Abstract
Objectives
To (i) validate the JIA parent global assessment (parent global) as a health-related quality of life (HRQoL) instrument; (ii) evaluate measurement properties of accepted HRQoL measures relative to those of the parent global; and (iii) assess causal pathways determining parent global scores.
Methods
Data from the Research in Arthritis in Canadian Children emphasizing outcomes (ReACCh-Out) cohort were used. Measurement properties were assessed in 344 patients at enrolment and 6 months later. Causal pathways were tested by structural equation modelling to understand root causes and mediators leading to parent global scores.
Results
Construct validity was supported by Spearman correlations of 0.53–0.70 for the parent global with the Juvenile Arthritis Quality of Life Questionnaire, Quality of My Life health scale (HRQoML), Pediatric Quality of Life Inventory (PedsQL)-Parent, and Child Health Questionnaire (CHQ)-Physical. Exceptions were PedsQL-Child (0.44) and CHQ-Psychosocial (0.31). Correlations were lower (0.14–0.49) with disease activity measures (physician global assessment of disease activity, active joint count, ESR). Responsiveness of the parent global to improvement according to parent ratings (0.51) was acceptable and within the range (0.32–0.71) of that of other measures. Reliability estimates and measurement errors for all measures were unsatisfactory, likely due to the prolonged time between assessments. Causal pathways for the parent global matched those previously reported for HRQoML.
Conclusions
Our results offer support for the parent global as a valid measure of HRQoL for JIA. If confirmed, existing studies using the parent global may be re-interpreted, enhancing our knowledge of HRQoL in children with JIA.
Keywords: Parent global assessment, JIA, health-related quality of life
Rheumatology key messages.
Our results provide preliminary support for the parent global as a measure of HRQoL in JIA.
Further studies are required to confirm our findings.
If confirmed, existing studies using the parent global may be re-interpreted in this light.
Introduction
The JIA parent global assessment (parent global) was introduced with the Childhood HAQ (CHAQ) in 1994 [1]. This is a visual analogue scale (VAS) anchored by the words 0 very well and 10 very poor, and headed by the instruction: ‘Considering all the ways that arthritis affects your child, rate how your child is doing by placing a mark on the line’. The parent global has been included in numerous cohort studies [2–5] and owing to its inclusion in the ACR core set of measures, it has been utilized extensively in clinical trials [6–9]. The parent global is also a component of the Juvenile Arthritis Disease Activity Score (JADAS) and the Juvenile Arthritis Multidimensional Assessment Report (JAMAR) [10, 11]. The formulation of the parent global can be traced in most instances to that provided in the CHAQ or Filocamo’s similarly worded 21-circle numerical rating scale [1, 12].
Despite its extensive use, there has been no formal conceptualization or validation of the parent global, resulting in uncertainty as to what it measures. The term ‘well-being’ was first introduced in the publication of the JIA ACR core set of measures and has been used most often to describe the parent global [2, 6, 9, 10, 12–14]. In turn, well-being may be linked to health, as health is defined by the World Health Organization (WHO) as a ‘state of complete physical, mental and social well-being’ [15]. Abiding by this definition, health-related quality of life (HRQoL) instruments such as the Juvenile Arthritis Quality of life Questionnaire (JAQQ), the Child Health Questionnaire (CHQ), and the Paediatric Quality of Life inventory (PedsQL) assess physical, mental and social domains of health [16–18]. We propose that the parent global [1, 12] is also an assessment of a child’s health as affected by arthritis and should be considered a disease-specific HRQoL measure.
The objectives of the present analyses were: (i) to validate the parent global as a measure of HRQoL in a JIA inception cohort; (ii) to investigate the measurement properties of recognized HRQoL measures in the same cohort to provide context for the performance of the parent global; and (iii) to investigate causal pathways leading to parent global scores.
Methods
Patients and data
Data from the Research in Arthritis in Canadian Children emphasizing Outcomes (ReACCh-Out) cohort were used. Children fulfilling International League of Associations for Rheumatology JIA criteria [19] were enrolled within one year of diagnosis from 2005 to 2010 at 16 Canadian centres. Physician assessments, patient/parent-completed questionnaires, and clinical information were collected at study visits at enrolment, then 6-monthly to 2 years, and yearly thereafter up to 5 years [20]. The ReACCh-Out study was approved by research ethics boards at all participating institutions and was carried out according to the Declaration of Helsinki. Written informed consent and assent as applicable were obtained from parents and patients. The present analyses were approved by the University of British Columbia and Children’s and Women’s Health Centre of British Columbia Research Ethics Board (H21-03353).
Parents and patients completed the following questionnaires: parent global measured on a 10 cm horizontal VAS, CHAQ-disability index (CHAQ-DI), and a 10-cm VAS for pain severity [1]; the JAQQ [16]; the CHQ (PF50 version) [14, 17, 21]; PedsQL parent and child generic core scales (used with permission from J Varni) [18]; and the preference-based Quality of My Life questionnaire health VAS (HRQoML) [22]. Parents completed the questionnaires for patients <9 years and patients ≥9 years completed their own with or without their parents’ help. The complete set of questionnaires was scheduled for the first five study visits for patients enrolled during the first two years of the ReACCh-Out study. The PedsQL and CHQ were omitted thereafter. Physicians completed an active joint count and a 10-cm VAS for physician’s global assessment of disease activity (PGADA) at each study visit.
Patients with completed parent global and PedsQL-Parent measures at enrolment were selected for the present analyses. Data from enrolment and 6-month visits were used for estimating measurement properties. Additional analyses compared available data for the 18 and 24-month visits. Causal pathway analyses included all patients who completed a parent global within 28 days after diagnosis, and those who completed it 3–9 months after diagnosis (details provided in Supplementary Data S1, available at Rheumatology online).
Evaluation of measurement properties
The COnsensus‐based Standards for the selection of health Measurement INstruments (COSMIN) checklist for evaluation of measurement properties of patient reported outcome measures was followed where applicable, omitting steps related to measure development, multi-item measures, and comparison to a gold standard [23]. Measurement properties were assessed in the same manner for the JAQQ, HRQoML, CHQ-physical, CHQ-psychosocial, PedsQL-Parent and PedsQL-Child.
