Abstract
The hybG gene product from Escherichia coli has been identified as a chaperone-like protein acting in the maturation of hydrogenases 1 and 2. It was shown that HybG forms a complex with the precursor of the large subunit of hydrogenase 2. As with HypC, which is the chaperone-like protein involved in hydrogenase 3 maturation, the N-terminal cysteine residue is crucial for complex formation. Introduction of a deletion into hybG abolished the generation of active hydrogenase 2 but only quantitatively reduced hydrogenase 1 activity since HypC could replace HybG in this function. In contrast, HybG could not take over the role of HypC in a ΔhypC genetic background. Overproduction of HybG, especially of the variants with the replaced N-terminal cysteine residue, strongly interfered with hydrogenase 3 maturation, apparently by titrating some other component(s) of the maturation machinery. The results indicate that the three hydrogenase isoenzymes not only are interacting at the functional level but are also interconnected during the maturation process.
Under anaerobic growth conditions, Escherichia coli is able to synthesize three [NiFe] hydrogenases, designated hydrogenase 1, 2, and 3 (2, 3, 28, 29). Hydrogenases 1 and 2 are uptake hydrogenases which couple H2 oxidation to fumarate reduction, whereas hydrogenase 3 is a gas-evolving isoenzyme and an operational component of the formate hydrogenlyase complex (25, 28). The structural genes for hydrogenases 1 and 2 are located in the hya and hyb transcriptional units (22–24), whereas those coding for hydrogenase 3 are members of the hyc operon (5). Genes for a fourth hydrogenase (hyf) have been identified (1) but they do not appear to be expressed under the experimental conditions tested (27).
Apart from the structural genes, a set of seven genes has been identified whose products are involved in the synthesis and insertion of the [NiFe] metal center and maturation of the enzymes. Six of them were designated hyp (for hydrogenase pleiotropically acting genes) since mutations in most of them (hypB, hypD, hypE, and hypF) affect the maturation of all three hydrogenases (15, 18). The product of the seventh maturation gene is an endopeptidase which removes a C-terminal extension from each of the large subunits. As the cleavage reaction is a specific process, three different endopeptidases (HyaD, HybD, and HycI) are involved in the maturation of the large subunits of hydrogenases 1, 2, and 3, respectively (11, 24, 26).
When the genes coding for HypA and HypC were inactivated it was found that the mutations only affected the maturation of hydrogenase 3 (15). It was speculated that this apparent nonpleiotropic function could be due to the existence of two genes in the hyb operon, namely, hybF and hybG, whose derived amino acid sequences displayed similarity to those of hypA and hypC, respectively (15, 22). HybF thus may be the homolog of HypA and HybG may be that of HypC, and they could be involved in the maturation of hydrogenases 1 and 2.
The function of the hypC gene product has attracted considerable attention recently (10, 19). It was found that HypC is able to form a stable complex with the precursor of the large subunit (HycE) during the maturation process (10). The formation of the complex required the function of two residues, namely, the N-terminal cysteine residue of HypC (the N-terminal methionine is posttranslationally removed) and the first cysteine residue of the N-terminal motif (Cys241) involved in [NiFe] coordination (19). Since HypC forms a complex only with the precursor of the large subunit of hydrogenase 3 and not with the mature one, its function was tentatively envisioned as that of a specific chaperone, keeping the protein in a folding state amenable to metal insertion (10). Because the metal center of [NiFe] hydrogenases is located at the interface between the small and the large subunits (12, 30), an additional function of HypC could also reside in the prevention of the association during the maturation process (20). A putative catalytic role, however, is also possible.
In the present study, the function of HybG during formation of the three hydrogenase isoenzymes from Escherichia coli was analyzed. It is shown that HybG is the specific chaperone-like protein of hydrogenases 1 and 2 and that there is cross-interaction of the activities of HybG and HypC and also with other components of the hydrogenase maturation machinery.
MATERIALS AND METHODS
Bacterial strains, plasmids, and mutagenesis.
