Background:
There is a real unmet need for primary biliary cholangitis (PBC) treatments that can improve quality of life impacting symptoms. In this post hoc analysis, we evaluated potential effects of the NADP oxidase 1/4 inhibitor, setanaxib, on patient-reported quality of life from a phase 2 trial in PBC.
Patients and Methods:
The underpinning double-blind, randomized, placebo-controlled trial (NCT03226067) recruited 111 patients with PBC and inadequate response/intolerance to ursodeoxycholic acid. Patients self-administered oral placebo (n=37), setanaxib 400 mg once daily (OD; n=38), or setanaxib 400 mg twice daily (BID; n=36), in addition to ursodeoxycholic acid for 24 weeks. Quality of life outcomes were assessed using the validated PBC-40 questionnaire. Patients were stratified post hoc by baseline fatigue severity.
Results:
At week 24, patients treated with setanaxib 400 mg BID reported greater mean (SE) absolute reductions from baseline in PBC-40 fatigue domain score [–3.6 (1.3)] versus those receiving setanaxib 400 mg OD [–0.8 (1.0)]) or placebo [0.6 (0.9)]. Similar observations were made across all PBC-40 domains except itch. In the setanaxib 400 mg BID arm, patients with moderate-to-severe fatigue at baseline had a greater reduction in mean fatigue score at week 24 [–5.8 (2.1)] versus those with mild fatigue [–0.6 (0.9)]; results were similar across all domains. Reduced fatigue was correlated with emotional, social, symptom, and cognitive improvements.
Conclusions:
These results support further investigation of setanaxib as a treatment for patients with PBC, particularly for those with clinically significant fatigue.
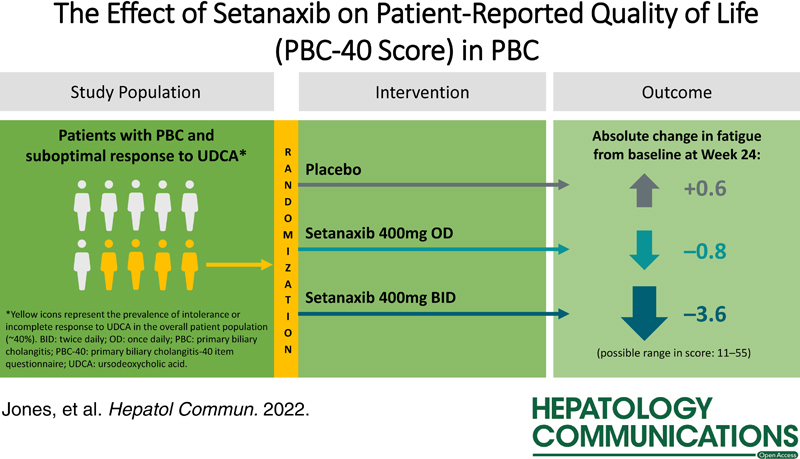
INTRODUCTION
Primary biliary cholangitis (PBC) is a progressive cholestatic liver disease characterized by immune-associated damage to, and ultimately destruction of, the intralobular bile ducts.1 Even before the potential appearance of liver cirrhosis and resulting complications, patients with PBC frequently experience a range of symptoms including fatigue, pruritus, sicca syndrome, abdominal discomfort, and joint or bone pain, all of which can greatly impact patients’ overall quality of life (QoL).2–8 The most common of these are fatigue (with more than half of patients experiencing at least moderate levels)9,10 and pruritus (which persists in more than one third of patients).11 These symptoms, particularly fatigue, can affect patients’ ability to perform day-to-day activities, and may significantly impact patients’ mental health, social life, family life, sexual life, and job performance.5,12 QoL-impacting symptoms are therefore important treatment targets for patients with PBC.
The only currently approved treatments for PBC are ursodeoxycholic acid (UDCA; first line) and obeticholic acid (OCA; second line add-on), which both target cholestasis.13–16 However, randomized controlled trials for UDCA and OCA have failed to prove efficacy of either drug in managing QoL symptoms such as fatigue and pruritus.8,13–17 In fact, OCA treatment has been reported to exacerbate pruritus in some patients, and fatigue and pruritus are listed among its common adverse effects.8,16,18 Consequently, patients may continue to experience debilitating symptoms despite PBC treatment.
Fibrates, including bezafibrate, are sometimes used off-label to treat PBC but are not approved for PBC at the time of writing. Although bezafibrate may have positive effects on pruritus,19 impact on fatigue has yet to be proven using a validated method of measurement.20 A recent study of seladelpar (CymaBay Therapeutics Inc.), an investigational selective peroxisome proliferator-activated receptor delta agonist in development for the second-line treatment of PBC, has reported substantial improvements in pruritus among PBC patients, which was correlated with improvements in patient-reported sleep disturbance and fatigue.21 However, from this study alone it cannot be distinguished whether seladelpar has a direct impact on fatigue in PBC, or whether alleviation of fatigue was due to improvements in pruritus leading to less disturbed sleep. Thus, there remains an unmet clinical need for medications that can effectively treat PBC as well as addressing and managing accompanying QoL-related symptoms of the disease, particularly fatigue.
A phase 2 trial for setanaxib (Calliditas Therapeutics Suisse SA), an investigational first-in-class selective inhibitor of NADP oxidase (NOX) isoforms 1 and 4, was recently completed, in which QoL outcomes were assessed as a secondary endpoint. The original study did not account for factors such as baseline levels of fatigue; in this post hoc analysis, we set out to evaluate the potential effects of setanaxib on QoL outcomes in further detail and draw comparisons between patients with and without clinically significant fatigue at baseline.
PATIENTS AND METHODS
Study design
This double-blind, randomized, placebo-controlled, multi-center, parallel group phase 2 trial (NCT03226067) took place between September 2017 and April 2019 (from the date of first patient enrollment to last patient’s final visit). Eligible patients with PBC and persistently elevated serum alkaline phosphatase (ALP) were randomized 1:1:1 to placebo, setanaxib 400 mg once daily (OD) or setanaxib 400 mg twice daily (BID) treatment arms. Patients self-administered setanaxib or placebo, in addition to UDCA (unless intolerant), orally for a total of 24 weeks. The study design (including randomization method), primary results, and safety data have been reported previously (Invernizzi P., unpublished manuscript).22,23
Ethics approval statement
This study was performed in accordance with the provisions of the 2013 Declaration of Helsinki, and in accordance with USA Food and Drug Administration (FDA) regulations. Ethics approval was granted by the relevant central and regional ethics committees; a full list of these committees has been made available in a previous publication (Invernizzi P., unpublished manuscript). The trial was conducted in agreement with the International Conference on Harmonization (ICH) Guidelines on Good Clinical Practice (GCP). Written informed consent was obtained from each patient included in the study.
