Abstract
Particulate and gaseous microemboli (GME) are side effects of cardiac surgery that interfere with postoperative recovery by causing endothelial dysfunction and vascular blockages. GME sources during surgery are multiple, and cardiopulmonary bypass (CPB) is contributory to this embolic load. Hematic antegrade repriming (HAR) is a novel procedure that combines the benefits of repriming techniques with additional measures, by following a standardized procedure to provide a reproducible hemodilution of 300 ml. To clarify the safety of HAR in terms of embolic load delivery, a prospective and controlled study was conducted, by applying Doppler probes to the extracorporeal circuit, to determine the number and volume of GME released during CPB. A sample of 115 patients (n = 115) was considered for assessment. Both groups were managed under strict normothermia, and similar clinical conditions and protocols, receiving the same open and minimized circuit. Significant differences in GME volume delivery (control group [CG] = 0.28 ml vs. HAR = 0.08 ml; p = 0.004) and high embolic volume exposure (>1 ml) were found between the groups (CG = 30.36% vs. HAR = 4.26%; p = 0.001). The application of HAR did not represent an additional embolic risk and provided a four-fold reduction in the embolic volume delivered to the patient (coefficient, 0.24; 95% CI, 0.08–0.72; p = 0.01), which appears to enhance GME clearance of the oxygenator before CPB initiation.
Keywords: cardiac surgery, cardiopulmonary bypass, extracorporeal circuits, gaseous microemboli, hematic antegrade repriming, pulsed Doppler
Hematic antegrade repriming (HAR) is a multidisciplinary approach to decrease the hemodilution and impact of cardiopulmonary bypass (CPB), in a standardized manner, to enhance the replicability of the results and avoid the heterogeneity in practice.1 Despite being inspired by the retrograde autologous priming (RAP) technique, which was initially described by Panico and Neptune2 and modified by numerous authors,3–6 it has been improved with some features to reduce the hemodilutional impact of CPB initiation to a constant amount of 300 ml. Thus, HAR combines some benefits of RAP2 and venous antegrade priming (VAP)7 with the advantages of using an open minimized circuit (similar to a MiECC Class IV),8 vacuum-assisted venous drainage (VAVD), and CPB initiation with an empty venous line. A preliminary study (n > 400 patients) pointed out that HAR was able to reduce the odds of transfusion requirements without an increase in postoperative complications.9 By avoiding the sudden hemodilution of the “crystalloid embolism” occurring during CPB initiation, HAR permits the preservation of hemoglobin concentration and oncotic pressure, favoring oxygen delivery and endothelial preservation.10–12
The delivery of solid and gaseous microemboli (GME) is an inherent side effect of cardiac surgery, with special relevance in valvular procedures.13 Nevertheless, a certain amount of emboli related to CPB can be prevented.14,15 It has been observed that while larger bubbles usually embolize cerebral arterioles, causing focal ischemia and neuronal injury, small emboli navigate along the vessels, provoking an insult to the endothelium and triggering an inflammatory response, microvascular dysfunction, and cognitive decline.16–18
Evidence suggests that priming with crystalloid solutions clears the air contained in the circuit only partially, leading to a variable bubble release during CPB.15,19,20 Maneuvers altering the density and pressure in the circuit like CPB initiation, cardioplegia delivery, hemofiltration procedures, and line clamping may result in extrication of microbubbles contained in the oxygenator toward the aortic cannula.21–23 Additionally, some authors have indicated that VAVD management and CPB initiation with an empty venous line may increase small-size GME release to the bloodstream.22
It is known that GME have a detrimental effect on the recovery of the patient.10,24,25 In addition, the concerns about the embolic influence of VAVD and CPB initiation with an empty venous line in a conventional setup are legitimate. Thus, it seems mandatory to evaluate the embolic behavior in real time19 under the application of HAR, to determine the impact and overall benefits of this standardized procedure.
Material and Methods
The current study was conducted as part of NCT03720184 Clinical Trial,26 which was approved by the ethical committee of the hospital (2018-5-1) and the Spanish Agency of Clinical Investigation (AEMPS).
A prospective sample of 300 adult patients, proposed for elective surgery involving valve repair or replacement under CPB, was recruited after signing the informed consent. Emergent, septic, and redo cases were excluded.
Patients were randomly assigned to two groups, forming an HAR group (HAR) and a control group (CG). Computerized randomization was applied with [R].27 Assignation of the group was revealed to the perfusionist just before surgery. Blinding was maintained for the observer of the Doppler measurements and for the patient, surgeon, and anesthesiologist by simulating the HAR application even during control cases.
After the assignation, 185 patients dropped out at different stages of the study because the treatment was not completed according to the protocol (n = 18), blinding was revealed (n = 6), or low reliability of the measurements was evidenced by an acoustic coupling control (ACC) of Doppler probes lower than 60% (n = 10).
During recruitment, the postoxygenator probe was accidentally damaged. Malfunctioning was suspected because ACC was constantly below 60%, and detection of GMEpost was discordant. “In vitro” tests with intentional air injections to the circuit were performed confirming the dysfunction, and recruitment was interrupted until obtaining a replacement.
