Abstract
Antimicrobial stewardship programmes in the outpatient setting have recently become an area of focus in an effort to improve antimicrobial prescribing. The Centers for Disease Control and Prevention and The Joint Commission have recently addressed this concern and provided a framework for the implementation of an outpatient stewardship programme. This manuscript offers detailed guidance on how to design and implement an outpatient antimicrobial stewardship programme and reviews the literature on current strategies. Challenges related to initiating and maintaining outpatient stewardship efforts are also discussed.
This article is part of the Antibiotic stewardship Special Issue: https://www.drugsincontext.com/special_issues/antimicrobial-stewardship-a-focus-on-the-need-for-moderation
Keywords: antimicrobial stewardship, feedback, outpatient
Introduction
Antimicrobial stewardship is a key tool in optimizing antimicrobial prescribing. Whilst stewardship programmes can improve antimicrobial prescribing in both the acute and ambulatory care settings, the latter has only recently become an area of focus.1,2 In 2020, The Joint Commission (TJC) released the R3 Report Issue 23 addressing antimicrobial stewardship in the outpatient setting.2 In addition, the Centers for Disease Control and Prevention (CDC) offer core elements of outpatient antibiotic stewardship, augmenting their hospital antibiotic stewardship programme recommendations.1 These reports offer a backbone for the implementation of outpatient stewardship programmes but challenges persist stemming from deep-rooted prescribing habits and unique differences from the inpatient setting. Lack of dedicated personnel (including pharmacists), incomplete documentation (such as antibiotics not being matched with a diagnosis code) and insufficient tracking capabilities may make antimicrobial stewardship programmes in the ambulatory care settings particularly challenging and dissimilar from inpatient antimicrobial stewardship.
Outpatient antimicrobial prescribing accounts for up to 95% of all antibiotics prescribed for human utilization globally.3 One study in the United Kingdom found that an average of 30.1% of adult patients registered with primary care practices were prescribed at least one antibiotic per year.4 In the United States in 2015, there were enough antibiotic courses dispensed to treat five out of every six people in the country.3
Outpatient antimicrobial stewardship programmes are still in the early phases of development across the healthcare continuum, and there is not a one-size-fits-all approach regarding to implementation. The Society of Infectious Diseases Pharmacists describes potential metrics that can be used to assess the effectiveness of stewardship interventions.5 They also outline areas where pharmacists can play key roles within the outpatient stewardship setting.5 Antibiotic prescribing and guideline adherence are benchmarks worth tracking and reporting, but the attitudes towards prescribing antibiotics must also be addressed. A group recently discussed this issue via a qualitative survey with eight focus groups across four major cities in the United States to assess prescribers’ feelings towards antimicrobial resistance and the feasibility of outpatient stewardship.6 Common themes included feelings that “antibiotic resistance is an issue, but not for my patients” as well as the feeling that antibiotic resistance is seen as less important than other public health issues faced by primary care providers such as opioid stewardship or obesity management. These attitudes surrounding antibiotic prescribing must not be ignored and are a key component of any stewardship programme.
Described herein are common concepts that will help identify the individual needs of an institution to assist in the implementation process, while also focusing on the attitudes surrounding antibiotic prescribing. There is a continued need to address the public health threat posed by organisms with continually developing resistance against antimicrobials not only in the acute but also in the ambulatory setting. The purpose of this review is to detail a ‘how-to’ for the implementation of an outpatient stewardship programme through a stepwise approach (Figure 1).
Figure 1.
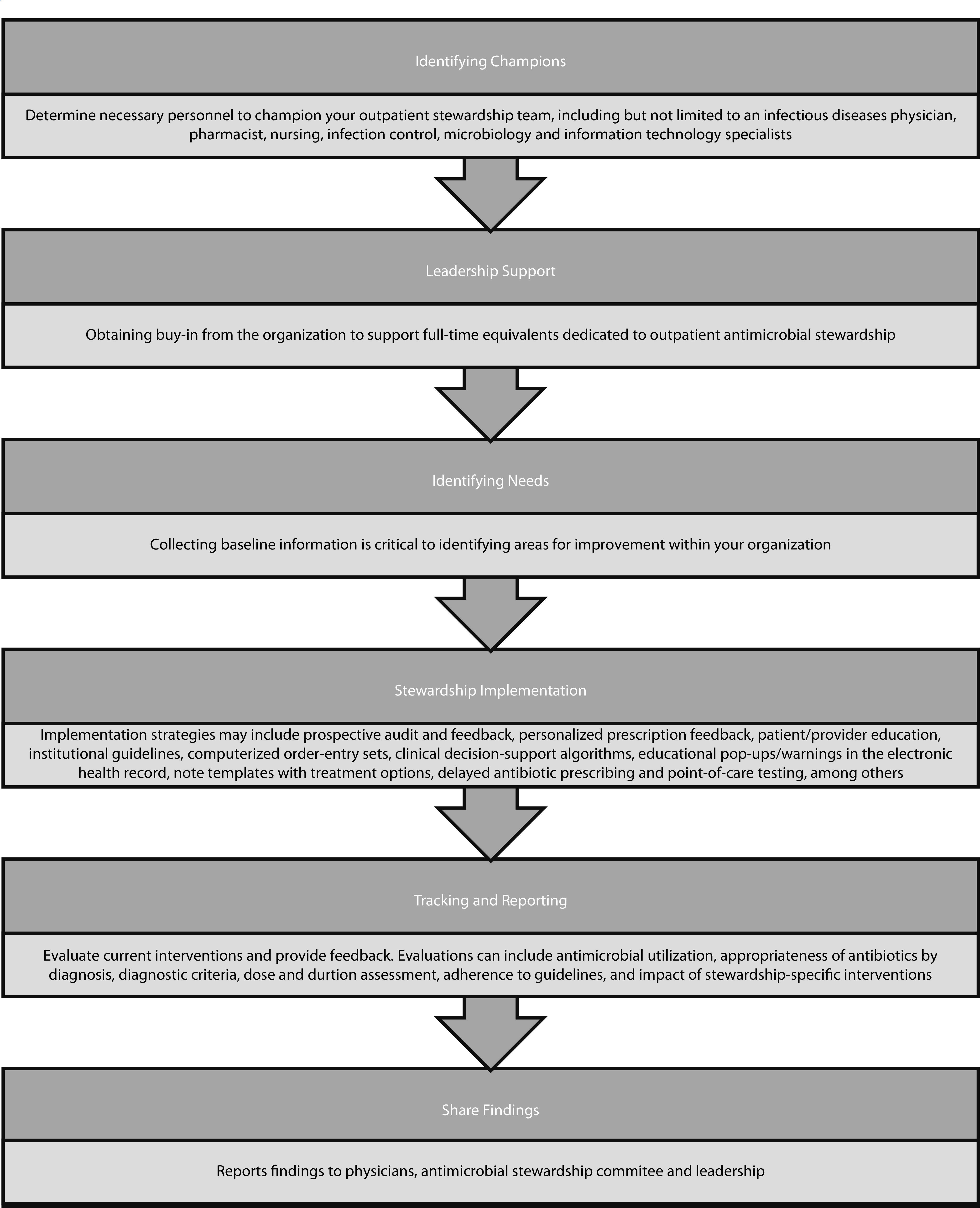
Steps to outpatient antimicrobial stewardship implementation.
This narrative review identified pertinent literature in the English language from January 1997 through March 2022, using PubMed and bibliography review to gather information about outpatient antimicrobial stewardship in both the adult and paediatric settings.
Identifying champions
