Abstract
Iron deficiency is the most common nutrient deficiency in the world, affecting over twenty percent of premenopausal women worldwide. Oral iron supplementation is often the first line treatment for the acute and chronic management of iron deficiency due to its ease and accessibility. However, there is no consensus on the optimal formulation or dosing strategy, or which patients should be preferentially treated with intravenous iron. Management of iron deficiency is complicated by the hepcidin-ferroportin iron regulatory pathway, which has evolved to prevent iron overload and thereby creates an inherent limit on gastrointestinal iron uptake and efficacy of oral iron. Unabsorbed iron propagates many of the side effects that complicate oral iron use including dyspepsia and constipation, all of which can thus be exacerbated by excessive oral iron doses. Daily low dose and every other day dosing protocols have attempted to bypass this physiologic bottleneck to allow for effective absorption and limit side effects; however, this approach has still resulted in low fractional iron absorption. In the following manuscript, we review the pathophysiology of iron absorption and current evidence for various preparations of oral iron. Lastly, we highlight opportunities for further study to advance the care of individuals affected by iron deficiency.
Keywords: iron deficiency, oral iron, anemia, blood loss
Introduction:
Iron deficiency is the most prevalent diagnosis in women’s health,1 and is most often secondary to blood loss or upper gastrointestinal pathology.2 Frequently, iron deficiency can result in numerous symptoms including chronic fatigue, poor concentration, impaired exercise performance, and poor quality of life.3 If persistent, iron deficiency will progress to microcytic anemia and occasionally thrombocytosis, although the clinical significance of this finding is unclear.4 Despite the prevalence of iron deficiency, readily available oral iron treatments are limited by suboptimal efficacy and frequent side effects. This is in part due to the nature of the human iron regulatory system which favors the long-term prevention of iron overload and prohibits the rapid correction of iron deficiency with oral therapy.5 Excessive reliance on oral iron can delay effective treatment via intravenous (IV) iron and also lead to significant side effects including dyspepsia and constipation.6 Observation studies have additionally suggested that physicians have significant knowledge gaps in diagnosing and treating iron deficiency.7 This is in part due to inequities in the United States healthcare system that have led to an absence of clear consensus guidelines regarding the treatment of iron deficiency and a resultant lack of practice uniformity.8
In the following manuscript we outline the pathophysiology of iron absorption, the available options for oral iron repletion, and potential side effects. We review the current evidence surrounding ideal oral iron repletion strategies and identify clinical scenarios in which patients would preferentially benefit from IV iron. Lastly, we highlight knowledge gaps and unmet needs in the science of iron repletion.
The pathophysiology of iron absorption:
Ingested iron may be categorized as either heme-iron (originating from animal proteins) or non-heme iron (originating from plant matter and non-biologic sources). The mechanisms underlying heme-iron absorption are poorly understood and appear to occur non-competitively with non-heme iron, potentially through separate pathways. The fractional absorption of non-heme iron is lower than heme iron. Absorption of non-heme iron occurs in the duodenum and upper jejunum via the divalent metal transporter 1 (DMT1). To be absorbed, iron must either be bound to a protein (e.g. heme), or in the ferrous or bivalent state (Fe 2+). The acidic pH of gastric acid allows the duodenal cytochrome B enzyme (Dcytb), on the brush border of the enterocytes, to convert the insoluble ferric, or trivalent form of iron (Fe 3+), to a more absorbable ferrous form.5 After absorption into the enterocyte, iron enters the circulation through the iron export channel ferroportin on the basolateral side of enterocytes. Ferroportin is also present on hepatocytes, which serve as the primary storage pool for iron, and on macrophages, which play an integral role in iron recycling and regulation.9 While we lack a full understanding of the iron sensing mechanisms, it is known that hepcidin is a key regulator of iron homeostasis and its production is tightly regulated by iron, inflammation, and erythroid activity.10,11 Hepcidin, in turn, binds to ferroportin and inhibits iron transport, ultimately leading to ferroportin degradation (Figure 1).10,12 The clinical potency of hepcidin is illustrated by type 2 (juvenile) hemochromatosis, in which dysfunction of the HAMP gene, which encodes hepcidin, leads to abnormally low hepcidin expression and iron overload in childhood.13 Clinical trials of oral iron in younger women with iron deficiency have shown that hepcidin synthesis is stimulated within hours of oral iron supplementation.14 This increased hepcidin expression is a key driver limiting the efficacy of oral iron, as evidenced by the fact that iron bioavailability is diminished by 35–45% on the second day after oral iron supplementation.14 Ultimately, the hepcidin-ferroportin axis creates diminishing returns from oral supplementation and allows only a small fraction of oral iron to be absorbed.15 For example, in the absence of a preceding dose, the absolute iron absorption from a dose of 60 mg of elemental iron has been shown to be 13.8 mg.14 The amount of absolute iron absorbed would decrease to 8.8 mg when given after a single iron dose administered on the preceding day, and to only 5.9 mg following twice-daily iron dosing on the preceding day.14 Similarly, the total iron absorbed was 23.6 mg if three 60 mg doses were administered within 24 hours compared with 22.6 mg when only two doses were given.14 This suggests that high doses of oral iron are likely to cause increased side effects with minimal additional benefit and requires consideration when identifying the optimal oral iron dosing strategy.16
Figure 1. Hepcidin (HAMP) regulation of systemic iron absorption.
