Abstract
Although adults aged 65 years or older make up a strong majority of cancer patients, their underrepresentation in cancer clinical trials leads to the lack of representative data to guide evidence-based therapeutic decisions in this patient population. The Trial Design Working Group, convened as part of the workshop titled, Engaging Older Adults in the National Cancer Institute Clinical Trials Network: Challenges and Opportunities, recommended study designs and design elements that could improve accrual of older adults in National Cancer Institute–funded clinical trials. These include trials that are specifically designed to enroll older adults, trials that include a cohort of older patients (parallel cohort, stratified cohort, or embedded cohort), and trials with pragmatic design elements to facilitate enrollment of older adults. This manuscript provides brief descriptions of the recommended designs, examples of successful trials, and considerations for implementation of these designs.
As with any clinical trial, the scientific questions and trial objectives should drive the study design, the selection of endpoints and intervention, and eligibility criteria. When designing trials that include older adults, the heterogeneity of fitness levels is an important consideration as fitness can influence accrual rates and outcomes. Appropriately incorporating geriatric assessments can help identify the optimal subset of older patients for inclusion and minimize selection bias. Incorporating pragmatic design elements to reduce the burden on trial participants as well as on accruing sites and retaining essential elements to ensure that the main goal of the trial can be accomplished can enhance enrollment without compromising the integrity of trials.
Older adults are underrepresented in cancer clinical trials. Studies conducted over several decades have demonstrated that although a strong majority of cancers occur in individuals aged 65 years or older, only approximately one-third of participants in trials are in this age group (1-4). Consequently, practitioners lack representative data for evidence-based treatment development and care plans. Care teams may not always have optimal data guiding therapeutic decisions in older patients. To this end, the American Society of Clinical Oncology (ASCO) convened a subcommittee to develop recommendations to improve evidence base for treatment for older adults. Two of the subcommittee’s recommendations included the use of clinical trials and leverage research designs (5). Despite these efforts, older adults continue to be underrepresented in cancer clinical trials.
More recently, the National Cancer Institute (NCI) convened a Trial Design Working Group to consider and recommend specific trial designs and design features that could facilitate and enhance accrual of older adults to NCI-funded clinical trials. The overarching goal was to consider and recommend trial designs and design elements that may positively impact accrual of older adults. The working group developed a set of guiding principles and recommendations, which are summarized in the current manuscript.
As the National Institutes of Health (NIH) policy states, individuals of all ages (including children and older adults) must be included in human subjects research supported by NIH unless an acceptable justification for their exclusion is provided. It is not expected that every study will include all age groups; however, exclusion based on age should only be considered when inclusion of certain age groups is precluded by the scientific aims of the study. As such, applicants for NIH funding should clearly describe and fully justify the distribution of individuals who will be included in the research. We strongly urge clinical investigators and sponsors to consider the design strategies recommended in this article to enhance older adult participation in clinical trials to ensure that they are well represented in cancer clinical trials to generate evidence base for treatment for benefitting this priority population.
Guiding Principles
Whenever older patients are relevant to the disease under study, use of specific trial designs that have been shown to be effective at increasing accrual of older adults should be a priority. The scientific questions and trial objectives should drive the study design, the selection of endpoints and intervention, and eligibility criteria. The heterogeneity of fitness levels must be considered in trial design as fitness can influence relative accrual rates and outcomes. Incorporation of the ASCO and Friends of Cancer Research guidelines for modernizing eligibility criteria where appropriate can promote pragmatic design elements, may enhance accrual for older patients, and make trial results more generalizable (6). Clinical trials design should also consider the role of geriatric assessment (GA) measures for capturing important data, especially when a hypothesis can be tested for better understanding the impact on outcomes.
