Study Design:
Prospective randomized Food and Drug Administration investigational device exemption clinical trial.
Objective:
The purpose of the present study is to report the 1-year clinical and radiographic outcomes and safety profile of patients who underwent lumbar facet arthroplasty through implantation of the Total Posterior Spine System (TOPS) device.
Summary of Background Data:
Lumbar facet arthroplasty is one proposed method of dynamic stabilization to treat grade-1 spondylolisthesis with stenosis; however, there are currently no Food and Drug Administration-approved devices for facet arthroplasty.
Methods:
Standard demographic information was collected for each patient. Radiographic parameters and patient-reported outcome measures were assessed preoperatively and at regular postoperative intervals. Complication and reoperation data were also collected for each patient.
Results:
At the time of this study, 153 patients had undergone implantation of the TOPS device. The mean surgical time was 187.8 minutes and the mean estimated blood loss was 205.7cc. The mean length of hospital stay was 3.0 days. Mean Oswestry Disability Index, Visual Analog Score leg and back, and Zurich Claudication Questionnaire scores improved significantly at all postoperative time points (P>0.001). There were no clinically significant changes in radiographic parameters, and all operative segments remained mobile at 1-year follow-up. Postoperative complications occurred in 11 patients out of the 153 patients (7.2%) who underwent implantation of the TOPS device. Nine patients (5.9%) underwent a total of 13 reoperations, 1 (0.6%) of which was for device-related failure owing to bilateral L5 pedicle screw loosening.
Conclusions:
Lumbar facet arthroplasty with the TOPS device demonstrated a statistically significant improvement in all patient-reported outcome measures and the ability to maintain motion at the index level while limiting sagittal translation with a low complication rate.
Key Words: lumbar facet arthroplasty, total posterior spine system, TOPS, ODI, VAS, Zurich Claudication Questionnaire, spondylolisthesis
Lumbar spinal fusion is commonly performed to treat a variety of spinal pathologies, including degenerative conditions that may result in neural compression, malalignment, and instability. The volume of elective lumbar spine fusions increased by nearly two-thirds from 2004 to 2015, with the largest increase occurring in patients with spondylolisthesis.1 The purpose of fusion is to eliminate motion at an intervertebral segment, thereby stabilizing the operative segment against further motion-induced degeneration. However, fusion causes the pathologic redistribution of motion to adjacent levels, accelerating adjacent level degeneration.2–5 Dynamic stabilization is an alternative to fusion that minimizes adjacent level degeneration by restoring segmental stability while maintaining motion at the affected segment.6 Lumbar facet arthroplasty is one proposed method of dynamic stabilization to treat grade 1 spondylolisthesis with stenosis; however, there are currently no Food and Drug Administration (FDA)-approved devices for facet arthroplasty.
The Total Posterior Spine System (TOPS) is a pedicle-screw-based lumbar facet arthroplasty construct consisting of two titanium alloy plates with an intervening capsule (Fig. 1). The titanium plates are connected by crossbars to 2 pedicle screws of the same vertebra, preventing micro-motion and minimizing the risk of screw loosening. Because the TOPS device is affixed with pedicle screws, an aggressive decompression of the neural elements can be performed. The intervening capsule contains an articulating construct made of titanium alloy and polycarbonate urethane that is intended to mimic the function of the facet joints, allowing movement between the titanium plates in axial rotation, lateral bending, flexion, and extension while blocking sagittal translation.
FIGURE 1.
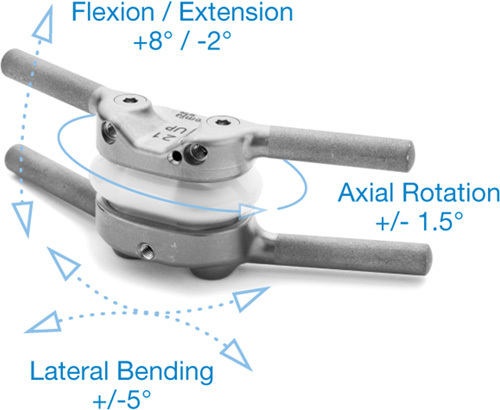
Schematic of the total posterior spine system device.
Biomechanical studies investigating the TOPS device have demonstrated its ability to restore ideal range of motion in all planes and maintain disc loads within a physiological range.7–9 Furthermore, the TOPS device places lower moments on the pedicle screws to which it is affixed compared with other pedicle-based dynamic stabilization systems such as the Dynesys System (Zimmer Spine, Warsaw, IN).8,10 This phenomenon can be explained by 2 important features of the TOPS device design: (1) the device is capable of a greater range of motion than other dynamic stabilization devices, allowing for improved load sharing between the device, disc, anterior longitudinal ligament, and posterior longitudinal ligament, and (2) the device is affixed to 2 pedicle screws at the same vertebral level rather than at 2 adjacent vertebral levels, like other dynamic stabilization devices. Since its first implantation in 2005, multiple prospective, nonrandomized clinical studies have demonstrated that wide surgical decompression followed by implantation of the TOPS device leads to a significant improvement in patient-reported outcome measures (PROMs) at both early and long-term follow-up with low rates of device-related complications.11–13 TOPS has been approved for clinical use in Europe (received CE Mark in August 2012) and is currently undergoing clinical trials in the United States to determine both its clinical efficacy and safety.
The purpose of the present study is to report the safety profile and 1-year clinical and radiographic outcomes of the investigational arm of a multicenter, prospective, randomized, controlled FDA investigational device exemption (IDE) trial comparing lumbar facet arthroplasty with the TOPS device to a control group of patients undergoing single-level transforaminal lumbar interbody fusion (TLIF). This information will help surgeons more accurately counsel patients regarding anticipated early clinical outcomes and complications following lumbar facet arthroplasty with the TOPS device.