Reliability
Because conditions for test-retest reliability were not set up within REACCh-Out, reliability was tested comparing enrolment to 6-month, and 18- to 24-month visits. Intraclass correlation coefficients (ICCs) were calculated when at the later visit the patient’s rating was ‘the same’ according to the question ‘Since the last time I was here my life is (much worse, a little worse, the same, a little better, much better)’, found in the QoML questionnaire [22] or the parent rating was ‘same’ according to the question ‘Relative to the last assessment do you feel your child is (much better, better, same, worse, much worse)’, found in the JAQQ, respectively [16]. ICCs were calculated using individual estimates from random effects models in STATA15 (StataCorp, College Station, TX, USA) as:
An ICC ≥0.7 was considered good reliability [23–25].
Measurement error
The same change questions were used as external anchors to calculate measurement error and minimal clinical important difference (MCID) over the enrolment to 6-month interval. Change was classified as much worse: –2; worse/a little worse: –1; same/the same: 0; better/a little better: +1; much better:+2. MCID was calculated as the mean change in absolute values of the parent global corresponding to +1 in the external anchor [25, 26]. Smallest detectable change (SDC) was calculated as:
Where SEM = standard error of measurement =√ (within subject variance) =√ (total variance – (1-ICC)) [24]. SDC < MCID was the accepted criterion of good measurement error [23].
Convergent construct validity
Cross-sectional data from enrolment and 6-month visits, respectively, were used to calculate Spearman correlation coefficients (Rs) of the parent global with HRQoL and other JIA measures. The following a priori hypotheses were based on a review of the literature followed by a survey and consensus of the authors (K.O., K.T-A., R.A.B., B.F.M., L.B.T., J.G.) [10, 12, 27–29].
If the parent global is an HRQoL measure, it will correlate with other HRQoL measures with Rs of ≥0.5 [23]. Specific hypothesized correlations were: 0.6–0.8, 0.5–0.7, 0.5–0.7, 0.6–0.8 and 0.7–0.8 for the JAQQ, CHQ-Physical, CHQ-Psychosocial, PedsQL and HRQoML, respectively.
If the parent global is an HRQoL measure it will have Rs of 0.3 to 0.5 with related JIA non-HRQoL measures, except for pain [23]. Specific hypothesized Rs were: 0.5–0.7, 0.4–0.5, 0.4–0.5, 0.3–0.5, 0.2–0.4 for pain VAS, active joint count, PGADA, CHAQ-DI and ESR, respectively.
Estimated correlations of the parent global with HRQoL measures will be higher than those with non-HRQoL measures but 95% confidence intervals (CIs) may overlap, as included non-HRQoL measures assess constructs also conceptualized as components of health (such as pain, function, symptoms) [15, 30].
CIs were calculated with bootstrapping in 1000 repetitions. For each correlation, overlap of the range of expected values and the 95% CI of observed values was accepted as fulfilling the hypothesis.
Discriminative construct validity
The a priori hypothesis was that values of the parent global at 6 months will differ significantly between patients with active and inactive disease. Inactive disease was an active joint count of 0, absence of systemic manifestations of systemic arthritis, absence of enthesitis in those with enthesitis-related arthritis or psoriatic arthritis, absence of uveitis and a PGADA <10 mm [20]. Differences in scores between patients with active and inactive disease were tested with a Mann–Whitney U test and significance was set at P < 0.05.
Responsiveness
The standardized response mean (SRM) was used to estimate responsiveness. Internal responsiveness estimating change over time was the mean change in scores from enrolment to 6 months divided by the standard deviation of changes [31]. External responsiveness relating change to an external standard [31] was calculated separately for patients who improved or worsened according to answers to the above change questions (better/a little better/much better and worse/a little worse/much worse, respectively). SRM for improvement was the mean change in those who improved divided by the standard deviation of change in those who improved, and SRM for worsening was the mean change in those who worsened divided by the standard deviation of change in those who worsened [31, 32]. Sensitivity analyses were performed using a change score of ≥10 mm in PGADA as alternative physician-based external standard to define improvement or worsening [33]. Responsiveness statistics were calculated as the mean change in those who improved or worsened, respectively, divided by the standard deviation of change in patients deemed to be the same [31]. We adopted generally used cut-off values for SRM and responsiveness statistic: 0.20: small; 0.50: moderate; 0.80: large [34]. Values of ≥0.5 were considered acceptable responsiveness.
Path analysis
The a priori hypothesis was that causal pathways determining parent global scores will be similar to those previously identified for HRQoML [35]. Methods are detailed in Supplementary Data S1, available at Rheumatology online. Structural equation modelling was performed using previously published models for ‘at diagnosis’ and at 3–9 months after diagnosis, substituting parent global for HRQoML [35]. Models were tested in the same subjects included in our previous analysis and final models were selected based on clinical plausibility and fit statistics [35]. Path analyses were conducted with the lavaan package in R software [36], and all other analyses were conducted using STATA15 (StataCorp).
Results
Patients
A total of 344 ReACCh-Out patients met criteria for inclusion to assess measurement properties (Supplementary Fig. S1, available at Rheumatology online). Patients were enrolled at a median of 1.25 (25, 75th centile, 0, 4.7) months after diagnosis with median parent global of 1.3 (0.2, 3.4) cm and median active joint count of 2 (1, 5) (Table 1). For the causal pathway analysis, 561 patients were included ‘at diagnosis’ and 887 at 3–9 months after diagnosis.
Table 1.
Baseline characteristics of patients included in the study
| Characteristic | Participants with data | Value |
|---|---|---|
| Age at onset, years | 341 | 7.9 (3.3, 11.8) |
| Female sex, n (%) | 344 | 220 (63.9) |
| JIA category, n (%) | 344 | |
| Oligoarthritis | 129 (37.5) | |
| Polyarthritis RF-negative | 67 (19.5) | |
| Enthesitis related Arthritis | 46 (13.4) | |
| Systemic arthritis | 29 (8.4) | |
| PsA | 27 (7.8) | |
| Polyarthritis RF-positive | 12 (3.5) | |
| Undifferentiated | 34 (9.9) | |
| Diagnosis to enrolment, months | 344 | 1.25 (0, 4.7) |
| Onset to enrolment, months | 341 | 6.9 (3.8, 12.3) |
| cJADAS10 | 343 | 7.1 (3, 14) |
| Active joints, number | 344 | 2 (1, 5) |
| PGADA, cm | 343 | 2.6 (1, 5.2) |
| ESR, mm/h | 309 | 19 (8, 38) |
| CRP, mg/L | 260 | 2 (0.01, 12.7) |
| CHAQ-DI score | 332 | 0.37 (0.05, 0.875) |
| Pain intensity in last week, cm | 344 | 1.9 (0.5, 5) |
| Parent global assessment, cm | 344 | 1.3 (0.2, 3.4) |
| JAQQ total score | 335 | 2.5 (1, 3.5) |
| HRQoML, cm | 329 | 8.2 (5.4, 9.5) |
| CHQ-Physical score | 311 | 42.9 (31.4, 51.3) |
| CHQ-Psychosocial score | 311 | 51.3 (42.9, 56.8) |
| PedsQL-Parent total score | 344 | 78.1 (61.2, 89) |
| PedsQL-Parent Physical | 344 | 71.9 (51.6, 90.6) |
| PedsQL-Parent Psychosocial | 344 | 80.5 (65, 90.4) |
| PedsQL-Child total score | 239 | 77.2 (60.9, 88) |
Values are median (25th, 75th centiles) unless otherwise specified. Pain was measured on a 10 cm visual analogue scale. cJADAS10: clinical juvenile arthritis disease activity score with up to 10 joints (0 best to 30 worst) [37]; PGADA: physician’s global assessment of disease activity (0–10); CHAQ-DI: childhood health assessment questionnaire disability index (0–3); JAQQ: juvenile arthritis quality of life questionnaire (1–7); HRQoML: health scale of the quality of my life questionnaire (10–0); CHQ: child health questionnaire (100–0, 50 is the mean of healthy children); PedsQL: paediatric quality of life inventory (100–0).