One of the first areas to consider when determining the steps to take in developing an outpatient antimicrobial stewardship programme is identifying champions who will help turn the programme from just an idea into reality. An ideal outpatient stewardship committee would include a ‘champion’ physician and pharmacist, ideally those with infectious diseases training. It should also include nursing representation and infection control and microbiology personnel. Finally, a stewardship committee would not be successful without information technology, data/statistics and administration support to the team. In the setting of a health system with many clinics, a shared committee may be helpful.
Reported in the R3 report published by TJC entitled Antimicrobial Stewardship in Ambulatory Health Care, the first requirement is identification of those responsible for stewardship activities.2 For this to happen efficiently and effectively, there should be buy-in from a champion within the organization. The Agency for Healthcare Research and Quality suggests that this champion should be at the director level within the organization.7 Their role primarily consists of building support within the institution and bringing awareness to the efforts being made to address antimicrobial prescribing. One other role this champion may play is in the identification of what resources are needed to create and support the programme. One of the key questions a champion may need to answer to get the programme up and running is the staff required. Greene et al. attempted to answer the question of how many full-time equivalents (FTE) are needed in stewardship-specific activities via summarizing literature and suggesting minimum staffing requirements.8 Most of their efforts were focused on the inpatient setting, concluding that, for every 250 patient beds, there should be 1 FTE of antimicrobial stewardship programme (ASP) support. By having this information ready for the programme champion to present to organizational leadership, stewardship activities can be framed for success before they have started. There is currently no available literature suggesting the FTE support necessary for a successful outpatient antimicrobial stewardship programme.
Tracking and reporting
Tracking and reporting comprise one of the four Core Elements of Outpatient Antibiotic Stewardship released by the CDC.1 This element is a useful step in identifying areas for improvement, evaluating current interventions and providing feedback within current or planned programmes. Tracked items can include antimicrobial utilization, appropriateness of antibiotics by diagnosis, diagnostic criteria, dose and duration assessment, adherence to guidelines, and impact of stewardship-specific interventions. The data collected can then be utilized to report provider feedback and may also be useful to determine the acceptability of particular interventions.1 Selecting what to analyse can be driven by the specific high priorities of the institution; this may require automatic electronic medical record extraction or manual periodic chart reviews.1
Outcomes may be analysed on an individual provider level and/or as an aggregate of the facility as a whole; both strategies have unique benefits. Individualized feedback is preferred and may be beneficial in motivating prescribers by seeing how they compare to their peers.1 This approach of tracking provider outcomes and reporting back on antibiotic prescribing practices for high-priority diseases has reduced inappropriate antibiotic prescribing in multiple cases.1,9,10 Another successful strategy is to determine the number of antibiotics prescribed by a single clinician in comparison to their total number of visits.1 Facility-wide data may be used to demonstrate the impact of antimicrobial stewardship interventions on a larger scale. Continued re-evaluation of needs is imperative and ongoing quality improvement to determine activities with greatest success is crucial.11
Other ways in which antimicrobials can be evaluated are by tracking Clostridioides difficile infections or other antibiotic-related adverse effects, drug–drug interactions, and facility-wide resistance patterns.1 Although less specific, these data can be utilized to pinpoint institution-level needs for improvement. Tracking data can not only identify success within an antimicrobial stewardship programme but can also be used to demonstrate achievements to leadership. For example, reducing C. difficile infection and multidrug-resistant organisms can impact hospitalizations, cost and mortality. Justifying the benefits of antimicrobial stewardship can also garner the buy-in and support needed for additional dedicated personnel.
