Abstract
Background
Older adults are a large and growing proportion of cancer cases in the United States, but concerns persist about whether older adults are adequately represented in the cancer clinical trials that test new options for treatment and cancer care.
Methods
This paper describes adult patient enrollments by age group to the National Cancer Institute’s National Clinical Trials Network (NCTN) from 2016 to 2021, compares patient enrollment by age with the estimated incident cancer population across cancer types, and explores possible associations between patient age and patient race, ethnicity, and sex.
Results
This analysis found that patients aged 18 to 69 years were overrepresented in NCTN trials, whereas patients aged 70 years and older were underrepresented compared with the estimated incident cancer population. Underrepresentation of older patients was seen across cancer types. Older patients who enrolled to NCTN trials were more likely to be non-Hispanic White than the estimated incident cancer population.
Conclusions
Compared with earlier analyses, NCTN trials are enrolling greater proportions of older adults, primarily driven by higher enrollment among patients aged 65 to 74 years. There is still significant room for improvement, however, especially among patients aged 75 years and older. Additionally, patient demographics should not be viewed in isolation: older Hispanic patients, for instance, were particularly underrepresented among patients enrolled to NCTN trials. The intersection between trial enrollment and age, race, and ethnicity warrants further study so that more targeted enrollment enhancement efforts can be developed that enhance trial diversity across demographic groups.
Older adults are a large and growing proportion of cancer cases in the United States, but concerns persist about whether older adults are adequately represented in the cancer clinical trials that test new options for treatment and cancer care (1-4). Older adult enrollment data from 2001 to 2011 for the National Cancer Institute (NCI)–supported adult trials were presented in 2012 (5). This paper describes updated and expanded data for adult patient enrollments by age group from 2016 to 2021 to clinical trials in NCI’s largest clinical trials network, the National Clinical Trials Network (NCTN). The analysis includes a comparison of patient enrollment by age to the estimated incident cancer population, overall and within certain cancer types.
There are also significant concerns regarding disparities in the enrollment of racial and ethnic minorities to NCI-supported trials (2,6). It is important to analyze and develop interventions to address disparities with an understanding of the potential for intersectionality with other patient characteristics and experiences (7). This analysis also explores possible associations between patient age and other patient demographic information that is systematically collected across NCTN trials: race, ethnicity, and sex.
Methods
Data
NCI supports the infrastructure for the conduct of national cancer treatment and advanced imaging clinical trials in adults, adolescents and young adults, and children through the NCTN (8,9). Cancer patients are enrolled to approximately 200 NCTN trials each year from more than 2000 academic and community oncology sites around the United States and internationally. For this analysis, data for enrollments to NCTN clinical trials between 2016 and 2021 were obtained from the web-based registration system for NCTN trials. The dataset included patient enrollments to trial interventions for trials enrolling participants aged 18 years and older and led by the adult NCTN groups. Enrollments outside of the United States were excluded.
Key variables for the analysis were patient age at time of enrollment, patient race, patient ethnicity, patient sex, enrollment year, and cancer type. Given the focus of this analysis on older adults, age groupings were used to separate younger (18 to 64 years) and older (65 years and older) patients and explore enrollment within older adult age groups. For patients aged 65 to 84 years, 5-year age groupings were used to align with the census categories. The mutually exclusive age groupings used were 18-64 years, 65-69 years, 70-74 years, 75-79 years, 80-84 years, and 85 years and older. For the cancer type analysis, cancer type was based on the main cancer type being enrolled in the trial. Race and ethnicity variables were recoded to mutually exclusive groupings reported in the NCI Surveillance, Epidemiology, and End Results (SEER) Program data.
Cancer incidence rates were obtained from the SEER Program data from the SEER*Stat Database for incidence based on cases diagnosed between 2013 and 2018 and reported across 21 registries (SEER 21) (10). Diagnosis years 2013-2018 were used because they are the most recent 6-year time period available in the SEER data. Registries included in the SEER 21 data cover 36.4% of the US population (11).
