Abstract
As the USA faces a worsening overdose crisis, improving access to evidence-based treatment for opioid use disorder (OUD) remains a policy priority. Federal regulatory changes in response to the COVID-19 pandemic substantially expanded flexibilities on take-home doses for methadone treatment for OUD. These changes have fuelled questions about the effect of new regulations on OUD outcomes and the potential effect on health of permanently integrating these flexibilities into treatment policy going forward. To aide US policy makers as they consider implementing permanent methadone regulatory changes, we conducted a review synthesising peer-reviewed research on the effect of the flexibilities of methadone take-home policies introduced during COVID-19 on methadone programme operations, OUD patient and provider experiences, and patient health outcomes. We interpret the findings in the context of the federal rule-making process and discuss avenues by which these findings can be incorporated and implemented into US policies on substance use treatment going forward.
Introduction
One million lives have been lost to the overdose crisis that has ravaged US communities for two decades.1 Exacerbated by the COVID-19 pandemic, 2021 was the deadliest year of this crisis to date, with over 100 000 deaths caused by overdose.2, 3, 4 A central challenge of the overdose crisis, both before and during the pandemic, has been limited access to life-saving treatments with medications for opioid use disorder (MOUD), particularly methadone and buprenorphine.5 These pharmacological treatments are highly effective at reducing risk of overdose6 and improving many other health and social outcomes.7 Of all MOUD, methadone has the most extensive evidence base and has been used successfully for treatment of opioid use disorder (OUD) since the 1960s.8 However, in 2020, only 311 000 people received methadone; less than 5% of the 7·6 million individuals estimated to have OUD.9, 10
The limited utilisation of methadone is largely attributed to the rigid and burdensome structure by which methadone treatment is regulated and delivered in the USA. Heavily influenced by the racialised drug-war rhetoric during the 1970s, the US system only allows methadone to be delivered via specialty opioid treatment programmes (OTPs) that are subject to stringent regulations by the Drug Enforcement Administration and the Substance Abuse and Mental Health Services Administration (SAMHSA).11, 12 Citing concern for misuse, diversion, and risk of overdose, these regulations limit the number of take-home doses given to patients and go even further to prohibit entities, such as pharmacies, from having a role13—an approach that differs from that of the UK, Australia, and Canada.14
The result is a system that requires patients to make almost daily visits to an OTP to receive medication, except on days that the clinic is closed and patients can take a dose home. Patients can qualify for additional take-home doses, but authorisation can take months or years and often depends on many subjective factors decided upon by clinic staff.12 This system is especially burdensome for patients who live far from OTPs, do not have transportation, or have competing work or childcare responsibilities.15 Additionally, this system disproportionately affects racially minoritised communities who have less access to office-based buprenorphine.16 Decades of research document experiences of patients who describe the OTP system, and the daily visits in particular, as burdensome, degrading, and dehumanising, often acting as a deterrent from initiating or staying in treatment.15, 17
In response to the COVID-19 pandemic, federal regulators in the USA issued a suite of policy changes to support physical distancing in health care.18 In mid-March, 2020, SAMHSA issued guidance allowing states to request flexibility for OTPs to give additional take-home doses of methadone. Under this policy, patients considered stable could receive 28 days of medication and patients considered less stable could receive up to 14 days of medication.19 In 2021, SAMHSA announced plans to make the pandemic flexibilities permanent.
Long-standing federal policy states that as agencies prepare to issue new rules, they should draw upon the best reasonably obtainable scientific information to inform their policies (Executive Order 12866).20 A common challenge regulators face in justifying proposed policy changes is not having data. In this case, SAMHSA can benefit from dozens of studies that explored the effects of the pandemic flexibilities for methadone take-home doses. In this Health Policy review, we aim to: (1) extract, review, and synthesise published research on the effects of the flexibilities introduced during the COVID-19 pandemic regarding methadone take-home doses on the operations of OTPs, patient and provider experiences, and patient health outcomes; (2) interpret research findings in the context of the US federal rule-making process; and (3) discuss avenues by which findings can be incorporated and implemented into updated federal regulations.