The CDC is not alone in this charge. In 2001, the European Surveillance of Antimicrobial Consumption was established to collect antibiotic use data internationally throughout Europe in the inpatient and outpatient settings in response to increasing rates of resistance.12 As of January 2020, TJC requirements now include five elements of performance related to antimicrobial stewardship for ambulatory healthcare settings,2 including collecting, analysing, and reporting data. Suggested data to evaluate include antimicrobial prescribing patterns, resistance patterns, or assessment of antimicrobial stewardship initiatives.2
Implementation strategies
A variety of strategies have been successfully implemented by inpatient antimicrobial stewardship programmes for years. Many of these methods can and have been adapted for use in outpatient clinics, including targeting commonly prescribed antibiotics in the outpatient setting, providing personalized feedback to providers, and evaluating prescriptions prospectively.13–16 Table 1 describes some of these strategies. Below, we discuss the currently available literature for a variety of ways to implement outpatient antimicrobial stewardship initiatives at a variety of practice sites.
Table 1.
Strategies and methods of outpatient antimicrobial stewardship programmes.
| Category | Methods of implementation | Strengths | Challenges |
|---|---|---|---|
| Prospective audit and feedback |
|
|
|
| Personalized prescription feedback |
|
|
|
| Patient education |
|
|
|
| Provider education |
|
|
|
| Institutional guidelines |
|
|
|
| Computerized order-entry sets |
|
|
|
| Clinical decision-support algorithms |
|
|
|
| Educational pop-ups/warnings in the electronic health record |
|
|
|
| Note templates with treatment options |
|
|
|
| Delayed antibiotic prescribing |
|
|
|
| Point of care testing |
|
|
|
Prospective audit and feedback
Prospective audit and feedback form prominent method of improving antibiotic prescribing in the inpatient setting that has also been successful in the outpatient arena. Although it is time consuming and requires ongoing dedicated personnel to review prescriptions, this method gives providers specific, frequent feedback on recommendations for improvement. One example of a successful implementation of a pharmacist-led audit and feedback intervention was conducted at a single primary care office and targeted upper respiratory tract infections (URTIs) and urinary tract infections (UTIs).17 This retrospective pilot study evaluated antibiotic prescribing over time following a bi-weekly audit of antibiotic prescriptions for URTIs and UTIs. An ambulatory care pharmacist devoted one half-day per week to review prescriptions and provide specific feedback, with the antimicrobial stewardship pharmacist and physician available for questions and guidance. Guideline-concordant prescribing of antibiotic selection and duration increased during the 7-month study period for both URTIs and UTIs. There was also a large reduction, from 25% to 2.6%, of asymptomatic patients receiving antibiotics for bacteriuria. This study demonstrated the success of prospective audit and feedback in a primary care clinic, led by a pharmacist.
Personalized prescription feedback
Personalized prescription feedback is another method frequently used by inpatient antimicrobial stewardship programmes that has been studied in the outpatient setting. This process involves gathering antibiotic data for a specific provider over a period of time and sharing the results with the provider. Often, the results are shared in comparison with other providers in the healthcare system. This requires less day-to-day effort by a single individual than prospective audit and feedback and may be ideal for institutions with limited dedicated personnel. Two studies have explored this method in primary care with mixed results. Hemkins et al. provided quarterly personalized antibiotic prescription feedback by mail and online for 2 years after a one-time guideline in a nationwide randomized parallel group trial in Switzerland.15 While some patient age groups had small changes in antibiotics prescribed, overall, an impact on antibiotic prescribing between the intervention and control groups was not seen. Gerber et al. reported a similar attempt at personalized, quarterly feedback for primary care paediatricians.10 This cluster-randomized trial evaluated inappropriate antibiotic prescribing for acute respiratory tract infections (ARIs), comparing physicians who received an 1-hour education session followed by quarterly feedback to those who did not. Feedback included compliance with guideline-based prescribing for the individual, the practice site, and the network of enrolled practices. Broad-spectrum prescriptions decreased from baseline in the intervention group by 12.5% as compared to 5.8% in the control group (p=0.01). Additionally, a decrease by 75% in off-guideline antibiotic prescribing for pneumonia was identified. This study combined prescriber education with personalized prescription feedback and was specifically limited to paediatric primary care offices and targeted specific infections, which may have contributed to its successful outcomes. It is noteworthy that the authors assessed durability for 18 months after discontinuation of the feedback intervention and demonstrated an increase in broad-spectrum antibiotics over time.18 The sustainability of outpatient antimicrobial stewardship programmes is discussed further below.