The bacterial strains and plasmids employed in this study are listed in Table 1. E. coli DH5α was used as a host for plasmid construction and maintenance. The primers listed in Table 2 were used for the generation of plasmids carrying the hybG gene or derivatives thereof. For this purpose, the hybG gene was amplified via PCR with the aid of the primers HybF1 and YghU1 using chromosomal DNA of strain MC4100 as the template. The resulting PCR products were cloned into the EcoRV-restricted vectors pACYC184 and pBR322, leading to plasmids pAHBG and pBHBG, respectively. On these plasmids, the expression of the hybG gene is under the control of the tet promoter. To replace the cysteine residue of HybG with alanine and serine, site-directed mutagenesis was performed on plasmid pBHBG as described previously (19) using the primer pairs HybGC2A-HybG1 and HybGC2S-HybG1, respectively. The fusion between the hybG gene and the sequence coding for the StreptagII was generated by PCR amplification of hybG on the plasmid pAHBG with the oligonucleotides HybF1 and HybG-StrepII. The resulting PCR product was then cloned into the EcoRV restriction site of pBR322.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype | Reference |
|---|---|---|
| Strains | ||
| DH5α | F− (φ80dlacZΔM15) recA1 endA1 gyrA96 thi-1 hsdR17 (rK− mK+) supE44 relA1 deoR Δ(lacZYA-argF)U169 | 32 |
| MC4100 | F−araD139 Δ(argF-lac)U169 ptsF25 deoC1 relA1 flbB5301 rpsL150 λ− | 7 |
| HD705 | MC4100 ΔhycE | 27 |
| HDK103 | MC4100 Δhya (Kmr) ΔhycABCDEFGH | 15 |
| HDK203 | MC4100 Δhyb (Kmr) ΔhycABCDEFGH | 15 |
| HDK200 | MC4100 Δhyb (Kmr) | M. Sauter (unpublished data) |
| DHP-C | MC4100 ΔhypC | 15 |
| NHC | DHP-C ΔhybG | This work |
| DHB-G | MC4100 ΔhybG | This work |
| CDH103 | HDK103 ΔhybG | This work |
| Plasmids | ||
| pMAK700 | Cmr | 13 |
| pACYC184 | Cmr Tcr | 8 |
| pBR322 | Apr Tcr | 6 |
| pJA1021 | pACYC184 hypC Cmr | 15 |
| pJA16 | pACYC184 hypBCDE Cmr | 18 |
| pMΔHBG | pMAK700 with 225-bp deletion in hybG; Cmr | This work |
| pAHBG | pACYC184 hybG Cmr | This work |
| pAΔHBG | pACYC184 with 225-bp deletion in hybG; Cmr | This work |
| pBHBG | pBR322 hybG Apr | This work |
| pBHBG-Strep | pBR322 hybG-StreptagII Apr | This work |
| pBC2A | pBR322 hybG[C2A] Apr | This work |
| pBC2S | pBR322 hybG[C2S] Apr | This work |
| pBC2A-Strep | pBR322 hybG-StreptagII[C2A] Apr | This work |
| pBC2S-Strep | pBR322 hybG-StreptagII[C2S] Apr | This work |
TABLE 2.
Oligonucleotide primers used in this work
| Designation | Sequencea |
|---|---|
| HybF1 | 5′-CAAGGATCCTGCGTTGAGGAGAGCGCCGT-3′ |
| YghU1 | 5′-CGAACGTCCGGCGGTGAAACGTGG-3′ |
| HybG-StrepII | 5′-TCACTTTTCAAACTGAGGGTGGCTCCACGCGCTGGTAATG-3′ |
| HybG3′ | 5′-TATGACGTCTATACCAGCGCGTGATGA-3′ |
| HybG5′ | 5′-ATAGACGTCATAGCCAATACACATTAT-3′ |
| HybGC2A | 5′-GCCTGGAACGCCAATAGCCATTATTAACTCCGG-3′ |
| HybGC2S | 5′-GCCTGGAACGCCAATGCTCATTATTAACTCCGG-3′ |
| HybG1 | 5′-ATTGGCGTTCCAGGCCAGGTGCTGGCTGTC-3′ |
Bases changed in comparison to the wild-type sequence are underlined; newly generated restriction sites are shown in boldface type.