Methods
Search strategy and selection criteria
We searched for peer-reviewed studies published between March 1, 2020, and Sept 6, 2022. We searched PubMed, PsycInfo, and Google Scholar with combinations of the following terms: “COVID-19”, “pandemic”, “methadone”, “take-home”, “methadone maintenance therapy/MMT”, “opioid treatment program/OTP”, “opioid-related disorder”, and “opiate substitution treatment” (see appendix p 1 for search strategy). We also reviewed reference lists from included articles for relevant studies not identified by the database search. We included articles that were published in English, based in the USA, original research, and focused on measuring the role or effect of the SAMHSA's COVID-19 pandemic guidance. We excluded any articles focusing solely on pre-pandemic outcomes. Using Covidence, a subscription-based systematic review tool,21 we removed duplicates, screened titles and abstracts for relevance, and reviewed the full text to assess eligibility on the basis of inclusion criteria. The full study team conferred to select the final list of eligible articles. We then extracted findings on six research questions with policy relevance: how the new methadone take-home flexibilities (1) were implemented; (2) influenced perceptions and experiences of methadone patients; (3) influenced perceptions and experiences of methadone providers; (4) affected overdose risk; (5) affected illicit drug use and methadone non-adherence or diversion; and (6) affected the initiation and retention of methadone treatment.
Synthesis of findings in the context of federal rule making
We first reviewed and synthesised findings related to each of the research questions, considering the different samples, study designs, analytical methods used, and limitations and strengths of each study. We then assessed the implications of the findings for the upcoming SAMHSA rule making. In the USA, to promulgate a new rule, federal regulators must generally follow particular steps set out in the Administrative Procedure Act: issue a proposed rule, take public comment, and then issue a final rule (US Code title 5 section 553).22 In a proposed rule, the regulator explains proposed changes and provides legal, policy, economic, and other justifications for the changes. The regulator's task is not merely to describe the regulatory change, but also to explain why the change is consistent with the law and in the public interest. Our findings are therefore organised using the instructions for regulators outlined in Executive Order 12866,20 with a subsequent discussion of implications and implementation considerations.
Results
Characteristics of reviewed studies
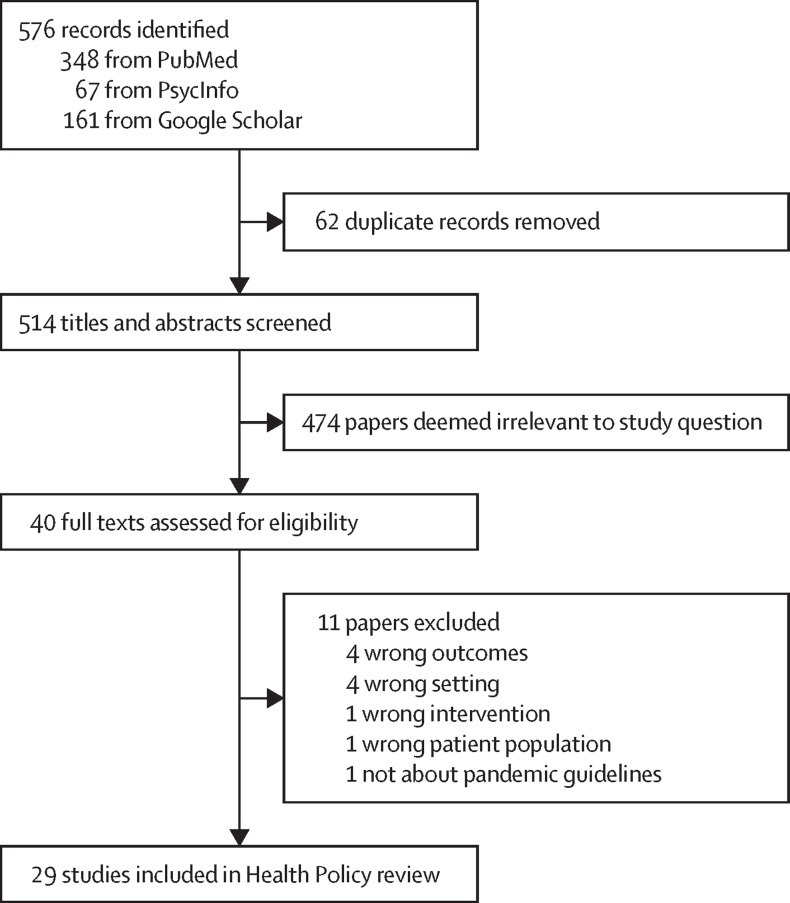
The search resulted in a total of 576 articles, of which 29 met the full criteria for this review (figure ). Descriptive characteristics of the 29 articles (table ) and detailed characteristics and outcomes by study are available (appendix p 2). Most studies were qualitative (11 [38%] of 29) or observational outcome studies (12 [41%]), and took place across multiple OTPs (13 [45%]) and multiple US states (11 [38%]). The most common outcome assessed was implementation of new take-home flexibilities (17 [59%]), followed by methadone patient experiences (10 [34%]) and provider experiences (7 [24%]). To illustrate many of the patient and provider perspectives expressed, we include a subset of direct quotes extracted from qualitative studies conveying some of the main emerging themes regarding experiences and perceptions (appendix pp 5–6).23, 24, 25, 26, 27, 28, 29, 30, 31 Summaries of the primary findings by each of our six policy-relevant questions are presented in the panel .
