Abstract
Background/Aim: Several prognostic risk factors have been recognized when using cisplatin-based conventional chemotherapy for the treatment of advanced urothelial carcinoma (UC); these include performance status (PS), liver metastasis, hemoglobin (Hb) levels, time from prior chemotherapy (TFPC), and other systemic inflammation scores including neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR). However, the benefit of these indicators for predicting outcome of immune checkpoint inhibitors is not fully understood. Here, we investigated the predictive value of the indicators in patients who received pembrolizumab for the treatment of advanced UC.
Patients and Methods: Seventy-five patients who received pembrolizumab treatment for advanced UC were included. The Karnofsky PS, liver metastasis, hemoglobin levels, TFPC, NLR, and PLR were analyzed, and their relationship with overall survival (OS) was determined.
Results: All factors were highlighted as significant prognostic indicators for OS in the univariate proportional regression analysis (p<0.05 for each). Multivariate analysis revealed that Karnofsky PS and liver metastasis were independent prognostic indicators for OS (p<0.01) but were applicable only for a small number of patients. Notably, the combined analysis with low Hb levels and high PLR was significantly associated with OS in patients who could gain less benefit from pembrolizumab at a median of 6.6 [95% confidence interval (CI)=4.2-9.0] versus 15.1 (95% CI=12.4-17.8) months (p=0.002).
Conclusion: The combination of Hb levels and PLR may be a broadly applicable indicator for the outcome of pembrolizumab as second-line chemotherapy in patients with advanced UC.
Keywords: Urothelial carcinoma, pembrolizumab, hemoglobin level, PLR
The urothelial carcinoma (UC) includes bladder cancer and upper tract urothelial carcinoma. Bladder cancer is the 10th most common cancer in the world, as of 2018 (1). Approximately 25% of patients were presented with muscle invasive bladder cancer at the time of diagnosis. Immune checkpoint inhibitors (ICIs) targeting programmed cell death protein 1 (PD-1) or programmed cell death 1 ligand 1 (PD-L1) have demonstrated durable responses in patients with cisplatin-refractory metastasized bladder cancer (2). However, only the modest objective response rate (ORR) to pembrolizumab (21.1% in KEYNOTE-045) was achieved in patients with advanced bladder cancer (3). However, other than ICIs, potential drugs for platinum-refractory UCs in second-line chemotherapy are currently quite limited (4). Therefore, discrimination of patients who may benefit from pembrolizumab highlights the need for identifying a useful prognostic factor.
Regarding a second-line conventional chemotherapy for advanced UCs, it has been well illustrated that the presence of risk factors according to performance status (PS), hemoglobin (Hb) levels, liver metastasis, and time from prior chemotherapy (TFPC) could predict prognosis (5,6). However, the possible impact of these risk factors on the efficacy of ICIs in advanced UCs remains unclear. In line with this, the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR), related to the systemic inflammation score, have been recently reported as potential predictors of pembrolizumab in patients with advanced UC (7,8). Mechanistically, it has been recognized that neutrophils inhibit cytotoxic activity of lymphocytes and play a protumorigenic role in the tumor microenvironment (9,10). Platelets have also been shown to have protumorigenic involvement, protecting tumor cells against the immune system and facilitating metastasis, and promoting tumor growth with angiogenesis (11). In studies on UC, thrombocytosis has been shown to predict tumor invasiveness (12). Furthermore, tumor PD-L1 positivity of UC has been shown to be significantly associated with shorter overall survival (OS) in patients exhibiting increased platelet counts (13).
In this study, we aimed to improve the prognostic accuracy of risk factors in patients with advanced UC and assessed various candidate factors for selecting patients who may gain survival benefits from treatment with pembrolizumab.