The strains DHB-G (MC4100 ΔhybG), CDH103 (MC4100 Δhya Δhyc ΔhybG), and NHC (MC4100 ΔhybG ΔhypC) were constructed following the method of Hamilton et al. (13) with the aid of the pMAK plasmid system. An in-frame deletion of 225 bp was introduced into hybG by inverse PCR on pAHBG using the oligonucleotides HybG3′ and HybG5′ (Table 2), yielding the plasmid pAΔHBG. After restriction of pAΔHBG with BamHI a 525-bp fragment, which includes the mutant hybG gene, was subcloned into the BamHI-restricted pMAK700 vector. The resulting plasmid was designated pMΔHBG and was utilized to transfer the in-frame deletion onto the chromosome of the strains MC4100, HDK103, and DHP-C, leading to mutants DHB-G, CDH103, and NHC, respectively.
Growth conditions.
E. coli cells were grown anaerobically at 37°C in a buffered rich medium, TGYEP (4), containing 15 mM sodium formate. As supplements, 1 μM sodium molybdate, 1 μM sodium selenite, and 5 μM nickel chloride were used. When needed for maintenance of plasmids or strains, the medium was supplemented with 30 μg of chloramphenicol, 50 μg of ampicillin, or 50 μg of kanamycin sulfate per ml. At an optical density at 600 nm of 1.0 the cells were harvested and stored at −20°C. The crude extracts were prepared as described previously (10).
Polyacrylamide gel electrophoresis and Western blotting.
Proteins were separated on sodium dodecyl sulfate (SDS)–10% polyacrylamide gels according to the method of Laemmli (17) or on 10% nondenaturating gels as specified by Drapal and Böck (10). Thirty micrograms of total protein was applied per lane.
For Western blot analysis, the proteins were transferred onto nitrocellulose membranes and the blots were reacted with antibodies raised against the large subunit of hydrogenase 2 (HybC) (1:1,500) or hydrogenase 3 (HycE) (1:1,000). The polyclonal antiserum directed against StreptagII was purchased from the Institut für Bioanalytik (Göttingen, Germany) and used at a dilution of 1:2,000.
To visualize the H2-dependent benzyl viologen (BV) reduction activity, the nondenaturating gels were incubated in sodium phosphate buffer (100 mM, pH 7.2) containing 0.5 mM BV and 1 mM triphenyltetrazolium chloride in a glove box under an atmosphere of 95% N2 and 5% H2 for 24 h (3). One hundred micrograms of total protein was applied per lane.
RESULTS
HybG is required for maturation of hydrogenases 1 and 2.
The final step of the maturation process of hydrogenases consists of the proteolytic removal of a C-terminal extension from the precursor of the large subunit. The assessment of the processing status, therefore, can be taken as a measure of the function of the components of the maturation machinery (10, 26).
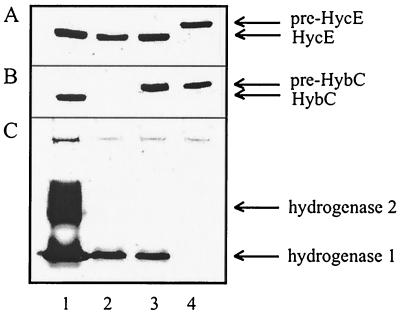
To study the in vivo maturation role of HybG by this approach, its gene was inactivated by the introduction of an in-frame deletion, yielding strain DHB-G. The ΔhybG lesion leads to the blockage of the maturation of the hydrogenase 2 (pre-HybC) without affecting processing of hydrogenase 3 (pre-HycE) (Fig. 1A and B, lanes 3). In the ΔhybG ΔhypC double mutant (NHC) (Fig. 1A and B, lanes 4) neither pre-HybC nor pre-HycE is processed.
FIG. 1.
Immunoblotting analysis of the precursor and mature forms of HycE and HybC in the wild-type strain MC4100 and in mutants with lesions in hybG and hypC. Crude extracts (30 μg protein) were subjected to SDS-polyacrylamide gel electrophoresis and reacted with antibodies raised against HycE (A) or HybC (B). Lane 1, MC4100; lane 2, HDK200 (Δhyb); lane 3, DHB-G (ΔhybG); lane 4, NHC (ΔhybG ΔhypC) (lane 4). (C) Hydrogenase activity staining of the same strains is displayed after nondenaturating polyacrylamide gel electrophoresis.