METHODS
The patient cohort evaluated in this study was from the investigational arm of the TOPS prospective, randomized IDE (FDA #G160168) clinical trial evaluating the safety and efficacy of the TOPS device. One hundred fifty-three patients were randomized to the investigational arm of this Institutional Review Board-approved, FDA-regulated, prospective, randomized multicenter trial, and underwent a single-level lumbar decompression and facet arthroplasty through implantation of the TOPS device. The indications for surgery were symptomatic grade 1 degenerative spondylolisthesis with moderate to severe lumbar spinal stenosis and thickening of the ligamentum flavum or scarring of the facet joint capsule at a single level between L2-3 and L4-L5. Clinical symptoms were radiculopathy or neurogenic claudication that were greater than axial back pain. Radiographic studies performed within 6 months preoperatively confirmed the presence of up to grade 1 degenerative spondylolisthesis,14 at least moderate lumbar spinal stenosis15 and facet degeneration. Additional inclusion criteria included age between 35 and 80 years, at least 6 months of failed conservative management, an Oswestry Disability Index (ODI) of at least 40/100 at baseline, pain predominantly located in the leg with a Visual Analog Score (VAS) of at least 40/100 at baseline, and neurogenic claudication. Exclusion criteria included >1 pathologic level requiring surgical intervention, the presence of a free fragment disc herniation or prior discectomy at the index level or either adjacent level, <4 mm of disc height at the index level, greater than grade 1 degenerative spondylolisthesis, nondegenerative etiologies for the spondylolisthesis, prior instrumented lumbar spine surgery at any level, scoliosis >10 degrees, body mass index (BMI) >40, autoimmune disease, pregnancy, and osteoporosis (based on <2 SDs below normal on dual-energy x-ray absorptiometry scan for subjects with risk factors for osteoporosis). Patients were also excluded if they had previously underwent any surgery at the index vertebral level or either adjacent vertebral level without instrumentation unless the previous intervention involved only the posterior elements (eg, rhizotomy, laminectomy, foraminotomy, and/or facetectomy).
All patients underwent a standard midline posterior approach to the lumbar spine followed by a wide decompression and bilateral facetectomies at the index level. Upon completion of the decompression, four surface-treated conical pedicle screws were placed in the 2 vertebrae adjacent to the pathologic segment. The TOPS device (Premia Spine, Netanya, Israel) was then affixed to these pedicle screws with proprietary alignment and insertion tools (Fig. 2).
FIGURE 2.
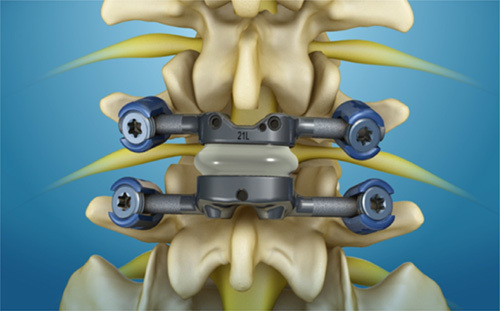
Schematic demonstrating the total posterior spine system device affixed to pedicle screws.
Standard demographic data were collected on all patients including sex, age, height, weight, body mass index, smoking history, and history of prior lumbar spine surgery. Standard surgical variables collected include operative levels, operative time, estimated blood loss (EBL), length of stay (LOS), and any operative complications. PROMs including ODI,16 VAS back and leg pain, and Zurich Claudication Questionnaire (ZCQ) scores17 were collected preoperatively at 6 weeks, 3 months, 6 months, and 12 months postoperatively. The percentage of patients achieving a minimum clinically important difference (MCID) in ODI,18 VAS,19 and ZCQ20 was determined based upon established criteria.
Preoperative imaging studies consisted of standing anteroposterior, lateral, left/right bending, and flexion/extension radiographs, and magnetic resonance imaging with T1, T2, and STIR sequences formatted in both axial and sagittal planes (Fig. 3). All patients underwent standing neutral anteroposterior and lateral radiographs before discharge from the hospital and at 6 weeks postoperatively. Radiographic parameters measured on preoperative and in-hospital postoperative neutral radiographs included disc height (anterior, posterior, and mean), segmental lordosis at the index level, global lumbar lordosis (from the inferior endplate of L1 to the superior endplate of L5), and magnitude and direction of spondylolisthesis (Fig. 4). Plain standing anteroposterior, lateral, left/right bending, and flexion/extension radiographs were then obtained at 3 months, 6 months, and 12 months postoperatively (Fig. 5). Radiographic parameters measured on dynamic radiographs preoperatively and at 12 months postoperatively included angular motion at the index level during left/right bending (lateral bending range of motion [ROM]), angular motion in flexion and extension (flexion/extension ROM), and translational motion in flexion and extension (flexion/extension translation) (Fig. 6). All radiographic measurements were performed by 2 independent fellowship-trained neuro-radiologists and an adjudicator in cases of disagreement between the primary reviewers.
FIGURE 3.
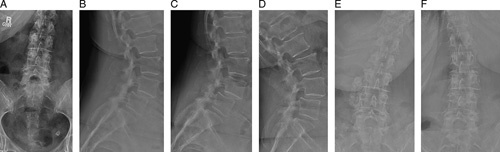
Preoperative standing radiographs in a representative patient before implantation of the total posterior spine system device, including A, Anteroposterior, B, lateral, C, flexion, D, extension, E, left bending, and F, right bending views.
FIGURE 4.
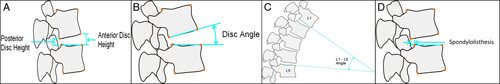
Schematic demonstrating the 4 measurements obtained on static radiographs preoperatively and at regular postoperative intervals, including A, disc height, B, disc angle, C, global lordosis, and D, spondylolisthesis.
FIGURE 5.
12-month postoperative standing radiographs in the same representative patient, including A, Anteroposterior, B, lateral, C, flexion, D, extension, E, left bending, and F, right bending views.
FIGURE 6.
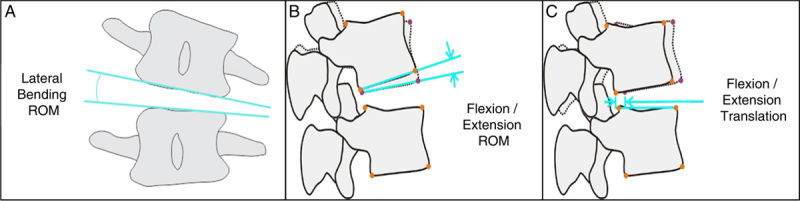
Schematic demonstrating the 3 measurements obtained on dynamic radiographs preoperatively and at 12 months postoperatively, including A, lateral bending ROM, B, flexion/extension ROM, and C, flexion/extension translation. ROM indicates range of motion.
