Background:
Osteoarthritis (OA) affects the entire joint structure. The most injured joints are the hands, knees, and hips. OA is a common disease all over the world, and a cause of disability in the elderly; hence, medicine is facing a steady challenge to find effective therapeutics to relieve the pain, improving symptoms for a better quality of life for patients.
Purpose:
To compare the results, in the recent literature, of intra-articular injection of platelet-rich plasma (PRP) and corticosteroids (CSs) in osteoarthritic knees at early and mid-term postinjection.
Methods:
A PubMed and CENTRAL (Cochrane Central Register of Controlled Trials) database search was performed. Initial screening yielded 108 randomized controlled trials, 17 results, and 17 others were added after updates. The final review includes nine randomized control trials, with outcome evaluating of knee OA by Western Ontario and McMaster Universities Arthritis Osteoarthritis Index, Knee Injury and Osteoarthritis Outcome Scale Index, and Visual Analog Scale.
Results:
PRP and CS intra-articular injections both are safe and effective treatments in knee OA for alleviating pain, and improving symptoms. It seems that PRP injections have prolonged and shown better improvement in some studies. However, the results do not prefer one method over the other.
Conclusion:
Up till now, it is not easy to draw firm conclusions about prioritizing PRP or CS injections for knee OA treatment due to the limitation of this review.
Keywords: corticostroids, intra-articular injections, knee osteoarthritis, PRP
HIGHLIGHTS
Knee osteoarthritis (OA) is a prevalent degenerative disease.
The prevalence of OA is increasing due to the rising prevalence of obesity and aging.
Medicine faces a steady challenge to find effective nonsurgical treatments to alleviate symptoms.
One of the nonsurgical treatments is intra-articular injections.
Many agents can be injected intra-articularly as a treatment of OA. The most traditional one is corticosteroid injection. One of the newly invented substances is platelet-rich plasma, which became popular.
Background
Osteoarthritis (OA) is a prevalent degenerative disease. It is the outcome of articular cartilage damage, subchondral bone restructured, and chronic synovitis in favor of catabolism and the release of inflammatory mediators in the extracellular matrix1. It may affect any joint in the body, especially the knee. The prevalence of OA is increasing due to the rising prevalence of obesity and aging. The medical community faces a steady challenge to find effective nonsurgical treatments to alleviate symptoms: persistent pain, joint stiffness, and limited joint mobility to improve the quality of life2.
Nonsurgical treatment involves intra-articular (IA) injections, which are viable when oral therapy is intolerant or ineffective or when attempting to delay or avoid surgery.
Many agents can be injected intra-articularly as a treatment of OA. The most traditionally accepted one is corticosteroid (CS) injection. A popular and new substance is platelet-rich plasma (PRP)3.
CSs mechanism of action
CSs have a complex anti-inflammatory and immunosuppressant effect. They disrupt the immune and inflammatory cascade at several levels and directly affect the nuclear steroid receptors. CSs reduce phagocytosis microvascular permeability while reducing the release of superoxide by neutrophils and the clustering of inflammatory cells. CSs also inhibit the synthesis and secretion of inflammatory mediators such as prostaglandins and leukotrienes4,5. This reduced redness, swelling, local heat, and tenderness of inflamed joints. Relative viscosity increases as a result of the increase in hyaluronic acid (HA) concentration in joints 4,6. CS injections are used to treat acute and chronic inflammation and are recommended for short-term management of acute attacks of OA7–10.
PRP mechanism of action
Alternatively, by injecting PRP, platelets are activated, leading to the release of fibrinogen, cytokines, growth factors, α-granules, interleukin-1 receptor antagonist, platelet-derived growth factors, tissue growth factors, and vascular endothelial growth factors, thus, diminish chondrocytes apoptosis and matrix loss11,12, revoke inflammatory mediators and enzymes, and stimulate chondrocytes proliferation, angiogenesis, cartilage molding, and mesenchymal stem cells propagation12–14.
Some authors propose PRP as a first choice for IA injections; nevertheless, its use is still considered controversial and orthopedic surgeons do not concur14.
Methods
This review compares the efficacy of PRP with CS injections in treating knee OA during the 3rd-month and 6th-month postinjection.
Search strategy
Both authors contributed to every process independently. The supervisor served as a reviewer for data extraction. This review was prepared by Preferred Reporting Items for Systemic Reviews and Meta-Analysis (PRISMA) statement15, Supplemental Digital Content 1, http://links.lww.com/MS9/A24 and Assessing the Methodological Quality of Systematic Reviews (AMSTAR) 2 methodological quality appraisal checklist16, Supplemental Digital Content 2, http://links.lww.com/MS9/A25.