Patients
Patients were assessed for eligibility during a 4-week screening period. Eligible patients were aged 18–80 years (inclusive) and had a diagnosis of PBC. Patients were also required to have received UDCA treatment for ≥6 months (with a stable dose for ≥3 mo) before their first visit, and to exhibit an intolerance or incomplete response to UDCA [defined by serum ALP and gamma-glutamyl transferase levels ≥1.5×upper limit of normal (ULN)]. UDCA treatment was continued, alongside setanaxib or placebo, throughout the duration of the trial. Full inclusion and exclusion criteria have been reported previously (Invernizzi P., unpublished manuscript).22,23
Patients from the intention-to-treat (ITT) population (all patients randomized to receive a treatment) were stratified post hoc by baseline PBC-40 fatigue score into mild, moderate, and severe groups using previously published PBC-40 fatigue domain cut-offs (Supplemental Table 1, http://links.lww.com/HC9/A127).24 Patients with moderate and severe fatigue (considered “clinically significant”)10 were grouped together. Separately, the ITT population were stratified by baseline liver stiffness (<9.6 kPa or ≥9.6 kPa) and serum ALP (<3 × ULN or ≥3 × ULN). Two values of ULN for ALP were used in this study depending on patient age: 125 U/L (19–59 y old) and 149 U/L (≥60 y old). Stratifications by liver stiffness and ALP were performed to evaluate whether changes in symptoms and QoL over time were related to greater liver disease severity (indicated by high liver stiffness or serum ALP at baseline).9,25,26
Study procedures and endpoints
Patient-reported QoL was assessed using the English version of the PBC-40 questionnaire (validated for use in patients with PBC), which includes the following domains: fatigue, emotional, social, symptom, itch, and cognitive (Supplemental Table 2, http://links.lww.com/HC9/A127).27 PBC-40 questionnaires were self-completed by patients at baseline, week 12, and week 24 of the randomized controlled trial period. The endpoints assessed in these post hoc analyses of the PBC-40 data, to evaluate the effect of setanaxib treatment on QoL outcomes, were: (1) change from baseline to week 24 in mean patient-reported score for each PBC-40 domain, and (2) the number and percentage of patients who achieved a meaningful response in each domain at week 24 (according to a predefined score reduction threshold). Liver stiffness was assessed through transient elastography (Fibroscan), and serum levels of markers of liver injury and inflammation [ALP, high-sensitivity C-reactive protein (hsCRP), and IL-6] were measured in venous blood samples collected at baseline and weeks 2, 6, 12, 18, and 24 (for ALP); 2, 4, 12, 18, 24, and 28 (for hsCRP); or 12 and 24 (for IL-6).
Statistical methods
Descriptive analyses of baseline disease characteristics and changes in score from baseline to week 12 and week 24 [reported as mean (SEM)] were performed for the 6 PBC-40 domains, stratified separately by treatment arm and fatigue severity subgroup. For each item of the PBC-40, there is a minimum score of 0 (for 6 items in the symptom, itch, and social domains; Supplemental Table 2, http://links.lww.com/HC9/A127) or 1 (for the remaining 34 items), and a maximum score of 5 (for all 40 items). This results in a different overall “floor value” (minimum total score) for each domain. In order to allow direct comparisons between changes in each domain from baseline to week 12 and week 24, the floor values were accounted for by calculating the “maximum dynamic range” [the maximum possible range between domain floor and domain ceiling (maximum total score)] for each domain (Supplemental Table 3, http://links.lww.com/HC9/A127). Changes in domain score are presented as (1) absolute mean change from baseline and (2) the absolute mean change in score from baseline as a percentage of the maximum dynamic range for the domain.
The percentage of patients who achieved a meaningful response in each domain at week 24 (defined by a 0.5-point change from baseline per item in the domain) are reported. The value used as a meaningful score reduction threshold was based on previous studies, which were powered to detect an average of a 0.5-point change per item in the PBC-40 fatigue domain (a change which had been demonstrated to be associated with significantly higher levels of social function).28,29 Pearson correlation coefficients were calculated (using proc corr in SAS) between improvement from baseline to week 24 in the fatigue domain and change from baseline in (1) the emotional, social, symptom, itch, and cognitive domains and (2) liver stiffness and serum ALP, log(hsCRP), and IL-6. Serum levels of hsCRP were log-transformed to ensure normal distribution. Correlations were described as weak (±0.00–0.39), moderate (±0.40–0.69), or strong (±0.70–1.0), adapted from previously published cut-offs.30 Post hoc analyses were based on an original statistical analysis plan, which was amended to include additional analyses upon availability of the final data. All statistical analyses were performed post hoc in SAS version 9.4.
RESULTS
Baseline patient PBC-40 scores
Of the 111 randomized patients, 37 received placebo, 38 received setanaxib 400 mg OD, and 36 received setanaxib 400 mg BID; patient disposition is summarized in Supplemental Figure 1 (http://links.lww.com/HC9/A127). Baseline characteristics have previously been reported by treatment group (Invernizzi P., unpublished manuscript).22,23 There were 56 patients (placebo: n=18; setanaxib 400 mg OD: n=17; setanaxib 400 mg BID: n=21) classified as having moderate-to-severe fatigue at baseline, compared with 55 patients (placebo: n=19; setanaxib 400 mg OD: n=21; setanaxib 400 mg BID: n=15) with mild fatigue. Baseline PBC-40 domain scores were similar for all domains across the treatment groups, both in the ITT population (Table 1) and in patients with moderate-to-severe fatigue (Supplemental Table 4, http://links.lww.com/HC9/A127). Higher mean baseline scores were observed in all PBC-40 domains for patients with moderate-to-severe fatigue compared with those who had mild fatigue at baseline (Table 2). Meanwhile, no differences were observed in baseline scores across the PBC-40 domains between patients stratified by higher and lower baseline liver stiffness (<9.6 kPa/≥9.6 kPa; Supplemental Table 5, http://links.lww.com/HC9/A127).
