Abstract
Background:
Obesity is associated with chronic, low-grade inflammation in tissues and predisposes to various complications, including inflammatory skin diseases. However, the link between obesity and contact hypersensitivity (CHS) is not fully understood.
Objectives:
We sought to determine the influence of obesity on T helper 1 (Th1)–mediated CHS.
Methods:
The activity/phenotype/cytokine profile of the immune cells was tested in vivo and in vitro. Using quantitative polymerase chain reaction (qPCR) and fecal microbiota transplantation (FMT), we tested the role of a high-fat diet (HFD)–induced gut microbiota (GM) dysbiosis in increasing the effects of CHS.
Results:
Exacerbated CHS correlates with an increased inflammation-inducing GM in obese mice. We showed a proinflammatory milieu in the subcutaneous adipose tissue of obese mice, accompanied by proinflammatory CD4+ T cells and dendritic cells in skin draining lymph nodes and spleen. Obese interleukin (IL)-17A−/−B6 mice are protected from CHS aggravation, suggesting the importance of IL-17A in CHS aggravation in obesity.
Conclusions:
Obesity creates a milieu that induces more potent CHS-effector cells but does not have effects on already activated CHS-effector cells. IL-17A is essential for the pathogenesis of enhanced CHS during obesity. Our study provides novel knowledge about antigen-specific responses in obesity, which may help with the improvement of existing treatment and/or in designing novel treatment for obesity-associated skin disorders.
Keywords: contact hypersensitivity, dendritic cells, high-fat diet–induced obesity, skin inflammation
1 |. INTRODUCTION
Contact hypersensitivity reaction (CHS) is a delayed hypersensitivity to haptens. During the sensitization phase in animal models, activated keratinocytes produce proinflammatory cytokines—interleukin 1β (IL-1β) and tumor necrosis factor α (TNF-α)—promoting migration and maturation of skin dendritic cells (sDCs).1 sDCs like Langerhans cells (LCs) and dermal dendritic cells (dDCs) present antigen during both sensitization (induction) and elicitation (effector) phases of CHS, to shape the adaptive immune response. LCs have regulatory and stimulatory functions during sensitization in CHS,2 whereas Langerin (CD207)–expressing dDCs exert only stimulatory effects.3,4 Increasing evidence from a number of studies suggests that effector cells that mediate CHS are interferon γ (IFN-γ)–producing CD4+T helper 1 (Th1) and CD8+T cytotoxic 1 (Tc1) lymphocytes.5,6 Moreover, IL-17 is also required for hapten-specific T-cell sensitization, especially for CD4+T cells,7 whereas IL-17-producing CD8+ T-cell effectors provoke CHS.8
Inflammation plays an important role in obesity, with immune cell infiltration in the adipose tissue (AT) of obese humans and mice induced by a high-fat diet (HFD).9 CD4+Th1 cells, CD8+ Tc1 effector cells, and macrophages were found in the infiltrates of HFD-induced obese (HFDIO) mice, whereas the number of regulatory T cells was decreased.10 DCs in AT were found to enhance inflammation by inducing Th17 cell development.11 HFD also causes significant changes in gut microbiota (GM), characterized by a decreased abundance of Bacteroidetes and a correspondingly increased abundance of Firmicutes.12 Some gut bacteria promote anti-inflammatory responses, whereas others induce inflammatory reactions.13,14 GM dysbiosis and proinflammatory conditions in obese individuals predispose to various complications, including type 2 diabetes,12 liver diseases,15 cardiovascular diseases,16 and autoimmune disorders.17 Recent studies have shown that obesity is a major risk factor for the development of inflammatory skin diseases.18 Katagiri et al. demonstrated that HFDIO partially impairs skin immunity in some mouse strains,19 and Watanabe et al. reported that nickel allergy is more common in obese patients.20 However, there is still a significant knowledge gap in understanding the mechanism by which obesity affects skin diseases. To fill this knowledge gap, we investigated HFDIO and CHS to 2,4,6-trinitrochlorobenzene (TNCB) in mouse models. We demonstrated that HFDIO aggravates CHS in C57BL/6 mice by altering GM composition and promoting a proinflammatory response in vivo. Our in vitro studies revealed that the proinflammatory response occurs in the skin-draining lymph nodes (sLNs), in spleen (SPL), and subcutaneous adipose tissue (scAT). Moreover, we found that IL-17A plays an important role in the CHS aggravation.
2 |. METHODS
2.1 |. Mice
Wild-type male C57BL/6 (H2b) mice were purchased from the Jackson Laboratory Bar Harbor, ME, and were maintained at Yale University School of Medicine and in the Chair of Biomedical Sciences Jagiellonian University Medical College. IL-17A−/−C57BL/6 (H2b) breeders were kindly provided by Dr. R. A. Flavell (Yale University) and the colony was expanded at Yale University School of Medicine. The procedures used in this study were approved by Institutional Animal Care and Use Committee (IACUC) of Yale University and 1st Local Ethical Committee on Animal Testing in Krakow.
2.2 |. Sensitization and elicitation of CHS in vivo
Mice were sensitized by the application of 150 μL of 5% TNCB in an acetone-ethanol mixture (1:3 ratio) to the shaved abdomen (positive groups). Unsensitized mice fed with HFD or a normal diet (ND) for 8 weeks were used as negative controls. Four days later, all the mice were challenged on both ears with 10 μL of 0.4% TNCB in olive oil–acetone mixture (1:1 ratio). The ear thickness was measured prior to testing with a micrometer (Mitutoyo, Tokyo, Japan), by an observer unaware of the experimental groups and then again at 24 hours after challenge. Ear thickness was calculated as (Ear thickness [μm] 24 hours after challenge) — (Ear thickness [μm] before challenge). The ear swelling was expressed in μm ± standard error of the mean (SEM). Sections were examined under an Olympus BX50 microscope (Olympus, Japan). Images were recorded using a DP-71 digital CCD camera (Olympus, Japan) coupled to an IBM PC-class computer equipped with the AnalySIS-FIVE (Soft Imaging System GmbH, Münster, Germany) image analysis system.
2.3 |. Adoptive transfer of CHS
CHS-immune effector cells were from the donor mice, fed with HFD or ND for 8 weeks prior to sensitization with 5% TNCB. Immune cells from axillary and inguinal lymph nodes (ALNs), as well as SPL were isolated 4 days after TNCB sensitization. In brief, the minced ALN and SPL tissue was filtered through a 70-μm nylon filter, the cells were washed with phosphate-buffered saline (PBS) supplemented with 1% fetal bovine serum (FBS) and centrifuged at 4°C at 300g for 10 minutes. The supernatant was decanted, and the remaining cell pellets were resuspended with PBS and counted, based on trypan blue exclusion. Next, 8 × 106 ALNC/recipient and 16 × 106 SPLC/recipient or 2 × 107 ALNC+SPLC/recipient were injected intravenously into naive syngeneic recipients fed with ND or HFD. All the animals were challenged with 10 μL of 0.4% TNCB in an olive oil–acetone mixture (1:1 ratio) the next day after cell transfer and tested for CHS 24 hours later. The baseline of ear thickness was measured before cell transfer. Mice in the negative control group did not receive any cells prior to the challenge and CHS test.
More information about the materials and methods used in this study can be found online in the supporting information tab for this article.