A PubMed and Cochrane Central Register of Controlled Trials (CENTRAL) database search of studies published in English was performed in April 2021 and was updated in February and September 2022 using the keywords shown in Appendix 1, Supplemental Digital Content 3, http://links.lww.com/MS9/A30.
The results were exported with Endnote X8, with screening conducted on an Excel spreadsheet with two stages: titles and abstracts screening and full-text screening. Duplicate and non-English publications were neglected with randomized control trials (RCTs) included according to inclusion criteria:
Population: patients with knee OA;
Procedure: PRP IA injections;
Comparator: CS IA injections;
Outcomes: clinical efficacy and adverse effects;
The minimum follow-up period: 6 months.
Research that did not meet the inclusion criteria was omitted. Initial screening yielded 108 RCTs; 17 results and 17 others were added after updates. The final review includes nine RCTs. The database search process is summarized in Figure 1. Risk of Bias was assessed according to the Cochrane Risk of Bias tool for RCTs. Deviations were assessed as ratings (high, low, or unclear) of individual items from five domains (selection, performance, attrition, reporting, and others). A summary of the Risk of Bias included studies is shown Figure 2.
Figure 1.
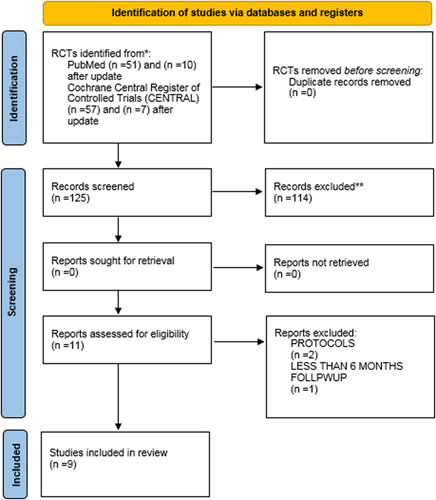
Preferred Reporting Items for Systemic Reviews and Meta-Analysis (PRISMA) 2020. RCT, randomized control trials.
Figure 2.
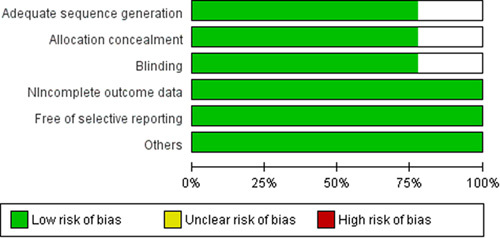
Risk of Bias in included studies.
Results
A total of 608 patients were included in nine RCTs and treated with PRP or CS injections, with a minimum 6 months follow-up. The grade of their lesions was classified according to Kellgren–Lawrence system.
Six17–22 out of nine studies used blinding approaches, single or double. Three studies19,22,23 used the intention-to-treat principle to analyze lost patients' results.
Characteristics of studies and patients were summarized in Table 1, including the number of randomized patients, number of analyzed patients, gender, BMI, age, grade of the osteoarthritic lesion, and follow-up period. Studies techniques were shown in Table 2, including injected agent, frequency of injections, and the use of ultrasound guidance of injection; studies designs and methods of assessments are shown in Tables 3 and 4, respectively.
Table 1.