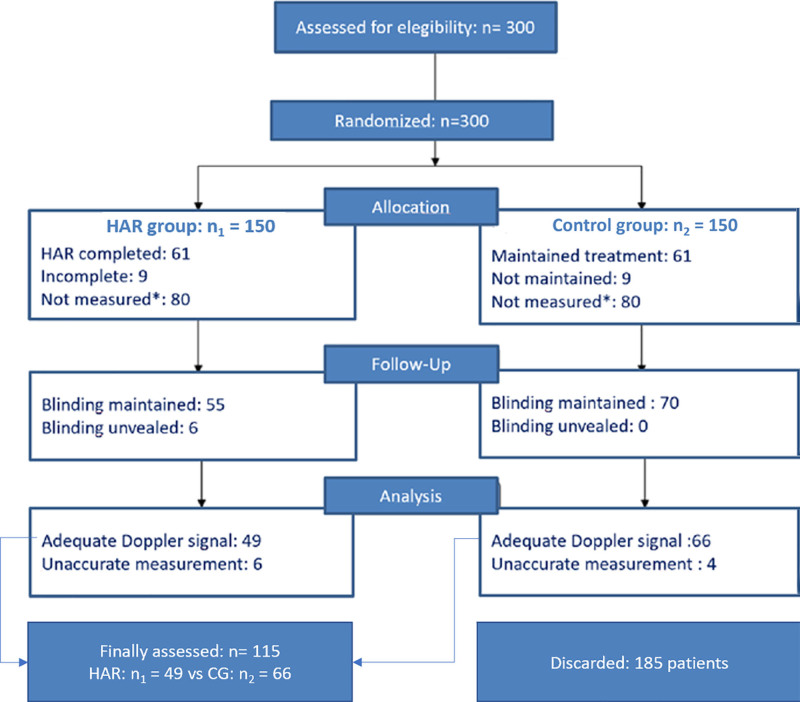
Thus, 49 patients received the HAR maneuvers before CPB initiation, whereas 66 patients were conventionally treated, using the same HAR standardized circuit,28 as the CG (n = 115) (Figure 1).
Figure 1.
Flow diagram of sample composition. *Repetitive Doppler measurements with low measurements and acoustic coupling control in postoxygenator probe suggested malfunction, which was verified “in vitro,” forcing to interrupt the experiment. HAR = hematic antegrade repriming (treatment group). Adapted from the study Mitchell and Gorman.16
General anesthesia was induced with propofol, remifentanil, and rocuronium, and maintained with sevoflurane and remifentanil in continuous perfusion before and after bypass. During CPB, hypnosis was maintained with boluses of propofol avoiding a bispectral index >60 (BIS, Medtronic, MN); 10 mg/kg of tranexamic acid was administered at induction, and a continuous infusion of 1 mg/kg/h was maintained during CPB. A bolus of 300 UI/kg of sodium heparin was used as an initial dose to achieve anticoagulation. Target-activated clotting time (ACT) during CPB was >440 sec (Hemochron Signature Elite, Werfen, Spain), so additional boluses of sodium heparin were added to maintain ACT in range. At the end of the procedure, heparin was reversed with protamine (1:1), and additional procoagulant actions were guided by thromboelastometry (TEG 6s, Haemonetics, MA).
CPB was performed using the HAR standard minimized circuit28 (MiECC Class IV29) and a Stöckert S5 heart-lung machine (Livanova PLC, London, UK). Patients with a body surface area (BSA) greater than 1.82 m2 were exposed to a Capiox FX25 oxygenator (Terumo Corporation, Tokyo, Japan). For smaller patients, the Inspire 6F oxygenator (Livanova PLC, London, UK) was used.
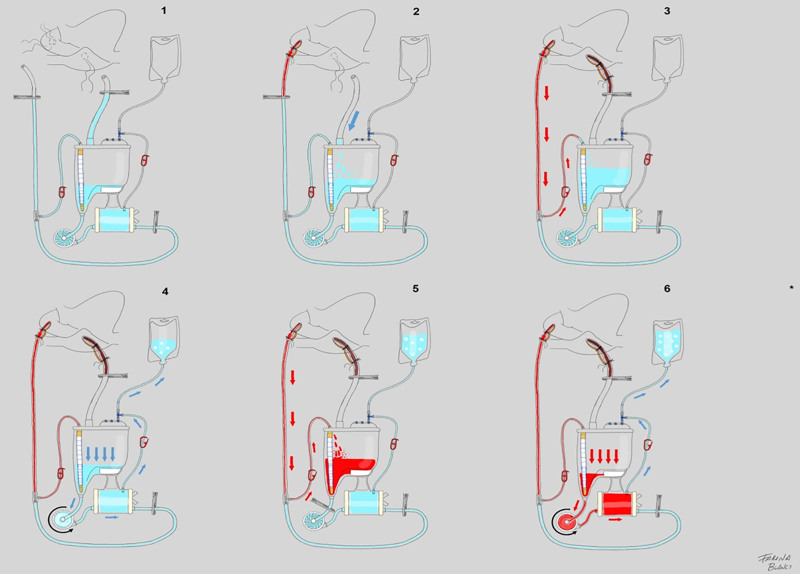
All circuits were initially primed with 1000 ml of isofundin (B.Braun, Melsungen, Germany), exceeding the minimum operating volume of the reservoir and following the instructions for use described for every oxygenator. Additionally, patients in the treatment group received HAR, according to the standard protocol “in 6 steps.”30 After connecting the arterial line to the patient, the crystalloid content was retrogradely collected, and the venous line content was anterogradely drained to the reservoir. Then, the excess of the crystalloid solution was anterogradely discarded into a collector bag through a recirculation line of the oxygenator. Then, blood was retrogradely drained from the aorta to the reservoir until obtaining enough volume to reprime the centrifugal pump and the oxygenator. Finally, both were anterogradely primed with autologous blood, and VAVD was enabled to initiate CPB (Figure 2). When required, phenylephrine boluses were administered by the anesthesiologist to avoid a mean arterial pressure <60 mmHg.