Analysis
SEER incidence rates were adjusted for age using US Census Bureau data from the 2015-2019 American Community Survey 5-Year Estimates. To perform the adjustment, SEER data on cancer incidence rates by age and cancer type were multiplied by the 2015-2019 population estimate within that age group and, if applicable, sex (for breast, ovarian, and prostate cancers and for the demographic subgroup analysis) to estimate the number of annual incident cases in the United States. We then calculated the proportion of incident cases by age group for comparison to the proportion of enrollments by age group.
We compared the proportion of patients in each age group for the enrolled patient population and the incident population. The primary analysis was across enrollments to all cancer sites. We also analyzed enrollments by cancer type within cancer types with at least 1500 enrollments included over the 6-year period. Subgroup analyses by sex, race, and ethnicity were limited to categories with at least 1000 enrollments in each subgroup. We present the relative differences for assessment of practical significance and clinical importance.
Results
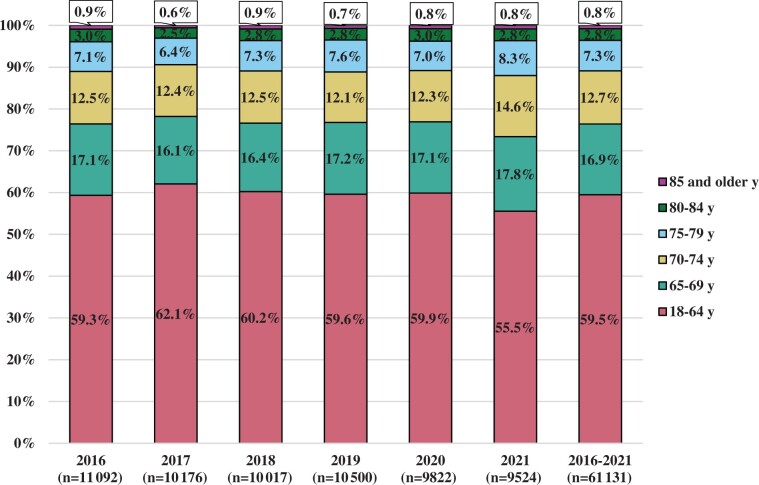
This analysis includes 61 131 adult patients enrolled in the United States to 300 trials between 2016 and 2021. There were between 9524 and 11 092 patients meeting these criteria enrolled annually from 2016 to 2021. Figure 1 shows the distribution of patient enrollments by age group and enrollment year. Enrollment by age group was similar in each of the included enrollment years, with slightly greater enrollment of older adults in 2021 compared with 2016 to 2020.
Figure 1.
Proportion of adult enrollments to the National Cancer Institute’s National Clinical Trials Network by age at enrollment and enrollment year.
Comparing the age distribution of patients enrolled to trials and the age distribution of incident cancer cases for all patient enrollments, there was overrepresentation of younger patients (59.5% of enrollments vs 42.0% of estimated incident cases) and patients aged 65 to 69 years (16.9% of enrollments vs 15.9% of cases; Table 1). Patients aged 70 years and older were generally underrepresented in trials, with the degree of underrepresentation increasing with each age group. Patients aged 70 to 74 years represented 12.7% of enrollments and 14.5% of incident cases—a 1.8% difference. This difference was 4.2% for patients aged 75 to 79 years, 5.4% for patients aged 80 to 84 years, and 7.1% for patients aged 85 years and older.
Table 1.
