Abstract
Background
The purpose of this study was to estimate the number of individuals living with metastatic breast, prostate, lung, colorectal, or bladder cancer or metastatic melanoma in the United States using population-based data.
Methods
A back-calculation method was used to estimate the number of individuals living with metastatic cancer for each cancer type from US cancer mortality and survival statistics from the Surveillance, Epidemiology, and End Results registries. The percentages of those living with metastatic cancer who advanced to metastatic disease from early stage cancer vs who were diagnosed with metastatic cancer de novo were calculated. One- and 5-year relative survival rates for de novo metastatic cancer were compared by year of diagnosis to assess time trends in survival.
Results
It is estimated that, in 2018, 623 405 individuals were living with metastatic breast, prostate, lung, colorectal, or bladder cancer, or metastatic melanoma in the United States. This number is expected to increase to 693 452 in 2025. In 2018, the percentage of metastatic cancer survivors who were initially diagnosed with early stage cancer and advanced to metastatic cancer ranged from 30% for lung cancer to 72% for bladder cancer.
Conclusions
This study demonstrates increasing numbers of individuals living with metastatic cancer of the 6 most common cancer types in the United States. This information is critical for informing the allocation of research efforts and healthcare infrastructure needed to address the needs of these individuals.
As of January 2022, it was estimated that more than 18 million individuals in the United States were living with a history of cancer (1). Because of the growth of the aging population alone, this number is projected to reach more than 22.1 million by 2030 (2). These estimates include cancer survivors at all disease stages and treatment phases of the cancer survivorship continuum, including survivors who are newly diagnosed and undergoing treatment as well as those who are long-term survivors and living without evidence of disease.
One understudied group of cancer survivors is those who have been diagnosed with or have progressed to metastatic cancer, the most advanced form of the disease. The needs of this subpopulation likely differ from the group of cancer survivors diagnosed with and treated for early stage disease. However, there remains a paucity of knowledge related to the needs, and ways to effectively address these needs, of individuals living with advanced or metastatic cancer (3).
Equally as important to addressing the evidence gaps related to the needs of those with metastatic cancer is better understanding the number of individuals living with metastatic cancer in the United States, as this information is critical to informing the allocation of research efforts and healthcare infrastructure needed to address the needs of these individuals (3). Cancer registry data can be used to estimate the number of survivors who were initially diagnosed with metastatic cancer (de novo), however, registries do not collect longitudinal data on recurrence or progression to metastatic disease. Thus, estimating the number of individuals who were initially diagnosed with early stage disease and who later have a recurrence (after being disease free) or progress (after never being disease free) to metastatic disease is challenging. With the number of cancer survivors increasing overall (2), as well as survival rates improving for many cancers (4), it is likely that the number of individuals who are living with metastatic cancer is also growing, as shown previously for breast cancer (5). However, data on the number of cancer survivors living with metastatic disease for cancer types other than breast are lacking.
Therefore, to better understand the current burden of metastatic cancer in the United States, we updated and extended the analysis by Mariotto et al. (5) to estimate the number of individuals living with metastatic disease (ie, the prevalence of metastatic cancer) for the 6 most common cancer types: breast, prostate, lung and bronchus (hereafter referred to as lung), colon and rectum (hereafter referred to as colorectal), bladder, and melanoma. We used national data on site-specific cancer mortality and distant stage site-specific survival from the Surveillance, Epidemiology, and End Results (SEER) registries to estimate the prevalence of metastatic cancer by cancer type, including those diagnosed with de novo metastatic cancer and those who are diagnosed with early stage cancer and experience a recurrence or progression to metastatic disease. From this point forward, we use the term metastatic recurrence to encompass a recurrence to metastatic disease after an early stage diagnosis (and disease-free period) and progression to metastatic disease after an early stage diagnosis (with no disease-free period). The term recurrence in this analysis does not include local or regional recurrence after an early stage diagnosis.