Figure.
Studies considered and selected in the Health Policy review
Table.
Characteristics of included studies
| n (%) of 29 studies | |
|---|---|
| Study design* | |
| Observational outcomes study | 12 (41·38%) |
| Qualitative study | 11 (37·93%) |
| Closed end survey | 4 (13·79%) |
| Randomised trial | 1 (3·45%) |
| Quantitative content analysis† | 2 (6·90%) |
| Setting | |
| Single OTP | 7 (24·14%) |
| Multiple OTPs | 13 (44·83%) |
| Other‡ | 9 (31·03%) |
| US region | |
| Northeast | 7 (24·14%) |
| South | 3 (10·34%) |
| Midwest | 1 (3·45%) |
| West | 6 (20·69%) |
| Multistate§ | 12 (41·38%) |
| Research question addressed* | |
| Implementation | 17 (58·62%) |
| Patient treatment experience and quality of life | 10 (34·48%) |
| Provider experiences and attitudes towards care | 7 (24·14%) |
| Overdose | 6 (20·69%) |
| Illicit drug use and diversion | 8 (27.59%) |
| Treatment initiation and retention | 1 (3·45%) |
OTP=Opioid treatment programme.
Not mutually exclusive categories because studies might have multiple outcomes or a mixed methods design.
Quantitative content analyses used natural language processing and machine learning to evaluate outcomes.
Studies from settings other than OTPs include Reddit forums (n=4); convenience sample of people who use drugs, clinicians, and US Government officials (n=3); and the National Poison Data System (n=1).
Multistate studies include participants from more than one state, including the studies that used Reddit and the National Poison Data System as data sources. Although Reddit is an international platform, we used findings from studies that referred to the US methadone treatment system.
Panel. Summary of findings by research question.
How did the new methadone take-home flexibilities become implemented in practice across opioid treatment programmes (OTPs)?
OTPs overall increased frequency of take-home doses, but only a minority of patients were granted the maximum allowable 14-day or 28-day take-home doses.9, 21, 30, 32, 33, 34, 35, 36, 37, 38, 39
Take-home eligibility was most often based on patient substance use, time in treatment, ability to safely store methadone, and risk of COVID-19.9, 26, 30, 32, 35, 37, 39, 40, 41
Barriers to implementation of flexibilities included concerns around managing take-home doses for patients with ongoing substance use, concerns around government oversight and liability, and concerns around financial sustainability.9, 28, 30, 31, 35, 39, 40
Facilitators to the implementation of flexibilities included change agents who encouraged the uptake of take-home doses, leveraging multidisciplinary clinical teams to determine take-home doses, telehealth and reduced toxicology testing to support a decrease in frequency of visits, and the use of medication lock boxes and other diversion prevention tactics.9, 29, 32, 35, 40, 41, 42
How did the new methadone take-home flexibilities influence perceptions and experiences of methadone patients?
Despite new flexibilities, some patients expressed a continued absence of or little access to methadone take-home privileges, which was often seen as unjust, burdensome, and a disincentive for methadone engagement.23, 24, 25, 27, 28, 43, 44
Many patients expressed that increased take-home doses supported their treatment experience by improving self-esteem and autonomy, reducing treatment burden, and avoiding negative triggers associated with clinic attendance.23, 24, 26, 27, 28, 43, 45
Some patients expressed that extended take-home flexibilities disrupted their routine and treatment stability, but incidences of diversion were rare.21, 23, 24, 27, 43
How did the new methadone take-home flexibilities influence perceptions and experiences of methadone providers?
Many providers expressed that take-home flexibilities allowed them to provide more patient-centred care, improved patient motivation, and reduced treatment burden.9, 28, 29, 30, 31, 40
Many providers expressed concern that pandemic flexibilities and less frequent contact with patients could be destabilising and lead to undesirable patient behaviours.28, 29, 30, 31, 39, 40
How did the new methadone take-home flexibilities impact overdose risk?
No significant increases in methadone overdoses were observed in relation to the implementation of take-home flexibilities.32, 35, 36, 46, 47, 48
How did the new methadone take-home flexibilities affect illicit drug use and methadone non-adherence?
Findings on changes in illicit substance use during the pandemic were mixed, but could not be necessarily attributable to new flexibilities in take-home doses.26, 46, 49
Findings on changes in methadone non-adherence during the pandemic were mixed, but could not be necessarily attributable to new flexibilities in take-home doses.36, 41, 46, 49, 50
How did the new methadone take-home flexibilities affect methadone treatment initiation and retention?