Differences in adult enrollments to National Cancer Institute’s National Clinical Trials Network (NCTN) vs estimated cancer incidence by age group and cancer type, 2016-2021a,b
| Cancer type | 18-64 y, % | 65-69 y, % | 70-74 y, % | 75-79 y, % | 80-84 y, % | 85 y and older, % |
|---|---|---|---|---|---|---|
| All cancer typesc | ||||||
| Enrollments (n = 61 131) | 59.5 | 16.9 | 12.7 | 7.3 | 2.8 | 0.8 |
| Incidence (n ≈ 1 723 000) | 42.0 | 15.9 | 14.5 | 11.5 | 8.2 | 7.9 |
| Difference | +17.5d | +1.0 | −1.8 | −4.2 | −5.4 | −7.1 |
| Bladder | ||||||
| Enrollments (n = 2579) | 31.2 | 18.9 | 19.3 | 16.3 | 10.1 | 4.1 |
| Incidence (n ≈ 77 000) | 23.5 | 15.3 | 17.2 | 16.3 | 13.5 | 14.2 |
| Difference | +7.7 | +3.6 | +2.1 | 0.0 | −3.4 | −10.1 |
| Breast | ||||||
| Enrollments (n = 16 534) | 80.5 | 11.0 | 5.5 | 2.1 | 0.7 | 0.2 |
| Incidence (n ≈ 256 000) | 52.8 | 14.8 | 12.6 | 8.9 | 5.7 | 5.2 |
| Difference | +27.7 | −3.8 | −7.1 | −6.8 | −5.0 | −5.0 |
| Colorectal | ||||||
| Enrollments (n = 1644) | 72.7 | 12.3 | 7.8 | 4.5 | 2.0 | 0.6 |
| Incidence (n ≈ 144 000) | 41.7 | 13.6 | 12.7 | 11.4 | 9.7 | 10.9 |
| Difference | +31.0 | −1.3 | −4.9 | −6.9 | −7.7 | −10.3 |
| Leukemia | ||||||
| Enrollments (n = 2415) | 54.2 | 19.1 | 15.2 | 8.1 | 2.9 | 0.6 |
| Incidence (n ≈ 50 000) | 37.3 | 13.9 | 14.2 | 12.9 | 10.4 | 11.2 |
| Difference | +16.9 | +5.2 | +1.0 | −4.8 | −7.5 | −10.6 |
| Lung | ||||||
| Enrollments (n = 10 962) | 41.2 | 21.6 | 20.0 | 12.0 | 4.4 | 0.9 |
| Incidence (n ≈ 212 000) | 27.3 | 16.9 | 18.8 | 16.3 | 11.5 | 9.1 |
| Difference | +13.9 | +4.7 | +1.2 | −4.3 | −7.1 | −8.2 |
| Melanoma and/or Skin | ||||||
| Enrollments (n = 2492) | 66.0 | 15.2 | 9.3 | 5.5 | 2.8 | 1.2 |
| Incidence (n ≈ 93 000) | 44.7 | 13.7 | 13.0 | 10.9 | 8.6 | 9.1 |
| Difference | +21.3 | +1.5 | −3.7 | −5.4 | −5.8 | −7.9 |
| Myeloma | ||||||
| Enrollments (n = 1817) | 53.2 | 20.3 | 17.2 | 6.5 | 2.2 | 0.5 |
| Incidence (n ≈ 28 000) | 34.0 | 16.6 | 16.0 | 14.0 | 10.6 | 8.8 |
| Difference | +19.2 | +3.7 | +1.2 | −7.5 | −8.4 | −8.3 |
| Ovarian | ||||||
| Enrollments (n = 2061) | 54.9 | 18.3 | 14.5 | 8.9 | 2.9 | 0.5 |
| Incidence (n ≈ 22 000) | 50.8 | 13.5 | 11.7 | 9.3 | 7.3 | 7.5 |
| Difference | +4.1 | +4.8 | +2.8 | −0.4 | −4.4 | −7.0 |
| Prostate | ||||||
| Enrollments (n = 4829) | 36.2 | 25.6 | 20.5 | 12.5 | 4.0 | 1.2 |
| Incidence (n ≈ 213 000) | 37.6 | 23.9 | 18.3 | 11.1 | 5.3 | 3.8 |
| Difference | −1.4 | +1.7 | +2.2 | +1.4 | −1.3 | −2.6 |
| Renal | ||||||
| Enrollments (n = 2491) | 61.6 | 15.6 | 12.1 | 7.3 | 3.0 | 0.4 |
| Incidence (n ≈ 62 000) | 47.1 | 16.4 | 14.0 | 10.6 | 6.5 | 5.4 |
| Difference | +14.5 | −0.8 | −1.9 | −3.3 | −3.5 | −5.0 |
Estimated annual incident cases and incidence rates adjusted by age group calculated using Surveillance, Epidemiology, and End Results (SEER) Program data from the SEER*Stat Database for incidence based on cases diagnosed between 2013 and 2018 and reported across 21 registries.
