Introduction and Importance:
Currently, coronavirus disease 2019 (COVID-19) and tuberculosis (TB) are among the most important causes of respiratory infections around the world. Both of them are sources of concern for human health and life safety. COVID-19 caused the deaths of millions of people, and many of them suffered from what has become known as ‘post-COVID squeal’. Immunosuppression is one of the most important of these symptoms that leave patients susceptible to severe infections like TB.
Case Presentation:
In these two cases, the authors observed the development of active TB after a period of COVID recovery. Two patients who were admitted to the hospital complained mainly, among other symptoms, of fever and a continuous cough after a period of COVID-19 recovery.
Clinical Discussion:
Radiological examination revealed a caving density in the two cases, and the Gene-Xpert test proved the presence of Mycobacterium tuberculosis bacteria despite the negative result of the Ziehl–Neelsen stain. The two patients were improved after standard TB treatment.
Conclusion:
Patients with post‐COVID‐19 chronic respiratory symptoms should be screened for TB, especially in TB-endemic areas, even though the result of the Ziehl–Neelsen stain was negative.
Keywords: activation, case report, coronavirus disease 2019, latent, tuberculosis
Highlights
Postcoronavirus disease 2019 (COVID-19) – a form of tuberculosis – may be neglected due to similar clinical presentations.
A respiratory viral infection increases the incidence of tuberculosis.
The COVID-19 therapy protocol with corticosteroids causes immunosuppression.
Latent tuberculosis infection could be activated after COVID-19 infection.
Introduction
Globally and for a long time, tuberculosis (TB) was the leading infectious disease killer in the world1. Nowadays, mortality due to the coronavirus disease 2019 (COVID-19) pandemic is exceeding annual rates of death from TB, as it has infected 256 million people between December 2019 and November 20212. This number was officially recorded, while the injuries are much greater.
At the same time, the WHO estimated that about a quarter of the world’s population has latent tuberculosis infection (LTBI)1. It is defined as a state of persistent immune response to stimulation by Mycobacterium tuberculosis antigens without evidence of clinically manifested active TB, which may remain ineffective until death without any damage3. In about 5–15% of cases of LTBI, patients may develop active TB disease at some stage of their lives.
COVID-19 and TB have many similarities, such as the route of transmission and attacking the lungs, causing cough, fever, and shortness of breath. However, there are differences in some symptoms, like acute sore throat and loss of smell, that distinguish COVID-19 from TB and vice versa. TB cough is usually accompanied by sputum and may be hemoptysis4.
In conjunction with the high prevalence of COVID-19, the diagnosis of post-COVID-19 TB may be neglected or delayed due to similar clinical presentation5. The data is needed to predict the impact of COVID-19 infection on the progress of TB, which is still rare. Studying such cases brings us closer to an accurate diagnosis and fast treatment. We report two cases of TB that activated in recovered COVID-19 patients in line with the SCARE guidelines6.
Case 1 presentation
A 68-year-old nonsmoking female presented to the emergency unit in Aleppo University Hospital with a dry cough for more than a month. At the time of admission, she complained of dyspnea, fever, chills, myalgia, and intermittent diarrhea. She denied any contact with anyone who was known or suspected to have TB. In the past medical history, she had diabetes mellitus type 2, hypertension, and she underwent a cholecystectomy 2 years ago.
The patient was diagnosed as being infected with COVID-19 by PCR 40 days ago, which required hospitalization, where she was treated with standard COVID-19 care (antipyretics, steroids, anticoagulants, and intravenous fluids).
Physical examination revealed crackles in the right lung, her oxygen saturations were 80% on ambient air, blood pressure was 118/80 and her pulse was 97 beats per minute. Laboratory investigations revealed white blood count of 33 000/mm3, C-reactive protein 194 mg/dl (Table 1).
Table 1.