Figure 2.
HAR: six steps that result in 300 ml of hemodilution. Step 1: the circuit is primed with 1000 ml of a balanced crystalloid solution. Then, venous and arterial lines are clamped. Step 2: venous line content is drained to the reservoir by activating vacuum-assisted venous drainage (VAVD) and removing the venous clamp. Step 3: removing the arterial line clamp that is proximal to the patient and opening the arterial line recirculation, autologous blood is retrogradely drained, pushing the crystalloid priming to the reservoir. Then, the arterial recirculation line clamp is closed to avoid blood mixing in the reservoir. Step 4: by opening the recirculation line of the oxygenator and setting the centrifugal pump (CP) to 2000 rpm, crystalloid priming is discarded into the collector bag until zero level in the reservoir is reached. Step 5: a clamp is placed after the reservoir and arterial line recirculation is opened again. Thus, retrogradely, 300 ml of arterial blood is sequestered into the reservoir (100–200 ml/min). Step 6: setting CP to 2000 rpm and opening the recirculation line of the oxygenator and removing the clamp after the reservoir, CP and oxygenator are reprimed with autologous blood, displacing the priming and GME to the collector bag reducing hemodilution to only 300 ml. *CPB is initiated with VAVD activation once the venous return is obtained. Adapted from the study Blanco-Morillo et al.30
Strict normothermia was preserved, and hyperglycemia (>180 mg/dl) was avoided in every case. Mean arterial pressure was maintained within the range of 50–80 mmHg, and pump index was between 2 and 2.4 L/min/m2. Field suction did not exceed 1 lpm. Myocardial protection was achieved with DelNido cardioplegia.31 If ultrafiltration was required to compensate for hemodilution, Renaflo II HF2000 hemofilter (Cantel Medical Corp, Little Falls, NJ) was used, and blood was returned to the high-efficiency filtration stage of the reservoir. CO2 was delivered in the surgical field during CPB.
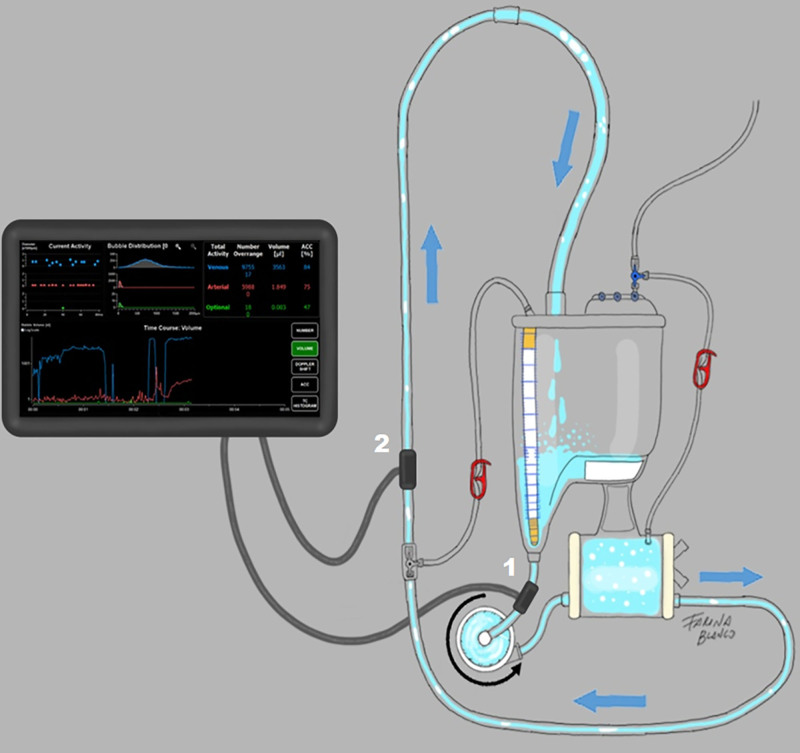
A GAMP BCC300 pulsed Doppler analyzer (GAMP mbH, Merseburg, Germany) was used to assess the GME activity in the extracorporeal circuit along with the CPB. Two probes were placed after and before the oxygenator, to assess the GME introduced to the circuit and delivered to the patient, respectively (Figure 3). Thus, outcome variates were the cumulative number and volume (ml) of GME detected by both probes. The filtration rates were calculated considering the reduction rate from probe 1 to probe 2, regarding to number and volume of GME (Figure 3). GME detection was performed after repriming in every case to evaluate the success of the priming procedure. Data acquisition was initiated simultaneously with CPB initiation.
Figure 3.
GAMPT BCC probes location. 1: preoxygenator probe counts the number and volume of bubbles entering the circuit. 2: postoxygenator probe determines the volume and number of GME delivered to the patient. Filtering rates of number and volume were automatically calculated by the device, considering the differences in measurements between both probes.
During CPB, a GME auditor supervised the procedure, logging in real-time different events that were expected to be related to potential GME activity, like CPB initiation, administration of cardioplegia, and the number of sample port injections, as well as the exposure to below-safety volume level in the reservoir, continuous ultrafiltration, hyperoxia PaO2 > 300 mmHg), cavitation of the field suckers, and sudden volume addition to the reservoir.23
Age, sex, BSA, variations of hemoglobin and temperature during CPB, pump indexes (minimum, average, and maximum), length of CPB, X-clamp duration, CPG, and sampling were used as continuous covariates and described using means and standard deviations. The exposure to ultrafiltration, mannitol, independent venous cannulation, FX25 as oxygenator, low level in the reservoir, hyperoxia, cavitation, and sudden volume addition during CPB was used as dichotomic covariates (Table 1).