In order to analyze the influence of hybG or hypC deletions on hydrogenase isoenzymes 1 and 2, cell extracts were separated by nondenaturing polyacrylamide gel electrophoresis and the gels were analyzed by hydrogenase activity staining (Fig. 1C). Under these conditions, hydrogenase 3 activity cannot be detected as previously reported (28). The results indicate that the hybG deletion abolishes hydrogenase 2 activity but not that of hydrogenase 1. Albeit reduced, its activity is on the order of the level displayed by a mutant (HDK200) carrying a deletion within the hyb operon.
The finding that there was still hydrogenase 1 activity present when the hyb operon was deleted or when hybG was inactivated suggested the involvement either of HypC or of some other unknown homolog in the maturation process. To follow this assumption, the NHC strain was also analyzed by hydrogenase activity staining (Fig. 1C, lane 4); the results show that deletion of both hybG and hypC completely abolished hydrogenase activity. Consequently, the hydrogenase 1 activity displayed by the Δhyb or ΔhybG strains is most likely due to the presence of the HypC protein.
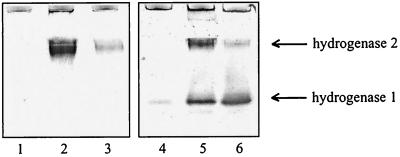
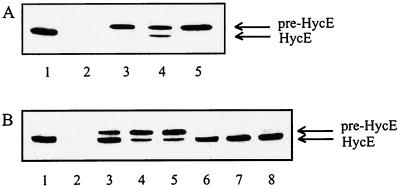
Since both HybG and HypC are able to participate in maturation of hydrogenase 1, it was important to study whether they also interact in the maturation of hydrogenases 2 and 3. For this purpose, the two strains CDH103 (Δhya Δhyc ΔhybG) and DHB-G (ΔhybG) were transformed with a plasmid carrying either hybG (pAHBG) or hypC (pJA1021), and the transformants were analyzed for restoration of hydrogenase activity (Fig. 2). It was found that expression of hybG from the plasmid led to full restoration of the activity of hydrogenase 2 (Fig. 2, lanes 2 and 5) and to an increase of the activity of isoenzyme 1 (Fig. 2, lane 5). Supply of hypC in trans resulted in an increase of both hydrogenase 1 and 2 activities (Fig. 2, lanes 3 and 6). Conversely, to analyze whether pre-HycE can be matured by HybG in the absence of HypC, the ΔhypC mutant (DHP-C) was transformed with plasmid pAHBG, and the transformants were analyzed by immunoblotting (Fig. 3A, lane 5). It is evident that no processing took place when hybG was expressed in trans. Intriguingly, however, expression of hypC in trans as a control was unable to fully complement the hypC mutation (Fig. 3A, lane 4; see below). The results support the contention that the predominant role of HybG resides in the maturation of pre-HybC and that maturation of isoenzyme 1 can be supported by both HybG and HypC.
FIG. 2.
Hydrogenase activity analysis of crude extracts of the ΔhybG strains CDH103 and DHB-G transformed with plasmids carrying hybG and hypC genes, respectively. Crude extracts (100 μg of protein) were subjected to nondenaturating polyacrylamide gel electrophoresis and stained for H2-dependent BV reduction activity as indicated in Materials and Methods. Lane 1, CDH103; lane 2, CDH103/pAHBG (hybG); lane 3, CDH103/pJA1021 (hypC); lane 4, DHB-G; lane 5, DHB-G/pAHBG (hybG); lane 6, DHB-G/pJA1021 (hypC).
FIG. 3.
Immunoblotting analysis of the precursor and mature forms of HycE. Crude extracts (30 μg protein) were subjected to SDS-polyacrylamide gel electrophoresis and reacted with antibodies raised against HycE. (A) Transformants of the ΔhypC strain (DHP-C) expressing either hypC or hybG genes from a plasmid were analyzed. Lane 1, MC4100; lane 2, HD705; lane 3, DHP-C; lane 4, DHP-C/pJA1021 (hypC); lane 5, DHP-C/pAHBG (hybG). (B) Transformants of MC4100 expressing hypC and different hybG variants from a plasmid were tested. Lane 1, MC4100; lane 2, HD705; lane 3, MC4100/pBHBG (hybG); lane 4, MC4100/pBC2A (hybG[C2A]); lane 5, MC4100/pBC2S (hybG[C2S]); lane 6, MC4100/pBHBG/pJA16 (hybG hypBCDE); lane 7, MC4100/pJA16 (hypBCDE); lane 8, MC4100/pJA1021 (hypC).