All patients were followed longitudinally for the development of postoperative complications including new neurological deficits, infection, dural tears, seroma or hematoma formation requiring surgical intervention, device-related complications, and the need for reoperation.
RESULTS
Demographics
As of August 15, 2021, one hundred fifty-three patients were enrolled in the investigational arm of this FDA-approved IDE clinical trial and underwent implantation of the TOPS device. The cohort consisted of 90 females (58.8%), and the mean age was 63.2 years. One hundred forty-five TOPS devices were implanted at L4-5 (94.8%), and 8 devices were implanted at L3-4 (5.2%) (Table 1). The mean surgical time was 187.8 minutes and the mean EBL was 205.7 cc. The mean length of hospital stay was 3.0 days (Table 2).
TABLE 1.
Demographics
| n=153 (%) | |
|---|---|
| Demographics | |
| Age (y) | 63.2±8.3 |
| Height (inches) | 66.8±4.0 |
| Weight (lbs) | 189.4±37.4 |
| BMI (kg/m2) | 29.7±4.8 |
| Sex (female) | 90 (58.8) |
| Use of nicotine products | |
| No, never smoked | 98 (64.1) |
| No, but prior history | 52 (34.0) |
| Current smoker | 3 (2.0) |
| Prior lumbar surgery | |
| Yes | 9 (5.9) |
| No | 144 (94.1) |
| Level implanted | n |
| L1/L2 | 0 |
| L2/L3 | 0 |
| L3/L4 | 8 (5.2) |
| L4/L5 | 145 (94.8) |
Continuous Variables are Displayed as mean±SD. Categorical variables are displayed as number (percent).
BMI indicates body mass index, lbs, pounds.
TABLE 2.
Surgical Variables
| n=153 (%) | |
|---|---|
| Surgical variables | |
| Time in surgery (mins) | 187.8±62.4 |
| Length of stay (days) | 3.0±4.1 |
| EBL (cc) | 205.7±154.3 |
| Estimated blood loss | |
| <100 cc | 28 (18.3) |
| 100-<250 cc | 75 (49.0) |
| 250-<400 cc | 31 (20.3) |
| ≥400 cc | 19 (12.4) |
Continuous variables are displayed as mean±SD. Categorical variables are displayed as number (percent).
EBL indicates estimated blood loss.
Safety Profile
Postoperative complications occurred in 11 of the 153 patients (7.2%), including 2 new neurological deficits, 2 dural tears, 2 retained drains, 1 pair of misplaced pedicle screws, 1 screw loosening, 1 infection, 1 seroma, and 1 hematoma (Table 3). The 2 patients (1.3%) reporting new neurological deficits postoperatively included 1 patient with a new foot drop and 1 patient with new numbness involving their left knee, both of whom experienced complete resolution of their neurological deficits by 1-year postoperatively.
TABLE 3.
Complications
| n=153 (%) | |
|---|---|
| # of Patients | |
| New neurological deficits | 2 (1.3) |
| Dural tear | 2 (1.3) |
| Infection | 1 (0.7) |
| Seroma | 1 (0.7) |
| Hematoma | 1 (0.7) |
| Implant loosening | 1 (0.7) |
| Misplaced instrumentation | 1 (0.7) |
| Retained drains | 2 (1.3) |
| Reoperation* | 9 (5.9) |
*9 Patients underwent a total of 13 Reoperations.
Nine patients (5.9%) required a total of 13 reoperations (Table 4). Two patients (1.3%) had retained drain specimens requiring reoperation for removal. One patient (0.7%) was noted to have symptomatic loosening of their pedicle screws at L5 nearly 17 months after implantation of the TOPS device at L4-5. This patient underwent removal of the TOPS device followed by an anterior lumbar interbody fusion (ALIF) at L4-5. One patient (0.7%) was found to have misplaced pedicle screws at L4 on routine in-hospital postoperative radiographs. This patient did not experience any neurological deficits and underwent revision surgery during the index hospitalization, at which time the L4 pedicle screws were repositioned and the TOPS device was reimplanted. One patient (0.7%) underwent hematoma evacuation 2 weeks after the index surgery, and 1 patient underwent seroma evacuation with the removal of the TOPS device nearly 3 months after the index surgery owing to suspected infection at the time of the seroma evacuation. One patient (0.7%) experienced a wound infection requiring irrigation and debridement on postoperative day 12. The debridement was complicated by an unrecognized dural tear, for which the patient subsequently underwent reoperation for removal of the TOPS device and repair of the dural tear followed by an ALIF without posterior instrumentation in a delayed fashion. One patient (0.7%) with a persistent exacerbation of their low back pain underwent reoperation 6 months postoperatively for removal of the TOPS device and conversion to a single-level posterolateral fusion. Finally, 1 patient (0.7%) underwent reoperation 2 weeks postoperatively owing to a persistent cerebrospinal fluid (CSF) leak, at which time the TOPS device was removed, the dural tear was identified and repaired, and the TOPS device was reimplanted. Nearly 1 year later, the patient continued to experience symptoms consistent with an ongoing CSF leak and underwent a second reoperation, which included removal of the TOPS device to permit exploration and identification of the tear, repair of a dural tear, and conversion to a single level posterolateral fusion. The patient required 2 subsequent reoperations owing to persistence of the CSF leak, ultimately requiring shunt implantation to address a pseudomeningocele.
TABLE 4.
Reoperations
| Patient ID | Index Level | Adverse Event | Time From Index Surgery to Reoperation (Months) | TOPS Removed? | Surgical Outcome |
|---|---|---|---|---|---|
| 1 | L4-5 | Retained drain | 1.7 | No | Removal of retained drain. Patient was offered removal during the index hospitalization but elected to delay removal until 1.7 m postoperatively. |
| 2 | L4-5 | Worse LBP | 6.6 | Yes | Removal of TOPS and conversion to single-level posterolateral fusion |
| 3 | L4-5 | Seroma | 2.5 | Yes | Wound exploration and TOPS removal |
| 4 | L4-5 | Dural Tear Persistent CSF Leak Persistent CSF Leak with severe leg pain Pseudomeningocele | 0.6 11.1 11.5 17.4 | Yes (replaced) Yes (convert to fusion) n/a n/a | Removal of TOPS, dural repair, placement of new TOPS Removal of TOPS, dural repair, conversion to single-level posterolateral fusion Wound exploration Wound exploration and shunt implantation |
| 5 | L4-5 | Loose pedicle screws at L5 | 17.2 | Yes | Two-stage revision including removal of TOPS followed by ALIF at L4-5 |
| 6 | L3-4 | Wound infection dural tear | 0.4 0.9 | No Yes | Irrigation and debridement Removal of TOPS, dural repair (subsequent ALIF at L3-4) |
| 7 | L3-4 | Hematoma | 0.4 | No | Hematoma evacuation |
| 8 | L4-5 | Retained drain | 0.1 | No | Removal of retained drain |
| 9 | L4-5 | Pedicle screw misplacement | 0.2 | No | Revision of L4 pedicle screws |
ALIF indicates anterior lumbar interbody fusion, CSF, cerebrospinal fluid; LBP, low back pain; TOPS, Total Posterior Spine System.