Treatment guidelines and clinical decision-support tools
Outpatient programmes that take a multi-faceted approach to the implementation of stewardship guidelines have repeatedly shown success. One programme found that clinician education, combined with electronic health record-embedded treatment recommendations and note templates, increased the rate of appropriate antibiotic treatment in women (18–64 years of age) diagnosed with either a UTI or pyelonephritis from 30/81 (37%) versus 58/81 (72%) (p<0.001).19 A three-arm, cluster-randomized trial found that provider education, patient education, and guideline audit and feedback led to a decline in antibiotic prescriptions for patients with acute bronchitis in primary care clinics when combined with clinical support models, deployed either in print (p=0.003) or computerized (p=0.014) form. Additionally, approximately one-third of providers at the intervention sites were found to have reduced their antibiotic prescription rates by more than 20% when comparing the baseline period to the intervention period.20
Another example of using multiple antimicrobial stewardship strategies simultaneously was described by Jenkins et al., who found that the implementation of a clinical treatment pathway combined with patient education materials reduced antimicrobial prescribing from 42.7% during the baseline period to 37.9% after implementation (p<0.0001).21 The same study set out to determine the impact of clinical pathways and patient education on a broader scale and evaluated the overall use of broad-spectrum antibiotics in the pre-intervention and post-intervention periods. Broad-spectrum antimicrobial prescribing decreased from 26.4% to 22.6%, respectively (p<0.0001), in the entire population. However, broad-spectrum prescribing did increase in the population with a UTI in the intervention group, with no evaluation of the appropriateness of therapy. This highlights the need for antimicrobial stewardship outcomes data to focus on the appropriateness of antimicrobial therapy use versus the reduction of antimicrobial therapy use.21 The REDUCE trial demonstrated that the combination of a training webinar, monthly feedback reports and an electronic decision-support tool led to a reduction in antimicrobial prescribing for patients aged 15–84 years with respiratory infections (adjusted rate ratio 0.84, 95% CI 0.75–0.95), associated with one antibiotic prescription per year avoided for every 62 patients (95% CI 40–200).22 Differences were not found in patients outside of this age range; however, it is important to note that the prescribing practices differed significantly across study sites and likely impacted the applicability of the study results. Importantly, no negative consequences of reduced antimicrobial prescribing were noted.22 These studies demonstrate the effective utilization of guidelines as an outpatient antimicrobial stewardship strategy; however, given the guideline deployment in conjunction with a variety of other interventions, it is difficult to ascertain the efficacy of any one intervention. Single-intervention studies may offer better insight to the efficacy of independent stewardship methods but they may be less likely to be successful. For example, Linder et al. implemented a clinical decision-support tool for the treatment of patients with ARIs.23 The tool was not implemented with any type of patient or provider education and did not auto-populate for use by the prescriber. The implementation was not effective in reducing antibiotic prescribing but was also only utilized in 742/11,954 (6%) visits. Based on a subgroup evaluation, the clinical decision-support tool would have impacted antimicrobial prescribing practices if utilized.23
Many of the previously discussed studies focus on single or multiple, specific disease states. Alternative to targeting certain infection sites, some outpatient stewardship methods have focused solely on reducing the use of a single antimicrobial or class of antimicrobials. The most common targets of these types of interventions are fluoroquinolone and azithromycin. Rattinger et al. aimed to reduce both inappropriate fluoroquinolone and azithromycin use in the management of ARIs.24 A computerized decision-support model that auto-populated when providers prescribed a targeted agent, in conjunction with provider education on how to maintain patient satisfaction without prescribing antibiotics, was found to decrease unnecessary prescriptions from 22% to 3.3% (p=0.0001). The proportion of visits with appropriate prescribing, as evaluated by adherence to guidelines, also increased significantly in the intervention population (0.63 pre-intervention versus 0.72 post-intervention; p=0.0001). No change in prescribing was found for antibiotics that were not specifically targeted.24 Lin et al. detail a staged approach to address fluroquinolone prescribing, including provider education, fluoroquinolone warning messages at time of ordering, urine culture ciprofloxacin susceptibility suppression for organisms with third-generation cephalosporin susceptibility, and ambulatory infectious diseases order-set implementation to guide therapy.25 The investigators did not find a significant change in prescribing practices after the first three interventions were employed but did find a significant decrease (39%; p<0.01) in total prescriptions per 1000 patient visits after the final intervention of order-sets were implemented. These results could indicate that order-sets were more-effective than the other three interventions but could also indicate that the combination of interventions is the true key to an efficacious programme. Additionally, the results seen after the final intervention could indicate that widespread shifts in prescribing culture and education within an institution can influence the prescribing practices of providers at an institution but can take time to develop.25
May et al. elected to employ a single-intervention (clinical decision-support order-set that auto populates at the time of ordering) to target azithromycin prescribing in primary care clinics.26 The investigators found that inappropriate azithromycin prescribing significantly decreased from pre-intervention to post-intervention (81.4% versus 68.8%, respectively; p<0.001). Key findings in this study were that inappropriate azithromycin prescriptions were largely written by attendings and were largely associated with non-academic settings. Although this intervention evinced success without specific educational interventions, the higher rates of inappropriate prescribing by attendings in non-academic settings suggests that updated education is imperative to outpatient antimicrobial stewardship practices.26
Many of the above interventions require electronic medical record support in creating clinical decision-support tools and integrating them into the workflow. It is critical to have information technology experts on the antimicrobial stewardship team to guide discussion on electronic health record capabilities and decision-support tool design and their creation. Each institution has unique electronic health record integration capabilities, so technology may differ.
