Background:
This systematic review and network meta-analysis aimed to provide a complete hepatotoxicity profile, hepatotoxicity spectrum, and safety ranking of immune checkpoint inhibitor drugs for cancer treatment.
Methods:
PubMed, Embase, Scopus, CINAHL, Web of Science, psycINFO, Cochrane Library, and ClinicalTrials.gov. websites were searched, and a manual search of relevant reviews and trials up to January 1, 2022, was undertaken. Head-to-head III randomized controlled trials comparing any 2 or 3 of the following treatments or different doses of the same immune checkpoint inhibitor drug were included: programmed death 1 (PD-1), programmed death ligand 1, and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) inhibitors and conventional therapy. We included 106 randomized trials (n=164,782) containing 17 treatment arms.
Results:
The overall incidence of hepatotoxicity was 4.06%. The rate of fatal liver adverse events was 0.07%. The programmed death ligand 1 inhibitor+targeted therapy drug+chemotherapy group had the highest risk of treatment-related increases in all-grade alanine aminotransferase and aspartate aminotransferase levels, and the differences were significant. For immune-related hepatotoxicity, no significant difference was found between PD-1 and CTLA-4 inhibitors for all-grade hepatotoxicity; however, CTLA-4 inhibitors were associated with a higher risk of grade 3–5 hepatotoxicity than PD-1 inhibitors.
Conclusions:
The highest incidence of hepatotoxicity and fatality was observed with triple therapy. The overall incidence of hepatotoxicity was similar between different dual regimens. For immune checkpoint inhibitor monotherapy, the overall risk of immune-mediated hepatotoxicity related to CTLA-4 inhibitors did not differ significantly from that of PD-1 inhibitors. There was no direct relationship between the risk of liver injury and drug dose, whether monotherapy or combination therapy was used.
INTRODUCTION
The rapid development of tumor therapy has brought hope for the survival of many patients with cancer, and immune checkpoint inhibitors (ICIs), including those of programmed cell death 1 (PD-1) and its ligand programmed cell death ligand 1 (PD-L1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4), have produced effective long-term responses in many tumors. CTLA-4 belongs to a class of co-stimulatory molecules expressed on the surfaces of T cells. PD-1 and PD-L1 are key proteins expressed on activated T cells, pro-B cells, and tumor cells. Both inhibit internal regulatory checkpoints and downregulate T-cell activation, thereby generating a therapeutic antitumor T-cell response and enhancing the effective immune response against tumor cells.1,2 At present, ICIs are widely used in the treatment of malignant tumors, such as melanoma, non–small cell lung cancer, renal cancer, other solid tumors, and hematological cancer. Previous studies have suggested that patients treated with ICIs have a better quality of life than those administered other treatment regimens.3,4 This might be related to the degree and frequency of adverse reactions to ICIs compared with other treatments. However, 1 study reported that immune-related adverse events (irAEs) might be more common than thought.5 Since 2011, the US Food and Drug Administration has approved 7 ICIs, including monotherapy, dual-targeted therapy, and combination therapy. With the promotion of dual and triple ICI regimens, it remains unclear whether combined therapy increases antitumor efficacy while also increasing drug-related adverse events (AEs).
Enhanced inflammatory cascades and complement activation are key mechanisms that trigger irAEs,6–8 which are associated with an increased immune response to tumor cells after ICI injection. AEs associated with ICIs include thyroid dysfunction, pneumonia, and colitis. The most common AEs are skin complications (eg, rash and pruritus), gastrointestinal discomfort (eg, diarrhea and colitis), hepatotoxicity, pneumonia, and endocrine disorders (such as hypothyroidism and hypophysitis). Serious irAEs have been reported in up to 27% of patients treated with anti-CTLA-4 agents and in 16% of those treated with anti-PD-1 drugs, and this might increase to 55% when both therapies are used concurrently.9
Hepatitis can be a fatal adverse reaction, and the main types of hepatitis are cholestatic, hepatocyte, and mixed damage.10 With the emergence of new ICIs, the incidence of immunotherapy-related hepatitis has been increasing, and the ever-increasing hepatic AEs are a concern. Therefore, the early monitoring of these side effects is crucial. The early diagnosis of more irAEs of lower severity (grade 1–2) and treatment with supportive measures can prevent AEs from progressing to higher toxicity levels and allow for the continuation of ICI therapy.11 Simultaneously, the cost of treating irAEs is lessened, which can reduce the cost of immunotherapy. Moreover, a reduction in the frequency and severity of AEs might help to improve the cost-effectiveness of the immunotherapy.12 However, hepatotoxicity caused by ICIs is asymptomatic in many patients; thus, clinicians often ignore its diagnosis in clinical practice. Therefore, we undertook a network meta-analysis to assess ICI-related liver toxicity by analyzing data from all published randomized clinical trials involving ICIs. Reliable conclusions on this topic are essential for increasing awareness, the early detection of hepatic AEs, and improving patient management.
METHODS
Data sources and searches
A comprehensive literature search was performed using PubMed, Embase, Scopus, CINAHL, Web of Science, PsycINFO, Cochrane Library, and ClinicalTrials.gov websites, in addition to manual searches of relevant literature or reference lists of relevant literature. The search period was from January 1, 2015, to January 1, 2022. Only articles published in English were included in this study. We prospectively registered the protocol at PROSPERO (ID: CRD42022325394). The search strategy is detailed in eTable 1 in the Supplement (http://links.lww.com/HC9/A156) and included the keywords anti-PD-1, PD-1 inhibitor, anti-PD-L1, PD-L1 inhibitor, nivolumab, pembrolizumab, avelumab, durvalumab, atezolizumab, ICIs, and cancer.
Study selection
The studies included in this meta-analysis were phase III multicenter trials that met the following inclusion criteria: (1) patients, those with cancer above 18 years of age; (2) intervention, at least 1 treatment group received an ICI, either as monotherapy or in combination with another ICI or traditional cancer therapy; (3) comparison, no intervention/placebo, traditional therapy, or any active intervention comprising ICIs; and (4) outcomes, we used common terminology criteria for adverse events version 3 or 4 to determine various liver indicators [alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), and bilirubin levels]. We excluded (1) nonrandomized and observational studies, (2) phase I or II trials, (3) single-center, single-arm trials, and (4) patients below 18 years of age.