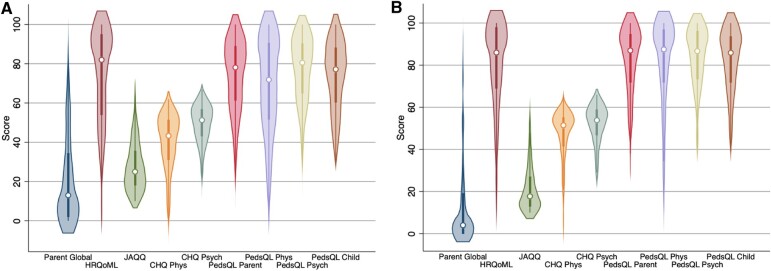
The distribution of observed scores for the parent global and the reference HRQoL instruments are shown in Fig. 1. As often seen in modern JIA cohorts, the distributions are skewed with scores concentrating at the optimal end of the scales. Exceptions are the CHQ scores, which are normalized to a reference population, meaning 50 points is the mean and 10 points is the standard deviation in healthy children.
Fig. 1.
Violin box plots for parent global and HRQoL measures at enrolment (A), 6 months (B)
Parent global, quality of my life health scale (HRQoML), and juvenile arthritis quality of my life questionnaire (JAQQ) scores were multiplied by 10. Range of scores are 0 (best) to 100 (worst) for parent global, 0 (worst) to 100 (best) for HRQoML, 10 (best) to 70 (worst) for JAQQ, 0 (worst) to 100 (best) for child health questionnaire (CHQ) and 50 is the mean of healthy children, 0 (worst) to 100 (best) for paediatric quality of life inventory (PedsQL). Phys: Physical; Psych: Psychosocial.
Reliability
The ICC estimate for the parent global at enrolment to 6 months as well as all HRQoL measures fell below the 0.7 threshold for good reliability (Table 2). Because test-retest reliability depends on the stability of respondents, ICC was also calculated for 18–24 months, a more stable time than the period immediately following diagnosis. At 18–24 months, point estimates of ICCs for parent global, HRQoML and the JAQQ were indeed higher than at the earlier time period, but only the ICC for the JAQQ using the parent change question reached the critical threshold of 0.7 (Table 2).
Table 2.
Intraclass correlation coefficients comparing scores in patients reporting no change
| External standard measure | Parent assessment of my child: ‘same’ |
Child assessment of my life: ‘the same’ |
||
|---|---|---|---|---|
| n | ICC (95% CI) | n | ICC (95% CI) | |
| Enrolment and 6 months | ||||
| Parent Global | 67 | 0.32 (0.09, 0.52) | 78 | 0.37 (0.16, 0.55) |
| JAQQ | 65 | 0.57 (0.38, 0.71) | 71 | 0.52 (0.30, 0.69) |
| HRQoML | 62 | 0.48 (0.27, 0.65) | 74 | 0.51 (0.32, 0.66) |
| CHQ-Physical | 51 | 0.57 (0.35, 0.73) | 51 | 0.63 (0.43, 0.77) |
| CHQ-Psychosocial | 51 | 0.51 (0.27, 0.68) | 51 | 0.59 (0.37, 0.74) |
| PedsQL-Parent | 56 | 0.49 (0.26, 0.66) | 62 | 0.45 (0.23, 0.63) |
| PedsQL-Parent Physical | 56 | 0.52 (0.30, 0.69) | 62 | 0.43 (0.20, 0.61) |
| PedsQL-Parent Psychosocial | 56 | 0.55 (0.33, 0.71) | 62 | 0.55 (0.35, 0.70) |
| PedsQL-Child | 36 | 0.64 (0.41, 0.80) | 39 | 0.64 (0.41, 0.80) |
| 18 and 24 monthsa | ||||
| Parent Global | 238 | 0.53 (0.43, 0.61) | 253 | 0.43 (0.32, 0.53) |
| JAQQ | 249 | 0.72 (0.66, 0.78) | 241 | 0.66 (0.58, 0.72) |
| HRQoML | 223 | 0.51 (0.41, 0.60) | 245 | 0.55 (0.45, 0.63) |
Scores between 18 and 24 months are for all patients in the ReACCh-Out cohort who had available scores for parent global, JAQQ or HRQoML at those visits. There were few patients with scores for other measures at those visits.
CHQ: child health questionnaire; HRQoML: health scale of the quality of my life questionnaire; ICC: intraclass correlation coefficient; JAQQ: juvenile arthritis quality of life questionnaire; PedsQL: paediatric quality of life inventory.
Measurement error
The requirement of SDC<MCID for good measurement error was not met by the parent global or any HRQoL measure (Supplementary Table S1, available at Rheumatology online). Because of their dependence on the respondents’ assessments over the first 6 months and the broad scales used (only two gradations for improvement), these results should also be viewed with caution.