Table 1.
Description of the Covariates
| Continuous | Control Group (n = 66) | Treated (HAR) (n = 49) | SMD | RV | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age* | 65.1 | 11.31 | 58.59 | 16.75 | 0.45 | 2.19 |
| BSA | 1.83 | 0.18 | 1.83 | 0.18 | 0.00 | 1.09 |
| Hgbpre | 12.9 | 1.95 | 12.99 | 1.40 | −0.07 | 0.51 |
| HgbNadir | 9.14 | 1.35 | 9.47 | 1.49 | −0.24 | 1.23 |
| Hgbfinal | 10.32 | 1.08 | 10.39 | 1.52 | −0.05 | 2.00 |
| TªNadir | 35.95 | 0.55 | 35.61 | 1.64 | 0.28 | 8.86 |
| Tªfinal | 36.40 | 0.36 | 36.08 | 1.52 | 0.29 | 17.57 |
| Pump-Indexmin | 1.92 | 0.14 | 1.95 | 0.18 | −0.19 | 1.65 |
| Pump-Indexavg | 2.12 | 0.21 | 2.13 | 0.21 | −0.05 | 0.95 |
| Pump-Indexmax | 2.28 | 0.27 | 2.26 | 0.25 | 0.06 | 0.90 |
| CPB length | 93 | 51.18 | 91.96 | 39.49 | 0.03 | 0.60 |
| X-clamp time | 70 | 36.44 | 67.16 | 33.26 | 0.08 | 0.83 |
| Sample port injections | 4.98 | 2.07 | 4.98 | 1.60 | 0.00 | 0.60 |
| Cardioplegia | 1.17 | 0.42 | 1.16 | 0.51 | 0.01 | 1.52 |
| Dichotomous | Proportion | SD | Proportion | SD | SMD | RV |
| Sex | 0.48 | 0.50 | 0.53 | 0.50 | −0.09 | 1.00 |
| Ultrafiltration* | 0.64 | 0.48 | 0.20 | 0.40 | 0.97 | 0.70 |
| Mannitol | 0.61 | 0.49 | 0.49 | 0.50 | 0.24 | 1.05 |
| OxyType | 0.56 | 0.50 | 0.59 | 0.49 | −0.06 | 0.98 |
| Independent cannulation | 0.35 | 0.48 | 0.29 | 0.45 | 0.13 | 0.90 |
| VAVD <−40 mmHg | 0.03 | 0.17 | 0.10 | 0.31 | −0.29 | 3.09 |
| Below safety level | 0.06 | 0.24 | 0.08 | 0.28 | −0.08 | 1.30 |
| Hyperoxia | 0.05 | 0.21 | 0.04 | 0.20 | 0.03 | 0.89 |
| Sudden volume addition | 0.12 | 0.33 | 0.10 | 0.31 | 0.07 | 0.85 |
| Cavitation of suckers | 0.09 | 0.29 | 0.14 | 0.35 | −0.16 | 1.47 |
Sex: 1 = male, oxygenator type: 1 = Fx25 oxygenator/0 = inspire 6F oxygenator, hyperoxia: arterial PO2 >300 mmHg.
*Significant differences were found for age and ultrafiltration (p < 0.05). No other significant differences were found. The lowest p-value obtained was 0.10 (TªFinal). A covariate was considered to be unbalanced if the SMD of the means was larger than 0.25 or if the highest-to-lowest RV was larger than 2.
%, percentage of the sample in this arm; Avg, average; BSA, body surface area (m2); CPB, cardiopulmonary bypass; Final, at the end of CPB; Hgb, hemoglobin; Max., maximum; Min, minimum; Nadir, minimum; Pre, previous to CPB; RV, ratio of variances; SMD, standardized mean difference; X-clamp, cross-clamp.
Quartiles were computed for all quantitative outcome variables and proportions for the dichotomous ones. GMEpost volume was dichotomized to identify the patients that received a cumulative amount greater than 1 ml. The effect of the HAR procedure was assessed with the median test, except for exposure to GME volume >1 ml, where the χ2 test was applied.
Considering that two different models of integrated-filter oxygenator were used, to reduce the risk of bias, GME number reduction and GME volume reduction were assessed for each type of oxygenator and compared with the median test.
To assess the influence of imbalances in our observations, the absolute standardized difference in means and the variances was computed. A covariate was considered unbalanced if the absolute standardized mean difference was larger than 0.25 or if the highest-to-lowest variance ratio was larger than 2 (Table 1). Then, a linear regression model was adjusted for the log of the number and volume of GMEpost to assess the effect of the HAR procedure while controlling for possible confounding factors. All tests were two-tailed, with a statistical significance level of p < 0.05. All the statistical analyses were performed with STATA/SE 14.2 (Texas, USA).32
Results
No major differences or misbalances were found between the groups regarding preoperative and intraoperative covariates (Table 1). Considering the registry of potentially embolic events during CPB, no significant differences were found between groups (p > 0.05). However, to preserve hemoglobin (Hgb) levels >8 mg/dl, ultrafiltration requirements were around three times more likely in the CG (CG = 64% vs. HAR = 20%). In addition to the significant differences in ultrafiltration usage and Age, Hgbfinal, TªNadir, Tªfinal, and exposure to high VAVD suction were found to be unbalanced (Table 1).