PROMs
Of the 153 patients enrolled in the investigational arm of this ongoing clinical trial, 105 patients (69%) reached their 1-year follow-up by the time of this interim analysis and are included in the PROMs at 1-year postoperatively (Table 5). Mean ODI scores improved from 56.9±12.4 preoperatively to 22.1±17.0 at 6 weeks postoperatively (P<0.001), and demonstrated maintenance of improvement at 3, 6, and 12 months postoperatively. At 12 months postoperatively, mean ODI scores were 11.5±14.9 and 93.2% of patients had achieved MCID (P<0.001) (Fig. 7).
TABLE 5.
Patient Reported Outcomes
| n=105 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ODI | VAS—Low Back Pain | VAS—Worst Leg Pain | ZCQ—Symptom Score | ZCQ—Physical Score | ZCQ—Satisfaction | |||||||
| Time Interval | Mean | MCID (%) | Mean | MCID (%) | Mean | MCID (%) | Mean | MCID (%) | Mean | MCID (%) | Mean | % <2.5 (%) |
| Preoperative | 56.9±12.4 | — | 67.2±24.4 | — | 83.9±13.2 | — | 3.76±0.64 | — | 2.95±0.40 | — | — | — |
| Week 6 | 22.1±17.0 | 83.7 | 15.9±16.7 | 79.6 | 12.0±20.4 | 91.3 | 1.96±0.63 | 94.2 | 1.64±0.59 | 85.6 | 1.42±0.49 | 96.2 |
| Month 3 | 14.3±16.2 | 92.2 | 13.4±19.8 | 79.6 | 12.4±21.3 | 94.1 | 1.86±0.74 | 95.1 | 1.45±0.62 | 90.3 | 1.43±0.61 | 91.3 |
| Month 6 | 14.0±17.5 | 89.3 | 15.1±23.4 | 80.6 | 13.3±23.4 | 90.3 | 1.78±0.71 | 94.2 | 1.43±0.64 | 89.3 | 1.40±0.65 | 91.3 |
| Month 12 | 11.5±14.9 | 93.2 | 12.7±21.8 | 83.5 | 11.5±22.7 | 94.2 | 1.74±0.74 | 93.2 | 1.39±0.56 | 93.2 | 1.34±0.64 | 91.3 |
| P | <0.001 | — | <0.001 | — | <0.001 | — | <0.001 | — | <0.001 | — | — | — |
Continuous variables are displayed as mean (SD).
MCID indicates minimum clinically important difference; ODI, Oswestry Disability Index; VAS, Visual Analog Score; ZCQ, Zurich Claudication Questionnaire.
FIGURE 7.

Bar graphs demonstrating (left) mean Oswestry Disability Index (ODI) scores at all measured time points and (right) the percentage of patients achieving minimum clinically important difference (MCID) in ODI.
Mean VAS for low back pain improved from 67.2±24.4 preoperatively to 15.9±16.7 at 6 weeks postoperatively (P<0.001). At 12 months postoperatively, VAS low back pain was 12.7 ±21.8 and 83.5% of patients had achieved MCID (P<0.001). Similarly, mean VAS for worst leg pain improved from 83.9±13.2 preoperatively to 12.0±20.4 at 6 weeks postoperatively, and remained low at 11.5±22.7 at 12 months postoperatively (P<0.001). Greater than 90% of patients achieved MCID in VAS worst leg pain at all postoperative time points (Fig. 8).
FIGURE 8.

Bar graphs demonstrating (left) mean Visual Analog Score (VAS) leg and back pain scores at all measured time points and (right) the percentage of patients achieving minimum clinically important difference (MCID) in VAS leg and pain.
ZCQ symptom scores improved from 3.76±0.64 to 1.96 ±0.63 at 6 weeks postoperatively and 1.74±0.74 at 12 months postoperatively (P<0.001). Similarly, ZCQ physical scores improved from 2.95±0.40 to 1.64±0.59 at 6 weeks postoperatively and 1.39±0.56 at 12 months postoperatively (P<0.001). Greater than 85% of patients achieved MCID in ZCQ symptom score and physical score at all postoperative time points. ZCQ satisfaction scores were 1.42±0.49 at 6 weeks postoperatively and were maintained at 12 months postoperatively (1.34±0.64). Greater than 90% of patients achieved ZCQ satisfaction scores <2.5 at all postoperative time points, indicating high levels of patient satisfaction (Fig. 9).
FIGURE 9.
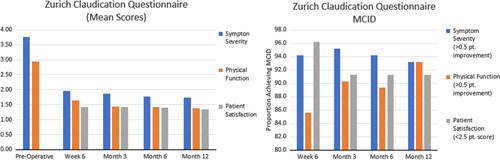
Bar graphs demonstrating (left) Mean Zurich Claudication Questionnaire (ZCQ) subscores at all measured time points and (right) the percentage of patients achieving minimum clinically important difference in ZCQ subscores.
Radiographic Parameters
All 153 patients who underwent implantation of the TOPS device had postoperative radiographs obtained before dismissal from the hospital for evaluation of static radiographic parameters. An additional 90 patients had completed 1 year of radiographic follow-up at the time of this interim analysis, allowing for comparison of preoperative and 1-year postoperative dynamic radiographic parameters (Table 6).
TABLE 6.