Patients and Methods
Patients and measurements. We retrospectively enrolled 75 patients with previously treated UC who received pembrolizumab treatment (200 mg pembrolizumab intravenously every 3 weeks) at Saitama Cancer Center from January 2018 to July 2022. This study was approved by the Ethics Committee of the Saitama Cancer Center (protocol number 1132 and 1254). All patients received cisplatin-based conventional chemotherapy, either as neoadjuvant or adjuvant therapy, before the introduction of pembrolizumab. The median follow-up time was 7.3 months (range=0.47-47.6 months). The levels of Hb, neutrophil counts, lymphocyte counts, and platelet counts were measured from the data of routine blood tests, prior to initiation of pembrolizumab therapy. The ratio of neutrophil-to-lymphocyte counts (NLR) and platelet-to-lymphocyte counts (PLR) were calculated from individual patients. The TFPC was determined from the last date of chemotherapy to the date of the first dose of pembrolizumab. OS was defined as the number of days from the first dose of pembrolizumab to the last follow-up or mortality. The ORR to pembrolizumab was assessed according to the Response Evaluation Criteria in Solid Tumors, version 1.1 (14). The optimal cut-off values for the PLR were determined using receiver operating characteristic (ROC) curve analysis (Mac ToukeiKaiseki, version 3.0; ESUMI Co., Ltd., Tokyo, Japan).
Statistical analysis. Continuous variables are reported as mean±standard deviation with the entire range of values. Cox proportional hazards regression models for univariate and multivariate analyses were used to estimate the hazard ratio (HRs) for OS with 95% confidence intervals (CIs), and p-Values in the candidate prognostic markers. The time-to-event outcomes were estimated using the Kaplan-Meier method with the log-rank test, which determine the probability of OS for two-group comparisons and the median of OS. Two-sided p-Values<0.05 were defined as statistically significant. All statistical analyses were performed using Mac ToukeiKaiseki version 3.0 (ESUMI Co., Ltd.).
Results
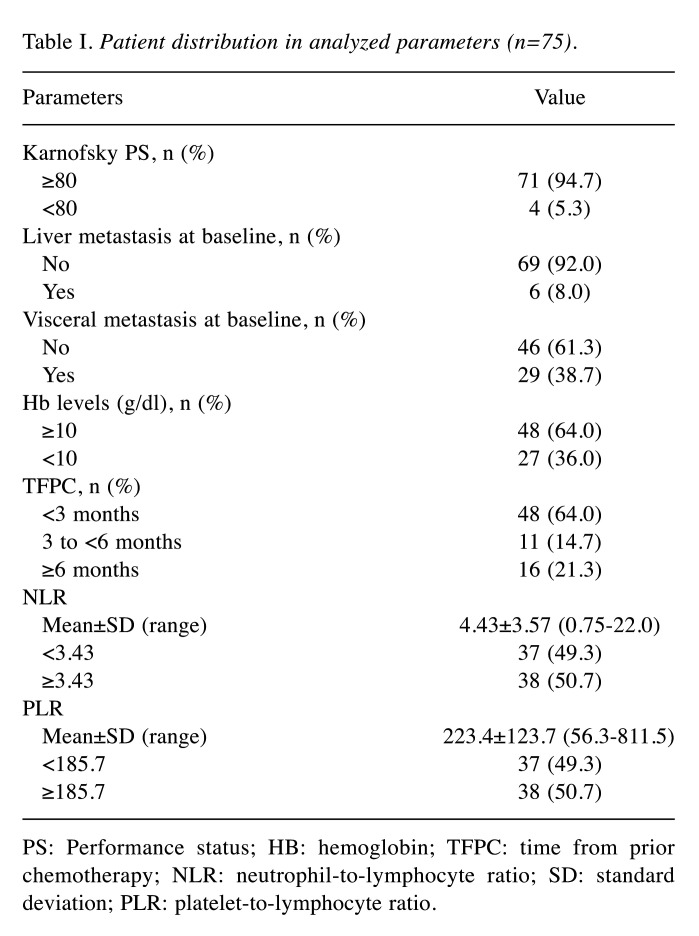
Prognostic impact of Hb levels in pembrolizumab treatment. The median OS and ORR in the total population after pembrolizumab initiation was 8.5 months (95% CI=6.4-10.7) and 21.3%, respectively. The clinical characteristics of 75 patients in the known adverse prognostic factors of platinum-based regimens are shown in Table I. Only four patients (5.3%) showed less than 80 scores of Karnofsky PS. Six patients (8.0%) had liver metastasis, whereas 29 patients (38.7%) had visceral metastasis that was defined as a metastasis to liver, lung and other organs except for lymph node and bone. Twenty-seven patients (36.0%) had Hb levels less than 10 g/dl. 48 patients (64.0%) and 59 patients (78%) started pembrolizumab less than 3 and 6 months from the last administration of prior chemotherapy (referred as TFPC), respectively (Table I).