Laboratory investigation findings of the indexed case
| Parameters | Patient 1 | Patient 2 |
|---|---|---|
| Complete blood counts | ||
| Total leukocyte count (×103/μl) | 33 | 12.7 |
| Neutrophils (%) | 91.1 | 75.9 |
| Lymphocytes (%) | 5.3 | 16.2 |
| Monocytes (%) | 3.6 | 7.2 |
| Platelets (×103/μl) | ||
| Total erythrocytes count (×106/μl) | 2.47 | 4.26 |
| Hemoglobin (g/dl) | 6.90 | 11.7 |
| Biochemistry | ||
| Glucose (mg/dl) | 121 | 95 |
| Creatinine (mg/dl) | 1.03 | 0.8 |
| Urea (mg/dl) | 40 | 20 |
| ALT (SGPT) (µl/l) | 22 | 21 |
| AST (SGOT) (µl/l) | 23 | 40 |
| Serology | ||
| CRP (mg/dl) | 194 | 169 |
ALT, alanine aminotransferase; AST, aspartate transaminase; CRP, C-reactive protein; SGOT, serum glutamic-oxaloacetic transaminase; SGPT, serum glutamic-pyruvic transaminase).
Chest radiography showed a caveated density in the upper lobe of the right lung, and computed tomography (CT) scan of thorax showed another density in the base of the right lung that was not seen in the previous radiograph (Fig. 1).
Figure 1.
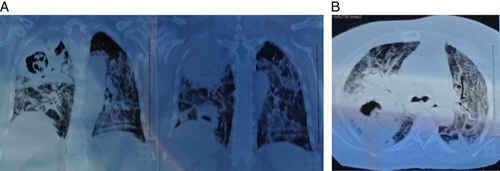
A computed tomography scan shows bilateral diffuse parenchymal densities. Two cavities appear in the right lung: the right upper lobe tuberculosis cavity (1) and the right lower lobe tuberculosis cavity (3), in addition to the residual ground-glass appearance due to previous coronavirus disease (2).
According to the previous finding, the differential diagnosis could be necrotizing pneumonia, lung abscess, TB disease, or a necrotic tumor. After a sputum culture and antibiotic sensitivity test, the patient was treated with Tazobatam and Tycoplanine, but she did not show a very favorable response.
To investigate TB infection, an early morning sputum specimen was examined using Ziehl–Neelsen stain to detect Koch bacilli, but the result was negative.
So, the patient underwent bronchoscopy, where complete obstruction of the right bronchi was found. Meanwhile, a biopsy was taken for histological examination, which did not reveal any abnormalities. Whereas a bronchoalveolar lavage sample examined by Gene-Xpert at the Tuberculosis Center proved the presence of tuberculosis bacilli.
On the next day of the bronchoscopy, she coughed up a piece of tissue, which was sent for pathology examination, and it appeared to be necrotic lung tissue without malignancy.
The patient started to improve after treatment with four-drug therapy (isoniazid, rifampin, ethambutol, and pyrazinamide) on the seventh day of hospitalization and was discharged to complete anti-TB medications with telephone follow-up.
Case 2 presentation
A 59-year-old heavy smoker presented to the emergency unit with a persistent cough, hemoptysis, and mid-grade fever (37.5C°). He was a businessman with no history of alcoholism or any other substance abuse. There was no history of any contact with TB in the family or among close contacts. In the past history of the patient, he had chronic obstructive pulmonary disease, and ischemic heart disease, and he was diagnosed with COVID-19 by PCR 1 year ago, for which he requires hospitalization. He was treated with standard COVID-19 care (antipyretics, steroids, anticoagulants, and intravenous fluids) until improvement.
Physical examination showed normal blood pressure and pulse; his oxygen saturation was 96 on ambient air. Laboratory investigation showed elevation in C-reactive protein value 169 mg/dl and increased white blood count (12 700/mm3) (Table 1). Chest auscultation revealed a crackle that was more severe in the left upper zone of his lung. Previous radiography showed the ground-glass appearance compatible with COVID-19 infection. The current imaging of CT scan of thorax showed resorption of ground-glass with appearance of round caveated density in the left upper lobe (Fig. 2).