Radiographic Parameters
| Parameters Assessed on Static Radiographs* | n=153 | |||
|---|---|---|---|---|
| Preoperative | Postoperative | Δ | P | |
| Disc Angle | ||||
| Index | 9.2±4.5 | 9.8±4.2 | 0.6±2.9 | 0.004 |
| Above index | 11.8±3.1 | 11.2±3.1 | -0.6±1.7 | 0.006 |
| Below index | 13.9±6.4 | 12.1±5.7 | -1.8±3.0 | <0.001 |
| Disc height | ||||
| Anterior | 10.6±3.0 | 11.5±2.8 | 0.9±1.3 | <0.001 |
| Posterior | 5.2±1.5 | 5.7±1.7 | 0.5±1.0 | <0.001 |
| Mean | 7.9±1.9 | 8.6±1.9 | 0.7±0.8 | <0.001 |
| Global lordosis (L1-L5) | 41.5±9.9 | 39.3±10.2 | -1.3±5.8 | 0.01 |
| Spondylolisthesis (mm)† | ||||
| Index | -4.7±2.5 | -3.1±2.6 | 1.6±1.9 | <0.001 |
| Above index | 1.4±1.5 | 1.4±1.5 | -0.1±0.6 | 0.13 |
| Below index | 1.3±1.6 | 1.3±1.6 | 0.0±0.4 | 0.44 |
| Parameters Assessed on Dynamic Radiographs* | n=90 | |||
| Preoperative | 12 mo Postoperative | Δ | P | |
| Flex/ex ROM | ||||
| Index | 3.9±2.9 | 3.7±3.0 | -0.2±3.4 | 0.55 |
| Above index | 3.2±3.0 | 3.8±3.5 | 0.6±3.4 | 0.13 |
| Below index | 5.4±3.6 | 6.9±4.1 | 1.5±4.1 | <0.001 |
| Flex/ex translation | ||||
| Index | 1.0±0.8 | 0.9±1.0 | -0.1±1.1 | 0.65 |
| Above index | 0.7±0.7 | 0.8±0.7 | 0.1±0.8 | 0.54 |
| Below index | 0.4±0.4 | 0.6±0.5 | 0.2±0.5 | 0.01 |
| Lateral bending ROM | 3.2±2.7 | 3.6±2.6 | 0.4±3.2 | 0.24 |
Continuous variables are displayed as mean (SD).
Static radiographs were obtained during the index hospitalization, before dismissal from the hospital. dynamic radiographs were obtained 12 months postoperatively.
Retrolisthesis reported as a positive value. anterolisthesis reported as a negative value.
mm indicates millimeters; ROM, range of motion..
Parameters assessed on neutral standing radiographs obtained preoperatively and postoperatively before dismissal from the hospital included global lordosis, disc height at the index level, and disc angle and spondylolisthesis at the index level and the levels above and below the index level. Disc angle increased at the index level from 9.2±4.5 degrees preoperatively to 9.8±4.2 degrees postoperatively (P=0.004), but decreased at the levels above (11.8±3.1 degrees to 11.2±3.1 degrees, P=0.006) and below (13.9±6.4 degrees to 12.1±5.7 degrees, P<0.001) the index level. Mean disc height at the index level increased from 7.9±1.9 mm preoperatively to 8.6±1.9 mm postoperatively (P<0.001). The index level spondylolisthesis improved from −4.7±2.5 mm preoperatively to −3.1±2.6 mm postoperatively (P<0.001). Global lordosis decreased from 41.5±9.9 degrees preoperatively to 39.3±10.2 degrees postoperatively (P=0.01).
Parameters assessed on dynamic standing radiographs obtained preoperatively and at 12 months postoperatively included flexion/extension ROM and translation at the index level and the levels above and below the index level and lateral bending ROM at the index level. Flexion/extension ROM demonstrated no significant change at the index level or the level above but increased from 5.4±3.6 degrees preoperatively to 6.9±4.1 degrees postoperatively at the level below the index level (P<0.001). Similarly, flexion/extension translation demonstrated no significant change at the index level or the level above but increased from 0.4±0.4 mm preoperatively to 0.6±0.5 mm postoperatively at the level below the index level (P=0.01). Lateral bending ROM was preserved from preoperatively to postoperatively at the index level. All operative segments remained mobile at 1-year follow-up without evidence of device failure.
DISCUSSION
This study presents an interim analysis of the safety profile and 1-year clinical and radiographic outcomes after implantation of the TOPS device as part of the investigational arm of an ongoing FDA-approved IDE clinical trial. The results presented demonstrate that the TOPS device compares favorably in all respects to outcomes reported in the literature after single-level TLIF. The mean surgical time in the TOPS cohort was 187.8±62.4 minutes. Surgical time for TLIF has been reported to range from 113.2 to 198.0 minutes, with a recent systematic review reporting the mean surgical time for TLIF to be 169 minutes.21–27 Given the novelty of the TOPS procedure, the authors would anticipate a decrease in surgical time as surgeons gain increased familiarity with the TOPS device. The mean EBL during the TOPS procedure was 205.7±154.3, which is substantially lower than the mean EBL of 350cc that was reported in a recent systematic review of patients undergoing single-level TLIF.27 Mean LOS following the TOPS procedure (3.0±4.1 d) was similar to reported LOS following TLIF (range 2.38–3.71 d).28,29
Complications occurred in 7.2% of patients in the TOPS cohort, which is similar in frequency to reported rates in the literature for patients undergoing single-level lumbar spine fusions.27 Ten of the 11 complications were not directly attributable to the TOPS device, but instead were inherent risks associated with the performance of a wide decompression and placement of pedicle screws. The 1.3% rate of new neurological deficit in the TOPS cohort is consistent with reported rates of new neurological deficits following single-level TLIF, which range from 0% to 3.3%.21–27,30–32 One patient (0.7%) in the TOPS cohort experienced a postoperative infection, which is comparable to reported rates in the literature following single-level TLIF (range 0–5.0%).21–26,31,32
A complete overview of the patients that required reoperation is provided in Table 4. Of note, only 1 patient required reoperation for a device-related complication (ie, pedicle screw loosening) and is described in detail below; the remaining 8 patients required reoperation for complications related to the decompression, pedicle screw placement, and retained drain specimens. The 5.9% overall rate of reoperation in this TOPS cohort is lower than reported 1-year reoperation rates in the literature following single-level lumbar fusions (13.5%) and similar to rates reported following single-level TLIF (4%).33 However, further exploration of the indication for reoperation reveals important differences. In a large database study of 71,456 patients undergoing one- or two-level lumbar fusions, Cummins et al found that 43.5% of the 9670 reoperations performed within 1-year from the index surgery were owing to adjacent segment degeneration, 37.65% were owing to mechanical failure, and 7.17% were owing to stenosis.33 No patient in the present study required reoperation for adjacent segment disease at 1 year postoperatively, and only 1 patient (0.7%) required reoperation owing to hardware complications. Biomechanical studies have demonstrated that preservation of the posterior ligamentous complex, which is not feasible with implantation of the TOPS device, may lead to decreased strain at the adjacent segments; long-term follow-up of the TOPS cohort will be necessary to determine whether the motion preserved by the TOPS device outweighs the potential downside of sacrificing the posterior ligamentous complex in terms of precipitating adjacent segment disease.34