Methods
Back-Calculation Method to Estimate the Prevalence of Metastatic Disease
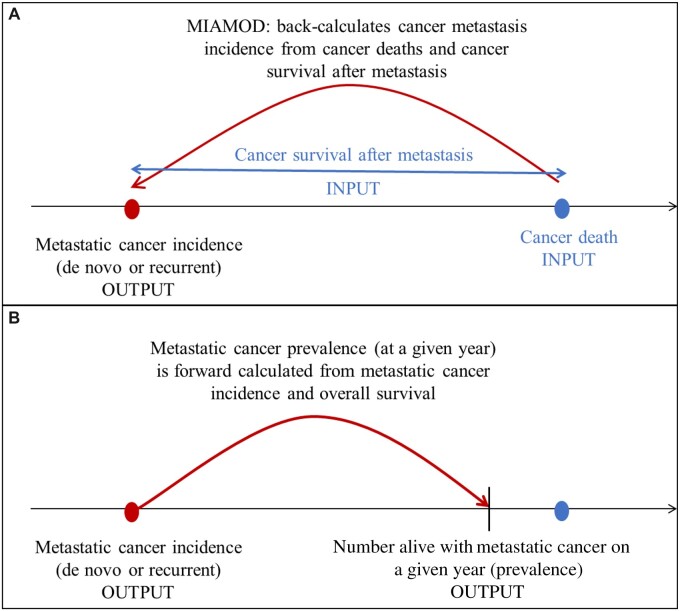
As in Mariotto et al. (5), we used the Mortality Incidence Approach Model (MIAMOD) to estimate prevalence of metastatic disease (6,7) (see Figure 1). MIAMOD assumes that each observed cancer death is the result of metastatic disease, either initially diagnosed as metastatic or a recurrence to metastatic disease after an early stage diagnosis. The main inputs to MIAMOD are US cancer deaths and cancer survival from metastatic disease. Other inputs include US all-cause deaths, to estimate overall survival, and US population counts. MIAMOD was applied by sex to each cancer type: breast [female only, to update Mariotto et al. (5)], prostate (male only), lung, colorectal, melanoma, and bladder. The MIAMOD software is available at www.eurocare.it/MiamodPiamod/tabid/60/Default.aspx.
Figure 1.
Illustration of MIAMOD back-calculation method. A) MIAMOD first back-calculates the incidence of metastatic cases (de novo or recurrent) diagnosed in a year from cancer deaths and cancer survival after metastasis. Cancer survival after metastasis is estimated from de novo metastatic survival and adjustments. B) The number of people living with metastatic cancer (ie, metastatic prevalence) is then estimated from the estimated metastatic cancer incidence and survival (overall) as . MIAMOD = Mortality Incidence Approach Model.
Cancer-Specific Mortality, All-Cause Mortality, and Population Data for the Back-Calculation Method
This analysis used US mortality data from the National Center for Health Statistics and US Census Bureau populations through 2018, both of which are available in the SEER*Stat software. We obtained US cancer-specific deaths for each cancer type, all-cause deaths, and populations from 1990-2018 by sex, single calendar year, single ages 0-84 years, and age group of 85 years or older. To project metastatic cancer prevalence into the future (2019-2025), we used the US Census Bureau July 1, 2016, population projections derived from the 2010 Census (8).
Survival Time from Metastatic Cancer
Survival time from metastatic cancer should reflect the mixture of survival after 1) de novo metastatic cancer and 2) recurrence to metastatic disease. Whereas survival from de novo metastatic cancer can be estimated from cancer registry data, survival after metastatic recurrence requires external data and assumptions.
To estimate survival from de novo metastatic cancer, we calculated relative survival for individuals diagnosed with distant stage cancer between 2000 and 2017 in the SEER-18 areas for each cancer type. Relative survival was calculated by age group (0-49, 50-64, 65-74, 75-84 years) and year of diagnosis (2000-2005, 2006-2011, 2012-2017). Distant stage at diagnosis for cancers diagnosed between 1998 and 2017 was defined using summary stage 2000. We included only invasive cancers that were the first primary tumor (sequence number 0 or 1) and excluded cancers diagnosed through death certificate or autopsy and cases lost to follow-up at the month of diagnosis. Joinpoint survival models were used to assess changes in relative survival over time (9).