Data extraction and quality assessment
Data extraction and quality assessment were performed by 2 independent reviewers (Caiyun Zheng and Baohui Hong). Any unresolved discrepancies in data extraction or outcome evaluation were resolved through discussion with a third reviewer (Shunmin Huang). The extracted content included study information (author, date, National Clinical Trial number, and trial name), patient baseline characteristics (sample size, median age, follow-up, cancer type, line of treatment, and disease stage), specific therapy details, and hepatotoxicity. Quality assessment was performed using Review Manager (RevMan) (version 5.3; Cochrane) software.
Types of outcome measures
The primary outcome was treatment-related hepatotoxicity, including ALT, AST, ALP, GGT, and bilirubin levels beyond the normal threshold. Various liver function indicators were further divided into any hepatotoxicity (all grades), severe hepatotoxicity (grades 3–5), and fatal hepatotoxicity (grade 5). The National Cancer Institute grades the severity of hepatotoxicity were in accordance with the common terminology criteria for adverse event version 4. This AE grading system was used for clinical trials on cancer treatment using AST, ALT, ALP, GGT, and total bilirubin measurements in multiples of the upper limit of the normal level. The common terminology criteria for adverse event grades severity on a scale of 1 to 5, where grade 5 refers to fatal hepatotoxicity.
Statistical analysis
Owing to expected differences between differing regimens, direct meta-analyses were performed using random-effects models to estimate pooled ORs and 95% CIs for within-study and between-study heterogeneity. Funnel plot asymmetry was used to assess publication bias. For incidence probability, a logit transformation (logit(z)=log(z) − log(1 − z)) was used. Furthermore, a normal distribution for the additive effects of study-level moderators was assumed to adjust for study-specific effects.13
Bayesian network meta-analysis was performed using a generalized linear model with random effects based on the Markov chain Monte Carlo method.14 Consistency models were constructed, and relative effect sizes of the treatments were calculated as the log OR and reported with their 95% CIs.15 To obtain the posterior distribution, each of the 4 chains was run simultaneously for 50,000 burn-in and 100,000 inference iterations. The Gelman-Rubin method was combined to detect the convergence of the model with a density plot and a tract plot.16 For all results, the evidence was summarized by plotting the network relationships. The hepatotoxicity in different treatment regimens was ranked according to the surface under the cumulative ranking curve.17 We used a tau-squared (t 2) test to evaluate the extent of heterogeneity for each outcome. In addition, subgroup analyses were performed using different outcome definitions, cancer types, and doses. Immune-mediated liver injury caused by checkpoint inhibitors (ILICI) is a unique type of DILI, which is caused by the indirect effects of ICIs on the liver through their immune-mediated mechanisms. It was determined based on a prespecified list of terms from the Medical Dictionary for Regulatory Activities, and the list was updated with each new version. Treatment-related AEs comprised any AE for which treatment could not be excluded as the cause. That is, they could be caused by chemotherapy, targeted therapy drug, or ICIs. We used Cochrane Review Manager version 5.3 (The Cochrane Collaboration, Copenhagen, Denmark) with R programming language, version 3.6.3 (R Foundation for Statistical Computing, Guangzhou, China) for direct comparisons, with p<0.05 indicating that the difference was statistically significant.
RESULTS
Study characteristics
In total, 56,174 studies were identified through an electronic search. After excluding duplicate studies, 5724 papers underwent title/abstract screening. Of these, 5523 studies were excluded owing to incomplete randomized clinical trials and title/abstract reviews, with 195 studies reviewed in full. At the end of the review process, 106 articles met the inclusion criteria (64,782 patients), and a qualitative comprehensive and quantitative meta-analysis was performed. A preferred reporting item for systematic reviews and meta-analyses (PRISMA) flow diagram is presented in eFigure 1i n the Supplement (http://links.lww.com/HC9/A156), and the characteristics of the included studies are provided in eTable 2 in the Supplement (http://links.lww.com/HC9/A156). Overall, of these 106 trials, 5 (5%) involved Chinese populations only, and the remaining trials included between 2 and 41 different countries. The median sample size was 617 (range, 4–792) patients. Seventy-four (70%) articles contained treatment-related hepatic AEs, and 41 (39%) articles contained data on immune-mediated AEs. All-grade and grade 3–5 AEs were reported in each article, except for 1 study.18 Among the 106 articles, 53 compared ICIs to chemotherapy (22 compared PD-1 inhibitors to chemotherapy, 17 compared PD-1 inhibitors+chemotherapy to chemotherapy, and 14 compared PD-L1 inhibitors to chemotherapy). Fifteen articles compared ICIs to placebo (10 compared PD-1 inhibitors to a placebo). The network plot is shown in Figure 1.
FIGURE 1.
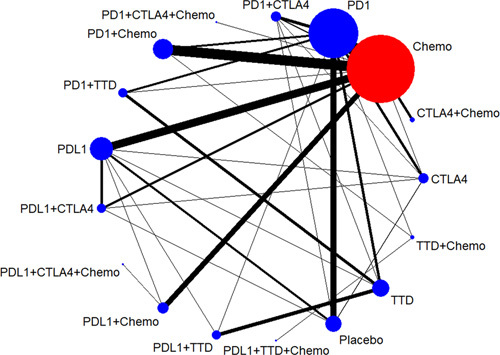
Network of included studies with available direct comparisons of hepatotoxicity. Abbreviations: Chemo, chemotherapy; CTLA-4, cytotoxic T-lymphocyte antigen 4 inhibitor; PD-1, programmed cell death 1 inhibitor; PD-L1, programmed cell death ligand 1 inhibitor; TTD, targeted therapy drug.
Risk of bias assessment and publication bias
The Cochrane systematic evaluation method was used for quality evaluation, and all included studies had a low risk of bias, with most studies having blinded testing of participants and staff (53.8%) and outcome assessments (50.9%). The risk of bias for each trial is shown in eFigure 2 in the Supplement (http://links.lww.com/HC9/A156). In addition, an examination of the comparison-adjusted funnel plots revealed no apparent asymmetry; thus, there was no significant risk of small-sample study effects (eFigure 3 in the Supplement, http://links.lww.com/HC9/A156).