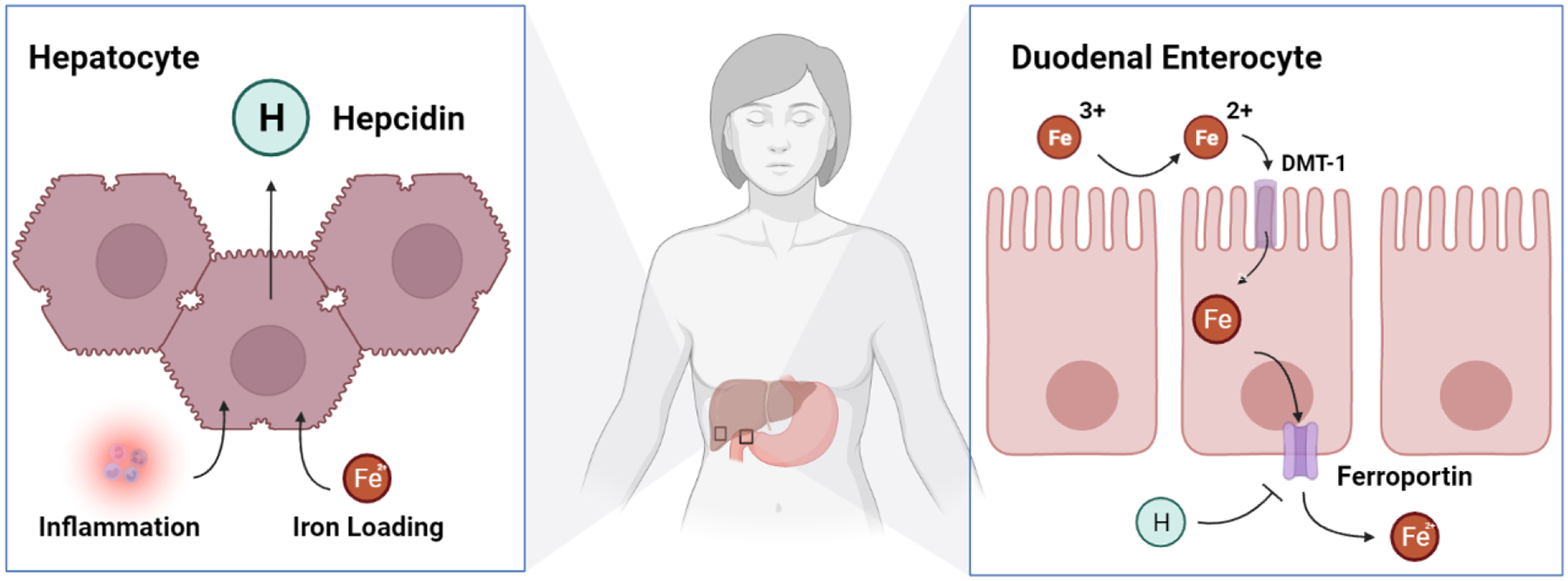
Following oral iron intake, reflexive upregulation of hepcidin expression leads to inhibition and degradation of the iron export channel ferroportin and loss of absorption supporting iron deficiency.
The side effects of oral iron:
A meta-analysis of 43 trials comprising 6,831 adult participants found that ferrous sulfate supplementation significantly increased the risk of gastrointestinal (GI) side-effects as compared to placebo or IV iron.6 The most commonly reported symptoms in the studies that parsed out individual side effects were constipation (12%), nausea (11%) and diarrhea (8%).6 Side effects appear to be dose dependent; at least one study comparing multiple dose and different preparations of iron reported that abdominal discomfort, nausea, vomiting, changes in bowel movements, and black stools were significantly more common with higher iron doses.16 A second meta-analysis of over 10,000 patients found high rates of GI side effects with all oral iron formulations including ferrous fumarate (43%), ferrous gluconate (31%), and ferrous sulfate (30%).17 Two factors have been suggested as drivers of the GI side effect profile of oral iron: 1) free radical generation through iron-induced redox cycling in the gut lumen and at the mucosal surface, which can promote inflammation, and 2) changes to the gut microbiota composition or metabolism.18 Indeed, iron-induced mucosal pathology is a common finding in the upper GI tract of patients taking oral iron therapy (detectable in 16% of patients in one series).19 It is important to consider these side effects on an individual’s quality of life, particularly in scenarios where oral iron administration is common, such as in pregnancy and in those with inflammatory bowel disease.20,21 Other notable side effects include the propensity for certain liquid iron formulations to stain tooth enamel.22 Some formulations can also provoke a displeasing metallic taste, while others can pass through the GI system undigested.23
Available Iron formulations:
There are numerous formulations of oral iron available (Table 1), the majority of which can be purchased without a prescription in the United States (with ferric maltol and iron hydroxyde adipate tartrate being exceptions).24 Although many small clinical studies examining the utility of various formulations exist in the literature, none strongly identify an ideal approach for oral iron repletion. Given this, there is not strong clinical evidence to suggest superiority of any specific iron preparation over another.25 Likewise, there are not well-designed clinical trials comparing liquid iron formulations to tablets in regards to efficacy and side effect profiles, and there is not a consensus on the ideal dosing strategy. Oral iron dosing is often confusing as many formulations report both a total iron dose (non-elemental + elemental iron) and a dose of elemental iron (for instance standard preparations of ferrous sulfate often contain 325mg of total iron and 65mg of elemental iron). Only elemental iron is readily absorbed, and its value should be the main consideration in selecting iron dosages. Further information regarding evidence-based dosing is outlined later in this review.
Table 1.