Figure 2.
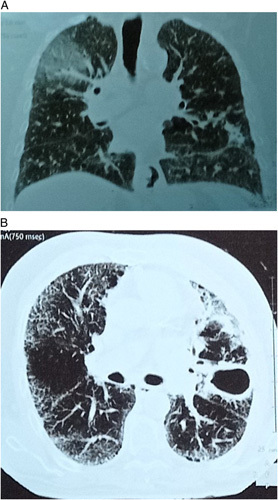
Chest computed tomography scan in emergency room showing left upper lobe tuberculosis cavity (1) and residual ground-glass appearance due to previous coronavirus disease 2019 (2).
A specimen of early morning sputum was examined using Ziehl–Neelsen stain to detect Koch bacilli, but the result was negative. However, a diagnostic bronchoscopy was performed. The intrabronchial macroscopic view was within normal limits. A bronchoalveolar lavage sample was examined by Gene Xpert at the Tuberculosis Center. The presence of M. tuberculosis bacteria was proven.
The patient was treated with four-drug therapy (isoniazid, rifampin, pyrazinamide, and ethambutol) and discharged within 7 days with telephone follow-up.
Discussion
As far as the authors know, these are the first documented cases of activated LTB in recovered COVID-19 patients in our country. Latent TB is present in populations everywhere, including Syria7.
Several factors may increase likelihood of activating TB like suppression immunity by HIV infection, tumor necrosis factor-α inhibitors, glucocorticoids, malnutrition, chemotherapy, or exposure to environmental factors, such as silica dust or cigarette smoke8.
On the other hand, more than one study described COVID-19 and active TB coinfections that increase mortality9. Some of these studies recorded the transformation of latent TB infection into active due to the influence of COVID-1910. The fact that respiratory viral infection increases the incidence of TB is well established, as with the Spanish flu pandemic of 1918–2020, which caused an increase in the number of lung TB cases. Patients with SARS or MERS infections were also found to develop lung TB11.
COVID-19 could cause a histological damage in lungs with involvement of other systems in human body as immune system. The COVID-19 infection could play a role in TB reactivation through many possible mechanisms, such as immunosuppression by corticosteroids12, cytokine storm13, and depletion and exhaustion of T-cell lymphocyte14. A significant depletion in T-cell lymphocyte counts was recorded in 76% of COVID-19 patients in a study from Wuhan; this depletion may promote the development of active TB in patients with LTBI14.
It is noteworthy that a very recent study suggests that dormant TB bacteria may exploit a novel altruistic stem cell defense mechanism against mouse coronavirus infection to replicate in the lung and cause pulmonary TB15.
As latent TB already affects a quarter of the world’s population, if the novel coronavirus activates a sizable proportion of these dormant infections. It could lead to the pandemic prevalence of TB in the same time that the global health is preoccupied with the of COVID-19. This situation can be handled with rapid and precise early detection. A chest CT may be useful in identifying active TB even if the chest radiograph is negative. Although chest CT is not the standard of care, it should be considered in doubtful cases16. On the other hand, using a nucleic acid amplification technique, the Gene-Xpert test can detect M. tuberculosis bacteria in suspected patients within ~2 h, ensuring early treatment and preventing transmission17.
Conclusion
These two cases showed that LTBI could be activated after COVID-19 infection due to deterioration of the patient’s immunity. This possibility should be considered, especially in TB-endemic areas. The patients with post‐COVID‐19 chronic respiratory symptoms should be screened for TB using the Gene-Xpert test even though the result of the Ziehl–Neelsen stain was negative.
Ethical approval
This is a case report; therefore, it did not require ethical approval from the ethics committee.
