Abstract
The use of biomimetic models of the glomerulus has the potential to improve our understanding of the pathogenesis of kidney diseases and to enable progress in therapeutics. Current in vitro models comprise organ-on-a-chip, scaffold-based and organoid approaches. Glomerulus-on-a-chip designs mimic components of glomerular microfluidic flow but lack the inherent complexity of the glomerular filtration barrier. Scaffold-based 3D culture systems and organoids provide greater microenvironmental complexity but do not replicate fluid flows and dynamic responses to fluidic stimuli. As the available models do not accurately model the structure or filtration function of the glomerulus, their applications are limited. An optimal approach to glomerular modelling is yet to be developed, but the field will probably benefit from advances in biofabrication techniques. In particular, 3D bioprinting technologies could enable the fabrication of constructs that recapitulate the complex structure of the glomerulus and the glomerular filtration barrier. The next generation of in vitro glomerular models must be suitable for high(er)-content or/and high(er)-throughput screening to enable continuous and systematic monitoring. Moreover, coupling of glomerular or kidney models with those of other organs is a promising approach to enable modelling of partial or full-body responses to drugs and prediction of therapeutic outcomes.
The global prevalence of kidney failure is increasing and this trend is expected to continue1,2. The primary causes of kidney failure include diabetes mellitus, hypertension, glomerulonephritis and cystic kidney disease2. Glomerulopathies are often direct causal or contributing factors in kidney failure and have limited treatment options3,4. Although haemodialysis can replicate aspects of kidney filtration function ex vivo and improve some of the symptoms of kidney failure, this intervention does not treat the root cause of the problem and is associated with very high morbidity and mortality as well as poor quality of life. The lack of therapeutic options to prevent the onset and progression of glomerular disease is related to limited understanding of the pathological mechanisms5. Efforts to investigate these mechanisms are hampered by a lack of suitable in vitro models, which makes the glomerulus extremely difficult to study. A grand challenge lies in closely mimicking the cellular and architectural complexity of the tissue6.
As the kidney is a site of excretion of drugs and their metabolites, kidney function directly influences drug disposition and indirectly affects drug efficacy and safety7. A reliable in vitro model of the glomerulus would therefore not only enable progress in the field of kidney therapeutics, but also in pharmacology in general. The development of precise in vitro human glomerular models will probably improve our understanding of the cells that comprise the kidney filters and facilitate the generation of disease models to guide therapeutic discovery and treatment options. Appropriate models will also enable high(er)-throughput screening (HTS) for glomerular toxicity, particularly for toxicants that act on podocytes or glomerular endothelial cells.
Tissue engineering has emerged as a promising tool to develop new and improved in vitro models. This technology combines synthetic materials, biologic compounds and cellular components to emulate the physiological functions of a tissue or organ8. Organ-on-a-chip technology has been used to model the glomerulus, study the physiology of the nephron, test drug safety and model diseased nephrons9. Strategies such as 3D scaffolding (including culture of glomerular cells on both sides of biocompatible membranes) and the generation of organoids are promising approaches to replicating complex glomerular structures. In this Review, we discuss current approaches to the replication of the glomerulus in vitro with a focus on organ-on-a-chip, scaffolding and organoid technologies. We also provide an outlook on future directions of research, including the use of emerging 3D biofabrication technologies.
The renal corpuscle
The kidney filters the blood to excrete toxins and toxicants, maintains acid–base and body volume homeostasis, blood pressure, electrolyte balance, calcium and phosphate metabolism and erythropoiesis10. The normal human kidney contains approximately one million nephrons, although this number can vary over a broad range10. The initial component of the human nephron, the glomerulus, is a specialized bundle of capillaries that serves as a barrier for filtration of plasma proteins (FIG. 1a)11. The glomerular filter retains blood cells and high molecular weight proteins in the circulation but allows smaller molecules such as water, urea, glucose and electrolytes to be freely filtered12. To function properly, each glomerulus must be integrated with the circulation. The afferent arteriole carries blood into the glomerulus and leads into the afferent vascular chamber (AVC), a space 1.6-fold greater than the efferent vascular chamber (EVC). From the AVC, non-branching conduit vessels deliver blood into filtration capillaries13. First-order vessels converge into the EVC, which leads to the efferent arteriole13. The efferent arteriole supplies the tubule with oxygen and nutrients, before draining into the kidney venous system and ultimately the vena cava inferior. The vascular–tubular relationships are complex with the efferent vessels often contributing to the peritubular capillaries of a number of nephrons.
Fig. 1 |. The glomerulus in health and disease.

a | The healthy adult glomerulus encapsulated in Bowman’s capsule. The juxtaglomerular apparatus (JGA) is located next to the vasculature of the glomerulus, enabling macula densa cells to detect sodium chloride concentrations within the distal tubule and communicate accordingly with the granular cells of the afferent arteriole to regulate renin release and hence filtration rates and blood pressure. The afferent arteriole delivers blood to a capillary network that is surrounded in large part by the glomerular basement membrane (GBM). There are regions of the capillaries, however, that have no GBM separating them from the mesangium. Mesangial cells and the matrix they produce provide structural support for the glomerular tuft. The endothelial layer is fenestrated and is covered by a negatively charged glycocalyx made up of proteoglycans and adsorbed plasma proteins. The tri-layered filtration barrier consists of podocyte foot processes, GBM and capillary endothelial cells. Neighbouring podocyte foot processes interdigitate and are connected by slit diaphragms to form a barrier that restricts the passage of large molecules from the capillaries into the Bowman’s space. b | In patients with diabetic kidney disease, the glomerulus is characterized by reduced resistance of the afferent capillary and increased resistance of the efferent arteriole (not shown) as well as a thickened GBM, podocyte foot process effacement (that is, loss of structural features that results in spreading out), mesangial cell proliferation, increased mesangial matrix deposition and some loss of glycocalyx components. c | Patients with glomerular hypertension show a decreased capillary diameter, GBM thickening, podocyte foot process effacement, mesangial cell proliferation, increased mesangial matrix deposition and glycocalyx abnormalities. d | Patients with glomerulonephritis also show a decreased capillary diameter, podocyte foot process effacement, mesangial cell proliferation, increased mesangial matrix deposition and glycocalyx abnormalities as well asthepresence of neutrophilsand, insomeforms, depositionofimmune (antigen–antibody) complexes. The antigen that is involved in immune complexes can be either a circulating antigen or a component of the GBM or podocytes. In disease states, loss of podocytes results in regions of glomeruli that lack a filtration barrier. This loss manifests clinically as proteinuria.
The juxtaglomerular apparatus is located between the afferent arteriole and the distal tubule and is important for tubuloglomerular feedback14. It is composed of macula densa cells, granular cells and the mesangium, which consists of mesangial cells and the matrix that they produce. Macula densa cells detect chloride concentration in the distal tubule lumen and signal to granular cells to release renin, which is synthesized in these cells15. The mesangial cells are irregularly shaped with processes that extend towards the glomerular basement membrane (GBM), a basal layer between the podocytes and vascular endothelium16. The mesangium provides structural support to the glomerular tuft and the matrix fills the spaces between mesangial cells and the GBM17. Microfibrils in the matrix contribute to the contractile properties of the mesangial cells and enable modulation of capillary flow and glomerular ultrafiltration surface area18. The genes Gata3, Pdgfrb and less commonly Plvap, Prkca, Art3 and Nt5e have been used to identify mature mesangial cells19,20 whereas expression of platelet-derived growth factor-β has been used to identify immature mesangial cells21. Damage to the mesangium can cause severe illness characterized by mesangiolysis and glomerular sclerosis. Mesangiolysis, degeneration of mesangial cells and dissolution of the mesangial matrix as well as glomerular sclerosis caused by thickening of the basement membrane and expansion of the mesangial matrix are characteristics of diabetic kidney disease22. The mesangial area also undergoes thickening in response to the increased glomerular pressure that characterizes glomerular hypertension23.
Together, podocytes, the GBM and capillary endothelial cells form the glomerular filtration barrier (GFB). This highly selective structure filters molecules based on size, charge and transcapillary pressure24. Small molecules are freely filtered, whereas those >70 kDa are not filtered and cations are more permeable than anions. The endothelial surface of the glomerular capillary wall is fenestrated with transcytoplasmic holes of 70–100 nm. The glomerular endothelial cells express markers such as CD31, Emcn, Flt1, Fbln2, Pecam1 and Ehd3, among others19,20. The filtration rate is dependent on the area of fenestration, as the size of the hole restricts protein passage25, and on a charge barrier that results from the glycocalyx that lines the endothelium. Damage to the endothelium can result in proteinuria and ultimately kidney failure even if the other layers of the filtration barrier are initially intact26.
The GBM is a sheet of complex mesh consisting of laminin-11 (also known as laminin α5β2γ1), collagen type IV network, nidogen and heparan sulfate proteoglycans27 that forms a selective barrier that separates the vasculature from the urinary space28. GBM assembly is a complex process that involves several steps of maturation in which substitution of laminin and collagen isoforms occurs in a coordinated but temporally and spatially separated manner29. Mutations of collagen or laminin genes result in protein leakage across the GBM (Alport syndrome and Pierson syndrome, respectively)30. Glomerular hypertension has also been associated with GBM alterations that lead to proteinuria23.
Podocytes are atypical epithelial cells with a large cell body, major extending processes and foot processes. These highly polarized elements appear during cell differentiation and account for podocyte function31. The foot processes interdigitate with those of adjacent podocytes to form a filtration barrier. Slit diaphragms (basolateral cell junctions ~40 nm in width) between the foot processes are important for the selectivity of the barrier32. Approximately 40 signature podocyte genes and proteins that are important for maintenance of the GFB have been identified, including nephrin (NPHS1), podocin (NPHS2), Wilms tumour protein (WT1) and short transient receptor potential channel 6 (TRPC6)20,31,33. Congenital nephrotic syndrome (CNS) can be caused by mutations in NPHS1, NPHS2 or WT1 that affect GFB integrity, resulting in proteinuria and albuminuria34. Upon injury, podocytes undergo foot process effacement due at least in part to disruption of their actin structure or actin-binding proteins35. Diseases such as diabetes, hypertension and glomerulonephritis are associated with structural changes to the glomerulus (FIG. 1b–d).
The glomerulus is enclosed in Bowman’s capsule, which is lined on its parietal side with squamous epithelial cells containing actin filaments that have important roles in many glomerular diseases36. The visceral lining is composed of podocytes that surround the glomerular tuft. Filtrate from the blood collects in the Bowman’s space between the visceral and parietal epithelial layers36. The glomerular filtration rate (GFR) reflects the flow of ultrafiltrate of plasma from the glomerulus into Bowman’s space, and is the average of the sum of the approximately two million glomeruli of the two adult kidneys. Filtration is driven by a counterbalance between the mean transcapillary hydraulic pressure gradient and the mean transcapillary oncotic pressure. The difference in hydraulic pressure inside the glomerular capillary and the Bowman’s space creates the transcapillary hydraulic pressure gradient. This force, generated by pumping of the heart, pushes water and other solutes out of the capillaries. The mean transcapillary oncotic pressure is determined by the difference in plasma protein concentration inside and outside the capillary. As large proteins remain inside the normal capillary, this force opposes filtration.
