Abstract
Novel diagnostic stewardship in infectious disease consists of interventions that modify ordering, processing, and reporting of diagnostic tests to provide the right test for the right patient, prompting the right action. The interventions work upstream and synergistically with traditional antimicrobial stewardship efforts. As diagnostic stewardship continues to gain public attention, it is critical that antimicrobial stewardship programmes not only learn how to effectively leverage diagnostic testing to improve antimicrobial use but also ensure that they are stakeholders and leaders in developing new diagnostic stewardship interventions within their institutions. This review will discuss the need for diagnostic and antimicrobial stewardship, the interplay of diagnostic and antimicrobial stewardship, evidence of benefit to antimicrobial stewardship programmes, and considerations for successfully engaging in diagnostic stewardship interventions.
This article is part of the Antibiotic stewardship Special Issue: https://www.drugsincontext.com/special_issues/antimicrobial-stewardship-a-focus-on-the-need-for-moderation
Keywords: antimicrobial stewardship, diagnostic stewardship
Introduction
Diagnostic stewardship and antimicrobial stewardship: two peas in a pod
Although laboratory stewardship techniques have been used in clinical microbiology for decades, the modern definition and practice of diagnostic stewardship is relatively new, first being described in 2017 by Morgan et al.1 Diagnostic stewardship is an extension of principles of improving diagnosis through preventing diagnostic error as detailed by the National Academies.2 At its core, diagnostic stewardship refers to the modification of the process of ordering, performing and reporting of diagnostic test results to improve diagnostic accuracy, optimize treatment and improve overall patient outcomes.3,4
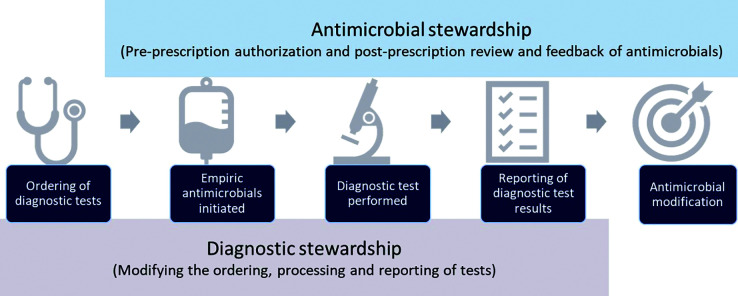
Modern diagnostic stewardship in infectious diseases works synergistically with antimicrobial stewardship (AMS). In fact, the CDC and Prevention Core Elements for AMS Programs designate diagnostic stewardship as a core part of AMS activities.5 Diagnostic stewardship often works upstream of traditional AMS efforts (Figure 1). Based on the terminology provided by Messacar et al.,6 diagnostic stewardship aims to provide the right test, for the right patient, at the right time, whilst AMS aims to provide the right (test) interpretation, providing the right antimicrobial, at the right time. Many of the core elements and key features needed for successful diagnostic stewardship interventions also directly align with those in AMS. In 2021, the CDC Division of Healthcare Quality Promotion, along with thought leaders, released a white paper on the importance of diagnostic stewardship for healthcare- associated infections, antimicrobial resistance, and sepsis management.7 This paper stressed the importance of an interdisciplinary approach that engages as many stakeholders as possible and includes AMS programme as core members of diagnostic stewardship teams.
Figure 1.
Relationship between diagnostic and antimicrobial stewardship.
Methods
This is a literature review using PubMed and Google Scholar for English-language papers on infectious diseases diagnostic stewardship and overdiagnosis of common healthcare-associated infections. The following keywords were used for the review of diagnostic stewardship: “antimicrobial stewardship”, “antibiotic stewardship”, “diagnosis”, “diagnostic stewardship”, “overdiagnosis”, “rapid diagnostics” and “stewardship”. Studies were limited to those most common to the healthcare setting, which had published literature on interventions, including epidemiology and treatment of asymptomatic bacteriuria (ASB) and urinary tract infections (UTIs), Clostridioides difficile infections (CDIs), bloodstream infections (BSIs), and lower respiratory tract infections (LRTIs). These were included as additional search terms. Given the novel nature of diagnostic stewardship, the search was limited to 2010–2022, and relevant papers reporting outcomes of the intervention on antibiotic utilization were selected for emphasis. The intended audience for this review includes healthcare professionals practicing in acute care settings; other settings, such as ambulatory care or outpatient, are not comprehensively addressed. Additionally, unless otherwise specified, interventions are focused to the adult patient population.
Review
The need for diagnostic stewardship
Whilst identifying a causative organism for an infectious syndrome allows for appropriate diagnosis and optimization of treatment, overdiagnosis in the field of infectious diseases has been increasingly recognized. Reliance on a sole diagnostic test, whether culture or non-culture based, leads to false-positive results, excessive antimicrobial exposure and associated downstream negative consequences, such as adverse drug events, CDI and selective pressure leading to development of resistance.8
Perhaps the most common cases of overdiagnosis and overtreatment occur in the setting of ASB. As defined in the 2019 Infectious Diseases Society of America guidelines, ASB is the presence of one or more bacteria in the urine in the absence of signs and symptoms of infection that are attributable or referable to the urinary tract.9 The incidence of ASB varies significantly: from 5–15% in healthy individuals to 30–60% amongst elderly patients residing in communities versus long-term care facilities, respectively, to upwards of 100% amongst those with chronic urinary catheters.10–12 Antimicrobial therapy for ASB is exceedingly common, occurring in upwards of 50% of cases documented in clinical literature.13 Additionally, a survey of hospital resident physicians found that only 33.7% were able to differentiate ASB from true infection and, importantly, after correct identification of ASB, approximately 50% reported still prescribing antimicrobial therapy despite no clear indication.14 Additional surveys have highlighted overcautiousness or risk perceptions, institutional culture and social norms, and being overworked as associated with overtreatment.15–18 Amongst primary care clinicians, 71% indicated that they would prescribe antimicrobial therapy in the setting of ASB without a clear indication.19 Clinical inertia often leads to continuation of antimicrobial therapy in the setting of ASB. For instance, in a 43-hospital cohort study of patients presenting to the emergency department with positive urine cultures but no documented signs or symptoms of a UTI, 74.4% received antimicrobial therapy.20 Factors associated with antimicrobial prescribing in the emergency department consisted of the presence of altered mental status, leucocytosis and abnormal results on urinalysis. Importantly, once antimicrobials were started, they often continued into patients’ hospital stay for at least 3 days; this was associated with longer hospitalization and development of CDI. Thus, inappropriate antimicrobial prescribing frequently stems from inappropriate urine culturing.21,22
CDIs are amongst the most common healthcare-associated infections and result in significant morbidity, mortality, hospital readmissions and attributable healthcare costs of approximately $6.3 billion USD annually.23–26 Diagnosis is through a combination of laboratory detection and clinical signs and symptoms of infection.27 In the 2010s, PCR for the detection of the genes responsible for toxin A and B production were released with sensitivities greater than 90%.28 Not long after, however, concerns emerged about the risk of ‘false-positive’ PCR-driving diagnosis and treatment.29 Importantly, adults can become asymptomatically colonized with C. difficile, with reported rates of 10% and 50% in patients who are acutely hospitalized or in long-term care, respectively, and colonization is 5–10 times more common than infection.29–32 A prospective study demonstrated the importance of differentiating infection versus colonization and the overreliance on PCR results.33 Amongst hospitalized adults tested for C. difficile, those who were positive in both toxin and PCR had longer durations of diarrhoea (median 3 days versus 2 days; p=0.003), higher rates of CDI complications (7.6% versus 0%), and higher rates of recurring CDI-attributable mortality (8.4% versus 0.6%; p=0.001) compared with those who were only positive in PCR. In fact, those who were toxin negative and PCR positive had clinical courses comparable to patients without a diagnosis of C. difficile. In a more recent study evaluating outcomes of patients who were PCR positive/toxin negative, results were similar in two time periods when PCR was reported, where patients were largely treated compared with a time period when toxin was reported and patients were largely untreated. Treatment for CDI in these PCR-positive/toxin-negative patients decreased from 91.5% to 15.1% after implementation, with no significant differences in unresolved diarrhoea at 7-day or 30-day mortality, suggesting substantial overtreatment of CDI occurring on the basis of PCR results alone.34 Overdiagnosis of CDI leads to overtreatment, which leads to concern of overgrowth of vancomycin-resistant enterococci, development of antimicrobial resistance and increased risk of future development of CDI through gut dysbiosis.35–38