Convergent construct validity
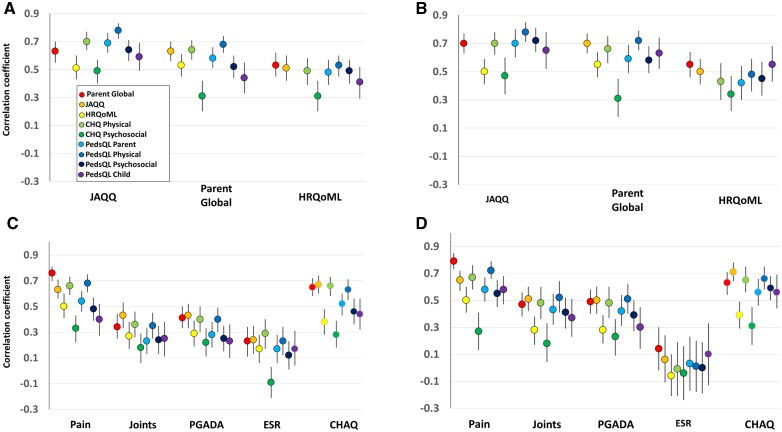
Correlations of the parent global with measures of HRQoL at enrolment and 6 months are shown in Fig. 2 and Supplementary Table S2, available at Rheumatology online. Spearman correlations of the parent global were 0.63 and 0.70 with the JAQQ, 0.58 and 0.59 with PedsQL-Parent, and 0.64 and 0.66 with CHQ-Physical, at enrolment and 6 months, respectively, and 0.63 with PedsQL-Child at 6 months. These correlations fulfilled the test hypotheses. Lower than predicted correlations were found with HRQoML, 0.53 and 0.55, and with CHQ-Psychosocial, 0.31 and 0.31, respectively, and PedsQL-Child at enrolment, 0.44. Correlations with PedsQL-Psychosocial were 0.52 and 0.58, respectively.
Fig. 2.
Construct validity of the parent global as a measure of health-related quality of life
Spearman correlations (95% confidence intervals) of parent global with measures of heath related quality of life at diagnosis (A), at 6 months (B); correlations with other related JIA measures at diagnosis (C), at 6 months (D). Pain was measured on 10-cm visual analogue scale. CHAQ: childhood HAQ disability index; CHQ: child health questionnaire; HRQoML: quality of my life health scale; JAQQ: juvenile arthritis quality of life questionnaire; Joints: active joint count; PedsQL: paediatric quality of life inventory; PGADA: physician’s global assessment of disease activity.
Among non-HRQoL measures, observed correlations of the parent global with traditional measures of disease activity including PGADA (0.41 and 0.49 at enrolment and 6 months, respectively), active joint count (0.34 and 0.47) and ESR (0.23 and 0.14) were all <0.5, and all fulfilled the test hypotheses (Fig. 2 and Supplementary Table S3, available at Rheumatology online). The correlations of parent global with CHAQ-DI (0.65 and 0.63) and with pain (0.79 at 6 months) were higher than predicted. Other HRQoL measures had similar patterns of low correlations with disease activity measures and high correlations with pain and CHAQ-DI (Fig. 2 and Supplementary Table S3, available at Rheumatology online). Exceptions were correlations of JAQQ with active joint count (0.51) and PGADA (0.50) at 6 months.
Discriminative construct validity
The parent global and all other HRQoL measures, except CHQ-Psychosocial, met criteria for discriminative validity as their values were significantly better during inactive than during active disease (Supplementary Table S4, available at Rheumatology online). For the parent global, median values were 0.1 (25th, 75th centiles 0, 0.4) during inactive and 1.1 (0.2, 3.4) during active disease (P < 0.0001).
Responsiveness
Internal responsiveness for overall change from enrolment to 6 months regardless of individual direction of change was low for all measures with the exception of the JAQQ, which just reached a moderate SRM of 0.50, the cut-off for acceptability (Table 3). External responsiveness to improvement defined by parent change ratings was moderate (SRM 0.50–0.79) for the parent global, JAQQ, CHQ-Physical and PedsQL-Child, and small (SRM 0.20–0.49) for HRQoML, PedsQL-Parent and CHQ-Psychosocial. Based on child ratings, responsiveness to improvement was small for the parent global, HRQoML, CHQ-Psychosocial and PedsQL-Parent and moderate for the JAQQ, CHQ-Physical and PedsQL-Child.
Table 3.
Responsiveness of parent global and health-related quality of life measures
| External standard | Measure | Direction of change | Participants with data | Mean change | s.d. of change | SRM: Mean/s.d. of change |
|---|---|---|---|---|---|---|
| None | Parent Global | Not applicable, all subjects with scores at enrolment and 6 months are included in calculation | 297 | 0.67 | 2.54 | 0.26 |
| JAQQ | 274 | 0.60 | 1.20 | 0.50 | ||
| HRQoML | 283 | –0.73 | 2.48 | –0.29 | ||
| CHQ-Physical | 173 | –5.08 | 13.3 | –0.38 | ||
| CHQ-Psychosocial | 173 | –2.52 | 9.70 | –0.26 | ||
| PedsQL-Parent | 209 | –6.18 | 18.53 | –0.33 | ||
| PedsQL-Parent Physical | 209 | –8.85 | 24.65 | –0.36 | ||
| PedsQL-Parent Psychosocial | 209 | –5.01 | 16.14 | –0.31 | ||
| PedsQL-Child | 133 | –7.23 | 16.74 | –0.43 | ||
| Parent rating | Parent Global | Better | 185 | 1.13 | 2.23 | 0.51 |
| Worse | 22 | –2.13 | 2.67 | –0.80 | ||
| JAQQ | Better | 183 | 0.81 | 1.14 | 0.71 | |
| Worse | 22 | –0.38 | 1.04 | –0.37 | ||
| HRQoML | Better | 178 | –0.92 | 2.30 | –0.40 | |
| Worse | 20 | 0.92 | 3.23 | 0.28 | ||
| CHQ-Physical | Better | 101 | –7.46 | 12.22 | –0.61 | |
| Worse | 13 | 6.22 | 14.4 | 0.43 | ||
| CHQ-Psychosocial | Better | 101 | –2.92 | 9.23 | –0.32 | |
| Worsea | 13 | –3.41 | 8.63 | –0.39 | ||
| PedsQL-Parent | Better | 128 | –7.75 | 18.14 | –0.43 | |
| Worsea | 16 | –8.27 | 17.24 | –0.48 | ||
| PedsQL-Parent Physical | Better | 128 | –11.93 | 22.2 | –0.54 | |
| Worse | 16 | 0.08 | 36.79 | 0.00 | ||
| PedsQL-Parent Psychosocial | Better | 126 | –6.67 | 15.44 | –0.43 | |
| Worsea | 16 | –8.4 | 14.6 | –0.58 | ||
| PedsQL-Child | Better | 77 | –11.07 | 16.46 | –0.67 | |
| Worse | 12 | 7.02 | 16.30 | 0.43 | ||
| Child rating | Parent Global | Better | 190 | 1.04 | 2.46 | 0.42 |
| Worse | 26 | –0.54 | 2.85 | –0.19 | ||
| JAQQ | Better | 178 | 0.69 | 1.22 | 0.57 | |
| Worsea | 22 | 0.20 | 1.1 | 0.18 | ||
| HRQoML | Better | 184 | –0.80 | 2.4 | –0.33 | |
| Worsea | 24 | –0.57 | 3.09 | –0.18 | ||
| CHQ-Physical | Better | 108 | –6.87 | 13.8 | –0.50 | |
| Worse | 13 | 2.01 | 17.9 | 0.11 | ||
| CHQ-Psychosocial | Better | 108 | –3.69 | 9.10 | –0.40 | |
| Worsea | 13 | –2.86 | 15.44 | –0.19 | ||
| PedsQL-Parent | Better | 128 | –6.94 | 17.95 | –0.39 | |
| Worsea | 16 | –3.62 | 19.95 | –0.18 | ||
| PedsQL-Parent Physical | Better | 128 | –9.78 | 22.75 | –0.43 | |
| Worsea | 16 | –1.06 | 33.13 | –0.03 | ||
| PedsQL-Parent Psychosocial | Better | 126 | –6.68 | 15.34 | –0.44 | |
| Worsea | 16 | –2.66 | 17.81 | –0.15 | ||
| PedsQL-Child | Better | 84 | –9.30 | 16.61 | –0.56 | |
| Worse | 10 | 3.13 | 19.94 | 0.16 |
External standards were parent’s or child’s ratings of better (better/a little better, much better) or worse (worse/a little worse, much worse) at 6 months relative to enrolment. For the quality of my life questionnaire health scale (HRQoML), child health questionnaire (CHQ) and paediatric quality of life inventory (PedsQL) negative values for change reflect improvement.