Oral Iron Formulations
| Formulations | Pros | Cons |
|---|---|---|
| Ferrous Iron Salts (Ferrous sulfate, Ferrous fumarate, Ferrous gluconate) | Inexpensive, widely available | Tendency to cause gastrointestinal side effects |
| Polysaccharide-Iron Complex | Lower risk of gastrointestinal intolerance, better taste profile | Less effective than ferrous salts in at least one randomized clinical trial of children. |
| Heme Iron | Higher absorption rate | Expensive. Not be a viable option for vegetarian or vegan individuals. |
| Carbonyl Iron | Inexpensive | No clear efficacy or side effect benefit as compared |
| Iron Protein Succinylate | Some data to suggest there may be better tolerability and efficacy as compared to ferrous salts. | Unsuitable for individuals with hypersensitivity to milk protein |
| Iron Amino Acid Chelates (Ferrous Bisglycinate, Ferric Trisglycinate) | Less susceptible to dietary interactions. | May be more expensive than ferrous salts |
Ferrous Iron Salts
Ferrous Iron Salts (ferrous sulfate, ferrous fumarate, and ferrous gluconate) are the most ubiquitous and commonly used oral iron preparations.26 For example, in 2012 over 97% of the 6.8 million prescriptions for oral iron filled in England were for ferrous salts.27 Ferrous, or bivalent iron (Fe2+), is more commonly used in oral iron preparations then ferric, or trivalent iron (Fe 3+).26 Ferric iron is 3–4 times less bioavailable than ferrous iron, as ferric iron has poor solubility in alkaline media and must be converted to ferrous iron in vivo to be absorbed.26 Ferrous salts are relatively inexpensive compared to other available formulations and numerous varieties are available over the counter. However, they embody many of the commonly ascribed side effects of oral iron including the potential for poor taste and gastrointestinal toxicity. Given this, ferrous salts are commonly used as the comparator arm in clinical trials evaluating novel iron preparations.
Polysaccharide-Iron Complex (PIC)
Polysaccharide-Iron Complex (PIC) is a synthetic complex of ferric iron and carbohydrate. Spectroscopy studies have shown the polysaccharide-Iron complex itself is spherical, 3–10 nm in diameter, and contains a ferrihydrite core similar to the structure of ferritin. PIC has been theorized to have an improved side effect profile (specifically a better taste and GI side effect profile), however a randomized trial in children found that the PIC has similar GI toxicity with less efficacy compared to ferrous sulfate.28
Heme Iron
Heme Iron is principally found in carnivorous diets in the form of hemoglobin or myoglobin, and is absorbed non-competitively with non-heme iron directly into enterocytes.29 The absorption of heme iron is not well understood and whether hepcidin affects heme iron absorption is largely unknown.29 Oral preparations of heme iron, generally prepared from animal blood or tissue, are available over the counter in the United States. Small trials comparing heme-iron supplements to ferrous iron salts have not suggested any notable clinical superiority.30,31 Given that heme-iron and non-heme iron appear to have non-competing absorption pathways, small clinical trials have assessed the combination of heme- and non-heme iron against ferrous salts, however no obvious benefit has been elucidated with combination therapy.32,33 Anecdotally, patients with iron deficiency commonly inquire on the addition of animal proteins to their diet, which provide a dietary source of heme-iron that can be used in parallel with oral supplements. We counsel that there is some data to suggest that adding meat protein to one’s diet may additionally augment the absorption of non-heme iron.34 The potential risks of colon cancer associated with heme-iron have been previously raised, as red meat and processed meat consumption has been linked to the incidence of colon cancer in certain epidemiologic studies and in some preclinical studies using mouse models.35 While there are not definitive studies on this topic, studies that parse out the source of dietary heme-iron have found this association is specific to heme iron derived from red meat (with heme-iron from white meats being protective), suggesting that a heterogenous group of risk factors specific to red meat may underlie this observation.36 We have generally counseled patients that if a risk exists, it is unlikely to be different from the risks associated with maintaining a diet that contains red meat.
Carbonyl Iron
Carbonyl Iron is available over the counter in the United States. The term “Carbonyl” refers to the manufacturing process in which the controlled heating of vaporized iron pentacarbonyl leads to the deposition of uncharged, elemental iron as submicroscopic crystals that form microscopic spheres of less than 5 μm in diameter.37 The conversion of particulate carbonyl iron to soluble ionized iron that can be absorbed by DMT1 is dependent on the acidic environment of the stomach and is limited by the rate of gastric acid production.38 There is currently no significant clinical data to suggest any benefits to carbonyl iron over iron salts.25
Iron Protein Succinylate (IPS)
Iron Protein Succinylate (IPS) is generally composed of a ferric iron bound to succinylated casein.39 Conceptually, the iron in IPS remains bound to the protein matrix in the acidic environment of the stomach.40 The iron is then released in the less acidic environment within the duodenum and the proximal jejunum. Given this, there may be a theoretical benefit to the use of IPS in patients with hypochlorhydria either from increasing age or medication use. At least one large meta-analysis has suggested that IPS may have a more favorable side effect profile then other iron formulations, although this claim has to be considered in the context of the limitations of the clinical trials that compose the analysis.39 Various formulations of IPS are available over the counter in the United States.
Iron Amino Acid Chelates
Iron Amino Acid Chelates (Ferrous Bisglycinate, Ferric Trisglycinate) are composed of a conjugate of ferrous (Fe +2) or ferric (Fe +3) iron with amino acids (generally glycine).41 Absorption of chelated iron appears to occur in a similar manner as other non-heme iron preparations, however chelated iron may be less affected by enhancers and inhibitors of iron absorption compared to ferrous salts.42 Chelated irons pass through the acidic environment of the stomach without being susceptible to interactions with dietary phosphates, phytates and fibers, and are subsequently absorbed directly into duodenal enterocytes.42 Despite these potential benefits, clinical data has not shown chelated iron to be superior to iron salts.43
Iron Hydroxyde Adipate Tartrate (IHAT)
Iron Hydroxyde Adipate Tartrate (IHAT) is a tartrate-modified, nano-dispersed Fe(III) oxo-hydroxide designed to have similar functional properties to ferrihydrite, the iron form found in the core of natural plant ferritin.44 IHAT is unique in that it does not require solubilization in the stomach for absorption. IHAT is instead absorbed as whole nanoparticles by duodenal enterocytes via endocytosis, challenging the traditional dogma of iron absorption.45 These unique properties are suggested to offer improved efficacy and protect against GI toxicity—potential benefits that are currently being evaluated in later phase clinical trials comparing IHAT to ferrous sulphate.46 While not directly available in the United States, IHAT is approved by the European Food Standards Agency for distribution in Europe.