Studies characteristics
| Elksniņš-Finogejevs et al.23 | Forogh et al.17 | Uslu Güvendi et al.18 | Huang et al.24 | Jubert et al.19 | ||
|---|---|---|---|---|---|---|
| Follow-up period (months) | 12 | 6 | 6 | 12 | 6 | |
| Randomized participants, N | ||||||
| PRP | 20 | 24 | Single: 19 | 40 | 35 | |
| Three: 14 | ||||||
| CS | 20 | 24 | 17 | 40 | 30 | |
| Analyzed, N | ||||||
| PRP | 19 | 23 | Single: 19 | 40 | 34 | |
| Three: 14 | ||||||
| CS | 17 | 16 | 17 | 40 | 30 | |
| Gender, M : F, n | ||||||
| PRP | 17 : 03 | 07 : 17 | Single: 1 : 18 | 21 : 19 | 12 : 23 | |
| Three: 1 : 13 | ||||||
| CS | 15 : 05 | 09 : 15 | 02 : 15 | 25:15:00 | 06 : 24 | |
| Age, years, mean/SD or N (%) | ||||||
| PRP | 66.4±8.4 | 59.13±7.03 | Single: 62.3±1.6 | 54.5±1.2 | 65.56±8.6 | |
| Three: 60.4±1.7 | ||||||
| CS | 70.2±9.2 | 61.13±6.7 | 62.8±1.7 | 54.3±1.4 | 68±7.17 | |
| BMI, mean/SD or N (%) | ||||||
| PRP | 28.6±5.0 | 28.9±2.8 | Single: 31.4±0.7 | 25.23±4.15 | 31.20±4.36 | |
| three: 31.0±1.0 | ||||||
| CS | 30.5±5.8 | 29.2±3.4 | 31.0±1.0 | 24.56±3.62 | 30.98±4.16 | |
| K–L degree, n | ||||||
| PRP | II: 5 | II: 7 | III | I/II | III: 10 | |
| III: 15 | III: 17 | IV: 25 | ||||
| CS | II: 6 | II: 8 | III | I/II | III: 17 | |
| III: 14 | III: 16 | IV: 13 | ||||
| Naderi Nabi et al.20 | Freire et al.21 | Khan et al.25 | Nunes-Tamashiro et al.22 | |||
| Follow-up period (months) | 6 | 6 | 6 | 12 | ||
| Randomized participants, N | ||||||
| PRP | 36 | 25 | 52 | 34 | ||
| CS | 36 | 25 | 51 | 33 | ||
| Analyzed, N | ||||||
| PRP | 33 | 25 | 52 | 34 | ||
| CS | 34 | 25 | 51 | 33 | ||
| Gender, M : F, n | ||||||
| PRP | 05 : 28 | 42 were females | 13 : 38 | 30 : 4 | ||
| CS | 07 : 27 | 12 : 39 | 30 : 3 | |||
| Age, years, mean/SD or N (%) | ||||||
| PRP | 59.09±7.79 | 64.15±8.02 | 50.912±13.07 | 67.6 (7.4) | ||
| CS | 58.55±8.79 | 60.21±5.92 | 52.089±12.1 | 65.8 (6.1) | ||
| BMI, mean/SD or N (%) | ||||||
| PRP | 28.4±2.78 | 19 patients were obese, 6 were not | 28±4 | 29.22 (3.2) | ||
| CS | 27.78±3.29 | 22 patients were obese, 3 were not | 26±5 | 29.59 (4.5) | ||
| K–L degree, n | ||||||
| PRP | I: 9 | I: 0 | II: 10 | II | II: 14 | |
| II: 24 | III: 11 | IV: 4 | III: 20 | |||
| CS | I: 11 | I: 1 | II: 10 | II | II: 16 | |
| II: 23 | III: 14 | IV: 0 | III: 17 | |||
CS, corticosteroid; K–L, Kellgren–Lawrence; PRP, platelet-rich plasma.
Table 2.
The technique used
| Elksniņš-Finogejevs et al.23 | Forogh et al.17 | Uslu Güvendi et al.18 | Huang et al.24 | Jubert et al.19 | |
|---|---|---|---|---|---|
| Frequency of injections | Single | Single | Single PRP: one PRP injection; three PRP: three PRP injections with 1-week interval | PRP group: (3 times, 4 ml, every 3 weeks); CS group: single | Single |
| Use of ultrasound guidance for verification of injection | Yes | No | No | No | No |
| Agent injected | |||||
| PRP | 8 ml of PRP | 5 ml of PRP was activated by adding 0.5 ml of a calcium gluconate solution (1 g/10 ml); PRP platelet count was 1501×10³ | Venous blood (18 ml) + 2 ml citrate dextrose was separated as plasma | IA-PRP (3 times, 4 ml, every 3 weeks) | 4 ml autologous PRP, a median value of 0.99×106 platelets/ml |
| CS | Triamcinolone acetonide (Kenalog) (1 ml of 40 mg/ml) plus lidocaine (5 ml of 2%) in a single syringe | 1 ml of Depo-Medrol (containing 40 mg of methylprednisolone acetate) | 1 ml suspension containing 6.43 mg of betamethasone dipropionate (equivalent to 5.0 mg of betamethasone) and 2.63 mg of betamethasone sodium phosphate (equivalent to 2.0 mg betamethasone). | IA-CS (1 ml) | 2 ml betamethasone: 6 mg betamethasone sodium phosphate and betamethasone acetate 6 mg (Merck) and 2 ml bupivacaine 0.25% (B. Braun) |
| Naderi Nabi et al.20 | Freire et al.21 | Khan et al.25 | Nunes-Tamashiro et al.22 | ||
| Frequency of injections | Once a month, for 3 consecutive months | Single | Twice, the second injection was given after 2 months | Single | |
| Use of ultrasound guidance for verification of injection | Yes | No | No | No | |
| Agent injected | |||||
| PRP | 5 ml of PRP | 5 ml of PRP | 5 ml of PRP | Platelet value of 2.5–5 times the number of platelets in 45–50 ml of collected blood | |
| CS | 40 mg triamcinolone | 2.5 ml of triamcinolone acetate | 1 ml (40 mg) of triamcinolone acetonide and 4 ml of 1% lidocaine hydrochloride mix | 40 mg (2 ml) of triamcinolone hexacetonide | |
CS, corticosteroid; IA, intra-articular; PRP, platelet-rich plasma.