In vitro glomerular modelling
The application of advanced in vitro human tissue models in research provides exceptional insight into the behaviour of cellular microenvironments37. In contrast to in vivo models, the conditions in which an in vitro model develops can be easily manipulated. These models provide the potential to perform a vast number of studies and easily measure their outcomes. In addition, newly developed technologies employed in the creation of in vitro models have enhanced their accuracy, enabling them to be used in drug screening and disease modelling. Nonetheless, the accuracy of a model depends on several variables. For instance, the cells employed in a glomerular model have great relevance to its physiological characteristics as they will account for the filtration properties of the model.
Ideally, cells will not only be derived from healthy tissue, but also from the tissue of patients with pathologies of interest to the study. Primary cells closely resemble the physiological state and recapitulate mature stages without additional genetic modifications but are difficult to obtain and expand in culture while maintaining the differentiated phenotype38. Immortalized cells are easier to culture and can proliferate indefinitely, but might not replicate the physiological state and often show lower expression of key genes than do primary cells10,39. Human primary cells and human pluripotent stem cells (hPSCs)40 are therefore preferred to conditionally immortalized cells41–44, although the use of both cell types in the same model is not unusual6,45. hPSCs have an almost indefinite capacity for self-renewal and are genotypically representative of the donor. However, further research is needed to achieve their total differentiation into mature phenotypes. The use of cells from animals, such as mice and rats, has also been evaluated46,47; however, species differences limit their application for human modelling48. These differences are evident at the genetic and morphological levels. Moreover, knockout mice do not fully recapitulate the effects of mutations that underlie human genetic kidney diseases49. No ideal cell source for glomerular models exists, and researchers must determine the optimal source and characteristics for each model and consider the limitations of the cells used when interpreting their results.
Podocytes are often the limiting factor in glomerular modelling owing to their lack of proliferative capacity and high specificity; therefore, a reliable method for obtaining physiologically relevant podocytes is necessary to accelerate research. The development of a method for the differentiation of hPSCs into podocytes was an important step towards the establishment of a source of human podocytes for glomerular models9. Subsequent studies have focused on fine-tuning podocyte differentiation from hPSCs by identifying and targeting the signalling pathways that are involved in the intermediate steps of this process50. The resulting differentiated podocytes show higher expression of NPSH1 and NPSH2 genes than immortalized podocyte cell lines50, suggesting that they might be more physiologically relevant for the replication of GFB structure and function. Nevertheless, further analyses are required to ensure that these cells show the differentiation and maturation that is required to enable their use in glomerular modelling. Filtration quality and specificity depends on the cells that form the GFB and the GBM. Disruptions or alterations in endothelial fenestration size, glycocalyx and GBM composition, podocyte primary and secondary foot processes or slit diaphragm size could have major consequences for the filtration process. Thus, special attention must be paid to model and properly characterize cells in vitro.
The interactions between cells within the model, as well as the way that they arrange in the volumetric space and interact with the extracellular matrix (ECM), also influence model performance. In the past, static 2D culture was the predominant model system. 2D cultures are easier to maintain than 3D cultures but can more easily evoke appreciable deviations in cells than their in vivo equivalents (TABLE 1). A lack of reliability caused by cell dedifferentiation and senescence in 2D culture is a major drawback of planar models51,52. In addition, the 2D static environment causes the cells to lose key functional components such as transporters and enzymes with roles in energy metabolism. The results obtained using static 2D systems are therefore not readily applicable to cells in the native tissues.
Table 1 |.
Advantages and disadvantages of current in vitro models of the glomerulus
| Model type | Advantages | Disadvantages |
|---|---|---|
| 2D | Well-established Cost-effective |
No dynamic stimuli Poor predictive accuracy Physiologically inaccurate |
| On-a-chip | Improved physiological accuracy through compartmentalization Can simulate fluid flows and other dynamic cues Potential for multi-organ modelling |
Limited 3D cell–cell and cell–ECM interactions Poor variety of biomaterials for fabrication Insufficient match of structural properties |
| 3D scaffolds | Fully 3D structures with biological relevance Can accurately replicate the GBM and ECM microenvironment Can use ECM-derived biomaterials |
No dynamic stimuli Limited physiological resemblance Poorly developed for the glomerulus |
| Organoids | 3D structures with physiological relevance Biological accuracy |
No dynamic stimuli Poor maturation resulting in resemblance to early stages of kidney development Limited cell–ECM interactions |
ECM, extracellular matrix; GBM, glomerular basement membrane.
3D culture settings have advantages such as the use of ECM-like biomaterials that promote cell support, signalling and morphogenesis and help maintain cell phenotypes to more closely resemble the native tissue53. Nevertheless, conventional 3D cell culture has many challenges, including optimization of the tissue–tissue interface, the development of biomaterials with suitable mechanical properties and ensuring the correct distributions of nutrients and waste products54–57. In the past decade, research has focused on the development of newer technologies to overcome these problems and create high-fidelity structures. On-a-chip platforms, biomimetic membranes, 3D hydrogel-based scaffolds, organoids and combinations of these approaches hold great promise for mimicking the complexity of the glomerulus in vitro (Supplementary Table 1).
On-a-chip platforms
The intrinsic ability of on-a-chip platforms to simulate fluid flows is an important asset when modelling the glomerulus. Tissues that are cultured on one or both sides of a thin surface, such as a porous membrane coated with ECM-like proteins, are often referred to as 2.5D models58,59. In these models, the cells are not fully encapsulated as in 3D cultures, but an additional spatial component exists compared with 2D cultures. 2.5D models have added value as the flow can generate cyclic mechanical forces and shear stresses like those that occur in the glomerulus due to blood pressure and flow of filtrate through the barrier45,60. Shear forces are important for all regions of the nephron, as shown in kidney tubule on-a-chip models61. Podocytes are highly sensitive to shear stress62 and appear to change their shape and cytoskeletal architecture in a singular manner to regulate shear forces within the glomerulus under altered conditions. Pressure gradients affect filtration rates28 and shear stresses affect cell polarization and its functions within the capillary tuft and the filtration barrier6.
In 2.5D systems, fluid flows can be adjusted to model the physiological parameters of transcapillary hydraulic and oncotic pressures that regulate GFR in vivo. Furthermore, the creation of multilayer systems enables filtration barriers to be added between channels, providing a good simulation of permeability. An additional value of microfluidic systems is the ability to successfully maintain glomerular cultures in the long term (up to 1 month)6,63,64. This success is closely related to the fluidic dynamic forces that provide the mechanical cues necessary to avoid cellular dedifferentiation and advancing polarization. Nevertheless, this technology still very much resembles a planar culture as the cells are seeded in 2D in each of the layers or compartments. Although these models are usually described as glomerulus-on-a-chip, they depict a simplified biomimetic version of the GFB formed by podocytes and endothelial cells. They do not incorporate the complex in vivo architecture of the glomerulus or the mesangium.
In one glomerulus-on-a-chip model, mouse endothelial cells and podocytes were co-cultured by seeding them on opposite sides of a porous polycarbonate membrane that was coated with basement membrane extract, which included laminin, collagen type IV, entacin and heparan sulfate65 (FIG. 2a). A microfluidic channel with increasing diameter in the inlet–outlet direction was created to enable building of pressure in a physiological-like manner to model glomerular hypertension and enable investigation of the mechanisms that lead to hypertension-induced glomerular sclerosis. Mimicking hypertension caused the podocytes to change their shape towards a spindle, fibroblast-like form. In addition, pathological increases in fluid flow damaged the permeability of the filtration barrier due to disruption of the slit diaphragms (characterized by assessment of NPHS1 and NPHS2 expression). This study provided proof of concept for the creation of a GFB-on-a-chip in healthy states and states characterized by glomerular hypertension.
Fig. 2 |. On a chip models of the glomerulus.

a | A classic chip assembly in which podocytes and endothelial cells are cultured on opposite sides of an artificial membrane. In the glomerulus, effective filtration pressure depends on the capillary hydrostatic and oncotic pressures and Bowman’s capsule pressure, which is normally very low. Endothelial cells are also affected by blood flow shear force. This chip device can recreate capillary pressure by supplying perfusion flow in the upper microchannel and introducing mechanical forces. Modification of pressure enables this device to be used to model healthy states and conditions associated with glomerular hypertension65. b | An alternative approach that avoids the need for an artificial membrane involves placing whole glomeruli in a chip63. In response to time in culture and fluidic stimulation, the glomeruli lose their spherical shape, spread out across the gel and begin to synthesize new basement membrane. c | A glomerulus on a chip has also been created by seeding glomerular endothelial cells directly on top of podocytes that are cultured on a collagen type I matrix6. The cultured cells synthesize basement membrane, and the collagen matrix separates the channel containing the cells from the filtrate collection channel. Part a adapted with permission from REF.65. Part b adapted with permission from REF.63.
Another model uses a four-channel chip composed of polydimethylsiloxane (PDMS)45. The two central channels are separated by a 50-µm-thick PDMS membrane with 7-µm pores to enable filtration. Cyclic suction is applied to the two lateral channels to create stretching forces to replicate the circulatory cyclic stress that is experienced by glomerular cells. Human primary endothelial cell-derived and hPSC-derived podocytes are seeded on opposite sides of the PDMS membrane in the central channels. The podocytes express markers that are characteristic of mature podocytes (NPHS1, NPHS2 and WT1) and the levels of these proteins increase in response to fluidic stimulation. The podocytes also show enhanced foot processes upon fluidic flow and cyclic mechanical strain. The expression levels of specific podocyte proteins are similar in hPSC-derived podocytes and immortalized podocyte lineages, but the distribution of proteins on the cell surface is more clustered in the hPSC-derived cells. The researchers also exposed their model to the anticancer agent doxorubicin to study drug-induced nephrotoxicity and showed dose-dependent disruption of the podocyte layer and cell extrusion in the luminal channel.
Glomerulus-on-a-chip models can also be created without using artificial material to simulate the filtration membrane. For example, one method incorporates several mouse glomeruli in a multichannel microfluidic chip63 (FIG. 2b). Individual glomeruli are placed on one of the PDMS channels to mimic the capillary lumen and the other is perfused with a low shear flow to resemble the capsular space. After 3 days of culture, the podocytes and endothelial cells respond to the shear stress by synthesizing new GBM. This model, which was exposed to high glucose to mimic diabetic kidney disease, provides a new approach to the synthesis and use of GBM-on-a-chip. Unfortunately, it did not recapitulate the 3D structure of the glomerulus because the glomeruli spread over the surface of the chip to form two flat layers. In addition, the use of mature glomeruli limits scalability.
An alternative approach to develop chips without a synthetic interface involves seeding human primary endothelial cells on top of human podocytes that are seeded on a collagen type I interface6 (FIG. 2c). This method allows the cells to synthesize basement membrane with size selectivity similar to that of native GBM. However, the model is limited by incomplete polarization of the podocytes as they are not exposed to a luminal space. In addition, slit diaphragm formation is not fully evidenced as nephrin is expressed throughout the surface of the podocytes and not only in the contact points between cells. The researchers used a commercially available system of 384-well plates containing arrays of three-channel chip configurations. This high-throughput system allowed them to analyse 2,000 independent chips. Their glomerulus-on-a-chip platform was applied successfully to model puromycin aminonucleoside nephrotoxicity, glucose-induced damage and membranous nephropathy.
A study that combined on-a-chip technology, 3D architecture and biomaterials, incorporated extruded topographic hollow fibres (h-FIBERs) to establish a dynamic culture of Lewis rat primary vein endothelial cells and mouse podocytes64. Key to the novelty and success of this study was the generation of hollow spindle-shaped nodes in the alginate microfibres. The nodes were designed to allow the co-culture of endothelial cells and podocytes with a microconvex topography that emulated the native topographical cues while allowing perfusion. The researchers showed enhanced podocyte interdigitation in the microtopographied areas of the nodes, supporting the key importance of microenvironmental cues for accurate cellular morphology and functions.