Delayed antibiotic prescribing
Delayed antibiotic prescribing is a method that can be implemented to potentially reduce antibiotic use in the outpatient setting. There is no one way to employ delayed prescribing, but it generally refers to either instructing the patient to call for a prescription if they are still experiencing symptoms after ‘X’ number of days or providing the patient with the written prescription and instructing them to wait to fill it per the same instructions. While reportedly common in practice, literature evaluating the efficacy and clinical outcomes of such practices are sparse. Additionally, the practice is not systematically defined and is employed via many different methods. Most available literature is more than 10 years old at this time. A systematic review and meta-analysis evaluating the use of delayed prescribing for patients with ARIs was published in 2017.27 The review included 11 studies (n=3555) and found that this prescribing method led to a significant reduction in antibiotic usage compared to immediate prescribing (odds ratio (OR) 0.04, 95% CI 0.03–0.05) with no difference in clinical outcomes or adverse effects. Delayed prescribing still leads to more antibiotic usage than refraining from prescribing at all. However, this study found that delayed prescribing is an effective way to maintain patient satisfaction as compared to not providing antibiotics. From a stewardship perspective, patient education would ultimately be preferred to this method.27–29
Challenges
Developing, implementing and maintaining an outpatient antimicrobial stewardship programme is not without challenges. First, leadership buy-in, team support, funding and necessary staffing are all hurdles that must be overcome at the starting line, prior to intervention implementation. Next, ensuring provider uptake and acceptance of antimicrobial stewardship initiatives, sustaining interventions and benefits, and ensuring appropriate patient education are all imperative to an effective programme.
Linder et al. published their efforts at implementing an antimicrobial stewardship tool, only to be met with low provider uptake.23 In this study, an electronic health record-integrated, clinical decision-support tool for ARIs — ARI Smart Form — was implemented. Of 262 clinicians, only 33% (86/262) used the ARI Smart Form at least once, in an estimated 6% of visits for ARIs. There was no improvement in antibiotic prescribing for ARIs in the intent-to-intervene analysis, which the authors attribute to the low uptake of the ARI Smart Form. Authors identified automatic decision support, unsatisfactory integration into the electronic health record and new methods of documentation (such as drop-down lists and check boxes) as potential reasons for poor provider uptake, and identified minimizing interruptions to workflow and thorough training of the ARI Smart Form prior to implementation as key areas for improvement.
Prescriber uptake and acceptance of antimicrobial stewardship opportunities is necessary but can prove difficult. The role that behaviour plays on the success of initiative acceptance and antimicrobial stewardship outcomes is complex but critical, and understanding current behaviour is an important initial step to determining how to approach changes.30,31 Implementation of a stewardship initiative must match the weaknesses of each specific facility. Lorencatto et al. make recommendations regarding approaching behaviour change such as identifying a specific problem of interest, investigating how and why interventions are successes or failures, identifying factors preventing behaviour change, reviewing available evidence, and avoiding implementing an intervention without full preparation.30 As interest in the role of behavioural and social theory on antimicrobial stewardship grows, further research in this area is warranted to increase understanding.11,30,31
Sustainability of antimicrobial stewardship programmes or specific interventions is another challenge to consider, with regard to both provider burnout and the lasting impact of benefits. The idea that antimicrobial stewardship is a marathon, not a sprint, was introduced by Heil and Bork in 2021 addressing sustainability in the intensive care unit, but the concept is also relevant to outpatient antimicrobial stewardship.11 The long-term sustainability of outpatient antimicrobial stewardship interventions has rarely been reported. Additionally, in the setting of COVID-19, burnout amongst antimicrobial stewardship leaders has increased, which may present additional challenges to sustaining interventions.32
As discussed above, practice change requires an understanding of current behaviours and motivators, which remains true when considering the longevity of interventions and practice change. The assumption cannot be made that following implementation of an intervention, initial benefits will be sustained and equally effective over time, especially without continued push. Multiple studies have demonstrated that discontinuation of stewardship efforts can lead to increases in antimicrobial utilization and increased expenditures.18,33
The fourth CDC Core Element is Education and Expertise. Providing education to patients and caregivers is crucial to antimicrobial stewardship and to ensuring an understanding of when antibiotics are and are not appropriate. Prescribers may feel pressure that patients are expecting antibiotics and may be dissatisfied to leave an appointment empty handed. Not only may patients feel as though they are not being heard but also that their illness is invalidated. In reality, antibiotics can do more harm than good, particularly when their illness does not require antibiotics for treatment.34
Ensuring that patients understand why antibiotics are being withheld is important. Colgan et al. list the following steps when having this discussion with a patient: explain the likely viral nature of the infection and lack of impact antibiotics have on symptoms, explain ways in which antibiotics may be harmful, including risk of resistance and unwanted adverse effects, empathize with the patient on the impact of their illness on their daily lives, provide educational materials, and recommend alternative therapies for symptom management.34 Patients are often most concerned about their symptoms leading to a physician visit; thus, providing symptom management instead of antibiotics may be a suitable substitute when antibiotics are not warranted or watchful waiting is appropriate.