Differences between the two types of oxygenator regarding the number and volume of GMEpre, GMEpost, and coefficients of reduction for both were not found to be significant (lowest p-value = 0.11).
Exposure to GMEpost volumes >1 ml during CPB was lower in the HAR group (CG = 29.82% [17/57] vs. HAR = 6.25% [3/48], p = 0.002). Concordantly, median volume of VGMEpost was significantly lower in the HAR group (CG = 0.28 ml, interquartile range [IQR], 0.05–1.21 vs. HAR = 0.08 ml, IQR, 0.02–0.26; p < 0.05) (Table 2).
Table 2.
Embolic Behavior During CPB
| Outcome variable | CG | HAR Group | p | ||
|---|---|---|---|---|---|
| N | Median (Q1–Q3) | N | Median (Q1–Q3) | ||
| Number of GMEpre | 64 | 36,200 (20,250–73,985) | 49 | 35,900 (16,000–91,500) | 0.95 |
| Volume of GMEpre | 64 | 13.37 (2.26–165.13) | 49 | 9.26 (2.58–86.70) | 0.63 |
| Number of GMEpost | 57 | 1,677 (461–4,695) | 48 | 655 (354–2,731) | 0.14 |
| Volume of GMEpost | 57 | 0.28 (0.05–1.21) | 48 | 0.08 (0.02–0.26) | 0.004 |
| GME number: reduction rate (%) | 57 | 99.97 (99.90–99.99) | 48 | 99.98 (99.93–99.99) | 0.21 |
| GME volume: reduction rate (%) | 57 | 99.99 (99.93–100) | 48 | 100 (99.97–100) | 0.63 |
Significant results mean P < 0.05.
CG, control group; HAR, hematic antegrade repriming; GME, gaseous microemboli; Post, after the oxygenator; Pre, before the oxygenator.
The regression model for volume of GMEpost confirmed the significant effect of HAR after adjusting for potential confounders (p = 0.01; see Table 3). No other covariates presented significant coefficients in the regression models for the outcome variables (Tables 3 and 4).
Table 3.
Influence of Covariates on the Effect of HAR Regarding the Embolic Volume Delivered
| logVGMEpost | Coefficient | Standard Error | 95% CI | p | Exponential (Coefficient) | 95% CI Exponential (Coefficient) |
|---|---|---|---|---|---|---|
| HAR | −1.42 | 0.55 | −2.51 to −0.33 | 0.01 | 0.24 | 0.08–0.72 |
| Age | −0.01 | 0.02 | −0.05 to 0.02 | 0.42 | 0.99 | 0.95–1.02 |
| Hgbfinal | 0.07 | 0.19 | −0.31 to 0.45 | 0.73 | 1.07 | 0.73–1.57 |
| TªNadir | −0.61 | 0.47 | −1.54 to 0.32 | 0.20 | 0.54 | 0.21–1.38 |
| Tªfinal | 0.63 | 0.52 | −0.41 to 1.67 | 0.23 | 1.88 | 0.67–5.29 |
| Ultrafiltration | −0.20 | 0.55 | −1.29 to 0.88 | 0.71 | 0.82 | 0.28–2.42 |
| VAVD <−40 mmHg | 0.54 | 0.98 | −1.41 to 2.49 | 0.59 | 1.71 | 0.24–12.02 |
Final, measured at the end of CPB; HAR, hematic antegrade repriming; Hgb, hemoglobin; Nadir, minimum in CPB; Tª, temperature; Ultrafiltration, use of continuous ultrafiltration; VAVD, vacuum-assisted venous drainage (The reference category for the regression model was the CG).
Table 4.
Influence of Covariates on the Effect of HAR Regarding the Number of Emboli Delivered
| logNGMEpost | Coefficient | Standard Error | 95% CI | p | Exponential (Coefficient) | 95% CI Exponential (Coefficient) |
|---|---|---|---|---|---|---|
| HAR | −0.61 | 0.39 | −1.39 to 0.18 | 0.13 | 0.55 | 0.25–1.19 |
| Age | −0.02 | 0.01 | −0.04 to 0.01 | 0.14 | 0.98 | 0.96–1.01 |
| Hgbfinal | 0.08 | 0.14 | −0.19 to 0.35 | 0.57 | 1.08 | 0.82–1.42 |
| TªNadir | −0.17 | 0.34 | −0.84 to 0.49 | 0.61 | 0.84 | 0.43–1.64 |
| Tªfinal | 0.17 | 0.37 | −0.57 to 0.91 | 0.65 | 1.19 | 0.57–2.49 |
| Ultrafiltration | 0.29 | 0.39 | −0.49 to 1.06 | 0.47 | 1.33 | 0.61–2.89 |
| VAVD <−40 mmHg | 0.95 | 0.70 | −0.45 to 2.34 | 0.18 | 2.58 | 0.64–10.42 |
Final, measured at the end of CPB; HAR, hematic antegrade repriming; Hgb, hemoglobin; Nadir, minimum in CPB; Tª, temperature; Ultrafiltration, use of continuous ultrafiltration; VAVD, vacuum-assisted venous drainage (The reference categoryfor the regression model was the CG was the CG).
Discussion
Despite concerns about the embolic potential of initiating CPB with an empty venous line and using VAVD support,22 we observed that, if both are applied following the HAR standard procedure, it does not represent an additional embolic risk. Moreover, our observations suggest that HAR can provide a four-fold reduction of the GME volume delivered through the circuit compared with a conventional CPB initiation. In addition, the percentage of patients exposed to GMEpost volume >1 ml was four times lower when applying HAR. This significant effect of HAR was confirmed after adjusting for potential confounders using logistic regression.