Data on survival after metastatic recurrence are largely unavailable for all cancer types considered except breast cancer. Thus, for bladder, colorectal, lung, melanoma, and prostate cancer, we assumed survival after metastatic recurrence following early stage diagnosis was equal to survival after de novo metastatic cancer. To estimate survival from breast cancer metastatic recurrence, we used an adjustment to de novo metastatic breast cancer survival reported in Mariotto et al. (5): . The adjustment was based on an MD Anderson Cancer Center study that showed, on average, the risk of death for recurrent relative to de novo metastatic breast cancer was 1.35 times higher (10). For breast cancer, survival from metastatic breast cancer was represented as a weighted sum of the de novo survival and recurrent survival: with (5). The weight was identified using the incidence-based mortality method, and further details can be found in Mariotto et al. (5).
Prevalence Projections and Estimates for All Ages
MIAMOD does not provide prevalence estimates for open age class intervals (eg, ages 85 years and older). To include survivors who are aged 85 years and older in the metastatic prevalence estimates, we assumed that the prevalence of metastatic cancer survivors aged 85 years and older is equal to the prevalence proportion among the oldest age category we are able to estimate (ages 80-84 years). The number of survivors aged 85 years and older living with metastatic cancer was calculated by multiplying the estimated prevalence proportion for ages 80-84 years and the number of individuals aged 85 years and older in the United States. This number was then added to the estimated number of metastatic cancer cases for those aged 0-84 years. Projections of the numbers of metastatic cancer cases from 2019 to 2025 assumed constant cancer mortality rates at 2018 levels, constant survival, and dynamic population size projections as estimated in the US Census Bureau population projections.
Prevalence of de Novo Metastatic Cancer Using the Counting Method
MIAMOD metastatic cancer prevalence estimates include both the prevalence of de novo and recurrent metastatic disease, and we are unable to distinguish them. To quantify how much of the total metastatic cancer prevalence represents survivors who had a recurrence to metastatic disease after an early stage diagnosis, we also calculated the prevalence of de novo metastatic cancer using cancer registry data and the counting method. We first calculated 18-year limited duration prevalence on January 1, 2018, by race (White or Unknown, Black, and Other) and 5-year age groups using SEER*Stat for those diagnosed with distant stage cancer between 2000 and 2017 in SEER-18 areas. We then multiplied these prevalence proportions by the respective 2018 US populations by race and age and summed across race, age, and sex to obtain US de novo metastatic cancer prevalence for each cancer type.