Only one study assessed treatment retention, finding that modest improvements in retention were associated with increased take-home doses.26
Implications for federal regulators: uptake of take-home flexibilities
Under EO 12866,20 the regulator begins by explaining the need for the proposed rule. Four findings on this subject emerged. First, all studies that explored the frequency of take-home doses observed some increase following the implementation of the COVID-19 pandemic guidance. The amount by which the proportion of patients who received take-home doses increased varied by study and clinic. Three studies directly asked OTP providers about changes to the volume of patient take-home doses. In multistate interviews with OTP clinicians, 72% indicated that their OTPs increased the number of take-home doses.30 Similarly, 25 (66%) of 38 of OTP directors surveyed in Pennsylvania noted they extended take-home supplies following the new flexibilities.51 In a survey of all eight OTPs in Connecticut, directors reported that the number of patients receiving one or no take-home doses decreased from 37·5% before to 9·6% after the introduction of the pandemic guidelines.32
Three surveys asked patients about take-home doses: in a survey of OTP patients in North Carolina, 87 (91·6%) said they received some take-home doses following the pandemic flexibilities compared with 69 (68·3%) who had received take-home doses before the pandemic.52 In a multistate survey, 185 (76%) of 243 OTP patients reported receiving more take-home doses since the pandemic.33 Across eight OTPs in New England, 79 (42%) of 188 patients surveyed reported receiving increased access to take-home medication.34 Other studies analysed OTP patient records directly before and after the introduction of the pandemic flexibilities. A study of five OTPs in New York, NY saw a reduction in the proportion of patients who came to the OTP 5–6 days per week from 47·2% to 9·4%.35 An OTP in Washington reported that over 90% of patients had increases in take-home doses, with an increase from an average of 11·4 to 22·3 monthly take-home doses, with increases sustained between the two samples analysed.36, 37 A study of patient records across Oregon's 20 OTPs found a 54% reduction in average monthly visits, with average take-home doses increasing from 5·8 to 11·3 per month.38 This uptake suggests many providers were able and willing to implement programme changes to increase take-home supply, even in a time of great uncertainty.
Second, despite some increase in take-home doses, providers did not uniformly grant patients the maximum supplies of 14 or 28 days. In a multistate survey of 170 OTP providers, 80 (47%) reported they routinely allowed 14 days of take-home doses for newly enrolled patients, 89 (52%) allowed 14 days of take-home doses for so-called less stable patients, and 112 (66%) allowed 28 days of take-home doses for so-called stable patients.39 State-specific studies support these findings: across OTPs in Connecticut, the proportion of patients receiving 14-day take-home doses increased from 14·2% to 26·8% and the proportion receiving 28-day take-home doses increased from 0·1% to 16·8%.32 Similarly, over 90% of OTPs in Pennsylvania noted that less than half of their patients received 14-day take-home doses, and 95% noted that less than a quarter of their patients received 28-day take-home doses.52 Two studies quantitatively compared take-home practices on the basis of OTP characteristics—including the size of OTP (ie, number of clients served), for-profit status, and urbanicity—and found no differences by provider characteristics.39, 51 Findings suggest that, even under a more permissive policy, providers will not necessarily permit all patients to receive maximum amounts of take-home supplies.
Third, some providers interviewed in qualitative studies expressed concern over patients continuing to use other drugs, such as sedatives.28, 35 Some expressed concerns about reduced vigilance or oversight of patients,40 or feared scrutiny of practices and outcomes by federal and state agencies.39 Many expressed concerns about how take-home dosing would reduce revenue.30, 39, 40, 51 Some noted concerns around legal liability for potential overdose or diversion of methadone, which made them apprehensive about take-home dosing over the long term.30, 31, 39 These findings suggest that provider uncertainty about the regulations, consequences for patients, and finances discouraged uptake.
Last, despite various uncertainties, providers in some studies described strategies to support effective uptake. These strategies included having OTP directors act as change agents to support the adoption of new processes and practices,40 or interdisciplinary teams to guide take-home decisions.35 Some studies found that providers were willing to provide additional take-home doses indefinitely: in a survey of OTP directors in Pennsylvania, 38 (79%) agreed with maintaining more flexibility on take-home length.51 Some providers believed that the criteria for establishing take-home doses before the COVID-19 pandemic were too strict, placing limits on providers’ clinical judgement.29 Many were supportive of retaining the flexibilities to improve access to and quality of care,28 or worried about returning to a more restrictive schedule if the pandemic flexibilities were rescinded.29 These findings support the idea that, if continued, flexibility for take-home doses could become a part of regular practice.