On-a-chip technology has great potential for drug testing and kidney disease modelling66. In particular, on-a-chip systems and co-cultures have achieved higher throughput, although this aspect has not yet been perfected. A limitation of currently available on-a-chip technologies is the use of only a few cell types. For example, the available models do not generally incorporate mesangial cells, which are key for structural support and endocrine glomerular cell communications67,68. Nonetheless, steps towards combining several cell types, 3D architecture and microfluidics have been taken. For example, a combined model was generated that included kidney organoids cultured in a microfluidic chip69. The fluidic shear stress exerted on the surfaces of the organoids improved the maturation of the podocytes and enhanced their glomerular vascularization. This method could potentially be a trend-setter in the next generation of kidney and glomerulus-on-a-chip models.
PDMS is currently the preferred substrate for microfluidic manufacturing because of its ease in handling, apparent biocompatibility, high gas permeability, chemical sensitivity, long shelf-life and low cost63,70. However, in the absence of treatment with plasma, surfactants or coatings, PDMS has drawbacks that hamper its widespread use in drug toxicity screening71. For example, the permeability and hydrophobicity of PDMS makes the material prone to absorption or adsorption of small molecules and certain biomolecules72. This issue limits reliable assessment of drug concentration exposures73. Although the cellular adhesive properties of this silicone-based polymer are not outstanding, they can often be enhanced by oxygen plasma treatment or coatings based on ECM components to which cells can bind (such as fibronectin or laminin)53,65,74. An alternative solution is the use of thermoplastic chips that are less prone to molecule absorption or adsorption than PDMS because the materials used possess inherent robustness with resistance to chemicals and mechanical deformation75,76. However, the unmatched mechanical properties between PDMS or thermoplastics and the kidney microenvironment directly influence the cell migration and differentiation characteristics on the artificial substrates.
Notably, there is a lack of variety in common manufacturing procedures for PDMS. Soft lithography is the most often used technique, but the architectures obtained are rather simple, consisting of microscale linear planar channels separated by thin membranes and thus limiting the final configuration77. Vat polymerization 3D printing has now been introduced as a way to fabricate complex 3D structures using PDMS and other polymers78. This technology could potentially replace soft lithography as the preferred approach for generation of organ-on-a-chip devices as it provides more flexibility and control over the manufactured structures.
Membranes and 3D scaffolds
Unlike most on-a-chip systems, 3D scaffold-based approaches provide cells with a basic biocompatible support to promote their growth, differentiation and proliferation. However, in many cases the addition of a third dimension leads to the risk of disregarding the importance of vascular fluidic dynamics, including the advantageous role of shear stress shown in on-a-chip devices. Research on 3D scaffolding has progressed in two directions. The first focuses on mimicking the glomerular micromembranes by direct replication of the different barrier layers that separate the cell types41–43. The second is based on the use of ECM-like biomaterials or decellularized ECM to build cell-embedding hydrogels44,79,80. Cell cultures on top of membranes and hydrogels are not always considered 3D cultures, as the cells tend to form a monolayer. However, podocytes and glomerular endothelial cells cultured within hydrogels have been shown to migrate and assemble into 3D colonies with cell–cell anatomical relationships similar to those seen in glomeruli43,44,79,80.
Progress in assembling a microporous biocompatible membrane to replicate the GFB has been made using electrospinning. This technique enables the development of a thin layer of interlaced nanoscale-diameter fibres that could accurately model the GBM (FIG. 3a). Very similar methodologies have been used to build electrospun membranes of collagen type I41 and bladder decellularized ECM42. Co-culture of human immortalized endothelial cells and podocytes on opposite sides of these membranes result in glomerular models in which podocytes developed appendages that resembled foot processes. Surprisingly, cells that were seeded on bladder decellularized ECM membrane do not remain as separated monolayers, but migrate through the membrane42. As these studies focused on the technological aspects of establishing membranes to enable co-culture of podocytes and glomerular endothelial cells, data on the characteristics of the cultured cells, including expression of genetic markers, slit diaphragms and polarization are limited.
Fig. 3 |. Membranes and 3D scaffolds for glomerular modelling.

a | Electrospinning can be used to build a porous biomimetic collagen membrane on which glomerular endothelial cells and podocytes can be seeded. The porosity and composition of the membrane enables intimate cell–cell interactions and cell migration41. b | The glomerulus can also be modelled by culturing endothelial cells, mesangial cells and podocytes in a collagen hydrogel44. The hydrogel is seeded with cells and then assembled by thermal-induced polymerization. The cultured cells assemble to form 3D glomerular morphology with foot processes.
Gelation and posterior vitrification of collagen or layer-by-layer assembly of collagen and polyacrylic acid have also been investigated as methods for producing GBM-mimicking membranes81,82. In one study, a collagen vitrigel membrane was used for co-culture of murine glomerular endothelial cells and mesangial cells81. Another study demonstrated that layer-by-layer assembly of an artificial GBM using polyacrylic acid and collagen enabled the permeability of the membrane to be modified by precise tuning of thickness, uniformity and nanoscale porosity82. However, the aim of this study was to manufacture an artificial GBM rather than a biomimetic model of the glomerulus.
One could conclude that the studies discussed above demonstrate methods for artificially manufacturing GBM, rather than mimicking glomerular tissue. However, the scope of these techniques becomes wider if the potential for the use of artificial GBMs in on-a-chip modelling systems is considered. Biological micromembranes could substitute for synthetic PDMS or polycarbonate barriers used in co-culture microfluidic systems to achieve a more native-like microenvironment with enhanced filtration properties and avoid the use of additional coatings. Culturing on both sides of a membrane also has limitations. For instance, cells will almost never achieve a 3D disposition and their crosstalk might be restricted by the existence of a physical barrier.
Bulk hydrogels are slightly more versatile than artificial porous membranes as they allow free movement of cells in a 3D environment. An added value of hydrogels is that more than two cell types can be co-cultured in the same setting with flexibility in their spatial arrangements (FIG. 3b). For example, a tri-culture of human condition ally immortalized glomerular endothelial cells, mesangial cells and primary podocytes was established in a collagen I–fibronectin hydrogel with the aim of identifying signalling routes between these cells44. The cells assembled into glomerular-like structures with podocyte foot processes encompassing endothelial cells, and the culture system was successfully used to mimic glomerulosclerosis mediated by transforming growth factor-β. However, the study did not provide insights into cell physiology or nuanced characterization of signature markers. Fibrin hydrogels reinforced by electrospun polyglycolic acid fibres have also enabled the co-culture of human podocytes and glomerular endothelial cells43. In this case, cells cultured within the bimodal scaffolds self-assembled into structures with glomerular-like architecture within 3 days of culture.
Hydrogels can be obtained quite straightforwardly by dissolving a hydrophilic polymer in an aqueous solvent and casting the solution79. A wide variety of synthetic and natural materials can be used as the polymeric basis of hydrogels. For tissue engineering in general, and kidney tissue engineering in particular, some polymeric biomaterials stand out owing to their biocompatibility and ability to reconstruct the microenvironment. For glomerular tissue engineering, it is pertinent to stress the importance of using ECM-derived materials.
Collagen and collagen/Matrigel hydrogels containing kidney cells have been used to engineer tissues that exhibit glomerular structures upon maturation44,80. However, improved cell adhesion and morphology have been reported with the use of decellularization approaches. Decellularization of whole organs has been proposed as a method for preserving the native cell microenvironment and hence improve the maintenance of in vivo cell properties in vitro83. This approach involves chemically or physically removing the cellular components of a tissue to leave only the components of the ECM. Decellularization conserves not only the micro-architecture and macro-architecture of the ECM, but also the protein domains to which cells establish connections. Scanning electron microscopic images obtained after kidney decellularization show that the glomerular ultrastructure is unaltered and can provide binding domains for glomerular cells84. As differentiation cues are also unaltered, recellularization with human kidney progenitor cells can potentially reconstruct the glomerulus. Moreover, decellularized kidney ECM and methacryloyl-modified kidney decellularized ECM have been used as a base for bioinks for 3D bioprinting of kidney tissue40 and proximal tubules85, offering a range of possibilities in the field of 3D glomerulus biofabrication that are currently under-explored.
Organoids
Organoids represent a scaffold-free approach to 3D cultures. They are self-organizing collections of cells in a spheroid shape that, upon maturation, resemble the native tissues and exhibit organ functions and 3D architecture. Organoids have some inherent advantages over conventional models including the fact that the cells are in close proximity to a diverse range of cell types in a complex 3D environment. Crosstalk among these cells can result in enhanced resemblance to podocytes in vivo compared with conventional 2D culturing methods. A 3D environment is not only crucial during differentiation but also important for the maintenance of the differentiated state, as organoid-derived podocytes dedifferentiate upon plating in a 2D environment51.
Organ functions that can be mimicked in organoids include, but are not limited to, specific signalling pathways and metabolic pathways. However, important functions of the glomerulus such as blood filtration across the GFB and regulation of blood flow rate and filtration surface by the glomerular arterioles and mesangium cannot be reproduced in organoids because they lack an inlet–outlet for the vasculature. Despite this limitation, organoids are a powerful tool that can be used as tissue models to elucidate key processes in glomerular development, disease and drug-induced toxicity, as well as drug efficacy testing. In addition, insight into their development might ultimately lead to regenerative therapies86. However, the restricted maturity of organoids presents a challenge that remains to be overcome.
Substantial progress has been made since the development of the first human kidney organoids in 2014–2015 (REFS87–90). Kidney organoids have been generated that contain glomerulus-like tissue, including fenestrated endothelial cells, podocytes and mesangial cells51,91–94. Histological analysis of these organoids showed the presence of a fused tri-laminar GBM separating endothelial cells from tightly interdigitated podocytes, surrounded by a Bowman’s capsule. The architecture and diameter were comparable to the capillary-loop stage of embryological kidney development in vivo. Organoids grown in a dish remain in this immature avascularized state that is highly similar to that of embryonic kidneys52,95 and the early developmental stage has been further confirmed by single-cell RNA sequencing96. Even prolonged culture does not result in increased maturation, suggesting the need for a different approach94. Characteristics of more mature kidneys, such as primary and secondary foot processes, apicobasal polarization with basal actin and nephrin accumulation and slit diaphragms, only appear upon further development of the model by implantation into a host91,93,94,97, the application of shear stress-inducing fluid flow69 or the addition of exogenous growth factors91,98 (FIG. 4). In addition to a certain degree of maturation and size increase, implantation of organoids leads to host vascular invasion of the glomerular tissue without the loss of the pre-existing vascular plexus in the organoids94.
Fig. 4 |. Glomerular tissue organoids with improved maturation and vascularization.