TABLE 1.
Baseline PBC-40 scores in the ITT population and across the three treatment groups
| Baseline PBC-40 score, mean (SEM) | ||||
|---|---|---|---|---|
| PBC-40 domain | ITT population (n=111) | Placebo (n=37) | Setanaxib 400 mg OD (n=38) | Setanaxib 400 mg BID (n=36) |
| Fatigue | 28.2 (1.1) | 28.4 (2.0) | 26.7 (1.7) | 29.5 (1.9) |
| Emotional | 7.6 (0.3) | 7.6 (0.5) | 7.1 (0.5) | 8.2 (0.6) |
| Social | 21.1 (0.9) | 22.2 (1.6) | 19.8 (1.3) | 21.3 (1.6) |
| Symptom | 14.4 (0.5) | 15.3 (0.8) | 14.1 (0.7) | 13.9 (0.9) |
| Itch | 4.4 (0.4) | 5.2 (0.8) | 4.4 (0.7) | 3.7 (0.6) |
| Cognitive | 12.5 (0.6) | 13.0 (1.0) | 11.7 (0.9) | 12.9 (1.1) |
| Marker of liver disease | Baseline measurement, median (min–max) unless otherwise stated | |||
| Liver stiffness, kPa, n,a median (min–max) | 91 8.1 (2.0–75.0) | 32 8.9 (3.0–35.0) | 33 8.0 (4.0–75.0) | 26 7.5 (2.0–16.0) |
| ALP, U/L | 269.0 (175.0–1091.0) | 251.0 (175.0–788.0) | 267.0 (179.0–809.0) | 319.5 (175.0–1091.0) |
| hsCRP, mg/L, n,b median (min–max) | 108 3.9 (0.0–29.0) | 37 3.9 (0.0–26.0) | 38 4.3 (1.0–29.0) | 33 3.1 (1.0–21.0) |
| IL-6, pg/mL, n,b mean (SEM) | 103 5.6 (0.5) | 36 5.9 (1.0) | 36 5.2 (0.6) | 31 5.7 (0.9) |
Only patients with liver stiffness values at both baseline and Week 24 visits are shown here.
Some patients had missing values at baseline.
Abbreviations: ALP, alkaline phosphatase; BID, twice daily; hsCRP, high-sensitivity C-reactive protein; ITT, intention-to-treat; max, maximum; min, minimum; OD, once daily; PBC, primary biliary cholangitis; PBC-40, primary biliary cholangitis-40 item questionnaire.
TABLE 2.
Baseline PBC-40 scores stratified by baseline fatigue severity
| Baseline PBC-40 score, mean (SEM) | ||
|---|---|---|
| PBC-40 domain | Mild fatigue (n=55) | Moderate-to-severe fatigue (n=56) |
| Fatigue | 18.4 (0.7) | 37.8 (0.8) |
| Emotional | 5.8 (0.4) | 9.4 (0.4) |
| Social | 15.8 (0.8) | 26.3 (1.2) |
| Symptom | 12.3 (0.6) | 16.5 (0.6) |
| Itch | 2.9 (0.4) | 5.9 (0.6) |
| Cognitive | 9.8 (0.6) | 15.1 (0.8) |
Abbreviation: PBC-40, primary biliary cholangitis-40 item questionnaire.
Change in PBC-40 scores by domain
Overall ITT population
Of the 111 randomized patients, 103 patients had PBC-40 data available for both baseline and week 24, and were therefore included in these analyses. Patients treated with setanaxib 400 mg BID reported reductions from baseline in mean PBC-40 scores across all domains except itch at week 24 (Figure 1). Meanwhile, patients treated with setanaxib 400 mg OD reported modest reductions in the fatigue, emotional, and itch domains, and patients treated with placebo reported increased scores in all domains except symptom. These changes were already apparent by week 12 (Supplemental Figure 2, http://links.lww.com/HC9/A127). To aid interpretation, mean changes from baseline are presented as percentages of the maximum dynamic range for the respective domain in Supplemental Figure 3 (http://links.lww.com/HC9/A127). In all domains except social and cognitive, a greater proportion of patients receiving either setanaxib regimen achieved a meaningful response (0.5-point reduction from baseline per item in the domain)24 at week 24 than patients receiving placebo (Figure 2). Although no effect was seen for meaningful response in cognitive function with setanaxib 400 mg OD treatment, 26.7% of patients receiving setanaxib 400 mg BID achieved a meaningful improvement in the cognitive domain (vs. 11.1% of those receiving placebo; Figure 2). The greatest difference in meaningful responses between the setanaxib treatment groups and placebo was observed in the emotional domain (setanaxib 400 mg OD: 35.1%; setanaxib 400 mg BID: 43.3%; placebo: 2.8%; Figure 2).
FIGURE 1.
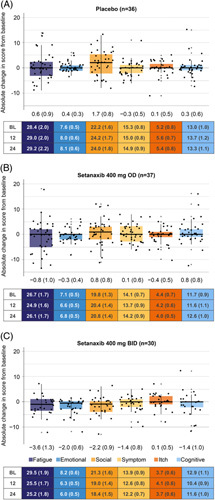
Absolute change in patient score from baseline to week 24 across the PBC-40 domains, stratified by treatment arm. (A) Placebo, (B) setanaxib 400 mg OD, (C) setanaxib 400 mg BID. All patients with PBC-40 data available at baseline and week 24 were included in these analyses (n=103); some patients may have stopped setanaxib treatment before the end of the treatment period. Reduced scores indicate improvements in symptom impact. Box plots show median (central line) and interquartile range; minimum and maximum values are depicted by vertical whiskers, with outliers indicated by crosses (+). Data labels depict mean (SEM) absolute change from baseline in score for each PBC-40 domain. Tables present mean (SEM) absolute scores in each PBC-40 domain at baseline (BL), week 12 and week 24 by treatment group. Abbreviations: BID, twice daily; OD, once daily; PBC-40, primary biliary cholangitis-40 item questionnaire.
FIGURE 2.