3 |. RESULTS
3.1 |. Obesity exacerbates CHS in mice
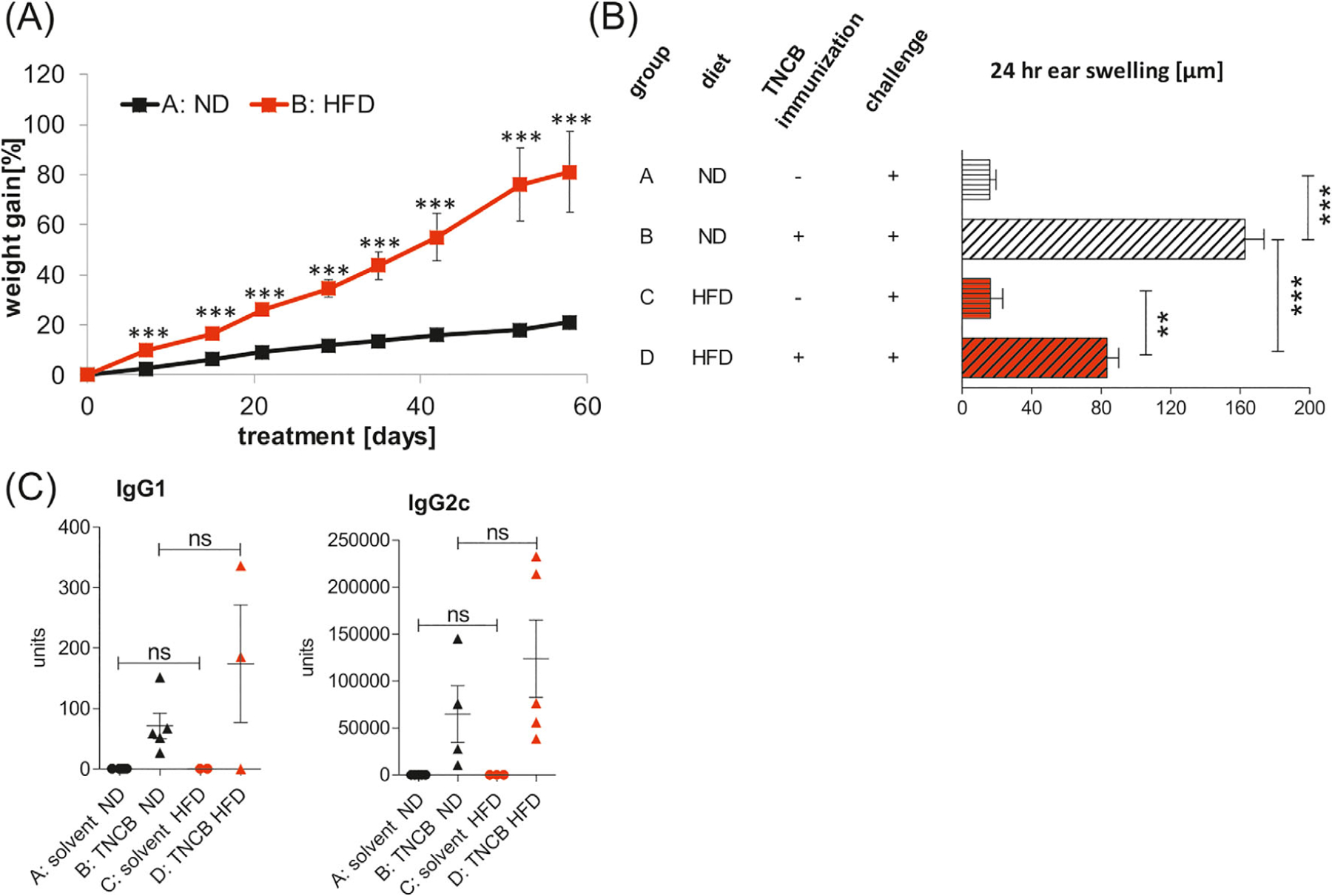
To determine the effect of obesity on CHS, we fed C57BL/6 mice with HFD or ND for 8 weeks prior to sensitization and challenge with TNCB. As expected, HFD-fed mice gained significantly more weight (Figure 1A) and, interestingly, developed enhanced CHS, indicated by ear swelling (Figure 1B, left panel, Group D vs B) and ear weight (Figure 1B, right panel, Group D vs B), when compared with control ND-fed mice. Histological examination in HFD-sensitized mice (Group D) revealed increased thickening of the edematous dermis; thickened, hyperplastic epidermis; as well as a significant accumulation of inflammatory cells (polymorphonuclear and mononuclear), mainly in dermis with formation of microabscesses in epidermis (Figure 1C, Group D vs B). Moreover, we found a strikingly elevated production of IL-17A, without obvious changes in IFN-γ and TNF-α secretion, by auricular lymph node cells (ELNCs) in HFD-fed mice, when compared with ND-fed mice (Figure 1D, Group C vs A and Group D vs B). It was shown previously that myeloperoxidase (MPO) promotes the induction of adaptive immune responses leading to DC activation and their migration from skin to draining lymph nodes and subsequent T cell priming.21 In our study, increased IL-17A secretion by ELNCs correlated with higher activity of MPO in ear tissue but only in obese mice, which were sensitized and challenged with the hapten (Figure 1E, Group D vs B). We also found a significant increase in the concentration of TNP-specific immunoglobulin 1 (IgG1) antibody in the obese mice when compared with the lean controls (Figure 1F, Group D vs B). TNP-specific IgG2c antibody was also elevated in the obese mice, but the changes were not significant (Figure 1G, Group D vs B).
FIGURE 1.
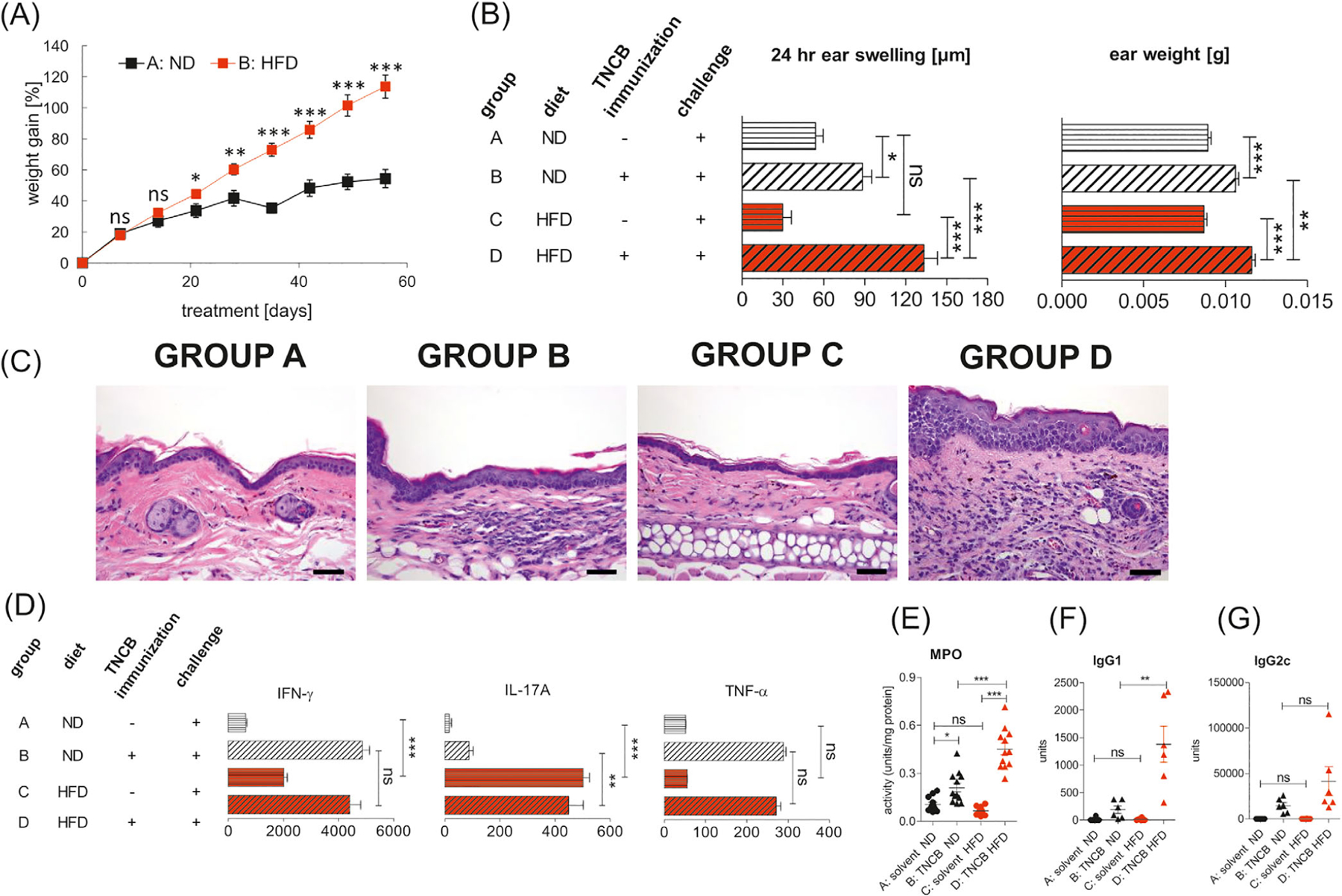
Enhanced CHS in obese C57BL/6 mice. CHS was induced after 8 weeks of feeding with HFD or ND. (A) Weight gain in mice fed with ND (group A) and HFD (group B); n = 12/group. (B) Ear swelling and ear weight in mice fed with ND (group A and B) or HFD (group C and D). Mice were sensitized (day 0) by application of 5% TNCB to the shaved abdomen (group B and D). Unsensitized mice fed with ND (group A) or HFD (group C) were used as negative controls. CHS and ear weight were measured 24 hours after challenge with TNCB (day +4); n = 12/group. (C) Histology of ear tissue after challenge with hapten TNCB in unsensitized mice on the ND (group A) and HFD (group C) and in sensitized mice fed with ND (group B) and HFD (group D). Hematoxylin and eosin staining. Scale bar = 50 μm. Representative images (magnification: ×40). (D) Concentrations of IFN-γ, IL-17A, and TNF-α in ELNC culture supernatants. Mice were fed with ND (group B) or HFD (group D) and subsequently sensitized and challenged with hapten TNCB. Negative control mice were fed with ND (Group A) or HFD (Group C) and subsequently challenged with hapten. ELNCs were isolated 24 hours after challenge and were cultured with antigen for 48 hours. The culture supernatants of ELNCs were collected and tested for cytokine concentration using an ELISA Kit; n = 5–7/group. (E), MPO activity in ear tissue after challenge with hapten TNCB in unsensitized mice (negative) on the ND (group A) and HFD (group C) and in TNCB-sensitized mice (positive) fed with ND (group B) and HFD (group D). MPO colorimetric activity assay was used to determine the activity of MPO in samples; n = 6–12/group. (F,G) Concentration of anti-TNP IgG1 and IgG2c antibodies in serum after challenge with hapten TNCB in unsensitized mice (negative) on the ND (group A) and HFD (group C) and in TNCB-sensitized (positive) mice fed with ND (group B) and HFD (group D). The collected sera were tested for antibodies concentration using an ELISA; n = 6–12/group. *P < .05, **P < .01, ***P < .001. Abbreviations: CHS, contact hypersensitivity reaction; ELNC, auricular lymph node cells; HFD, high fat diet; MPO, myeloperoxidase; ND, normal diet; TNCB, 2,4,6-trinitrochlorobenzene; TNP, 2,4,6-trinitrophenyl
3.2 |. HFD alters the bacterial population toward a proinflammatory profile but HFD-GM transfer does not affect CHS in the recipients
Studies support the notion of an association between the changes of GM composition and different autoimmune diseases22 and skin disorders.18 To determine if the exacerbated CHS response in HFD-fed mice was associated with dysbiosis, we assessed the abundance of some common GM by qPCR after 8 weeks of feeding with HFD. Our results showed that some species of GM were significantly altered in the obese mice compared to lean mice (Figure 2B), whereas others remained unchanged (Figure 2A). Among the tested species, the relative abundance of Bifidobacterium spp., Lactobacillus, Clostridium cluster IV, and Clostridium coccoides (cluster XIVa) was comparable between HFD-fed and ND-fed mice (Figure 2A, Group B vs A), whereas HFD significantly increased the relative abundance of Enterococcus spp., segmented filamentous bacteria (SFB), and Clostridium coccoides – E. rectale (cluster XIVab) (Figure 2B, Group B vs A). In contrast, the relative abundance of Bacteroidetes was significantly decreased in HFD-fed obese mice compared to the ND-fed mice (Figure 2B far right panel, Group B vs A). It is known that SFB induce IL-17–mediated inflammation23 and, interestingly, the abundance of SFB was significantly increased in HFD-fed mice, which is in line with the increase of IL-17A in ELNCs (Figure 1D). Further FMT transfers were performed to test whether an HFD-associated microbiota modulates CHS in lean recipients. The luminal contents of large intestine were harvested from the donors after 8 weeks of feeding with ND or HFD. The fecal supernatant was orally inoculated into the recipient mice, twice a week for 2 weeks, prior to CHS induction. To test the efficacy of FMT, the gut content of recipients were harvested 14 days after the first FMT. The results presented in Figure 2C showed in recipients of HFD-modified GM (Group B) the same trends in relative abundance of SFB, Clostridium coccoides – E. rectale (cluster XIVab) and Bacteroidetes, but the significant difference was found only in Clostridium cluster XIVab. Moreover, 2-week transfer of HFD-modified GM promoted a proinflammatory state in the scAT of the recipient mice with an increase in IL-6 and a decrease in IL-10 concentration (Figure 2D). Our previous study showed that antibiotic-induced GM dysbiosis downregulated CHS, which could be transferred to naive recipients, causing suppression of CHS.24 Of interest, HFD-induced GM dysbiosis did not transfer exacerbated CHS in the current study (Figure S1A, Group C vs B) or change the bodyweight of the 2-week FMT HFD recipients (Figure S1B).