Incidence of liver AEs
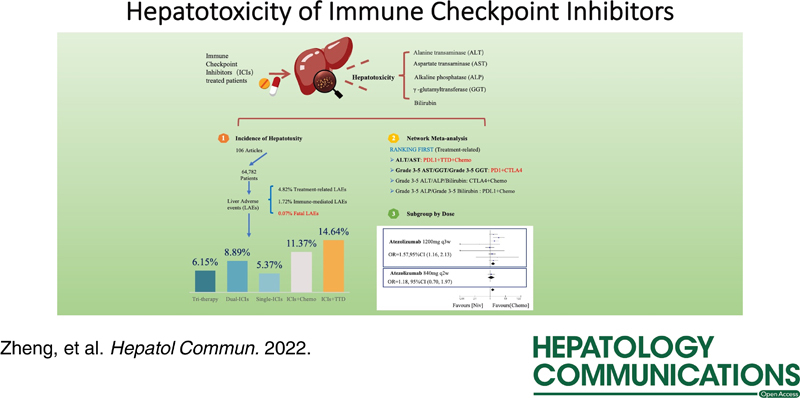
The incidence of treatment-related hepatotoxicity was higher than that of ILICI (4.82%, 95% CI, 4.33%–5.31% and 1.72%, 95% CI, 1.31%–2.13%, respectively). The incidences of all-grade and grade 3–5 treatment-related hepatotoxicity were 8.09% (95% CI, 7.27%–8.91%) and 1.53% (95% CI, 1.18%–1.89%), respectively. The incidences of all-grade and grade 3–5 ILICI were 2.77% (95% CI, 2.05%–3.49%) and 0.67% (95% CI, 0.40%–0.95%), respectively. The treatment-related hepatotoxicity with the highest incidence was an all-grade ALT elevation (10.38%, 95% CI, 8.86%–11.90%) and that with the lowest incidence was a grade 3–5 GGT elevation (0.64%, 95% CI, 0.26%–1.03%) (Figure 2A). The incidence of ILICI was similar, with an all-grade ALT elevation having the highest incidence at 4.73% (95% CI, 2.88%–6.57%) and a grade 3–5 bilirubin elevation having the lowest incidence at 0.05% (95% CI, 0%–0.12%) (Figure 2B). Overall, the incidence of hepatotoxicity in the triple therapy groups (PD-L1 inhibitor+targeted therapy drug (TTD)+chemotherapy and PD-1/PD-L1 inhibitor+CTLA-4 inhibitor+chemotherapy) was 6.28% (95% CI, 5.01%–7.56%). The incidence of hepatotoxicity in the dual-ICI group was 8.47% (95% CI, 5.14%–11.79%; PD-1/PD-L1 inhibitor+CTLA-4 inhibitor). The incidence of hepatotoxicity with single-agent ICIs was 5.77% (95% CI, 4.70%–6.85%). The incidence of hepatotoxicity for ICIs+chemotherapy was 13.62% (95% CI, 10.44%–16.79%), and the incidence of hepatotoxicity for ICIs+TTD was 16.07% (95% CI, 12.07%–20.08%) (Figure 2C). The incidences of AEs among different types of treatment regimens according to the treatment group are shown in Figure 2D–G. Of these, the highest incidence of treatment-related all-grade hepatotoxicity was observed with PD-1 inhibitors+chemotherapy (21.03%, 95% CI, 15.96–26.10). The highest incidence of treatment-related grade 3–5 hepatotoxicity was observed with CTLA-4 inhibitors+chemotherapy (8.18%, 95% CI, 0–17.41). The highest incidence of immune-mediated all-grade and grade 3–5 hepatotoxicity was observed for PD-1 inhibitors+CTLA-4 inhibitors (8.95%, 95% CI, 0%–19.01% and 3.32%, 95% CI, 0%–8.05%, respectively). The incidence of fatal liver AEs was 0.07% (95% CI, 0.03%–0.11%).
FIGURE 2.
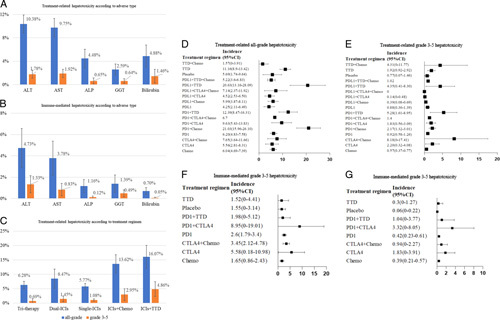
Incidence of liver adverse events. (A) Incidence of treatment-related hepatotoxicity according to the adverse event type. (B) Incidence of immune-mediated hepatotoxicity according to the adverse event type. (C) Incidence of treatment-related hepatotoxicity according to the treatment regimen. (D) Incidence of treatment-related all-grade hepatotoxicity according to the treatment regimen. (E), Incidence of treatment-related grade 3–5 hepatotoxicity according to the treatment regimen. (F) Immune-mediated all-grade hepatotoxicity according to the treatment regimen. (G) Immune-mediated grade 3–5 hepatotoxicity according to the treatment regimen. Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Chemo, chemotherapy; CTLA-4, cytotoxic T-lymphocyte antigen 4 inhibitor; GGT, gamma-glutamyl transferase; ICIs, immune checkpoint inhibitors; PD-1, programmed cell death 1 inhibitor; PD-L1, programmed cell death ligand 1 inhibitor; TTD, targeted therapy drug.
Conventional pairwise meta-analysis
The results of the paired meta-analysis are presented in eFigure 4 in the Supplement (http://links.lww.com/HC9/A156). PD-1 inhibitors+chemotherapy versus chemotherapy showed significant differences in treatment-related ALT, AST, and GGT levels. PD-1 inhibitors+chemotherapy versus chemotherapy showed significant differences in treatment-related ALT, AST, and GGT elevation. CTLA-4 inhibitors+chemotherapy versus chemotherapy had significant differences in treatment-related grade 3–5 ALT, AST, GGT, and immune-mediated ALT and AST. PD-L1 inhibitors+chemotherapy versus chemotherapy had significant differences in the risk of treatment-related ALT, AST, and ALP elevation.
There was no significant difference between PD-1 and CTLA-4 inhibitors in treatment-related all-grade hepatotoxicity; however, CTLA-4 inhibitors had a higher risk of grade 3–5 hepatotoxicity than PD-1 inhibitors. PD-1 inhibitors+CTLA-4 inhibitors versus PD-1 inhibitors significantly increased the risk of hepatotoxicity. PD-1 inhibitors+CTLA-4 inhibitors versus CTLA-4 inhibitors also increased the risk of hepatotoxicity, with significant differences.