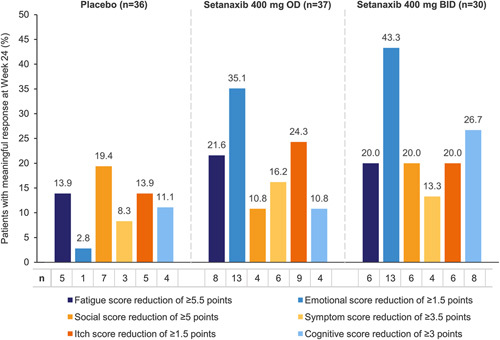
Meaningful responses to setanaxib treatment in the 6 PBC-40 domains within each treatment arm. All patients with PBC-40 data available at baseline and week 24 were included in these analyses (n=103); some patients may have stopped setanaxib treatment before the end of the treatment period. Meaningful responses to setanaxib treatment at week 24 were defined by a 0.5-point change from baseline per item in the domain (for example, in the fatigue domain, a change in score of ≥5.5 points would be considered a meaningful response). Abbreviations: BID, twice daily; OD, once daily; PBC-40, primary biliary cholangitis-40 item questionnaire.
Fatigue severity subgroups
Patients with moderate-to-severe fatigue receiving setanaxib 400 mg OD (n=17) and setanaxib 400 mg BID (n=17) had a larger reduction in mean fatigue score from baseline at week 24 compared with those in the corresponding treatment arms who had mild fatigue (n=20 and n=13; Figures 3 and 4). In the moderate-to-severe fatigue group, absolute changes in fatigue [mean (SEM)] were greatest among patients receiving setanaxib 400 mg BID [−5.8 (2.1) vs. placebo: 1.0 (1.5) and setanaxib 400 mg OD: −3.1 (1.3); Figure 4]. As a percentage of the maximum dynamic range for fatigue, 44 (Supplemental Table 3, http://links.lww.com/HC9/A127), these changes were –13.2% (4.8) versus 2.3% (3.4) and –7.0% (2.9), respectively (Supplemental Figure 4B2, http://links.lww.com/HC9/A127). Similar observations were made in the emotional domain (Figure 4 and Supplemental Figure 4B, http://links.lww.com/HC9/A127). For patients with moderate-to-severe fatigue receiving setanaxib 400 mg BID, a moderate decrease in mean score was also seen across all other domains except itch, whereas an increase (or no change) was seen for all domains in those receiving placebo (Figure 4 and Supplemental Figure 4B, http://links.lww.com/HC9/A127). These changes were mostly consistent with those seen at week 12 (Supplemental Figure 4A, http://links.lww.com/HC9/A127 and Supplemental Figure 5, http://links.lww.com/HC9/A127).
FIGURE 3.
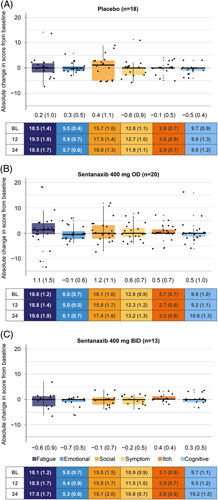
Absolute change in score from baseline to week 24 across the PBC-40 domains in patients with mild fatigue at baseline, by treatment arm. (A) Placebo, (B) setanaxib 400 mg OD, (C) setanaxib 400 mg BID. All patients with mild fatigue at baseline and PBC-40 data available at baseline and week 24 were included in these analyses (n=51); some patients may have stopped setanaxib treatment before the end of the treatment period. Reduced scores indicate improvements in symptom impact. Box plots show median (central line) and interquartile range; minimum and maximum values are depicted by vertical whiskers, with outliers indicated by crosses (+). Data labels depict mean (SEM) absolute change from baseline in score for each PBC-40 domain. Tables present mean (SEM) absolute scores in each PBC-40 domain at baseline (BL), week 12 and week 24 by treatment group. Abbreviations: BID, twice daily; OD, once daily; PBC-40, primary biliary cholangitis-40 item questionnaire.
FIGURE 4.
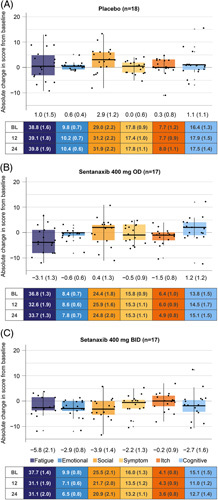
Absolute change in score from baseline to week 24 across the PBC-40 domains in patients with moderate-to-severe fatigue at baseline, by treatment arm. (A) Placebo, (B) setanaxib 400 mg OD, (C) setanaxib 400 mg BID. All patients with moderate-to-severe fatigue at baseline and PBC-40 data available at baseline and Week 24 were included in these analyses (n=52); some patients may have stopped setanaxib treatment before the end of the treatment period. Reduced scores indicate improvements in symptom impact. Box plots show median (central line) and interquartile range; minimum and maximum values are depicted by vertical whiskers, with outliers indicated by crosses (+). Data labels depict mean (SEM) absolute change from baseline in score for each PBC-40 domain. Tables present mean (SEM) absolute scores in each PBC-40 domain at baseline (BL), Week 12 and week 24 by treatment group. Abbreviations: BID, twice daily; OD, once daily; PBC-40, primary biliary cholangitis-40 item questionnaire.
Change in fatigue domain scores by baseline liver stiffness and serum ALP
Changes in fatigue domain scores across treatment arms for patients with lower (<9.6 kPa) and higher liver stiffness (≥9.6 kPa) followed a similar pattern to the ITT population, except patients with liver stiffness ≥9.6 kPa treated with setanaxib 400 mg OD who reported an increased mean absolute score from baseline to week 24 (Figure 5). Regardless of liver stiffness, patients treated with setanaxib 400 mg BID experienced the greatest reduction in fatigue among the treatment groups; in fact, those receiving placebo reported a mean increase from baseline in fatigue scores at week 24 (Figure 5). This was consistent with results observed at week 12 (Supplemental Figure 6, http://links.lww.com/HC9/A127). These changes are presented in the context of maximum dynamic range for the fatigue domain in Supplemental Figure 7 (http://links.lww.com/HC9/A127). Mean absolute changes from baseline in fatigue domain score at week 24 were also similar in patients with baseline ALP <3 × ULN [–1.4 (0.7); n=84] and those with ALP ≥3 × ULN [–0.2 (2.0); n=19]. At week 12, these were –1.8 (0.7; n=88) and 0.3 (1.8; n=21), respectively.
FIGURE 5.