Sucrosomial Iron (SI)
Sucrosomial Iron (SI) similarly is not available in the United States, but is widely available in Europe and is available in 60 countries worldwide. Sucorsomial iron is a newer oral formulation consisting of ferric pyrophosphate protected by a phospholipid bilayer plus a sucrester matrix (sucrosome). This formulation is is absorbed through para-cellular and trans-cellular routes (M cells).47 As a result, the absorption is mostly independent of hepcidin. Given its increased bioavailability and gastrointestinal tolerance, it is recommended by some to be the most appropriate first line recommendation for oral iron supplementation. Several studies have even suggested it as a suitable alternative to IV iron in particular patients with severe anemia and inflammatory conditions.48–51
Clinical use of oral iron:
Who should preferentially receive IV iron instead of oral iron:
Individuals who have undergone foregut surgery or have active foregut pathology (e.g., the majority of patients who have had bariatric surgery, those with active celiac disease, or inflammatory bowel disease) are unlikely to benefit from oral iron due to poor GI absorption and should preferentially be treated with IV iron.52 Specifically, in patients with inflammatory bowel disease, there is data to suggest that oral iron worsens quality of life and adversely impacts gastrointestinal inflammation and the microbiome.53,54 We also offer IV iron preferentially to individuals who have previously undergone treatment with oral iron, but failed to replete their iron stores after 6–12 weeks, or individuals that found the side effects of oral iron to be untenable.3,55 In addition, individuals with active ongoing blood loss may be unable to effectively replete their iron stores with oral supplementation; in such cases the clinician must use their judgement regarding the cadence of bleeding and the clinical need for rapid iron repletion. Lastly, we also consider IV iron in anemic elderly patients, for preoperative anemia, and in patients with severe iron deficiency anemia, especially those with end-stage chronic kidney disease, congestive heart failure with reduced ejection fractions, and cancer patients.
How should patients be instructed to take oral iron:
Given the absence of compelling data to suggest the superiority of any specific iron preparation, our practice is to counsel patients on the various iron formulations available and encourage them to select an over-the-counter iron product (or try different preparations to assess for taste and side effects). We have found that this guidance allows for the greatest amount of patient autonomy and is often less expensive than formal prescriptions depending on the patient’s insurance coverage. In our experience the vast majority of patients initially trial a ferrous salt.
The absorption of oral iron preparations, with the possible exception of chelated iron, is negatively impacted by phytates (in bran, oats, rye fiber), polyphenols56 (in tea, some vegetables and cereals), and soy proteins.57,58 Likewise, medications which lower the gastric pH (antacids, proton pump inhibitors) inhibit the absorption of the most common forms of oral iron.59 For patients who chose to consume animal proteins, they should be counseled that factors in meat (fish and beef) appear to aid the absorption of non-heme iron, independent of heme-iron.58 As such, consuming oral iron in conjunction with a protein rich meal may facilitate iron absorption.
Vitamin C has been proposed to create a more acidic environment in the stomach and prevent the oxidization of ferrous iron to ferric iron, and has long been touted as a means to improve oral iron repletion.60 There is also data to suggest that vitamin C neutralizes the negative effects of certain inhibitors of iron absorption, including phytate, polyphenols, and calcium.61 There is conflicting data, however, regarding the efficacy of vitamin C to aid in the absorption of iron; a recent randomized clinical trial did not find a benefit of vitamin C co-administration with iron.60,62 Nevertheless, many patients choose to supplement vitamin C with their oral iron (in the form of juice, compounded vitamin C-Iron tablets, or separately purchased supplemental vitamin C).
To our knowledge, co-administration of iron with empiric laxatives is not an evidence-based practice and should be reserved for patients who develop side effects of constipation but prefer to continue oral iron as opposed to IV iron supplementation. Diarrhea is experienced by a minority of individuals taking oral iron and is a potential side effect that should also be considered in patients undergoing oral iron supplementation.63
How should iron be dosed?
Given that higher doses of oral iron have diminishing returns due to induction of hepcidin expression and may cause increasing GI side effects, we agree with many experts that oral iron should be limited to a single dose administered each day, or every other day (Figure 2).64 The rationale for every other day (QOD) dosing is based on recent prospective clinical trials which demonstrated that QOD dosing minimized hepcidin expression and increased the fractional absorption of iron.65,66 Despite this, the total amount of iron absorbed is slightly lower with QOD dosing as opposed to daily dosing (for example, the cumulative total iron absorption was projected to be 261 mg versus 175 mg after 28 days), thus daily supplementation may be considered for those with absent or tolerable side effects.65 Plasma hepcidin follows a circadian rhythm and typically increases over the day, and thus oral iron is best given as a single dose in the morning.67,68 Doses higher then 60–120mg of elemental iron per day or every other day are unlikely to offer significant benefit in terms of iron repletion and may cause unintended side effects; likewise, patients may benefit from even lower dosages if they develop side effects.16,67
Figure 2.