Table 3.
Studies designs
| Elksniņš-Finogejevs et al.23 | Forogh et al.17 | Uslu Güvendi et al.18 | Huang et al.24 | Jubert et al.19 |
|---|---|---|---|---|
| Single-center prospective randomized controlled study (RCT) | Double-blind, randomized clinical trial | Single-center, prospective, randomized, single-blind study | Prospective, randomized study | Prospective randomized, double-blind, parallel-group, active-controlled study |
| Naderi Nabi et al.20 | Freire et al.21 | Khan et al.25 | Nunes-Tamashiro et al.22 | |
| RCT | A randomized, controlled, longitudinal, double-blind, comparative, descriptive, and analytical study | Randomized control trial | An RCT, with blinded patients and an assessor | |
Table 4.
Assessments
| Elksniņš-Finogejevs et al.23 | VAS, IKDC, and KSS scales at 1 week, 5 weeks, 15 weeks, 30 weeks, and 1 year after treatment |
| Forogh et al.17 | KOOS, the 20 MW test, ROM, flexion contracture, and VAS were assessed before, 2 months, and 6 months after interventions |
| Uslu Güvendi et al.18 | VNS, WOMAC, Lequesne index, and the HAD Scale pretreatment, and at 2 and 6 months posttreatment |
| Huang et al.24 | WOMAC score prior to the first injection and then at 3, 6, 9, and 12 months; VAS pain prior to treatment and after 12 months |
| Jubert et al.19 | VAS at 1 month, KOOS and SF-36 at 1, 3, and 6 months after treatment |
| Naderi Nabi et al.20 | VAS and KOOS monthly for 3 consecutive months, as well as 6 months after the treatment |
| Freire et al.21 | KSS and WOMAC preinjection and after 1 month and 6 months of intervention |
| Khan et al.25 | WOMAC every 2 weeks for 6 months |
| Nunes-Tamashiro et al.22 | Patients were assessed at baseline and after 4, 8, 12, and 52 weeks with VAS, WOMAC, Timed to Up and Go test, 6-min walk test, percentage of improvement, goniometry, quality of life SF-36 questionnaire, Likert scale and K–L radiographic scale (only at baseline and 52 weeks) |
20 MW test, 20 meters walk test; HAD Scale, Hospital Anxiety and Depression Scale; IKDC, International Knee Documentation Committee; KOOS, Knee Injury and Osteoarthritis Outcome Scale; KSS, Knee Society Score; K–L, Kellgren–Lawrence; ROM, range of motion; SF-36, Short Form-36; VAS, Visual Analog Scale; VNS, Visual Numeric Scale; WOMAC, Western Ontario and McMaster Universities Arthritis Osteoarthritis Index.
Outcome assessment
We compared the results of the included studies according to the most frequent outcome scales, which were Visual Analog Scale (VAS) Index, Knee Injury and Osteoarthritis Outcome Scale (KOOS) Index, and Western Ontario and McMaster Universities Arthritis Osteoarthritis (WOMAC) Index. The outcomes were assessed by different scales, as shown in Table 5.
Table 5.