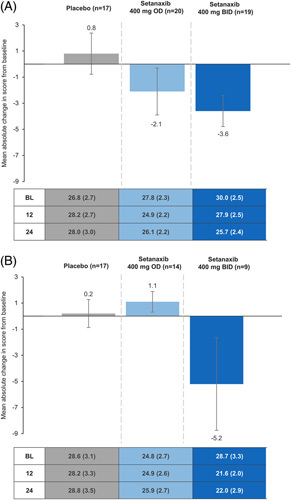
Mean absolute change in PBC-40 fatigue domain score from baseline to week 24 in patients stratified by baseline liver stiffness. (A) <9.6 kPa (n=56), (B) ≥9.6 kPa (n=40). All patients with known baseline liver stiffness and PBC-40 data available at baseline and week 24 were included in these analyses (n=96); some patients may have stopped setanaxib treatment before the end of the treatment period. Reduced scores indicate improvements in symptom impact. Error bars denote SEM. Values in table indicate mean (SEM) absolute scores in each PBC-40 domain at baseline (BL), week 12 and week 24 by treatment group. Abbreviations: BID, twice daily; OD, once daily; PBC-40, primary biliary cholangitis-40 item questionnaire.
Correlations between improvement in fatigue and other factors
Improvement in fatigue from baseline at week 24 in the ITT population showed moderate positive correlation with improvements in the emotional, social, symptom, and cognitive domains (Figure 6). A weak positive correlation with improvement in the itch domain was also observed (Figure 6). Almost no correlation was observed between improvement in fatigue and markers of liver disease (liver stiffness, ALP, hsCRP, and IL-6; Supplemental Figure 8, http://links.lww.com/HC9/A127).
FIGURE 6.
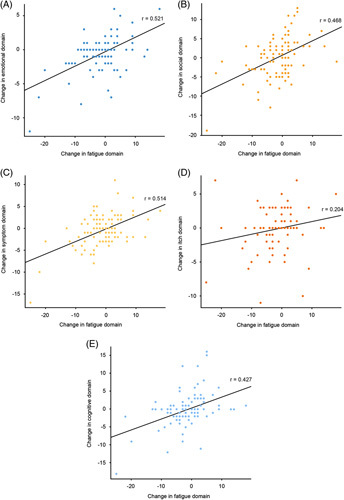
Distribution of correlations between improvement in fatigue at week 24 compared with baseline and change from baseline in other PBC-40 domains (n=103). (A) Emotional, (B) social, (C) symptom, (D) itch, (E) cognitive. All patients with PBC-40 data available at baseline and week 24 were included in these analyses (n=103); some patients may have stopped setanaxib treatment before the end of the treatment period. Correlation coefficients (r values) were generated using proc corr in SAS (Ver. 9.4). Abbreviation: PBC-40, primary biliary cholangitis-40 item questionnaire.
DISCUSSION
This phase 2 trial was the first to investigate, in this post hoc analysis, the effect of setanaxib, a first-in-class selective NOX1/4 inhibitor, on QoL in patients with PBC. The results suggest that setanaxib 400 mg BID may reduce fatigue caused by PBC, and may also alleviate impacts on emotional, social, and cognitive function in patients with moderate-to-severe fatigue. The 400 mg BID dose of setanaxib was most effective at improving fatigue and other PBC-40 domain scores in both the overall ITT population and in the moderate-to-severe fatigue subgroup, supporting the exploration of higher dosages of setanaxib in treating the QoL symptoms of PBC in a future phase 2b/3 trial and supplementing previously reported primary results from this study (Invernizzi P., unpublished manuscript).22,23
In the ITT population, treatment with setanaxib 400 mg BID led to reductions in patient-reported PBC-40 scores across the fatigue, emotional, social, symptom, and cognitive domains. Previous evidence indicates that emotional, social, and cognitive symptoms are associated with fatigue, whereas itch is not,4 supporting the possibility that improvements in the fatigue-linked domains in the present study were due to a real effect of setanaxib on fatigue in PBC patients. This notion is reinforced by the moderate positive correlations observed between improvements in fatigue and scores in each of these fatigue-linked domains (emotional, social, and cognitive). There was also no observable placebo effect for any domain; in fact, mean scores worsened from baseline to week 24 for all domains except symptom in patients receiving placebo.
Reductions in fatigue in the ITT population were not related to baseline values or changes over time in liver stiffness or serum ALP, according to stratification and correlation analyses. Similarly, correlation analyses did not indicate a relationship between improvements in fatigue and change from baseline in IL-6 or hsCRP levels. Previous research suggests that fatigue symptoms in patients with PBC are unrelated to stage of disease and may have an extrahepatic etiology.7,31,32 Thus, the absence of a relationship between improvement in fatigue and markers of liver disease in the current study suggest that the observed effects of setanaxib on fatigue are PBC-specific, rather than due to the alleviation of general fatigue related to cirrhosis.
Recent studies have reported that treatment with UDCA does not reduce relative risk of fatigue in patients with PBC; fatigue was even given the highest symptom burden score by patients receiving UDCA (followed by emotional, social, and symptom PBC-40 domains).31,33 The only trial to date that assessed the effects of OCA treatment on fatigue did not show alleviation of symptoms.8 Liver transplantation, a last resort for some patients with end-stage PBC and not a treatment option for fatigue alone, has been reported to result in some alleviation and reduction in risk of fatigue, although not to normal levels.31,34 This suggests that none of the currently approved interventions for PBC are able to target the underlying cause of fatigue. Although the exact pathophysiology of fatigue in PBC is poorly understood, it is considered to have both peripheral and central components.35 Whereas UDCA and OCA primarily target cholestasis, setanaxib inhibits NOX isoforms 1 and 4. The NOX family of enzymes produce reactive oxygen species which can trigger muscle wasting and fatigue.36 Oxidative stress has also been suggested as a potential mechanism of fatigue in PBC.37 The results of the present study may therefore support the theory that setanaxib has a direct impact on peripheral fatigue in PBC; however, this would need to be investigated in a future study that is optimized to evaluate changes in fatigue. As proposed by Phaw et al.,10 ideally such a study would distinguish between populations of patients with central versus peripheral fatigue endotypes.