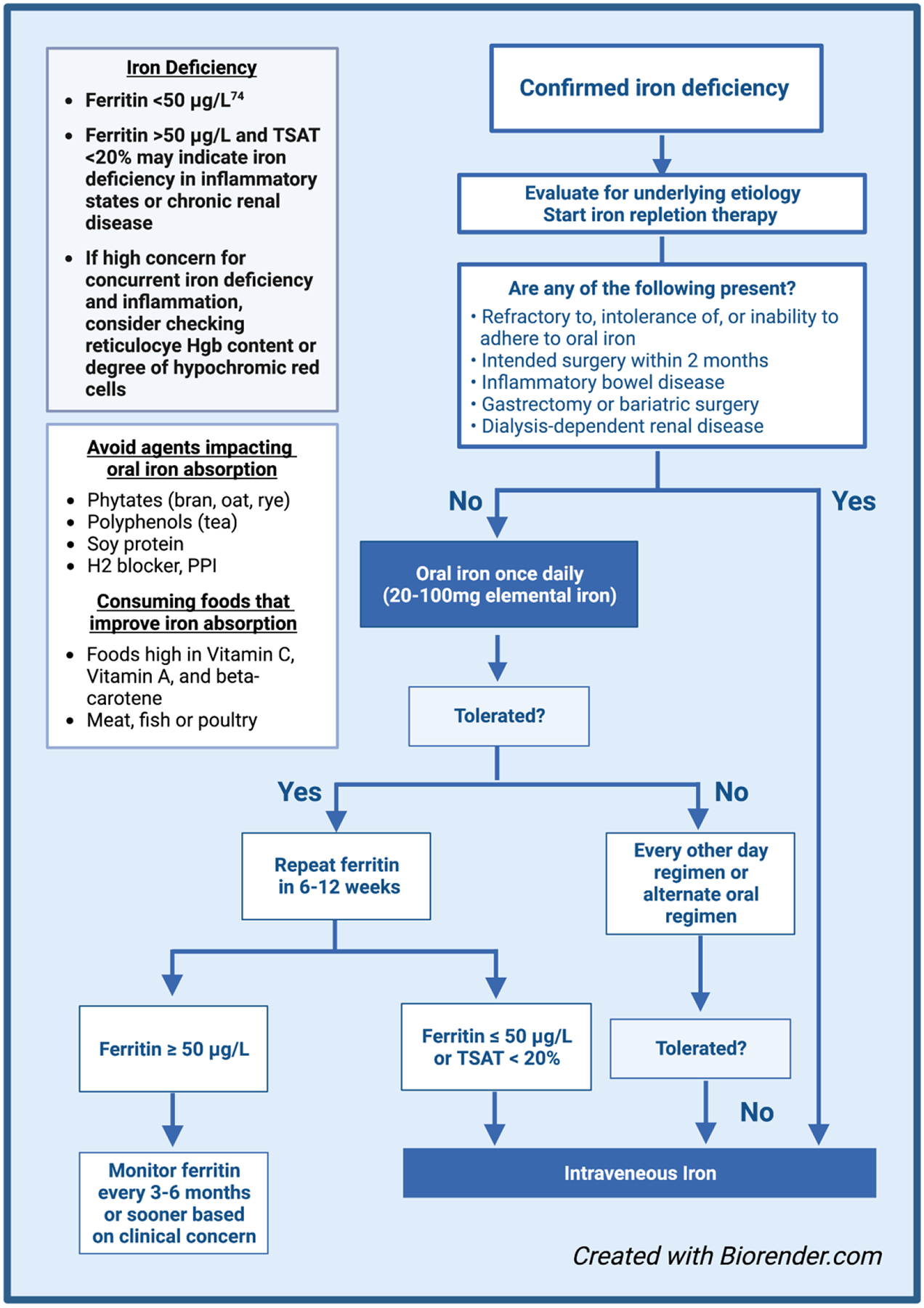
Proposed algorithm for treating iron deficiency.74
How long should oral iron be trialed before considering IV iron:
There is no consensus in the literature on the appropriate duration of a trial of oral iron before a patient is deemed refractory. In our practice, we define iron repletion as a ferritin ≥ 50 ng/ml, based on two clinical trials suggesting this to be the threshold by which the symptoms of iron deficiency are resolved.69,70 We agree with experts who recommend assessing ferritin response 6 to 12 weeks after initiating oral iron in stable patients without significant anemia. Individuals who are unable to replete their iron stores by 6 to 12 weeks are unlikely to achieve adequate levels moving forward and should be considered for IV iron administration.55,64 Likewise, in those with significant anemia, a hemoglobin response <1.0 g/dL at day 14 of oral iron identifies subjects who are unlikely to adequately respond to oral iron.71
Should oral iron be continued in individuals receiving IV iron:
A prior large meta-analysis suggests that chronic oral iron use improves quality of life in younger women, but at the cost of increased GI side effects.72,73 This should be considered in the context of each patient and balanced with the comparative efficacy of IV iron, which has been shown to rapidly correct iron deficiency with a reduced side effect profile.55 In our practice most patients choose to stop oral iron after failing oral repletion and proceeding to IV iron. In select women who prefer to limit further doses of IV iron and tolerate oral iron well, we recommend continuing oral iron indefinitely (or until menopause). The utility of this approach should be individualized and evaluated at each clinical follow up encounter.
Conclusions:
Despite the prevalence of iron deficiency in premenopausal women, optimal selection of oral iron formulations, dosing regimens, and monitoring protocols remain obstruse. There is an unmet need to develop more effective oral iron formulations as the fraction of absorbed iron is modest at best and is frequently limited by side effects. Lastly, there is a paucity of evidence-based guidelines on the appropriate use of oral iron in terms of optimal dosing and monitoring protocols; however daily or every other day dosing regimens increase absorption compared to more frequent dosing schedules. The well intentioned, but unnecessary, use of high dose oral iron is likely to result in toxicity without added benefit, thus education on the effective and appropriate use of oral iron is essential. With optimal use, there is strong clinical data to suggest that routine oral iron supplementation can improve the quality of life of women worldwide.