Antimicrobial and diagnostic stewardship are also important for the prompt and appropriate management of BSIs, which are often associated with sepsis. Studies have shown the importance of diagnostics to improve the time to appropriate therapy in sepsis and its association with improved mortality.39,40 On the other hand, however, diagnostic stewardship can help optimize blood culture practices and reduce unnecessary treatment of contaminated blood cultures. Overall, blood cultures are associated with an extremely low yield, as only approximately 10% grow organisms. As Fabre et al. indicated, this low yield is partially caused by frequent ordering of blood cultures in those at low risk of a BSI.41 Additionally, though false positives can be caused by the blood culture identification instrument, this only accounts for less than 1% of bottles.42 More commonly, false positives are caused by contamination, with upwards of 50% of positive blood cultures being possibly contaminated.43,44 These contaminated cultures frequently result in prolonged hospital stays, antimicrobial therapy, and additional diagnostic testing.45–47 Similarly, amongst true Gram-negative BSIs, it has been shown that unnecessary follow-up blood cultures also result in prolonged antimicrobial administration and hospital stay.48,49
In the setting of intensive care units (ICUs), antimicrobial use is primarily driven by suspected ventilator-associated pneumonia (VAP), which has a reported incidence of more than 50%.50 This aligns with the significant morbidity and mortality of VAP, where delays in appropriate antimicrobial therapy have been directly associated with poor outcomes.51,52 These concerns were illuminated by a qualitative survey of healthcare providers that identified fear of missing a diagnosis as the primary driver of overdiagnosis and excessive antimicrobial therapy.53 Further compacting the issue is the wide array of non-specific diagnostic criteria and overreliance on microbiological culturing to determine a diagnosis because there is a lack of other reliable methods, such as biomarkers or scoring systems, to aid in the diagnosis.54 As such, it is speculated that VAP misdiagnosis is a common occurrence amongst patients in the ICU. For example, in a study of 231 patients with possible VAP, 58.4% were determined to not have this diagnosis based on external multidisciplinary committee review of day 1 symptomatology.55 Additionally, antimicrobials were continued in over 75% of patients determined not to have VAP on day 3, and researchers estimated patients received over 1100 excess antimicrobial days of therapy (DOT). The overreliance on respiratory culturing to diagnose VAP can be seen in the recent cohort study by Albin et al., who evaluated patients on mechanical ventilation with new abnormal white blood cell (WBC) count or temperature but without radiographic evidence, ventilatory changes or purulent secretion.56 Amongst the 534 patients studied, 21.2% had respiratory tract sampling for microbial cultures. This respiratory tract culturing was strongly associated with prolonged unnecessary antimicrobial use (OR 2.32; 95% CI 1.23–4.24), including broad-spectrum antimicrobial use (OR 2.23; 95% CI 1.33–3.73). This over-reliance on respiratory culturing and misconception that the presence of bacteria is synonymous with infection led Albin et al. to coin the term “asymptomatic bacterisputia” as an aid in promoting VAP stewardship efforts.57 How to decrease detection and treatment of asymptomatic bacterisputia in patients with VAP remains an area with research gaps that need to be addressed before widespread measures can be implemented.58
Examples of the impact of diagnostic stewardship on antimicrobial use
Ordering phase
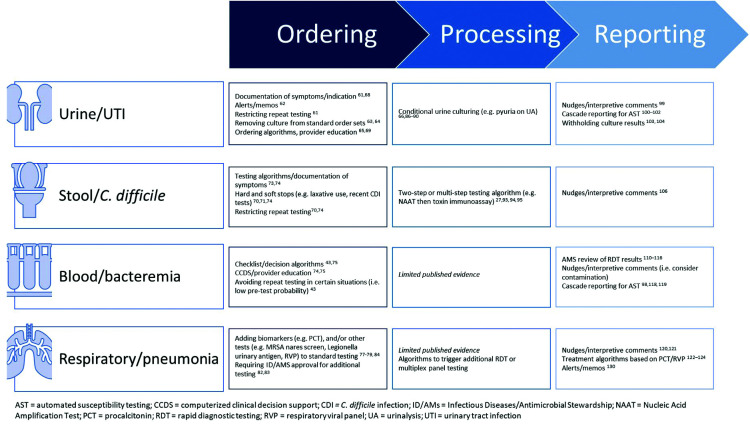
Several stewardship interventions have focused on the preanalytic, or ordering, phase of diagnostic testing, which includes the process of placing an order for the test. In the Choosing Wisely Campaign, the American Board of Internal Medicine and the American Society of Clinical Pathology highlight the need for appropriate test selection and timing.59,60 Both underutilization and overutilization of diagnostic tests are important as well as optimizing the timing of test ordering and performance. In the case of overutilization, interventions may focus on removing certain tests from routine order sets, reducing duplicative laboratory orders/testing, limiting repeat testing, providing decision support to guide appropriate testing, and/or educating clinicians about appropriate indications for diagnostic testing (Figure 2).61,62 Underutilization of appropriate diagnostic testing can lead to unnecessary diagnostic delays, increased unnecessary empirical antimicrobial use, longer hospitalization or worse patient outcomes due to missed diagnoses.61
Figure 2. Evidence-based diagnostic stewardship examples impacting antimicrobial use.
AST, automated susceptibility testing; CCDS, computerized clinical decision support; CDI, C. difficile infection; ID/AMs, Infectious Diseases/Antimicrobial Stewardship; NAAT, Nucleic Acid Amplification Test; PCT, procalcitonin; RDT, rapid diagnostic testing; RVP, respiratory viral panel; UA, urinalysis; UTI, urinary tract infection
A variety of best practices have been proposed to optimize testing for UTIs.62 These have been evaluated for their impact in several different populations and settings and may substantially reduce urine culture orders, UTI rates, costs, and antimicrobial use.63–70 For example, in a cluster-randomized controlled trial, a multifaceted intervention including educational sessions and a diagnostic/treatment algorithm was implemented in nursing homes. The intervention was associated with a 31% reduction in antimicrobials prescribed for suspected UTIs.70 More recently, an educational intervention in concert with computerized clinical decision support (CCDS) resulted in a reduction in urine cultures as well as antimicrobial use in a cluster-randomized trial in Canadian long-term care facilities.68 UTI antimicrobial prescriptions declined from 3.2 to 2.5 per 1000 resident days with no increase in hospital admissions; all-cause mortality declined in the intervention facilities (0.2 per 1000 resident days compared with baseline). Interventions focused on urine culture ordering in the critical care setting have also been successful. In one study, a reflex to culture strategy was adopted in the adult ICUs of an academic medical centre, whereby culturing was only performed based on the presence of pyuria in urinalysis (WBC count >10/hpf).67 This resulted in a 30% reduction in urine cultures performed immediately after the protocol started and a 6% monthly decline during the following year. The use of urinary antimicrobial agents in ICUs decreased from 449 DOT per 1000 patient days (DOT/1000 PD) preintervention to 425 DOT/1000 PD postintervention but, overall, there was not a significant trend to demonstrate a month-over-month decline during the 12-month postintervention period compared with baseline. In a random subset of patients, there was a significant decrease in new antimicrobial starts based on urine culture results (41% preintervention phase versus 23% postintervention; p<0.002).
Similarly, diagnostic stewardship interventions have optimized the workup of C. difficile, leading to reductions in CDI rates and antimicrobial use. Most publications reporting the impact of CDI test ordering interventions on antimicrobial use describe the implementation of clinical decision support tools or questions posed to clinicians at the time of test ordering.71–74 One centre used a ‘soft stop’, whereby the clinician can continue to place an order after viewing alerts reminding them of appropriate criteria, followed by a ‘hard stop’ that prevents the order from being placed unless certain criteria are met. They were able to demonstrate a decrease in test ordering of 24% as well as a decrease in oral vancomycin use.71 These strategies were compared in a multicentre collaborative study that initiated hard (nine institutions) or soft stops (four institutions) on orders for patients who had recently received laxatives or had a recent CDI test performed.75 Two additional institutions employed a human interaction approach, whereby members of AMS would encourage the cancellation of CDI test orders for inappropriate indications. This resulted in an adjusted 33% and 23% reduction in CDI test ordering at hard-stop and soft-stop sites, respectively, and a 21% reduction at the human interaction sites. All three interventions were associated with a lower rate of CDI cases and with significantly lower use of antimicrobials for CDI treatment, with reductions in oral vancomycin and fidaxomicin use of 23% for hard-stop, 15% for soft-stop and 27% for human interaction sites. CCDS tools have even been successfully extended to haematology/oncology populations, who are at high risk for C. difficile colonization and infection.74 A CCDS CDI testing algorithm requiring the presence of symptoms (leukocytosis, abdominal pain, ≥3 loose stools in 24 hours) and no laxative use within 72 hours was implemented in haematology/oncology wards as well as in a haematopoietic stem cell transplant unit of an academic medical centre. The centre also had a best practice alert (BPA) with a soft stop to prevent testing in the presence of laxative use within the previous 72 hours as well as C. difficile test positivity within the past 14 days. This intervention resulted in a 63% decrease in CDI rate, a 55% decrease in monthly CDI events, a 57% decrease in mean monthly use of oral vancomycin on these units, a 77% decline in vancomycin-resistant enterococci colonization, and an 86% decline in vancomycin-resistant enterococci hospital-onset bacteraemia over 22 months of follow-up.