A challenge when designing trials inclusive of older patient is the assignment of fitness or functional status. This is often a subjective assessment, and opportunities to develop tools for objective fitness measures are needed to advance this component of clinical trials design. Validation of such tools requires a range of less intense to more intense therapeutics and a range of less fit to more fit patients participating in the clinical trials. Additionally, clinical trial endpoints vary depending on the purpose of the trial and the patient population to be studied. Using a multidomain assessment of multiple factors relevant to older patients can be incorporated into the designs to describe enrolled patients with greater granularity or to define trial eligibility. Further, GA measures can be included to guide treatment allocation, to evaluate outcomes important to older adults, and to identify older adults at highest risk for adverse events or symptomatic toxicity. There is no consensus on a minimum dataset because the preferred GA measures to include in a study depend on the intended use of the assessment, the goals of the trial, the patient population, and the treatment setting. However, a general guiding principle is, whenever possible, to use established and validated measures and to minimize patient burden. Magnuson et al. (7) provide more comprehensive guidance for incorporating GA into NCI clinical trials.
Recommended Trial Designs and Design Features
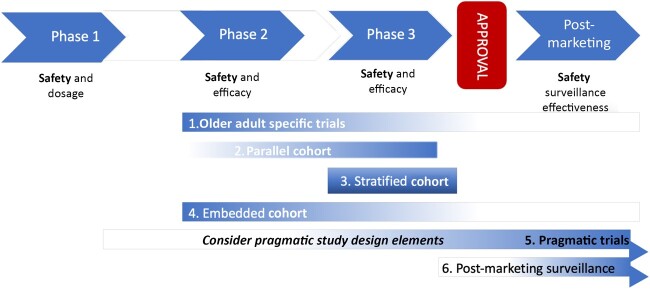
The working group identified 5 trial design strategies that could help facilitate improved enrollment and evaluation of therapies in older patients. These include 1) trials that are specifically designed to enroll older adults; 2) trials that include a parallel cohort of older patients; 3) trials that include stratified (or blocked) randomization; 4) trials with an embedded cohort of older patients; and 5) trials with pragmatic design elements to facilitate enrollment of older adults. Most of these designs focus mainly on the phase II to phase III spectrum of the typical drug or therapeutic development process (Figure 1). Depending on the phase of study, the focus may be more on safety or on efficacy. Therefore, the age-related issues must be viewed in the context of the design considerations relevant to the purpose of the trial. The best design to use depends on the scientific question and the clinical setting of interest.
Figure 1.
Possible points of integration of recommended study designs.
Older Adult–Specific Trials
Older adult–specific trials are trials that enroll only older adults above a specified age level and can conceivably employ a variety of study designs. In addition to the age requirement, statistical design considerations are essentially the same as for all clinical trials. Depending on the scientific question to be answered, the trial may be a single-arm trial or a randomized trial comparing outcomes between treatment arms. Single-arm and randomized trials may be focused on establishing whether a treatment is superior to some other treatment or whether a treatment of interest is good enough compared with a known treatment and could be used instead of the known treatment. Such design considerations are age agnostic.
An advantage of older adult–specific trials appears to be in accruing a greater proportion of those with more advanced age (Table 1). For example, a comparison of Alliance for Clinical Trials in Oncology breast cancer trials that focused only on older patients showed that in the older adult–specific trials, those aged 75 years and older represented 26% of enrolled patients, whereas they represented less than 10% of older patients enrolled onto age‐unspecified trials (8).
Table 1.
