology, 53(1), 27–51.doi: 10.1146/annurev.psych.53.100901.135231. [DOI] [PubMed] [Google Scholar]
- Apkarian A.V., Sosa Y., Krauss B.R., et al. (2004). Chronic pain patients are impaired on an emotional decision-making task. Pain, 108(1–2), 129–36.doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Bastian B., Jetten J., Ferris L.J. (2014). Pain as social glue: shared pain increases cooperation. Psychological Science, 25(11), 2079–85.doi: 10.1177/0956797614545886. [DOI] [PubMed] [Google Scholar]
- Berkowitz L. (1993). Pain and aggression: some findings and implications. Motivation and Emotion, 17(3), 277–93.doi: 10.1007/BF00992223. [DOI] [Google Scholar]
- Beyer F., Münte T.F., Erdmann C., Krämer U.M. (2014). Emotional reactivity to threat modulates activity in mentalizing network during aggression. Social Cognitive and Affective Neuroscience, 9(10), 1552–60.doi: 10.1093/scan/nst146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodhun C., Borelli E., Weiss T. (2021). Influence of acute pain on valence rating of words. PLos One, 16(3), e0248744.doi: 10.1371/journal.pone.0248744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., et al. (2013). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–90.doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss A.H., Perry M. (1992). The aggression questionnaire. Journal of Personality and Social Psychology, 63(3), 452–9.doi: 10.1037/0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- Carlsson A.M. (1983). Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain, 16(1), 87–101.doi: 10.1016/0304-3959(83)90088-X. [DOI] [PubMed] [Google Scholar]
- Chen L.-F., Yen Y.-S. (2007). Taiwanese Facial Expression Image Database. Taipei: Brain Mapping Laboratory, Institute of Brain Science, National Yang-Ming University. [Google Scholar]
- Chester D.S., Lynam D.R., Milich R., DeWall C.N. (2018). Neural mechanisms of the rejection–aggression link. Social Cognitive and Affective Neuroscience, 13(5), 501–12.doi: 10.1093/scan/nsy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy O., Raine A., Hamilton R.H. (2018). Stimulation of the prefrontal cortex reduces intentions to commit aggression: a randomized, double-blind, placebo-controlled, stratified, parallel-group trial. The Journal of Neuroscience, 38(29), 6505–12.doi: 10.1523/JNEUROSCI.3317-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro E.F., Fanning J.R., Keedy S.K., Lee R.J. (2016). Social cognition in intermittent explosive disorder and aggression. Journal of Psychiatric Research, 83, 140–50.doi: 10.1016/j.jpsychires.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J.R., Henry J.D. (2004). The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. British Journal of Clinical Psychology, 43(3), 245–65.doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Dodge K.A., Crick N.R. (1990). Social information-processing bases of aggressive behavior in children. Personality & Social Psychology Bulletin, 16(1), 8–22.doi: 10.1177/0146167290161002. [DOI] [Google Scholar]
- Eccleston C., Crombez G. (1999). Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychological Bulletin, 125(3), 356–66.doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Inagaki T.K., Mashal N.M., Irwin M.R. (2010). Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain, Behavior, and Immunity, 24(4), 558–63.doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I., Lieberman M.D. (2004). Why rejection hurts: a common neural alarm system for physical and social pain. Trends in Cognitive Sciences, 8, 294–300.doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Elliott R., Agnew Z., Deakin J.F.W. (2010). Hedonic and informational functions of the human orbitofrontal cortex. Cerebral Cortex, 20(1), 198–204.doi: 10.1093/cercor/bhp092. [DOI] [PubMed] [Google Scholar]
- Ferris L.J., Jetten J., Molenberghs P., Bastian B., Karnadewi F. (2016). Increased pain communication following multiple group memberships salience leads to a relative reduction in pain-related brain activity. PLoS One, 11(9), e0163117.doi: 10.1371/journal.pone.0163117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgeron P.A., King S., Stinson J.N., McGrath P.J., MacDonald A.J., Chambers C.T. (2010). Social functioning and peer relationships in children and adolescents with chronic pain: a systematic review. Pain Research and Management, 15(1), 27–41.doi: 10.1155/2010/820407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgeron P.A., McGrath P., Stevens B., et al. (2011). Social information processing in adolescents with chronic pain: my friends don’t really understand me. Pain, 152(12), 2773–80.doi: 10.1016/j.pain.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Gatchel R.J., Peng Y.B., Peters M.L., Fuchs P.N., Turk D.C. (2007). The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychological Bulletin, 133(4), 581–624.doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- Gilam G., Abend R., Gurevitch G., et al. (2018). Attenuating anger and aggression with neuromodulation of the vmPFC: a simultaneous tDCS-fMRI study. Cortex, 109, 156–70.doi: 10.1016/j.cortex.2018.09.010. [DOI] [PubMed] [Google Scholar]