FIGURE 2.
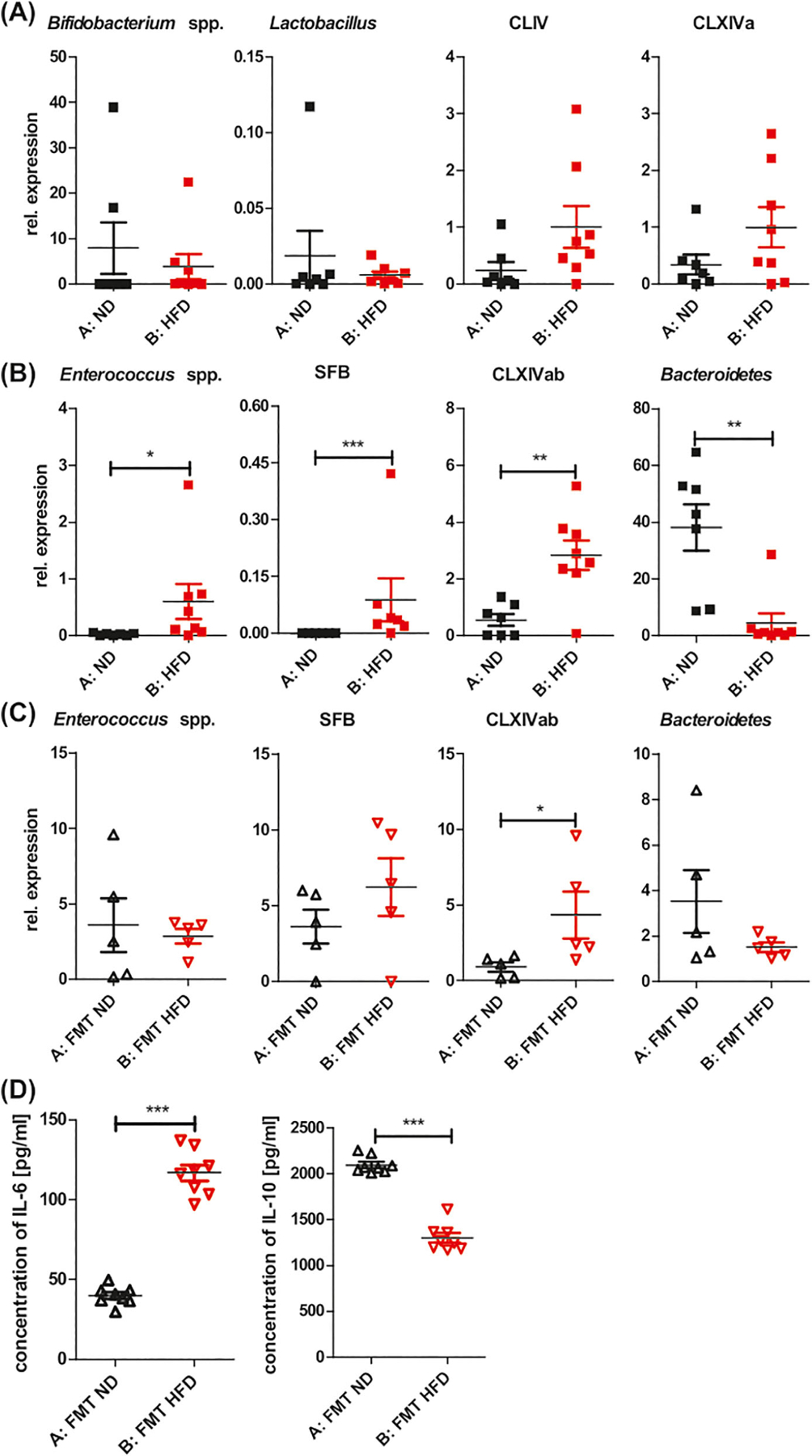
HFD modifies GM toward bacteria that induce a proinflammatory profile. Relative abundance of bacterial conserved 16S rDNA fragments in gut contents. qPCR was used to evaluate the alteration of gut bacteria. (A) Unchanged relative abundance of Bifidobacterium spp, Lactobacillus, Clostridium cluster IV (CLIV), and Clostridium cluster XIVa (CLXIVa) in mice fed with HFD vs ND for 8 weeks. (B) Increase in relative abundance of Enterococcus spp, SFB, and Clostridium cluster XIVab (CLXIVab) in mice fed with HFD vs ND for 8 weeks. Decrease in relative abundance of Bacteroidetes in mice fed with HFD vs ND for 8 weeks. (C) Relative abundance of Enterococcus spp, SFB, and Clostridium cluster XIVab (CLXIVab), Bacteroidetes in recipients of 2-week FMT HFD vs FMT ND. (D) Concentration of IL-6 and IL-10 in scAT of recipients of 2-week FMT HFD and FMT ND. The culture supernatants of scAT were tested for cytokine concentration using an ELISA; n = 6–8/group. *P < .05, **P < .01, ***P < .001. Abbreviations: FMT, fecal microbiota transplantation; GM, gut microbiota; qPCR, quantitative polymerase chain reaction; SFB, segmented filamentous bacteria
3.3.|. Obesity promotes a proinflammatory profile in T cells and dendritic cells (DCs)
CHS can be transferred into naive syngeneic recipients by intravenous injection of T effector cells.25 To determine if the exacerbated CHS seen in HFDIO mice is transferable, we performed adoptive cell-transfer experiments. Naive recipient mice were challenged with TNCB after receiving axillary and inguinal lymph node cells (ALNCs) or splenocytes (SPLCs) from TNCB-sensitized ND-fed lean donors or TNCB-sensitized HFD-fed obese donors. As shown in Figure 3A, the recipients of ALNCs and SPLCs from obese donors developed increased CHS, compared to the mice that received ALNCs or SPLCs from lean donors (Group C vs B and Group E vs D). Our results demonstrate that HFDIO increased T effector cell function as they transferred aggravated CHS into naive syngeneic recipients. We also found that CHS was significantly less in obese recipients after transfer of CHS-effector cells, isolated from TNCB-sensitized lean mice, when compared to CHS in lean and obese recipients after transfer of CHS-effector cells from TNCB-sensitized HFD-fed donors (Figure 3B, Group D vs C and E, respectively). These results suggest that HFDIO created the milieu for induction of more potent T effector cells, but not for the T cells already activated in lean donors.
FIGURE 3.