Unlike standard fusion constructs, the purpose of the TOPS device is to maintain motion at a previously pathologic intervertebral segment. This is in contrast to a single-level fusion, in which consolidation of the fusion mass is intended to entirely eliminate motion, leading to decreasing strain on the pedicle screws over time. As such, the primary theoretical concern is that ongoing motion through the TOPS device may lead to increased rates of pedicle screw loosening. In the present study, 1 patient (0.7%) demonstrated loosening of their bilateral L5 pedicle screws at 17 months postoperatively, requiring a 2-stage revision to remove the TOPS device and perform an L4-5 ALIF. This low rate of hardware-related complications compares favorably to reported rates following TLIF (range 0–6.1%).21–27,30–32 Furthermore, the 0.7% rate of pedicle screw loosening in the TOPS cohort is substantially lower than the 9.6% rate of loosening at 1-year postoperatively reported for the Dynesys System.10 This difference in pedicle screw loosening rates is likely attributable to the design of the TOPS device, which relies on crossbars that connect pedicle screws at the same vertebral level, rather than connecting pedicle screws at adjacent levels as seen in the Dynesys System. In sequential retrospective reviews of 10 patients who underwent implantation of the TOPS device, Anekstein et al11 found no clinical or radiographic evidence of pedicle screw loosening at 5-year, 7-year, or 11-year follow-up.13,35 In addition, there was no evidence of spontaneous fusion, and the TOPS device remained mobile on flexion/extension radiographs in these 3 studies. Continued long-term follow-up of the patients in the present clinical trial will more definitively establish the ability of the TOPS device to maintain long-term segmental motion while avoiding mechanical complications such as pedicle screw loosening and device failure.
Patients who underwent implantation of the TOPS device demonstrated a significant improvement in all PROMs at all postoperative time points. Of particular note, >79% of patients reported MCID in all PROMs at all postoperative time points, and >90% achieved MCID in ODI, VAS worst leg pain, ZCQ symptom score, and ZCQ physical score at 12-month postoperative follow-up. These results compare favorably to reported rates of patient-reported improvement following single-level TLIF. In their retrospective review of 24 patients who underwent single-level TLIF for lumbar instability, Sakeb et al25 reported an improvement in ODI from 56.71 preoperatively to 7.46 postoperatively and in VAS from 59.4 preoperatively to 18.3 postoperatively. Yang et al26 reported an improvement in ODI from 49.6 preoperatively to 14.1 postoperatively and in VAS from 47.2 preoperatively to 13.3 postoperatively in the cohort of patients who underwent TLIF as part of their randomized control trial comparing TLIF to PLIF. Following implantation of the TOPS device, patients report high rates of clinical improvement in all PROMs, which are similar to reported rates of improvement following single-level TLIF.
Early postoperative assessment of static radiographic parameters demonstrated increased index disc angle and disc height with a reduction in the magnitude of spondylolisthesis. Comparison of dynamic radiographic parameters from preoperative to 12 months postoperative demonstrated increased flexion/extension ROM and translation. Although these changes proved to be statistically significant, the magnitude of change is small and unlikely to be of clinical significance. These findings suggest that the TOPS device maintains motion at the index surgical level while preventing further sagittal translation. In their small case series, Smorgick et al13 demonstrated that the TOPS device maintained motion without evidence of worsening sagittal translation, pedicle screw loosening, or device failure at 11 years postoperatively. Longitudinal follow-up of the TOPS cohort enrolled in this clinical trial will more definitively establish the ability of the TOPS device to maintain motion and limit sagittal translation in the long term.
This study has several limitations. First, this study analyzes outcomes in only the investigational arm of an ongoing clinical trial owing to strict guidelines requiring that outcomes in the control arm not be reported until the conclusion of the trial. As such, the results reported herein are uncontrolled and can only be interpreted in comparison to previously published cohorts of fusion patients in the literature. Although the uncontrolled nature of this analysis limits the strength of conclusions that can be drawn, the present study utilizes PROMs that are well validated in the literature and can be easily compared with previously published cohorts of fusion patients. Second, the present study analyzes the complication profile and reoperation rates in the entire cohort of patients who have undergone the TOPS procedure as part of the ongoing clinical trial as of August 15, 2021. Consequently, only two-thirds of the TOPS investigational arm of this study has completed 1 year of follow-up. Although most of the reoperations were performed within 3 months postoperatively, the short follow-up in one-third of the patients may underestimate the 1-year reoperation rate following the TOPS procedure. Furthermore, long-term complications inherent to a motion-preserving lumbar facet arthroplasty device cannot be determined by this cohort, in which the longest follow-up remains <5 years. Third, this study identified a slight decrease in lumbar lordosis measured on static radiographs obtained during the index hospitalization in this cohort of patients undergoing implantation of the TOPS device. Although this small decrease in lumbar lordosis is likely clinically insignificant, the study protocol does not call for this parameter to be remeasured until the 2-year postoperative radiographs. As such, this 1-year follow-up study is unable to determine whether long-term lumbar lordosis is impacted by placement of the TOPS device.
CONCLUSIONS
Lumbar facet arthroplasty with the TOPS device demonstrated a low complication rate, significant improvement in all PROMs, and the ability to maintain motion at the index level while limiting sagittal translation. The complication rates and improved postoperative PROMs are similar to those reported in the literature following single-level TLIF. However, no patient in the TOPS cohort required reoperation within the first year owing to adjacent segment degeneration, whereas this is the most frequently reported indication for reoperation within the first year for patients who have undergone a single-level lumbar fusion. The final comparison of the TOPS cohort to patients undergoing single-level TLIF at the conclusion of the ongoing clinical trial will be necessary to draw firm conclusions regarding clinical performance of the investigational TOPS device.