Several recent studies have evaluated the potential effects of diagnostic stewardship interventions on blood culture orders, but not all have measured the direct impact of these on antimicrobial use.43,76 The use of a checklist and decision algorithm to guide decision-making by clinicians regarding blood culture orders was evaluated in a 36-bed paediatric ICU (PICU).76 The intervention was associated with a 46% reduction in blood culture collection rate with no increase in in-hospital mortality or readmissions. Consensus recommendations for when to avoid blood cultures in patients in the PICU were developed by an expert panel, and then subsequently studied with a quality improvement intervention in 14 PICUs.76,77 This resulted in a 33% relative reduction in blood cultures and a 13% relative reduction in the use of broad-spectrum antimicrobials, with no increase in mortality, length of stay, readmission, or clinical diagnoses such as sepsis/severe sepsis.
Stewardship interventions aimed at optimizing test ordering for the evaluation of LRTIs have been deployed using different strategies. In the ICU setting, a protocol was implemented to allow pharmacists to order diagnostic tests (influenza PCR, a respiratory viral panel, Legionella urine antigen test and procalcitonin (PCT)) based on certain criteria for patients with community-acquired pneumonia.78 This increased the performance of recommended diagnostic testing, with a 1.5-fold increase in the identification of a pathogen and more than a two-fold increase in antimicrobial de-escalations without any apparent increase in adverse patient outcomes. In patients with suspected pneumonia, several studies have evaluated adding a PCT order to standard of care and leveraging those results in antimicrobial de-escalation.79,80 However, this has not been universally successful so needs to be considered in the context of turn-around times and resources available at each facility.81 For instance, PCT levels that suggest a non-bacterial cause of infection do not necessarily result in timely antibiotic discontinuation in the majority of patients.82 Increasing availability of multiplex respiratory virus panels (RVPs) has added to the complexity of diagnostic test options available to clinicians, with the potential for more rapid results but at increased cost and, in some cases, may be duplicative with other tests. Several methods might be employed to optimize ordering of these tests, including reflexive or stepwise testing based on other results, diagnostic algorithms/CCDS, prior authorization from ID/AMS teams for testing and educational programmes.6,83,84 In the case of LRTIs, the addition of a multiplex panel with rapid results-directed standardized antimicrobial recommendations (within 5 hours of testing) shortened the duration of inappropriate antimicrobial therapy by 38.6 hours, without apparent adverse consequences on clinical stability, length of stay, or readmission rate.85
Processing phase
Clinical microbiology laboratories have established criteria for various types of specimens to ensure reliable test performance.3,86 These criteria and testing algorithms largely go unnoticed by clinicians. Diagnostic stewardship interventions aimed at processing of specimens exist in addition to these established testing criteria and can also have a significant downstream impact on antimicrobial use. However, evidence is largely limited to urine and stool/C. difficile testing.
Conditional urine reflex testing is the practice of cancelling urine cultures when specific criteria are not met by urinalysis.62 Several studies have demonstrated that implementation of conditional urine testing significantly decreases the number of urine cultures being processed, which has a substantial impact on laboratory workflow.67,87–91 Less data, however, are available to demonstrate the impact of this procedure on antimicrobial use. In a recent prospective pilot study of over 4000 patients, authors examined a matrix of urinalysis criteria (either alone or in combination) and their impact on urine culture positivity, UTIs, and inappropriate antimicrobial use.92 The authors reported that implementation of conditional urine reflex testing has the potential to decrease inappropriate antimicrobial use from 45% to 9%. In a recent quasi-experimental study in the emergency department setting, implementation of conditional urine reflex testing resulted in a significant decrease in inappropriate treatment of ASB from 10.2% to 1.9% (p=0.01).93
The Updated Infectious Diseases Society of America C. difficile Guidelines recommend multistep testing to aid in diagnostics of CDI.27,94 This assists in decreasing false-positive results, which are often caused by the combination of high sensitivity of nucleic acid amplification tests and colonization of the gastrointestinal tract with C. difficile.33 Implementation of a two-step testing algorithm for CDI at an 800-bed regional medical centre was associated with significant reductions in hospital-onset and community-onset CDI cases as well as with a 58% reduction in initiation of metronidazole (IV and oral) as treatment for CDI.95 A similar study found that, though PCR-positive C. difficile results did not change after implementation of two-step testing, the incidence of reportable CDI significantly decreased (54/10,000 admissions versus 15/10,000 admissions; p<0.0001). Importantly, use of oral vancomycin also decreased significantly, from 16 DOT/1000 PD to 9 DOT/1000 PD (p=0.0002).96
Reporting phase
Antimicrobial stewardship programmes frequently collaborate with clinical microbiology laboratories to alter the reporting of culture-and non-culture-based results to influence prescribing practices and decrease inappropriate antimicrobial use.6,97 The term ‘nudge’ has been used to describe these types of interventions and originated from behavioural economic theory.98 Nudges are interventions meant to guide decision-making through choice architecture whilst maintaining autonomy. The recent scoping review from Langford et al., entitled NIMBLE, described the most common type of nudges used in diagnostic and antimicrobial stewardship to affect antimicrobial use.99
There are examples of nudges within an electronic medical record (EMR) to alter not only urine culture ordering but also antimicrobial prescribing. Use of a BPA to remind providers that positive urine cultures, without signs or symptoms referred to the urinary tract, do not require treatment was able to decrease antimicrobial utilization from an average of 6.3 days to 2.2 days (p<0.001) without subsequent adverse events.100 Selective or cascade reporting of antimicrobials has also successfully altered prescribing practices for suspected UTIs.101–103 Given the successful impact of these interventions, they have become recommended by experts as core urine culture diagnostic stewardship practices.62 The most extreme form of selective reporting is to completely restrict reporting of all results from the urine culture, instead requiring clinicians to call the clinical microbiology laboratory for results if concerns for true infections persisted. In a proof-of-concept study, Leis et al.104 restricted urine culture results for all patients without a catheter. Amongst the specimens collected in the postintervention period, 89% of patients with positive cultures did not meet criteria for UTIs. The rate of antimicrobial prescribing for ASB decreased by 36% (95% CI 15–57%). A subsequent randomized, parallel, unblinded, superiority trial, again in patients without a catheter, demonstrated that restrictive reporting significantly improved the appropriateness of antimicrobial prescribing compared to traditional reporting (80% versus 52.7%; p=0.002).105
Limited data exist with respect to altering reporting for CDIs.106 Similar to BSI, AMS interventions often occur after reporting rapid diagnostic test (RDT) results. Most data relate to diagnostic stewardship interventions to prevent unnecessary test ordering.106 One recent study leveraged multistep processing of C. difficile nucleic acid amplification tests and toxin A/B testing in conjunction with a nudge to consider colonization when the toxin enzyme immunoassay test was negative.107 After implementation of this nudge, authors reported significantly decreased mean duration of CDI therapy (13.6−7.9 days (−5.8 days); 95% CI −3.9 to −7.6) and an increase in the proportion of patients who did not receive any CDI therapy (OR 4.5; 95% CI 2.3–8.7). Importantly, there was no change in the number of patients who later developed toxin positivity or experienced mortality.
Two main types of reporting interventions are commonly used by AMS programmes to improve antimicrobial use in BSIs: cascade reporting and AMS review and feedback on blood culture rapid diagnostics. RDTs for BSIs can identify key causative organisms hours to days sooner than traditional microbiological testing methods, with a high degree of sensitivity.108–110 In both Gram-positive and Gram-negative BSI, studies have shown the importance of AMS intervention on RDT results to improve time to appropriate antimicrobials and clinical outcomes.111–113 In one of a seminal paper by Banerjee et al., using a randomized trial design, those assigned to AMS review had significantly decreased time to therapy optimization compared with traditional testing or RDT with use of a templated comment. This includes both time to antimicrobial de-escalation (34 hours control versus 38 hours RDT versus 21 hours RDT plus AMS; p<0.001) and antimicrobial escalation (24 hours control versus 6 hours RDT versus 5 hours RDT plus AMS; p=0.04).114 In a systematic review and meta-analysis, Timbrook et al. also demonstrated that AMS involvement in review and feedback on RDT results can improve patient outcomes; in particular, studies with AMS involvement showed a decreased odds of all-cause mortality compared with those without AMS (OR 0.64; 95% CI 0.51–0.79).115 Finally, AMS involvement with RDT results has also been shown to be cost-effective.116,117 In decision-analytic models, RDTs with AMS review have a probability of being 80% cost-effective and have substantially improved incremental cost-effectiveness ratios compared with other identification methods or RDT alone.116,117 Given the important impact of AMS on patient outcomes, it is crucial that AMS programmes are integral in the implementation of diagnostic strategies for BSI at their local facilities.118
Cascade reporting is a more passive intervention often employed by AMS to alter prescribing practices by first reporting limited-spectrum agents and only reporting secondary, more broad-spectrum agents if resistance to those limited-spectrum agents is present.119 Cascade reporting is most commonly employed for blood and urine cultures.99 Several studies have reported on the impact of cascade reporting on the management of BSI, including across eight Veterans’ Health Affairs Medical Centres. In this study, AMS developed reporting testing algorithms by genus for BSI caused by Enterobacterales or Pseudomonas aeruginosa.120 After implementation of cascade reporting, there was a 24% decrease in mean DOT/1000 PD for meropenem use (p=0.005).