Outcome scales
| References | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Elksniņš-Finogejevs et al.23 | Forogh et al.17 | Uslu Güvendi et al.18 | Huang et al.24 | Jubert et al.19 | Naderi Nabi et al.20 | Freire et al.21 | Khan et al.25 | Nunes-Tamashiro et al.22 | |
| WOMAC Index | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| KOOS Index | ✓ | – | – | ✓ | ✓ | – | – | ||
| VAS | ✓ | ✓ | – | ✓ | ✓ | ✓ | – | ✓ | ✓ |
| VNS, WOMAC, Lequesne index, and the HAD Scale0 | – | – | ✓ | – | – | – | – | – | – |
| IKDC | ✓ | – | – | – | – | – | – | – | – |
| KSS | ✓ | – | – | – | – | – | ✓ | – | – |
| 20 MW test active and passive knee ROMs and flexion contracture | – | ✓ | – | – | – | – | – | – | – |
| SF-36 | – | – | – | – | ✓ | – | – | – | ✓ |
| Timed Up and Go test, 6-min walk test, percentage of improvement, goniometry, and Likert scale | – | – | – | – | – | – | – | – | ✓ |
20 MW test, 20 meters walk test; IKDC, International Knee Documentation Committee; KOOS, Knee Injury and Osteoarthritis Outcome Scale; KSS, Knee Society Score; ROMs, range of motions; SF-36, Short Form-36; VAS, Visual Analog Scale; VNS, Visual Numeric Scale; WOMAC, Western Ontario and McMaster Universities Arthritis Osteoarthritis Index.
At 3rd month
Variables in Elksniņš-Finogejevs et al.’s23 study were analyzed using the intention-to-treat principle. According to the authors, the best improvement of the VAS was in the 3rd month in the PRP group (mean of −4.6±1.6; −77%).
No evaluation was done in 3rd month by Forogh et al.’s17 study.
According to Huang et al.’s24 study, WOMAC pain scores in 3rd month showed similar and significant improvement (P>0.05).
According to Jubert et al.’s19 study, KOOS outcomes tended to be better in the PRP group with no significant difference. The results in the KOOS–Quality of Life score in the 3rd month enhanced significantly in the PRP in comparison to the CS group (mean, 17.77 vs. 4.91, P=0.05) in the 3rd month.
According to Naderi Nabi et al.’s20 study, results showed beneficial effects on pain reduction based on VAS. The pain was relieved in both groups during the first 3 months.
At 6th month
According to Elksniņš-Finogejevs et al.’s23 study, the best improvement of VAS was at the 6th month in CS group (−3.4±1.2; −58%).
Forogh et al.’s17 study showed significant improvement in the PRP treatment group, including quality of life, daily activities, and pain relief, Whereas no difference was noted in sporting ability. With regard to pain relief, CS injections were more effective in the 2nd month, while in the PRP group the efficacy was noted at the 2nd and the 6th months. A significant improvement was noted in the VAS scale in the 6th month in the PRP group in comparison to the CS group.
According to Uslu Güvendi et al.’s18 study, the WOMAC pain score was improved with a significant difference noted in favor of PRP groups (P<0.05) with no difference between single PRP and three PRP in the 6th month.
According to Huang et al.24, WOMAC results at the 6th, 9th, and 12th months in the PRP group showed a significantly superior improvement to the HA and CS groups. No VASs were measured in the 3rd and 6th months.
According to Jubert et al.’s19 study, KOOS outcomes tended to be better in the PRP group with no significant difference. The results in the KOOS–Quality of Life score in the 6th month enhanced significantly in the PRP in comparison to the CS group (mean, 17.77 vs. 4.91 in the 3rd month and 16.88 vs. 3.56, P=0.03) in 6 months.
According to Naderi Nabi et al.’s20 study, pain reduction continued to the 6th month in the PRP group; it increased in CSs group at the 6th month but was still lower than the baseline, also Naderi Nabi et al.’s20 study showed significant improvement in KOOS outcomes in PRP treatment, including life quality, daily activities, and pain relief (related to disease grade), especially at the 6th month.
Freire et al.’s21 study demonstrated a reduction in pain of WOMAC scale scores which were more overt in the 6th month postinjection when using PRP with no significant difference between them, and both were effective.
According to Khan et al.’s25 study, WOMAC results in the 6th month of follow-up were equal in both groups in improving pain and function (P=0.001). The CS group showed better results in improving stiffness (P<0.001), while in the PRP group, this difference was not statistically significant. Pain VAS scores improved in both groups with statistically significant differences (P<0.001).
At 1 year
According to Elksniņš-Finogejevs et al.’s23 study, VAS score changes at 1 year showed a higher mean change from baseline in the PRP group than the CS group (PRP − 3.1±2.0, 52%; CS − 0.8±1.8, 14%) with significant difference between groups.