Network meta-analysis
The main outcomes for hepatotoxicity are shown in Figure 3. The network of comparisons between treatment-related hepatotoxicity and ILICI is shown in eFigure 5 in the Supplement (http://links.lww.com/HC9/A156). The surface under the cumulative ranking and rankings for treatment and ILICI are shown in eFigure 6 and eTables 3−4 in the Supplement (http://links.lww.com/HC9/A156).
FIGURE 3.
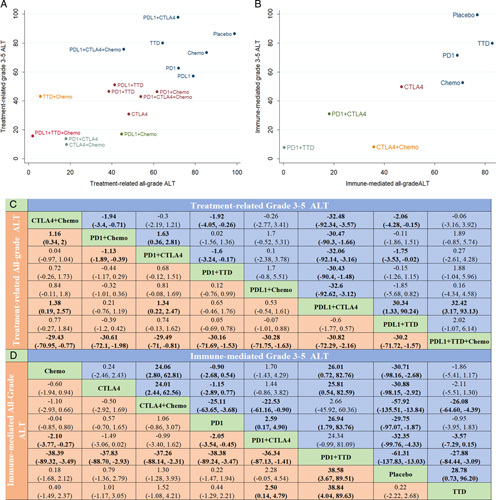
Main network meta-analysis results of hepatotoxicity. (A) Distribution of treatment-related surface under the cumulative ranking (SUCRA) values for ALT elevation. (B) Distribution of immune-mediated SUCRA values for ALT elevation. (C) Network estimates of main treatment comparisons for treatment-related ALT elevation. (D) Network estimates of treatment comparisons for immune-mediated ALT elevation. Abbreviations: ALT, alanine aminotransferase; Chemo, chemotherapy; CTLA-4, cytotoxic T-lymphocyte antigen 4 inhibitor; ICIs, immune checkpoint inhibitors; PD-1, programmed cell death 1 inhibitor; PD-L1, programmed cell death ligand 1 inhibitor; TTD, targeted therapy drug.
In the network meta-analysis, the PD-L1 inhibitor+TTD+chemotherapy group had the highest risk of treatment-related all-grade ALT and AST elevations, and the differences were significant (except for TTD+chemotherapy). However, PD-1 inhibitors+CTLA-4 inhibitors ranked first in the risk of treatment-related grade 3–5 AST, GGT, and Grade 3-5 GGT elevation. CTLA-4 inhibitors+chemotherapy ranked first in terms of risk of all-grade ALP, bilirubin, and grade 3–5 ALT elevation, and for treatment-related all-grade ALP elevation, there were significant differences in each treatment regimen. PD-L1 inhibitors+chemotherapy ranked first in terms of risk of grade 3–5 ALP and grade 3–5 bilirubin elevation, but there was no significant difference between PD-L1 inhibitors+chemotherapy and any other treatment group (p>0.05).
Concerning treatment-related hepatotoxicity, PD-1 inhibitors+chemotherapy showed significant differences in terms of the risk of increased ALT and AST levels compared with PD-1 inhibitors+CTLA-4 inhibitors. There was no significant difference in the incidence of ALT and AST elevation between PD-L1 inhibitors+chemotherapy and PD-L1 inhibitor+CTLA-4 inhibitor groups, and there was no significant difference in the incidence of hepatotoxicity between CTLA-4 inhibitor+chemotherapy and PD-1 inhibitor+CTLA-4 inhibitor groups (except all-grade ALP). There was no significant difference in the incidence of hepatotoxicity between CTLA-4 inhibitors+chemotherapy and PD-L1 inhibitors+CTLA-4 inhibitors (except all-grade ALT and grade 3–5 ALT). PD-1 inhibitors+TTD ranked first in all-grade, grade 3–5 ALT, and all-grade AST for ILICI, with significant differences compared with those in any treatment group (p>0.05). PD-1 inhibitors+CTLA-4 inhibitors ranked first in ALP, GGT, and bilirubin elevation but only showed significant differences when compared with some treatment groups.
Fatal liver AEs
The ranking of fatal liver AEs is shown in eFigure 7 and eTable 5 in the Supplement (http://links.lww.com/HC9/A156). PD-1 inhibitors+CTLA-4 inhibitors+chemotherapy had the highest mortality rate, with an incidence of 0.56%; however, only PD-1 inhibitors+TTD, PD-L1 inhibitors+TTD, placebo, and TTD differed significantly. After PD-L1 inhibitor+CTLA-4 inhibitor+chemotherapy treatment, the incidence was 0.38%; however, it was only significantly different from that of PD-L1 inhibitor+TTD, placebo, and TTD groups.
Hepatotoxicity network meta-analysis according to cancer type
According to tumor type, we performed a subgroup analysis of treatment-related all-grade ALT and grade 3–5 ALT elevations, which we divided into respiratory system cancer, urogenital system cancer, skin cancer, head and neck cancer, and digestive system cancer. The network plot is shown in eFigure 8 in the Supplement (http://links.lww.com/HC9/A156). Surface under the cumulative ranking values are shown in Figure 4 and eTable 6 in the Supplement (http://links.lww.com/HC9/A156). The highest risk of treatment-related ALT elevation in the urogenital system was observed with CTLA-4 inhibitors, but this was only significantly different from that of chemotherapy in terms of treatment-related all-grade ALT. The highest risk of treatment-related ALT elevation in skin cancer was observed with CTLA-4 inhibitors+chemotherapy, but this was only significantly different from that with chemotherapy in terms of treatment-related all-grade and grade 3–5 ALT. The highest risk of treatment-related all-grade ALT and grade 3–5 ALT elevation in head and neck cancer and digestive system cancer was observed with TTD+chemotherapy, and there were significant differences with that in other treatment groups in terms of treatment-related all-grade ALT elevation, except for PD-L1 inhibitors+chemotherapy. The highest risk of treatment-related ALT elevation in the respiratory system was observed with PD-1 inhibitors+CTLA-4 inhibitors, which was significantly different from that in chemotherapy, CTLA-4 inhibitor, CTLA-4 inhibitor+chemotherapy, PD-1 inhibitor, and PD-1 inhibitor+chemotherapy groups for treatment-related all-grade ALT.
FIGURE 4.