Antimicrobial stewardship programmes have shown the power of collaborating with clinical microbiology to alter reporting of sputum, tracheal aspirate and bronchoalveolar lavage cultures with simple nudges regarding the presence or absence of certain organisms.121,122 For example, Musgrove et al.121 implemented a nudge comment that highlighted the absence of methicillin-resistant Staphylococcus aureus or Pseudomonas spp. in the final culture results. This comment, coupled with AMS provider education, was able to decrease broad-spectrum antimicrobial prescribing from a median of 7 to 5 days (p<0.001) and was independently associated with a 5.5-fold increased odds of de-escalation. The widespread use of multiplex RVP PCRs has provided AMS programmes with opportunities to intervene on unnecessary antimicrobial therapy. CDSS tools that flag patients with positive RVP test results sent to AMS pharmacists for prospective audit and feedback have been shown to facilitate significant and substantial decreases in unnecessary therapy.123–125 The biomarker PCT has also been studied in randomized trials as a tool to differentiate a viral from bacterial aetiology of LRTI.79,126–128 However, adherence to algorithms in real-world practice tends to be less than optimal.129,130 Nevertheless, the combination of RVP, PCT and AMS involvement can be highly valuable in guiding providers to appropriate management or viral LRTIs.84 For example, Moradi et al.131 implemented an AMS alert for adult patients with positive RVP, low PCT and at least one systemic antimicrobial agent ordered that urged providers to reassess the continued need for therapy. After implementing this BPA, mean DOT decreased by 2.2 days and there was a significant decrease in antimicrobial use at discharge (47.8% versus 20%; p<0.001).
Discussion
Pitfalls and considerations of implementing diagnostic stewardship
There are many pitfalls to avoid when leveraging diagnostic stewardship to optimize antimicrobial use. These have been reported on to some extent in the literature but additional local considerations may be identified and addressed by including multidisciplinary groups with various perspectives in the planning stages. Designing these interventions can be hampered by a lack of consensus around certain testing criteria and marked variability between labs in specimen handling and processing.132 Engaging physician champions and understanding the local culture within an institution are helpful to navigating these challenges. Implementation of testing protocols may also be sub-optimal when the reported reference range for a test is not consistent with AMS protocols to guide interpretation as has been described with PCT.133
An important consideration when undertaking diagnostic stewardship interventions is the need for administrative support, and it is imperative to include health information technology and other administrative personnel in the diagnostic stewardship team in order to ensure successful implementation and maintain positive impact. Many of these interventions involve programming EMR systems to provide CCDS and nudge comments on test results to guide antimicrobial selection or de-escalation. Too many alerts may lead to disruptions in clinician workflow, alert fatigue and information overload.7,61,134 However, user-centred EMR design could make it easier for clinicians to appropriately order diagnostic tests. For example, showing recent laxative use in an order panel or dates/results of prior CDI testing has been shown to facilitate appropriate ordering of CDI tests.73 There is increasing recognition of the challenges inherent in making one group responsible for antimicrobial use within an institution or health system and a call to broaden this responsibility to individual prescribers. By harnessing CCDS, educational tools, local syndrome-based guidelines, established frameworks for decision-making and other stewardship strategies, programmes can engage prescribers in taking ownership of diagnostic and antimicrobial stewardship to drive appropriate use of diagnostic tests and antimicrobial agents.135
Additionally, the adoption of new broad syndromic diagnostic panels without careful attention may lead to unintended consequences. For example, molecular testing for C. difficile is included in several comprehensive stool testing panels; therefore, if performed in addition to other CDI testing, these could be unnecessarily duplicative and could yield conflicting results.136 Low-yield scenarios for implementation of syndromic panels should be considered and, if multiplex panels are adopted for higher-yield use cases, these diagnostic tests should be carefully stewarded.7 Furthermore, implementation of highly sensitive non-culture-based diagnostic tests might identify organisms that did not appear in cultures and do not definitively indicate the organism is responsible for the symptoms the patient is experiencing nor do they distinguish colonization from infection.
Conclusions
There is a need to address overdiagnosis of infectious diseases, particularly in acute care settings, as this often leads to excessive antimicrobial use. As AMS programmes continue to strive to improve antimicrobial use and patient outcomes, novel diagnostic stewardship interventions should be considered. Core AMS principles, such as prospective audit and feedback, remain central components of a successful AMS programme. By leveraging diagnostic stewardship and strong relationships with clinical microbiology laboratories as well as interdisciplinary teams, AMS programmes will be able to affect positive changes throughout the diagnostic process and will not be limited to interventions that occur after diagnostic decision-making. These diagnostic interventions will complement AMS activities. Recognizing the limitations of our current knowledge and technology in relation to diagnostic methods, we must engage in continuous quality improvement to build on what we know and optimize diagnosis and treatment for patients with infectious syndromes.
Acknowledgements
None.
Footnotes
Contributions: The authors (KCC and MDJ) contributed equally to the development and writing of this work. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: KCC reports speaking and service for bioMérieux, research funding from Centers from Disease Control and Prevention and Merck & Co. KCC has served on advisory boards for bioMérieux, Melinta Therapeutics, La Jolla Pharmaceuticals and AbbVie. MDJ has received consulting fees from Astellas, Cidara, Merck, Entasis, Paratek, Pfizer and Theratechnologies, author royalties from UpToDate, and research grants to her institution from Astellas, Scynexis, Charles River Laboratories and Merck & Co, and has served on the Board of the Society of Infectious Diseases Pharmacists. She also has a patent pending for gene expression–based classifiers of fungal infection. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2023/01/dic.2022-9-5-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2023 Claeys KC, Johnson MD. https://doi.org/10.7573/dic.2022-9-5. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Article URL: https://www.drugsincontext.com/leveraging-diagnostic-stewardship-within-antimicrobial-stewardship-programmes
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 6 Green Lane Business Park, 238 Green Lane, New Eltham, London, SE9 3TL, UK.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Morgan DJ, Malani P, Diekema DJ. Diagnostic stewardship – leveraging the laboratory to improve antimicrobial use. JAMA. 2017;318(7):607–608. doi: 10.1001/jama.2017.8531. [DOI] [PubMed] [Google Scholar]
- 2.Balogh EP, Miller BT, Ball JR, editors. Committee on Diagnostic Error in Health Care, Board on Health Care Services, Institute of Medicine, The National Academies of Sciences, Engineering, and Medicine. Improving Diagnosis in Health Care. Washington, DC: National Academies Press; 2015. [DOI] [PubMed] [Google Scholar]
- 3.Patel R, Fang FC. Diagnostic stewardship: opportunity for a laboratory-infectious diseases partnership. Clin Infect Dis. 2018;67(5):799–801. doi: 10.1093/cid/ciy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bearman G, Morgan DJ, Murthy RK, Hota S, editors. Infection Prevention: New Perspectives and Controversies. Cham, Switzerland: Springer International Publishing; 2022. [DOI] [Google Scholar]
- 5.CDC. Core Elements of Hospital Antibiotic Stewardship Programs. Antibiotic Use. [Accessed August 16, 2018]. https://www.cdc.gov/antibiotic-use/healthcare/implementation/core-elements.html .