In addition, while seladelpar has been shown to improve pruritus, sleep disturbance, and fatigue (according to the pruritus visual analogue scale, the 5-D itch scale, and the PBC-40 itch and fatigue domains) among patients with PBC in an uncontrolled, phase 2 study, these results were not independent of each other, suggesting that the fatigue improvements were not central to seladelpar.21 In contrast, the post hoc results of the present phase 2 trial for setanaxib in PBC demonstrate improvements in scores in the PBC-40 fatigue domain for patients receiving setanaxib 400 mg BID, in the absence of improvements in the itch domain. This suggests that any effect setanaxib may have on fatigue in PBC is unrelated to pruritus-related sleep disturbance.
Symptomatic presentation (including fatigue) at PBC onset has previously been linked to faster-progressing and UDCA-resistant disease with reduced survival rates.38,39 The patients in the moderate-to-severe fatigue subgroup in the present study therefore represent clinically significant symptomatic patients who are most in need of innovative treatments for PBC. Greater improvements in the fatigue, emotional, social, and symptom domains were observed in patients with moderate-to-severe fatigue following setanaxib 400 mg BID treatment compared to the mildly fatigued group. These improvements were accompanied by alleviation of cognitive dysfunction in the moderate-to-severe fatigue group, again supporting the effectiveness of setanaxib 400 mg BID in reducing fatigue-related symptoms in patients with clinically relevant fatigue. Meanwhile, setanaxib 400 mg BID treatment did not seem to alleviate social or cognitive dysfunction in patients who had mild fatigue at baseline, possibly due to low symptom burden at baseline compared with those with moderate-to-severe fatigue (Table 2). It has been previously demonstrated that fatigue has the greatest impact on patient-reported QoL when accompanied by symptoms of social dysfunction,40 therefore if setanaxib 400 mg BID is able to alleviate symptoms of both fatigue and social dysfunction in patients with clinically-severe fatigue, this could result in a substantial improvement in overall QoL. These results highlight the unmet need in patients with clinically severe fatigue; however, these data should be interpreted with caution as the number of patients in each treatment arm stratified by fatigue severity were small [(mild/moderate-to-severe) placebo: n=18/n=18; setanaxib 400 mg OD: n=20/n=17; setanaxib 400 mg BID: n=13/n=17].
As the mechanism of fatigue in PBC is still poorly understood, there are many outstanding questions regarding the potential effect of setanaxib on fatigue-related symptoms. Future studies may explore this further, once the pathogenesis of PBC-related fatigue has been elucidated or suitable biomarkers have been identified. The post hoc results reported at this time are therefore purely descriptive but may be used to inform future investigations.
Strengths and limitations
The strengths of the study included the use of the PBC-40 questionnaire, which was developed and verified for patients with PBC, thus the findings are valid for this patient population. Use of the PBC-40 questionnaire also enabled the investigation of the impact of fatigue on patient QoL in the context of other important symptoms of PBC. This study also included 111 patients across 9 countries over a 24-week period, with regular assessments of outcomes of interest; it was therefore one of the largest and longest phase 2 trials conducted for this indication, and provided a large data set that was representative of patients across multiple geographies. The demographics of the ITT population were similar to those observed in clinical settings (Invernizzi P., unpublished manuscript).23 It may therefore be possible to extrapolate results from the ITT population to patients in a real-world setting.
Of the patients included in this study, 50.5% had moderate-to-severe fatigue at baseline, which is reflective of the general patient population.10 However, due to the small number of patients with moderate-to-severe fatigue in each treatment group, the results in these subgroups should not be assumed for all patients with clinically significant fatigue. Finally, this phase 2 trial was not powered for the interpretation of secondary endpoints, such as QoL data, and as such these post hoc data are purely descriptive and should be interpreted accordingly.
CONCLUSIONS
In conclusion, the post hoc results of this 24-week phase 2 trial suggest that setanaxib 400 mg BID treatment may be associated with improved fatigue and emotional function in patients with PBC; plus improved social, emotional, and cognitive function in those with clinically significant fatigue. These results support the further investigation of setanaxib, used in addition to the current standard of care, UDCA, for patients experiencing QoL impairments, particularly those with clinically significant levels of fatigue who therefore have the greatest unmet need. Recruitment is currently underway for a phase 2b/3 trial for setanaxib, which will aim to evaluate efficacy, safety, and QoL outcomes in patients with PBC following treatment with higher dosages of setanaxib in addition to UDCA.41
AUTHOR CONTRIBUTIONS
David Jones: conceptualization, methodology, validation, writing—review and editing. Marco Carbone, Pietro Invernizzi, Frederik Nevens, Mark Swain, and Cynthia Levy: conceptualization, validation, writing—review and editing. Nicola Little: conceptualization, formal analysis, methodology, validation, writing—review and editing. Philippe Wiesel: conceptualization, data curation, investigation, methodology, project administration, resources, supervision, validation, writing—review and editing. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
ACKNOWLEDGMENTS
The authors thank the patients, the investigators and their teams who took part in this study. The authors also acknowledge Krassimir Mitchev, MD, PhD, Calliditas Therapeutics AB, London, UK, for publication coordination and Tara Groves, BSc, and Sarah Jayne Clements, PhD, from Costello Medical, Cambridge, UK, for medical writing and editorial assistance based on the authors’ input and direction. The statistical analysis protocol was designed and carried out by Alvin Ng, BSc, from Costello Medical, Singapore, in collaboration with Calliditas Therapeutics AB. Pietro Invernizzi, Marco Carbone, and Frederik Nevens are members of the European Reference Network on Hepatological Diseases (ERN RARE-LIVER). The authors thank AMAF Monza ONLUS and AIRCS for the unrestricted research funding. Pietro Invernizzi and Marco Carbone acknowledge that this research was partially supported by the Italian Ministry of University and Research (MIUR) Department of Excellence project PREMIA (PREcision MedIcine Approach: Bringing Biomarker Research to Clinic).