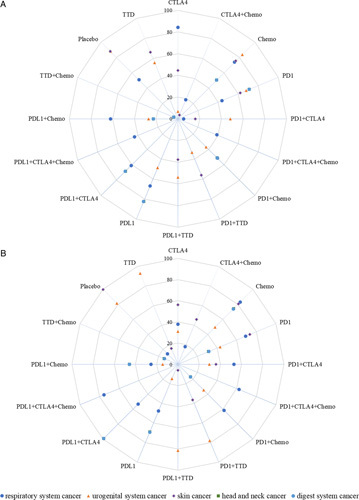
Distribution of treatment-related ALT surface under the cumulative ranking values stratified by cancer type. (A) All-grade ALT. (B) Grade 3–5 ALT. Abbreviations: ALT, alanine aminotransferase; Chemo, chemotherapy; CTLA-4, cytotoxic T-lymphocyte antigen 4 inhibitor; PD-1, programmed cell death 1 inhibitor; PD-L1, programmed cell death ligand 1 inhibitor; TTD, targeted therapy.
Dose-dependent ICI-induced hepatotoxicity
We next performed a subgroup analysis according to ICI dose (eFigure 9 in the Supplement, http://links.lww.com/HC9/A156). We extracted data only for nivolumab compared with chemotherapy. The results showed that there was no significant difference in hepatotoxicity between the 3 mg/kg (q2w) and 240 mg (q2w) regimens [3 mg/kg (q2w): OR: 1.27, 95% CI, 0.85–1.92, I 2=0%, p=0.25; 240 mg (q2w): OR: 1.18 95% CI, 0.41–3.44, I 2=0%, p=0.76]. The control group comprising atezolizumab was the conventional treatment group (treatment without atezolizumab), and the results showed that a 1200 mg (q3w) treatment regimen increased the risk of hepatotoxicity compared with conventional treatment (OR: 1.57, 95% CI, 1.16–2.13, I 2=17%; p=0.004); however, that with the 840 mg q2w regimen did not differ significantly when compared with that in the control group (OR: 1.18, 95% CI, 0.70–1.97; p=0.65). There was no significant difference in hepatotoxicity between durvalumab 10 mg/kg (q2w) and 20 mg/kg (q4w) regimens [10 mg/kg (q2w): OR: 0.49, 95% CI, 0.19–1.24, p=0.13; 20 mg (q4w): OR: 0.68, 95% CI, 0.30–1.57, p=0.37]. High-dose ipilimumab [10 mg/kg (q3w)] significantly increased liver toxicity, but low-dose ipilimumab showed no significant difference had a significantly increased risk of hepatotoxicity, whereas the other regimens showed no significant difference [10 mg/kg (q3w): OR: 3.48, 95% CI, 1.54–7.84, I 2=54%, p=0.003; 3 mg/kg (q3w): OR: 2.28, 95% CI, 0.65–7.93, I 2=84%, p=0.20; and 1 mg/kg (q6w): OR: 1.27, 95% CI, 0.87–3.38, p=0.12]. Pembrolizumab [2 mg/kg (q3w)] resulted in a significantly increased risk of hepatotoxicity, whereas the other regimens showed no significant difference [10 mg/kg (q2w): OR: 1.24, 95% CI, 0.51–2.99, p=0.63; 10 mg/kg (q3w): OR: 0.40, 95% CI, 0.12–1.32, p=0.13; 200 mg (q3w): OR: 1.15, 95% CI, 0.86–1.54, I 2=66%, p=0.34; and 2 mg/kg (q3w): OR: 3.04, 95% CI, 1.73–5.36, p=0.0001].
Transitivity, consistency, and convergence analysis
An assessment of transitivity for hepatotoxicity indicated that the sample size, median age, and sex ratio across treatment comparisons and tumor types were relatively similar, and thus, no threats to the transitivity assumption were identified (Figure 5). The consistency results are presented in eTable 7 in the Supplement (http://links.lww.com/HC9/A156), using node split analysis to assess whether direct and indirect evidence was consistent. There were no significant differences between the different treatments. This indicated that the consistency model was acceptable. According to the funnel plot, there was no significant publication bias among the included studies. In addition, the potential scale reduction factor was limited to 1, indicating a good degree of model convergence.
FIGURE 5.
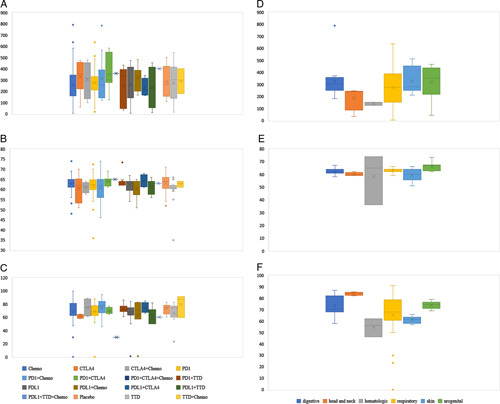
Assessment of transitivity among all included trials. (A) Number of patients by treatment comparison. (B) Age by treatment comparison. (C) Male ratio by treatment comparison. (D) Number by tumor types. (E) Age by tumor type. (F) Male ratio by tumor type. Abbreviations: Chemo, chemotherapy; CTLA-4, cytotoxic T-lymphocyte antigen 4 inhibitor; PD-1, programmed cell death 1 inhibitor; PD-L1, programmed cell death ligand 1 inhibitor; TTD, targeted therapy.
DISCUSSION
This systematic review and network meta-analysis of hepatotoxicity in adults receiving ICI therapy included data from 106 clinical trials (n=64,782 patients with tumors who were randomized to 17 different treatment regimens, Supplement eTable 8, http://links.lww.com/HC9/A156). This study is the first to comprehensively investigate adverse liver events in patients receiving ICI therapy; these AEs manifest as abnormal liver function indicators, such as the abnormal elevation of ALT, AST, ALP, GGT, and bilirubin levels, and are divided into all-grade, grades 3–5, and lethal hepatotoxicity (grade 5). At the same time, we also explored the effect of different treatment regimens and different doses of ICIs on the risk of hepatic AEs in patients with tumors of various systems.
It is worth mentioning that we divided adverse liver events into treatment-related hepatotoxicity and immune-related hepatotoxicity, as TRAEs can warn clinicians about the risk of hepatotoxicity associated with various treatment options to better identify and prevent the occurrence of hepatotoxicity in advance. The mechanism of immune-related hepatotoxicity is different from that of other hepatotoxicities. It has to be isolated for analysis, and steroid therapy needs to be started early to alleviate the progression of toxicity. Furthermore, clinicians can distinguish between TRAEs and ILICI by whether initiating steroid therapy is effective or not.