Current evidence indicates that hemodilution and GME load are directly related to both glycocalyx degradation and endothelial dysfunction.10,14 Thus, the hemodilution and embolic reduction provided by HAR9,30 may result in better preservation of the microvascular system. This should be evaluated in further studies focusing on hyaluronan, syndecan-1, and heparan sulfate shedding.33,34
BCC300 permitted to observe in real time that embolic release was lower during CPB initiation after the HAR procedure, representing the majority of the GMEpost volume detected. Other sources of small bubbles were found to increase the GME number during the CPB like hyperoxia, cavitation of field suckers, VAVD <−40 mmHg, sample manifold injection, or low level on the reservoir, as described in previous investigations.23 However, we did not find these sources to be significantly relevant to the embolic number and volume delivered to the patient.
When looking for the underlying mechanism of the GME volume reduction provided by HAR, we may hypothesize two main ways. First, this could be related to the rupture of GME in smaller bubbles during HAR. On the other hand, antegrade repriming of the circuit with autologous blood may have forced the elimination of the air that persisted in the oxygenation chamber. However, considering the nonsignificant differences found between the groups in terms of the volume and number of GMEpre, GMEpost number, and the reduction rates for both, we can discard the hypothesis of bubble rupture.
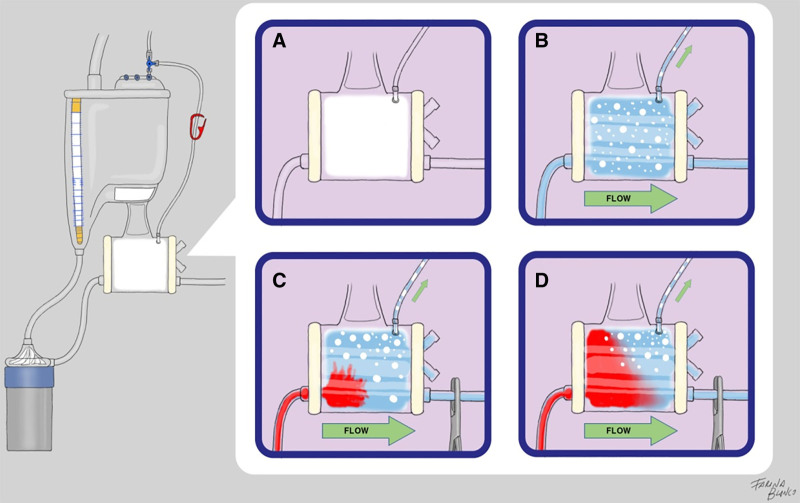
Previous literature pointed out that, after priming a circuit with crystalloid solutions, a certain amount of GME remains adhered to the oxygenation chamber, being released into the bloodstream during CPB initiation.15,20,23 Arterial filters are specifically designed to eliminate GME from CPB circuits due to the pressure difference generated by the filter and the buoyancy of bubbles that divert them through the recirculation line.35 During HAR maneuvers, the only output of the oxygenation chamber is the recirculation line conducting to a collector bag. Pressure increases, induced by the difference in diameter between the flow input (3/8″) and the recirculation output (Luer-lock), favor the GME elimination capacities of the integrated filters. Considering that increases in viscosity augment the embolic release during CPB,36 the antegrade blood entrance to the oxygenator during HAR also enhances the elimination of remaining GME to the collector bag. Both phenomena seem to play a major role in this remarkable embolic volume reduction. Thus, repriming the circuit with HAR appears to provide protective capacities against GME that are not transferrable to autologous priming when it is applied in a retrograde manner. This hypothesis could be tested by hydrodynamical experimental models in future studies (Figure 4).
Figure 4.
Hypothesized behavior of GME in the oxygenation chamber during HAR. (A) When the circuit is not primed, it is full of air. (B) After crystalloid priming, a certain amount of bubbles remains adhered to the low-flow areas of the oxygenator. (C) When the arterial line is clamped and flow is initiated with the recirculation line open, internal pressure in the oxygenation chamber raises, and some bubbles are diverted to the collector bag. (D) Blood entrance to the oxygenator represents an increase in density that enhances the elimination of bubbles to the collector bag and reduces the embolic load delivered to the patient during CPB initiation is reduced.
Current evidence indicates that better preservation of cognitive performance might be expected postoperatively when GME is reduced.18 Despite a threshold of GMEpost volume to avoid the occurrence of postoperative cognitive decline not being established,37–39 our results suggest that the HAR procedure may contribute to reducing such clinical outcomes. These clinical effects on postoperative cognitive performance are being explored in further stages of this clinical trial.26,40
Due to the innovative nature of the research, there were no previous data about the effect size; thus, initial sample size (n = 300) was arbitrary. Considering that when the postoxygenator probe was damaged, we decided to analyze the available data of patients with an ACC > 80%. Due to the relevance of our preliminary observations, we decided to interrupt the experiment.
The sample reduction was not related to the treatment assignation and similarly affected both groups during different stages of the study. Despite some authors indicating that this should not be considered a bias,41 to obtain significance when assessing the number of GMEpost, a bigger sample size should be recruited. Thus, future prospective studies are required to validate our observations.