Park et al. addressed this topic by conducting a pilot survey study to evaluate patient involvement and understanding of their antibiotic therapy.35 Amongst 112 survey respondents with current or previous antimicrobial use for at least 3 days, the majority were women and most were college graduates. Less than half (46%) of the patients knew the name of their antibiotic and 68% believed that they were properly prescribed. Similarly, 46% knew the adverse effects or precautions of their antibiotics but only 25% knew how to manage them, and even less (19%) knew when they were expected to follow up. Patient education is a critical component of outpatient antimicrobial stewardship programme success and this study highlights room for improvement in this area.
Shared decision-making with patients is another strategy that may improve patient autonomy and patient satisfaction.36 A review specifically assessing the impact of shared decision-making on antibiotic prescribing for ARIs in the primary care setting was performed.37 This study found a significant reduction in antibiotic prescribing for ARIs when prescribers utilized shared decision-making interventions as compared to standard of care. This is another potential way to overcome dissatisfaction through education and autonomy.
Not only is it important that patients are provided education but ensuring clinician expertise or access to experts in infectious diseases can be challenging. The CDC Core Elements recommend providing educational training and other continuing education opportunities to providers related to infectious diseases and antimicrobial stewardship.1 They also highlight the need for education related specifically to communication strategies regarding appropriate antibiotic prescribing to utilize with patients to ensure patient understanding and satisfaction.1 One example of a specific communication training programme is the Dialogue Around Respiratory Illness Treatment (DART) programme; this programme offers both a communication tutorial and antibiotics tutorial as a series of video trainings for providers.38 Successful implementation of the DART programme has been described, specifically in paediatric patients with acute respiratory tract infection.39 Uptake of the communication training was also evaluated by parents demonstrating implementation of the behaviours.40
Finally, pharmacists or other specialty provider consultants can be useful in aiding appropriate antibiotic prescribing and such needs should be determined based on the facility.1
Conclusion
A shift in antimicrobial stewardship has occurred from a predominately inpatient emphasis to a shared focus with outpatient antimicrobial prescribing. This modification occurred simultaneously, with efforts from TJC and the CDC highlighting its importance. Optimizing antimicrobial prescribing practices requires many steps, which are reviewed in this paper. Creating a team, gaining leadership buy-in, and determining staffing and funding are a challenging but crucial starting place.
Evaluating the workforce, training staff and assessing individualized needs are also necessary. Only then should this be followed by selecting antimicrobial stewardship interventions appropriate for a given institution and evaluating the impact of the contribution. Specific areas to focus on may vary depending on need and available resources. Identifying high-priority conditions for intervention, determining gaps in knowledge or other barriers resulting in suboptimal outcomes, and establishing specific clinical practice guidelines for antibiotics are important initial steps.1 It is recommended that at least one of the following strategies are implemented as part of initial antimicrobial stewardship initiatives: providing communication skills training to healthcare providers, requiring diagnosis or other written justification in the medical record for antibiotic prescribing, providing clinical decision-support tools, and using consulting or triage systems to reduce unnecessary clinic visits such as for common viral infections. While much alike inpatient antimicrobial stewardship programmes, the outpatient setting provides unique challenges as discussed above. Of note, in addition to resources available from the CDC and TJC, resources from other national organizations are available.1,2,5,41
Acknowledgements
None.
Footnotes
Contributions: All authors contributed equally to the preparation of this manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2023/01/dic.2022-8-2-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2023 Drwiega EN, Griffith N, Herald F, Badowski ME. https://doi.org/10.7573/dic.2022-8-2. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Article URL: https://www.drugsincontext.com/how-to-design-and-implement-an-outpatient-antimicrobial-stewardship-programme
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 6 Green Lane Business Park, 238 Green Lane, New Eltham, London, SE9 3TL, UK.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(RR-6):1–12. doi: 10.15585/mmwr.rr6506a1. [DOI] [PubMed] [Google Scholar]
- 2.R3 report: requirement rationale reference; antimicrobial stewardship in ambulatory health care. [Accessed February 9, 2023]. https://www.jointcommission.org/-/media/tjc/documents/standards/r3-reports/r3_23_antimicrobial_stewardship_amb_6_14_19_final2.pdf .