Results
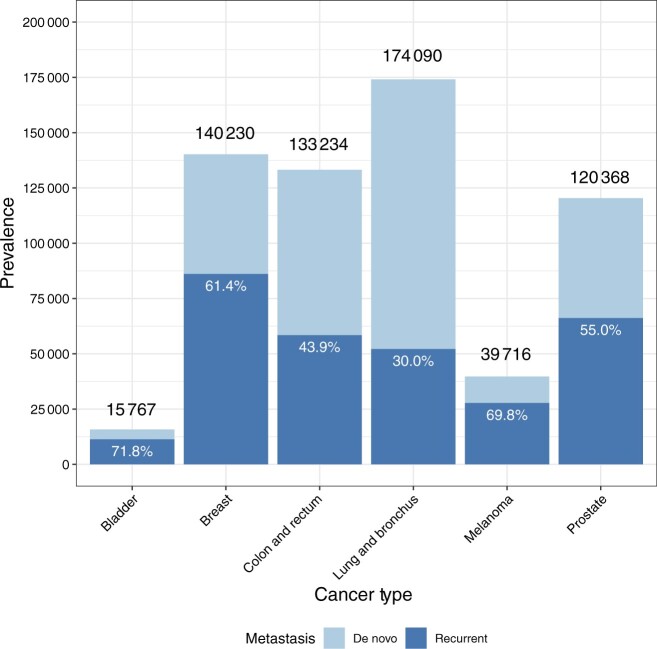
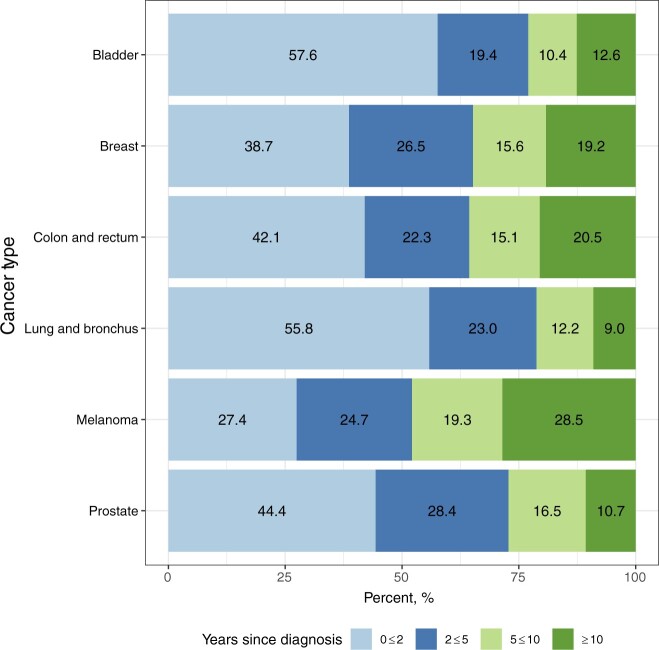
In 2018, the last year with observed SEER data, it is estimated that 623 405 individuals were living with metastatic breast, prostate, lung, colorectal, or bladder cancer, or metastatic melanoma, in the United States (Table 1). The percentage of metastatic cancer survivors initially diagnosed with early stage cancer who later experienced a metastatic recurrence ranged from 30% for lung cancer to 71.8% for bladder cancer (Figure 2). Most survivors with metastatic cancer of each type, except for melanoma, are estimated to have been living less than 5 years with metastatic disease (range: 52.1% for melanoma to 78.8% for lung cancer). Almost 30% of individuals diagnosed with metastatic melanoma and one-fifth of individuals diagnosed with metastatic colorectal or metastatic breast cancer are estimated to have been living with metastatic disease for 10 or more years (Figure 3; Supplementary Table 1, available online).
Table 1.
Estimates of deaths and incidence and prevalence of metastatic cancer including de novo and recurrent metastatic disease in the United States for 2018a
| Cancer type | Number in US population in 2018 | Number of cancer deaths |
Number of new metastatic cancer cases (incidence) |
Number of individuals living with metastatic disease on January 1, 2018 (prevalence) |
|||
|---|---|---|---|---|---|---|---|
| Observed | Estimated | De novo (observed) | De novo and recurrence (estimated) | De novo (observed) | De novo and recurrence (estimated) | ||
| Lung and bronchus | 326 687 501 | 142 211 | 142 069 | 95 723 | 148 533 | 121 943 | 174 090 |
| Breast (female) | 165 801 767 | 39 974 | 40 029 | 15 800 | 46 666 | 54 117 | 140 230 |
| Colon and rectum | 326 687 501 | 48 154 | 47 857 | 32 015 | 55 162 | 74 784 | 133 234 |
| Prostate (male) | 160 885 734 | 26 172 | 25 881 | 18 469 | 40 388 | 54 207 | 120 368 |
| Melanoma | 326 687 501 | 7693 | 7586 | 3928 | 9468 | 11 979 | 39 716 |
| Bladder | 326 687 501 | 14 193 | 14 140 | 4070 | 15 874 | 4446 | 15 767 |
Estimates are presented for males and females together (all ages) unless otherwise specified.
Figure 2.
Percentages of estimated metastatic cancer cases (on January 1, 2018) who were initially diagnosed with metastasis (de novo) or who experienced recurrent metastatic disease after being initially diagnosed with early stage disease. Numbers above each bar represent total estimated metastatic prevalence for each cancer type.
Figure 3.
Percentage of estimated metastatic prevalence (on January 1, 2018) by years since diagnosis for each cancer type.