Cancer type subgroups are limited to those with at least 1500 NCTN patient enrollments between 2016 and 2021.
“All cancer types” includes all incident cases and all enrollments and is not limited to the cancer types shown in this table.
The “+” symbol indicates that the proportion of enrollments was greater than the proportion of incident cases and the age group was overrepresented in trial enrollments. The “−” symbol indicates that the proportion of enrollments was less than the proportion of incident cases and the age group was underrepresented in trial enrollments.
There were 10 cancer types with at least 1500 enrollments from 2016 to 2021: bladder (n = 2579), breast (n = 16 534), colorectal (n = 1644), leukemia (n = 2415), lung (n = 10 962), melanoma (n = 2492), myeloma (n = 1817), ovarian (n = 2061), prostate (n = 4829), and renal (n = 2491). Patient age distribution varied for both enrollments and estimated incident cases across cancer types. Bladder cancer had the smallest estimated incident population aged 18 to 64 years, and bladder cancer trials had the lowest proportion of enrollments in this youngest age group, although these patients were still overrepresented (31.2% of enrollments vs 23.5% of estimated incident cases). Bladder cancer trials had the greatest proportion of enrollments among the oldest patients aged 85 years and older, although this still underrepresented the incident patient population (4.1% of enrollments vs 14.2% of cases). Breast cancer had the largest estimated incident population aged 18 to 64 years, and this age group was also overrepresented in breast cancer trials (80.5% of enrollments vs 52.8% of cases).
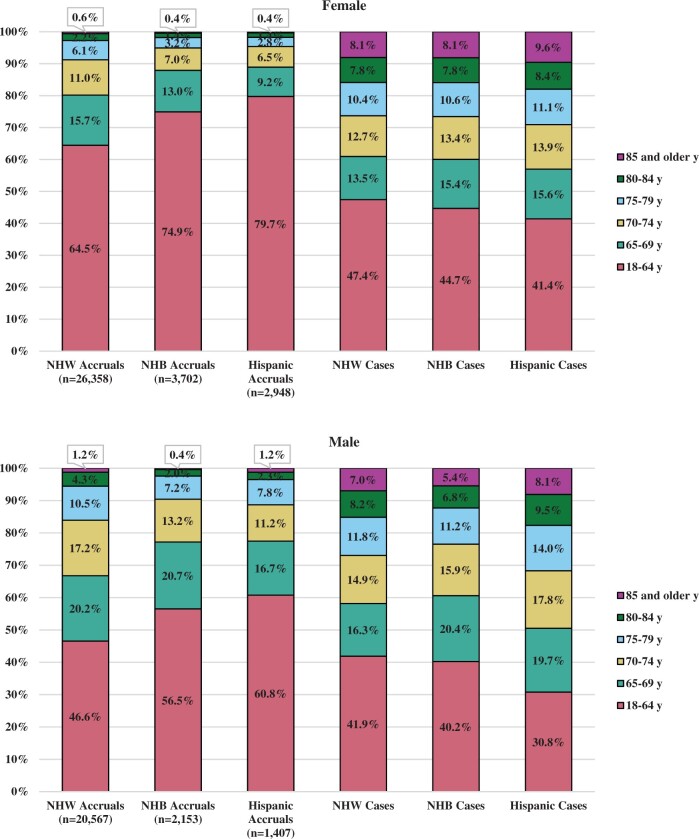
Age distribution also varied based on patient demographics. Given the race and origin variable available via SEER, there were 3 groups with at least 1000 patient enrollments among both male and female patients over the 6-year period: non-Hispanic White, non-Hispanic Black, and Hispanic. There was greater enrollment to trials of older patients who were non-Hispanic White compared with patients who were non-Hispanic Black or Hispanic (Figure 2). Among female patients enrolled to trials, 35.5% of non-Hispanic White patients were aged 65 years and older compared with 25.1% of non-Hispanic Black patients and 20.3% of Hispanic patients. A similar trend is seen for male patients. This trend was not reflective of the incident patient population. Based on the estimated incident cancer cases, a greater proportion of Hispanic patients was expected to be diagnosed with cancer at ages 65 years and older. Older patients were underrepresented in trials in every demographic subgroup. However, Hispanic patients aged 65 years and older were especially underrepresented in trials (female patients: 20.3% of enrollments vs 58.6% of estimated incident cases; male patients: 39.2% of enrollments vs 69.2% of cases).