Recommended designs or design features
| Study design and features | Recommended for trials where | Pros | Cons |
|---|---|---|---|
| Older patient–specific | |||
|
|
|
|
| Parallel cohort | |||
|
|
|
|
| Stratified (or blocked) randomization | |||
|
|
|
|
| Embedded cohort | |||
|
|
|
|
| Pragmatic clinical trial | |||
|
|
|
|
Older adult–specific trials may be important because sometimes the treatments being evaluated are different than for younger populations. For example, in a randomized controlled trial, the therapy used for the control arm in younger patients may not be appropriate for older patients. Older adult–specific trials may be required to adequately evaluate safety and tolerability of regimens that are already approved but that have limited data for older adult populations. Evaluation of GA-guided treatment may the best leverage for the older adult–specific trial. For example, GA can be tested as a tool to guide treatment intensification or de-intensification while maintaining patient-centric therapeutic goals within an older adult–specific trial. Older adult–specific trials may not be necessary if standard-of-care treatment for the older and younger adult population does not substantially differ, and no differential treatment effects in toxicity or in other clinical outcomes between younger and older individuals are expected. However, given slower accrual rates for older patients compared with younger, efforts to ensure adequate representation is required if not using an older adult–specific trial. Additionally, whenever the outcome differs by age group, older adult–specific trials may be preferred.
An example of successful age-specific randomized controlled clinical trials is illustrated by the approach taken in the NCI Clinical Trials Network (NCTN) chronic lymphocytic leukemia portfolio. The median age for chronic lymphocytic leukemia is 71 years, and only 5%-11% are younger than age 55 years (9). However, the accrual rate for the younger patients is substantially higher, and completion of accrual to similarly sized studies is typically sooner than for the older age group. Moreover, up until 2019, the preferred standard initial chemotherapy for older and younger patients differed substantially. Thus, the NCTN launched 2 separate trials. E1912 accrued patients aged 18-70 years and compared standard fludarabine, cyclophosphamide, and rituximab with ibrutinib (10). Simultaneously, the NCTN developed and launched protocol A041202 for those aged 65 years and older (11). In A041202, the control arm was bendamustine with rituximab, a regimen suited for older patients, whereas fludarabine, cyclophosphamide, and rituximab regimen is not. The underlying assumptions for effect size differ in the younger populations, and this informed the statistical parameters for the 2 trials. E1912 accrued 354 patients over 39 months, and A041202 accrued 547 patients over 42 months. Additionally, follow-up time differed between the 2 trials. The time to reach the primary progression-free survival endpoint for the younger patients in E1912 was 33.6 months compared with 38 months for the older patients in A041202. Although these studies could have been done as 1 trial, designing a single trial with sufficient power and incorporating different control arms would have required substantially more efforts, and the trial conduct would have been more operationally challenging. The study results would have been potentially delayed waiting for accrual and endpoint maturation for all patients. Thus, separate older patient–specific trials can in some cases have clear advantages over other approaches when definitive treatment-defining information is the study goal.
Another example of a successful older patient–specific trial is the Cancer and Leukemia Group B (CALGB, now part of the Alliance for Clinical Trials in Oncology) trial 49907, a randomized trial evaluating the noninferiority of capecitabine compared with standard chemotherapy (either cyclophosphamide with methotrexate and fluorouracil or cyclophosphamide with doxorubicin) as adjuvant therapy for older women with early stage breast cancer (12). This trial was designed specifically to assess the effect of adjuvant chemotherapy in older women and evaluated an oral agent that would be preferable to older women compared with younger women. Of patients enrolled on CALGB 49907, 60% were aged 70 years or older, a much higher number than would be expected in an age-unspecified trial. Additionally, data related to quality of life, functional status, patient preferences, and treatment adherence collected on CALGB 49907 shed light on previously limited knowledge on experience of older women undergoing chemotherapy for breast cancer.