- 6.Messacar K, Parker SK, Todd JK, Dominguez SR. Implementation of rapid molecular infectious disease diagnostics: the role of diagnostic and antimicrobial stewardship. J Clin Microbiol. 2017;55(3):715–723. doi: 10.1128/JCM.02264-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curren EJ, Lutgring JD, Kabbani S, et al. Advancing diagnostic stewardship for healthcare-associated infections, antibiotic resistance, and sepsis. Clin Infect Dis. 2021;74(4):723–728. doi: 10.1093/cid/ciab672. [DOI] [PubMed] [Google Scholar]
- 8.Hollenbeck BL, Hoffman M, Fang CJ, Counterman K, Cohen S, Bell CA. Elimination of routine urinalysis before elective orthopaedic surgery reduces antibiotic utilization without impacting catheter-associated urinary tract infection or surgical site infection rates. Hip Pelvis. 2021;33(4):225–230. doi: 10.5371/hp.2021.33.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):e83–e110. doi: 10.1093/cid/ciy1121. [DOI] [PubMed] [Google Scholar]
- 10.Sedor J, Mulholland SG. Hospital-acquired urinary tract infections associated with the indwelling catheter. Urol Clin North Am. 1999;26(4):821–828. doi: 10.1016/s0094-0143(05)70222-6. [DOI] [PubMed] [Google Scholar]
- 11.Ipe DS, Sundac L, Benjamin WH, Moore KH, Ulett GC. Asymptomatic bacteriuria: prevalence rates of causal microorganisms, etiology of infection in different patient populations, and recent advances in molecular detection. FEMS Microbiol Lett. 2013;346(1):1–10. doi: 10.1111/1574-6968.12204. [DOI] [PubMed] [Google Scholar]
- 12.Saint S, Chenoweth CE. Biofilms and catheter-associated urinary tract infections. Infect Dis Clin North Am. 2003;17(2):411–432. doi: 10.1016/s0891-5520(03)00011-4. [DOI] [PubMed] [Google Scholar]
- 13.Spivak ES, Burk M, Zhang R, et al. Management of bacteriuria in veterans affairs hospitals. Clin Infect Dis. 2017;65(6):910–917. doi: 10.1093/cid/cix474. [DOI] [PubMed] [Google Scholar]
- 14.Lee MJ, Kim M, Kim NH, et al. Why is asymptomatic bacteriuria overtreated?: a tertiary care institutional survey of resident physicians. BMC Infect Dis. 2015;15:289. doi: 10.1186/s12879-015-1044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drekonja DM, Abbo LM, Kuskowski MA, Gnadt C, Shukla B, Johnson JR. A survey of resident physicians’ knowledge regarding urine testing and subsequent antimicrobial treatment. Am J Infect Control. 2013;41(10):892–896. doi: 10.1016/j.ajic.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Trautner BW, Petersen NJ, Hysong SJ, Horwitz D, Kelly PA, Naik AD. Overtreatment of asymptomatic bacteriuria: identifying provider barriers to evidence-based care. Am J Infect Control. 2014;42(6):653–658. doi: 10.1016/j.ajic.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Grigoryan L, Naik AD, Horwitz D, et al. Survey finds improvement in cognitive biases that drive overtreatment of asymptomatic bacteriuria after a successful antimicrobial stewardship intervention. Am J Infect Control. 2016;44(12):1544–1548. doi: 10.1016/j.ajic.2016.04.238. [DOI] [PubMed] [Google Scholar]
- 18.Eyer MM, Läng M, Aujesky D, Marschall J. Overtreatment of asymptomatic bacteriuria: a qualitative study. J Hosp Infect. 2016;93(3):297–303. doi: 10.1016/j.jhin.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Baghdadi JD, Korenstein D, Pineles L, et al. Exploration of primary care clinician attitudes and cognitive characteristics associated with prescribing antibiotics for asymptomatic bacteriuria. JAMA Netw Open. 2022;5(5):e2214268. doi: 10.1001/jamanetworkopen.2022.14268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petty LA, Vaughn VM, Flanders SA, et al. Assessment of testing and treatment of asymptomatic bacteriuria initiated in the Emergency Department. Open Forum Infect Dis. 2020;7(12):ofaa537. doi: 10.1093/ofid/ofaa537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drekonja DM, Gnadt C, Kuskowski MA, Johnson JR. Urine cultures among hospitalized veterans: casting too broad a net? Infect Control Hosp Epidemiol. 2014;35(5):574–576. doi: 10.1086/675829. [DOI] [PubMed] [Google Scholar]
- 22.Dougherty DF, Rickwa J, Guy D, et al. Reducing inappropriate urine cultures through a culture standardization program. Am J Infect Control. 2020;48(6):656–662. doi: 10.1016/j.ajic.2019.09.028. [DOI] [PubMed] [Google Scholar]
- 23.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deshpande A, Pant C, Olyaee M, Donskey CJ. Hospital readmissions related to Clostridium difficile infection in the United States. Am J Infect Control. 2018;46(3):346–347. doi: 10.1016/j.ajic.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 25.Desai K, Gupta SB, Dubberke ER, Prabhu VS, Browne C, Mast TC. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infect Dis. 2016;16:303. doi: 10.1186/s12879-016-1610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang S, Palazuelos-Munoz S, Balsells EM, Nair H, Chit A, Kyaw MH. Cost of hospital management of Clostridium difficile infection in United States—a meta-analysis and modelling study. BMC Infect Dis. 2016;16(1):447. doi: 10.1186/s12879-016-1786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin Infect Dis. 2018;66(7):e1–e48. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deshpande A, Pasupuleti V, Rolston DDK, et al. Diagnostic accuracy of real-time polymerase chain reaction in detection of Clostridium difficile in the stool samples of patients with suspected clostridium difficile infection: a meta-analysis. Clin Infect Dis. 2011;53(7):e81–e90. doi: 10.1093/cid/cir505. [DOI] [PubMed] [Google Scholar]
- 29.Dubberke ER, Burnham CAD. Diagnosis of Clostridium difficile Infection: treat the patient, not the test. JAMA Intern Med. 2015;175(11):1801–1802. doi: 10.1001/jamainternmed.2015.4607. [DOI] [PubMed] [Google Scholar]
- 30.Arvand M, Moser V, Schwehn C, Bettge-Weller G, Hensgens MP, Kuijper EJ. High prevalence of Clostridium difficile colonization among nursing home residents in Hesse, Germany. PLoS One. 2012;7(1):e30183. doi: 10.1371/journal.pone.0030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponnada S, Guerrero DM, Jury LA, et al. Acquisition of Clostridium difficile colonization and infection after transfer from a veterans affairs hospital to an affiliated long-term care facility. Infect Control Hosp Epidemiol. 2017;38(9):1070–1076. doi: 10.1017/ice.2017.140. [DOI] [PubMed] [Google Scholar]
- 32.Fleming MS, Hess O, Albert HL, et al. Test stewardship, frequency and fidelity: impact on reported hospital- onset Clostridioides difficile. Infect Control Hosp Epidemiol. 2019;40(6):710–712. doi: 10.1017/ice.2019.63. [DOI] [PubMed] [Google Scholar]
- 33.Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med. 2015;175(11):1792–1801. doi: 10.1001/jamainternmed.2015.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogan CA, Hitchcock MM, Frost S, et al. Clinical outcomes of treated and untreated C. difficile PCR-positive/toxin-negative adult hospitalized patients: a quasi-experimental noninferiority study. J Clin Microbiol. 2022;60(6):e0218721. doi: 10.1128/jcm.02187-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes HY, Odom RT, Michelin AV, et al. A retrospective cohort study of antibiotic exposure and vancomycin-resistant Enterococcus recolonization. Infect Control Hosp Epidemiol. 2019;40(4):414–419. doi: 10.1017/ice.2019.15. [DOI] [PubMed] [Google Scholar]
- 36.Kelly SG, Yarrington M, Zembower TR, et al. Inappropriate Clostridium difficile testing and consequent overtreatment and inaccurate publicly reported metrics. Infect Control Hosp Epidemiol. 2016;37(12):1395–1400. doi: 10.1017/ice.2016.210. [DOI] [PubMed] [Google Scholar]