Overproduction of HybG interferes with the activity of HypC in maturation of hydrogenase 3.
Overproduction of HypC in the ΔhypC genetic background did not fully substitute for the function of a hypC copy located at its indigenous chromosomal site (Fig. 3A, lane 4). This unexpected but intriguing finding could be the consequence of a polarity effect of the mutation on the expression of some downstream gene(s) or the effect of titration of some other component(s) of the maturation machinery. To differentiate between these possibilities, strain MC4100 was transformed with plasmids carrying either hypC, hybG, or one of the hybG variants constructed (see below), and processing of pre-HycE was studied by immunoblotting (Fig. 3B). It is evident that expression of hybG in trans impeded pre-HycE processing (Fig. 3B, lane 3) and the inhibition was augmented when the N-terminal cysteine of HybG was replaced by alanine or serine (Fig. 3B, lanes 4 and 5). Introduction of a second plasmid carrying the hypBCDE genes counteracted the inhibition exerted by the overproduction of HybG (Fig. 3B, lane 6). In contrast, expression of hypC from a plasmid did not interfere with pre-HycE maturation when the chromosomal hypC gene copy was present (Fig. 3B, lane 8).
HybG forms a complex with pre-HybC.
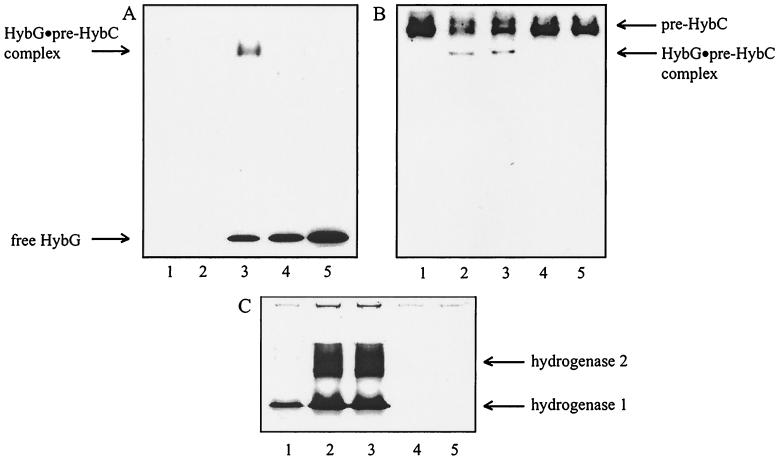
To analyze whether HybG indeed has a chaperone-like function in the maturation of the large subunit of hydrogenase 2, its ability to form a stable complex with pre-HybC was studied. Since antibodies directed against HybG were not available, a fusion between hybG at its 3′ end and the StreptagII sequence was constructed, and a commercially available anti-StreptagII serum was used to localize the gene fusion product in nondenaturating polyacrylamide gels (Fig. 4). A HybG–pre-HybC complex could be detected both by anti-StreptagII antibodies (Fig. 4A, lane 3) and by anti-HybC antibodies (Fig. 4B, lanes 2 and 3). HybG was unable to enter the complex when its N-terminal cysteine was replaced by an alanine or serine residue (Fig. 4A and B, lanes 4 and 5). These HybG variants were also unable to generate hydrogenase 1 and 2 activities (Fig. 4C, lanes 4 and 5).
FIG. 4.
Immunoblotting analysis of HybG–pre-HybC complex formation in transformants expressing different HybG variants from a plasmid. (A and B) Crude extracts (30 μg protein) were analyzed by Western blotting after nondenaturating polyacrylamide gel electrophoresis with antisera directed against StreptagII (A) and HybC (B). (C) Corresponding hydrogenase activity staining. Lanes 1, DHB-G; lanes 2, DHB-G/pBHBG; lanes 3, DHB-G/pBHBG-Strep; lanes 4, DHB-G/pBC2A-Strep; lanes 5, DHB-G/pBC2S-Strep.