Table I. Patient distribution in analyzed parameters (n=75).
PS: Performance status; HB: hemoglobin; TFPC: time from prior chemotherapy; NLR: neutrophil-to-lymphocyte ratio; SD: standard deviation; PLR: platelet-to-lymphocyte ratio.
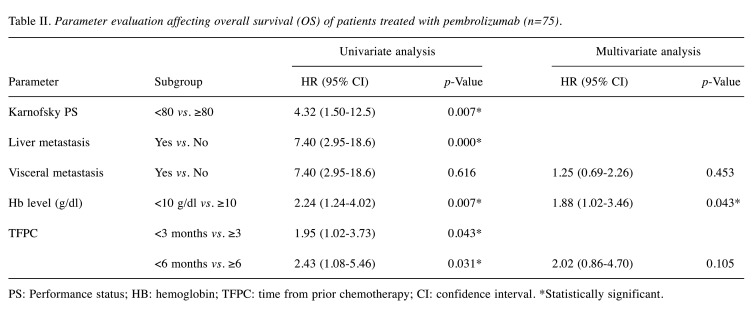
Next, univariate analyses were performed to investigate whether the known adverse prognostic factors of treatment with conventional second-line chemotherapy in advanced UCs could be applicable to the patients treated with pembrolizumab as a significant factor. As shown in Table II, scores of Karnofsky PS <80, presence of liver metastasis, and pretreatment levels of Hb <10 g/dl were prognostic factors for decreased survival (p<0.01 for all). TFPC <3 months and TFPC <6 months were also significant as poor prognostic factors (p<0.05). In contrast, visceral metastasis had no statistical significance in OS (p=0.616). As the predictive ability of Karnofsky PS and liver metastasis was applicable to a small population (5.3% and 8.0%, respectively) of the enrolled patients (Table I), they were likely to have only a modest benefit for selecting a therapy other than pembrolizumab. For this reason, the visceral metastasis patients that accounted for 38.7% of all patients were used for further multivariate analysis. As shown in Table II, a Hb <10 g/dl was highlighted as a significant prognostic factor for worse OS in the multivariate analysis (HR=1.88, 95% CI=1.02−3.46, p=0.043).
Table II. Parameter evaluation affecting overall survival (OS) of patients treated with pembrolizumab (n=75).
PS: Performance status; HB: hemoglobin; TFPC: time from prior chemotherapy; CI: confidence interval. *Statistically significant.