Potential benefits of the proposed rule
The next step in regulatory analysis explores benefits and costs of the proposal. First, many patients described that receiving more take-home doses and being given the responsibility to manage their medication resulted in feelings of pride, accomplishment, and self-confidence that supported treatment goals and sobriety and helped build a stronger relationship with their providers.26, 27, 28, 43 Patients described additional take-home doses as liberating24 and valued how additional take-home doses, and reduced OTP visits, provided them with a sense of normalcy and stability27 and reduced stigma associated with frequent attendance at the clinic.45
Patients also reported that reduced travel to the clinic gave them more time to attend to aspects of their lives, such as jobs, school, caregiving, and recreation.23, 26, 28, 45 Some described how liberating it was to not have to arrange child care or get up early and commute before or after work, to have more time for family, and to spend less time and money driving to and from the clinic on a daily basis.27 Patients also described that additional take-home doses allowed them to avoid the clinic and triggers for use: fewer clinic visits reduced exposure to so-called less stable individuals in recovery and other potential triggers.26, 27 Others described that having fewer people in the waiting room and reduced crowding created a healthier atmosphere for mental health and was beneficial in preventing transmission of COVID-19 and other infections to family members.26
In addition to positive patient experiences, one study found that additional take-home doses were associated with a lower probability of treatment discontinuation. Of three groups examined by the number of days in treatment at the start of the study period (ie, <90 days, 90–180 days, or 180+ days), only individuals with at least 180 days of treatment received additional take-home doses and this group saw reduced odds of treatment discontinuation for every increase in take-home dosing above expected prepandemic regimens. Only patients in treatment fewer than 90 days—who did not receive additional take-home doses—were more likely to discontinue treatment in the COVID-19 period (13% before COVID-19 vs 26% after COVID-19).26
Beyond patient benefits, many providers expressed appreciation for the new flexibility to make better and more equitable decisions to support the needs of their patients.28, 29, 30, 40 Some providers also noted that the additional take-home doses during the pandemic permitted greater adherence to treatment and improved autonomy and motivations to change.29 Providers expressed that over-regulation of methadone undermined patient-centred care, impeded methadone access, and was a waste of resources.32 In a survey of OTP directors in Pennsylvania, 96% agreed that take-home methadone is less burdensome for accessing treatment.51 Some OTPs were willing to try different protocols and technologies to improve flexibility, such as telehealth32 and reduced toxicology testing35 to support decreased frequency of visits.
Potential costs of the proposed rule
Some studies attempted to elucidate potential costs of flexibility for take-home doses, including concerns about overdose, patient destabilisation, and diversion. Indeed, studies found that providers worried that less frequent contact with patients would lead to patient destabilisation28, 39 or difficulty building rapport.30 A few expressed concerns regarding overdose risk associated with misuse.31, 40 Other concerns centred on having less control over what were seen as undesired behaviours, such as patients not taking methadone as prescribed, diverting medications, or continuing to use other substances.29, 31, 40 However, these concerns rarely precipitated.
Our Health Policy review finds no evidence of increased methadone overdose risk as a result of the guidance. Six studies assessed the effect of the pandemic flexibilities on methadone-related overdoses: three used OTP patient records to assess overdose events, none of which found substantial increases. In a study of 3600 patients at OTPs, six non-fatal and no fatal overdoses were reported in a 3-month period following the introduction of the COVID-19 flexibilities, compared with two non-fatal and one fatal overdose in a 3-month period before the flexibilities were introduced.46 In another study, only one (0·8%) of 129 patients at an OTP reported an overdose in a 1-month period after the pandemic compared with three (2·3%) in a 4-month period prepandemic.46 A study of 183 patients found no significant changes in emergency department overdose visits (16 [8·7%] in a 270-day period prepandemic vs 15 [8·2%] in a 270-day period post pandemic).36 A study assessing mortality data in Connecticut before and after the changes made as a result of the pandemic found that neither methadone-only nor methadone-involved fatalities increased in the 5-month period in 2020 compared with earlier years, after accounting for the increase in overall fatal overdoses.32
Two studies analysed data at the national level. The first analysed data on calls to 55 poison-control centres across the USA and found that, although the number of yearly adult intentional exposures involving methadone increased by 5·3 percentage points (1199 to 1262), there was no significant change in reported methadone-involved hospitalisations or deaths.47 The second study analysed national data on overdose deaths and found that the proportion of methadone-involved deaths did not increase following the COVID-19 changes, despite an increase in the overall number of overdose deaths during that time period.48 In a few qualitative studies, providers admitted that they did not observe overdoses as a consequence of additional take-home doses, despite what they had anticipated.28, 29, 30
Another concern expressed was that take-home doses would disrupt patient care routines and lead to adverse patient outcomes. Our review revealed that these experiences varied substantially across patients and were less commonly expressed than positive sentiments. Some patients reported feeling overwhelmed, not trusting themselves, feared the temptation to overuse their medication,27, 43 or thought take-home doses fractured their daily routine and sense of stability.23 Some expressed difficulty adhering to the prescribed dosing regimen and feared that admitting this difficulty to the OTP would result in losing their new take-home privileges.23
Other studies assessed patient stability by looking at patient non-adherence with methadone, as established by urine toxicology. One study found no significant change in the number of OTP patients with a negative methadone screen (15·8% before vs 16·9% after the pandemic period),36 and another found no significant change in positive tests for methadone (92% before vs 96% after the pandemic period).46 Only one OTP study found a significant increase in the percentage of tests negative for methadone (1·9% before vs 4% after the pandemic period).49 Despite these mixed findings, across all studies, methadone non-adherence remained rare even after implementing take-home flexibilities.