DISCUSSION
The complex between HypC and the precursor of the large subunit of hydrogenase 3 has been demonstrated to be the key intermediate in the maturation process (10). Its formation is an early step since mutants defective in each other gene involved in maturation accumulate the complex (10) and since its dissolution only precedes the final step, namely, endoproteolytic removal of the C-terminal extension of pre-HycE (20). In view of the high sequence similarity between HypC and HybG (∼77%), a similar function was assumed for HybG in the maturation of hydrogenase 2 and possibly also of isoenzyme 1 (15, 22).
The in silico predictions now have been proven biochemically: HybG forms a complex with the precursor of the large subunit of hydrogenase 2 and as with HypC the N-terminal cysteine residue appears to be directly involved in complex formation. It is still open whether this residue solely has a structural role in the interaction of the two proteins or whether its role is catalytic, e.g., in some redox reaction taking place during insertion of the metal(s) or the addition of the CO or CN ligands identified in the active site of hydrogenases (9, 14, 31).
Intriguingly, HybG has a dual function in that it is also required for maturation of hydrogenase 1. However, whereas the function of HybG is indispensable for the maturation of hydrogenase 2, its involvement in hydrogenase 1 maturation can be partially taken over by HypC. The evidence is that a ΔhybG mutant contains significant levels of hydrogenase 1 activity which can be augmented by overproducing HypC in trans. Also, a double mutant lacking both HybG and HypC is unable to form the three hydrogenase isoenzymes in a processed and active form.
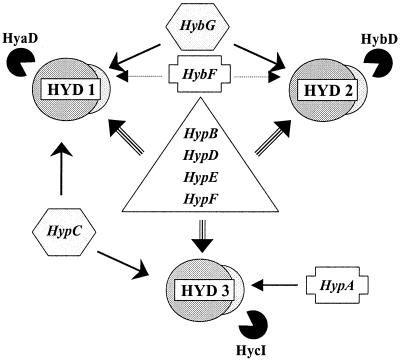
The specificity and the interactions of the various maturation components are summarized in Fig. 5. HypB, HypD, HypE, and HypF are required for the synthesis of all three hydrogenase isoenzymes, since they are possibly involved in general reactions like nickel acquisition and donation (21) or CO-CN ligand biosynthesis. Others, such as HybG and HypC, are shared between two of the systems or they are specific like the endopeptidases (HyaD, HybD, and HycI), processing the precursors of the large subunits after the metals have been inserted. It will be interesting to study the consequences of these interconnections under different physiological conditions.
FIG. 5.
Network of involvement of the auxiliary proteins in the formation of hydrogenases 1, 2, and 3. Dotted lines leading away from HybF indicate postulated functions.
The results of this study are also relevant for the discussion of the physiological role of the three hydrogenase isoenzymes. Whereas the function of hydrogenase 2 as an uptake enzyme and that of hydrogenase 3 as a fermentative gas-evolving one are undisputed, that of hydrogenase 1 is not fully understood (3, 16). If it is indeed H2 recycling the results would add another argument for such a role since the two enzymes would not only be interacting functionally but also be connected via a common component during the maturation process.
When overexpressed in trans, hybG interfered with hydrogenase 3 maturation in an otherwise wild-type genetic background: the inhibition was particularly pronounced in the case of the HybG variants in which the N-terminal cysteine was altered. A plausible explanation is that the overproduced HybG sequesters some other component(s) of the processing machinery, thereby forming unproductive processing intermediates in the case of the N-terminally altered variants. The fact that coexpression of the hypBCDE genes alleviated the inhibition by the HybG variants provides support for this contention.
ACKNOWLEDGMENTS
We thank A. Paschos and E. Zehelein for the kind donation of antiserum directed against HybC.
This work was supported by a research fellowship from the Alexander von Humboldt Foundation to A.M. and by grants from the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie to A.B.