Figure 2.
Proportion of adult enrollments to the National Cancer Institute’s National Clinical Trials Network and estimated cancer incidence by age group, sex, race and ethnicity. All groups are mutually exclusive, limited to categories with at least 1000 enrollments in each subgroup. NHB = non-Hispanic Black; NHW = non-Hispanic White.
Discussion
This analysis found that there was overrepresentation of those aged 18 to 69 years and underrepresentation of those aged 70 years and older among patients enrolled to NCTN trials from 2016 to 2021. Underrepresentation of older patients was seen across cancer types. Older patients who enrolled to NCTN trials were more likely to be non-Hispanic White than the estimated incident cancer population. A key limitation to this analysis is that the incident cancer population is estimated based on SEER data; these data are the most comprehensive compilation of incident cancer cases available but may not be truly reflective of the US incident cancer population. Additionally, the patient demographic information is limited to what is routinely collected across NCTN trials, which includes key variables required by the National Institutes of Health to monitor inclusion of women and minorities but do not capture potentially important patient characteristics such as whether patients are from rural or urban communities (12).
From 2001 to 2011, an average of 68% of patients enrolled each year in adult NCI Cooperative Group phase II and III treatment trials were aged younger than 65 years, 23% were between ages 65 and 74 years, and 9% were aged 75 years and older (5). In these updated data from 2016 to 2021, 59.5% of patients enrolled in adult NCTN trials were aged younger than 65 years, 29.6% were between ages 65 and 74 years, and 10.9% were aged 75 years and older. Based on these overarching data, it appears that NCTN trials are enrolling greater proportions of older adults than in the earlier time period. Enhancing enrollment of older patients has been a priority for the NCI and the NCTN, with several activities potentially contributing to the greater enrollment, including broadening clinical trial inclusion criteria to reduce the unnecessary exclusion of patients with well-managed comorbidities, which may have disproportionately excluded older patients (13,14). NCTN trials do not include older age limits in eligibility criteria unless there is a compelling scientific and medical justification. However, there is still significant room for improvement in the enrollment of older adults to NCTN trials.
Some of the change in enrollment seen here may also reflect a shift in enrollment by disease area in the intervening years. In 2001-2011 and 2016-2021, breast cancer had the highest proportion of enrollments of younger patients, with approximately 80% of patients enrolled to breast cancer trials aged younger than 65 years. These breast cancer enrollments represented 40% of the overall enrollment from 2001 to 2011 compared with 27% of the enrollment from 2016 to 2021.
It is important to note that the characteristics of patients enrolled in clinical trials in any given year or disease area will depend greatly on the available trial portfolio. The overall enrollment within the NCTN was relatively stable from 2016 to 2019, with somewhat lower enrollment in 2020 and 2021, likely due to the COVID-19 pandemic, but the trials available for enrollment were constantly changing. In some trials, enrollment of a younger patient population is justified. As was noted for the 2016 to 2021 enrollments, a greater proportion (80.5%) of patients enrolled to trials in breast cancer were aged younger than 65 years compared with the average (59.5%) across all disease areas. One of the highest-enrolling breast cancer studies during this time period was Alliance for Clinical Trials in Oncology trial A011502, studying aspirin as adjuvant therapy in breast cancer patients (15,16). Because of the risks of aspirin in older patients, this trial was open only to patients aged 69 years and younger, and the enrolled patient population was largely younger. This analysis included enrollments to 26 breast cancer trials; among the included enrollments, the median proportion of patients aged 18 to 64 years across these breast cancer trials was 79.7%. Because this is less than a percentage point lower than the overall proportion reported for breast cancer trials, it suggests that the breast cancer analysis is not being skewed by particularly high enrollment of younger patients to a small number of trials.