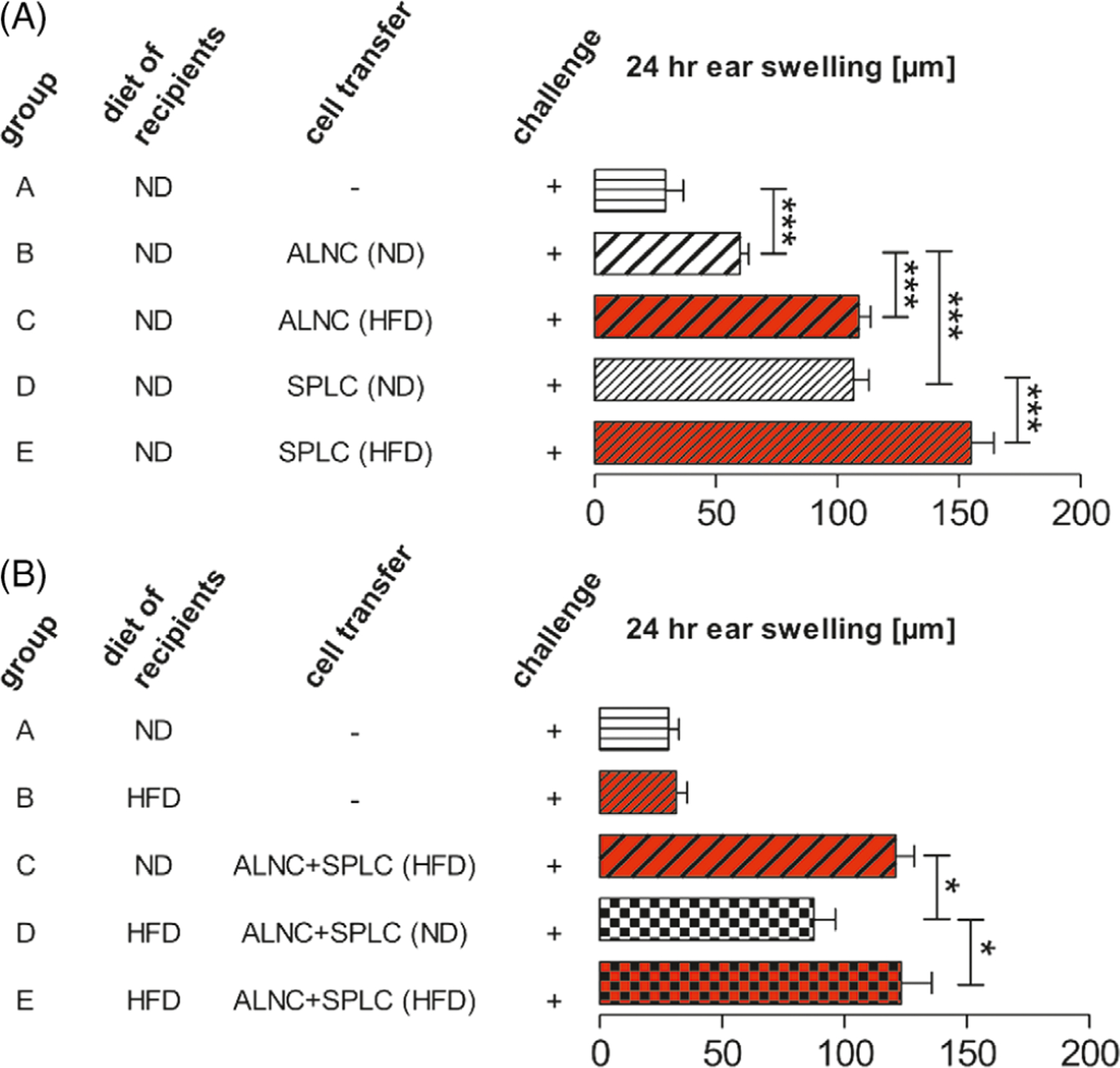
ALNCs and SPLCs from HFDIO donors transfer elevated CHS into naive syngeneic recipients. (A) Transfer of ALNCs (group B) and SPLCs (group D) from donors fed with ND and TNCB-sensitized. Transfer of ALNCs (group C) and SPLCs (group E) from donors fed with HFD for 8 weeks and TNCB-sensitized. Number of transferred 4-day immune cells: 8 × 106 ALNC/recipient and 16 × 106 SPLC/recipient. Negative control mice did not receive any cells (group A). All mice were challenged with hapten TNCB. CHS was measured 24 hours after challenge with TNCB. (B) HFDIO does not influence T-cell function in the effector phase of CHS. Transfer of ALNCs+SPLCs from 8-week HFD-fed and TNCB-sensitized donors into ND-fed recipients (group C) or 8-week HFD-fed recipients (group E). Transfer of ALNCs+SPLCs from ND-fed and TNCB-sensitized donors into 8-week HFD-fed recipients (group D). Number of transferred 4-day immune cells: 2×107. Negative control mice did not receive any cells (group A and group B). All mice were challenged with TNCB. CHS was measured 24 hours after challenge with TNCB. n = 7–16/group. *P < .05, ***P < .001. Abbreviations: ALNC, axillary and inguinal lymph node cells; HFDIO, HFD-induced obesity/HFD-induced obese; SPLC, splenocytes
It is known that CHS responses in mice are mediated by either MHC class II-restricted CD4+Th1 or MHC class I-restricted CD8+Tc1 cells, through locally released IFN-γ and/or IL-17,26,27 and these cytokines recruit more inflammatory infiltrates and direct cytotoxic damage to local keratinocytes respectively.28,29 To investigate the cellular mechanism involved in the aggravation of CHS in obese mice, we examined the phenotype of T cells in axillary and inguinal lymph nodes ALNs and spleen SPL after TNCB sensitization (induction phase of CHS). We found a significantly higher percentage of CD4+IL-17A+ cells in ALNs and SPL from HFD-fed mice (Figure 4A,B, far left panel) and increased frequency of CD4+CCR7+ ALNCs (Figure 4A, far right panel). The frequency of CD4+IFN-γ+ in SPLCs, but not in ALNCs, was significantly increased in HFDIO mice (Figure 4B, second panel from the left). Furthermore, we found that obese mice had a significantly lower frequency of IL-4– and IL-10–producing CD4+ T cells in SPL (Figure 4B, middle panel and second panel from the right), but, interestingly, not in ALNs (Figure 4A, middle panel and second panel from the right). In contrast, the proportion of CD4+CD25+FoxP3+ Treg cells from obese TNCB-sensitized mice was similar to the TNCB-treated lean mice (data not shown). Apart from the CHS T-effector cells, DETCs play an important role in CHS. TCRγδ cells, including DETCs, are crucial regulators of immune responses to contact allergens. Although some studies have suggested that TCRγδ cells might play an anti-inflammatory role during CHS,30,31 the majority of studies point to a proinflammatory role of TCRγδ cells in CHS.32 It was found that TCRγδ cells assist TCRαβ cells in the transfer of CHS from sensitized to naïve mice,33–37 as adoptive transfer of only TCRαβ cells was not sufficient to transfer CHS. Of interest, the TCRγδ cells that assist the TCRαβ cells were shown to be DETCs.33,34 Because our work showed involvement of IL-17A in aggravation of CHS in obese mice, we decided to evaluate the percentage of IL-17A+TCRγδ+ cells in peripheral immune organs. However, the percentage of IL-17A+TCRγδ+ cells in ALNCs and SPLCs from the obese mice was similar to that observed in the lean control mice (data not shown).
FIGURE 4.
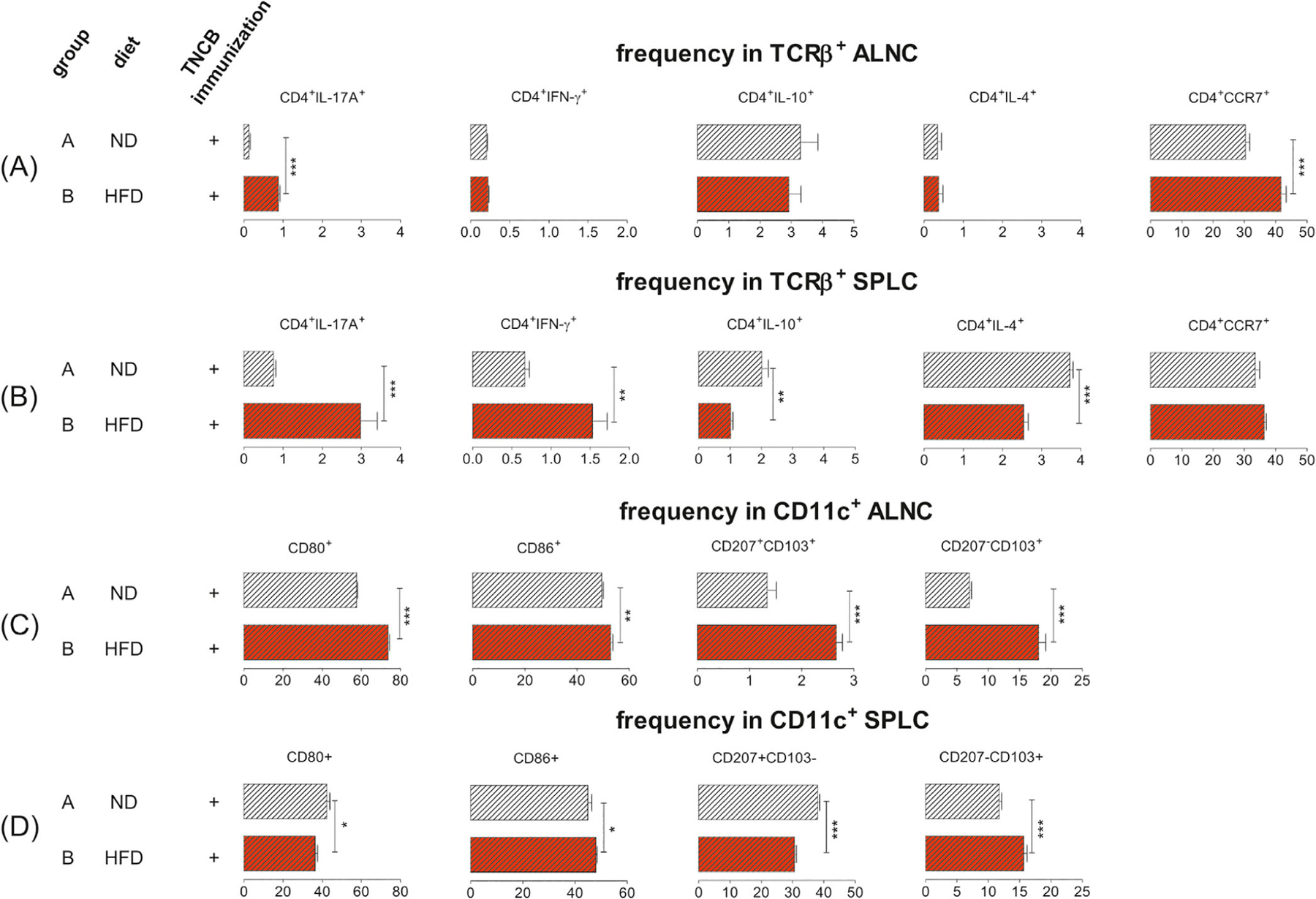
HFDIO promotes pro-inflammatory T cells and DCs in obese (group B) vs lean mice (group A) 4 days after TNCB sensitization. (A) The frequency of CD4+IL-17A+, CD4+IFN-γ+, CD4+IL-10+, CD4+IL-4+, and CD4+CCR7+ T cells in the TCRβ+ population in ALNCs. (B) The frequency of CD4+IL-17A+, CD4+IFN-γ+, CD4+IL-10+, CD4+IL-4+, and CD4+CCR7+ T cells in the TCRβ+ population in SPLCs. (C) The expression of CD80 and CD86, and frequency of CD207+CD103+ and CD207−CD103+ cells in the CD11c+ population in ALNCs. (D) The expression of CD80 and CD86, and frequency of CD207+CD103− and CD207−CD103+ cells in the CD11c+ population in SPLCs. Samples were analyzed in a flow cytometer. Gating information is presented as Supplementary Information. n = 5/group. *P < .05, **P < .01, ***P < .001.