What is the new aspect of your work?
This review is the first to summarize the underlying mechanisms and clinical data surrounding the use of oral iron to treat iron deficiency.
What is the central finding of your work?
We outline the physiology of iron absorption; the body will preferentially avoid iron overload by allowing only a small quantity of oral iron to absorbed per day. This concept should drive the appropriate use of oral iron, as increasing doses can cause gastrointestinal toxicity while offering minimal clinical benefit.
What is (or could be) the specific clinical relevance of your work?
Daily low dose and every other day oral iron dosing protocols represent a rational approach to the initial treatment of iron deficiency in select patients. Many individuals will require intravenous iron to achieve adequate iron repletion. We suggest an evidence-based workflow for the treatment of iron deficiency with oral elemental iron to guide healthcare providers in the management of this common condition.
Financial Support:
JJ. Shatzel is supported by the National Heart, Lung, and Blood Institute/National Institutes of Health (R01HL151367).
Footnotes
Disclosures:
JJ. Shatzel reports receiving consulting fees from Aronoa Inc. The remaining authors have nothing to disclose.
Data Availability:
The data used to support the findings of this study are included within the article.
References:
- 1.Warner MJ, Kamran MT. Iron Deficiency Anemia. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright © 2022, StatPearls Publishing LLC.; 2022. [PubMed] [Google Scholar]
- 2.Scott DE, Pritchard JA. Iron deficiency in healthy young college women. Jama. 1967;199(12):897–900. [PubMed] [Google Scholar]
- 3.Elstrott B, Khan L, Olson S, Raghunathan V, DeLoughery T, Shatzel JJ. The role of iron repletion in adult iron deficiency anemia and other diseases. Eur J Haematol. 2020;104(3):153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song AB, Kuter DJ, Al-Samkari H. Characterization of the rate, predictors, and thrombotic complications of thrombocytosis in iron deficiency anemia. Am J Hematol. 2020. [DOI] [PubMed] [Google Scholar]
- 5.Ems T, St Lucia K, Huecker MR. Biochemistry, Iron Absorption. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright © 2022, StatPearls Publishing LLC.; 2022. [PubMed] [Google Scholar]
- 6.Tolkien Z, Stecher L, Mander AP, Pereira DI, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015;10(2):e0117383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Read AJ, Waljee AK, Sussman JB, et al. Testing Practices, Interpretation, and Diagnostic Evaluation of Iron Deficiency Anemia by US Primary Care Physicians. JAMA Netw Open. 2021;4(10):e2127827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dugan C, MacLean B, Cabolis K, et al. The misogyny of iron deficiency. Anaesthesia. 2021;76 Suppl 4:56–62. [DOI] [PubMed] [Google Scholar]
- 9.Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105(2):260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pagani A, Nai A, Silvestri L, Camaschella C. Hepcidin and Anemia: A Tight Relationship. Front Physiol. 2019;10:1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girelli D, Nemeth E, Swinkels DW. Hepcidin in the diagnosis of iron disorders. Blood. 2016;127(23):2809–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kowdley KV, Gochanour EM, Sundaram V, Shah RA, Handa P. Hepcidin Signaling in Health and Disease: Ironing Out the Details. Hepatol Commun. 2021;5(5):723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphree CR, Nguyen NN, Raghunathan V, Olson SR, DeLoughery T, Shatzel JJ. Diagnosis and management of hereditary haemochromatosis. Vox Sang. 2020;115(4):255–262. [DOI] [PubMed] [Google Scholar]
- 14.Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126(17):1981–1989. [DOI] [PubMed] [Google Scholar]
- 15.Ginzburg YZ. Hepcidin-ferroportin axis in health and disease. Vitam Horm. 2019;110:17–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rimon E, Kagansky N, Kagansky M, et al. Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am J Med. 2005;118(10):1142–1147. [DOI] [PubMed] [Google Scholar]
- 17.Cancelo-Hidalgo MJ, Castelo-Branco C, Palacios S, et al. Tolerability of different oral iron supplements: a systematic review. Curr Med Res Opin. 2013;29(4):291–303. [DOI] [PubMed] [Google Scholar]
- 18.Kortman GA, Raffatellu M, Swinkels DW, Tjalsma H. Nutritional iron turned inside out: intestinal stress from a gut microbial perspective. FEMS Microbiol Rev. 2014;38(6):1202–1234. [DOI] [PubMed] [Google Scholar]
- 19.Kaye P, Abdulla K, Wood J, et al. Iron-induced mucosal pathology of the upper gastrointestinal tract: a common finding in patients on oral iron therapy. Histopathology. 2008;53(3):311–317. [DOI] [PubMed] [Google Scholar]
- 20.Peña-Rosas JP, De-Regil LM, Gomez Malave H, Flores-Urrutia MC, Dowswell T. Intermittent oral iron supplementation during pregnancy. Cochrane Database Syst Rev. 2015;2015(10):Cd009997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Resál T, Farkas K, Molnár T. Iron Deficiency Anemia in Inflammatory Bowel Disease: What Do We Know? Front Med (Lausanne). 2021;8:686778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asgari I, Soltani S, Sadeghi SM. Effects of Iron Products on Decay, Tooth Microhardness, and Dental Discoloration: A Systematic Review. Archives Of Pharmacy Practice. 2020;11(1):60–72. [Google Scholar]