In other disease areas, there is a strong scientific and clinical rationale for conducting trials in patients within certain age ranges because of observed differences in the disease progression or treatment response. For instance, the National Comprehensive Cancer Network guidelines offer distinct principles for the management of acute myeloid leukemia in patients aged older than 60 years and for the treatment of acute lymphoblastic leukemia in adults aged 65 years and older (17,18). Many leukemia trials in the NCTN have been designed specifically to answer questions in older or younger patient populations (19). SWOG Cancer Research Network trial S1318 was a phase II trial evaluating use of blinatumomab as induction and consolidation therapy in newly diagnosed acute lymphoblastic leukemia patients aged 65 years and older (20,21). The median age of the 29 patients in this study was 75 years. As more NCTN leukemia studies focused on older patient populations report out, we expect to see similarly older age distributions.
This monograph features several articles with recommendations and considerations for continuing to enhance the enrollment of older adults to NCTN clinical trials. A particularly important takeaway from this analysis is that patient demographics should not be viewed in isolation. As this analysis shows, Black and Hispanic patients enrolled to adult NCTN cancer trials tended to be younger than the non-Hispanic White patients enrolled, even though the Black and Hispanic incident cancer population tended to be older. As we consider ways to increase enrollment of older adults to trials, we must be sure that this is not associated with lower enrollment of non-White patients. In fact, as the estimated incidence data showed, more representative trial enrollment should likely include more older patients who are Black and Hispanic.
Older Hispanic patients were particularly underrepresented among patients enrolled to NCTN trials. The NCI has sought to reduce barriers to trial enrollment among Hispanic patients, including by expanding consent translation services to provide Spanish translations of informed consent documents for all NCTN trials starting in 2015. Additionally, the NCI Community Oncology Research Program seeks to bring NCTN trials to diverse patients in their communities (22). However, as these findings emphasize, more needs to be done to overcome barriers to trial enrollment for non-White patients (23). The intersection between trial enrollment and age, race, ethnicity, and other patient characteristics such as geographic area and rurality warrants further study so that more targeted enrollment enhancement efforts can be developed that improve trial diversity across demographic groups.
Funding
No funding was used for this study.
Notes
Role of the funder: Not applicable.
Disclosures: No disclosures to report.
Author contributions: GEM: Conceptualization, Methodology, Formal Analysis, Data Curation, Writing—Original Draft, Writing—Review & Editing, Visualization, Project Administration. AMD, AFB, And RFL: Conceptualization, Methodology, Writing—Review & Editing.
Disclaimers: This article represents the opinions of the authors and not those of the National Cancer Institute.
Contributor Information
Grace E Mishkin, Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Rockville, MD, USA.
Andrea M Denicoff, Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Rockville, MD, USA.
Ana F Best, Biometric Research Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Rockville, MD, USA.
Richard F Little, Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Rockville, MD, USA.
Data Availability
The data underlying this article cannot be shared to protect the privacy of the clinical trial participants. Information to support the findings of this analysis are available from the corresponding author upon reasonable request.
References
- 1. Trimble EL, Carter CL, Cain D, et al. Representation of older patients in cancer treatment trials. Cancer. 1994;74(suppl 7):2208-2214. [DOI] [PubMed] [Google Scholar]
- 2. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720-2726. [DOI] [PubMed] [Google Scholar]
- 3. Scher KS, Hurria A. Under-representation of older adults in cancer registration trials: known problem, little progress. J Clin Oncol. 2012;30(17):2036-2038. [DOI] [PubMed] [Google Scholar]
- 4. Sedrak MS, Freedman RA, Cohen HJ, et al. ; for the Cancer and Aging Research Group (CARG). Older adult participation in cancer clinical trials: a systematic review of barriers and interventions. CA Cancer J Clin. 2021;71(1):78-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mooney M. Older Patients in NCI-sponsored Clinical Treatment Trials. https://meetinglibrary.asco.org/record/68859/video. Published 2012. Accessed August 8, 2022.
- 6. Unger JM, Hershman DL, Osarogiagbon RU, et al. Representativeness of Black patients in cancer clinical trials sponsored by the National Cancer Institute compared with pharmaceutical companies. JNCI Cancer Spectr. 2020;4(4):pkaa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alvidrez J, Greenwood GL, Johnson TL, et al. Intersectionality in public health research: a view from the National Institutes of Health. Am J Public Health. 2021;111(1):95-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Cancer Institute. Division of Cancer Treatment and Diagnosis Programs and Initiatives 2013-2017. https://dctd.cancer.gov/NewsEvents/2017DCTDprograms.pdf. Published October 2018. Accessed August 8, 2022.