Key findings and comparison with other studies
In ICI therapy, extensive activation of T cells, coupled with the depletion of regulatory T cells, leads to attacks on various organ systems and a series of AEs known as irAEs.19 Of the hepatotoxicities of ICIs, a substantial proportion of patients present with a cholestatic injury (60%), whereas 30% have a hepatocellular injury and 10% have a mixed injury.20 Previous meta-analyses have investigated the risk of hepatotoxicity associated with ICIs, but these studies have focused on single cancers or single ICIs.21–24 Furthermore, these studies did not explicitly examine the risk of hepatotoxicity with different ICI regimens, which might vary according to the cancer type or dose.
Studies have shown that patients treated with PD-1/PD-L1 inhibitors alone have a higher risk of increased hepatotoxicity incidence than those treated with chemotherapy.25–28 Similar to that in our study, all other regimens showed a significantly higher risk of treatment-related hepatic AEs than the chemotherapy arm (except placebo). Among treatment-related liver AEs, PD-L1 inhibitors+TTD+chemotherapy significantly increased the risk of all-grade ALT and AST elevations compared with other treatment regimens. The incidence of treatment-related grade 3–5 ALT elevation for PD-L1 inhibitors+CTLA-4 inhibitors was significantly higher than that in the other treatment groups. The risk of treatment-related grade 3–5 ALT elevation in the PD-L1 inhibitor+CTLA-4 inhibitor group was significantly higher than that in the other treatment groups. Concerning ILICI, the risk of ALT and AST elevation with PD-1 inhibitors+TTD was significantly higher than that in the other treatment groups (including the dual-ICI regimen). One reason for this might be the different mechanisms of ICIs, chemotherapy, and targeted therapy. Conventional chemotherapeutics and TTD induce hepatotoxicity by damaging the hepatocytes.29 Another reason could be the synergistic effects of ICI therapy and chemotherapy. Chemotherapy has been reported to enhance PD-L1 expression, thereby enhancing the antitumor activity of ICIs in combination with immunotherapy and chemotherapy.30,31
In addition, studies on lung cancer and other solid tumors have shown that the combined use of ICIs has a higher risk of toxicity than monotherapy.32,33 Chang et al34 showed that in melanoma immunotherapy, nivolumab (1 mg/kg, q3w) combined with ipilimumab (3 mg, q3w) has the highest risk of serious irAEs compared with ipilimumab, pembrolizumab, or nivolumab alone. Facchinetti and colleagues found that irAEs that occurred when PD-1/PD-L1 ICIs were added to chemotherapy had a smaller effect on overall toxicity and were only slightly increased in the experimental group (OR: 2.89, 95% CI, 1.13–7.38; p=0.03). Furthermore, there was no significant difference in grade 3–4 AEs (OR: 1.08, 95% CI, 0.86–1.36; p=0.51). Serious irAEs were observed in only a minority of patients.35 In addition, PD-1 inhibitors combined with CTLA-4 inhibitors triggered more irAEs than PD-1 alone (55%–60% vs. 10%–20% high-grade events).36,37 This is similar to the results of our study; however, in our meta-analysis, we included several large studies discussing liver AEs involving a variety of different tumor types. We found a higher risk of treatment-related all-grade ALT elevation with combination therapy (PD-1 inhibitors+chemotherapy, PD-1 inhibitors+CTLA-4, PD-1 inhibitors+TTD) compared with PD-1 inhibitors. Compared with PD-L1 inhibitors, combination therapy (PD-L1 inhibitors+chemotherapy, PD-L1 inhibitors+CTLA-4 inhibitors+chemotherapy, PD-L1 inhibitors+TTD, PD-L1 inhibitors+TTD+chemotherapy) had a higher risk of treatment-related all-grade ALT elevation. A possible explanation is that although both anti-CLTA-4 and anti-PD-1 drugs restore antitumor immunity, they do so in different ways. CTLA-4 inhibitors have an early regulatory role in immune responses in lymphoid organs. In contrast, PD-1 inhibitors play a late regulatory role in T-cell activation in peripheral tissues. When drugs with different mechanisms are combined, the incidence of common AEs increases.38
Similarly, among immune-mediated hepatic AEs, PD-1 combination therapy (PD-1 inhibitors+CTLA-4 inhibitors, PD-1 inhibitors+TTD) significantly increased the risk of elevated ALT, AST, GGT, and bilirubin levels compared with PD-1 inhibitors. However, there was no significant difference in the risk of grade 3–5 AEs. This result suggests that although the ILICI caused by the combination therapy increases, the possibility of rechallenge is also greater. These toxic effects remain a major challenge in clinical care and a barrier to the development of more aggressive combinations. Our study provides more details on the toxicity of ICIs for clinicians choosing treatment options. Clinicians need to obtain an accurate medical history, assess liver status before administering combined immunotherapy, and then conduct appropriate follow-up tests of liver function. It is noteworthy that our meta-analysis found that the hepatotoxicity of dual-ICI therapy (PD-1 inhibitors+CTLA-4 inhibitors and PD-L1 inhibitors+CTLA-4 inhibitors) was not greater than that of ICIs combined with TTD therapy or chemotherapy. Patients who do not respond to monotherapy or have poor liver function can thus be prioritized.
Fatal liver AEs
In the 64,782 treated patients, 40 liver-related fatal events were reported. The results of our study showed that PD-1 inhibitors+TTD+chemotherapy had the highest risk of fatal liver AEs, but there was no significant difference compared with the other regimens. The incidence of fatal hepatic AEs was significantly higher in the CTLA-4 inhibitor+chemotherapy, PD-1 inhibitor+chemotherapy, PD-1 inhibitor+CTLA-4 inhibitor, and PD-1 inhibitor+CTLA-4 inhibitor+chemotherapy groups than in the placebo group. The most common fatal AE associated with PD-1 inhibitors is pulmonary toxicity, and ipilimumab has significant dose-dependent lethal toxicity.39 This suggests that the lethal risk of immune-related hepatotoxicity might be dose related.
Subgroups according to cancer type
Previous studies have shown that the incidence of irAEs varies among patients with different solid tumors.40 Similarly, our results showed that compared with patients with other tumors, the highest incidence of ALT elevation was associated with skin cancer (10.39%), head and neck cancer (9.90%), respiratory system cancer (9.56%), and urogenital system cancer (8.20%). To date, the mechanism underlying this result has not been well elucidated. However, some studies have reported high PD-1 expression in melanoma (skin cancer).41 When PD-1/PD-L1 inhibitors block the binding of these receptors to their ligands, the inhibitory signal is strongly suppressed, and the host antitumor response is more likely to be effectively enhanced.42 Simultaneously, normal liver tissue cells are attacked more, leading to an increased risk of hepatotoxicity in cancer patients.23 Therefore, our findings suggest that the incidence of immune-mediated liver dysfunction is related to the tumor type and provide a basis for clinicians to choose appropriate treatment options for patients with advanced tumors.