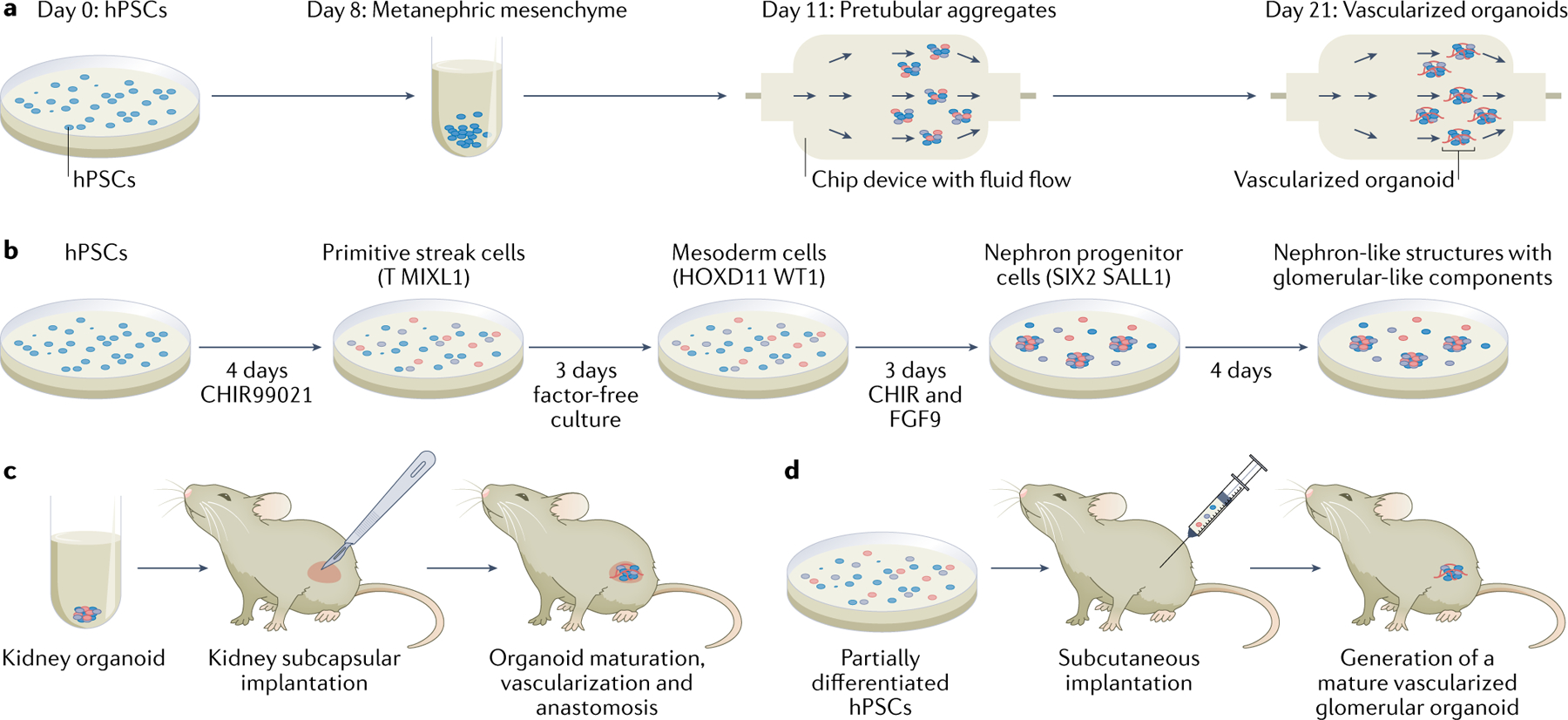
a | Organoids containing glomeruli, tubules and stroma have been derived from human pluripotent stem cells (hPSCs) by subjecting them to a differentiation process using small molecules and growth factors101. The intermediate organoids were exposed to different superfusion flow rates to induce the development of various degrees of vascularization69. A clear difference in the degree of vascularization could be seen between days 8 and 21. The final organoids included glomeruli and tubular regions of the nephron. b | Another protocol to obtain organoids containing glomeruli-like structures involves culturing the cells with a Wnt-signalling activator (that is, an inhibitor of glycogen synthase kinase 3 (CHIR99021)) and recombinant human fibroblast growth factor-9 (FGF9) for set exposure times99. This protocol enables fine-tuning of Wnt signalling, which controls the secretion of vascular endothelial growth factor A by podocytes and results in an on-demand percentage of each segment of the organoid. c | After a certain degree of maturation has been obtained, organoids can be implanted under the kidney capsule of mice to achieve further vascularization and maturation99. d | Vascularized glomerular organoids can also be generated in mice by subcutaneous implantation of partially differentiated hPSCs91. Part a adapted from REF.69.
A study in which hPSC-derived kidney progenitor cells were implanted subcutaneously into mouse hosts showed the development of kidney organoids containing vascularized glomerular tissue91 (FIG. 4d). The presence of red blood cells inside the capillaries of these organoids confirmed that they are vascularized. With the development of blood vessels, the organoid tissue achieved a unique podocyte–capillary interface. The function of the organoids was examined by assessing the route of low-molecular-weight dextran injected into the host circulation. Dextran was found inside a small blood vessel and along the apical surface of the proximal tubule cells, which could indicate effective blood filtration and reabsorption. However, the collecting ducts were sparse or not present in the organoid tissue and a ureter and larger arteries were lacking. Improved maturation and vascularization has also been achieved by implanting organoids under the kidney capsule of mice99 (FIG. 4c). Host implantation is a promising option to enhance maturation beyond the embryological stages and incorporate vascular flow, although the need for an animal host limits the translational potential. Moreover, the extent to which intraspecies differences in embryological development influence the maturation of the organoids is yet to be determined.
An alternative host-free approach to organoid maturation involves application of fluid shear stress by combining organoid culture with organ-on-a-chip methods69 (FIG. 4a). hPSC-derived organoids were cultured on a gelatin and fibrin-coated chip with superfusion of circulating medium. This method increased the abundance of vasculature as well as the maturation of tubular and glomerular cells69. A similar role of flow in glomerular development has been found in zebrafish models100. The ability to promote flow-enhanced development in vitro opens new avenues for investigating kidney organogenesis. However, this method does not ensure that the microvascular networks in the organoids will be perfusable, meaning that the in vitro vascularization is not equivalent to that obtained by transplantation into a host74.
Generation of kidney organoids with a de novo vascular network without the use of a host, exogenous growth factors or shear stress, has been achieved by precisely regulating Wnt signalling to adjust the relative proportions of proximal versus distal segments of the nephron (FIG. 4b)99. Regulation of Wnt signalling in turn regulates podocyte secretion of vascular endothelial growth factor A, which stimulates endothelial cells to form a defined vascular network. The glomerular tissue showed capillary tufts of human origin and size-selective dextran handling, and thus approached a functional filtration barrier. Over time the expression of platelet endothelial cell adhesion molecule increased and the stem cell markers CD34 and KDR decreased, suggesting further differentiation. Although these findings represent a step in the right direction, full maturation of kidney organoids remains an elusive goal.
Organoids rely on their self-organizing capacity to form intricate structures, closely mimicking embryogenesis. This process makes them an attractive model for studying kidney development and congenital glomerular abnormalities by introducing mutations using CRISPR–Cas9 techniques86 or by using patient-derived hPSCs51,101. Several podocytopathies have been modelled using organoids, including autosomal dominant focal segmental glomerulosclerosis owing to podocalyxin mutations87,92, CNS51 and autosomal recessive polycystic kidney disease (ARPKD)99. One such study used organoids generated from patient-derived hPSCs to model Finnish-type CNS caused by a missense mutation in NPHS1 (REF.102). The researchers showed that this mutation resulted in the absence of slit diaphragms in the patient-derived organoids. However, correction of the mutation in the hPSCs using CRISPR–Cas9 gene editing enabled the organoids to form slit diaphragms, confirming the hypothesis that this mutation causes the disease phenotype.
Proof of principle has also been provided for the use of kidney organoids for drug screening. For example, organoids generated from hPSCs derived from patients with ARPKD were used to test the efficacies of thapsigargin and a cystic fibrosis transmembrane conductance regulator inhibitor99. Both drugs blocked
ARPKD cystogenesis in the organoids. These results are consistent with those of previous experiments in other tissue models, strongly demonstrating the potential of organoids for drug screening. In another study, hPSC-derived kidney organoids were cultured and then sieved to isolate organoid-derived glomeruli for further culturing51. The glomeruli were then exposed to increasing doses of doxorubicin for toxicity screening. Following treatment, the glomeruli exhibited a dose-dependent size reduction probably due to apoptosis. The generation of glomerular organoids that mimic glomerular functions and express distinct cellular markers is required to optimize their applicability for pharmacological studies.
Thus organoid technology has the potential to replicate the complexity of the glomerular 3D microenvironment and crosstalk between different cell types. To date, they are the only modelling approach that can self-assemble and mimic the 3D architecture of the kidney and cellular interactions without the need for a scaffold. However, organoids are currently not an optimal in vitro model owing to the inaccessibility of the inner ‘capillary’ areas of the glomerular-like structures and the limited ability to assess their filtration capacity103. This lack of perfusability together with the immaturity of the tissue limit the ability to study the organoid GFB in vitro. This problem will be hard to overcome at the current stage of organoid tissue development. One approach to overcoming some of these limitations would be to use organoids in combination with other technologies such as microfluidic devices69,104 composed of different chambers or submodules that hold separate organoids and allow vascularization, perfusion of the vasculature and measurement of characteristics such as glomerular filtration105.
Alternatively, glomerulus function has been assessed in mouse-implanted organoids that show a certain degree of connection with the vasculature of the host91,99,106. Combining a glass window placed above the implanted organoid with the use of fluorescein-tagged molecules enables imaging of glomerular filtration in real time. Moreover, by adjusting the size of the label molecules used, filtration uptake assays could be used to assess the size selectivity of the GFB99,106. In functional measurements, glomerular tissue organoids are lagging behind on-a-chip approaches in which albumin and inulin permeability assays are already being performed6,45,63–65. Measurement of GFR and single nephron GFR in organoids would require reproduction of glomerular capillary function with an entry and exit for the vessels as well as a way to collect the filtrate for analysis.
Other challenges in the field of kidney organoids include the current dependence on Matrigel as the culture matrix and the variability between protocols in the degree of definition of the medium and in gene expression associated with maturation, nephron pattering and cell proportions. Matrigel supports the growth and long-term culture of organoids, but its murine tumour origin results in inter-batch variability and limits the clinical application of the organoids. However, use of Matrigel is not the only option for organoid culture90,103 and alternative synthetic and bio-derived materials have been proposed107,108. The use of these latter biomaterials could facilitate the translational potential of organoids by reducing costs and minimizing inter-batch variability, advancing research on cell responses to environmental cues and enabling the combination of organoids with other approaches such as organ-on-a-chip and biofabrication109. Many, but not all82,84,88, of the current protocols for organoid culture include the addition of murine embryonic spinal cord to the medium and rely on undefined secreted factors for organoid development83,89. Feeder cells such as mouse embryonic fibroblasts are effective for nurturing the cultures and can be neglected after expansion110. However, their contribution is not fully understood and potentially leads to undesired variability. Efforts are being made to unravel the signalling established between cultured cells and feeder cells with the aim of isolating the secreted factors and using them to supplement the culture medium. Moreover, inter-laboratory, inter-experimental, and inter-clonal variability can have substantial effects on organoid gene expression. High(er)-throughput systems might mitigate the effects of this variability and be a great asset for drug screening48,98. For example, use of a 3D bioprinter to extrude organoids and eliminate the manual process of micro-mass formation has been reported52. This method increases reproducibility and throughput and therefore results in an increased organoid yield.
Another important factor to consider is the long-term stability of organoids. Glomerular tissue-derived organoids have been cultured for longer (52 days)94 than on-a-chip models (30 days)6,64 and cultures using biocompatible membranes or 3D scaffolds (<7 days)43,44. However, longer-term culture does not necessarily ensure higher functionality or maturation and can lead to loss of vascular gene expression as a consequence of loss of podocyte VEGF production as well as hypoxia and metabolic deficit owing to the larger sizes of the generated structures94.