According to Huang et al.24, the PRP group showed significantly lower WOMAC scores at 12 months after treatment (P<0.05). PRP and CSs groups showed significant efficiency in VAS pain scales at the 12th month compared to pretreatment.
According to Nunes-Tamashiro et al.22, the Triamcinolone Hexacetonide group (at baseline 5.29±2.06 and 2.91±2.73 at 52 weeks), the PRP group (at baseline 5.39±1.96 and 2.57±2.52 at 52 weeks), so the Triamcinolone Hexacetonide did not differ from the PRP group. There was no difference among the three groups for the other variables assessed, including the VAS score of pain on movement.
Complications and side effects
Not all studies mentioned or recorded the complications and side effects in either or both groups. Based on the available data, we can estimate that both PRP and CS IA injections are safe options for knee OA treatment. Complications and side effects were reported in Table 6.
Table 6.
Complications and side effects
| Complications | |||
|---|---|---|---|
| References | PRP | CS | Remarks |
| Elksniņš-Finogejevs et al.23 | Mild synovitis was recorded by 15 patients (75%) | No adverse events were recorded | |
| Forogh et al.17 | No information | ||
| Uslu Güvendi et al.18 | One case of mild rash declined within 6 h after applying cold compresses | No information | |
| Huang et al.24 | Pain, nausea, and dizziness, which were relieved after 24 or 48 h, were observed in five patients (4.2%) | Pain, nausea, and dizziness, which were relieved after 24 or 48 h, were observed in three patients (2.5%) | No records of any development of low-grade fever, deep vein thrombosis, or sepsis |
| Jubert et al.19 | No patients experienced side effects during injection or during follow-up | ||
| Naderi Nabi et al.20 | Mild to moderate pain at the injection site, which was not considered a major complication | ||
| Freire et al.21 | PRP therapy has fewer side effects and does not record information about them | Not mentioned | |
| Khan et al.25 | There is no assessment of the adverse effect | ||
| Nunes-Tamashiro et al.22 | No adverse effects were observed in any patients of any group | ||
CS, corticosteroid; PRP, platelet-rich plasma.
Discussion
CSs and PRP are widely used for the treatment of OA. Their injections are considered safe and effective options for knee OA treatment. Even though some studies showed that PRP injections were superior to CSs, it cannot be asserted that PRP injections are the best option.
Costa et al.26, conducted a systematic review and meta-analysis of 40 RCTs indicated that PRP was as effective as other therapies in pain, function, and stiffness and more effective in some studies at 6 months follow-up. However, the evidence is of low or very low quality and is based on studies with a high Risk of Bias and high heterogeneity.
Anil et al.27, in a network meta-analysis of 79 RCTs found that the treatment with the highest P-score at 6 months postinjection for WOMAC score was PRP (P-score=0.7676) in comparison with other IA injectables such as CSs and HA.
Singh et al.28 conducted a systematic review and network meta-analysis that demonstrated that all injectable agents (HA, PRP, and plasma rich in growth factors, except CSs resulted in statistically significant improvements in outcomes compared to placebo. Due to the large effect size, PRP showed clinically meaningful differences in functional improvement compared with CS and placebo.
Also, McLarnon et al.29 conducted a systematic review and meta-analysis of eight studies showed that PRP was significantly better compared with CS injections in reducing OA symptoms (pain, stiffness, functionality) at 3, 6, and 9 months postintervention (P<0.01).
The American College of Rheumatology strongly recommends weight loss and exercise as nonpharmacological cures for knee OA. Oral and topical nonsteroidal anti-inflammatory drugs and IA glucocorticoid injections are strongly recommended, whereas there is no recommendation about PRP injections30.
There are five injectable CSs approved by Food and Drug Administration label for IA injections, including methylprednisolone acetate, triamcinolone acetate, betamethasone acetate, and betamethasone sodium phosphate, triamcinolone hexacetonide, and dexamethasone. Some research has been published comparing results after different IA CS injections; however, the results were indecisive. While we need more research, it appears that each compound has similar potency as long as it is used for the correct indication, dosage, timing, and application1.
The variety of applicable PRP systems, including PRP collection volumes and preparation protocols, reflects an absence of consistency among trials. Cell membrane receptors are limited, so very high concentrations of growth factors probably have no beneficial effect on cell stimulatory processes. Additionally, the limited biological half-life of many growth factors in PRP can explicate, at least in part, the variance seen with PRP treatment results. Considerable differences in baseline platelet counts among individual patients and differences between PRP preparation procedures may prevent the demonstration of the relation between platelet dose and concentration as well as activated growth factor concentration. However, more research is needed to clear mechanistic understanding and unanimity regarding the standardization of PRP procedure to achieve the best result clinical outcomes31.