change in score suggests improvement, not worsening. Change: score at enrolment minus score at 6 months; mean change: average of individual changes in score; JAQQ: juvenile arthritis quality of life questionnaire; SRM: standardized response mean.
SRMs for worsening were based on fewer patients (12–26) and tended to be lower than those for improvement, except for the parent global when using parent ratings (0.80 for worsening, 0.51 for improvement) (Table 3). When improvement was defined as a ≥10 mm decrease in PGADA, SRMs were in the moderate range for the parent global, JAQQ and CHQ-Physical, and small for HRQoML, CHQ-Psychosocial, PedsQL-Parent and PedsQL-Child (Supplementary Table S5, available at Rheumatology online). Responsiveness statistics for parent rating of much better were just below moderate for the parent global and HRQoML (both 0.49), large for the JAQQ, small for CHQ-Psychosocial and moderate for the remaining HRQoL measures (Supplementary Table S6, available at Rheumatology online). Values for child rating of much better were moderate for all measures except CHQ-Physical (large) and HRQoML and PedsQL-Parent (both small) (Supplementary Table S7, available at Rheumatology online).
Considering all responsiveness indicators, the parent global SRMs for improvement were at the median relative to other HRQoL measures, but its responsiveness statistic for much better improvement was relatively lower.
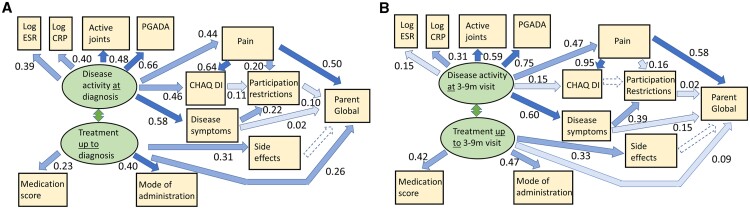
Path analysis
The a posteriori models for causal pathways leading to parent global scores had good fit statistics when using the same pathways previously published for HRQoML (Fig. 3 and Supplementary Table S8, available at Rheumatology online) [35]. However, the effect of pain was greater on parent global (path coefficient 0.50 and 0.58 at diagnosis and 3–9 months, respectively) than on HRQoML (0.24 and 0.27 as previously reported [35]).
Fig. 3.
Path analysis for parent global at diagnosis (A), 3–9 months after diagnosis (B)
Structural equation modelling results after substituting parent global in models previously reported for HRQoML [35]. See Supplementary Data S1, available at Rheumatology online for details. At diagnosis: ≤28 days after actual diagnosis date; 3–9 months: 3–9 months after actual diagnosis date; dashed lines: paths not supported; numbers: path coefficients; colour shades: light, path coefficients <0.2; medium, 0.2–0.49; dark, ≥0.5; medication score: weighted sum of potency of treatments; mode of administration: 1, oral to 4, repeated intravenous injection; joints: active joint count; PGADA: physician’s global assessment of disease activity; CHAQ DI: childhood HAQ disability index.
Discussion
Our study presents the following preliminary findings supporting the validity of the parent global as a measure of HRQoL in children with JIA: (i) construct validity was supported by moderate-to-high correlations with accepted HRQoL measures and correlations with non-HRQoL measures largely as predicted; (ii) discriminant validity was supported by significant differences in scores during active and inactive disease; (iii) responsiveness to change was acceptable; (iv) the performance of other HRQoL measures was similar to that of the parent global; and (v) causal pathways leading to parent global scores mirrored those for HRQoML. On the other hand, reliability and measurement error were unsatisfactory for parent global and most other measures. The lack of good reliability in our study is likely due to the prolonged time interval of six months. Good reliability has been reported previously for the CHQ, and a recent study showed good test-retest reliability for the parent global when administered over a 1-to-2-week interval [38–40].
Responsiveness depends on the way change is assessed [41]. Our findings showed acceptable moderate SRMs of the parent global for external responsiveness to improvement assessed by parents and to clinical improvement assessed by physicians. While internal responsiveness of the parent global was similar to that previously reported after intraarticular injection, it was lower than that reported following methotrexate initiation or a disease flare [42–44].
Although most of the hypotheses for construct validity of the parent global as an HRQoL measure were fulfilled, correlations with pain and CHAQ were higher, and those with HRQoML and CHQ-Psychosocial were lower than predicted. The first may be explained on a conceptual basis as both pain and physical function may be conceived as components of health [15, 30]. An additional explanation for the high correlation with pain may be the proximity of the parent global to the pain VAS on the CHAQ questionnaire [45]. The low correlation with HRQoML may relate to the parent global being a disease-specific measure and the HRQoML a generic measure, or to a difference in the respondents implied by the wording of the questions (parent for parent global, child for HRQoML).