Parallel Cohort Design
Parallel cohort design allows inclusion of a separate cohort (or cohorts) of patients of special interest that may not be considered appropriate for inclusion in the study primary analysis but instead conducted in parallel to the primary trial and analyzed separately. In the context of improving accrual of older adults, a cohort of older adult patients could be included and designed as a single-arm study or a randomized study, with modified dosing or schedule, to evaluate safety and feasibility and to estimate clinical outcomes of this patient population. Such a strategy can provide evidence in the case of a positive randomized trial that the parallel cohort of older adults not included in the primary analysis may benefit from the new treatment. Importantly, this strategy can inform the toxicity and dose adjustments required for best tolerance to a treatment. Similar to the older adult–specific trials, this design should be considered when differences in tolerance to the treatment are expected and that outcomes may differ from the primary study population. For example, this design may be appropriate when limited data exist for older adults on prior therapies, and thus a different dosing or schedule of the regimen may be needed for older adults. The parallel cohort design may also promote inclusion of GA and endpoints of specific importance for older adults. A parallel cohort allows for separate design (can be similar or different from the primary cohort), conduct, and reporting of results, so that completion of the reporting of 1 cohort does not necessarily depend on the other. It allows for flexibility of research aims and endpoints with appropriate power for the endpoints of interest.
An example to highlight the parallel cohort approach is the Alliance trial A041703: a phase II study of inotuzumab ozagamicin followed by blinatumomab for PH-negative CD22-positive B-lineage acute lymphoblastic leukemia in newly diagnosed older adults or adults with relapsed or refractory disease (NCT03739814). This phase II trial evaluates the safety and activity of the regimen in 2 cohorts. One cohort is adult patients with refractory disease. A second cohort is for patients aged 60 years or older with newly diagnosed disease. The rationale for the 2 cohorts was that there was limited data for older patients because of historical exclusion from trials and a need for more tolerable regimens in the first-line setting for older adults who were transplant ineligible. The parallel cohort design was appropriate because of the necessity for different eligibility criteria needed for the 2 cohorts and expectations that tolerability and outcomes may be dissimilar. This separation into 2 cohorts allowed for appropriate power in both cohorts to evaluate 1-year event-free survival. Additionally, there is an operational efficiency in this case where enrolling sites can open 1 trial instead of 2 trials. The Study Design Working group considers this design to be a valuable option, particularly when enrolling older adults for a specific disease or regimen is expected to be challenging.
A common situation amenable to parallel cohort studies is one where patients who are ineligible for the primary study could instead be included in the research by enrolling into a parallel cohort. This can allow for modified treatment or other adoptive approaches to minimize toxicity and preserve adequate dosing if possible. An example of a trial using this parallel cohort approach is the GO2 phase III randomized trial, which evaluated reduced intensity chemotherapy in older and frail patients with advanced gastroesophageal cancer (13). The main trial cohort included patients deemed fit for chemotherapy who were randomly assigned to 1 of the 3 dose levels of oxaliplatin and capecitabine, and patients whose indication for chemotherapy was determined uncertain by the patient and the clinician were enrolled into a parallel cohort and were randomly assigned to either best standard of care or the lowest dose level of oxaliplatin and capecitabine of the main cohort. The parallel cohort design for patients who are otherwise ineligible can provide needed data on safety and efficacy of modified treatment regimens for more diverse populations. This applies not only to older adults but also to those with comorbidities or those from underrepresented backgrounds who are more commonly excluded because of comorbidities or other health conditions.
Stratified (or Blocked) Randomization
In randomized phase II and III studies, stratified or blocked randomization by key factors minimizes the imbalance in these factors between the treatment arms. Relevant to trials that include older adults, where age and fitness status of the patients are known prognostic factors, randomization should be stratified by age and fitness status to ensure balance between the study arms. Validated fitness tools with known cut points should be used for stratification. In addition to minimizing imbalances between arms because of these factors, the design creates important opportunity to explore outcomes by random assignment between patient groups defined by age or levels of fitness. Because the study sample size is determined to satisfy the primary endpoint requirements for the totality of the trial, these additional questions may not be powered to address hypotheses specifically about older adult patients. This design may, however, be used to enable improved accrual of older patients if a required stratum-specific enrollment target for older adult patients were to be specified up front. One potential limitation to this strategy is that the trial would need to remain active to enrollment, and the study analysis would, accordingly, need to wait until stratum-specific accrual was achieved. This could delay trial conclusion and thus, potentially, the dissemination of the study results. Therefore, the specification that a study must meet age or fitness stratum–specific accruals should only be considered if there is strong rationale to support stratum-specific accruals and that doing so would improve study validity, even at the potential cost of longer time to trial completion.