- 23.Hashemi H, Noori M, Noori M. Susceptibility artifact induced by undigested iron tablets (sustained release carbonyl iron). European Journal of Radiology Extra. 2009;71(3):e135–e138. [Google Scholar]
- 24.Khoury A, Pagan KA, Farland MZ. Ferric Maltol: A New Oral Iron Formulation for the Treatment of Iron Deficiency in Adults. Ann Pharmacother. 2021;55(2):222–229. [DOI] [PubMed] [Google Scholar]
- 25.Gamad N, Saha PK, Sharma P, Suri V, Chakrabarti A, Saha L. A randomized controlled trial comparing the efficacy, tolerability, and cost of oral iron preparations in iron-deficiency anemia in pregnancy. J Obstet Gynaecol Res. 2021;47(11):3828–3841. [DOI] [PubMed] [Google Scholar]
- 26.Santiago P Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: a clinical overview. TheScientificWorldJournal. 2012;2012:846824–846824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tolkien Z, Stecher L, Mander AP, Pereira DIA, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PloS one. 2015;10(2):e0117383–e0117383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powers JM, Buchanan GR, Adix L, Zhang S, Gao A, McCavit TL. Effect of Low-Dose Ferrous Sulfate vs Iron Polysaccharide Complex on Hemoglobin Concentration in Young Children With Nutritional Iron-Deficiency Anemia: A Randomized Clinical Trial. Jama. 2017;317(22):2297–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.West A-R, Oates P-S. Mechanisms of heme iron absorption: current questions and controversies. World journal of gastroenterology. 2008;14(26):4101–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barraclough KA, Brown F, Hawley CM, et al. A randomized controlled trial of oral heme iron polypeptide versus oral iron supplementation for the treatment of anaemia in peritoneal dialysis patients: HEMATOCRIT trial. Nephrol Dial Transplant. 2012;27(11):4146–4153. [DOI] [PubMed] [Google Scholar]
- 31.Abdelazim IA, Abu-Faza M, Shikanova S, Zhurabekova G, Maghrabi MM. Heme-bound iron in treatment of pregnancy-associated iron deficiency anemia. Journal of family medicine and primary care. 2018;7(6):1434–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frykman E, Bystrom M, Jansson U, Edberg A, Hansen T. Side effects of iron supplements in blood donors: superior tolerance of heme iron. J Lab Clin Med. 1994;123(4):561–564. [PubMed] [Google Scholar]
- 33.Eskeland B, Malterud K, Ulvik RJ, Hunskaar S. Iron supplementation in pregnancy: is less enough? A randomized, placebo controlled trial of low dose iron supplementation with and without heme iron. Acta Obstet Gynecol Scand. 1997;76(9):822–828. [DOI] [PubMed] [Google Scholar]
- 34.Cook JD, Monsen ER. Food iron absorption in human subjects. III. Comparison of the effect of animal proteins on nonheme iron absorption. Am J Clin Nutr. 1976;29(8):859–867. [DOI] [PubMed] [Google Scholar]
- 35.Bastide NM, Pierre FH, Corpet DE. Heme iron from meat and risk of colorectal cancer: a meta-analysis and a review of the mechanisms involved. Cancer Prev Res (Phila). 2011;4(2):177–184. [DOI] [PubMed] [Google Scholar]
- 36.Luo H, Zhang NQ, Huang J, et al. Different forms and sources of iron in relation to colorectal cancer risk: a case-control study in China. Br J Nutr. 2019;121(7):735–747. [DOI] [PubMed] [Google Scholar]
- 37.Gordeuk VR, Brittenham GM, McLaren CE, Hughes MA, Keating LJ. Carbonyl iron therapy for iron deficiency anemia. Blood. 1986;67(3):745–752. [PubMed] [Google Scholar]
- 38.Huebers HA, Brittenham GM, Csiba E, Finch CA. Absorption of carbonyl iron. J Lab Clin Med. 1986;108(5):473–478. [PubMed] [Google Scholar]
- 39.Martínez Francés A, Leal Martínez-Bujanda J. Efficacy and tolerability of oral iron protein succinylate: a systematic review of three decades of research. Curr Med Res Opin. 2020;36(4):613–623. [DOI] [PubMed] [Google Scholar]
- 40.Cremonesi P, Caramazza I. Chemical and biological characterization of iron-protein succinylate (ITF 282). Int J Clin Pharmacol Ther Toxicol. 1993;31(1):40–51. [PubMed] [Google Scholar]
- 41.Franklin M, Rohse WG, Huerga Jdl, Kemp CR. CHELATE IRON THERAPY. Journal of the American Medical Association. 1958;166(14):1685–1693. [DOI] [PubMed] [Google Scholar]
- 42.Pizarro F, Olivares M, Hertrampf E, et al. Iron bis-glycine chelate competes for the nonheme-iron absorption pathway. The American Journal of Clinical Nutrition. 2002;76(3):577–581. [DOI] [PubMed] [Google Scholar]
- 43.Abdel Moety GAF, Ali AM, Fouad R, Ramadan W, Belal DS, Haggag HM. Amino acid chelated iron versus an iron salt in the treatment of iron deficiency anemia with pregnancy: A randomized controlled study. Eur J Obstet Gynecol Reprod Biol. 2017;210:242–246. [DOI] [PubMed] [Google Scholar]
- 44.Pereira DI, Bruggraber SF, Faria N, et al. Nanoparticulate iron(III) oxo-hydroxide delivers safe iron that is well absorbed and utilised in humans. Nanomedicine. 2014;10(8):1877–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pereira DI, Mergler BI, Faria N, et al. Caco-2 cell acquisition of dietary iron(III) invokes a nanoparticulate endocytic pathway. PLoS One. 2013;8(11):e81250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pereira DIA, Mohammed NI, Ofordile O, et al. A novel nano-iron supplement to safely combat iron deficiency and anaemia in young children: The IHAT-GUT double-blind, randomised, placebo-controlled trial protocol. Gates Open Res. 2018;2:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gómez-Ramírez S, Brilli E, Tarantino G, Muñoz M. Sucrosomial(®) Iron: A New Generation Iron for Improving Oral Supplementation. Pharmaceuticals (Basel). 2018;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ciudin A, Simó-Servat O, Balibrea JM, et al. Response to oral sucrosomial iron supplementation in patients undergoing bariatric surgery. The BARI-FER study. Endocrinol Diabetes Nutr (Engl Ed). 2018;65(1):17–20. [DOI] [PubMed] [Google Scholar]