Developments in biofabrication
New biofabrication technologies could potentially be used for automated generation of complex glomerular models using cell-laden biomaterials. 3D bioprinting enables controlled delivery of cells, biomaterials and soluble factors in complex 3D patterns, whereas laser ablation improves the structural accuracy of models with high resolution.
3D bioprinting.
3D bioprinting is being investigated as an approach to assemble microfluidic-based and scaffold-based models with precise cell and biomaterial distributions. Compared with traditional scaffolding technologies, such as plain cell encapsulation in which cells are suspended in a liquid hydrogel precursor that is later moulded and crosslinked, 3D bioprinting offers unprecedented versatility and the ability to pattern cell-laden biomaterials with precise control over their composition, spatial distribution and architecture111–114. 3D bioprinting can therefore achieve detailed and personalizable recapitulation of the fine shapes, structures and architecture of target tissues and organs. Encouraging progress has been made in applying this technology to the fabrication of complex structures such as kidney proximal tubules115 and lung alveoli116. These successes should motivate the application of 3D bioprinting for glomerular modelling.
Bioprinting is an extremely versatile technique that can provide a consistent, repeatable, automated and higher-throughput approach to cell patterning in biomedical engineering applications117 and therefore improve reproducibility in comparison to traditional scaffolding40,44 and organoid cultures52,118. In general, the technology is cost-effective and time-efficient, and stands out for its flexibility in terms of manufacturing and biomaterials119–122. These features make 3D bioprinting an attractive alternative for the fabrication of glomerulus-like structures. However, the strongest attribute of this technology is the capacity to create complex, predefined architectures. Using computer-aided design software, 3D models of the glomerulus can be created and sent to a 3D bioprinter to reproduce the same defined geometric pattern.
Medical imaging techniques such as micro-computed tomography (CT) scans have been combined with artificial intelligence approaches to create models of various organs123. In the future, this approach could be used to create accurate designs of the glomerulus. A micro-CT imaging method for the glomerulus with a resolution of 10 × 10 × 10 μm has been developed124. Scans created using this method could potentially be combined with high-resolution bioprinting to improve the accuracy of glomerular models.
A variety of bioprinting strategies, including inkjet, extrusion-based and light-based technologies117,39, have been used to engineer different types of tissue substitutes and models. This diversity enables the generation of platforms that integrate 3D culture, different materials and mechanical properties and mechanical stimuli. Many of these approaches have been used to reproduce various structures of the human body that contain channels that can be perfused and are composed of different materials and cell types125. Modifying, combining or adapting the methods used to print these tissues could enable the fabrication of a physiologically relevant glomerulus structure.
A method of extrusion bioprinting with sacrificial ink (Pluronic F127) was used to produce a proximal tubule model115 (FIG. 5a). Fugitive filaments were printed at room temperature using the ink and then cast with an ECM-like material composed of fibrinogen and gelatin. The construct was then cooled to 4 °C to liquefy the ink, thereby creating open channels that were subsequently seeded with human proximal tubule cells. The channels were perfused with medium to expose the cells to flow, thereby combining the benefits of 3D culture and on-a-chip devices. This method could potentially be used to improve glomerular models as complex hollow channels like those of the glomerulus could be created using materials laden with mesangial cells or podocytes and then seeded with vascular cells.
Fig. 5 |. 3D bioprinting and multi-photon ablation techniques that could be used to build glomerular models.

Several techniques that have been used to create models of other tissues could be adapted to glomerulus bioprinting. a | Extrusion 3D bioprinting with sacrificial ink involves printing a mould and then casting with an extracellular matrix-like material. The sacrificial ink is then liquefied to create channels that can be seeded with cells. This technology has been used to build models of the proximal tubule by culturing human proximal tubule epithelial cells in a matrix composed of gelatin and fibrinogen115. b | Extrusion bioprinting can also be used to print multiple cell types or materials at the same time with high precision. This technique has been used to build bladder models125. c | Vat polymerization-based bioprinters can print complex hollow structures by exposing a light-sensitive bioink to sequential light patterns to start a photo-crosslinking reaction. d | Multi-photon ablation has been used to precisely remove collagen hydrogel and form a vessel network structure and Bowman’s capsule as a model of the glomerulus141. e | Post-printing processing approaches such as immersion of a hyaluronic acid methacrylate hydrogel structure in a polyion solution of net opposite charge (such as chitosan solution) can be used to shrink constructs to achieve smaller channel sizes that closely emulate the vasculature or other cannular tissues136. Part a adapted from REF.115.
Another approach is to use extrusion or inkjet bioprinting of cell-laden bioinks to create 3D structures126,127 (FIG. 5b). These methods enable bioprinting of bioinks with various viscosity profiles128 and potentially bioprinting of multiple cell types or materials in the same defined space or at the same time using a single bioprinter with multiple bioinks129–131. These techniques have been used to fabricate multi-material bladder constructs125 and multi-cell lineage tissue constructs132. When applied to modelling of the glomerulus, multi-material bioprinting could enable the incorporation of podocytes, mesangial cells and vascular and parietal endothelial cells in a single 3D microenvironment, enabling their simultaneous co-culture.
Given the complexity of glomerular structure, few models have attempted to replicate the cup-like Bowman’s capsule or the capillary tuft. However, advances in light-based biofabrication could enable the manufacture of complex tissue designs, including uneven and concave surfaces6,122. For example, vat photo-polymerization bioprinters can build structures with precise curves and hollow channels116 (FIG. 5c) by projecting images onto the biomaterial or hydrogel precursor to start a photocrosslinking reaction. These bioprinters also have the potential to print with cell-laden bioinks and create multi-material structures133. The final constructs can therefore include bioinks with different mechanical properties and/or cell lines134,135, similar to those reported for extrusion bioprinting modalities. This technique has been used to create small, intertwined channels to model the vascular network of lung alveoli116 and could also potentially be used to create channels resembling the size and entanglement of glomerular capillaries.
Post-processing techniques can be used to achieve better mechanical properties of biomaterials or higher-resolution constructs that more closely resemble physiological structures. For example, we have developed a method to shrink hyaluronic acid methacrylate hydrogel structures with channels that emulate cannular tissues by immersing them in a chitosan solution for up to 24 h. This method achieved a reduction in microchannel diameter of around 49%136 (FIG. 5e). Many bioinks include mixtures of different hydrogels with multiple crosslinking mechanisms; the primary material improves printability, whereas the secondary material undergoes crosslinking after bioprinting to ensure mechanical fidelity137.
Some limitations of 3D bioprinting remain to be overcome before printing of a fully functional glomerular structure can be achieved122,138. For example, extrusion bioprinting is limited by the mechanical strength and viscosity of the bioink, can cause damage to cells due to mechanical and thermal stress137, has low resolution and can be slow. Light-based bioprinting with UV or high-energy lasers can also be harmful to cells, the equipment can be extremely complex and difficult to operate, and the resulting constructs can be small and fragile (potentially resulting in collapse of channels during printing). Moreover, there is a lack of standardized cytocompatible bioinks.
Another challenge is that the diameter of the human glomerulus is only ~200 µm, which demands a tiny feature resolution11,119,139,140. The resolutions of current bioprinting techniques range from several hundred micrometres for extrusion bioprinting to 20 µm achieved by some light-assisted systems119. Only two-photon lithography has achieved accuracy below the micrometre scale, but the resulting constructs are limited in size and the interior architecture resembles cells encapsulated in a 3D environment rather than complex native tissue39,119. As the GBM has a thickness of 325–375 nm39, higher resolutions will be required to achieve a physiologically relevant renal corpuscle model with accurate capillary diameters. However, integration of 3D-bioprinted structures with microfluidic and on-a-chip technologies has the potential to enable the creation of physiologically relevant and accurate glomerulus models.
Laser ablation.
Multi-photon ablation is a technique that uses a laser to remove material. The most structurally accurate biofabricated model of a glomerulus to date was created using multi-photon ablation of channels as small as 10 µm in a collagen hydrogel containing two parallel vessel-like structures that were previously cellularized with human umbilical vein endothelial cells (HUVECs)141 (FIG. 5d). Afferent and efferent channels were also ablated to connect the model glomerulus to the parallel vessels and a pressure differential (~1 mmHg) was applied to induce migration of HUVECs into the lumen of the glomerular channels, resulting in the creation of a fully cellularized glomerular-like structure. However, this approach has not yet been shown to be suitable for use with other cell types such as podocytes and mesangial cells.
In the near future, use of combinations of bioprinting and ablation technologies could lead to the creation of a precise and fully functional model of a human glomerulus that supports cell–cell and cell–ECM interactions and the integration of flows. Such a model could enable crosstalk between cell types in a 3D microenvironment that replicates the native microstructures of the glomerulus. The potential impact of such a model on the understanding of glomerular physiology, as well as the study of pharmacodynamics, pharmacokinetics and toxicity, remains to be determined.
Scalability and standardization
Success in widespread application of in vivo models relies on their scalability and standardization. The generation of models that are suitable for HTS and high-content screening (HCS) is a necessary step towards the large(r)-scale application of tissue-engineered glomerular constructs. The feasibility of HTS is dependent on three main factors, production, maturation and readouts. HCS relies mostly on the combination of automated microscopic imaging analysis and high-throughput techniques.
Production.
The ease and speed of model production is dependent on the specific technique used. In general, microfluidic devices will be the easiest to manufacture and handle, followed by organoids and 3D scaffolded structures. Organoid52 and organ-on-a-chip115 technologies enhanced by bioprinting are somewhat more complex but strong progress is being made. Use of bioprinting to manufacture chips would enable a variety of biological materials to be used instead of the current synthetic alternatives. Bioprinting has already been used to create on-a-chip models of convoluted kidney tubules, demonstrating the suitability of this technique for building perfusable channels compatible with kidney models115. In the case of organoids, bioprinting can aid in standardization of the manufacturing processes52 and permit tight control of the size, geometry and cellular density of the spheroids, leading to improved reproducibility of results118,142. Use of bioprinting could also enable different tissue-specific organoids to be combined to create more sophisticated and larger constructs. Moreover, organoids can be included within bioinks to build constructs in which the cellular building blocks are already organized into semi-mature structures142. Scalability and translation towards personalized medicine will also require standardization of protocols for obtaining and including human cells in any of the proposed models.
Maturation.
Once models are manufactured and seeded with cells, time is required for the cells to mature. Maturation is an essential step but represents a bottleneck regardless of the specific technique used. A more mature tissue will more closely resemble the in vivo tissue but may require a longer maturation period. While the cells are maturing, sufficient nutrients need to be supplied while maintaining O2 and CO2 levels and temperature, and waste products have to be eliminated. Models with an inlet and an outlet are easier to handle and are, therefore, preferred. In addition, the process can be sped up by automating as much as possible, for example by using a liquid handling robot98.
Readouts.
Conventional analytical tools rely on manual sample collection and lead to inevitable system disturbance. Ideally, monitoring systems would be fully integrated into models and analysed continuously in a fully automated fashion over long periods of time143. Multiplexed probes and CRISPR–Cas-9-based approaches can be used for HCS of complex 3D culture systems144. Efforts have also been made to integrate sensors into glomerular models, for example, CRISPR–Cas9-mediated fluorescent tagging of SIX2 and NPHS1 genes has been used to monitor differentiation and maturation of organoids in situ145.