ACKNOWLEDGMENTS
The authors thank the surgeons of TOPS Study Investigator Group for their contribution to the study: Joshua Ammerman; Joshua Wind (Sibley Memorial Hospital); Neel Anand (Cedars-Sinai Medical Center Los Angeles); Theodore Belanger; Akwasi Boah (Texas Back Institute); Harel Deutsch (Rush University Medical Center); Mark Gerber (Neuroscience and Spine Associates); Ken Hsu, James Zucherman (St. Mary’s Medical Center San Francisco); Babak Kalantar (MedStar Georgetown University Hospital); Christopher Kong (Cedars-Sinai Medical Center); Andy Kranenburg (Southern Oregon Orthopedics); Ajit Krishnaney; Douglas Orr (Cleveland Clinic Foundation); Mitchell Levine; Jonathon Oren (Lenox Hill Hospital); James Lindley (Memorial University Medical Center Savannah); Kent New (Ascension St. Vincent’s Southside Jacksonville); Michael Oh (University of CA Irvine Medical Center); Mark Oppenlander; Osama Kashlan (University of Michigan); Ashvin Patel (Kennedy-White Orthopaedic Center Sarasota); Vikas Patel; Evalina Burger (University of Colorado); Nick Phan (Marshall Health, Huntington); Eric Potts; Jean-Pierre Mobasser (Goodman Campbell Brain and Spine Indianapolis); Alan Villavicencio; Sharad Rajpal (Boulder Neurosurgical & Spine Associates); Scott Webb (Florida Spine Institute Clearwater); Gregory Wiggins (Bronson Neuroscience Center Kalamazoo); Yizhar Floman (Israel Spine Center); Nathan Lebwohl (University of Miami); and Larry Khoo (The Spine Clinic of Los Angeles).
Footnotes
The authors have no financial or other influential relationships to disclose. Funding for the ongoing FDA-approved investigational device exemption trial is provided by Premia Spine.
Approval obtained from the Institutional Review Board at each participating site.
The authors declare no conflict of interest.
Contributor Information
Zachariah W. Pinter, Email: pinter.zachariah@mayo.edu.
Brett A. Freedman, Email: freedman.brett@mayo.edu.
Ahmad Nassr, Email: nassr.ahmad@mayo.edu.
Arjun S. Sebastian, Email: sebastian.arjun@mayo.edu.
Domagoj Coric, Email: dom@cnsa.com.
William C. Welch, Email: william.welch@uphs.upenn.edu.
Michael P. Steinmetz, Email: steinmm@ccf.org.
Stephen E. Robbins, Email: stephenrobbinsmd@wiscboneandjoint.com.
Jared Ament, Email: jament@sierraneuro.org.
Neel Anand, Email: neelanand@mac.com.
Paul Arnold, Email: pmarnold36@gmail.com.
Eli Baron, Email: eli.baron@cshs.org.
Jason Huang, Email: jason.huang@bswhealth.org.
Robert Whitmore, Email: rgwhitmore@gmail.com.
Donald Whiting, Email: donald.whiting@ahn.org.
David Tahernia, Email: dtahernia@gmail.com.
Faheem Sandhu, Email: drfasandhu@gmail.com.
Ali Chahlavi, Email: chahlavi@me.com.
Joseph Cheng, Email: chengj6@ucmail.uc.edu.
John Chi, Email: jchi@bwh.harvard.edu.
Stephen Pirris, Email: pirrsm@gmail.com.
Michael Groff, Email: mgroff@bwh.harvard.edu.
Alain Fabi, Email: fabialain63@gmail.com.
Scott Meyer, Email: smeyer@altairhealth.com.
Vivek Kushwaha, Email: vivekkushwaha@sbcglobal.net.
Roland Kent, Email: r.kent@axisspinecenter.com.
Steven DeLuca, Email: delucasteven@me.com.
Yossi Smorgick, Email: ysmorgick@gmail.com.
Yoram Anekstein, Email: nuritan@zahav.net.il.
REFERENCES
- 1.Martin BI, Mirza SK, Spina N, et al. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine. 2019;44:369–376. [DOI] [PubMed] [Google Scholar]
- 2.Cheh G, Bridwell KH, Lenke LG, et al. Adjacent segment disease followinglumbar/thoracolumbar fusion with pedicle screw instrumentation: a minimum 5-year follow-up. Spine. 2007;32:2253–2257. [DOI] [PubMed] [Google Scholar]
- 3.Eck JC, Humphreys SC, Hodges SD. Adjacent-segment degeneration after lumbar fusion: a review of clinical, biomechanical, and radiologic studies. Am J Orthop. 1999;28:336–340. [PubMed] [Google Scholar]
- 4.Kim TH, Lee BH, Moon SH, et al. Comparison of adjacent segment degeneration after successful posterolateral fusion with unilateral or bilateral pedicle screw instrumentation: a minimum 10-year follow-up. Spine J. 2013;13:1208–1216. [DOI] [PubMed] [Google Scholar]
- 5.Schlegel JD, Smith JA, Schleusener RL. Lumbar motion segment pathology adjacent to thoracolumbar, lumbar, and lumbosacral fusions. Spine. 1996;21:970–981. [DOI] [PubMed] [Google Scholar]
- 6.Mulholland RC, Sengupta DK. Rationale, principles and experimental evaluation of the concept of soft stabilization. Eur Spine J. 2002;11:S198–S205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heuer F, Schmidt H, Käfer W, et al. Posterior motion preserving implants evaluated by means of intervertebral disc bulging and annular fiber strains. Clin Biomech. 2012;27:218–225. [DOI] [PubMed] [Google Scholar]
- 8.Meyers K, Tauber M, Sudin Y, et al. Use of instrumented pedicle screws to evaluate load sharing in posterior dynamic stabilization systems. Spine J. 2008;8:926–932. [DOI] [PubMed] [Google Scholar]
- 9.Wilke HJ, Schmidt H, Werner K, et al. Biomechanical evaluation of a new total posterior-element replacement system. Spine. 2006;31:2790–2796. [DOI] [PubMed] [Google Scholar]
- 10.Stoll TM, Dubois G, Schwarzenbach O. The dynamic neutralization system for the spine: a multi-center study of a novel non-fusion system. Eur Spine J. 2002;11(Suppl 2):S170–S178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anekstein Y, Floman Y, Smorgick Y, et al. Seven years follow-up for total lumbar facet joint replacement (TOPS) in the management of lumbar spinal stenosis and degenerative spondylolisthesis. Eur Spine J. 2015;24:2306–2314. [DOI] [PubMed] [Google Scholar]