- 37.Lee HS, Plechot K, Gohil S, Le J. Clostridium difficile: diagnosis and the consequence of over diagnosis. Infect Dis Ther. 2021;10(2):687–697. doi: 10.1007/s40121-021-00417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isaac S, Scher JU, Djukovic A, et al. Short- and long-term effects of oral vancomycin on the human intestinal microbiota. J Antimicrob Chemother. 2017;72(1):128–136. doi: 10.1093/jac/dkw383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marquet K, Liesenborgs A, Bergs J, Vleugels A, Claes N. Incidence and outcome of inappropriate in-hospital empiric antibiotics for severe infection: a systematic review and meta-analysis. Crit Care. 2015;19(1):63. doi: 10.1186/s13054-015-0795-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lodise TP, Zhao Q, Fahrbach K, Gillard PJ, Martin A. A systematic review of the association between delayed appropriate therapy and mortality among patients hospitalized with infections due to Klebsiella pneumoniae or Escherichia coli: how long is too long? BMC Infect Dis. 2018;18:625. doi: 10.1186/s12879-018-3524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fabre V, Carroll KC, Cosgrove SE. Blood culture utilization in the hospital setting: a call for diagnostic stewardship. J Clin Microbiol. 2022;60(3):e0100521. doi: 10.1128/JCM.01005-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamy B, Dargère S, Arendrup MC, Parienti JJ, Tattevin P. How to optimize the use of blood cultures for the diagnosis of bloodstream infections? A state-of-the art. Front Microbiol. 2016;7:697. doi: 10.3389/fmicb.2016.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fabre V, Klein E, Salinas AB, et al. A diagnostic stewardship intervention to improve blood culture use among adult nonneutropenic inpatients: the DISTRIBUTE study. J Clin Microbiol. 2020;58(10):e01053–20. doi: 10.1128/JCM.01053-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dempsey C, Skoglund E, Muldrew KL, Garey KW. Economic health care costs of blood culture contamination: a systematic review. Am J Infect Control. 2019;47(8):963–967. doi: 10.1016/j.ajic.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 45.Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA. 1991;265(3):365–369. [PubMed] [Google Scholar]
- 46.Alahmadi YM, Aldeyab MA, McElnay JC, et al. Clinical and economic impact of contaminated blood cultures within the hospital setting. J Hosp Infect. 2011;77(3):233–236. doi: 10.1016/j.jhin.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 47.Doern CD. The confounding role of antimicrobial stewardship programs in understanding the impact of technology on patient care. J Clin Microbiol. 2016;54(10):2420–2423. doi: 10.1128/JCM.01484-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in Gram-negative bacteremia: are they needed? Clin Infect Dis. 2017;65(11):1776–1779. doi: 10.1093/cid/cix648. [DOI] [PubMed] [Google Scholar]
- 49.Buzzalino LG, Mease J, Bernhardi CL, Bork JT, Johnson JK, Claeys KC. Follow-up blood culture practices for Gram-negative bloodstream infections in immunocompromised hosts at a Large Academic Medical Center. Open Forum Infect Dis. 2022;9(5):ofac173. doi: 10.1093/ofid/ofac173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rimawi RH, Mazer MA, Siraj DS, Gooch M, Cook PP. Impact of regular collaboration between infectious diseases and critical care practitioners on antimicrobial utilization and patient outcome. Crit Care Med. 2013;41(9):2099–2107. doi: 10.1097/CCM.0b013e31828e9863. [DOI] [PubMed] [Google Scholar]
- 51.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33(10):2184–2193. doi: 10.1097/01.ccm.0000181731.53912.d9. [DOI] [PubMed] [Google Scholar]
- 52.Zilberberg MD, Nathanson BH, Puzniak LA, Zilberberg NWD, Shorr AF. Inappropriate empiric therapy impacts complications and hospital resource utilization differentially among different types of bacterial nosocomial pneumonia: a cohort study, United States, 2014–2019. Crit Care Explor. 2022;4(4):e0667. doi: 10.1097/CCE.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kenaa B, O’Hara LM, Richert ME, et al. A qualitative assessment of the diagnosis and management of ventilator-associated pneumonia among critical care clinicians exploring opportunities for diagnostic stewardship. Infect Control Hosp Epidemiol. 2022;43(3):284–290. doi: 10.1017/ice.2021.130. [DOI] [PubMed] [Google Scholar]
- 54.Browne E, Hellyer TP, Baudouin SV, et al. A national survey of the diagnosis and management of suspected ventilator-associated pneumonia. BMJ Open Resp Res. 2014;1(1):e000066. doi: 10.1136/bmjresp-2014-000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nussenblatt V, Avdic E, Berenholtz S, et al. Ventilator-associated pneumonia: overdiagnosis and treatment are common in medical and surgical intensive care units. Infect Control Hosp Epidemiol. 2014;35(3):278–284. doi: 10.1086/675279. [DOI] [PubMed] [Google Scholar]
- 56.Albin OR, Saravolatz L, Petrie J, Henig O, Kaye KS. Rethinking the “Pan-Culture”: clinical impact of respiratory culturing in patients with low pretest probability of ventilator-associated pneumonia. Open Forum Infect Dis. 2022;9(6):ofac183. doi: 10.1093/ofid/ofac183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Albin OR, Pogue JM, Petty LA, Kaye KS. Asymptomatic bacterisputia: rethinking diagnostic stewardship in pneumonia. Infect Control Hosp Epidemiol. 2021;42(6):737–739. doi: 10.1017/ice.2021.109. [DOI] [PubMed] [Google Scholar]
- 58.Kenaa B, Richert ME, Claeys KC, et al. Ventilator-associated pneumonia: diagnostic test stewardship and relevance of culturing practices. Curr Infect Dis Rep. 2019;21(12):50. doi: 10.1007/s11908-019-0708-3. [DOI] [PubMed] [Google Scholar]
- 59.Morgan DJ, Croft LD, Deloney V, et al. Choosing Wisely in healthcare epidemiology and antimicrobial stewardship. Infect Control Hosp Epidemiol. 2016;37(7):755–760. doi: 10.1017/ice.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.American Society for Clinical Pathology. [Accessed September 25, 2022];Choosing Wisely. https://www.choosingwisely.org/societies/american-society-for-clinical-pathology . [Google Scholar]
- 61.Hueth KD, Prinzi AM, Timbrook TT. Diagnostic stewardship as a team sport: interdisciplinary perspectives on improved implementation of interventions and effect measurement. Antibiotics. 2022;11(2):250. doi: 10.3390/antibiotics11020250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Claeys KC, Trautner BW, Leekha S, et al. Optimal urine culture diagnostic stewardship practice- results from an expert modified-delphi procedure. Clin Infect Dis. 2021;75(3):382–389. doi: 10.1093/cid/ciab987. [DOI] [PubMed] [Google Scholar]
- 63.Keller SC, Feldman L, Smith J, Pahwa A, Cosgrove SE, Chida N. The use of clinical decision support in reducing diagnosis of and treatment of asymptomatic bacteriuria. J Hosp Med. 2018;13(6):392–395. doi: 10.12788/jhm.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Munigala S, Jackups RR, Poirier RF, et al. Impact of order set design on urine culturing practices at an academic medical centre emergency department. BMJ Qual Saf. 2018;27(8):587–592. doi: 10.1136/bmjqs-2017-006899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Munigala S, Rojek R, Wood H, et al. Effect of changing urine testing orderables and clinician order sets on inpatient urine culture testing: Analysis from a large academic medical center. Infect Control Hosp Epidemiol. 2019;40(3):281–286. doi: 10.1017/ice.2018.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175(7):1120–1127. doi: 10.1001/jamainternmed.2015.1878. [DOI] [PubMed] [Google Scholar]
- 67.Sarg M, Waldrop GE, Beier MA, et al. Impact of changes in urine culture ordering practice on antimicrobial utilization in intensive care units at an Academic Medical Center. Infect Control Hosp Epidemiol. 2016;37(4):448–454. doi: 10.1017/ice.2015.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pasay DK, Guirguis MS, Shkrobot RC, et al. Antimicrobial stewardship in rural nursing homes: Impact of interprofessional education and clinical decision tool implementation on urinary tract infection treatment in a cluster randomized trial. Infect Control Hosp Epidemiol. 2019;40(4):432–437. doi: 10.1017/ice.2019.9. [DOI] [PubMed] [Google Scholar]
- 69.Watson KJ, Trautner B, Russo H, et al. Using clinical decision support to improve urine culture diagnostic stewardship, antimicrobial stewardship, and financial cost: a multicenter experience. Infect Control Hosp Epidemiol. 2020;41(5):564–570. doi: 10.1017/ice.2020.37. [DOI] [PubMed] [Google Scholar]
- 70.Loeb M, Brazil K, Lohfeld L, et al. Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: cluster randomised controlled trial. BMJ. 2005;331(7518):669. doi: 10.1136/bmj.38602.586343.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mizusawa M, Small BA, Hsu YJ, et al. Prescriber behavior in Clostridioides difficile testing: a 3-hospital diagnostic stewardship intervention. Clin Infect Dis. 2019;69(11):2019–2021. doi: 10.1093/cid/ciz295. [DOI] [PubMed] [Google Scholar]
- 72.Sperling K, Priddy A, Suntharam N, Feuerhake T. Optimizing testing for Clostridium difficile infection: a quality improvement project. Am J Infect Control. 2019;47(3):340–342. doi: 10.1016/j.ajic.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 73.Rock C, Perlmutter R, Blythe D, et al. Impact of Statewide Prevention and Reduction of Clostridioides difficile (SPARC), a Maryland public health–academic collaborative: an evaluation of a quality improvement intervention. BMJ Qual Saf. 2022;31(2):153–162. doi: 10.1136/bmjqs-2021-014014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nix CD, Messer WB, Hale ML, Lewis JS, Strasfeld LM. Impact of a Clostridioides difficile testing computerized clinical decision support tool on an adult stem cell transplantation and hematologic malignancies unit. Transplant Cell Ther. 2021;27(1):94. doi: 10.1016/j.bbmt.2020.10.005. e1–94.e5. [DOI] [PubMed] [Google Scholar]