Microfluidic devices can also be designed to enable continuous measurements. We have developed a modular, fully automated system to enable constant monitoring of the biochemical and biophysical parameters of organ-on-a-chip models146,147. A breadboard controls the fluid flow, a label-free electrochemical immune-biosensor records the presence of soluble biomarkers (including albumin, glutathione S-transferase-α and creatine kinase–myocardial band), an optical sensing unit measures changes in pH, temperature and O2 and a mini-microscope is used to monitor morphology. Biomarker sensing was selective and highly sensitive, and we provided proof of concept by applying the system to drug toxicity testing. This system is universal and can be used for a wide range of biomarkers with minor adaptations.
Standardization.
Before high-throughput model systems can be established for applications such as drug testing, an urgent need exists for standardization. Complex models must be stable and well-characterized, and the cells employed must be properly differentiated. Great heterogeneity exists in the cellular markers that are used to classify kidney glomerular cells, particularly those that have undergone a differentiation process. This variety of markers partly reflects the lack of uniformity in cell sources, harvesting procedures, dedifferentiation and re-differentiation protocols and culture conditions. Other than podocin and nephrin, no consensus on characterization markers exists6,9,45,51,52,65,84,120,148. Moreover, diverse signature genes have been reported for mouse20 and human glomerular cells143 and for human organoids50,51,93. The field of glomerular modelling would greatly benefit from the adoption and widespread assessment of consensus markers for future work.
Future perspectives
Despite substantial progress, tissue engineering of complex organs such as the kidney, remains challenging. Kidney and glomerular tissue engineering could potentially benefit from advances in the engineering of other barrier tissues for which 3D modelling is more developed. For example, liver-on-a-chip models have replicated flows through the hepatic lobules by mimicking the hepatic sinusoidal structure149 and a similar setting could be employed for the fenestrated vessels of the capillary tuft. Lung-on-a-chip systems rely on lateral pneumatic chambers to stretch the cultured cells and mimic the effects of inhalation and exhalation150. Similar chips have been used to mimic the pulsations of the glomerular capillaries45,151 and further adaptation could be used to exert pressure on the walls of the vascular chambers and thus mimic glomerular diseases. One of the distinctive aspects of on-a-chip models is their capacity to provide a continuous shear stress and supply of oxygen and nutrients, which increases the probability of obtaining mature and stable cultures of the glomerulus in the long term. However, when upscaling the complexity and the size of tissue analogues, as occurs in 3D cultures and organoids, it is of paramount importance to ensure that oxygen and nutrients are provided by vascularization instead of superperfusion.
Moving from chips towards 3D structures, non-planar membranes have been used for long-term culture of proximal tubule cells in bioartificial kidneys152,153, proving not only that 3D architectures are suitable for prolonged cultures but that this approach may be advantageous for mimicking the glomerulus. The complex interior architecture of the renal corpuscle could benefit from the high resolution of light-based 3D bioprinting techniques115. However, the suitability of 3D bioprinting for glomerular tissue engineering has not yet been extensively assessed.
Importantly, the glomerulus is only a part of the kidney and integrating kidney glomerulus and tubular regions on sequential chips could be of great interest. Filtrate and blood flows could be established simultaneously to mimic glomerular filtration followed by tubular reabsorption. Incorporating mesangial cells in glomerulus chips, either by indirect co-culture or by adding cells directly to the model on the outside of the capillary channel, would also be highly advantageous. Such an approach could potentially result in enhanced stability of the GFB-forming cells and enable autologous modulation of the capillary flow. The inclusion of mesangial cells might also result in more accurate models for diabetes, hypertension and glomerulonephritis, among other genetic and non-genetic diseases affecting the glomerulus. In addition, a Bowman’s capsule-on-a-chip is yet to be accomplished. Such a model might be achieved by combining chips with multiple channels for the GFB and Bowman’s space. A combined approach bringing together a glomerular model with a model of tubular function could ultimately lead to the development of a more complete kidney-on-a-chip that mimics the entire urine production process.
To fully recapitulate the effects of a drug on the human body and evaluate potential toxicity, the effects on key drug-processing organs should be assessed concurrently. A body-on-a-chip that includes models of the blood–brain barrier, brain, lung, liver, heart, skin, gut and kidney has been developed74. Use of such a model provides a promising approach to systemic predictive drug screening in human tissue in vitro.
Conclusions
Kidney glomerular modelling is advancing towards accurate depiction of physiological and pathophysiological states. On-a-chip models provide a dynamic environment for establishing the GFB and enable the application of flow and shear stress to avoid cell dedifferentiation and enhance the expression of nephrin and podocin. However, the GFB is only a small part of the glomerulus and modelling of the GBM is often over-simplified by the use of synthetic materials. Electrospinning has been proposed as a cell-friendly, natural approach to create models of the GBM. Although the resulting membranes are promising in terms of micro-organization and porosity, few examples of the use of this technique exist. Hydrogels containing embedded cells have also been used as a scaffold for developing glomerular tissues. ECM-like and ECM-derived polymers show great promise to sustain glomerular constructs in which both the GFB and the mesangium can be replicated. Organoids can self-assemble to mimic the native 3D architecture of the glomerulus. Host implantation, fine-tuning of Wnt signalling and fluid flow are potential strategies for overcoming maturation issues and achieve vascularization of organoids. The organoid approach is unique because not only the glomerular tissue, but also the other segments of the nephron are replicated. However, ex vivo organoids currently lack inflow and outflow of blood supply and tubular drainage, which are critical for modelling glomerular function.
Glomerular modelling approaches could be combined to avoid forfeiting a dynamic microenvironment for 3D complexity. For example, engineered native-like membranes could be used in on-a-chip systems and organoids can be cultured within chips. Nevertheless, the current lack of standardization and limited scalability in addition to intrinsic technique-related limitations, continue to restrict the potential of these approaches. The use of novel technologies, such as 3D bioprinting, could help overcome these challenges. Application of well-characterized and accurate glomerular models could lead to improved understanding of disease characteristics and the genetic and non-genetic processes that result in pathobiology. In addition, such models could be used for HTS of drug efficacy and toxicity and eventually inform the use of precision medicine approaches for patients with kidney diseases.
Supplementary Material
Key points.
The prevalence of kidney failure is increasing worldwide, and glomerulopathy is often a causal or contributing factor.
The available in vitro models of the glomerulus are suboptimal, which limits their use for interrogation of pathological mechanisms and testing of drugs.
The complexity of the renal corpuscle results in a fragile balance of constituents that can be easily disturbed in pathological situations, leading to irreparable damage.
Initiatives for improving current in vitro biomimetic models of the glomerulus focus on replication of the 3D microenvironment using on-a-chip technology, scaffolds and organoids containing glomerular tissue.
The suitability of emerging biofabrication techniques, such as 3D bioprinting, for glomerular modelling is yet to be fully assessed.
Larger-scale applications of glomerular models will rely on their standardization and effective utility for high(er)-throughput and/or high(er)-content screening.
Drug disposition
In pharmacology, drug disposition refers to the processes of drug distribution and elimination of a drug once it has entered the body.
Microfluidics
The technology used to manufacture devices containing chambers and tunnels in the microscale through which fluids flow or are confined. These devices, commonly known as microfluidic chips, permit fluids to be precisely directed, mixed, separated or manipulated to attain multiplexing, automation and high-throughput systems.
Oxygen plasma treatment
A surface treatment in which oxygen plasma is used to remove impurities and contaminants, as well as to create functional groups to increase hydrophilicity and enhance bonding properties.
Thermoplastics
A group of polymers that melt at high temperatures and harden upon cooling. They can be melted and recast almost indefinitely so are highly recyclable.
Soft lithography
A group of techniques that use elastomeric stamps and moulds to shape materials with micrometre or nanometre resolution.
Vat polymerization 3D printing
A 3D printing technique in which each layer of the construct is created by projecting a different image onto a liquid resin contained in a vat to start the crosslinking reaction.
Hydrogel
Highly crosslinked hydrophilic polymer network that is heavily swollen with water and can be used as a dynamic, tunable, degradable material for growing cells and tissues.
Electrospinning
A manufacturing technique based on the creation of small diameter fibres (nanometres to micrometres) by exposing a polymeric solution to a high-power electric field during its ejection.
Bioinks
Solutions of natural and/or synthetic biomaterials used to create tissue constructs by bioprinting; bioinks usually encapsulate cells.
Extrusion bioprinting
A technique used to create organized tissue constructs by continuously dispensing or extruding a bioink through a nozzle, resulting in 3D structures.
Inkjet bioprinting
A technique used to create organized tissue constructs by rapidly dispensing small droplets in the picolitre volume range.
Feature resolution
The smallest feature size upon which a structure can be built.
Two-photon lithography
A light-assisted bioprinting technique in which excitation for the polymerization of the material is achieved by a femtosecond pulsed near-infrared laser. This technology typically creates high-resolution features with sizes of ~50–100 nm.
Breadboard
In the microfluidic context, a breadboard is a piece of hardware containing built-in microfluidic channels and valves that enable modular assembly of microfluidic chips and fluid routing.
Acknowledgements
The authors gratefully acknowledge research funding from the National Institutes of Health (R00CA201603, R21EB025270, R21EB026175, R21EB030257, R01EB028143, R01HL153857, R01DK72381, R03EB027984, R37DK39773 and UH3TR002155), the National Science Foundation (CBET-EBMS-1936105), the Brigham Research Institute, the Hofvijverkring Visiting Scientist Program, the Dutch Kidney Foundation (18KVP01), Utrecht Institute for Pharmaceutical Sciences and European Union’s Horizon 2020 research and innovation programme WIDESPREAD-05-2018-TWINNING Remodel.
Footnotes
Competing interests
Y.S.Z. sits on the Advisory Board of Allevi, Inc., which however, did not support this work. J.V.B. is co-founder and holds equity in Goldfinch Bio. The interests of Y.S.Z. and J.V.B. were reviewed and are managed by Brigham and Women’s Hospital and Mass General Brigham in accordance with their conflict of interest policies. The other authors declare no competing interests.
Peer review information
Nature Reviews Nephrology thanks the anonymous reviewers for their contribution to the peer review of this work.
Supplementary information
The online version contains supplementary material available at https://doi.org/10.1038/s41581-021-00528-x.