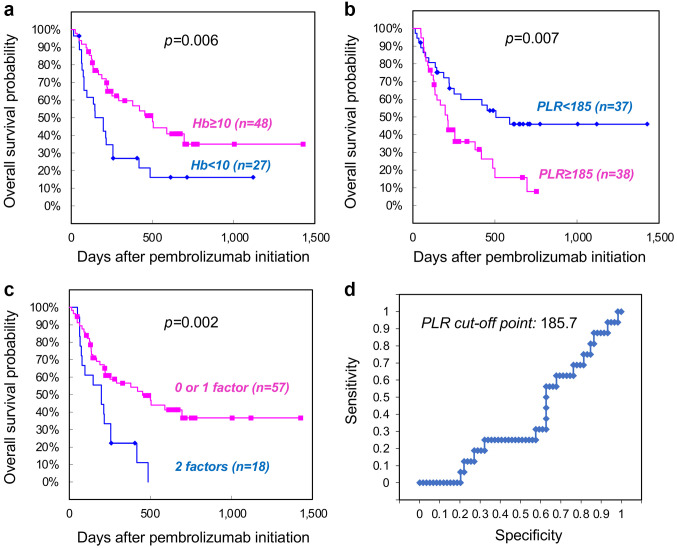
Predictive ability of the combination of Hb levels and PLR in OS of patients treated with pembrolizumab. To improve the predictive ability in patients who may benefit from pembrolizumab, we assessed a possible combination of predictive factors with the Hb levels. For this purpose, we first investigated the potential predictive ability of NLR and PLR. The total population was divided into two groups, based on the median values of NLR and PLR (3.43 and 185.7, respectively, Table I). Notably, a PLR ≥185.7 was a significant prognostic factor for worse OS in the univariate analysis (HR=2.25, 95% CI=1.22-4.15, p=0.009), while NLR ≥3.43 was not (HR=1.21, 95% CI=0.68-2.16, p=0.052). These results were consistent with our previous findings in 54 patients, with an overlapping population of the present study (7). Interestingly, univariate analysis using continuous variables of NLR improved the statistical significance for the prognostic value in OS (HR=1.00, 95% CI=1.00-1.01, p=0.021), but to a lesser extent than that of PLR (HR=1.10, 95% CI=1.03-4.19, p=0.008). Because of the statistical superiority of PLR, we next attempted to evaluate whether the Hb levels in combination with PLR could improve the prognostic accuracy for OS in patients who received pembrolizumab. The Kaplan-Meier curves showed significantly lower survival of patients with Hb <10 g/dl than of those with Hb ≥10 g/dl at a median of 6.6 (95% CI=3.0-10.1) versus 16.7 (95% CI=13.9-19.4) months (p=0.006, Figure 1a). Moreover, a PLR ≥185.7 had a significant impact for worse OS at a median of 7.1 (95% CI=4.9-9.2) versus 16.8 (95% CI=13.2-20.5) months (p=0.007, Figure 1b). Notably, combination of Hb <10 g/dl and PLR ≥185.7 (referred to as two factors) improved the ability to discriminate patients who could gain less benefit from pembrolizumab than 0 or 1 factors at a median of 6.6 (95% CI=4.2-9.0) versus 15.1 (95% CI=12.4-17.8) months (p=0.002, Figure 1c). Furthermore, ROC curve analysis revealed that the optimal cut-off value of PLR based on the ORR, as partial or complete response and no response (progressive disease or stable), was exactly the same as the median value of 185.7 in the total population, confirming the expedient means of PLR as a prognostic marker (Figure 1d).
Figure 1. Kaplan-Meier analysis of overall survival (OS) in patient subgroups stratified by (a) Hb level, (b) platelet-to-lymphocyte ratio (PLR) and (c) number of factors. (d) Receiver operating characteristic (ROC) curve analysis of PLR in 75 patients based on objective response rate (ORR) to pembrolizumab.
Discussion
In the present study, potential prognostic predictors of pembrolizumab in patients with cisplatin-refractory advanced UC were investigated. The PS, Hb levels, liver metastasis, and TFPC are listed as known adverse prognostic factors of treatment with conventional chemotherapy in the second-line setting, all of which have the potential to predict OS in patients treated with pembrolizumab (5,6). The results of multivariate analysis revealed that Karnofsky PS and liver metastasis were independent prognostic factors, indicating that patients who possessed these adverse prognostic factors gained less benefit from a second-line chemotherapy including pembrolizumab. Currently, the potential drugs for platinum-refractory UCs in second-line chemotherapy are quite limited, with ICIs representing the only widely accepted treatment strategy (4). In the KEYNOTE-361 trial, first-line pembrolizumab plus platinum-based chemotherapy did not significantly improve progression-free survival and OS in patients with advanced UC as compared to chemotherapy alone (15). Candidate prognostic biomarkers need to be validated, considering the future requirement for improving the efficacy of pembrolizumab in combination with standard chemotherapies as well as multiple targeted drugs. Enfortumab vedotin plus pembrolizumab is expected to be a promising combination for first-line therapy in patients who are ineligible for cisplatin and is currently being tested in an ongoing phase III study (NCT04223856) (16). Additionally, considerable efforts have led to the successful development of Erdafitinib, a potent tyrosine kinase inhibitor of the gene encoding fibroblast growth factor receptor for treating patients with advanced UC (17).