Difficulties in enrolling older patients may occur if differences in the burden of trial participation are prevalent in the specific disease and treatment setting. In such cases, if stratum-specific enrollment targets are included in the design, incentives to alleviate the direct and indirect costs of trial participation may be incorporated.
Embedded Cohorts
An embedded cohort design is another strategy to consider. This approach typically involves the inclusion of a single-arm substudy cohort within the main study or a separate study alongside a randomized trial. Although random assignment of the embedded substudy cohort is technically possible, extreme caution is needed to ensure that the second random assignment does not inadvertently bias or confound the results of the main study. Patients enrolled in the embedded cohort may be required to provide an additional assessment, additional data, biospecimen collection, or more follow-up care compared with nontrial care. This design is especially advantageous for correlative studies, to study feasibility of enrolling and providing protocol-specified care, or to try to determine the prevalence of 1 or more adverse events. For example, CALGB 361006 (14) and CALGB 361101 (15) used embedded cohorts to evaluate the feasibility of performing GA and explore the association between baseline GA and clinical outcomes in older adults with acute myeloid leukemia. This strategy is generally not intended to establish efficacy of a new treatment given that typically no randomized comparison to a control treatment is included, though there are certain situations where single-arm designs can accomplish this. Moreover, as with any protocol that involves eligibility criteria and patient consent, selection bias may be influential in the interpretation of study findings.
Streamlining Trials and Pragmatic Trial Elements
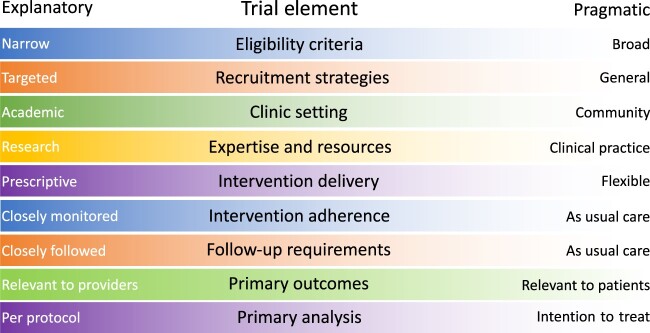
It is essential that trials be developed that are flexible, faster, simpler, and less expensive yet produce high-impact results that seamlessly integrate with clinical practice. This entails decreasing the regulatory burden, increasing efficiency of data collection, and focusing on the essential endpoints. For older patients, incorporating these features into a given trial can translate into easier participation. Pragmatic elements relevant to older patients include decreasing traveling time and time spent in the cancer center and considering procedures that can be conducted remotely vs those that must be conducted at the site. This allows for increased flexibility and enhances generalizability of results by virtue of enrolling and retaining older participants in well-designed and focused trials. The Pragmatic Explanatory Continuum Indicator Summary 2 highlights trial design characteristics that are considered pragmatic (16). These include broad eligibility criteria; broad recruitment strategies (eg, using electronic health records); generalizable settings (eg, community clinics); limited research organization or resources required (ie, studying the intervention in the context of routine clinical care); flexibility in intervention schedules and adherence; required follow-up as per usual care; focused patient-relevant endpoints; and primary analysis based on intention to treat (Figure 2). Focus on essential endpoints can help keep sample sizes reasonable to achieve timely trial completion. The NCI Clinical Trials and Translational Research Advisory Committee has set forth recommendations consistent with the working group recommendations. Strategies such as remote or virtual consent options, use of test results obtained during clinical care, limiting research-only biospecimens, including adaptive design elements in trials, and streamlining operational burdens could help increase accrual of older adults. These approaches have been taken during the COVID-19 pandemic, and some are recommended to be considered for continuation.