The relatively low correlation of the parent global with CHQ-Psychosocial has been noted previously [12], and may suggest that the parent global does not capture the mental and social components of health satisfactorily, or alternatively that the CHQ-Psychosocial does not detect the early psychosocial impacts of JIA well. We favour the second explanation because scores of the CHQ-Psychosocial at enrolment were close to the range of scores for healthy children [14, 46], scores were similar in patients with inactive or active disease, correlations were also low with the JAQQ and the HRQoML, and the PedsQL-Psychosocial had correlations >0.5 with the parent global.
Van Dijkhuizen et al. have recently proposed that the parent global measures constructs beyond disease activity ‘including all aspects of the disease burden affecting’ HRQoL [40]. Disease activity in JIA has been defined as those aspects that are potentially reversible measuring ‘signs and symptoms related to inflammation’ [47]. Disease activity measures such as ESR, active joint count and PGADA can be thought of as indicators of the inflammatory pathogenic process, while the consequences of this biologic activity on the individual include effects on function and ultimately HRQoL as illustrated in our path analysis. The results of our study are more in keeping with the view of the parent global as a measure of HRQoL rather than a measure of disease activity by showing higher correlations with HRQoL measures in contrast to lower correlations with measures of disease activity, and causal pathways very similar to those for other HRQoL measures [35].
The ReACCh-Out study did not collect data for formal assessment of face and cross-cultural validity. Face validity of the parent global as a measure of health may be surmised from multiple studies in which it is referred to as a measure of well-being; while its cross-cultural validity as a component of the JAMAR was found to be satisfactory [2, 9–11, 13, 48, 49]. However, future studies are necessary to confirm face validity, including qualitative studies with parents and patients to ascertain what they consider when completing the parent global.
The main strengths of the present study are the large representative sample of children with JIA, the availability of established HRQoL measures collected simultaneously with the parent global, and the assessment of their measurement properties in the same manner. The present analyses have several limitations. First, conditions for testing reliability and measurement error were suboptimal. In particular, it is likely patients and parents assessed change relative to their preceding clinic visit rather than the preceding study visit 6 months earlier when the first set of measures were collected. Some patients considered ‘the same’ since their previous clinic appointment could have changed significantly since the earlier study visit, increasing within-subject variance. Second, respondents could not be confidently separated into child and parent because the questionnaires were often completed by both together. Lastly, although our results are derived from a representative Canadian JIA cohort, their generalizability to other settings needs confirmation.
This study has explored the validity of the parent global as a measure of HRQoL in children with JIA. Although our findings support this concept, further studies in other settings are needed, especially to reassess its test-retest reliability, confirm its construct validity as a measure of HRQoL, and assess its face validity both for researchers and parents/patients. Measurement properties of accepted HRQoL measures for JIA should be assessed simultaneously in a comprehensive manner to ensure appropriate validation. Confirmation of the parent global as an HRQoL measure would allow a re-interpretation of the many existing studies using this measure and increase our understanding of HRQoL in JIA.
Supplementary Material
Acknowledgements
The authors are grateful to Thomas M. Loughin, Department of Statistics and Actuarial Science, Simon Fraser University, Burnaby, British Columbia, Canada, for assistance with the structural equation modelling, to the patients and parents who participated in the ReACCh-Out study, and to the following ReACCh-Out investigators for their contributions to the ReACCh-Out cohort study: Roxana Bolaria, David Cabral, Katherine Gross, Kristin Houghton, Kimberly Morishita, Ross Petty, Stuart E. Turvey, University of British Columbia, Vancouver, British Columbia; Janet Ellsworth, the Stollery Children’s Hospital and University of Alberta, Edmonton, Alberta; Susanne Benseler, Nicole Johnson, Paivi Miettunen, Heinrike Schmeling, the Alberta Children’s Hospital and University of Calgary, Calgary, Alberta; Maggie Larche, McMaster University, Hamilton, Ontario; Bonnie Cameron, Ronald M. Laxer, Lynn Spiegel, Rayfel Schneider, Earl Silverman, Rae S. M. Yeung, Deborah M Levy, Shirley Tse, Hospital for Sick Children and University of Toronto, Toronto, Ontario; Michele Gibbon, Johannes Roth, Karen Watanabe Duffy, Children’s Hospital of Eastern Ontario and University of Ottawa, Ottawa, Ontario; Anne-Laure Chetaille, Jean Dorval, Centre Hospitalier Universitaire de Laval and Université Laval, Quebec City, Quebec; Gilles Boire, Alessandra Bruns, Centre Hospitalier Universitaire de Sherbrooke and Université de Sherbrooke, Sherbrooke, Quebec; Sarah Campillo, Gaelle Chedeville, Claire LeBlanc, Rosie Scuccimarri, McGill University Health Centre and McGill University, Montreal, Quebec; Elie Haddad, Claire St. Cyr, Debbie Feldman, CHU Ste. Justine and Université de Montreal, Montreal, Quebec; Bianca Lang, Suzanne E. Ramsey, Elizabeth Stringer, Adam M Huber, IWK Health Centre and Dalhousie University, Halifax, Nova Scotia; Paul Dancey, Memorial University, St. John’s, Newfoundland and Labrador, Canada.
Funding: The REACCh-Out study was supported by a New Emerging Team research grant from the Canadian Institutes of Health Research [QNT 69301] and the Fast Foundation; J.G. is supported by a Clinical Investigator Award from the BC Children’s Hospital Research Institute, Vancouver, Canada.
Disclosure statement: The authors have declared no conflicts of interest.
Contributor Information
Kiem Oen, Department of Pediatrics and Child Health, University of Manitoba, Winnipeg.
Karine Toupin-April, School of Rehabilitation Sciences and Department of Pediatrics, University of Ottawa, Children's Hospital of Eastern Ontario Research Institute and Institut du Savoir Montfort, Ottawa.
Brian M Feldman, Division of Rheumatology, The Hospital for Sick Children, and Departments of Pediatrics and Medicine, and Institute of Health Policy, Management, and Evaluation, University of Toronto, Toronto.
Roberta A Berard, Pediatric Rheumatology, Children’s Hospital, London Health Sciences Centre, London.
Ciẚran M Duffy, Children’s Hospital of Eastern Ontario and Department of Pediatrics, University of Ottawa, Ottawa.
Lori B Tucker, Division of Pediatric Rheumatology, British Columbia Children’s Hospital, and Department of Pediatrics, University of British Columbia, Vancouver.