- 49.Mafodda A, Giuffrida D, Prestifilippo A, et al. Oral sucrosomial iron versus intravenous iron in anemic cancer patients without iron deficiency receiving darbepoetin alfa: a pilot study. Support Care Cancer. 2017;25(9):2779–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karavidas A, Troganis E, Lazaros G, et al. Oral sucrosomial iron improves exercise capacity and quality of life in heart failure with reduced ejection fraction and iron deficiency: a non-randomized, open-label, proof-of-concept study. Eur J Heart Fail. 2021;23(4):593–597. [DOI] [PubMed] [Google Scholar]
- 51.Giordano G, Napolitano M, Di Battista V, Lucchesi A. Oral high-dose sucrosomial iron vs intravenous iron in sideropenic anemia patients intolerant/refractory to iron sulfate: a multicentric randomized study. Ann Hematol. 2021;100(9):2173–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Auerbach M, Ballard H. Clinical Use of Intravenous Iron: Administration, Efficacy, and Safety. Hematology. 2010;2010(1):338–347. [DOI] [PubMed] [Google Scholar]
- 53.Powell JJ, Cook WB, Hutchinson C, et al. Dietary fortificant iron intake is negatively associated with quality of life in patients with mildly active inflammatory bowel disease. Nutrition & metabolism. 2013;10(1):9–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee T, Clavel T, Smirnov K, et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut. 2017;66(5):863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Auerbach M, Deloughery T. Single-dose intravenous iron for iron deficiency: a new paradigm. Hematology. 2016;2016(1):57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hurrell RF, Reddy M, Cook JD. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br J Nutr. 1999;81(4):289–295. [PubMed] [Google Scholar]
- 57.Zijp IM, Korver O, Tijburg LB. Effect of tea and other dietary factors on iron absorption. Crit Rev Food Sci Nutr. 2000;40(5):371–398. [DOI] [PubMed] [Google Scholar]
- 58.Bæch SB, Hansen M, Bukhave K, et al. Nonheme-iron absorption from a phytate-rich meal is increased by the addition of small amounts of pork meat. The American Journal of Clinical Nutrition. 2003;77(1):173–179. [DOI] [PubMed] [Google Scholar]
- 59.Hamano H, Niimura T, Horinouchi Y, et al. Proton pump inhibitors block iron absorption through direct regulation of hepcidin via the aryl hydrocarbon receptor-mediated pathway. Toxicol Lett. 2020;318:86–91. [DOI] [PubMed] [Google Scholar]
- 60.Cook JD, Reddy MB. Effect of ascorbic acid intake on nonheme-iron absorption from a complete diet. The American Journal of Clinical Nutrition. 2001;73(1):93–98. [DOI] [PubMed] [Google Scholar]
- 61.Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91(5):1461s–1467s. [DOI] [PubMed] [Google Scholar]
- 62.Li N, Zhao G, Wu W, et al. The Efficacy and Safety of Vitamin C for Iron Supplementation in Adult Patients With Iron Deficiency Anemia: A Randomized Clinical Trial. JAMA Network Open. 2020;3(11):e2023644–e2023644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bloor SR, Schutte R, Hobson AR. Oral Iron Supplementation—Gastrointestinal Side Effects and the Impact on the Gut Microbiota. Microbiology Research. 2021;12(2):491–502. [Google Scholar]
- 64.Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91(1):31–38. [DOI] [PubMed] [Google Scholar]
- 65.Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4(11):e524–e533. [DOI] [PubMed] [Google Scholar]
- 66.Stoffel NU, Zeder C, Brittenham GM, Moretti D, Zimmermann MB. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica. 2020;105(5):1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stoffel NU, von Siebenthal HK, Moretti D, Zimmermann MB. Oral iron supplementation in iron-deficient women: How much and how often? Molecular Aspects of Medicine. 2020;75:100865. [DOI] [PubMed] [Google Scholar]
- 68.Schaap CC, Hendriks JC, Kortman GA, et al. Diurnal Rhythm rather than Dietary Iron Mediates Daily Hepcidin Variations. Clinical Chemistry. 2013;59(3):527–535. [DOI] [PubMed] [Google Scholar]
- 69.Vaucher P, Druais P-L, Waldvogel S, Favrat B. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2012;184(11):1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keller P, von Känel R, Hincapié CA, et al. The effects of intravenous iron supplementation on fatigue and general health in non-anemic blood donors with iron deficiency: a randomized placebo-controlled superiority trial. Sci Rep. 2020;10(1):14219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okam MM, Koch TA, Tran MH. Iron Supplementation, Response in Iron-Deficiency Anemia: Analysis of Five Trials. Am J Med. 2017;130(8):991.e991–991.e998. [DOI] [PubMed] [Google Scholar]
- 72.Mintz J, Mirza J, Young E, Bauckman K. Iron Therapeutics in Women’s Health: Past, Present, and Future. Pharmaceuticals (Basel, Switzerland). 2020;13(12):449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Low MS, Speedy J, Styles CE, De-Regil LM, Pasricha SR. Daily iron supplementation for improving anaemia, iron status and health in menstruating women. Cochrane Database Syst Rev. 2016;4:Cd009747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DeLoughery TG. Microcytic anemia. N Engl J Med. 2014;371(14):1324–1331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