- 75.Rock C, Abosi O, Bleasdale S, et al. Clinical decision support systems to reduce unnecessary Clostridioides difficile testing across multiple hospitals. Clin Infect Dis. 2022;75(7):1187–1193. doi: 10.1093/cid/ciac074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Woods-Hill CZ, Fackler J, Nelson McMillan K, et al. Association of a clinical practice guideline with blood culture use in critically ill children. JAMA Pediatr. 2017;171(2):157–164. doi: 10.1001/jamapediatrics.2016.3153. [DOI] [PubMed] [Google Scholar]
- 77.Woods-Hill CZ, Colantuoni EA, Koontz DW, et al. Association of diagnostic stewardship for blood cultures in critically ill children with culture rates, antibiotic use, and patient outcomes: results of the bright STAR collaborative. JAMA Pediatr. 2022;176(7):690–698. doi: 10.1001/jamapediatrics.2022.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bianchini ML, Mercuro NJ, Kenney RM, et al. Improving care for critically ill patients with community-acquired pneumonia. Am J Health Syst Pharm. 2019;76(12):861–868. doi: 10.1093/ajhp/zxz068. [DOI] [PubMed] [Google Scholar]
- 79.Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498. doi: 10.1002/14651858.CD007498.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Broyles MR. Impact of procalcitonin-guided antibiotic management on antibiotic exposure and outcomes: real-world evidence. Open Forum Infect Dis. 2017;4(4):ofx213. doi: 10.1093/ofid/ofx213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379(3):236–249. doi: 10.1056/NEJMoa1802670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peña K, Cooper M, Greer N, Elders T, Septimus E. Process analysis of procalcitonin monitoring within community hospitals. Am J Health Syst Pharm. 2020;77(8):632–635. doi: 10.1093/ajhp/zxaa028. [DOI] [PubMed] [Google Scholar]
- 83.Timbrook T, Maxam M, Bosso J. Antibiotic discontinuation rates associated with positive respiratory viral panel and low procalcitonin results in proven or suspected respiratory infections. Infect Dis Ther. 2015;4(3):297–306. doi: 10.1007/s40121-015-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Covert K, Bashore E, Edds M, Lewis PO. Utility of the respiratory viral panel as an antimicrobial stewardship tool. J Clin Pharm Ther. 2021;46(2):277–285. doi: 10.1111/jcpt.13326. [DOI] [PubMed] [Google Scholar]
- 85.Darie AM, Khanna N, Jahn K, et al. Fast multiplex bacterial PCR of bronchoalveolar lavage for antibiotic stewardship in hospitalised patients with pneumonia at risk of Gram-negative bacterial infection (Flagship II): a multicentre, randomised controlled trial. Lancet Respir Med. 2022;10(9):877–887. doi: 10.1016/S2213-2600(22)00086-8. [DOI] [PubMed] [Google Scholar]
- 86.Burd EM, Kehl KS. A Critical appraisal of the role of the clinical microbiology laboratory in the diagnosis of urinary tract infections. J Clin Microbiol. 2011;49(Suppl 9):S34–S38. doi: 10.1128/JCM.00788-11. [DOI] [Google Scholar]
- 87.Humphries RM, Dien Bard J. Point-counterpoint: reflex cultures reduce laboratory workload and improve antimicrobial stewardship in patients suspected of having urinary tract infections. J Clin Microbiol. 2016;54(2):254–258. doi: 10.1128/JCM.03021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Claeys KC, Zhan M, Pineles L, et al. Conditional reflex to urine culture: evaluation of a diagnostic stewardship intervention within the Veterans’ Affairs and Centers for Disease Control and Prevention Practice-Based Research Network. Infect Control Hosp Epidemiol. 2021;42(2):176–181. doi: 10.1017/ice.2020.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lynch CS, Appleby-Sigler A, Bork JT, et al. Effect of urine reflex culturing on rates of cultures and infections in acute and long-term care. Antimicrob Resist Infect Control. 2020;9(1):96. doi: 10.1186/s13756-020-00762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Epstein L, Edwards JR, Halpin AL, et al. Evaluation of a novel intervention to reduce unnecessary urine cultures in intensive care units at a Tertiary Care Hospital in Maryland, 2011–2014. Infect Control Hosp Epidemiol. 2016;37(5):606–609. doi: 10.1017/ice.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Howard-Anderson JR, Ashraf S, Overton EC, Reif L, Murphy DJ, Jacob JT. Sustained decrease in urine culture utilization after implementing a reflex urine culture intervention: a multicenter quasi-experimental study. Infect Control Hosp Epidemiol. 2020;41(3):369–371. doi: 10.1017/ice.2020.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ourani M, Honda NS, MacDonald W, Roberts J. Evaluation of evidence-based urinalysis reflex to culture criteria: impact on reducing antimicrobial usage. Int J Infect Dis. 2021;102:40–44. doi: 10.1016/j.ijid.2020.09.1471. [DOI] [PubMed] [Google Scholar]
- 93.Patel UC, Ismail G, Suda KJ, Sabzwari R, Pacheco SM, Bhoopalam S. Evaluating the impact of a urinalysis to reflex culture process change in the Emergency Department at a Veterans Affairs Hospital. Fed Pract. 2022;39(2):76–81. doi: 10.12788/fp.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Madden GR, Poulter MD, Sifri CD. Diagnostic stewardship and the 2017 update of the IDSA-SHEA Clinical Practice Guidelines for Clostridium difficile Infection. Diagnosis. 2018;5(3):119–125. doi: 10.1515/dx-2018-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walker M, Mitchell M, Mellor B, Buras S, Young M, O’Neal CS. 2363. Implementation of a multi-step diagnostic algorithm for C. difficile infection. Open Forum Infect Dis. 2019;6(Suppl 2):S814. doi: 10.1093/ofid/ofz360.2041. [DOI] [Google Scholar]
- 96.Bork J, Claeys KC, Johnson JK, et al. 2362. Back to the future: the impact of multi-step algorithm C. difficile testing at a Large Tertiary Medical Center. Open Forum Infect Dis. 2019;6(Suppl 2):S813–S814. doi: 10.1093/ofid/ofz360.2040. [DOI] [Google Scholar]
- 97.MacVane SH, Hurst JM, Steed LL. The role of antimicrobial stewardship in the clinical microbiology laboratory: stepping up to the plate. Open Forum Infect Dis. 2016;3(4):ofw201. doi: 10.1093/ofid/ofw201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thaler RH, Sunstein CR. Nudge: Improving Decisions about Health, Wealth, and Happiness. New Haven, CT: Yale University Press; 2008. [Google Scholar]
- 99.Langford BJ, Leung E, Haj R, et al. Nudging in MicroBiology Laboratory Evaluation (NIMBLE): a scoping review. Infect Control Hosp Epidemiol. 2019;40(12):1400–1406. doi: 10.1017/ice.2019.293. [DOI] [PubMed] [Google Scholar]
- 100.Linares LA, Thornton DJ, Strymish J, Baker E, Gupta K. Electronic memorandum decreases unnecessary antimicrobial use for asymptomatic bacteriuria and culture-negative pyuria. Infect Control Hosp Epidemiol. 2011;32(7):644–648. doi: 10.1086/660764. [DOI] [PubMed] [Google Scholar]
- 101.Coupat C, Pradier C, Degand N, Hofliger P, Pulcini C. Selective reporting of antibiotic susceptibility data improves the appropriateness of intended antibiotic prescriptions in urinary tract infections: a case-vignette randomised study. Eur J Clin Microbiol Infect Dis. 2013;32(5):627–636. doi: 10.1007/s10096-012-1786-4. [DOI] [PubMed] [Google Scholar]
- 102.Hall BC, Alexander JS, Anderson SS, et al. Limited impact of selective susceptibility reporting of Escherichia coli and Klebsiella isolates from concurrent blood and urine cultures. Infect Control Hosp Epidemiol. 2020;42(5):647–648. doi: 10.1017/ice.2020.308. [DOI] [PubMed] [Google Scholar]
- 103.Langford BJ, Daneman N, Diong C, et al. Antibiotic susceptibility reporting and association with antibiotic prescribing: a cohort study. Clin Microbiol Infect. 2021;27(4):P568–P575. doi: 10.1016/j.cmi.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 104.Leis JA, Rebick GW, Daneman N, et al. Reducing antimicrobial therapy for asymptomatic bacteriuria among noncatheterized inpatients: a proof-of-concept study. Clin Infect Dis. 2014;58(7):980–983. doi: 10.1093/cid/ciu010. [DOI] [PubMed] [Google Scholar]
- 105.Daley P, Garcia D, Inayatullah R, Penney C, Boyd S. Modified reporting of positive urine cultures to reduce inappropriate treatment of asymptomatic bacteriuria among nonpregnant, noncatheterized inpatients: a randomized controlled trial. Infect Control Hosp Epidemiol. 2018;39(7):814–819. doi: 10.1017/ice.2018.100. [DOI] [PubMed] [Google Scholar]