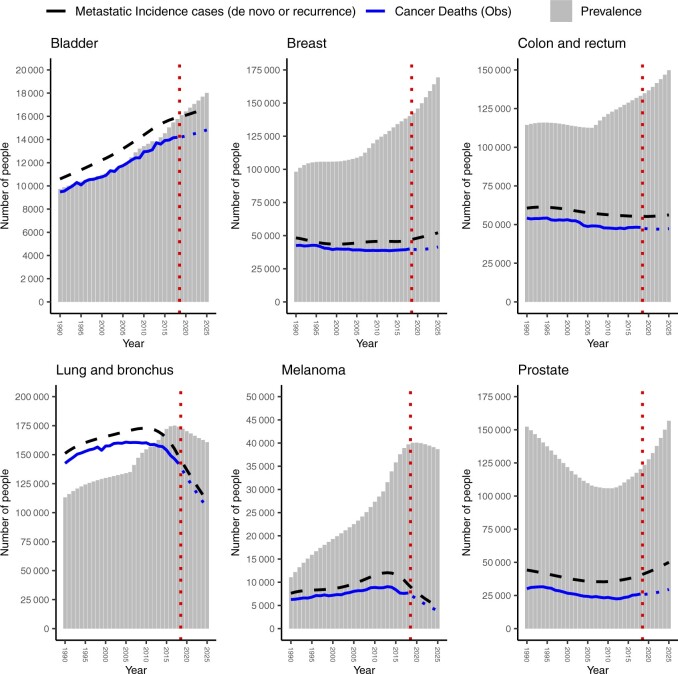
By January 1, 2025, the following numbers of individuals are projected to be living with metastatic cancer in the United States: breast, 169 347; lung, 160 743; prostate, 156 812; colorectal, 149 840; melanoma, 38 691; and bladder, 18 019 (Table 2; Figure 4). For all cancer types except prostate, the metastatic cancer prevalence has increased linearly from 1990 to 2018, with the greatest percentage increase observed for melanoma (258%) and bladder cancer (62%) (Figure 4). The number of individuals living with metastatic cancer is projected to increase from 2019 to 2025 for breast, bladder, colorectal, and prostate cancer; conversely, the number of individuals living with metastatic cancer is projected to decrease for lung and melanoma over these 7 years.
Table 2.
Projections of deaths and incidence and prevalence of metastatic cancer including de novo and recurrent metastatic disease in the United States for 2025a
| Cancer type | Projected number in US population in 2025 | Projected number of cancer deaths | Projected number of new metastatic (de novo and recurrence) cancer cases (incidence) | Projected number of individuals living with metastatic disease (de novo and recurrence) on January 1, 2025 (prevalence) |
|---|---|---|---|---|
| Lung and bronchus | 344 114 571 | 104 363 | 110 132 | 160 743 |
| Breast (female) | 174 407 303 | 41 434 | 52 126 | 169 347 |
| Colon and rectum | 344 114 571 | 47 355 | 56 227 | 149 840 |
| Prostate (male) | 169 707 268 | 29 567 | 50 055 | 156 812 |
| Melanoma | 344 114 571 | 3900 | 4810 | 38 691 |
| Bladder | 344 114 571 | 14 826 | 16 720 | 18 019 |
Estimates are presented for males and females together (all ages) unless otherwise specified.
Figure 4.
Estimates and projections of metastatic cancer incidence and prevalence for both sexes combined in the United States from 1990 to 2025 (gray bars) by cancer type. Dashed black line is estimated number of new incidence cases with metastatic cancer, including de novo and recurrent metastatic cancer. Solid blue line is observed number of cancer deaths (all stages) from 1990 to 2018. Dotted blue line is projected number of cancer deaths (all stages) from 2019 to 2025. Dotted red line indicates projections from 2019 to 2025.