FUNDING INFORMATION
This study was sponsored by Genkyotex. Support for third-party writing assistance for this article from Costello Medical, UK, was funded by Calliditas Therapeutics in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
CONFLICT OF INTEREST
David Jones: consultancy and grant funding from Intercept; speaker fees from Abbott and Falk; consultancy fees from Calliditas Therapeutics AB and GSK. Marco Carbone: consultancy fees from CymaBay Therapeutics, Echosens, Genkyotex, Intercept Pharmaceuticals, Mayoly Spindler, Moderna Inc., and Perspectum; grant/research support from Genetic SpA, and Intercept Pharmaceuticals. Pietro Invernizzi: grant/research support from Bruschettini SRL, Gilead Sciences, and Intercept Pharmaceuticals. Nicola Little: consultancy fees from Calliditas Therapeutics AB. Frederik Nevens: consultancy fees from AbbVie, AgomAb therapeutics, Biotest, BMS, Chemomab Therapeutics, Cook Medical, Durect, Dynacure, Ferring, Fundplus, Genkyotex, Gilead, Gore, Intercept, Ipsen, Mayoly Spindler, MedPartners, MSD, Promethera, and Twin Pharma. Mark G. Swain: clinical trial funding from Astra Zeneca, Celgene, CymaBay, Galectin Therapeutics, Genfit, Genkyotex, Gilead, GSK, Intercept, Novartis, Novo Nordisk, and Pfizer (funded investigator initiated studies for Gilead and Intercept); membership on advisory boards: Gilead, Intercept, and Roche; membership on speakers’ bureaus: Abbott, Gilead, and Intercept. Philippe Wiesel: employee of AgomAb Therapeutics; former stockholder and employee of Genkyotex. Cynthia Levy: grant/research support from Alnylam Pharmaceuticals, Calliditas Therapeutics AB, Cara Therapeutics, CymaBay Therapeutics, Genkyotex, GENFIT, Gilead Sciences, GSK, HighTide Therapeutics, Intercept Pharmaceuticals, Mirum Pharmaceuticals, Mitsubishi Tanabe Pharma Corporation, Novartis, Pliant Therapeutics, and Zydus Pharmaceuticals; consultancy fees from Calliditas Therapeutics AB, Cara Therapeutics, GENFIT, GSK, Mirum Pharmaceuticals Inc., Pliant Therap eutics, and Teva Pharmaceuticals.
DATA SHARING STATEMENT
Study data, including individual participant data, will not be made available to others after publication. The study protocol is available for access at https://clinicaltrials.gov/ct2/show/study/NCT03226067.
Author names in bold designate shared co-first authorship.
Footnotes
Abbreviations: ALP, alkaline phosphatase; BID, twice daily; FDA, Food and Drug Administration; GCP, Good Clinical Practice; hsCRP, high-sensitivity C-reactive protein; ICH, International Conference on Harmonization, ITT: intention-to-treat; OCA, obeticholic acid; OD, once daily; NOX, NADP oxidase; PBC, primary biliary cholangitis; PBC-40, primary biliary cholangitis-40 item questionnaire; QoL, quality of life; UDCA, ursodeoxycholic acid; ULN, upper limit of normal.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.hepcommjournal.com.
Contributor Information
David Jones, Email: David.Jones@newcastle.ac.uk.
Marco Carbone, Email: marco.carbone@unimib.it.
Pietro Invernizzi, Email: pietro.invernizzi@unimib.it.
Nicola Little, Email: nicola.little@calliditas.com.
Frederik Nevens, Email: frederik.nevens@uzleuven.be.
Mark G. Swain, Email: swain@ucalgary.ca.
Philippe Wiesel, Email: philippe.wiesel@agomab.com.
Cynthia Levy, Email: clevy@med.miami.edu.
REFERENCES
- 1.Lleo A, Leung PSC, Hirschfield GM, Gershwin EM. The pathogenesis of primary biliary cholangitis: a comprehensive review. Semin Liver Dis. 2020;40:34–48. [DOI] [PubMed] [Google Scholar]
- 2.Rannard A, Buck D, Jones DE, James OF, Jacoby A. Assessing quality of life in primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2004;2:164–74. [DOI] [PubMed] [Google Scholar]
- 3.Onofrio FQ, Hirschfield GM, Gulamhusein AF. A practical review of primary biliary cholangitis for the gastroenterologist. Gastroenterol Hepatol (N Y). 2019;15:145–54. [PMC free article] [PubMed] [Google Scholar]
- 4.Purohit T, Cappell MS. Primary biliary cirrhosis: pathophysiology, clinical presentation and therapy. World J Hepatol. 2015;7:926–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin XY, Khan TM. Quality of life among patients suffering from cholestatic liver disease-induced pruritus: a systematic review. J Formos Med Assoc. 2016;115:689–702. [DOI] [PubMed] [Google Scholar]
- 6.Hirschfield GM, Dyson JK, Alexander GJM, Chapman MH, Collier J, Hübscher S, et al. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut. 2018;67:1568–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selmi C, Gershwin ME, Lindor KD, Worman HJ, Gold EB, Watnik M, et al. Quality of life and everyday activities in patients with primary biliary cirrhosis. Hepatology. 2007;46:1836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375:631–43. [DOI] [PubMed] [Google Scholar]
- 9.Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69:394–419. [DOI] [PubMed] [Google Scholar]
- 10.Phaw NA, Dyson JK, Mells G, Jones D. Understanding fatigue in primary biliary cholangitis. Dig Dis Sci. 2021;66:2380–6. [DOI] [PubMed] [Google Scholar]
- 11.Hegade VS, Mells GF, Fisher H, Kendrick S, DiBello J, Gilchrist K, et al. Pruritus is common and undertreated in patients with primary biliary cholangitis in the United Kingdom. Clin Gastroenterol Hepatol. 2019;17:1379.e1373. [DOI] [PubMed] [Google Scholar]
- 12.Khanna A, Hegade VS, Jones DE. Management of fatigue in primary biliary cholangitis. Curr Hepatol Rep. 2019;18:127–33. [Google Scholar]
- 13.Lindor KD, Dickson ER, Baldus WP, Jorgensen RA, Ludwig J, Murtaugh PA, et al. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology. 1994;106:1284–90. [DOI] [PubMed] [Google Scholar]
- 14.Heathcote EJ, Cauch-Dudek K, Walker V, Bailey RJ, Blendis LM, Ghent CN, et al. The Canadian multicenter double-blind randomized controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology. 1994;19:1149–56. [PubMed] [Google Scholar]
- 15.Combes B, Carithers RL, Maddrey WC, Lin D, McDonald MF, Wheeler DE, et al. A randomized, double-blind, placebo-controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology. 1995;22:759–66. [PubMed] [Google Scholar]