Figure 2.
Pragmatic trial spectrum.
An example of successful incorporation of some of these features is the NCI-funded University of Rochester Cancer Center 13059 trial, entitled A Geriatric Assessment Intervention for Patients Aged 70 and Over Receiving Chemotherapy or Similar Agents for Advanced Cancer: Reducing Toxicity in Older Adults (17). In this study, older adults with aging-related conditions, as measured by GA, were enrolled onto a cluster randomized study (practices were randomized) to evaluate whether a GA-based care model could reduce symptomatic toxicities compared with usual care. The trial successfully completed accrual of 718 older patients as planned. The study research question was important and appropriate for older patients with aging-related conditions. Also, there were patient-centered procedures (eg, patients were able to complete patient-reported outcomes over several days at home). Ongoing and continuous input from patient and community oncology partners and inclusion of investigators with aging-related expertise help ensure that a trial is developed that meets the needs of those affected by cancer.
Accrual of older adults to clinical trial is essential to the goal of decreasing suffering and death due to cancer. Specific trial design considerations pertinent to the aims of any given study can be employed to foster enhanced older patient accrual. Appropriately incorporated GAs can help identify patient subsets and characteristics for focused study and minimize selection bias. The recommendations provided in this paper are to provide insights into how clinical trials designs can be used to increase older adult accrual across the entire clinical trials enterprise. Incorporation of modernized eligibility criteria and GAs are tools that should be carefully selected in the context of specific study goals. Minimizing burdensome barriers is essential. These include number of clinic visits and duration and the financial impact of the trial on the patient and their family. Some degree of pragmatism should be considered while retaining essential elements to ensure that the main goal of the study can be accomplished. GAs can be performed in the office to understand better the frailness, fitness, and vulnerability of a patient and could be an initial time point to discuss potential clinical trials with the patients. Results of the assessment can provide objective criteria for patient selection and reduce potential selection bias inherent in subjective fitness assignment.
From the meeting workshop, input from stakeholders clearly reminded the working group of the important impacts that cancer has on a person’s individual life across a broad range of areas. This includes the core of physical, psychosocial, spiritual, sexual, and cultural experience important to life’s meaning. For older-adult cancer patients, the question of quality and longevity should not be minimized. Therefore, understanding the impact of using GAs and how a specific research design meets those needs is critical, if cancer care for older patients is to be meaningfully addressed for these patients.
From the patients’ perspective, incorporating pragmatic features to increase flexibility will make trial enrollment more attractive to older patients. Specific examples include increasing flexibility in how treatments are delivered (eg, providing more options for dose reduction) or increasing flexibility in terms of where the trial is offered (eg, although the main site might be an academic center, it allows patients to go to a local site to have their blood collected, for imaging or even for an infusion, or to have a home nurse perform some of the infusions). There are many more ways to make trials more flexible that would really help increase enrollment, and many cancer patients do not have the luxury of patience, so let’s make this a reality sooner rather than later.
Although the NCI does not fund phase IV trials, postmarketing studies may be conducted by NCI as confirmatory studies for agents that have provisional or accelerated marketing approval status by the US Food and Drug Administration. Studies with specific regulatory intent are conducted in collaboration with an industry partner. However, most studies conducted by NCI with approved or experimental agents do not have specific regulatory intent but rather are intended to inform the optimal treatment approach for specific disease settings or specific patient population such as older adults with cancer.
Funding
No funding was used for the generation of this manuscript.
Notes
Role of the funder: Not applicable.
Disclosures: None exist.
Author contributions: Conceptualization: JLR, SM, JU, MH, JF, SL, JP, ED, ME, KD, WT, HK, TMW, MSS, AJ, RFL; Writing: JLR, SM, JU, MH, JF, SL, JP, ED, ME, KD, WT, HK, TMW, MSS, AJ, RFL.