- Goulden N., Khusnulina A., Davis N.J., et al. (2014). The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. NeuroImage, 99, 180–90.doi: 10.1016/j.neuroimage.2014.05.052. [DOI] [PubMed] [Google Scholar]
- Groman S.M., Keistler C., Keip A.J., et al. (2019). Orbitofrontal circuits control multiple reinforcement-learning processes. Neuron, 103(4), 734–746.e3. doi: 10.1016/j.neuron.2019.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi M.A., McHugh G.A., Closs S.J. (2019). Impact of chronic pain on patients’ quality of life: a comparative mixed-methods study. Journal of Patient Experience, 6(2), 133–41.doi: 10.1177/2374373518786013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G., Xiao P., Hung Y.S., Iannetti G.D., Zhang Z.G., Hu L. (2013). A novel approach to predict subjective pain perception from single-trial laser-evoked potentials. NeuroImage, 81, 283–93.doi: 10.1016/j.neuroimage.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Levy D.J., Glimcher P.W. (2012). The root of all value: a neural common currency for choice. Current Opinion in Neurobiology, 22(6), 1027–38.doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeWinn K.Z., Strigo I.A., Connolly C.G., et al. (2018). An exploratory examination of reappraisal success in depressed adolescents: preliminary evidence of functional differences in cognitive control brain regions. Journal of Affective Disorders, 240, 155–64.doi: 10.1016/j.jad.2018.07.020. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure & Function, 214(5–6), 655–67.doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modir J.G., Wallace M.S. (2010). Human experimental pain models 3: heat/capsaicin sensitization and intradermal capsaicin models. In: Analgesia: Methods and Protocols, Methods in Molecular Biology. A. Szallasi, ed. 169–74. Totowa, NJ: Humana Press. [DOI] [PubMed] [Google Scholar]
- Navratilova E., Porreca F. (2014). Reward and motivation in pain and pain relief. Nature Neuroscience, 17(10), 1304–12.doi: 10.1038/nn.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G., Richter A., Gessner M., et al. (2000). Functional dissociation between medial and lateral prefrontal cortical spatiotemporal activation in negative and positive emotions: a combined fMRI/MEG study. Cerebral Cortex, 10(1), 93–107.doi: 10.1093/cercor/10.1.93. [DOI] [PubMed] [Google Scholar]
- O’Doherty J., Kringelbach M.L., Rolls E.T., Hornak J., Andrews C. (2001). Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience, 4(1), 95–102.doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C., Cai X. (2011). The orbitofrontal cortex and the computation of subjective value: consolidated concepts and new perspectives. Annals of the New York Academy of Sciences, 1239, 130–7.doi: 10.1111/j.1749-6632.2011.06262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva P., Wirth J.H., Williams K.D. (2011). The consequences of pain: the social and physical pain overlap on psychological responses. European Journal of Social Psychology, 41(6), 681–7.doi: 10.1002/ejsp.837. [DOI] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–56.doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience, 12(3), 154–67.doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sip K.E., Smith D.V., Porcelli A.J., Kar K., Delgado M.R. (2015). Social closeness and feedback modulate susceptibility to the framing effect. Social Neuroscience, 10(1), 35–45.doi: 10.1080/17470919.2014.944316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Groep I.H., Bos M.G.N., Jansen L.M.C., Achterberg M., Popma A., Crone E.A. (2021). Overlapping and distinct neural correlates of self-evaluations and self-regulation from the perspective of self and others. Neuropsychologia, 161, 108000.doi: 10.1016/j.neuropsychologia.2021.108000. [DOI] [PubMed] [Google Scholar]
- Wagner A.D., Maril A., Bjork R.A., Schacter D.L. (2001). Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. NeuroImage, 14(6), 1337–47.doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- Wang C., Gao J., Ma Y., Zhu C., Dong X.W. (2018). Physical pain increases interpersonal trust in females. European Journal of Pain, 22(1), 150–60.doi: 10.1002/ejp.1111. [DOI] [PubMed] [Google Scholar]
- Wang C., Zhang T., Shan Z., Liu J., Yuan D., Li X. (2019). Dynamic interpersonal neural synchronization underlying pain-induced cooperation in females. Human Brain Mapping, 40(11), 3222–32.doi: 10.1002/hbm.24592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Bao C., Gao J., Gu Y., Dong X.W. (2020). Pain modulates neural responses to reward in the medial prefrontal cortex. Human Brain Mapping, 41(5), 1372–81.doi: 10.1002/hbm.24882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A.C.D.C., Craig K.D. (2016). Updating the definition of pain. Pain, 157(11), 2420–3.doi: 10.1097/j.pain.0000000000000613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets are available on request. The raw data and generated data during analyses supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.