REFERENCES
- 1.Andrews S C, Berks B C, McClay J, Ambler A, Quail M A, Golby P, Guest J R. A 12-cistron Escherichia coli operon (hyf) encoding a putative proton-translocating formate hydrogenlyase system. Microbiology. 1997;143:3633–3647. doi: 10.1099/00221287-143-11-3633. [DOI] [PubMed] [Google Scholar]
- 2.Ballantine S P, Boxer D H. Isolation and characterisation of a soluble active fragment of hydrogenase isoenzyme 2 from the membranes of anaerobically grown Escherichia coli. Eur J Biochem. 1986;156:277–284. doi: 10.1111/j.1432-1033.1986.tb09578.x. [DOI] [PubMed] [Google Scholar]
- 3.Ballantine S P, Boxer D H. Nickel-containing hydrogenase isoenzymes from anaerobically grown Escherichia coli K-12. J Bacteriol. 1985;163:454–459. doi: 10.1128/jb.163.2.454-459.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Begg Y A, Whyte J N, Haddock B A. The identification of mutants of Escherichia coli deficient in formate dehydrogenase and nitrate reductase activities using dye indicator plates. FEMS Microbiol Lett. 1977;2:47–50. [Google Scholar]
- 5.Böhm R, Sauter M, Böck A. Nucleotide sequence and expression of an operon in Escherichia coli coding for formate hydrogenlyase components. Mol Microbiol. 1990;4:231–243. doi: 10.1111/j.1365-2958.1990.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 6.Bolivar F, Rodriguez R L, Greene P J, Betlach M C, Heyneker H L, Boyer H W. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 7.Casadaban M J, Cohen S N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci USA. 1979;76:4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang A C, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Lacey A L, Hatchikian E C, Volbeda A, Frey M, Fontecilla-Camps J C, Fernandez V M. Infrared-spectroelectrochemical characterization of the [NiFe] hydrogenase of Desulfovibrio gigas. J Am Chem Soc. 1997;119:7181–7189. [Google Scholar]
- 10.Drapal N, Böck A. Interaction of the hydrogenase accessory protein HypC with HycE, the large subunit of Escherichia coli hydrogenase 3 during enzyme maturation. Biochemistry. 1998;37:2941–2948. doi: 10.1021/bi9720078. [DOI] [PubMed] [Google Scholar]
- 11.Fritsche E, Paschos A, Beisel H G, Böck A, Huber R. Crystal structure of the hydrogenase maturating endopeptidase HybD from Escherichia coli. J Mol Biol. 1999;288:989–998. doi: 10.1006/jmbi.1999.2719. [DOI] [PubMed] [Google Scholar]
- 12.Garcin E, Vernede X, Hatchikian E C, Volbeda A, Frey M, Fontecilla-Camps J C. The crystal structure of a reduced [NiFeSe] hydrogenase provides an image of the activated catalytic center. Struct Fold Des. 1999;7:557–566. doi: 10.1016/s0969-2126(99)80072-0. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton C M, Aldea M, Washburn B K, Babitzke P, Kushner S R. New method for generating deletions and gene replacements in Escherichia coli. J Bacteriol. 1989;171:4617–4622. doi: 10.1128/jb.171.9.4617-4622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Happe R P, Roseboom W, Pierik A J, Albracht S P, Bagley K A. Biological activation of hydrogen. Nature. 1997;385:126. doi: 10.1038/385126a0. [DOI] [PubMed] [Google Scholar]
- 15.Jacobi A, Rossmann R, Böck A. The hyp operon gene products are required for the maturation of catalytically active hydrogenase isoenzymes in Escherichia coli. Arch Microbiol. 1992;158:444–451. doi: 10.1007/BF00276307. [DOI] [PubMed] [Google Scholar]
- 16.King P W, Przybyla A E. Response of hya expression to external pH in Escherichia coli. J Bacteriol. 1999;181:5250–5256. doi: 10.1128/jb.181.17.5250-5256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Lutz S, Jacobi A, Schlensog V, Böhm R, Sawers G, Böck A. Molecular characterization of an operon (hyp) necessary for the activity of the three hydrogenase isoenzymes in Escherichia coli. Mol Microbiol. 1991;5:123–135. doi: 10.1111/j.1365-2958.1991.tb01833.x. [DOI] [PubMed] [Google Scholar]