Jiahao Tian, Department of Statistics and Actuarial Science, Simon Fraser University, Burnaby.
Dax G Rumsey, Paediatric Rheumatology, Stollery Children’s Hospital and Department of Pediatrics, University of Alberta, Edmonton, Canada.
Jaime Guzman, Division of Pediatric Rheumatology, British Columbia Children’s Hospital, and Department of Pediatrics, University of British Columbia, Vancouver.
ReACCh-Out investigators:
Roxana Bolaria, David Cabral, Katherine Gross, Kristin Houghton, Kimberly Morishita, Ross Petty, Stuart E Turvey, Janet Ellsworth, Susanne Benseler, Nicole Johnson, Paivi Miettunen, Heinrike Schmeling, Maggie Larche, Bonnie Cameron, Ronald M Laxer, Lynn Spiegel, Rayfel Schneider, Earl Silverman, Rae S M Yeung, Deborah M Levy, Shirley Tse, Michele Gibbon, Johannes Roth, Karen Watanabe Duffy, Anne-Laure Chetaille, Jean Dorval, Gilles Boire, Alessandra Bruns, Sarah Campillo, Gaelle Chedeville, Claire LeBlanc, Rosie Scuccimarri, Elie Haddad, Claire St Cyr, Debbie Feldman, Bianca Lang, Suzanne E Ramsey, Elizabeth Stringer, Adam M Huber, and Paul Dancey
Data availability statement
The data underlying this article are available in the article and in its online supplementary material.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Singh G, Athreya BH, Fries JF, Goldsmith DP.. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis Rheum 1994;37:1761–9. [DOI] [PubMed] [Google Scholar]
- 2. Shoop-Worrall SJW, Hyrich KL, Wedderburn LR, Thomson W, Geifman N; CAPS the CLUSTER Consortium. Patient-reported wellbeing and clinical disease measures over time captured by multivariate trajectories of disease activity in individuals with juvenile idiopathic arthritis in the UK: a multicentre prospective longitudinal study. Lancet Rheumatol 2021;3:e111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prince FH, Twilt M, ten Cate R. et al. Long-term follow-up on effectiveness and safety of etanercept in juvenile idiopathic arthritis: the Dutch national register. Ann Rheum Dis 2009;68:635–41. [DOI] [PubMed] [Google Scholar]
- 4. Minden K, Horneff G, Niewerth M. et al. Time of disease-modifying antirheumatic drug start in juvenile idiopathic arthritis and the likelihood of a drug-free remission in young adulthood. Arthritis Care Res 2019;71:471–81. [DOI] [PubMed] [Google Scholar]
- 5. Nordal E, Zak M, Aalto K. et al. Ongoing disease activity and changing categories in a long-term Nordic cohort study of juvenile idiopathic arthritis. Arthritis Rheum 2011;63:2809–18. [DOI] [PubMed] [Google Scholar]
- 6. Giannini EH, Ruperto N, Ravelli A. et al. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum 1997;40:1202–9. [DOI] [PubMed] [Google Scholar]
- 7. Ruperto N, Martini A.. Networking in paediatrics: the example of the Paediatric Rheumatology International Trials Organisation (PRINTO). Arch Dis Child 2011;96:596–601. [DOI] [PubMed] [Google Scholar]
- 8. Brunner HI, Rider LG, Kingsbury DJ. et al. Pediatric Rheumatology Collaborative Study Group - over four decades of pivotal clinical drug research in pediatric rheumatology. Pediatr Rheumatol Online J 2018;16:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lovell DJ, Dare JA, Francis-Sedlak M. et al. A 6-month, multicenter, open-label study of fixed dose naproxen/esomeprazole in adolescent patients with juvenile idiopathic arthritis. Pediatr Rheumatol Online J 2018;16:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Consolaro A, Ruperto N, Bazso A. et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum 2009;61:658–66. [DOI] [PubMed] [Google Scholar]
- 11. Filocamo G, Consolaro A, Schiappapietra B. et al. A new approach to clinical care of juvenile idiopathic arthritis: the Juvenile Arthritis Multidimensional Assessment Report. J Rheumatol 2011;38:938–53. [DOI] [PubMed] [Google Scholar]
- 12. Filocamo G, Davì S, Pistorio A. et al. Evaluation of 21-numbered circle and 10-centimeter horizontal line visual analog scales for physician and parent subjective ratings in juvenile idiopathic arthritis. J Rheumatol 2010;37:1534–41. [DOI] [PubMed] [Google Scholar]
- 13. Magni-Manzoni S, Ruperto N, Pistorio A. et al. Development and validation of a preliminary definition of minimal disease activity in patients with juvenile idiopathic arthritis. Arthritis Rheum 2008;59:1120–7. [DOI] [PubMed] [Google Scholar]
- 14. Ruperto N, Ravelli A, Pistorio A. et al. Cross-cultural adaptation and psychometric evaluation of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ) in 32 countries. Review of the general methodology. Clin Exp Rheumatol 2001;19(4 Suppl 23):S1–9. [PubMed] [Google Scholar]
- 15. Post MW. Definitions of quality of life: what has happened and how to move on. Topics Spinal Cord Injury Rehabil 2014;20:167–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duffy CM, Arsenault L, Duffy KN, Paquin JD, Strawczynski H.. The Juvenile Arthritis Quality of Life Questionnaire–development of a new responsive index for juvenile rheumatoid arthritis and juvenile spondyloarthritides. J Rheumatol 1997;24:738–46. [PubMed] [Google Scholar]
- 17. Landgraf JM, Maunsell E, Speechley KN. et al. Canadian-French, German and UK versions of the Child Health Questionnaire: methodology and preliminary item scaling results. Qual Life Res 1998;7:433–45. [DOI] [PubMed] [Google Scholar]
- 18. Varni JW, Seid M, Kurtin PS.. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001;39:800–12. [DOI] [PubMed] [Google Scholar]
- 19. Petty RE, Southwood TR, Manners P. et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. [PubMed] [Google Scholar]
- 20. Guzman J, Oen K, Tucker LB. et al. The outcomes of juvenile idiopathic arthritis in children managed with contemporary treatments: results from the ReACCh-Out cohort. Ann Rheum Dis 2015;74:1854–60. [DOI] [PubMed] [Google Scholar]
- 21. Landgraf JM, Abetz L, Ware JE.. The CHQ user's manual, 1st edn. Boston, Massachusetts: The Health Institute, New England Medical Center, 1996. [Google Scholar]
- 22. Feldman BM, Grundland B, McCullough L, Wright V.. Distinction of quality of life, health related quality of life, and health status in children referred for rheumatologic care. J Rheumatol 2000;27:226–33. [PubMed] [Google Scholar]
- 23.COSMIN methodology for systematic reviews of patient-reported outcome measures (PROMs). User Manual. Version 1.0. 2018. https://www.cosmin.nl/wp-content/uploads/COSMIN-syst-review-for-PROMs-manual_version-1_feb-2018-1.pdf (8 March 2021, date last accessed).