- 12.McAfee P, Khoo LT, Pimenta L, et al. Treatment of lumbar spinal stenosis with a total posterior arthroplasty prosthesis: implant description, surgical technique, and a prospective report on 29 patients. Neurosurg Focus. 2007;22:E13. [DOI] [PubMed] [Google Scholar]
- 13.Smorgick Y, Mirovsky Y, Floman Y, et al. Long-term results for total lumbar facet joint replacement in the management of lumbar degenerative spondylolisthesis. J Neurosurg Spine. 2019;32:1–6. [DOI] [PubMed] [Google Scholar]
- 14.Meyerding HW. Spondyloptosis. Surg Gynecol Obstet. 1932;54:371–377. [Google Scholar]
- 15.Lurie JD, Tosteson AN, Tosteson TD, et al. Reliability of readings of magnetic resonance imaging features of lumbar spinal stenosis. Spine. 2008;33:1605–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fairbank JC, Couper J, Davies JB, et al. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- 17.Stucki G, Daltroy L, Liang MH, et al. Measurement properties of a self-administered outcome measure in lumbar spinal stenosis. Spine. 1996;21:796–803. [DOI] [PubMed] [Google Scholar]
- 18.Carragee EJ, Cheng I. Minimum acceptable outcomes after lumbar spinal fusion. Spine J. 2010;10:313–320. [DOI] [PubMed] [Google Scholar]
- 19.Kovacs FM, Abraira V, Royuela A, et al. Minimal clinically important change for pain intensity and disability in patients with nonspecific low back pain. Spine. 2007;32:2915–2920. [DOI] [PubMed] [Google Scholar]
- 20.Fukushima M, Oka H, Oshima Y, et al. Evaluation of the minimum clinically important differences of the zurich claudication questionnaire in patients with lumbar spinal stenosis. Clin Spine Surg. 2020;33:E499–E503. [DOI] [PubMed] [Google Scholar]
- 21.de Kunder S, Rijkers K, Van Hemert W, et al. Transforaminal versus posterior lumbar interbody fusion as operative treatment of lumbar spondylolisthesis, a retrospective case series. Interdiscip Neurosurg. 2016;5:64–68. [Google Scholar]
- 22.Han S-Y, Xiao Q, Zhu G-T, et al. Comparison between transforaminal lumbar interbody fusion and posterior lumbar interbody fusion in treatment of lumbar spondylolisthesis. Int J Clin Exp Med. 2016;9:3932–3938. [Google Scholar]
- 23.Liu J, Deng H, Long X, et al. A comparative study of perioperative complications between transforaminal versus posterior lumbar interbody fusion in degenerative lumbar spondylolisthesis. Eur Spine J. 2016;25:1575–1580. [DOI] [PubMed] [Google Scholar]
- 24.Park J-S, Kim Y-B, Hong H-J, et al. Comparison between posterior and transforaminal approaches for lumbar interbody fusion. J Korean Neurosurg Soc. 2005;37:340–344. [Google Scholar]
- 25.Sakeb N, Ahsan K. Comparison of the early results of transforaminal lumbar interbody fusion and posterior lumbar interbody fusion in symptomatic lumbar instability. Ind J Orthop. 2013;47:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang EZ, Xu JG, Liu XK, et al. An RCT study comparing the clinical and radiological outcomes with the use of PLIF or TLIF after instrumented reduction in adult isthmic spondylolisthesis. Eur Spine J. 2016;25:1587–1594. [DOI] [PubMed] [Google Scholar]
- 27.de Kunder SL, van Kuijk SMJ, Rijkers K, et al. Transforaminal lumbar interbody fusion (TLIF) versus posterior lumbar interbody fusion (PLIF) in lumbar spondylolisthesis: a systematic review and meta-analysis. Spine J. 2017;17:1712–1721. [DOI] [PubMed] [Google Scholar]
- 28.Kerolus MG, Yerneni K, Witiw CD, et al. Enhanced recovery after surgery pathway for single-level minimally invasive transforaminal lumbar interbody fusion decreases length of stay and opioid consumption. Neurosurgery. 2021;88:648–657. [DOI] [PubMed] [Google Scholar]
- 29.Siemionow K, Pelton MA, Hoskins JA, et al. Predictive factors of hospital stay in patients undergoing minimally invasive transforaminal lumbar interbody fusion and instrumentation. Spine. 2012;37:2046–2054. [DOI] [PubMed] [Google Scholar]
- 30.Asil K, Yaldiz C. Retrospective comparison of radiological and clinical outcomes of PLIF and TLIF techniques in patients who underwent lumbar spinal posterior stabilization. Medicine. 2016;95:e3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fariborz S, Gharedaghi M, Khosravi AF, et al. Comparison of results of 4 methods of surgery in grade 1 lumbosacral spondylolisthesis. Neurosurg Quart. 2016;26:14–18. [Google Scholar]
- 32.Yan D-l, Pei F-x, Li J, et al. Comparative study of PILF and TLIF treatment in adult degenerative spondylolisthesis. Eur Spine J. 2008;17:1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cummins D, Hindoyan K, Wu H-H, et al. Reoperation and mortality rates following elective 1 to 2 level lumbar fusion: a large state database analysis. Glob Spine J. 2021;00:2192568220986148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang YP, Du CF, Cheng CK, et al. Preserving posterior complex can prevent adjacent segment disease following posterior lumbar interbody fusion surgeries: a finite element analysis. PLoS ONE. 2016;11:e0166452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haleem S, Ahmed A, Ganesan S, et al. Mean 5-year follow-up results of a facet replacement device in the treatment of lumbar spinal stenosis and degenerative spondylolisthesis. World Neurosurg. 2021;152:e645–e651. [DOI] [PubMed] [Google Scholar]