Abbreviation: DCs, dendritic cells
In the initiation phase of CHS, both LCs and dDCs migrate into sLNs and present antigen to naïve T lymphocytes.38,39 It is conceivable that the exacerbated CHS response in obese mice was due to the proinflammatory phenotype of DCs. To test this hypothesis, we examined the DCs phenotype in ALNs and SPL. We found a higher expression of costimulatory molecules (CD80 and CD86) on CD11c+ DCs in the ALNs of the sensitized obese mice, compared to the lean controls (Figure 4C, far left panel and second panel from the left). The expression of CD86 in splenic DCs of the obese mice was also significantly increased (Figure 4D, second panel from the left). However, the expression of CD80 in splenic CD11c+ cells was decreased (Figure 4D, far left panel). Of interest, we found an increased frequency of CD103+ DCs, regardless of CD207 expression, in ALNs (Figure 4C, the two panels on the right), whereas the increased CD103+ DCs in the spleen were CD207− (Figure 4D, far right panel) in TNCB-immunized obese mice, compared to lean control mice. We also found decreased frequency of CD207+CD103− DCs in SPL from TNCB-immunized obese mice when compared with lean control mice (Figure 4D, second panel from the right). The gating strategies are presented in Figures S2-S5.
3.4 |. Proinflammatory milieu in scAT of obese mice
Chen et al. reported that CD11c+ cells from epididymal AT are proinflammatory ATDCs not macrophages.11 To investigate the potential role for ATDCs in the enhanced CHS, we analyzed cell infiltrates in the scAT, which is adjacent to the skin. In line with the weight gain shown in Figure 1A, the weight of scAT from obese mice was also significantly increased when compared to the lean controls (Figure 5A, Group C vs A and Group D vs B). The proinflammatory cytokine IL-6 was significantly higher in the scAT of both naive and TNCB-sensitized obese mice compared to lean controls (Figure 5B, left panel, Group C vs A and Group D vs B, respectively). In contrast, the anti-inflammatory cytokine IL-10 was markedly decreased in scAT of the TNCB-sensitized obese mice, compared to the lean counter-parts (Figure 5B, right panel, group D vs B). In addition, we found a significant decrease in the proportion of Treg cells (Figure 5C, left panel, Group D vs B) but a higher percentage of TCRβ+CD4+ (Figure 5C, middle panel, Group D vs B) and TCRβ+CD4− cells expected as CD8+ T lymphocytes (Figure 5C, right panel, Group D vs B) in scAT of the obese mice. The expression of CD80 and CD86 (Figure 5D) in CD11c+ cells was also increased in the obese and sensitized mice compared with the lean controls (Group D vs C). The gating strategies are presented in Figures S6-S8. Our results suggest that obesity promotes an inflammatory milieu in the scAT, which attracts more immune cells and aggravates the inflammation.
FIGURE 5.
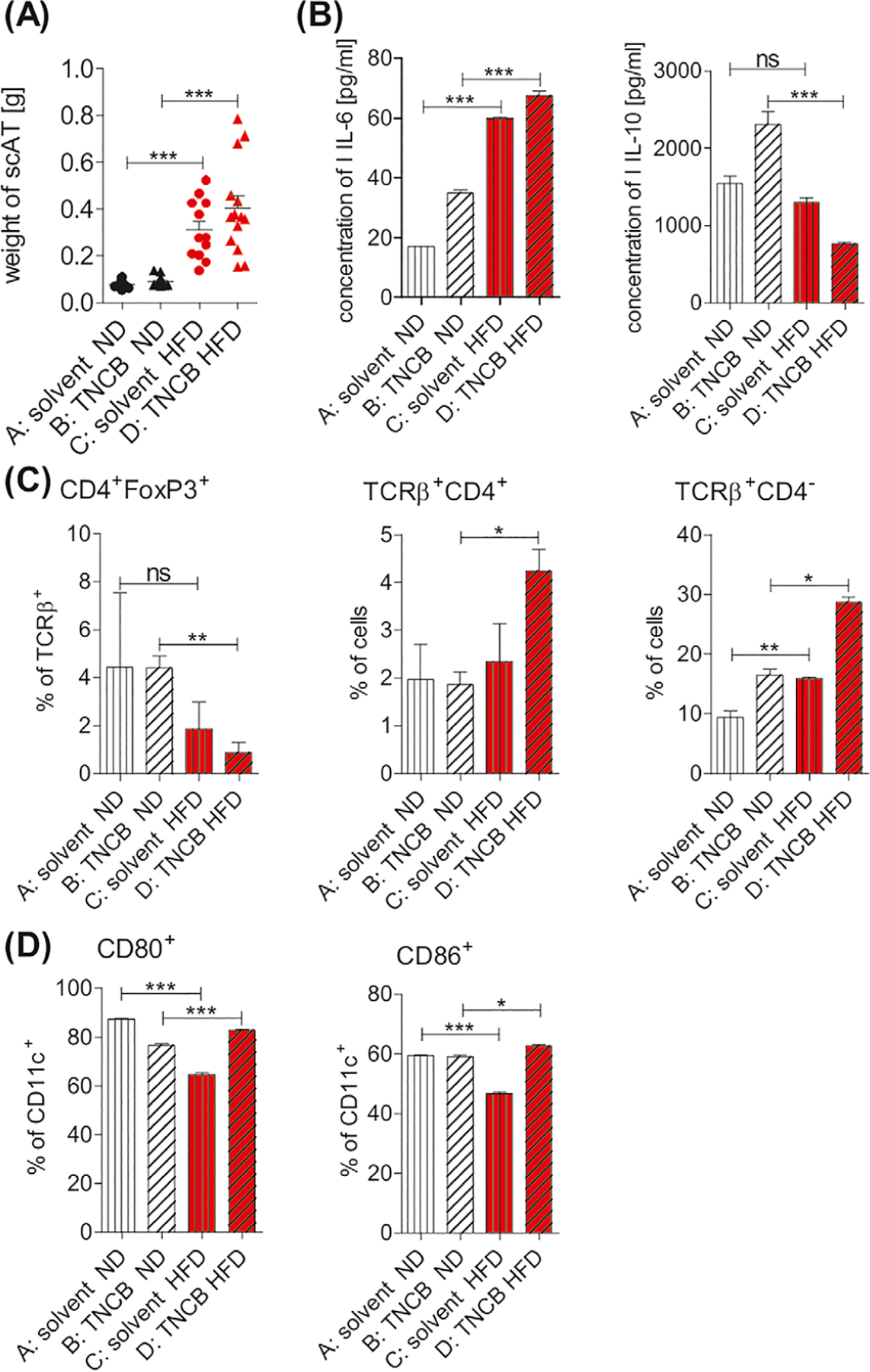
Mice with HFDIO have a proinflammatory milieu in scAT. Mice were fed with ND (group A and B) or HFD (group C and D). Immunization with hapten TNCB was performed in group B and group D (positive groups). (A) Weight of scAT; n = 10–14/group. (B) Concentration of IL-6 and IL-10 in scAT tissue culture supernatants using an ELISA; n = 2–7/group. (C) Frequency of CD4+FoxP3+, TCRβ+CD4+, and TCRβ+CD4− (expected as TCRβ+CD8+ T cells); n = 2–5/group. (D) Frequency of CD80+ and CD86+ among CD11c+ cells; n = 2–5/group. Samples were analyzed in a flow cytometer. Gating information is presented as Supplementary Information. *P < .05, **P < .01, ***P < .001. Abbreviation: scAT, subcutaneous adipose tissue
3.5 |. IL-17A is required for the exacerbated CHS in obese mice
Given that IL-17A is the highest upregulated inflammatory cytokine in our model system (Figure 1D; Figure 4A,B), we hypothesized that IL-17A is essential in aggravated CHS in the HFDIO mice. To test our hypothesis, we fed IL-17A−/−B6 mice with HFD for 8 weeks prior to CHS induction. As presented in Figure 6, IL-17A−/−B6 mice fed with HFD gained significantly more weight (Figure 6A, Group B vs A). In contrast to C57BL/6 mice, obese IL-17A−/−B6 mice did not develop enhanced CHS indicated by ear swelling (Figure 6B, Group D vs B). Moreover, the TNP-specific IgG1 antibody level was not statistically different, although elevated in the obese IL-17A−/−B6 mice, compared with the lean IL-17A−/−B6 mice (Figure 6C, Group D vs B in the left panel). There was also no difference in TNP-specific IgG2c antibody level (Figure 6C, Group D vs B in the right panel).
FIGURE 6.