Among the above-mentioned adverse prognostic factors in a second-line conventional chemotherapy, we highlighted that pretreatment Hb levels may be broadly applicable to identify the patients who will benefit from pembrolizumab. Further improvements in the predictive impact were obtained from a combination of Hb levels and PLR. A median of PLR in the total population was applied as a cut-off value based on the ORR, because the optimal value for PLR in relation to OS remains to be investigated. Notably, ROC curve analysis confirmed that the median value of PLR was the same as the optimal cut-off value in the total population, suggesting the usefulness of the PLR in selecting patients who may benefit from pembrolizumab as a second-line therapy. In contrast, a median NLR of 3.43 did not show predictive significance for OS, although continuous variables of NLR were significant. Considering that the median NLR in this study was quite comparable with an optimal cut-off value (2.9, 3 or 3.5) described in several studies on patients who received ICIs for advanced UC (18-21), an optimal NLR cut-off value may be defined in a broad-range depending on the population. Consistently, other groups have shown that an NLR ≥5.0 is an independent predictor of no clinical benefit based on objective response and worse survival to ICIs in metastatic UC (22,23). Furthermore, 1-month change of NLR ≥+43% at post-treatment with pembrolizumab is highlighted as a significant predictor for poor OS (21); therefore, the sequential measurement of these indicators is also valuable for future investigation.
Conclusion
Collectively, although a potential reason for the discrepancy in our findings between the PLR and NLR remains to be elucidated, Hb levels in combination with PLR are likely to be valuable for predicting patients who will gain less benefit from pembrolizumab as a second-line therapy. However, the OS of the total study population was 8.5 months, which was shorter than that reported in the KEYNOTE-045 trial (10.3 months) due to the shorter follow-up time, while the ORRs were comparable (21.3% and 21.1%, respectively). Therefore, an extended follow-up duration might be required for evaluating the optimal cut-off values of PLR and NLR. Further consecutive studies will improve the clinical benefit in the combination of Hb and PLR as a useful biomarker for selecting patients who may gain a survival benefit from pembrolizumab-based treatments.
Conflicts of Interest
None of the Authors declare any conflicts of interest.
Authors’ Contributions
RK and KA conceived and designed this study. RK, MI, RM, KM, YM and YK collected samples and recorded the general data and the observational indicators of patients. KA analyzed the data and wrote the first draft of the article. RK, MI, HT, MH, RO, YM, TK and YK critically reviewed and corrected the article. All Authors reviewed and approved the final version of the article.
Acknowledgements
The Authors thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this article.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Apolo AB. Determining superior first-line therapy in metastatic bladder cancer. Nat Rev Urol. 2019;16(12):698–699. doi: 10.1038/s41585-019-0245-8. [DOI] [PubMed] [Google Scholar]