Conclusions
Although the HAR procedure combines the use of measures like VAVD and CPB initiation with an empty venous line, it did not represent an increase in embolic load. On the contrary, the antegrade manner of repriming the circuit with autologous blood seems to reduce the embolic volume released during CPB initiation and the exposure to cumulative GMEpost volumes greater than 1 ml. Specific training in the use of Doppler measurement and HAR practice is paramount to the correct, standardized application of the HAR technique and to improve the replicability of these results. Further stages of this study aimed to evaluate the clinical impact of our findings.
Acknowledgments
The authors would like to thank the Latin American Perfusion Association (ALAP) and the Spanish Perfusion Association (AEP) for facilitating the outreach of this investigation during pandemics.
Footnotes
Disclosure: The authors have no conflicts of interest to report.
References
- 1.Blanco-Morillo J, Sornichero-Caballero A, Farina P, et al. : Haematic antegrade repriming procedure to initiate a safer cardiopulmonary bypass. 2020. Cited 2020 Nov 16. Available at: https://zenodo.org/record/4276132#.X7K8AchKgRk.
- 2.Panico FG, Neptune WB: A mechanism to eliminate the donor blood prime from the pump-oxygenator. Surg Forum 10: 605–609, 1960. [PubMed] [Google Scholar]
- 3.Balachandran S, Cross MH, Karthikeyan S, Mulpur A, Hansbro SD, Hobson P: Retrograde autologous priming of the cardiopulmonary bypass circuit reduces blood transfusion after coronary artery surgery. Ann Thorac Surg 73: 1912–1918, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Rosengart TK, DeBois W, O’Hara M, et al. : Retrograde autologous priming for cardiopulmonary bypass: A safe and effective means of decreasing hemodilution and transfusion requirements. J Thorac Cardiovasc Surg 115: 426–38; discussion 438, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Hagedorn C, Glogowski K, Valleley M, McQuiston L, Consbruck K; Illustrations by Oyer A: Retrograde autologous priming technique to reduce hemodilution during cardiopulmonary bypass in the pediatric cardiac patient. J Extra Corpor Technol 51: 100–103, 2019. [PMC free article] [PubMed] [Google Scholar]
- 6.Vandewiele K, Bové T, De Somer FM, et al. : The effect of retrograde autologous priming volume on haemodilution and transfusion requirements during cardiac surgery. Interact Cardiovasc Thorac Surg 16: 778–783, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teman N, Delavari N, Romano M, Prager R, Yang B, Haft J: Effects of autologous priming on blood conservation after cardiac surgery. Perfusion 29: 333–339, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Anastasiadis K, Murkin J, Antonitsis P, et al. : Use of minimal invasive extracorporeal circulation in cardiac surgery: Principles, definitions and potential benefits. A position paper from the Minimal invasive Extra-Corporeal Technologies international Society (MiECTiS). Interact Cardiovasc Thorac Surg 22: 647–662, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanco-Morillo J, Arribas JM, Lopez J, Tormos E, Sornichero A, Cánovas SJ. (apellido): To HAR or not to HAR: Identificación de predictores transfusionales en Circulación Extracorpórea. Revista Española de Perfusión 61: 9–14, 2016. [Google Scholar]
- 10.Myers GJ, Wegner J: Endothelial glycocalyx and cardiopulmonary bypass. J Extra Corpor Technol 49: 174–181, 2017. [PMC free article] [PubMed] [Google Scholar]
- 11.Puis L, Milojevic M, Boer C, et al. : 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Interact CardioVasc Thorac Surg. Cited 2019 Nov 20. Available at: https://academic.oup.com/icvts/advance-article/doi/10.1093/icvts/ivz251/5579824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranucci M, Carboni G, Cotza M, Bianchi P, Di Dedda U, Aloisio T; Surgical and Clinical Outcome Research (SCORE) Group: Hemodilution on cardiopulmonary bypass as a determinant of early postoperative hyperlactatemia. PLoS One 10: e0126939, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martens S, Dietrich M, Doss M, Deschka M, Keller H, Moritz A: Behavior of gaseous microemboli in extracorporeal circuits: Air versus CO2. Int J Artif Organs 29: 578–582, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Lou S, Ji B, Liu J, Yu K, Long C: Generation, detection and prevention of gaseous microemboli during cardiopulmonary bypass procedure. Int J Artif Organs 34: 1039–1051, 2011. [DOI] [PubMed] [Google Scholar]
- 15.DeFoe GR, Dame NA, Farrell MS, Ross CS, Langner CW, Likosky DS: Embolic activity during in vivo cardiopulmonary bypass. J Extra Corpor Technol 46: 150–156, 2014. [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell S, Gorman D: The pathophysiology of cerebral arterial gas embolism. J Extra Corpor Technol 34: 18–23, 2002. [PubMed] [Google Scholar]
- 17.Giacinto O, Satriano U, Nenna A, et al. : Inflammatory response and endothelial dysfunction following cardiopulmonary bypass: Pathophysiology and pharmacological targets. Recent Pat Inflamm Allergy Drug Discov 13: 158–173, 2019. [DOI] [PubMed] [Google Scholar]
- 18.Patel N, Minhas JS, Chung EM: Risk factors associated with cognitive decline after cardiac surgery: A systematic review. Cardiovasc Psychiatry Neurol 2015: 370612, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch JE, Wells C, Akers T, et al. : Monitoring microemboli during cardiopulmonary bypass with the EDAC quantifier. J Extra Corpor Technol 42: 212–218, 2010. [PMC free article] [PubMed] [Google Scholar]
- 20.Husebråten IM, Fiane AE, Ringdal MIL, Thiara APS: Measurement of gaseous microemboli in the prime before the initiation of cardiopulmonary bypass. Perfusion 33: 30–35, 2018. [DOI] [PubMed] [Google Scholar]
- 21.Stehouwer MC, de Vroege R, Hoohenkerk GJF, et al. : Carbon dioxide flush of an integrated minimized perfusion circuit prior to priming prevents spontaneous air release into the arterial line during clinical use. Artif Organs 41: 997–1003, 2017. [DOI] [PubMed] [Google Scholar]
- 22.Groom RC, Quinn RD, Lennon P, et al. ; Northern New England Cardiovascular Disease Study Group: Detection and elimination of microemboli related to cardiopulmonary bypass. Circ Cardiovasc Qual Outcomes 2: 191–198, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Blanco-Morillo JB, López SC, Ruiz ET, et al. : Embolia: el enemigo silente. Estudio multicéntrico anónimo para la descripción de eventos embólicos evitables en circulación extracorpórea. Revista de la Asociación Española de Enfermería Quirúrgica 41:55–63, 2018. [Google Scholar]
- 24.Ge Y, Ma Z, Shi H, Zhao Y, Gu X, Wei H: [Incidence and risk factors of postoperative cognitive dysfunction in patients underwent coronary artery bypass grafting surgery]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 39: 1049–1055, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Glumac S, Kardum G, Karanovic N: Postoperative cognitive decline after cardiac surgery: A narrative review of current knowledge in 2019. Med Sci Monit 25: 3262–3270, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanco-Morillo J: Haemo-autologous antegrade repriming (HAR) as minimum impact perfusion strategy for cardiopulmonary bypass. ClinicalTrials.gov. 2018. Available at: https://clinicaltrials.gov/ct2/show/NCT03720184.