Lack of increased CHS in obese IL-17A−/−B6 mice. CHS was induced after 8 weeks of feeding with HFD or ND. (A) Weight gain in mice fed with ND (group A) and HFD (group B); n = 5–8/group. (B) Ear swelling in mice fed with ND (group A and B) or HFD (group C and D). Mice were sensitized by application of 5% TNCB on the shaved abdomen (group B and D). Unsensitized mice fed with HFD (group C) or ND (group A) were used as negative controls. CHS was measured 24 hours after challenge with TNCB. (C) Concentration of anti-TNP IgG1 and IgG2a antibodies in serum after challenge with hapten TNCB in unsensitized mice (negative) on the ND (group A) and HFD (group C) and in TNCB-sensitized mice (positive) fed with ND (group B) and HFD (group D). The collected sera were tested for antibodies concentration using an ELISA; n = 2–8/group. ***P < .001
4 |. DISCUSSION
Our study has revealed the following findings: (1) HFDIO leads to exacerbated T cell–dependent CHS in C57BL/6 mice; (2) HFDIO promotes a proinflammatory profile of GM and creates the milieu for induction of more potent CHS-effector cells; (3) HFDIO does not modulate the function of effector cells in the CHS effector phase; and (4) exacerbated CHS in obese mice is IL-17A dependent. Our findings support results reported by others. The increased ear swelling correlating with histological changes in obese mice in our study is in line with earlier studies reporting an elevated inflammatory response to haptens and nonspecific irritants in obese mice.40 Recent study published by Rühl-Muth et al. showed that HFD increases the initial reaction of mice to TNCB manifested by higher neutrophil migration and overproduction of IL-6 and TNF-α in the ear tissue. In addition, the increase in saturated fatty acids of the HFD and its control diet created the inflammatory milieu resulting in the induction of CHS independent of TLR2 and TLR4.41 Moreover, the human study also reported that obesity exacerbated inflammatory skin diseases, including eczema, atopic dermatitis, and psoriasis.42 Of interest, another human study showed an association of diet-induced weight loss with reduced delayed-type hypersensitivity (DTH) responsiveness in obese humans.43 Our results are in contrast with those reported by Katagiri et al., showing that C57BL/6 mice but not BALB/c mice fed with HFD for 4 weeks or longer became obese and showed impaired CHS response, although both strains of mice showed enhanced irritant response to TNCB, an elevated inflammatory response to haptens, and non-specific irritants in obese mice.19 The discrepancy may arise from (1) the use of a different protocol to study CHS; (2) the use of different sex of mice; and (3) the use of HFD with oleic acid–rich oil, which attenuates inflammation in obese mice.44 The protocol for CHS in our study differs significantly from that used by Katagiri et al. in many aspects, including the dose of hapten and the composition of the solvents used for both sensitization and elicitation, as well as the time of CHS test after sensitization. However, we believe that the strong influencing factor for the difference between our and Katagiri et al. observation was likely to be the dose of TNCB used. We used a 30 times higher sensitization dose of the hapten than that used by Katagiri et al.19 It is clear that further investigations and standardization of the experimental protocols, including the mouse use (strain and sex) are needed.
Obesity is associated with chronic, low-grade inflammation in tissues and promotes the development of numerous diseases. It has been reported that B cells play a pathogenic role in the development of adipose tissue inflammation during obesity, causing increased plasma concentrations of IgG1 antibody.45,46 The obese mice in our study have increased levels of TNP-specific IgG1 antibody, the isotype associated with aggravated CHS to TNCB.47 Several studies have indicated the role of IL-17A in the onset and/or progression of chronic inflammatory diseases in obese mice and humans.48–50 Our in vitro experiments revealed higher production of IL-17A by ELNCs in obese mice, whereas IFN-γ and TNF-α were unchanged when compared with lean mice. Moreover, the higher IL-17A secretion was accompanied by an increased MPO activity in ear homogenates. Of interest, we found significantly increased IL-17A concentration in ELNC supernatants from naive HFD-fed mice when compared to naive mice receiving ND. This observation may suggest that HFDIO alone promotes inflammation after hapten application and that IL-17A mediates neutrophil recruitment into the inflammation site.51 The role of IL-17A in upregulated CHS during obesity in our study was further confirmed in IL-17A−/−B6 mice, which exhibited normal CHS to TNCB despite the presence of obesity.
We found that the stronger CHS in obese mice was transferable to lean recipients by CHS-effector cells from obese donors, whereas CHS-effector cells from lean donors failed to induce stronger CHS in the obese recipients. These results suggest a role for obesity in the induction of potent CHS-effector cells, whereas obesity does not affect function of preexisting CHS-effector cells during the effector phase of CHS. Phenotypic analysis revealed that effector cells were predominantly proinflammatory CD4+ T cells in the obese mice, including (1) increased frequency of CD4+IL-17A+T cells in both ALNs and SPL; (2) upregulated frequency of CD4+IFN-γ+ in SPL, and (3) lower frequency of CD4+IL-4+ and CD4+IL-10+ in SPL. There might be at least two reasons that the pattern of immune response differs in ALNs and SPL. First, SPL is not the major skin-draining lymphoid tissue like ALNs. Second, SPL, as a systemic and major lymphoid organ, has a large repertorie of different immune cells including various cells with regulatory activity.52 Our data are in agreement with a previous study reporting an increased number of CD4+IL-17+ cells in the draining lymph nodes of obese mice compared to lean mice whereas the CD4+IFN-γ+ T cell compartment was unaffected by HFDIO.53
During inflammation, dDCs are mobilized rapidly from the skin and home to draining lymph nodes within 48 hours, preceding the arrival of LCs.54 We have shown that the enhanced CHS response in TLR- and MyD88-deficient NOD mice is associated with an enriched proportion of Th1-inducing CD207−CD103+ dDCs and a decreased proportion of tolerizing CD207+CD103− LCs in ALNs.47 Our results in the current study showed that the increased CHS in obese mice was accompanied by reduced frequency of CD207+CD103− DCs, without affecting splenic Treg cells, although CD207+ dDCs or CD103− dDCs can induce Treg cells in C57BL/6 mice.55–57 Nagao et al. suggested that LCs and CD207+ dDCs act as negative regulators of Th1 cellular responses, since mice lacking both LCs and CD207+ dDCs have an increased number of CD4+IFN-γ+ SPLCs.58 Our data are in line with these findings, as we found a higher frequency of CD4+IFN-γ+, along with a lower abundance of CD207+CD103− cells in SPL of TNCB-sensitized obese mice. CD207+CD103+ and CD207−CD103+ dDCs also play a role in CD4+ T cell–mediated autoimmune diseases,59 and we found more abundant populations of CD207+CD103+ and CD207−CD103+ DCs in ALNs and CD207−CD103+ DCs in SPL in TNCB-sensitized obese mice. It is possible that the enhanced CHS response in obese C57BL/6 mice was associated with increased proportions of CD207+CD103+ and CD207−CD103+ DCs, which induced subsequently higher abundance of CD4+IL-17A+ and CD4+IFN-γ+ cells. The higher expression of the costimulatory molecules, CD80 and CD86, in CD11c+ cells in obese mice lends support to this possibility. Taken together, our results suggest that the enhanced CHS response in obese mice is associated with proinflammatory phenotype of DCs and T cells.
CCR7 is a key regulator of T lymphocyte migration from the skin to the draining lymph nodes.60 Förster et al. found that impaired migration of T cells to ALNs was due to a lack of CCR7 on T lymphocytes, and the reduction of the T cell–mediated CHS reaction.61 A recent study suggests that CCR7 plays a causal role in maintaining innate and adaptive immunity in obesity.46 Our current study demonstrated that obesity resulted in a higher expression of CCR7 on CD4+T cells in local ALNs but not in systemic SPL after TNCB sensitization. We also demonstrated a proinflammatory milieu in the scAT of TNCB-sensitized obese mice, suggesting that the immune cell traffic is likely through adipose tissue depots during acute inflammation.62 Our finding supports the notion that AT inflammation occurs in obesity, accompanied by a higher number and activation of proinflammatory cells.10,63 The proinflammatory state in our study was manifested by (1) increased production of IL-6 but decreased secretion of IL-10; (2) a higher frequency of CD4+ and CD8+ T cells but a lower frequency of CD4+FoxP3+ cells; and (3) elevated expression of costimulatory molecules such as CD80 and CD86 by DCs. Zhong et al. showed that B7 costimulation reduced adipose inflammation by maintaining the number of regulatory T cells in adipose tissue. The authors also demonstrated that decreased B7 expression in obesity appeared to directly impair Treg proliferation and function that led to excessive proinflammatory macrophages and the development of insulin resistant.64 We also found a lower percentage of Treg cells, which is in line with the lower expression of CD 80 and CD86 markers in the scAT of obese mice (Figure 5B,C). It should be pointed out that in our model, unsensitized obese mice did not show a higher expression of CD80 and CD86 by CD11c+ cells, as reported previously.11 The imbalance between a proinflammatory and anti-inflammatory state in scAT in our study confirmed the presence of obesity-related local and systemic inflammation, which may have enhanced T cell–dependent CHS in the obese mice.