- 3.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi A, Gerritsen W, Gurney H, Quinn DI, Culine S, Sternberg CN, Mai Y, Poehlein CH, Perini RF, Bajorin DF, KEYNOTE-045 Investigators Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Liu J, Fang K, Ke C, Jiang Y, Wang G, Yang T, Chen T, Shi X. Second-line treatment strategy for urothelial cancer patients who progress or are unfit for cisplatin therapy: a network meta-analysis. BMC Urol. 2019;19(1):125. doi: 10.1186/s12894-019-0560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellmunt J, Choueiri TK, Fougeray R, Schutz FA, Salhi Y, Winquist E, Culine S, von der Maase H, Vaughn DJ, Rosenberg JE. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28(11):1850–1855. doi: 10.1200/JCO.2009.25.4599. [DOI] [PubMed] [Google Scholar]
- 6.Sonpavde G, Pond GR, Fougeray R, Choueiri TK, Qu AQ, Vaughn DJ, Niegisch G, Albers P, James ND, Wong YN, Ko YJ, Sridhar SS, Galsky MD, Petrylak DP, Vaishampayan UN, Khan A, Vogelzang NJ, Beer TM, Stadler WM, O’Donnell PH, Sternberg CN, Rosenberg JE, Bellmunt J. Time from prior chemotherapy enhances prognostic risk grouping in the second-line setting of advanced urothelial carcinoma: a retrospective analysis of pooled, prospective phase 2 trials. Eur Urol. 2013;63(4):717–723. doi: 10.1016/j.eururo.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurashina R, Ando K, Inoue M, Izumi K, Maruyama R, Mitani K, Takenobu H, Haruta M, Iizuka T, Kamijo T, Kageyama Y. Platelet-to-lymphocyte ratio predicts the efficacy of pembrolizumab in patients with urothelial carcinoma. Anticancer Res. 2022;42(2):1131–1136. doi: 10.21873/anticanres.15576. [DOI] [PubMed] [Google Scholar]
- 8.Yanagisawa T, Mori K, Katayama S, Mostafaei H, Quhal F, Laukhtina E, Rajwa P, Motlagh RS, Aydh A, König F, Grossmann NC, Pradere B, Miki J, Kimura T, Egawa S, Shariat SF. Pretreatment clinical and hematologic prognostic factors of metastatic urothelial carcinoma treated with pembrolizumab: a systematic review and meta-analysis. Int J Clin Oncol. 2022;27(1):59–71. doi: 10.1007/s10147-021-02061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.el-Hag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol. 1987;139(7):2406–2413. [PubMed] [Google Scholar]
- 10.Rice CM, Davies LC, Subleski JJ, Maio N, Gonzalez-Cotto M, Andrews C, Patel NL, Palmieri EM, Weiss JM, Lee JM, Annunziata CM, Rouault TA, Durum SK, McVicar DW. Tumour-elicited neutrophils engage mitochondrial metabolism to circumvent nutrient limitations and maintain immune suppression. Nat Commun. 2018;9(1):5099. doi: 10.1038/s41467-018-07505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu L, Guo Y, Chang Z, Zhang D, Zhang S, Pei H, Pang J, Zhao ZJ, Chen Y. Bidirectional interaction between cancer cells and platelets provides potential strategies for cancer therapies. Front Oncol. 2021;11:764119. doi: 10.3389/fonc.2021.764119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Can C, Baseskioglu B, Yılmaz M, Colak E, Ozen A, Yenilmez A. Pretreatment parameters obtained from peripheral blood sample predicts invasiveness of bladder carcinoma. Urol Int. 2012;89(4):468–472. doi: 10.1159/000343278. [DOI] [PubMed] [Google Scholar]
- 13.Miyama Y, Morikawa T, Miyakawa J, Koyama Y, Kawai T, Kume H, Fukayama M. The prognostic value of PD-L1 expression in upper tract urothelial carcinoma varies according to platelet count. Cancer Med. 2018;7(9):4330–4338. doi: 10.1002/cam4.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Powles T, Csőszi T, Özgüroğlu M, Matsubara N, Géczi L, Cheng SY, Fradet Y, Oudard S, Vulsteke C, Morales Barrera R, Fléchon A, Gunduz S, Loriot Y, Rodriguez-Vida A, Mamtani R, Yu EY, Nam K, Imai K, Homet Moreno B, Alva A, KEYNOTE-361 Investigators Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(7):931–945. doi: 10.1016/S1470-2045(21)00152-2. [DOI] [PubMed] [Google Scholar]