A final set of studies considered destabilisation by assessing changes in illicit substance use. The findings were also mixed, with one finding no statistically significant change between prepandemic and post pandemic periods in the positive test detection for non-prescribed opioids (39% prepandemic to 36% post pandemic) or other illicit drugs (45% prepandemic to 40% post pandemic),46 whereas another study found an increase in positive tests for opioids (14% prepandemic to 22% post pandemic), benzodiazepines (6·3% prepandemic to 11% post pandemic), and methamphetamine (10% prepandemic to 16% post pandemic).49 A study analysing changes in drug use by the time that the patient was in treatment found only patients in treatment between 90 and 180 days, who did not receive an increase in take-home doses during the study period, saw an increase in other drug use from 19% to 33%.26 These data imply increases in illicit substance use are not necessarily attributable to additional take-home doses.
Relatedly, some studies explored whether increasing the number of take-home doses increases diversion. Diversion generally means the selling, trading, sharing, or giving away, either voluntarily or involuntarily (eg, by way of theft), of a prescription medication to someone to whom it was not prescribed.50 Only one reviewed study surveyed OTP patients directly about diversion of methadone take-home doses. Only 15 patients (14%) of 104 reported knowing someone who gave away doses, most commonly noted to be as a result of needing money or drugs (40, 38%), helping someone else (39, 38%), or saving up for travel (30, 29%).52 In some instances, providers admitted that their concerns about diversion did not materialise.29, 32 Many studies also described the tactics that OTPs implemented to reduce diversion, such as medication lock boxes41, 42, 51 and medication callbacks.29, 51
Alternatives
The regulator should also work through alternative formulations of a proposed change and consider their implications. One core issue for SAMHSA's rule will be defining which patients could receive additional take-home supplies. One option would be that SAMHSA declines to restrict the amount of take-home supplies.53 This approach would default to providers adopting a medical standard of care rather than proscriptive federal rules. Our review suggests that dosing-decision freedoms would be treated with caution by providers; most OTPs declined to provide maximum take-home supplies to patients in the context of the pandemic. Therefore, that providers would default to longer take-home doses, even if that might be the preference of harm-reduction advocates, is not clear. Another option would be for SAMHSA to propose an undefined standard, such as stable or less stable, that stops short of deferring to the medical standard of care but allows providers to exercise subjective judgement. This would mirror the first 20 months after SAMHSA initially provided take-home flexibilities and before it issued guidance with more specific criteria. A final option would be for SAMHSA to provide detailed definitions of stable and less stable in regulation. In November, 2021, SAMHSA issued additional guidance that provided more explicit criteria, including the requirement for 60 days of negative toxicological screening. SAMHSA might therefore be expected to propose these additional criteria to continue the pandemic policy.
A second and related issue is whether patients should have recourse to appeal decisions regarding take-home flexibilities. When regulators craft policies on the basis of subjective criteria (eg, stable or less stable) that will result in some patients receiving more flexible treatment options than others, patients might reasonably have concerns about whether they are being treated fairly. Our review reveals that, in the context of pandemic flexibilities, patients often viewed their inability to access take-home doses as unjust, discriminatory, burdensome, and expressed concern at being required to come to the clinic daily in the midst of a pandemic.24, 43, 44 Some patients with ongoing substance use or who lacked housing were frustrated that they were excluded from take-home policies, and others voiced frustrations about being given additional take-home doses that were subsequently rolled back.24, 27, 28 Some were denied additional doses and were not believed by staff when their doses had spilled, or were lost or stolen from them.23, 24 As a result of such frustrations, some individuals felt they had to self-manage withdrawal or reported that they wished to stop methadone treatment altogether.43, 44
In designing its new take-home rules, SAMHSA has an opportunity to help address these patient concerns about fair treatment and day-to-day frustrations, which align with long-running patient complaints about care provided by OTPs.14 For example, SAMHSA could allow patients to appeal an OTP's decision on take-home flexibilities or otherwise request a second opinion for aspects of their care. Because of the scarcity of OTPs and how tightly methadone is regulated, patients do not always have realistic options for alternative providers if they are dissatisfied. Although adding more methadone-provider and treatment-setting options (eg, office-based methadone prescribing) might ultimately be the best way to give patients options, an oversight or appeals process for patient-care decisions is an alternative that could mitigate problematic behaviour of providers and therefore give patients confidence that they are being treated fairly.