References
- 1.Kambham N, Markowitz GS, Valeri AM, Lin J & D’Agati VD Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 59, 1498–1509 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Saran R et al. US Renal Data System 2018 annual data report: epidemiology of kidney disease in the United States. Am. J. Kidney Dis 73, A7–A8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naqvi R Glomerulonephritis contributing to chronic kidney disease. Urol. Nephrol. Open Access. J 5(4), 00179 (2017). [Google Scholar]
- 4.Ziółkowska H, Adamczuk D, Leszczyńska B & Roszkowska-Blaim M Glomerulopathies as causes of end-stage renal disease in children [Polish]. Pol. Merkur. Lekarski 26, 301–305 (2009). [PubMed] [Google Scholar]
- 5.Moxey-Mims MM et al. Glomerular diseases: registries and clinical trials. Clin. J. Am. Soc. Nephrol 11, 2234–2243 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrosyan A et al. A glomerulus-on-a-chip to recapitulate the human glomerular filtration barrier. Nat. Commun 10, 3656 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonventre JV, Vaidya VS, Schmouder R, Feig P & Dieterle F Next-generation biomarkers for detecting kidney toxicity. Nat. Biotechnol 28, 436–440 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cieslinski DA & David Humes H Tissue engineering of a bioartificial kidney. Biotechnol. Bioeng 43, 678–681 (1994). [DOI] [PubMed] [Google Scholar]
- 9.Musah S, Dimitrakakis N, Camacho DM, Church GM & Ingber DE Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a glomerulus chip. Nat. Protoc 13, 1662–1685 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sánchez-Romero N, Schophuizen CMS, Giménez I & Masereeuw R In vitro systems to study nephropharmacology: 2D versus 3D models. Eur. J. Pharmacol 790, 36–45 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Vimtrup B On the number, shape, structure, and surface area of the glomeruli in the kidneys of man and mammals. Am. J. Anat 41, 123–151 (1928). [Google Scholar]
- 12.D’Amico G & Bazzi C Pathophysiology of proteinuria. Kidney Int 63, 809–825 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Neal CR et al. Novel hemodynamic structures in the human glomerulus. Am. J. Physiol. -Ren. Physiol 315, F1370–F1384 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capasso G, Trepiccione F & Zacchia M in Critical Care Nephrology 3rd edn Ch. 8 (eds Ronco C, Bellomo R, Kellum JA & Ricci Z) 42–48.e1 (Elsevier, 2019). [Google Scholar]
- 15.Arendshorst WJ & Bello-Reuss E in Handbook of Cell Signaling 2nd edn Ch. 318 (eds Bradshaw RA& Dennis EA) 2707–2731 (Academic, 2010). [Google Scholar]
- 16.Maezawa Y, Cina D & Quaggin SE in Seldin and Giebisch’s The Kidney 5th edn Ch. 22 (eds Alpern RJ, Moe OW & Caplan M) 721–755 (Academic, 2013). [Google Scholar]
- 17.Miner JH Renal basement membrane components. Kidney Int 56, 2016–2024 (1999). [DOI] [PubMed] [Google Scholar]
- 18.Rabelink TJ, Heerspink HJL & de Zeeuw D in Chronic Renal Disease Ch 9 (eds Kimmel PL& Rosenberg ME) 92–105 (Academic, 2015). [Google Scholar]
- 19.Chung JJ et al. Single-cell transcriptome profiling of the kidney glomerulus identifies key cell types and reactions to injury. J. Am. Soc. Nephrol 31, 2341–2354 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karaiskos N et al. A single-cell transcriptome atlas of the mouse glomerulus. J. Am. Soc. Nephrol 29, 2060–2068 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arar M et al. Platelet-derived growth factor receptor β regulates migration and DNA synthesis in metanephric mesenchymal cells. J. Biol. Chem 275, 9527–9533 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Kamel KS & Halperin ML in Fluid, Electrolyte and Acid-Base Physiology 5th edn Ch. 9 (eds Kamel KS & Halperin ML) 215–263 (Elsevier, 2017). [Google Scholar]
- 23.Iversen BM & Ofstad J The effect of hypertension on glomerular structures and capillary permeability in passive Heymann glomerulonephritis. Microvascular Res 34, 137–151 (1987). [DOI] [PubMed] [Google Scholar]
- 24.Briggs JP, Kriz W & Schnermann JB in National Kidney Foundation’s Primer on Kidney Diseases 6th edn (eds Gilbert SJ, Weiner DE, Gipson DS, Perazella MA & Tonelli M) 2–18 (Elsevier, 2014). [Google Scholar]
- 25.Satchell SC & Braet F Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am. J. Physiol. Ren. Physiol 296, F947–F956 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haraldsson B & Nyström J The glomerular endothelium: new insights on function and structure. Curr. Opin. Nephrol. Hypertens 21, 258–263 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Dean DF & Molitoris BA in Critical Care Nephrology 3rd edn Ch. 7 (eds Ronco C, Bellomo R, Kellum JA & Ricci Z) 35–42.e2 (Elsevier, 2019). [Google Scholar]
- 28.Pollak MR, Quaggin SE, Hoenig MP & Dworkin LD The glomerulus: the sphere of influence. Clin. J. Am. Soc. Nephrol 9, 1461–1469 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abrahamson DR, st. John PL, Stroganova L, Zelenchuk A & Steenhard BM Laminin and type IV collagen isoform substitutions occur in temporally and spatially distinct patterns in developing kidney glomerular basement membranes. J. Histochem. Cytochem 61, 706–718 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feher J Quantitative Human Physiology 2nd edn 705–714 (Academic, 2017). [Google Scholar]
- 31.Garg P A review of podocyte biology. Am. J. Nephrol 47, 3–13 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Oddsson Á, Patrakka J & Tryggvason K Glomerular filtration barrier: From Molecular Biology to Regulation Mechanisms. Reference Module in Biomedical Sciences (Elsevier, 2014). [Google Scholar]
- 33.Garg P & Rabelink T Glomerular proteinuria: a complex interplay between unique players. Adv. Chronic Kidney Dis 18, 233–242 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moorthy AV & Blichfeldt TC in Pathophysiology of Kidney Disease and Hypertension (ed. Moorthy AV) 1–15 (Saunders, 2009). [Google Scholar]
- 35.Reiser J & Altintas MM Podocytes. F1000Res 5, 114 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kriz W & Elger M in Comprehensive Clinical Nephrology 4th edn Ch. 1 (eds Floege J, Johnson RJ & Feehally J) 3–14 (Mosby, 2010). [Google Scholar]
- 37.Nikolic M, Sustersic T & Filipovic N In vitro models and on-chip systems: biomaterial interaction studies with tissues generated using lung epithelial and liver metabolic cell lines. Front. Bioeng. Biotechnol 6, 120 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang K, Shindoh H, Inoue T & Horii I Advantages of in vitro cytotoxicity testing by using primary rat hepatocytes in comparison with established cell lines. J. Toxicol. Sci 27, 229–237 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Wragg NM, Burke L & Wilson SL A critical review of current progress in 3D kidney biomanufacturing: advances, challenges, and recommendations. Ren. Replace. Ther 5, 18 (2019). [Google Scholar]
- 40.Ali M et al. A photo-crosslinkable kidney ECM-derived bioink accelerates renal tissue formation. Adv. Healthc. Mater 8, e1800992 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slater SC et al. An in vitro model of the glomerular capillary wall using electrospun collagen nanofibres in a bioartificial composite basement membrane. PLoS ONE 6, e20802 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Z et al. Solution fibre spinning technique for the fabrication of tuneable decellularised matrix-laden fibres and fibrous micromembranes. Acta Biomater 78, 111–122 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Tuffin J et al. A composite hydrogel scaffold permits self-organization and matrix deposition by cocultured human glomerular cells. Adv. Healthc. Mater. 8, 1900698 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Waters JP et al. A 3D tri-culture system reveals that activin receptor-like kinase 5 and connective tissue growth factor drive human glomerulosclerosis. J. Pathol 243, 390–400 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musah S et al. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat. Biomed. Eng 1, 0069 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Becker GJ & Hewitson TD Animal models of chronic kidney disease: useful but not perfect. Nephrol. Dial. Transplant 28, 2432–2438 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Hewitson TD, Ono T & Becker GJ Small animal models of kidney disease: a review. Methods Mol. Biol 466, 41–57 (2009). [DOI] [PubMed] [Google Scholar]
- 48.Shankland SJ The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 69, 2131–2147 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Shankland SJ, Pippin JW, Reiser J & Mundel P Podocytes in culture: past, present, and future. Kidney Int 72, 26–36 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Yoshimura Y et al. Manipulation of nephron-patterning signals enables selective induction of podocytes from human pluripotent stem cells. J. Am. Soc. Nephrol 30, 304–321 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hale LJ et al. 3D organoid-derived human glomeruli for personalised podocyte disease modelling and drug screening. Nat. Commun 9, 5167 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Higgins JW et al. Bioprinted pluripotent stem cell-derived kidney organoids provide opportunities for high content screening. Preprint at bioRxiv 10.1101/505396 (2018). [DOI] [Google Scholar]
- 53.Edmondson R, Broglie JJ, Adcock AF & Yang L Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay. Drug Dev. Technol 12, 207–218 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaicharoenaudomrung N, Kunhorm P & Noisa P Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J. Stem Cell 11, 1065–1083 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmid J et al. A perfusion bioreactor system for cell seeding and oxygen-controlled cultivation of three-dimensional cell cultures. Tissue Eng. Part C. Methods 24, 585–595 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lovett M, Lee K, Edwards A & Kaplan DL Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev 15, 353–370 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu H & Humphreys BD Single cell sequencing and kidney organoids generated from pluripotent stem cells. Clin. J. Am. Soc. Nephrol 15, 550–556 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pebworth M-P, Cismas SA & Asuri P A novel 2.5D culture platform to investigate the role of stiffness gradients on adhesion-independent cell migration. PLoS ONE 9, e110453 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langhans SA Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol 9, 6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reiser J & Sever S Podocyte biology and pathogenesis of kidney disease. Annu. Rev. Med 64, 357–366 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jang KJ & Suh KY A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab. Chip 10, 36–42 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Friedrich C, Endlich N, Kriz W & Endlich K Podocytes are sensitive to fluid shear stress in vitro. Am. J. Physiol. Ren. Physiol 291, F856–F865 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Wang L et al. A disease model of diabetic nephropathy in a glomerulus-on-a-chip microdevice. Lab. Chip 17, 1749–1760 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Xie R et al. h-FIBER: microfluidic topographical hollow fiber for studies of glomerular filtration barrier. ACS Cent. Sci 6, 903–912 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou M et al. Development of a functional glomerulus at the organ level on a chip to mimic hypertensive nephropathy. Sci. Rep 6, 31771 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilmer MJ et al. Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol 34, 156–170 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Toda N et al. Crucial role of mesangial cell-derived connective tissue growth factor in a mouse model of anti-glomerular basement membrane glomerulonephritis. Sci. Rep 7, 42114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tung C-W, Hsu Y-C, Shih Y-H, Chang P-J & Lin C-L Glomerular mesangial cell and podocyte injuries in diabetic nephropathy. Nephrology 23, 32–37 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Homan KA et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 16, 255–262 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ashammakhi N, Wesseling-Perry K, Hasan A, Elkhammas E & Zhang YS Kidney-on-a-chip: untapped opportunities. Kidney Int 94, 1073–1086 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanyeri M & Tay S Viable cell culture in PDMS-based microfluidic devices. Methods Cell Biol 148, 3–33 (2018). [DOI] [PubMed] [Google Scholar]
- 72.Gokaltun A, Yarmush ML, Asatekin A & Usta OB Recent advances in nonbiofouling PDMS surface modification strategies applicable to microfluidic technology. Technology 5, 1–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pourmand A et al. Fabrication of whole-thermoplastic normally closed microvalve, micro check valve, and micropump. Sens. Actuators B Chem 262, 625–636 (2018). [Google Scholar]
- 74.Novak R et al. Robotic fluidic coupling and interrogation of multiple vascularized organ chips. Nat. Biomed. Eng 4, 407–420 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shaegh SAM et al. Rapid prototyping of whole-thermoplastic microfluidics with built-in microvalves using laser ablation and thermal fusion bonding. Sens. Actuators B Chem 255, 100–109 (2018). [Google Scholar]