Consent
Written informed consent was obtained from the patient for the publication of these case reports and accompanying images. A copy of the written consents is available for review by the Editor-in-Chief of this journal on request.
Sources of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
All authors have significantly contributed to the investigation, development, and writing of this article.
Conflicts of interest disclosure
The authors declare no conflict of interest.
Research registration unique identifying number (UIN)
NA.
Guarantor
Dr Abdullah Khouri, Department of Thoracic Medicine, Faculty of Medicine, Aleppo University, Syria.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 1 February 2023
Contributor Information
Rawaa S. Al-kayali, Email: rawaa.kayali@wpu.edu.sy;rawah67@hotmail.com.
Mohamad F. Kashkash, Email: Fatehaminkashkash@gmail.com.
Azzam H. Alhussein Alhajji, Email: azzaamalhajji@gmail.com.
Abdullah Khouri, Email: akhourir6@gmail.com.
References
- 1. WHO. Global tuberculosis report 2020. Accessed 15 August 2021. https://www.who.int/publications/i/item/9789240013131
- 2. WHO. Weekly epidemiological update on COVID-19. Accessed 23 November 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19
- 3. Kiazyk S, Ball TB. Latent tuberculosis infection: an overview. Can Commun Dis Rep 2017;43:62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Visca D, Ong CWM, Tiberi S, et al. Tuberculosis and COVID-19 interaction: a review of biological, clinical and public health effects. Pulmonology 2021;27:151–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zimmer AJ, Klinton JS, Oga-Omenka C, et al. Tuberculosis in times of COVID-19. J Epidemiol Community Health 2022;76:310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agha RA, Franchi T, Sohrabi C, et al. for the SCARE Group. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int J Surg 2020;84:226–30. [DOI] [PubMed] [Google Scholar]
- 7. Al-Kayali R, Almekhlef AM, Khouri A. Risk factors for latent tuberculosis infection in close contacts of active tuberculosis patients in Aleppo City. Eur J Biomed Pharm 2018;5:124–8. [Google Scholar]
- 8. Ai JW, Ruan QL, Liu QH, et al. Updates on the risk factors for latent tuberculosis reactivation and their managements. Emerg Microbes Infect 2016;5:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mishra A, George AA, Sahu KK, et al. Tuberculosis and COVID-19 co-infection: an updated review. Acta Biomed 2020;92(1)):e2021025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee G, Stoll JJ, El Husseini I. Rare case of latent tuberculosis reactivation in the setting of COVID-19 infection. Am J Respir Crit Care Med 2021;203:1188–1990.33347378 [Google Scholar]
- 11. Ong CWM, Migliori GB, Raviglione M. Epidemic and pandemic viral infections: impact on tuberculosis and the lung. Eur Respir J 2020;56:2001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gopalaswamy R, Subbian S. Corticosteroids for COVID-19 therapy: potential implications on tuberculosis. Int J Mol Sci 2021;22:3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boni FG, Hamdi I, Koundi LM, et al. Cytokine storm in tuberculosis and IL-6 involvement. Infect Genet Evol 2022;97:105166. [DOI] [PubMed] [Google Scholar]
- 14. Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol 2020;11:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pathak L, Gayan S, Pal B, et al. Coronavirus activates an altruistic stem cell–mediated defense mechanism that reactivates dormant tuberculosis: implications in coronavirus disease 2019 pandemic. Am J Pathol 2021;191:1255–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nachiappan AC, Rahbar K, Shi X, et al. Pulmonary tuberculosis: role of radiology in diagnosis and management. RadioGraphics 2017;37:52–72. [DOI] [PubMed] [Google Scholar]
- 17. Kabir S, Parash MTH, Emran NA, et al. Diagnostic challenges and Gene-Xpert utility in detecting Mycobacterium tuberculosis among suspected cases of pulmonary tuberculosis. PLoS One 2021;16:e0251858. [DOI] [PMC free article] [PubMed] [Google Scholar]