- 3.King LM, Fleming-Dutra KE, Hicks LA. Advances in optimizing antibiotic prescribing in outpatient settings. BMJ. 2019;363:k3047. doi: 10.1136/bmj.k3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shallcross L, Beckley N, Rait G, Hayward A, Petersen I. Antibiotic prescribing frequency amongst patients in primary care: a cohort study using electronic health records. J Antimicrob Chemother. 2017;72:1818–1824. doi: 10.1093/jac/dkx048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klepser ME, Dobson EL, Pogue JM, et al. A call to action for outpatient antibiotic stewardship. J Am Pharm Assoc. 2017;57(4):457–463. doi: 10.1016/j.japh.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Zetts RM, Stoesz A, Garcia AM, et al. Primary care physicians’ attitudes and perceptions towards antibiotic resistance and outpatient antibiotic stewardship in the USA: a qualitative study. BMJ Open. 2020;10:e034983. doi: 10.1136/bmjopen-2019-034983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toolkit 1 start an antimicrobial stewardship program. [Accessed February 9, 2023]. https://www.ahrq.gov/nhguide/toolkits/implement-monitor-sustain-program/toolkit1-start-program.html .
- 8.Greene MH, Nesbitt WJ, Nelson GE. Antimicrobial stewardship staffing: how much is enough? Infect Control Hosp Epidemiol. 2020;41(1):102–112. doi: 10.1017/ice.2019.294. [DOI] [PubMed] [Google Scholar]
- 9.Meeker D, Knight TK, Friedberg MW, et al. Nudging guideline-concordant antibiotic prescribing: a randomized controlled trial. JAMA Intern Med. 2014;174(3):425–431. doi: 10.1001/jamainternmed.2013.14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized clinical trial. JAMA. 2013;309(22):2345–2352. doi: 10.1001/jama.2013.6287. [DOI] [PubMed] [Google Scholar]
- 11.Heil EL, Bork JT. It is a marathon, not a sprint – sustainability of stewardship in the ICUs. Crit Care Med. 2021;49(1):159–161. doi: 10.1097/CCM.0000000000004714. [DOI] [PubMed] [Google Scholar]
- 12.Coenen S, Ferech M, Haaijer-Ruskamp FM, et al. European Surveillance of Antimicrobial Consumption (ESAC): quality indicators for outpatient antibiotic use in Europe. Qual Saf Health Care. 2007;16:440–445. doi: 10.1136/qshc.2006.021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balinskaite V, Johnson AP, Holmes A, Aylin P. The impact of a national antimicrobial stewardship program on antibiotic prescribing in primary care: an interrupted time series analysis. Clin Infect Dis. 2019;69(2):227–232. doi: 10.1093/cid/ciy902. [DOI] [PubMed] [Google Scholar]
- 14.Dinh A, Duran C, Davido B, et al. Impact of an antimicrobial stewardship programme to optimize antimicrobial use for outpatients at an emergency department. J Hosp Infect. 2017;97:288–293. doi: 10.1016/j.jhin.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Hemkins L, Saccilotto R, Reyes SL, et al. Personalized prescription feedback using routinely collected data to reduce antibiotic use in primary care: a randomized clinical trial. JAMA Intern Med. 2017;177(2):176–183. doi: 10.1001/jamainternmed.2016.8040. [DOI] [PubMed] [Google Scholar]
- 16.Li DX, Ferrada MA, Avdic E, Tamma PD, Cosgrove SE. Sustained impact of an antibiotic stewardship intervention for community-acquired pneumonia. Infect Control Hosp Epidemiol. 2016;37(10):1243–1246. doi: 10.1017/ice.2016.165. [DOI] [PubMed] [Google Scholar]
- 17.Burns KW, Johnson KM, Pham SN, Egwuatu NE, Dumkow LE. Implementing outpatient antimicrobial stewardship in a primary care office through ambulatory care pharmacist-led audit and feedback. J Am Pharm Assoc. 2020;60(6):e246–e251. doi: 10.1016/j.japh.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Gerber JS, Prasad PA, Fiks AG, et al. Durability of benefits of an outpatient antimicrobial stewardship intervention after discontinuation. JAMA. 2014;312(23):2569–2570. doi: 10.1001/jama.2014.14042. [DOI] [PubMed] [Google Scholar]
- 19.McCormick JZ, Cardwell SM, Wheelock C, Wong CM, Vander Weide LA. Impact of ambulatory antimicrobial stewardship on prescribing patterns for urinary tract infections. J Clin Pharm Ther. 2020;45(6):1312–1319. doi: 10.1111/jcpt.13210. [DOI] [PubMed] [Google Scholar]
- 20.Gonzales R, Anderer T, MuCulloch CE, et al. A cluster-randomized trial of decision support strategies for reducing antibiotic use for acute bronchitis. JAMA Intern Med. 2013;173(4):267–273. doi: 10.1001/jamainternmed.2013.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins TC, Irwin A, Coombs L, et al. Effects of clinical pathways for common outpatient infections on antibiotic prescribing. Am J Med. 2013;126(4):327–335. doi: 10.1016/j.amjmed.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gulliford MC, Prevost AT, Charlton J, et al. Effectiveness and safety of electronically delivered prescribing feedback and decision support on antibiotic use for respiratory illness in primary care: REDUCE cluster randomised trial. BMJ. 2019;364:l236. doi: 10.1136/bmj.l236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linder JA, Schnipper J, Tsurikova R, et al. Documentation-based clinical decision support to improve antibiotic prescribing for acute respiratory infections in primary care: a cluster randomised controlled trial. Inform Prim Care. 2009;17:231–240. doi: 10.14236/jhi.v17i4.742. [DOI] [PubMed] [Google Scholar]