- 24. Beckerman H, Roebroeck ME, Lankhorst GJ. et al. Smallest real difference, a link between reproducibility and responsiveness. Qual Life Res 2001;10:571–8. [DOI] [PubMed] [Google Scholar]
- 25. Streiner D, Norman G, Cairney J.. Health measurement scales: a practical guide to their development and use. Oxford: Oxford University Press, 2015. [Google Scholar]
- 26. Jaeschke R, Singer J, Guyatt GH.. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clin Trials 1989;10:407–15. [DOI] [PubMed] [Google Scholar]
- 27. van Mater HA, Williams JW Jr, Coeytaux RR, Sanders GD, Kemper AR.. Psychometric characteristics of outcome measures in juvenile idiopathic arthritis: a systematic review. Arthritis Care Res 2012;64:554–62. [DOI] [PubMed] [Google Scholar]
- 28. Oen K, Duffy CM, Tse SM. et al. Early outcomes and improvement of patients with juvenile idiopathic arthritis enrolled in a Canadian multicenter inception cohort. Arthritis Care Res 2010;62:527–36. [DOI] [PubMed] [Google Scholar]
- 29. Oen K, Guzman J, Dufault B. et al. Health-Related Quality of Life in an Inception Cohort of Children With Juvenile Idiopathic Arthritis: a Longitudinal Analysis. Arthritis Care Res 2018;70:134–44. [DOI] [PubMed] [Google Scholar]
- 30. Ware JE. Jr. Conceptualization and measurement of health-related quality of life: comments on an evolving field. Arch Phys Med Rehabil 2003;84:S43–51. [DOI] [PubMed] [Google Scholar]
- 31. Husted JA, Cook RJ, Farewell VT, Gladman DD.. Methods for assessing responsiveness: a critical review and recommendations. J Clin Epidemiol 2000;53:459–68. [DOI] [PubMed] [Google Scholar]
- 32. Middel B, van Sonderen E.. Statistical significant change versus relevant or important change in (quasi) experimental design: some conceptual and methodological problems in estimating magnitude of intervention-related change in health services research. Int J Integr Care 2002;2:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brunner HI, Klein-Gitelman MS, Miller MJ. et al. Minimal clinically important differences of the childhood health assessment questionnaire. J Rheumatol 2005;32:150–61. [PubMed] [Google Scholar]
- 34. Brunner HI, Ravelli A.. Developing outcome measures for paediatric rheumatic diseases. Best Pract Res Clin Rheumatol 2009;23:609–24. [DOI] [PubMed] [Google Scholar]
- 35. Oen K, Tian J, Loughin TM. et al. Causal pathways to health-related quality of life in children with juvenile idiopathic arthritis: results from the ReACCh-Out cohort. Rheumatology 2021;60:4691–702. [DOI] [PubMed] [Google Scholar]
- 36. Rosseel Y. lavaan: an R Package for Structural Equation Modeling. J Stat Softw 2012;48:1–36. [Google Scholar]
- 37. McErlane F, Beresford MW, Baildam EM. et al. Validity of a three-variable Juvenile Arthritis Disease Activity Score in children with new-onset juvenile idiopathic arthritis. Ann Rheum Dis 2013;72:1983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ruperto N, Ravelli A, Pistorio A. et al. The Italian version of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ). Clin Exp Rheumatol 2001;19(4 Suppl 23):S91–5. [PubMed] [Google Scholar]
- 39. Nugent J, Ruperto N, Grainger J. et al. The British version of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ). Clin Exp Rheumatol 2001;19(4 Suppl 23):S163–7. [PubMed] [Google Scholar]
- 40. van Dijkhuizen EHP, Ridella F, Naddei R. et al. Validity and reliability of four parent/patient reported outcome measures for juvenile idiopathic arthritis remote monitoring. Arthritis Care Res 2022;doi:10.1002/acr.24855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beaton DE, Bombardier C, Katz JN, Wright JG.. A taxonomy for responsiveness. J Clin Epidemiol 2001;54:1204–17. [DOI] [PubMed] [Google Scholar]
- 42. Moretti C, Viola S, Pistorio A. et al. Relative responsiveness of condition specific and generic health status measures in juvenile idiopathic arthritis. Ann Rheum Dis 2005;64:257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ruperto N, Ravelli A, Falcini F. et al. Responsiveness of outcome measures in juvenile chronic arthritis. Italian Pediatric Rheumatology Study Group. Rheumatology 1999;38:176–80. [DOI] [PubMed] [Google Scholar]
- 44. Magni-Manzoni S, Cugno C, Pistorio A. et al. Responsiveness of clinical measures to flare of disease activity in juvenile idiopathic arthritis. Clin Exp Rheumatol 2005;23:421–5. [PubMed] [Google Scholar]
- 45. Garcia-Munitis P, Bandeira M, Pistorio A. et al. Level of agreement between children, parents, and physicians in rating pain intensity in juvenile idiopathic arthritis. Arthritis Rheum 2006;55:177–83. [DOI] [PubMed] [Google Scholar]
- 46. Oliveira S, Ravelli A, Pistorio A. et al. Proxy-reported health-related quality of life of patients with juvenile idiopathic arthritis: the Pediatric Rheumatology International Trials Organization multinational quality of life cohort study. Arthritis Rheum 2007;57:35–43. [DOI] [PubMed] [Google Scholar]
- 47. Luca NJ, Feldman BM.. Disease activity measures in paediatric rheumatic diseases. Int J Rheumatol 2013;2013:715352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Consolaro A, Ruperto N, Pistorio A. et al. Development and initial validation of composite parent- and child-centered disease assessment indices for juvenile idiopathic arthritis. Arthritis Care Res 2011;63:1262–70. [DOI] [PubMed] [Google Scholar]
- 49. Bovis F, Consolaro A, Pistorio A. et al. Cross-cultural adaptation and psychometric evaluation of the Juvenile Arthritis Multidimensional Assessment Report (JAMAR) in 54 languages across 52 countries: review of the general methodology. Rheumatol Int 2018;38:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.