- 19.Magalon A, Böck A. Analysis of the HypC-HycE complex, a key intermediate in the assembly of the metal center of the Escherichia coli hydrogenase 3. J Biol Chem. 2000;275:21114–21220. doi: 10.1074/jbc.M000987200. [DOI] [PubMed] [Google Scholar]
- 20.Magalon A, Böck A. Dissection of the maturation reactions of the [NiFe] hydrogenase 3 from Escherichia coli taking place after nickel incorporation. FEBS Lett. 2000;473:254–258. doi: 10.1016/s0014-5793(00)01542-8. [DOI] [PubMed] [Google Scholar]
- 21.Maier T, Lottspeich F, Böck A. GTP hydrolysis by HypB is essential for nickel insertion into hydrogenases of Escherichia coli. Eur J Biochem. 1995;230:133–138. [PubMed] [Google Scholar]
- 22.Menon N K, Chatelus C Y, Dervartanian M, Wendt J C, Shanmugam K T, Peck H D, Jr, Przybyla A E. Cloning, sequencing, and mutational analysis of the hyb operon encoding Escherichia coli hydrogenase 2. J Bacteriol. 1994;176:4416–4423. doi: 10.1128/jb.176.14.4416-4423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menon N K, Robbins J, Peck H D, Jr, Chatelus C Y, Choi E S, Przybyla A E. Cloning and sequencing of a putative Escherichia coli [NiFe] hydrogenase-1 operon containing six open reading frames. J Bacteriol. 1990;172:1969–1977. doi: 10.1128/jb.172.4.1969-1977.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menon N K, Robbins J, Wendt J C, Shanmugam K T, Przybyla A E. Mutational analysis and characterization of the Escherichia coli hya operon, which encodes [NiFe] hydrogenase 1. J Bacteriol. 1991;173:4851–4861. doi: 10.1128/jb.173.15.4851-4861.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peck H D, Gest H. Formic dehydrogenase and the hydrogenlyase enzyme complex in coli-aerogenes bacteria. J Bacteriol. 1957;73:706–721. doi: 10.1128/jb.73.6.706-721.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossmann R, Maier T, Lottspeich F, Böck A. Characterisation of a protease from Escherichia coli involved in hydrogenase maturation. Eur J Biochem. 1995;227:545–550. doi: 10.1111/j.1432-1033.1995.tb20422.x. [DOI] [PubMed] [Google Scholar]
- 27.Sauter M, Böhm R, Böck A. Mutational analysis of the operon (hyc) determining hydrogenase 3 formation in Escherichia coli. Mol Microbiol. 1992;6:1523–1532. doi: 10.1111/j.1365-2958.1992.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 28.Sawers R G, Ballantine S P, Boxer D H. Differential expression of hydrogenase isoenzymes in Escherichia coli K-12: evidence for a third isoenzyme. J Bacteriol. 1985;164:1324–1331. doi: 10.1128/jb.164.3.1324-1331.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawers R G, Boxer D H. Purification and properties of membrane-bound hydrogenase isoenzyme 1 from anaerobically grown Escherichia coli K-12. Eur J Biochem. 1986;156:265–275. doi: 10.1111/j.1432-1033.1986.tb09577.x. [DOI] [PubMed] [Google Scholar]
- 30.Volbeda A, Charon M H, Piras C, Hatchikian E C, Frey M, Fontecilla-Camps J C. Crystal structure of the nickel-iron hydrogenase from Desulfovibrio gigas. Nature. 1995;373:580–587. doi: 10.1038/373580a0. [DOI] [PubMed] [Google Scholar]
- 31.Volbeda A, Garcin E, Piras C, de Lacey A L, Fernandez V M, Hatchikian E C, Frey M, Fontecilla-Camps J C. Structure of the [NiFe] hydrogenase active site: evidence for biologically uncommon Fe ligands. J Am Chem Soc. 1996;118:12989–12996. [Google Scholar]
- 32.Woodcock D M, Crowther P J, Doherty J, Jefferson S, DeCruz E, Noyer-Weidner M, Smith S S, Michael M Z, Graham M W. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 1989;17:3469–3478. doi: 10.1093/nar/17.9.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]