Dose-dependent analysis
Previous studies have shown that clinical factors, such as dose, weight, and exposure, can be used to predict an increased risk of ILICI.28,43 Previous studies have shown that the incidence of hepatotoxicity in patients receiving high-dose ipilimumab (16%) is higher than that in patients receiving low-dose ipilimumab (4.5%).44 Zhao et al45 comprehensively reviewed the safety of AEs across body weight groups and predicted exposure quartiles; they showed that neither body weight nor exposure was associated with AEs. Similar to the results of our study, there was no significant difference in the risk of ALT elevation between nivolumab at 3 mg/kg (q2w) and 240 mg (q2w). Ipilimumab at 10 mg/kg (q3w) has a greater risk of hepatotoxicity than other regimens [ipilimumab 3 mg/kg (q3w); ipilimumab 1 mg/kg (q6w)]. Therefore, low-dose ICI monotherapy is initially recommended to avoid irAEs in patients with preexisting autoimmune disease, those undergoing radiotherapy, and those who are active smokers.
Limitations
To the best of our knowledge, this is the largest and most comprehensive study to compare the risk of hepatic AEs with ICI-based therapy. Overall, relatively little heterogeneity was observed in this meta-analysis. In addition, we provide data concerning the incidence and risk of hepatotoxicity after administering ICIs combined with conventional cancer therapy. As the diagnosis of elevated ALT and AST levels is based on liver function tests, no subjective factors are likely to have affected the results.
However, our meta-analysis had some limitations, mainly because our results covered treatment-related AEs, including those caused by chemotherapy, TTD, and ICIs. This may confuse clinicians between TRAEs and ILICI. Despite this, clinicians should be vigilant of all types of AEs caused by ICIs. Second, we analyzed different ICI categories rather than individual ICIs and specific doses, which might have led to differences in the study results. Similarly, different chemotherapeutic or targeted agents with different rates of hepatic AEs were defined as one class, which might be a source of heterogeneity. Nonetheless, it is not feasible to differentiate treatment regimens based on individual drugs and specific doses because of the limited number of samples.
CONCLUSIONS
The findings of this network meta-analysis showed that among patients with cancer, ICI-based therapy is associated with a higher risk of hepatotoxicity than chemotherapy. ICI combination therapy was associated with a higher risk of hepatotoxicity than ICI monotherapy. The toxicity of dual ICIs was not higher than that of ICIs combined with chemotherapy and TTD. These findings might have important implications for the individualized treatment of patients with cancer. In conclusion, enhanced surveillance is essential for early identification and intervention.
Supplementary Material
FUNDING INFORMATION
The Startup Fund for Scientific Research, Fujian Medical University (Grant number: 2020QH1346), the Startup Fund for Scientific Research, Fujian Medical University (Grant number: 2020QH1345), Fujian Undergraduate Education and teaching reform research Major project (FBJG20210119), Fujian Provincial health technology project (2021QNA018), and Natural Science Foundation of Fujian Province (2021J011304).
CONFLICT OF INTEREST
Nothing to report.
Footnotes
Abbreviations: AEs, adverse events; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTLA-4, cytotoxic T-lymphocyte–associated antigen 4; GGT, gamma-glutamyl transferase; ICI(s), immune checkpoint inhibitor(s); ILICI, immune-mediated liver injury caused by checkpoint inhibitors; irAEs, immune-related adverse events; PD-1, programmed death 1; PD-L1, programmed death ligand 1; SUCRA, surface under the cumulative ranking; TTD, targeted therapy drug.
Caiyun Zheng and Shunmin Huang contributed equally.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.hepcommjournal.com.
Contributor Information
Caiyun Zheng, Email: saiun1992@163.com.
Shunmin Huang, Email: 930481735@qq.com.
Meimei Lin, Email: lmm13645015310@163.com.
Baohui Hong, Email: 546877606@qq.com.
Ruping Ni, Email: 810838238@qq.com.
Hengfen Dai, Email: henfengdai2011@163.com.
Xiuqin Lin, Email: 453250204@qq.com.
Jing Yang, Email: wlz0965@fjmu.edu.cn.
REFERENCES
- 1.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11:805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long GV, Atkinson V, Ascierto PA, Robert C, Hassel JC, Rutkowski P, et al. Effect of nivolumab on health-related quality of life in patients with treatment-naïve advanced melanoma: results from the phase III CheckMate 066 study. Ann Oncol. 2016;27:1940–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reck M, Taylor F, Penrod JR, DeRosa M, Morrissey L, Dastani H, et al. Impact of Nivolumab versus Docetaxel on health-related quality of life and symptoms in patients with advanced squamous non-small cell lung cancer: results from the CheckMate 017 study. J Thorac Oncol. 2018;13:194–204. [DOI] [PubMed] [Google Scholar]
- 5.Toxicities associated with checkpoint inhibitor immunotherapy[EB/OL]. 2017. Accessed January 13, 2023. http://www.uptodate.com/contents/toxicities-associated-with-checkpoint-inhibitor-immunotherapy [Google Scholar]
- 6.Callahan MK, Yang A, Tandon S, Xu Y, Subudhi SK, Roman RA, et al. Evaluation of serum IL-17 levels during ipilimumab therapy: correlation with colitis. J Clin Oncol. 2011;29:2505–205. [Google Scholar]
- 7.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–68. [DOI] [PubMed] [Google Scholar]
- 8.Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jim HSL, Knoop H, Dicker AP. Immune checkpoint inhibitor therapy toxicities. JAMA. 2021;326:87. [DOI] [PubMed] [Google Scholar]
- 10.Losser MR, Payen D. Mechanisms of liver damage. Semin Liver Dis. 1996;16:357–67. [DOI] [PubMed] [Google Scholar]
- 11.Naing A. Being realistic and optimistic in curing cancer. J Immunother Precis Oncol. 2020;1:53–55. [Google Scholar]
- 12.Paracha N, Felizzi F. PCN194—Rate of hospitalization due to adverse event and length of stay for Atezolizumab in second and third line metastatic non-small lung cancer (NSCLC) using phase 3 Oak study. Value Health. 2017;20:A447. [Google Scholar]