- 76.Gomez-Sjoberg R, Leyrat AA, Houseman BT, Shokat K & Quake SR Biocompatibility and reduced drug absorption of sol-gel-treated poly(dimethyl siloxane) for microfluidic cell culture applications. Anal. Chem 82, 8954–8960 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paoli R & Samitier J Mimicking the kidney: a key role in organ-on-chip development. Micromachines 7, 126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhattacharjee N, Parra-Cabrera C, Kim YT, Kuo AP & Folch A Desktop-stereolithography 3D-printing of a polydimethylsiloxane)-based material with Sylgard-184 properties. Adv. Mater 30, e1800001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang YS & Khademhosseini A Advances in engineering hydrogels. Science 356, eaaf3627 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lü SH et al. Self-assembly of renal cells into engineered renal tissues in collagen/matrigel scaffold in vitro. J. Tissue Eng. Regen. Med 6, 786–792 (2012). [DOI] [PubMed] [Google Scholar]
- 81.Wang PC Reconstruction of renal glomerular tissue using collagen vitrigel scaffold. J. Biosci. Bioeng 99, 529–540 (2005). [DOI] [PubMed] [Google Scholar]
- 82.Pullela SR et al. Permselectivity replication of artificial glomerular basement membranes in nanoporous collagen multilayers. J. Phys. Chem. Lett 2, 2067–2072 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Finesilver G, Bailly J, Kahana M & Mitrani E Kidney derived micro-scaffolds enable HK-2 cells to develop more in-vivo like properties. Exp. Cell Res 322, 71–80 (2014). [DOI] [PubMed] [Google Scholar]
- 84.Du C et al. Functional kidney bioengineering with pluripotent stem-cell-derived renal progenitor cells and decellularized kidney scaffolds. Adv. Healthc. Mater 5, 2080–2091 (2016). [DOI] [PubMed] [Google Scholar]
- 85.Singh NK et al. Three-dimensional cell-printing of advanced renal tubular tissue analogue. Biomaterials 232, 119734 (2020). [DOI] [PubMed] [Google Scholar]
- 86.Yin X et al. Engineering stem cell organoids. Cell Stem Cell 18, 25–38 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Freedman BS et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat. Commun 6, 8715 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morizane R et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol 33, 1193–1200 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takasato M et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Taguchi A et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14, 53–67 (2014). [DOI] [PubMed] [Google Scholar]
- 91.Bantounas I et al. Generation of functioning nephrons by implanting human pluripotent stem cell-derived kidney progenitors. Stem Cell Rep 10, 766–779 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim YK et al. Gene-edited human kidney organoids reveal mechanisms of disease in podocyte development. Stem Cell 35, 2366–2378 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharmin S et al. Human induced pluripotent stem cell-derived podocytes mature into vascularized glomeruli upon experimental transplantation. J. Am. Soc. Nephrol 27, 1778–1791 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van den Berg CW et al. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Rep 10, 751–765 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Geuens T, van Blitterswijk CA & LaPointe VLS Overcoming kidney organoid challenges for regenerative medicine. NPJ Regen. Med 5, 8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Combes AN, Zappia L, Er PX, Oshlack A & Little MH Single-cell analysis reveals congruence between kidney organoids and human fetal kidney. Genome Med 11, 3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garreta E et al. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat. Mater 18, 397–405 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Czerniecki SM et al. High-throughput screening enhances kidney organoid differentiation from human pluripotent stem cells and enables automated multidimensional phenotyping. Cell Stem Cell 22, 929–940.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Low JH et al. Generation of human PSC-derived kidney organoids with patterned nephron segments and a de novo vascular network. Cell Stem Cell 25, 373–387.e9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Serluca FC, Drummond IA & Fishman MC Endothelial signaling in kidney morphogenesis: a role for hemodynamic forces. Curr. Biol 12, 492–497 (2002). [DOI] [PubMed] [Google Scholar]
- 101.Morizane R & Bonventre JV Generation of nephron progenitor cells and kidney organoids from human pluripotent stem cells. Nat. Protoc 12, 195–207 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tanigawa S et al. Organoids from nephrotic disease-derived iPSCs identify impaired NEPHRIN localization and slit diaphragm formation in kidney podocytes. Stem Cell Rep 11, 727–740 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morizane R & Bonventre JV Kidney organoids: a translational journey. Trends Mol. Med 23, 246–263 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim J, Koo B-K & Knoblich JA Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol 21, 571–584 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McGuigan AP & Sefton MV Vascularized organoid engineered by modular assembly enables blood perfusion. Proc. Natl Acad. Sci. USA 103, 11461–11466 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van den Berg CW, Koudijs A, Ritsma L & Rabelink TJ In vivo assessment of size-selective glomerular sieving in transplanted human induced pluripotent stem cell-derived kidney organoids. J. Am. Soc. Nephrol 31, 921–929 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ye S et al. A chemically defined hydrogel for human liver organoid culture. Adv. Funct. Mater 30, 2000893 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Curvello R, Alves D, Abud HE & Garnier G A thermo-responsive collagen-nanocellulose hydrogel for the growth of intestinal organoids. Mater. Sci. Eng C. 124, 112051 (2021). [DOI] [PubMed] [Google Scholar]
- 109.Agarwal T, Celikkin N, Costantini M, Maiti TK & Makvandi P Recent advances in chemically defined and tunable hydrogel platforms for organoid culture. Bio-Des. Manuf 4, 641–674 (2021). [Google Scholar]
- 110.Hynds RE, Bonfanti P & Janes SM Regenerating human epithelia with cultured stem cells: feeder cells, organoids and beyond. EMBO Mol. Med 10, 139–150 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nicodemus GD & Bryant SJ Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. Part B Rev 14, 149–165 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murphy SV & Atala A 3D bioprinting of tissues and organs. Nat. Biotechnol 32, 773–785 (2014). [DOI] [PubMed] [Google Scholar]
- 113.Zhang YS et al. 3D bioprinting for tissue and organ fabrication. Ann. Biomed. Eng 45, 148–163 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gungor-Ozkerim PS, Inci I, Zhang YS, Khademhosseini A & Dokmeci MR Bioinks for 3D bioprinting: an overview. Biomater. Sci 6, 915–946 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Homan KA et al. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci. Rep 6, 34845 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grigoryan B et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 364, 458–464 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mandrycky C, Wang Z, Kim K & Kim DH 3D bioprinting for engineering complex tissues. Biotechnol. Adv 34, 422–434 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lawlor KT et al. Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation. Nat. Mater 20, 260–271 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miri AK et al. Effective bioprinting resolution in tissue model fabrication. Lab. Chip 19, 2019–2037 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heinrich MA et al. 3D bioprinting: from benches to translational applications. Small 15, e1805510 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moroni L et al. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mater 3, 21–37 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li W et al. Recent advances in formulating and processing biomaterial inks for vat polymerization-based 3D printing. Adv. Healthc. Mater 9, 2000156 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu Z, Ng DWH, Park HS & McAlpine MC 3D-printed multifunctional materials enabled by artificial-intelligence-assisted fabrication technologies. Nat. Rev. Mater 6, 27–47 (2020). [Google Scholar]
- 124.Xie L et al. Micro-CT imaging and structural analysis of glomeruli in a model of adriamycin-induced nephropathy. Am. J. Physiol. Ren. Physiol 316, F76–F89 (2019). [DOI] [PubMed] [Google Scholar]
- 125.Zhang K et al. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: an in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater 50, 154–164 (2017). [DOI] [PubMed] [Google Scholar]
- 126.Zhang YS et al. 3D extrusion bioprinting methods. Nat. Rev. Methods Primers 1, 75 (2021). [Google Scholar]
- 127.Li X et al. Inkjet bioprinting of biomaterials. Chem. Rev 120, 10793–10833 (2020). [DOI] [PubMed] [Google Scholar]
- 128.Bishop ES et al. 3-D bioprinting technologies in tissue engineering and regenerative medicine: current and future trends. Genes Dis 4, 185–195 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ravanbakhsh H et al. Emerging technologies in multi-material bioprinting. Adv. Mater 33, 2104730 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu W et al. Rapid continuous multimaterial extrusion bioprinting. Adv. Mater 29(3), 1604630 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kolesky DB et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater 26, 3124–3130 (2014). [DOI] [PubMed] [Google Scholar]
- 132.Xu T et al. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials 34, 130–139 (2013). [DOI] [PubMed] [Google Scholar]
- 133.Miri AK et al. Microfluidics-enabled multimaterial maskless stereolithographic bioprinting. Adv. Mater 30, e1800242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ma X et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl Acad. Sci. USA 113, 2206–2211 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhu W et al. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials 124, 106–115 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gong J et al. Complexation-induced resolution enhancement of 3D-printed hydrogel constructs. Nat. Commun 11, 1267 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee JM & Yeong WY Design and printing strategies in 3D bioprinting of cell-hydrogels: a review. Adv. Healthc. Mater 5, 2856–2865 (2016). [DOI] [PubMed] [Google Scholar]
- 138.Duan B State-of-the-art review of 3D bioprinting for cardiovascular tissue engineering. Ann. Biomed. Eng 45, 195–209 (2017). [DOI] [PubMed] [Google Scholar]
- 139.Fogo AB & Kon V The glomerulus–a view from the inside–the endothelial cell. Int. J. Biochem. Cell Biol 42, 1388–1397 (2010). [DOI] [PubMed] [Google Scholar]
- 140.Aguado BA, Mulyasasmita W, Su J, Lampe KJ & Heilshorn SC Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng. Part A 18, 806–815 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rayner SG et al. Multiphoton-guided creation of complex organ-specific microvasculature. Adv. Healthc. Mater 10, e2100031 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brassard JA, Nikolaev M, Hübscher T, Hofer M & Lutolf MP Recapitulating macro-scale tissue self-organization through organoid bioprinting. Nat. Mater 20, 22–29 (2021). [DOI] [PubMed] [Google Scholar]
- 143.Zanella F, Lorens JB & Link W High content screening: seeing is believing. Trends Biotechnol 28, 237–245 (2010). [DOI] [PubMed] [Google Scholar]
- 144.Li S & Xia M Review of high-content screening applications in toxicology. Arch. Toxicol 93, 3387–3396 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Boreström C et al. A CRISP(e)R view on kidney organoids allows generation of an induced pluripotent stem cell-derived kidney model for drug discovery. Kidney Int 94, 1099–1110 (2018). [DOI] [PubMed] [Google Scholar]
- 146.Zhang YS et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl Acad. Sci. USA 114, E2293–E2302 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aleman J, Kilic T, Mille LS, Shin SR & Zhang YS Microfluidic integration of regeneratable electrochemical affinity-based biosensors for continual monitoring of organ-on-a-chip devices. Nat. Protoc 16, 2564–2593 (2021). [DOI] [PubMed] [Google Scholar]
- 148.Abdallah M et al. Influence of hydrolyzed polyacrylamide hydrogel stiffness on podocyte morphology, phenotype, and mechanical properties. ACS Appl. Mater. Interfaces 11, 32623–32632 (2019). [DOI] [PubMed] [Google Scholar]
- 149.Nakao Y, Kimura H, Sakai Y & Fujii T Bile canaliculi formation by aligning rat primary hepatocytes in a microfluidic device. Biomicrofluidics 5, 022212 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huh D et al. Reconstituting organ-level lung functions on a chip. Science 328, 1662–1668 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tran T et al. In vivo developmental trajectories of human podocyte inform in vitro differentiation of pluripotent stem cell-derived podocytes. Dev. Cell 50, 102–116.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ng CP, Zhuang Y, Lin AWH & Teo JCM A fibrin-based tissue-engineered renal proximal tubule for bioartificial kidney devices: development, characterization and in vitro transport study. Int. J. Tissue Eng 2013, 319476 (2013). [Google Scholar]
- 153.Chevtchik NV et al. Upscaling of a living membrane for bioartificial kidney device. Eur. J. Pharmacol 790, 28–35 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