The strengths of our review included RCTs only, assessment of Risk of Bias with the validated tool in these trials, and comparison between the validated frequent outcomes scales (WOMAC, KOOS, and VAS).
Limits involving the follow-up period do not detect the long-term efficacy of both treatment options, few studies are included, and their sample sizes are small, so results cannot be generalized. On the other hand, not all included randomized trials were blinded. Outcomes were not analyzed with the intention-to-treat principle except two studies so the results can be affected by bias. Also, a lack of VAS recordings at certain time points existed in at least one of the nine studies.
In addition to the different disease stages studied, different scales and subscales were used to assess disease, and different injection volumes, repeats, and intervals were used, so robust meta-analyses could not be performed. This review neglected whether PRP is leukocyte-rich or leukocyte-poor PRP.
Conclusion
This review demonstrated that PRP injections have favorable improvements when compared with CS injections in the management of knee OA such as pain alleviation and decreasing joint stiffness. This evidence does not reflect a certain judgment, and further research and investigation are required.
PRP or CS injections in the treatment of knee OA needs more research. Stronger medical evidence is required, such as conducting RCTs, following standardized scales to assess disease, and listing risk factors and confounding. Moreover, meticulous design and a priori involvement of a biostatistician would lead to higher quality and, consequently, more reliable clinical trials on this issue. Only then will we be able to draw firm conclusions to prefer specific IA injections.
Ethics approval and consent to participate
Not applicable.
Sources of funding
The authors received no financial support for the review and/or authorship of this article.
Author contribution
Both authors revised the initial draft multiple times and were involved in the final manuscript. M.S. acted as supervisor for the project.
Conflicts of interest disclosure
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Research registration unique identifying number (UIN)
None.
Guarantor
Both authors.
Consent for publication
Not applicable.
Data availability
Not applicable.
Supplementary Material
Acknowledgments
Special thanks go to Dr Asem Salma for reviewing and revising the manuscript for grammar and syntax (MD, Attending (consultant) Neurosurgeon, Mercy Health – St. Rita’s Medical Center, 770 W. High St, Suite 160B, Lima, Ohio 45801, USA).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 6 February 2023
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.annalsjournal.com.
Contributor Information
Fatima A. Idres, Email: fatemaedress@gmail.com.
Michel Samaan, Email: msamaan@albaath-univ.edu.sy.
References
- 1. Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop 2014;5:351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heidari B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: part I. Caspian J Intern Med 2011;2:205–12. [PMC free article] [PubMed] [Google Scholar]
- 3. Testa G, Giardina SMC, Culmone A, et al. Intra-articular injections in knee osteoarthritis: a review of literature. J Funct Morphol Kinesiol 2021;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ostergaard M, Halberg P. Intra-articular corticosteroids in arthritic disease: a guide to treatment. BioDrugs 1998;9:95–103. [DOI] [PubMed] [Google Scholar]
- 5. Creamer P. Intra-articular corticosteroid treatment in osteoarthritis. Curr Opin Rheumatol 1999;11:417–21. [DOI] [PubMed] [Google Scholar]
- 6. Jessar RA, Ganzell MA, Ragan C. The action of hydrocortisone in synovial inflammation. J Clin Invest 1953;32:480–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone 2012;51:249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ayral X, Pickering EH, Woodworth TG, et al. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis – results of a 1-year longitudinal arthroscopic study in 422 patients. Osteoarthr Cartil 2005;13:361–7. [DOI] [PubMed] [Google Scholar]
- 9. Roemer FW, Guermazi A, Felson DT, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheumdis 2011;70:1804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Conaghan PG, D’Agostino MA, Le Bars M, et al. Clinical and ultrasonographic predictors of joint replacement for knee osteoarthritis: results from a large, 3-year, prospective EULAR study. Ann Rheum Dis 2010;69:644–7. [DOI] [PubMed] [Google Scholar]
- 11. Peter I, Wu K, Diaz R, et al. Platelet-rich plasma. Phys Med Rehabil Clin 2016;27:825–53. [DOI] [PubMed] [Google Scholar]