Discussion
Our review provides key information for SAMHSA to consider as it takes steps to make permanent the methadone flexibilities it made available in response to the COVID-19 pandemic. Importantly, findings from research suggest this change did not result in increases in overdoses or other adverse effects among patients. On the contrary, data indicate that potentially improved treatment retention, substantial quality of life and self-efficacy improvements, reduced burden, and fewer stressful clinic encounters for patients were associated with greater take-home flexibility. Benefits were also described by treatment providers, including improved patient motivation and satisfaction in the ability to provide patient-centred care. Therefore, efforts to create a permanent policy to support increased flexibility of take-home doses, with the goal of improved patient outcomes, are well supported.
Once offered, many providers adopted this new flexibility, suggesting that they will use ongoing flexibility to benefit patients. However, providers did not default to providing maximum take-home supplies to patients, which should allay some concerns around implementation. Uncertainty tended to depress uptake, which suggests the importance of a permanent change. In proposing new rules to extend the benefits of greater take-home flexibilities more permanently, SAMHSA has two main choices: whether to dictate which patients qualify for additional flexibility and how this flexibility should be established, and whether patients have recourse to appeal decisions around their take-home flexibility. Our Health Policy review clarifies those choices: providers did not default to maximum take-home supplies, suggesting that a more relaxed policy does not necessarily lead to more risk of diversion or overdose, but also that patients might benefit from the availability of a second, independent opinion on this crucial care decision.
There are key implementation issues. First, the review suggests that SAMHSA would be well advised to expect and plan for provider uncertainty. Although many providers acknowledged the benefits of additional take-home doses and the flexibility it allotted them, many also expressed hesitancy about how the new take-home allowances would work. In a complex area of patient care that is subject to a wide range of different legal requirements, if SAMHSA's goal is to encourage uptake of additional flexibilities, providers might require technical assistance and implementation support to work through their concerns without fear of penalty.
Second, some states declined to implement the pandemic flexibility54 in some cases due to the change being temporary.55 Therefore, a long-term regulatory change could make it more likely that additional states and jurisdictions would take up the flexibility. Other states have objected to the flexibility on policy grounds, preferring to keep the status quo approach to the treatment of OUD with methadone.55, 56 Federal regulations give states a large role in overseeing OTPs.SAMHSA could consider how to proactively support and encourage the implementation of this policy at the state level,13 and how to oversee and ensure individual providers are complying with such regulations. Issuing durable policy rather than iterative guidance could reduce uncertainty. Providing technical assistance to address provider questions is another approach that could reduce uncertainty. A third approach could be to increase SAMHSA's oversight of the relevant state regulators that oversee OTPs.
Third, the utility of diversion-prevention tactics, such as the use of lock boxes, is another important consideration. These approaches are not without their problems. For example, lock boxes might not be feasible for indivduals who are homeless and over-reliance on urine drug tests can be burdensome and challenging for patient–provider trust.15 Additional studies, including pilot testing, could help SAMHSA make informed decisions about whether these emerging approaches acheive the right balance between equitable care and concerns about safety and diversion.
Last, although payment-policy issues are likely to be outside of SAMHSA's discretion, providers showed awareness of and sensitivity to financial disincentives and implications of changes to service delivery models. To the extent that SAMHSA can factor this issue into their consideration of alternatives, including making recommendations to other agencies to align their policies and payment systems, it might help to ensure that implementation aligns with policy goals. For example, introducing an appeal process could help patients feel more confident that take-home restrictions are grounded in medical decision making rather than the result of financial disincentives for fewer office visits.