Disclaimers: None.
Prior presentations: None.
Contributor Information
Jennifer Le-Rademacher, Division of Clinical Trials and Biostatistics, Mayo Clinic, Rochester, MN, USA.
Supriya Mohile, Department of Medicine, University of Rochester Medical Center, Rochester, NY, USA.
Joseph Unger, Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Matthew F Hudson, Prisma Health, Greenville, SC, USA.
Jared Foster, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Rockville, MD, USA.
Stuart Lichtman, Previously MSKCC, New York, NY, USA.
Jane Perlmutter, Gemini.
Efrat Dotan, Department of Hematology/Oncology, Fox Chase Cancer Center, Philadelphia, PA, USA.
Martine Extermann, Moffitt Cancer Center, Tampa, FL, USA.
Kevin Dodd, Division of Cancer Prevention, National Cancer Institute, Rockville, MD, USA.
William Tew, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Heidi Klepin, Department of Medicine, Wake Forest School of Medicine, Winston-Salem, NC, USA.
Tanya M Wildes, Cancer & Aging Research Group, St Louis, MO, USA.
Mina S Sedrak, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, CA, USA.
Aminah Jatoi, Department of Oncology, Mayo Clinic, Rochester, MN, USA.
Richard F Little, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Rockville, MD, USA.
Data Availability
Not applicable.
References
- 1. Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061-2067. [DOI] [PubMed] [Google Scholar]
- 2. Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22(22):4626-4631. [DOI] [PubMed] [Google Scholar]
- 3. Murthy VH, Krumholz HM, Gross CP, et al. Participation in cancer clinical trials race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720-2726. [DOI] [PubMed] [Google Scholar]
- 4. Unger JM, Coltman CA, Crowley JJ, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol. 2006;24(1):141-144. [DOI] [PubMed] [Google Scholar]
- 5. Hurria A, Levit L, Dale A, et al. ; for the American Society of Clinical Oncology. Improving the evidence base for treating older adults with cancer: American Society of Clinical Oncology statement. J Clin Oncol. 2015;33(32):3826-3833. [DOI] [PubMed] [Google Scholar]
- 6. Magnuson A, Bruinooge SS, Singh H, et al. Modernizing clinical trial eligibility criteria: recommendations of the ASCO-Friends of Cancer Research performance status work group. Clin Cancer Res. 2021;27(9):2424-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Magnuson A, VanderWalde N, McKoy JM, et al. Integrating geriatric assessment measures into National Cancer Institute clinical trials. J Natl Cancer Inst Monogr. 2022; doi: 10.1093/jncimonographs/lgac021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dao D, Zemla T, Jatoi A, et al. Older‐patient‐specific cancer trials: a pooled analysis of 2,277 patients (A151715). Oncologist. 2019;24(6):e284-e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Julio D, Neus V. Chronic lymphocytic leukemia in young individuals revisited. Haematologica. 2014;99(1):4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360(20):2055-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hall PS, Swinson D, Cairns DA, et al. ; for the GO2 Trial Investigators. Efficacy of reduced-intensity chemotherapy with oxaliplatin and capecitabine on quality of life and cancer control among older and frail patients with advanced gastroesophageal cancer: the GO2 phase 3 randomized clinical trial. JAMA Oncol. 2021;7(6):869-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klepin HD, Ritchie E, Major-Elechi B, et al. Geriatric assessment among older adults receiving intensive therapy for acute myeloid leukemia: report of CALGB 361006 (Alliance). J Geriatr Oncol. 2020;11(1):107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ritchie EK, Klepin HD, Storrick E, et al. Geriatric assessment for older adults receiving less-intensive therapy for acute myeloid leukemia: report of CALGB 361101. Blood Adv. 2022;6(12):3812-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. doi: 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]
- 17. Mohile SG, Mohamed MR, Xu H, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. Lancet. 2021;398(10314):1894-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.