- 12. Asjid R, Faisal T, Qamar K, et al. Platelet-rich plasma-induced inhibition of chondrocyte apoptosis directly affects cartilage thickness in osteoarthritis. Cureus 2019;11:e6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dai W-L, Zhou A-G, Zhang H, et al. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy 2017;33:659–70. [DOI] [PubMed] [Google Scholar]
- 14. Cook CS, Smith PA. Clinical update: why PRP should be your first choice for injection therapy in treating osteoarthritis of the knee. Curr Rev Musculoskelet Med 2018;11:583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 16. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Forogh B, Mianehsaz E, Shoaee S, et al. Effect of single injection of platelet-rich plasma in comparison with corticosteroid on knee osteoarthritis: a double-blind randomized clinical trial. J Sports Med Phys Fitness 2016;56:901–8. [PubMed] [Google Scholar]
- 18. Uslu Güvendi E, Aşkin A, Güvendi G, et al. Comparison of efficiency between corticosteroid and platelet rich plasma injection therapies in patients with knee osteoarthritis. Arch Rheumatol 2017;33:273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jubert NJ, Rodríguez L, Reverte-Vinaixa MM, et al. Platelet-rich plasma injections for advanced knee osteoarthritis: a prospective, randomized, double-blinded clinical trial. Orthop J Sports Med 2017;5:2325967116689386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naderi Nabi B, Sedighinejad A, Mardani-Kivi M, et al. Comparing the effectiveness of intra-articular platelet-rich plasma and corticosteroid injection under ultrasound guidance on pain control of knee osteoarthritis. Iran Red Crescent Med J 2018;20:e62157. [Google Scholar]
- 21. Freire MRdM, da Silva PMC, Azevedo AR, et al. Comparative effect between infiltration of platelet-rich plasma and the use of corticosteroids in the treatment of knee osteoarthritis: a prospective and randomized clinical trial. Rev Bras Ortop (Sao Paulo) 2020;55:551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nunes-Tamashiro JC, Natour J, Ramuth FM, et al. Intra-articular injection with platelet-rich plasma compared to triamcinolone hexacetonide or saline solution in knee osteoarthritis: a double blinded randomized controlled trial with one year follow-up. Clin Rehabil 2022;36:900–15. [DOI] [PubMed] [Google Scholar]
- 23. Elksniņš-Finogejevs AV, Vidal L, Peredistijs A. Intra-articular platelet-rich plasma vs corticosteroids in the treatment of moderate knee osteoarthritis: a single-center prospective randomized controlled study with a 1-year follow up. J Orthop Surg Res 2020;15:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang Y, Liu X, Xu X, et al. Intra-articular injections of platelet-rich plasma, hyaluronic acid or corticosteroids for knee osteoarthritis: a prospective randomized controlled study. Orthopade 2019;48:239–47. [DOI] [PubMed] [Google Scholar]
- 25. Khan AF, Gillani SFUHS, Khan AF. Role of intra-articular corticosteroid with xylocaine vs plate rich plasma for the treatment of early grade II knee osteoarthritis at Akhtar Saeed Teaching Hospital Lahore: a randomized controlled trail. Pakistan J Med Health Sci 2018;12:1432–35. [Google Scholar]
- 26. Costa LAV, Lenza M, Irrgang JJ, et al. How does platelet-rich plasma compare clinically to other therapies in the treatment of knee osteoarthritis? A systematic review and meta-analysis. Am J Sports Med 2022. doi: 10.1177/03635465211062243 [Epub ahead of print]. PMID: 35316112. [DOI] [PubMed] [Google Scholar]
- 27. Anil U, Markus DH, Hurley ET, et al. The efficacy of intra-articular injections in the treatment of knee osteoarthritis: a network meta-analysis of randomized controlled trials. Knee 2021;32:173–82. [DOI] [PubMed] [Google Scholar]
- 28. Singh H, Knapik DM, Polce EM, et al. Relative efficacy of intra-articular injections in the treatment of knee osteoarthritis: a systematic review and network meta-analysis. Am J Sports Med 2022;50:3635465211029659. [DOI] [PubMed] [Google Scholar]
- 29. McLarnon M, Heron N. Intra-articular platelet-rich plasma injections versus intra-articular corticosteroid injections for symptomatic management of knee osteoarthritis: systematic review and meta-analysis. BMC Musculoskelet Disord 2021;22:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/of Arthritis Foundation Guideline for the Management Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res (Hoboken) 2020;72:149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Szwedowski D, Szczepanek J, Paczesny Ł, et al. The effect of platelet-rich plasma on the intra-articular microenvironment in knee osteoarthritis. Int J Mol Sci 2021;22:5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