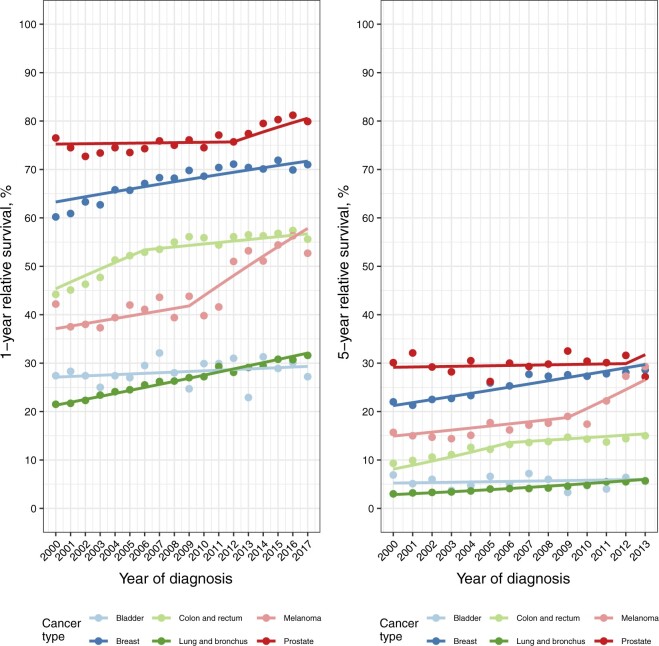
One- and 5-year relative survival estimates and corresponding joinpoint survival model fits by cancer type for individuals diagnosed with de novo metastatic cancer in the SEER-18 areas are shown in Figure 5. Increases in 1- and 5-year relative survival are observed over time for all cancer types except bladder cancer. For melanoma in particular, 1-year relative survival increased approximately 10% from 2000 to 2017, and 5-year relative survival increased almost 15% over the same period. One- and 5-year relative survival for the diagnosis periods 2000-2005, 2006-2011, and 2012-2017 are presented in Supplementary Tables 2 and 3 (available online), respectively.
Figure 5.
One-year and 5-year de novo metastatic relative survival (%) for each cancer type by year of diagnosis. Solid points correspond to cumulative relative survival. Solid lines correspond to predicted joinpoint survival model fits.
Discussion
To our knowledge, this is the first study to report on the prevalence of individuals living in the United States with metastatic cancer of the prostate, lung, colon and rectum, and bladder as well as metastatic melanoma; it also provides an update on the number of women living with metastatic breast cancer in the United States (5). Our study presents not only the overall number of metastatic cases for each cancer type but also the proportion of cases that arise from either de novo metastatic cancer or early stage cancer that later metastasizes. The prevalence counts for breast cancer differ slightly from those reported in Mariotto et al. (5). Mariotto et al. (5) calibrated the de novo metastatic prevalence estimated via MIAMOD to the de novo metastatic prevalence estimated via the counting method by adjusting metastatic survival in an ad hoc way. In our analysis, we did not calibrate the de novo metastatic prevalence estimate in this same way; the metastatic breast cancer prevalence estimate is based on the raw survival data.
Our findings indicate that, in general, the number of metastatic cancer survivors of the 6 cancer types examined have increased substantially since 1990, with the greatest percentage increases in melanoma and bladder cancer. For metastatic bladder cancer, the increase appears to be primarily because of an increased incidence of de novo metastatic diagnoses. For metastatic melanoma, the increase is likely due, in part, to the improvement in relative survival. In addition, the results show that a large percentage of cancer survivors living with metastatic disease were diagnosed with early stage cancer and later experienced a recurrence or progression to metastatic disease. This finding highlights the need to identify methods to capture individuals diagnosed with early stage cancers who have a recurrence or progression to metastatic disease in cancer registries and electronic medical records. These types of methods would enable accurate counts of this unique group of cancer survivors as well as advance research pertaining to metastatic cancer survivorship.