- 106.Boly FJ, Reske KA, Kwon JH. The role of diagnostic stewardship in Clostridioides difficile testing: challenges and opportunities. Curr Infect Dis Rep. 2020;22(3):7. doi: 10.1007/s11908-020-0715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Herman DJ, Sarabia A, Chan H, Graham C. Changing results to change results: nudging antimicrobial prescribing for Clostridium difficile. Open Forum Infect Dis. 2021;8(6):ofaa605. doi: 10.1093/ofid/ofaa605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bork JT, Leekha S, Heil EL, Zhao L, Badamas R, Johnson JK. Rapid testing using the Verigene Gram-negative blood culture nucleic acid test in combination with antimicrobial stewardship intervention against Gram-negative bacteremia. Antimicrob Agents Chemother. 2015;59(3):1588–1595. doi: 10.1128/AAC.04259-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Avdic E, Carroll KC. The role of the microbiology laboratory in antimicrobial stewardship programs. Infect Dis Clin North Am. 2014;28(2):215–235. doi: 10.1016/j.idc.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 110.Ledeboer NA, Lopansri BK, Dhiman N, et al. Identification of Gram-negative bacteria and genetic resistance determinants from positive blood culture broths by use of the Verigene Gram-negative blood culture multiplex microarray-based molecular assay. J Clin Microbiol. 2015;53(8):2460–2472. doi: 10.1128/JCM.00581-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wenzler E, Wang F, Goff DA, et al. An automated, pharmacist-driven initiative improves quality of care for staphylococcus aureus bacteremia. Clin Infect Dis. 2017;65(2):194–200. doi: 10.1093/cid/cix315. [DOI] [PubMed] [Google Scholar]
- 112.Claeys KC, Heil EL, Hitchcock S, Johnson JK, Leekha S. Management of Gram-negative bloodstream infections in the era of rapid diagnostic testing: impact with and without antibiotic stewardship. Open Forum Infect Dis. 2020;7(10):ofaa427. doi: 10.1093/ofid/ofaa427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rivard KR, Athans V, Lam SW, et al. Impact of antimicrobial stewardship and rapid microarray testing on patients with Gram-negative bacteremia. Eur J Clin Microbiol Infect Dis. 2017;36(10):1879–1887. doi: 10.1007/s10096-017-3008-6. [DOI] [PubMed] [Google Scholar]
- 114.Banerjee R, Teng CB, Cunningham SA, et al. Randomized trial of rapid multiplex polymerase chain reaction–based blood culture identification and susceptibility testing. Clin Infect Dis. 2015;61(7):1071–1080. doi: 10.1093/cid/civ447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Timbrook TT, Morton JB, McConeghy KW, Caffrey AR, Mylonakis E, LaPlante KL. The effect of molecular rapid diagnostic testing on clinical outcomes in bloodstream infections: a systematic review and meta-analysis. Clin Infect Dis. 2017;64(1):15–23. doi: 10.1093/cid/ciw649. [DOI] [PubMed] [Google Scholar]
- 116.Pliakos EE, Andreatos N, Shehadeh F, Ziakas PD, Mylonakis E. The cost-effectiveness of rapid diagnostic testing for the diagnosis of bloodstream infections with or without antimicrobial stewardship. Clin Microbiol Rev. 2018;31(3):e00095–17. doi: 10.1128/CMR.00095-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mponponsuo K, Leal J, Spackman E, Somayaji R, Gregson D, Rennert-May E. Mathematical model of the cost-effectiveness of the BioFire FilmArray Blood Culture Identification (BCID) Panel molecular rapid diagnostic test compared with conventional methods for identification of Escherichia coli bloodstream infections. J Antimicrob Chemother. 2022;77(2):507–516. doi: 10.1093/jac/dkab398. [DOI] [PubMed] [Google Scholar]
- 118.Wenzler E, Timbrook TT, Wong JR, Hurst JM, MacVane SH. Implementation and optimization of molecular rapid diagnostic tests for bloodstream infections. Am J Health Syst Pharm. 2018;75(16):1191–1202. doi: 10.2146/ajhp170604. [DOI] [PubMed] [Google Scholar]
- 119.Clinical Laboratory Stanards Instiute (CLSI) M100. Clinical and Laboratory Standards Institute; [Accessed April 7, 2019]. https://clsi.org/standards/products/free-resources/access-our-free-resources/ [Google Scholar]
- 120.Vissichelli NC, Orndahl CM, Cecil JA, et al. Impact of cascade reporting of antimicrobial susceptibility on fluoroquinolone and meropenem consumption at a Veterans’ Affairs medical center. Infect Control Hosp Epidemiol. 2022;43(2):199–204. doi: 10.1017/ice.2021.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Musgrove MA, Kenney RM, Kendall RE, et al. Microbiology comment nudge improves pneumonia prescribing. Open Forum Infect Dis. 2018;5(7):ofy162. doi: 10.1093/ofid/ofy162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mcbride J, Schulz L, Fox B, Dipoto J, Sippel N, Osterby K. Influence of a “No MRSA, No Pseudomonas” comment to a respiratory culture in antibiotic utilization during the treatment of lower respiratory tract infection. Open Forum Infect Dis. 2015;2(Suppl 1):1500. doi: 10.1093/ofid/ofv133.1053. [DOI] [Google Scholar]
- 123.Srinivas P, Rivard KR, Pallotta AM, et al. Implementation of a stewardship initiative on respiratory viral PCR-based antibiotic deescalation. Pharmacotherapy. 2019;39(6):709–717. doi: 10.1002/phar.2268. [DOI] [PubMed] [Google Scholar]
- 124.Lowe CF, Payne M, Puddicombe D, et al. Antimicrobial stewardship for hospitalized patients with viral respiratory tract infections. Am J Infect Control. 2017;45(8):872–875. doi: 10.1016/j.ajic.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 125.Weiss ZF, Cunha CB, Chambers AB, et al. Opportunities revealed for antimicrobial stewardship and clinical practice with implementation of a rapid respiratory multiplex assay. J Clin Microbiol. 2019;57(10):e00861–19. doi: 10.1128/JCM.00861-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Branche AR, Walsh EE, Vargas R, et al. Serum procalcitonin measurement and viral testing to guide antibiotic use for respiratory infections in hospitalized adults: a randomized controlled trial. J Infect Dis. 2015;212(11):1692–1700. doi: 10.1093/infdis/jiv252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saeed K, González Del Castillo J, Backous C, et al. Hot topics on procalcitonin use in clinical practice, can it help antibiotic stewardship? Int J Antimicrob Agents. 2019;54(6):686–696. doi: 10.1016/j.ijantimicag.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 128.Townsend J, Adams V, Galiatsatos P, et al. Procalcitonin-guided antibiotic therapy reduces antibiotic use for lower respiratory tract infections in a United States Medical Center: results of a clinical trial. Open Forum Infect Dis. 2018;5(12):ofy327. doi: 10.1093/ofid/ofy327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ammar AA, Lam SW, Duggal A, et al. Compliance with procalcitonin algorithm antibiotic recommendations for patients in Medical Intensive Care Unit. Pharmacotherapy. 2017;37(2):177–186. doi: 10.1002/phar.1887. [DOI] [PubMed] [Google Scholar]
- 130.Branche AR, Walsh EE, Jadhav N, et al. Provider decisions to treat respiratory illnesses with antibiotics: insights from a randomized controlled trial. PLoS One. 2016;11(4):e0152986. doi: 10.1371/journal.pone.0152986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moradi T, Bennett N, Shemanski S, Kennedy K, Schlachter A, Boyd S. Use of procalcitonin and a respiratory polymerase chain reaction panel to reduce antibiotic use via an EMR alert. Clin Infect Dis. 2019;71(7):1684–1689. doi: 10.1093/cid/ciz1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Prinzi AM, Parker SK, Curtis DJ, Ziniel SI. The pediatric endotracheal aspirate culture survey (PETACS): examining practice variation across pediatric microbiology laboratories in the United States. J Clin Microbiol. 2021;59(3):e02232–20. doi: 10.1128/JCM.02232-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nguyen CT, Li J, Occhipinti EA, Hand J. Challenges in procalcitonin implementation in the real-world. Open Forum Infect Dis. 2018;5(2):ofy012. doi: 10.1093/ofid/ofy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562–570. doi: 10.1001/jama.2016.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jenkins TC, Tamma PD. Thinking beyond the “core” antibiotic stewardship interventions: shifting the onus for appropriate antibiotic use from stewardship teams to prescribing clinicians. Clin Infect Dis. 2021;72(8):1457–1462. doi: 10.1093/cid/ciaa1003. [DOI] [PubMed] [Google Scholar]
- 136.Baghdadi JD, Coffey KC, Leekha S, Johnson JK, Diekema DJ, Morgan DJ. Diagnostic stewardship for comprehensive gastrointestinal pathogen panel tests. Curr Infect Dis Rep. 2020;22(6):15. doi: 10.1007/s11908-020-00725-y. [DOI] [Google Scholar]