- 13.Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5:1008–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gelman A, Rubin DB. Markov chain Monte Carlo methods in biostatistics. Stat Methods Med Res. 1996;5:339–55. [DOI] [PubMed] [Google Scholar]
- 15.Hong H, Wang C, Rosner GL. Meta-analysis of rare adverse events in randomized clinical trials: Bayesian and frequentist methods. Clin Trials. 2021;18:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7:434–55. [Google Scholar]
- 17.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–71. [DOI] [PubMed] [Google Scholar]
- 18.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–92. [DOI] [PubMed] [Google Scholar]
- 19.Yang W, Men P, Xue H, Jiang M, Luo Q. Risk of gastrointestinal adverse events in cancer patients treated with immune checkpoint inhibitor plus chemotherapy: a systematic review and meta-analysis. Front Oncol. 2020;10:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parlati L, Vallet-Pichard A, Batista R, Hernvann A, Sogni P, Pol S, et al. Incidence of grade 3-4 liver injury under immune checkpoints inhibitors: a retrospective study. J Hepatol. 2018;69:1396–7. [DOI] [PubMed] [Google Scholar]
- 21.Fu J, Li WZ, McGrath NA, Lai CW, Brar G, Xiang YQ, et al. Immune checkpoint inhibitor associated hepatotoxicity in primary liver cancer versus other cancers: a systematic review and meta-analysis. Front Oncol. 2021;11:650292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin LL, Lin GF, Yang F, Chen XQ. A systematic review and meta-analysis of immune-mediated liver dysfunction in non-small cell lung cancer. Int Immunopharmacol. 2020;83:106537. [DOI] [PubMed] [Google Scholar]
- 23.Deng S, Yang Q, Shu X, Lang J, Lu S. The relative risk of immune-related liver dysfunction of PD-1/PD-L1 inhibitors versus chemotherapy in solid tumors: a meta-analysis of randomized controlled trials. Front Pharmacol. 2019;10:1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balibegloo M, Nejadghaderi SA, Sadeghalvad M, Soleymanitabar A, Salehi Nezamabadi S, Saghazadeh A, et al. Adverse events associated with immune checkpoint inhibitors in patients with breast cancer: a systematic review and meta-analysis. Int Immunopharmacol. 2021;96:107796. [DOI] [PubMed] [Google Scholar]
- 25.O’Kane GM, Labbé C, Doherty MK, Young K, Albaba H, Leighl NB. Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist. 2017;22:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma K, Lu Y, Jiang S, Tang J, Li X, Zhang Y. The relative risk and incidence of immune checkpoint inhibitors related pneumonitis in patients with advanced cancer: a meta-analysis. Front Pharmacol. 2018;9:1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su Q, Zhang X, Shen X, Hou Y, Sun Z, Gao ZH. Risk of immune-related colitis with PD-1/PD-L1 inhibitors vs chemotherapy in solid tumors: systems assessment. J Cancer. 2018;9:1614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W, Lie P, Guo M, He J. Risk of hepatotoxicity in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis of published data. Int J Cancer. 2017;141:1018–28. [DOI] [PubMed] [Google Scholar]
- 29.Rosner MH, Perazella MA. Acute kidney injury in patients with cancer. N Engl J Med. 2017;376:1770–81. [DOI] [PubMed] [Google Scholar]
- 30.Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. Management of immunotherapy-related toxicities, version 1.2019. J Natl Compr Canc Netw. 2019;17:255–89. [DOI] [PubMed] [Google Scholar]
- 31.Peng J, Hamanishi J, Matsumura N, Abiko K, Murat K, Baba T, et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-κB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015;75:5034–45. [DOI] [PubMed] [Google Scholar]
- 32.Petrelli F, Grizzi G, Ghidini M, Ghidini A, Ratti M, Panni S, et al. Immune-related adverse events and survival in solid tumors treated with immune checkpoint inhibitors: a systematic review and meta-analysis. J Immunother. 2020;43:1–7. [DOI] [PubMed] [Google Scholar]
- 33.Su Q, Zhu EC, Wu JB, Li T, Hou YL, Wang DY, et al. Risk of pneumonitis and pneumonia associated with immune checkpoint inhibitors for solid tumors: a systematic review and meta-analysis. Front Immunol. 2019;10:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang CY, Park H, Malone DC, Wang CY, Wilson DL, Yeh YM, et al. Immune checkpoint inhibitors and immune-related adverse events in patients with advanced melanoma: a systematic review and network meta-analysis. JAMA Netw Open. 2020;3:e201611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Facchinetti F, Di Maio M, Tiseo M. Adding PD-1/PD-L1 inhibitors to chemotherapy for the first-line treatment of extensive stage small cell lung cancer (SCLC): a meta-analysis of randomized trials. Cancers (Basel). 2020;12:2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shoushtari AN, Friedman CF, Navid-Azarbaijani P, Postow MA, Callahan MK, Momtaz P, et al. Measuring toxic effects and time to treatment failure for Nivolumab plus Ipilimumab in melanoma. JAMA Oncol. 2018;4:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–82. [DOI] [PubMed] [Google Scholar]
- 39.Jiang Y, Zhang N, Pang H, Gao X, Zhang H. Risk and incidence of fatal adverse events associated with immune checkpoint inhibitors: a systematic review and meta-analysis. Ther Clin Risk Manag. 2019;15:293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang PF, Chen Y, Song SY, Wang TJ, Ji WJ, Li SW, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol. 2017;8:730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleffel S, Posch C, Barthel SR, Mueller H, Schlapbach C, Guenova E, et al. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell. 2015;162:1242–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson DB, Sullivan RJ, Ott PA, Carlino MS, Khushalani NI, Ye F, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol. 2016;2:234–40. [DOI] [PubMed] [Google Scholar]
- 44.Peeraphatdit TB, Wang J, Odenwald MA, Hu S, Hart J, Charlton MR. Hepatotoxicity from immune checkpoint inhibitors: a systematic review and management recommendation. Hepatology. 2020;72:315–29. [DOI] [PubMed] [Google Scholar]
- 45.Zhao X, Suryawanshi S, Hruska M, Feng Y, Wang X, Shen J, et al. Assessment of nivolumab benefit-risk profile of a 240-mg flat dose relative to a 3-mg/kg dosing regimen in patients with advanced tumors. Ann Oncol. 2017;28:2002–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