- 24.Rattinger GB, Mullins CD, Zuckerman IH, et al. A sustainable strategy to prevent misuse of antibiotics for acute respiratory infections. PLoS One. 2012;7(12):e51147. doi: 10.1371/journal.pone.0051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin K, Zahlanie Y, Ortwine JK, et al. Decreased outpatient fluoroquinolone prescribing using a multimodal antimicrobial stewardship initiative. Open Forum Infect Dis. 2020;7(6):ofaa182. doi: 10.1093/ofid/ofaa182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.May A, Hester A, Quairoli K, Wong JR, Kandiah S. Impact of clinical decision support on azithromycin prescribing in primary care clinics. J Gen Intern Med. 2021;36(8):2267–2273. doi: 10.1007/s11606-020-06546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spurling G, Del Mar C, Dooley L, Clark J, Askew D. Delayed antibiotics prescriptions for respiratory infections. Cochrane Database Syst Rev. 2017;9(9):CD004417. doi: 10.1002/14651858.CD004417.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Little P, Williamson I, Warner G, Gould C, Gantley M, Kinmonth AL. Open randomised trial of prescribing strategies in managing sore throat. BMJ. 1997;314(7082):722–727. doi: 10.1136/bmj.314.7082.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de la Poza Abad M, Dalmau GM, Bakedano MM, et al. Prescription strategies in acute uncomplicated respiratory infections A randomized clinical trial. JAMA Inter Med. 2016;176(1):21–29. doi: 10.1001/jamainternmed.2015.7088. [DOI] [PubMed] [Google Scholar]
- 30.Lorencatto F, Charani E, Sevdalis N, Tarrant C, Davey P. Driving sustainable change in antimicrobial prescribing practice: how can social and behavioural sciences help? J Antimicrob Chemother. 2018;73(10):2613–2623. doi: 10.1093/jac/dky222. [DOI] [PubMed] [Google Scholar]
- 31.Borek AJ, Santillo M, Wanat M, Butler CC, Tonkin-Crine S. How can behavioural science contribute to qualitative research on antimicrobial stewardship in primary care? JAC Antimicrob Resist. 2022;4(1):dlac007. doi: 10.1093/jacamr/dlac007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaughn VM, Dunn GE, Horowitz JK, McLaughlin ES, Gandhi TN. Duties, resources, and burnout of antibiotic stewards during the coronavirus disease 2019 (COVID-19) pandemic. Antimicrob Steward Healthc Epidemiol. 2021;1:e39. doi: 10.1017/ash.2021.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Standiford HC, Chan S, Tripoli M, Weekes E, Forrest GN. Antimicrobial stewardship at a large tertiary care academic medical center: cost analysis before, during, and after a 7-year program. Infect Control Hosp Epidemiol. 2012;33(4):338–345. doi: 10.1086/664909. [DOI] [PubMed] [Google Scholar]
- 34.Colgan R, Powers JH. Appropriate antimicrobial prescribing: approaches that limit antibiotic resistance. Am Fam Physician. 2001;64(6):999–1004. [PubMed] [Google Scholar]
- 35.Park S, Geum MJ, Choi HJ, et al. Validation of a questionnaire for patient awareness and the need for a community-based outpatient antimicrobial stewardship (O-ASP): a pilot study. Antibiotics. 2021;10(4):441. doi: 10.3390/antibiotics10040441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spatz ES, Spertus JA. Shared decision making: a path toward improved patient-centered outcomes. Circ Cardiovasc Qual Outcomes. 2012;5(6):e75–e77. doi: 10.1161/circoutcomes.112.969717. [DOI] [PubMed] [Google Scholar]
- 37.Coxeter P, Del Mar C, McGregor L, Beller E, Hoffmann T. Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care. Cochrane Database Syst Rev. 2015;11:CD010907. doi: 10.1002/14651858.CD010907.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dialogue Around Respiratory Illness Treatment. [Accessed October 19, 2022]. https://www.uwimtr.org/dart/
- 39.Kronman MP, Gerber JS, Grundmeier RW, et al. Reducing antibiotic prescribing in primary care for respiratory illness. Pediatrics. 2020;146(3):e20200038. doi: 10.1542/peds.2020-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mangione-Smith R, Robinson JD, Zhou C, et al. Fidelity evaluation of the dialogue around respiratory illness treatment (DART) program communication training. Patient Educ Couns. 2022;105(7):2611–2616. doi: 10.1016/j.pec.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barlam T, Cosgrove SE, Abbo L, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):1197–1202. doi: 10.1093/cid/ciw217. [DOI] [PubMed] [Google Scholar]