- 9. National Cancer Institute. An overview of NCI’s National Clinical Trials Network. https://ctep.cancer.gov/initiativesprograms/nctn.htm. Updated February 17, 2022. Accessed August 8, 2022.
- 10. National Cancer Institute Surveillance Research Program. Surveillance, Epidemiology, and End Results (SEER) Program SEER 21 Reg Research Data, Nov 2020 Sub (2000-2018). National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch. http://www.seer.cancer.gov. Published April 2021.
- 11. National Cancer Institute. Number of persons by race and Hispanic ethnicity for SEER participants (2020 Census Data). https://seer.cancer.gov/registries/data.html. Accessed August 8, 2022.
- 12. National Institutes of Health. NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research. https://grants.nih.gov/policy/inclusion/women-and-minorities/guidelines.htm. Updated December 6, 2017. Accessed August 8, 2022.
- 13. Denicoff A. CTAC Strategic Planning Working Group: CTEP Analysis of Implementing ASCO-Friends Broadened Eligibility Criteria in CTEP-Sponsored Trials. https://deainfo.nci.nih.gov/advisory/ctac/1121/Doroshow-Denicoff.pdf. Published November 10, 2021. Accessed August 8, 2022.
- 14. Habr D, McRoy L, Papadimitrakopoulou VA. Age is just a number: considerations for older adults in cancer clinical trials. J Natl Cancer Inst. 2021;113(11):1460-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alliance for Clinical Trials in Oncology. A randomized phase iii double blinded placebo controlled trial of aspirin as adjuvant therapy for HER2 negative breast cancer: the ABC trial. https://clinicaltrials.gov/ct2/show/NCT02927249. Updated January 10, 2022. Accessed August 8, 2022.
- 16. Chen WY, Ballman KV, Winer EP, et al. A randomized phase III, double-blinded, placebo-controlled trial of aspirin as adjuvant therapy for breast cancer (A011502): the Aspirin after Breast Cancer (ABC) Trial. J Clin Oncol. 2022;40(suppl 36):360922. [Google Scholar]
- 17. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Acute Lymphoblastic Leukemia Version 1. https://www.nccn.org/professionals/physician_gls/pdf/all.pdf. Published 2022. Accessed August 8, 2022.
- 18. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Acute Myeloid Leukemia Version 1. https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf. Published 2022. Accessed August 8, 2022.
- 19. National Cancer Institute. NCTN leukemia trials portfolio. https://ctep.cancer.gov/initiativesPrograms/docs/nctn_trials/NCTN_Leukemia_Trials.pdf. Updated July 15, 2022. Accessed August 8, 2022.
- 20. Advani AS, Moseley A, O’Dwyer KM, et al. SWOG 1318: a phase II trial of blinatumomab followed by POMP maintenance in older patients with newly diagnosed Philadelphia chromosome-negative B-cell acute lymphoblastic leukemia. J Clin Oncol. 2022;40(14):1574-1582. doi: 10.1200/jco.21.01766:Jco2101766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. SWOG Cancer Research Network. Blinatumomab and combination chemotherapy or dasatinib, prednisone, and blinatumomab in treating older patients with acute lymphoblastic leukemia. https://www.clinicaltrials.gov/ct2/show/NCT02143414. Updated June 30, 2022. Accessed August 8, 2022.
- 22. McCaskill-Stevens W, Lyss AP, Good M, et al. The NCI community oncology research program: what every clinician needs to know. Am Soc Clin Oncol Educ Book. 2013;33:e84-e89. doi: 10.14694/EdBook_AM.2013.33.e84. [DOI] [PubMed] [Google Scholar]
- 23. Hamel LM, Penner LA, Albrecht TL, et al. Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control. 2016;23(4):327-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article cannot be shared to protect the privacy of the clinical trial participants. Information to support the findings of this analysis are available from the corresponding author upon reasonable request.