- 16.Hoimes CJ, Flaig TW, Milowsky MI, Friedlander TW, Bilen MA, Gupta S, Srinivas S, Merchan JR, McKay RR, Petrylak DP, Sasse C, Moreno BH, Yu Y, Carret AS, Rosenberg JE. Enfortumab vedotin plus pembrolizumab in previously untreated advanced urothelial cancer. J Clin. 2022;Oncol:JCO2201643. doi: 10.1200/JCO.22.01643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loriot Y, Necchi A, Park SH, Garcia-Donas J, Huddart R, Burgess E, Fleming M, Rezazadeh A, Mellado B, Varlamov S, Joshi M, Duran I, Tagawa ST, Zakharia Y, Zhong B, Stuyckens K, Santiago-Walker A, De Porre P, O’Hagan A, Avadhani A, Siefker-Radtke AO, BLC2001 Study Group Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019;381(4):338–348. doi: 10.1056/NEJMoa1817323. [DOI] [PubMed] [Google Scholar]
- 18.Isobe T, Naiki T, Sugiyama Y, Naiki-Ito A, Nagai T, Etani T, Nozaki S, Iida K, Noda Y, Shimizu N, Tomiyama N, Banno R, Kubota H, Hamamoto S, Ando R, Kawai N, Yasui T. Chronological transition in outcome of second-line treatment in patients with metastatic urothelial cancer after pembrolizumab approval: a multicenter retrospective analysis. Int J Clin Oncol. 2022;27(1):165–174. doi: 10.1007/s10147-021-02046-z. [DOI] [PubMed] [Google Scholar]
- 19.Ito K, Kobayashi T, Kojima T, Hikami K, Yamada T, Ogawa K, Nakamura K, Sassa N, Yokomizo A, Abe T, Tsuchihashi K, Tatarano S, Inokuchi J, Tomida R, Fujiwara M, Takahashi A, Matsumoto K, Shimizu K, Araki H, Kurahashi R, Osaki Y, Tashiro Y, Uegaki M, Ogawa O, Kitamura H, Nishiyama H. Pembrolizumab for treating advanced urothelial carcinoma in patients with impaired performance status: Analysis of a Japanese nationwide cohort. Cancer Med. 2021;10(10):3188–3196. doi: 10.1002/cam4.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tural D, Ölmez ÖF, Sümbül AT, Özhan N, Çakar B, Köstek O, Ekenel M, Erman M, Coşkun HŞ, Selçukbiricik F, Keskin Ö, Türköz FP, Oruç K, Bayram S, Bilgetekin İ, Yıldız B, Şendur MAN, Paksoy N, Dirican A, Erdem D, Selam M, Tanrıverdi Ö, Paydaş S, Urakçı Z, Atağ E, Güncan S, Ürün Y, Alkan A, Kaya AO, Özyükseler DT, Taşkaynatan H, Yıldırım M, Sönmez M, Başoğlu T, Gündüz Ş, Kılıçkap S, Artaç M. Prognostic factors in patients with metastatic urothelial carcinoma who have treated with Atezolizumab. Int J Clin Oncol. 2021;26(8):1506–1513. doi: 10.1007/s10147-021-01936-6. [DOI] [PubMed] [Google Scholar]
- 21.Kadono Y, Kawaguchi S, Nohara T, Shigehara K, Izumi K, Kamijima T, Seto C, Takano A, Yotsuyanagi S, Nakagawa R, Miyagi T, Aoyama S, Asahi H, Fukuda R, Mizokami A. Blood cell count biomarkers predicting efficacy of pembrolizumab as second-line therapy for advanced urothelial carcinoma. Anticancer Res. 2021;41(3):1599–1606. doi: 10.21873/anticanres.14921. [DOI] [PubMed] [Google Scholar]
- 22.Banna GL, Di Quattro R, Malatino L, Fornarini G, Addeo A, Maruzzo M, Urzia V, Rundo F, Lipari H, De Giorgi U, Basso U. Neutrophil-to-lymphocyte ratio and lactate dehydrogenase as biomarkers for urothelial cancer treated with immunotherapy. Clin Transl Oncol. 2020;22(11):2130–2135. doi: 10.1007/s12094-020-02337-3. [DOI] [PubMed] [Google Scholar]
- 23.Nassar AH, Mouw KW, Jegede O, Shinagare AB, Kim J, Liu CJ, Pomerantz M, Harshman LC, Van Allen EM, Wei XX, McGregor B, Choudhury AD, Preston MA, Dong F, Signoretti S, Lindeman NI, Bellmunt J, Choueiri TK, Sonpavde G, Kwiatkowski DJ. A model combining clinical and genomic factors to predict response to PD-1/PD-L1 blockade in advanced urothelial carcinoma. Br J Cancer. 2020;122(4):555–563. doi: 10.1038/s41416-019-0686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]