Overall, the highest estimated prevalence of the cancer types examined was for metastatic lung cancer. Interestingly, it appears that the prevalence of individuals living with metastatic lung cancer may have peaked around 2016 and is beginning to decrease. This decrease may be because overall lung cancer mortality has been declining since the late 1980s and more markedly in the most recent years (11). Distant stage incidence has also been declining (https://seer.cancer.gov/explorer/). These declines are not surprising, given the fact that low-dose computed tomography was endorsed by the US Preventive Services Task Force in 2013 for lung cancer screening among adults aged 55 to 80 years who have a 30 pack-year smoking history and are either currently smoking or have quit in the past 15 years (12). These recommendations were subsequently updated in 2021 (13). Use of low-dose computed tomography in this high-risk population has likely shifted the stage at diagnosis distribution, with more lung cancers being diagnosed at earlier stages (14). Treatments for metastatic lung cancer have also improved with the development and use of newer immunotherapies and targeted therapies (11), although findings from this study suggest that 5-year relative survival for individuals with metastatic lung cancer has improved only modestly, from 3% in 2000-2005 to 7% in 2012-2017.
For cancer of the breast, colon and rectum, prostate, and bladder, estimates for the number of individuals living with metastatic disease are projected to continue to rise, as they have been, for the most part, over the past 3 decades. In addition, many individuals with metastatic cancer of these types are living longer. For example, our findings show that approximately 30% of metastatic melanoma survivors and approximately one-fifth of metastatic breast and colorectal cancer survivors have lived 10 or more years postdiagnosis. Unfortunately, despite the growing numbers of individuals living with metastatic cancer and those living longer postdiagnosis, there remains a dearth of knowledge about the needs of these survivors (3). Knowledge gaps identified in a 2021 National Cancer Institute meeting on the topic of metastatic cancer survivorship include the trajectory and mechanisms of symptoms, particularly associated with newer therapies, experienced by individuals living with metastatic cancer; the psychosocial needs for individuals living with metastatic cancer; and care patterns throughout the care trajectory (3). Filling these knowledge gaps is critical for delivering high-quality healthcare that addresses the needs of individuals living with metastatic cancer.
Several strengths of this study should be noted. The dataset used in the analysis was large and population based and included a long follow-up period, with the ability to compare survival estimates across multiple time periods. Further, consistent definitions of staging and other variables were used across time. The main limitation of this study is the absence of population-based survival estimates following progression to metastatic disease. Duplicating the method used in Mariotto et al. (5), we used a 1.35 higher risk of cancer death for recurrent metastatic breast cancer relative to de novo disease; however, this estimate was based on a single institution study (10). Had we instead assumed equal risk for recurrent metastatic breast cancer, it is likely that our prevalence estimate for metastatic cancer would be markedly higher. To our knowledge, there are no other studies for the other cancer types examined in this study comparing survival among individuals diagnosed with de novo vs recurrent metastatic disease; therefore, it was assumed in the analysis that survival was the same for both. Population-based studies estimating differences in survival between de novo and recurrent metastatic disease for the cancers analyzed in this study are needed and would improve the accuracy of the prevalence estimates. Prevalence estimates are impacted by model inputs (which can be affected by certain events such as the introduction of curative therapies); our estimates are based on the available data and do not reflect future changes in treatment or screening.
The methods described in this manuscript assume that before dying of cancer, patients were diagnosed or advanced to metastatic cancer. Progression to metastasis refers to a worsening of the cancer and progressing to a later stage without the patient being ever cancer free. Recurrence refers to the return of the cancer after treatment and a disease-free period. The cancer can return to the same organ (local recurrence), lymph nodes (regional recurrence), or distant organs (metastatic recurrence). In this paper, we do not distinguish between progression to metastasis or metastatic recurrence. Use of the counting method to estimate de novo metastatic prevalence in combination with MIAMOD allows us to quantify the proportions of patients who are de novo and recurrent metastatic. However, because of the lack of recurrence information in the SEER registries, it is difficult to determine whether any metastatic patients are disease free.