- 27.R Core Team (2020): R: A language and environment for statistical computing. Vienna. Austria. 2020. Cited 2020 Dec 23. Available at: http://www.R-project.org/. [Google Scholar]
- 28.Blanco-Morillo J, Sornichero-Caballero A, Tornos-Ruiz E, Verdú-Verdú A, Farina P, Arribas-Leal J, et al. : Mini-circuito extracorpóreo durante la aplicación de recebado anterógrado hemático: una descripción detallada. REB 4: 64–68, 2021. [Google Scholar]
- 29.Blanco-Morillo J, Sornichero-Caballero A, Arribas Leal JM, Farina P, Cánovas-López S: Description of the minimized extracorporeal circuit to perform haematic antegrade repriming in cardiopulmonary bypass. Puis L, editor. 2020. Cited 2020 Nov 14. Available at: https://zenodo.org/record/4273689#.X6-0hchKgRk [Google Scholar]
- 30.Blanco-Morillo J, Arribas-Leal JM, Farina P, et al. : Hematic antegrade repriming: A reproducible method to decrease the cardiopulmonary bypass insult. J Extra Corpor Technol 53: 75–79, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanco-Morillo J, Caballero AS, Verdú AV, Ruiz ET, Canovas S: Protocolo de protección miocárdica con cardioplegia de Del Nido en cirugía mínimamente invasiva i. Revista Española de Perfusión 41:25–8, 2016. [Google Scholar]
- 32.StataCorp LLC: Stata. Cited 2022 Feb 8. Available at: https://www.stata.com/new-in-stata/.
- 33.Dogné S, Flamion B: Endothelial glycocalyx impairment in disease: Focus on hyaluronan shedding. Am J Pathol 190: 768–780, 2020. [DOI] [PubMed] [Google Scholar]
- 34.Wu Q, Gao W, Zhou J, et al. : Correlation between acute degradation of the endothelial glycocalyx and microcirculation dysfunction during cardiopulmonary bypass in cardiac surgery. Microvasc Res 124: 37–42, 2019. [DOI] [PubMed] [Google Scholar]
- 35.Herbst DP: Effects of purge-flow rate on microbubble capture in radial arterial-line filters. J Extra Corpor Technol 48: 105–112, 2016. [PMC free article] [PubMed] [Google Scholar]
- 36.Miyamoto S, Soh Z, Okahara S, et al. : Neural network-based modeling of the number of microbubbles generated with four circulation factors in cardiopulmonary bypass. Sci Rep 11: 549, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiberg S, Vedel AG, Holmgaard F, et al. : Lack of association between gaseous microembolisms assessed by a single detection device and cerebral complications in cardiac surgery patients. J Cardiothorac Vasc Anesth 34: 1496–1503, 2020. [DOI] [PubMed] [Google Scholar]
- 38.Melnyk V, Fedorko L, Djaiani G: Microemboli on cardiopulmonary bypass: Should we care? J Cardiothorac Vasc Anesth 34: 1504–1505, 2020. [DOI] [PubMed] [Google Scholar]
- 39.Mukherjee D, Jani ND, Selvaganesan K, Weng CL, Shadden SC: Computational assessment of the relation between embolism source and embolus distribution to the circle of Willis for improved understanding of stroke etiology. J Biomech Eng 138, 2016. [DOI] [PubMed] [Google Scholar]
- 40.Blanco-Morillo J, Lallana JM, Morales-Ortiz A, et al. ; Revista En Bomba: Evaluación cognitiva del paciente sometido a circulación extracorpórea: Una herramienta determinar el impacto de la práctica. 2021. Cited 2021 Sep 25. Available at: https://www.revistaenbombaalap.org/index.php/bomba/article/view/143.
- 41.Higgins JP, Page MJ, Savović J: Assessing risk of bias in a randomized trial. Cited 2022 Feb 9. Available at: https://training.cochrane.org/handbook/current/chapter-08.