Increasing evidence demonstrates that the GM dysbiosis has an impact on immune responses and immune-related disorders.13,14 The GM composition can be influenced by several epigenetic factors.65 We have shown previously that 2-week oral administration of enrofloxacin disturbs the natural composition of GM and induces regulatory immune cells, which suppress CHS inflammatory response.24 In this study, we described GM dysbiosis after 8 weeks of HFD feeding in C57BL/6 mice. It has been reported that obese mice and humans have a proinflammatory bacterial community, with a decrease in Bacteroidetes and an increase in Firmicutes.66,67 Our results support these findings, as we observed a significantly higher abundance of Enterococus spp. (Firmicutes, class Bacilli) and a decreased abundance of Bacteroidetes in the gut of the obese mice. Moreover, it has been reported that Enterococcus faecalis CECT7121 induces the accumulation of splenic IFN-γ–producing cells in C57BL/6 mice,68 whereas Bacteroidetes fragilis increases the percentage of IL-10–producing Foxp3+ Treg cells in the gut.69 Of interest, in some obese patients, weight loss is associated with significant increase of Bacteroides-Prevotella abundance.70 Our results indicate a higher presence of Enterococcus spp and lower abundance of Bacteroidetes in obese mice likely promoted the augmented CHS, accompanied by an increase in CD4+IFN-γ+, and a decrease in CD4+IL-10+ T cells in lymphoid tissue. It is notable that we found a significant increase in relative abundance of SFB in obese mice, which is crucial for Th17 induction.23,71,72 The increase of SFB in obese mice in our model indeed correlated with a higher frequency of CD4+IL-17A+ cells in ALNs and SPL and elevated production of IL-17A by ELNC. GM dysbiosis in obese C57BL/6 was also characterized by changes in three clusters of the genus Clostridium. We did not find significant changes in relative abundance of Clostridium cluster IV and XIVa, which has been shown to promote Treg cell accumulation.73 However, Clostridium coccoides – Eubacterium rectale (cluster XIVab) was more abundant in the gut of obese mice, supporting the human study demonstrating its reduction in patients in whom weight loss exceeded 4 kg.70 We previously reported that 2-week FMT from antibiotic-treated donors downregulated CHS in the recipients.24 Other investigators also showed that 2-week FMT is sufficient to modify the microbiota and immune response in the recipients.74–76 However, in our model, 2-week FMT from the obese donors neither affected CHS nor changed the bodyweight of the lean recipients. Recent data showed that mice housed in SPF conditions are less likely to exhibit phenotypic changes after microbiota transplantation.77 Thus, we assume that our clean mouse housing conditions can influence the FMT effect. In summary, it is possible that (1) the increase in anti-inflammatory SCFA-producing Clostridium cluster XIVab (CLXIVab) could be responsible for the lack of apparent influence of the microbiota in the FMT HFD on CHS, (2) 2-week FMT is not sufficient; and (c) HFD-induced GM dysbiosis and obesity-related low-grade inflammation need to coexist for the development of aggravated CHS. Thus, HFD is required for the recipients prior to CHS test. It is clear that further investigation is required.
In summary, our study demonstrates that HFD modifies GM composition that promotes a proinflammatory state in scAT. HFDIO also promotes proinflammatory T cells and DCs locally and systemically. Moreover, IL-17A is essential in the pathogenesis of enhanced CHS. Our study provides novel knowledge related to obesity-associated exacerbated CHS and we hope our results will aid in improvement of existing treatment and/or in designing novel treatment for obesity-associated skin disorders.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Lucy Zhang and Dorota Woźniak, and Dominika Biała and Aneta Kiecka for taking care of the mouse colonies, and the members in LWʼs lab for technical help and scientific suggestions. This study was supported by grant from Ministry of Science and Higher Education IP2012 0443 72 and K/ZDS/007123 to MMS and National Institutes of Health (NIH) DK045735 to LW (Yale Diabetes Center Mouse Core).
Funding information
Foundation for the National Institutes of Health, Grant/Award Number: DK045735; Ministry of Science and Higher Education, Grant/Award Numbers: IP2012 0443 72, K/ZDS/007123
Abbreviations:
- ALNs
axillary and inguinal lymph nodes
- ALNCs
axillary and inguinal lymph node cells
- AT
adipose tissue
- ATDCs
adipose tissue dendritic cells
- CHS
contact hypersensitivity reaction
- DCs
dendritic cells
- dDCs
dermal dendritic cells
- ELNCs
auricular lymph node cells
- FMT
fecal microbiota transplantation
- FMT HFD
fecal microbiota transplantation from HFD-fed donors
- FMT ND
fecal microbiota transplantation from ND-fed donors
- GM
gut microbiota
- HFD
high-fat diet
- HFDIO
HFD-induced obesity/HFD-induced obese
- LCs
Langerhans cells
- LNCs
lymph node cells
- MPO
myeloperoxidase
- ND
normal diet
- scAT
subcutaneous adipose tissue
- sDCs
skin dendritic cells
- sLNs
skin draining lymph nodes
- SPL
spleen
- SPLC
splenocytes
- TNCB
2,4,6-trinitrochlorobenzene
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
Data are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Honda T, Egawa G, Grabbe S, Kabashima K. Update of immune events in the murine contact hypersensitivity model: toward the understanding of allergic contact dermatitis. J Invest Dermatol 2013;133(2):303–315. doi: 10.1038/jid.2012.284 [DOI] [PubMed] [Google Scholar]
- 2.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity 2005;23(6):611–620. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Bursch LS, Kissenpfennig A, Malissen B, Jameson SC, Hogquist KA. Langerin expressing cells promote skin immune responses under defined conditions. J Immunol 2008;180(7):4722–4727. [DOI] [PubMed] [Google Scholar]
- 4.Bursch LS, Wang L, Igyarto B, et al. Identification of a novel population of Langerin+ dendritic cells. J Exp Med 2007;204(13):3147–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang B, Fujisawa H, Zhuang L, et al. CD4+ Th1 and CD8+ type 1 cytotoxic T cells both play a crucial role in the full development of contact hypersensitivity. J Immunol 2000;165(12):6783–6790. [DOI] [PubMed] [Google Scholar]
- 6.Mori T, Kabashima K, Yoshiki R, et al. Cutaneous hypersensitivities to hapten are controlled by IFN-γ-upregulated keratinocyte Th1 chemokines and IFN-γ-downregulated langerhans cell Th2 chemokines. J Invest Dermatol 2008;128(7):1719–1727. doi: 10.1038/jid.2008.5 [DOI] [PubMed] [Google Scholar]
- 7.Nakae S, Komiyama Y, Nambu A, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 2002;17(3): 375–387. [DOI] [PubMed] [Google Scholar]
- 8.He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. CD8+ IL-17 producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J Immunol 2006;177(10):6852–6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112(12):1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrionol 2014;222(3):R113–R127. doi: 10.1530/JOE-14-0283 [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Tian J, Tian X, et al. Adipose tissue dendritic cells enhances inflammation by prompting the generation of Th17 cells. PLoS One 2014;9(3):e92450. doi: 10.1371/journal.pone.0092450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harsch IA, Konturek PC. The role of gut microbiota in obesity and type 2 and type 1 diabetes mellitus: new insights into “old” diseases. Med Sci (Basel) 2018;6(2):32. doi: 10.3390/medsci6020032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science 2012;336(6086):1268–1273. doi: 10.1126/science.1223490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosiewicz MM, Zirnheldand AL, Alard P. Gut microbiota, immunity, and disease: a complex relationship. Front Microbiol 2011;2:180. doi: 10.3389/fmicb.2011.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llorente C, Schnabl B. The gut microbiota and liver disease. Cell Mol Gastroenterol Hepatol 2015;1(3):275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miele L, Giorgio V, Alberelli MA, De Candia E, Gasbarrini A, Grieco A. Impact of gut microbiota on obesity, diabetes, and cardiovascular disease risk. Curr Cardiol Rep 2015;17(12):120. doi: 10.1007/s11886-015-0671-z [DOI] [PubMed] [Google Scholar]
- 17.Li B, Selmi C, Tang R, Gershwin ME, Ma X. The microbiome and autoimmunity: a paradigm from the gut–liver axis. Cell Mol Immunol 2018; 15(6):595–609. doi: 10.1038/cmi.2018.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salem I, Ramser A, Isham N, Ghannoum AM. The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol 2018;9:1459. doi: 10.3389/fmicb.2018.01459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katagiri K, Arakawa S, Kurahashi R, Hatano Y. Impaired contact hypersensitivity in diet-induced obese mice. J Dermatol Science 2007; 46(2):117–126. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe M, Masieri S, Costantini D, et al. Overweight and obese patients with nickel allergy have a worse metabolic profile compared to weight matched non-allergic individuals. PLoS One 2018;13(8): e0202683. doi: 10.1371/journal.pone.0202683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strzepa A, Gurski CJ, Dittel LJ, Szczepanik M, Pritchard KA, Dittel BN, et al. Neutrophil-derived myeloperoxidase facilitates both the induction and elicitation phases of contact hypersensitivity. Front Immunol 2021;11:608871. doi: 10.3389/fimmu.2020.608871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Opazo MC, Ortega-Rocha EM, Coronado-Arrázola I, et al. Intestinal microbiota influences non-intestinal related autoimmune diseases. Front Microbiol 2018;9:432. doi: 10.3389/fmicb.2018.00432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strzępa A, Majewska-Szczepanik M, Lobo FM, Wen L, Szczepanik M. Broad spectrum antibiotic enrofloxacin modulates contact sensitivity through gut microbiota in a murine model. J Allergy Clin Immunol 2017;140(1):121–133.e3. doi: 10.1016/j.jaci.2016.11.052 [DOI] [PubMed] [Google Scholar]