This study demonstrates the growing burden of metastatic cancer in the United States. Despite this growing burden, little is known about the needs of these survivors. Leveraging existing resources can help address research gaps in this understudied population; these resources include cancer registry–based datasets (eg, SEER-Medicare), foundation-based registries and datasets (eg, Susan Love Foundation Health of Women study), and electronic health record–based databases (eg, Flatiron, American Society of Clinical Oncology’s CancerLinq). Importantly, future studies, including those using existing resources, should account for the heterogeneity of individuals living with metastatic cancer, even within cancer type, by collecting detailed data on demographic characteristics including race and ethnicity, disease state, underlying comorbid conditions, treatments, and symptoms to help better identify needs. Further, engaging patients and advocates, as well as other stakeholders, can ensure that the issues and experiences most important to them are accurately reflected in research as well as implementation efforts in the healthcare setting. This study is a first step toward a more comprehensive understanding of an understudied, and often medically underserved, cancer survivor population.
Funding
No funding was used for this study.
Notes
Role of the funder: Not applicable.
Disclosures: No conflicts of interest exist. LG, a JNCI Associate Editor and co-author on this article, was not involved in the editorial review or decision to publish this manuscript.
Author contributions: Conceptualization: LG, MAM, AM; Formal analysis: TPD, AM; Visualization: TPD, AM; Writing-original draft: LG, TPD, MAM, AM; Writing-review & editing: ET.
Disclaimers: The article was prepared as part of the authors’ (LG, TPD, ET, MAM, AM) official duties as employees of the US Federal Government. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Cancer Institute.
Supplementary Material
Contributor Information
Lisa Gallicchio, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD, USA.
Theresa P Devasia, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD, USA.
Emily Tonorezos, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD, USA.
Michelle A Mollica, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD, USA.
Angela Mariotto, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD, USA.
Data Availability
The data underlying this article are available through the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program at https://seer.cancer.gov/data-software/datasets.html and the U.S. Census Bureau at https://www.census.gov/data/datasets/2017/demo/popproj/2017-popproj.html.
References
- 1. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2022-2024. Atlanta: American Cancer Society; 2022. [Google Scholar]
- 2. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA A Cancer J Clin. 2019;69(5):363-385. [DOI] [PubMed] [Google Scholar]
- 3. Mollica MA, Smith AW, Tonorezos E, et al. Survivorship for individuals living with advanced and metastatic cancers: National Cancer Institute meeting report. J Natl Cancer Inst. 2021;114(4):489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Surveillance Research Program, National Cancer Institute . SEERExplorer: An Interactive Website for SEER Statistics. https://seer.cancer.gov/statistics-network/explorer/. Accessed January 15, 2022.
- 5. Mariotto AB, Etzioni R, Hurlbert M, et al. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26(6):809-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Angelis G, De Angelis R, Frova L, et al. MIAMOD: a computer package to estimate chronic disease morbidity using mortality and survival data. Comput Methods Programs Biomed. 1994;44(2):99-107. [DOI] [PubMed] [Google Scholar]
- 7. Verdecchia A, Capocaccia R, Egidi V, Golini A. A method for the estimation of chronic disease morbidity and trends from mortality data. Stat Med. 1989;8(2):201-216. [DOI] [PubMed] [Google Scholar]
- 8. U.S. Census Bureau. 2017 National Population Projections Datasets. https://www.census.gov/data/datasets/2017/demo/popproj/2017-popproj.html. Published 2017. Accessed October 8, 2021.
- 9. Mariotto AB, Zhang F, Buckman DW, et al. Characterizing trends in cancer patients’ survival using the JPSurv software. Cancer Epidemiol Biomarkers Prev. 2021;30(11):2001-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dawood S, Broglio K, Ensor J, et al. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 2010;21(11):2169-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanoue LT, Tanner NT, Gould MK, et al. Lung cancer screening. Am J Respir Crit Care Med. 2015;191(1):19-33. [DOI] [PubMed] [Google Scholar]
- 13. Krist AH, Davidson KW, Mangione CM, et al. ; for the US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(10):962-970. [DOI] [PubMed] [Google Scholar]
- 14. Ganesh A, Katipally R, Pasquinelli M, et al. Increased disparities in patients diagnosed with metastatic lung cancer following lung CT screening in the United States. Clin Lung Cancer. 2022;23(2):151-158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available through the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program at https://seer.cancer.gov/data-software/datasets.html and the U.S. Census Bureau at https://www.census.gov/data/datasets/2017/demo/popproj/2017-popproj.html.