- 16.Hirschfield GM, Mason A, Luketic V, Lindor K, Gordon SC, Mayo M, et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148:751.e758. [DOI] [PubMed] [Google Scholar]
- 17.Rudic JS, Poropat G, Krstic MN, Bjelakovic G, Gluud C. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database Syst Rev. 2012;12:CD000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Food and Drug Administration. Highlights of prescribing information: Ocaliva® (obeticholic acid). Accessed June 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/207999s007lbl.pdf
- 19.De Vries E, Bolier R, Goet J, Parés A, Verbeek J, De Vree M, et al. Fibrates for itch (FITCH) in fibrosing cholangiopathies: a double-blind, randomized, placebo-controlled trial. Gastroenterology. 2021;160:734.e736. [DOI] [PubMed] [Google Scholar]
- 20.Corpechot C, Chazouillères O, Rousseau A, Le Gruyer A, Habersetzer F, Mathurin P, et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N Engl J Med. 2018;378:2171–81. [DOI] [PubMed] [Google Scholar]
- 21.Kremer AE, Mayo MJ, Hirschfield G, Levy C, Bowlus CL, Jones DE, et al. Seladelpar improved measures of pruritus, sleep, and fatigue and decreased serum bile acids in patients with primary biliary cholangitis. Liver Int. 2022;42:112–23. [DOI] [PubMed] [Google Scholar]
- 22.Huang J, Invernizzi P, Dalekos G, Nevens F, Van Vlierberghe H, Zigmond E, et al. The NOX1/4 inhibitor GKT831 achieves clinically meaningful reductions in liver stiffness, attenuates cholestasis, and improves quality of life in patients with primary biliary cholangitis [Abstract]. Hepatology. 2019;70(suppl):777A–8A. [Google Scholar]
- 23. https://clinicaltrials.gov/ct2/show/NCT03226067 ClinicalTrials.gov. Study to Assess Safety & Efficacy of GKT137831 in Patients With Primary Biliary Cholangitis Receiving Ursodiol. Accessed October 25, 2022. [Google Scholar]
- 24.Newton JL, Hudson M, Tachtatzis P, Sutcliffe K, Pairman J, Burt JA, et al. Population prevalence and symptom associations of autonomic dysfunction in primary biliary cirrhosis. Hepatology. 2007;45:1496–505. [DOI] [PubMed] [Google Scholar]
- 25.Corpechot C, Carrat F, Poujol-Robert A, Gaouar F, Wendum D, Chazouillères O, et al. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology. 2012;56:198–208. [DOI] [PubMed] [Google Scholar]
- 26.Lammers WJ, van Buuren HR, Hirschfield GM, Janssen HL, Invernizzi P, Mason AL, et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338.e1335. [DOI] [PubMed] [Google Scholar]
- 27.Jacoby A, Rannard A, Buck D, Bhala N, Newton J, James O, et al. Development, validation, and evaluation of the PBC-40, a disease specific health related quality of life measure for primary biliary cirrhosis. Gut. 2005;54:1622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jopson L, Newton JL, Palmer J, Floudas A, Isaacs J, Qian J, et al. RITPBC: B-cell depleting therapy (rituximab) as a treatment for fatigue in primary biliary cirrhosis: study protocol for a randomised controlled trial. BMJ Open. 2015;5:e007985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khanna A, Jopson L, Howel D, Bryant A, Blamire A, Newton JL, et al. Rituximab is ineffective for treatment of fatigue in primary biliary cholangitis: a phase 2 randomized controlled trial. Hepatology. 2019;70:1646–57. [DOI] [PubMed] [Google Scholar]
- 30.Overholser BR, Sowinski KM. Biostatistics primer: part 2. Nutr Clin Pract. 2008;23:76–84. [DOI] [PubMed] [Google Scholar]
- 31.Lee JY, Danford CJ, Trivedi HD, Tapper EB, Patwardhan VR, Bonder A. Treatment of fatigue in primary biliary cholangitis: a systematic review and meta-analysis. Dig Dis Sci. 2019;64:2338–50. [DOI] [PubMed] [Google Scholar]
- 32.Newton JL, Bhala N, Burt J, Jones DE. Characterisation of the associations and impact of symptoms in primary biliary cirrhosis using a disease specific quality of life measure. J Hepatol. 2006;44:776–83. [DOI] [PubMed] [Google Scholar]
- 33.Kaps L, Grambihler A, Yemane B, Nagel M, Labenz C, Ploch P, et al. Symptom burden and treatment response in patients with primary biliary cholangitis (PBC). Dig Dis Sci. 2020;65:3006–13. [DOI] [PubMed] [Google Scholar]
- 34.Carbone M, Bufton S, Monaco A, Griffiths L, Jones DE, Neuberger JM. The effect of liver transplantation on fatigue in patients with primary biliary cirrhosis: a prospective study. J Hepatol. 2013;59:490–94. [DOI] [PubMed] [Google Scholar]
- 35.Swain MG, Jones DEJ. Fatigue in chronic liver disease: new insights and therapeutic approaches. Liver Int. 2019;39:6–19. [DOI] [PubMed] [Google Scholar]
- 36.Pal R, Basu Thakur P, Li S, Minard C, Rodney GG. Real-time imaging of NADPH oxidase activity in living cells using a novel fluorescent protein reporter. PLoS One. 2013;8:e63989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar D, Tandon RK. Fatigue in cholestatic liver disease--a perplexing symptom. Postgrad Med J. 2002;78:404–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quarneti C, Muratori P, Lalanne C, Fabbri A, Menichella R, Granito A, et al. Fatigue and pruritus at onset identify a more aggressive subset of primary biliary cirrhosis. Liver Int. 2015;35:636–41. [DOI] [PubMed] [Google Scholar]
- 39.Jones DE, Al-Rifai A, Frith J, Patanwala I, Newton JL. The independent effects of fatigue and UDCA therapy on mortality in primary biliary cirrhosis: results of a 9 year follow-up. J Hepatol. 2010;53:911–7. [DOI] [PubMed] [Google Scholar]
- 40.Mells GF, Pells G, Newton JL, Bathgate AJ, Burroughs AK, Heneghan MA, et al. Impact of primary biliary cirrhosis on perceived quality of life: the UK-PBC national study. Hepatology. 2013;58:273–83. [DOI] [PubMed] [Google Scholar]
- 41.Jones D, Carlsson S, von Reedtz S, Levy C. A phase 2b/3 randomized, placebo-controlled, double-blind trial for treatment of primary biliary cholangitis (PBC) with setanaxib: objectives and study design [Abstract]. Hepatology. 2022;76(S1):S1461–S1462; Abstract 4700. [Google Scholar]