- 25.Majewska-Szczepanik M, Strzepa A, Marcińska K, Wen L, Szczepanik M. Epicutaneous immunization with TNP-Ig and zymosan induces TCRαβ+ CD4+ contrasuppressor cells that reverse skin-induced suppression via IL-17A. Int Arch Allergy Immunol 2014; 164(2):122–136. doi: 10.1159/000363446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, Balato A, Fishelevich R, Chapoval A, Mann DL, Gaspari AA. Th17/Tc17 infiltration and associated cytokine gene expression in elicitation phase of allergic contact dermatitis. Br J Dermatol 2009; 161(6):1301–1306. doi: 10.1111/j.1365-2133.2009.09400.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. IL-17 and IFN-gamma mediate the elicitation of contact hypersensitivity responses by different mechanisms and both are required for optimal responses. J Immunol 2009;183(2):1463–1470. doi: 10.4049/jimmunol.0804108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalish RS, Askenase PW. Molecular mechanisms of CD8+ T cell-mediated delayed hypersensitivity: implications for allergies, asthma and autoimmunity. J Allergy Clin Immunol 1999;103(2):192–199. [DOI] [PubMed] [Google Scholar]
- 29.Gocinski BL, Tigelaar RE. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J Immunol 1990;144(11):4121–4128. [PubMed] [Google Scholar]
- 30.Szczepanik M, Anderson LR, Ushio H, et al. γδ T cells from tolerized αβ T cell receptor (TCR)-deficient mice inhibit contact sensitivity-effector T cells in vivo, and their interferon-γ production in vitro. J Exp Med 1996;184(6):2129–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szczepanik M, Ptak W, Askenase PW. Role of interleukin-4 in down-regulation of contact sensitivity by γδ T cells from tolerized T-cell receptor α−/− mice. Immunology 1999;98(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mraz V, Geisler C, Bonefeld CM. Dendritic epidermal T cells in allergic contact dermatitis. Front Immunol 2020;11:874. doi: 10.3389/fimmu.2020.00874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dieli F, Asherson GL, Sireci G, et al. γδ cells involved in contact sensitivity preferentially rearrange the Vg3 region and require interleukin-7. Eur J Immunol 1997;27(1):206–214. [DOI] [PubMed] [Google Scholar]
- 34.Dieli F, Ptak W, Sireci G, et al. Cross-talk between Vb8+ and gd+ T lymphocytes in contact sensitivity. Immunology 1998;93(4):469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ptak W, Askenase PW. Gamma delta T cells assist alpha beta T cells in adoptive transfer of contact sensitivity. J Immunol 1992;149(11): 3503–3508. [PubMed] [Google Scholar]
- 36.Askenase PW, Szczepanik M, Ptak M, Palival V, Ptak W. γδ T cells in normal spleen assist immunized αβ T cells in the adoptive cell transfer of contact sensitivity. Effect of Bordetella pertussis, cyclophosphamide, and antibodies to determinants on suppressor cells. J Immunol 1995;154(8):3644–3653. [PubMed] [Google Scholar]
- 37.Ptak W, Szczepanik M, Ramabhadran R, Askenase PW. Immune or normal γδ T cells that assist αβ T cells in elicitation of contact sensitivity preferentially use Vγ5 and Vδ4 variable region gene segments. J Immunol 1996;156(3):976–986. [PubMed] [Google Scholar]
- 38.Edele F, Molenaar R, Gütle D, et al. Cutting edge: instructive role of peripheral tissue cells in the imprinting of T cell homing receptor patterns. J Immunol 2008;181(6):3745–3749. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan DH. In vivo function of langerhans cells and dermal DC. Trends Immunol 2010;31(12):446–451. doi: 10.1016/j.it.2010.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savetsky IL, Albano NJ, Cuzzone DA, et al. Lymphatic function regulates contact hypersensitivity dermatitis in obesity. J Invest Dermatol 2015;135(11):2742–2752. doi: 10.1038/jid.2015.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rühl-Muth AC, Maler MD, Esser PR, Martin SF. Feeding of a fatenriched diet causes the loss of resistance to contact hypersensitivity. Contact Dermatitis 2021;85(4):398–406. [DOI] [PubMed] [Google Scholar]
- 42.Nakamizo S, Honda T, Kabashima K. Obesity and inflammatory skin diseases. Trends Immunother 2019;3(1):50–57. [Google Scholar]
- 43.Stallone DD, Stunkard AJ, Zweiman B, Wadden TA, Foster GD. Decline in delayed-type hypersensitivity response in obese women following weight reduction. Clin Diag Lab Immunol 1994;1(2):202–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lima EA, Silveira LS, Masi LN, et al. Macadamia oil supplementation attenuates inflammation and adipocyte hypertrophy in obese mice. Mediators Inflamm 2014;2014:870634. doi: 10.1155/2014/870634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winer DA, Winer S, Shen L, et al. B lymphocytes promote insulin resistance through modulation of T lymphocytes and production of pathogenic IgG antibody. Nat Med 2011;17(5):610–617. doi: 10.1038/nm.2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hellmann J, Sansbury BE, Holden CR, et al. CCR7 maintains non-resolving lymph node and adipose inflammation in obesity. Diabetes 2016;65(8):2268–2281. doi: 10.2337/db15-1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szczepanik M, Majewska-Szczepanik M, Wong FS, Kowalczyk P, Pasare C, Wen L. Regulation of contact sensitivity in non-obese diabetic (NOD) mice by innate immunity. Contact Dermatitis 2018;79(4): 197–207. doi: 10.1111/cod.13046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jensen P, Skov L. Psoriasis and obesity. Dermatology 2016;232(6): 633–639. doi: 10.1159/000455840 [DOI] [PubMed] [Google Scholar]
- 49.Mathews JA, Wurmbrand AP, Ribeiro L, Neto FL, Shore SA. Induction of IL-17A precedes development of airway hyperresponsiveness during diet-induced obesity and correlates with complement factor D. Front Immunol 2014;5:440. doi: 10.3389/fimmu.2014.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chehimi M, Vidal H, Eljaafari A. Pathogenic role of IL-17-producing immune cells in obesity, and related inflammatory diseases. J Clin Med 2017;6(7):68. doi: 10.3390/jcm6070068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity 2011;34(2):149–162. doi: 10.1016/j.immuni.2011.02.012 [DOI] [PubMed] [Google Scholar]
- 52.Zhao L, Liu L, Guo B, Zhu B. Regulation of adaptive immune responses by guiding cell movements in the spleen. Front Microbiol 2015;6:645. doi: 10.3389/fmicb.2015.00645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winer S, Paltser G, Chan Y, et al. Obesity predisposes to Th17 bias. Eur J Immunol 2009;39(9):2629–2635. doi: 10.1002/eji.200838893 [DOI] [PubMed] [Google Scholar]
- 54.Tay SS, Roediger B, Tong PL, Tikoo S, Weninger W. The skin-resident immune network. Curr Dermatol Rep 2013;3(1):13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Azukizawa H, Döhler A, Kanazawa N, et al. Steady state migratory RelB1+ langerin1+ dermal dendritic cells mediate peripheral induction of antigen-specific CD4+CD25+Foxp3+ regulatory T cells. Eur J Immunol 2011;41(5):1420–1434. doi: 10.1111/cod.13046 [DOI] [PubMed] [Google Scholar]
- 56.Guilliams M, Crozat K, Henri S, et al. Skin-draining lymph nodes contain dermis-derived CD103(−) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood 2010;115(10):1958–1968. doi: 10.1182/blood-2009-09-245274 [DOI] [PubMed] [Google Scholar]
- 57.Idoyaga J, Fiorese C, Zbytnuik L, et al. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest 2013; 123(2):844–854. doi: 10.1172/JCI65260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagao K, Ginhoux F, Leitner WW, et al. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc Natl Acad Sci U S A 2009;106(9):3312–3317. doi: 10.1073/pnas.0807126106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.King IL, Kroenke MA, Segal BM. GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in Th effector cell differentiation after subcutaneous immunization. J Exp Med 2010;207(5):953–961. doi: 10.1084/jem.20091844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Debes GF, Arnold CN, Young AJ, et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol 2005;6(9):889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Förster R, Schubel A, Breitfeld D, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999;99(1):23–33. [DOI] [PubMed] [Google Scholar]
- 62.Kuan EL, Ivanov S, Bridenbaugh EA, et al. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. J Immunol 2015;194(11):5200–5210. doi: 10.4049/jimmunol.1500221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009;15(8):930–939. doi: 10.1038/nm.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhong J, Rao X, Braunstein Z, et al. T-cell costimulation protects obesity-induced adipose inflammation and insulin resistance. Diabetes 2014;63(4):1289–1302. doi: 10.2337/db13-1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown K, DeCoffe D, Molcan E, Gibson DL. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients 2012;4(8):1095–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolf KJ, Lorenz RG. Gut microbiota and obesity. Curr Obes Rep 2012;1(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 2009;1(6):6ra14. doi: 10.1126/scitranslmed.3000322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Molina MA, Díaz AM, Hesse C, et al. Immunostimulatory effects triggered by Enterococcus faecalis CECT7121 probiotic strain involve activation of dendritic cells and interferon-gamma production. PLoS One 2015;10(5):e0127262. doi: 10.1371/journal.pone.0127262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A 2010;107(27):12204–12209. doi: 10.1073/pnas.0909122107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nadal I, Santacruz A, Marcos A, et al. Shifts in clostridia, bacteroides and immunoglobulin coating fecal bacteria associated with weight loss in obese adolescents. Int J Obes (Lond) 2009;33(7):758–767. doi: 10.1038/ijo.2008.260 [DOI] [PubMed] [Google Scholar]
- 71.Bradley CP, Teng F, Felix KM, et al. Segmented filamentous bacteria provoke lung autoimmunity by inducing gut-lung axis Th17 cells expressing dual TCRs. Cell Host Microbe 2017;22(5):697–704. doi: 10.1016/j.chom.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schnupf P, Gaboriau-Routhiau V, Sansonetti PJ, Cerf-Bensussan N. Segmented filamentous bacteria, Th17 inducers and helpers in a hostile world. Curr Opin Microbiol 2017;35:100–109. doi: 10.1016/j.mib.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 73.Atarashi K, Tanoue T, Shima T, et al. Induction of colonic regulatory T cells by indigenous Clostridium Species. Science 2011;331(6015):337–341. doi: 10.1126/science.1198469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DʼAmato A, Di Cesare Mannelli L, Lucarini E, et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome 2020;8(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444(7122):1027–1031. [DOI] [PubMed] [Google Scholar]
- 76.Lee J, dʼAigle J, Atadja L, et al. Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ Res 2020; 127(4):453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaiser T, Nalluri H, Zhu Z, Staley C. Donor microbiota composition and housing affect recapitulation of obese phenotypes in a human microbiota-associated murine model. Front Cell Infect Microbiol 2021; 11:614218. doi: 10.3389/fcimb.2021.614218 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon reasonable request.