Our Health Policy review has some limitations. Our search was confined to peer-reviewed studies published before September, 2022, and might have missed more recent studies or studies published in the grey literature. For example, one study published after our review period quantified characteristics of individuals who experienced methadone-involved overdose deaths before and after the pandemic, which might include notable considerations.57 Furthermore, synthesis and interpretation of findings was limited by the wide variation in settings, outcomes, and methods used to answer research questions of interest. For instance, observational studies that used clinical data from OTPs varied greatly in size, the methods and types of data used to assess outcomes, and time periods assessed in relation to the implementation of the new COVID-19 regulations. Moreover, clinics that conducted their own evaluations or agreed to participate in research probably represent academic or research-oriented programmes, and their practices, providers, and patients might not represent OTPs broadly. Findings should also be interpreted while considering possible social desirability bias involved in self-report surveys and interviews and potential confounders not accounted for in quantitative data studies. Importantly, the quickly changing nature of the COVID-19 pandemic and the multiple associated social and structural changes make it difficult to attribute outcomes directly to SAMHSA's take-home guidance. This difficulty includes the potential influence of other changes to OTP practices, such as the use of virtual platforms for behavioural services and less urine drug screening, which were not explored in this Health Policy review. As such, none of the associations described in this review can be determined to be causal.
Despite these limitations, our report proposes concrete policy considerations based on evidence triangulated from across diverse research settings, geographies, populations, data sources, and stakeholder groups. Particularly important is the integration of the perspectives of methadone patients as gathered by qualitative research because patients at the centre of the substance-use treatment system are often excluded from these important policy conversations. Many questions remain around the effect of this policy change, including the role of other methadone delivery practices such as counselling and drug screening on the experiences and health of methadone patients, and how technologies such as safety lock boxes and virtual health platforms can aid new regulatory environments. There are also many discussions to be explored around federal versus state roles for regulators and the role of OTPs in the delivery of methadone more broadly. Another potential reform would expand methadone treatment to an office-based or pharmacy-dispensing delivery system, as is done in other countries,14 and understanding how that would affect access and the experience of patients is important.58 These questions should be the subject of further research and ongoing discussion but should not act as a deterrent to the timely implementation of changes for which there is evidence so far, including the many observed benefits and few drawbacks of increased take-home flexibilities, gathered by existing studies.
Conclusion
It took a pandemic to break through long-standing rules that have constrained patient access to methadone in the USA. Returning to the prepandemic status quo would forgo the considerable benefits discussed in this Health Policy review. Although any policy that makes it incrementally easier for patients to self-administer methadone opens the door to some risks, those risks should be considered in context with the substantial benefits afforded by increased access. Based on studies covered by this Health Policy review, a more flexible approach to take-home medication can be a benefit for patients and society as a whole, and is urgently needed during this ongoing overdose crisis.
Declaration of interests
NK declares providing expert testimony in an ongoing opioid litigation. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This Health Policy review was supported by a grant from the Pew Charitable Trusts.
Contributors
NK conceptualised the paper, reviewed and synthesised research for the review, and drafted the manuscript. BDR conducted the search strategy, reviewed research studies for the review, and edited the manuscript. EL reviewed research studies for the review and edited the manuscript. BCED conceptualised the paper, synthesised and interpreted findings in the context of the US federal policy process, and drafted and edited the manuscript. All authors contributed to the revision and writing of the final manuscript.
Supplementary Material
References
- 1.CDC Drug overdose deaths remain high. June 2, 2022. https://wonder.cdc.gov/mcd.html
- 2.Morin KA, Acharya S, Eibl JK, Marsh DC. Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treatment patients in Ontario, Canada. Int J Drug Policy. 2021;90 doi: 10.1016/j.drugpo.2020.103088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank D, Krawczyk N, Arshonsky J, Bragg MA, Friedman SR, Bunting AM. Covid-19-related changes to drug-selling networks and their effects on people who use illicit opioids. J Stud Alcohol Drugs. 2022 doi: 10.15288/JSAD.21-00438. published online Sept 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad FB, Rossen LM, Sutton P. Vital statistics rapid release—provisional drug overdose death counts. Jan 2, 2022. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- 5.Abraham AJ, Andrews CM, Harris SJ, Friedmann PD. Availability of medications for the treatment of alcohol and opioid use disorder in the USA. Neurotherapeutics. 2020;17:55–69. doi: 10.1007/s13311-019-00814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santo T, Clark B, Hickman M, et al. Association of opioid agonist treatment with all-cause mortality and specific causes of death among people with opioid dependence. JAMA Psychiatry. 2021;78:979–993. doi: 10.1001/jamapsychiatry.2021.0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leshner AI, Mancher M. Medications for opioid use disorder save lives. 2019. https://nap.nationalacademies.org/catalog/25310/medications-for-opioid-use-disorder-save-lives [PubMed]
- 8.Dole VP, Nyswander M. A medical treatment for diacetylmorphine (heroin) addiction: a clinical trial with methadone hydrochloride. JAMA. 1965;193:646–650. doi: 10.1001/jama.1965.03090080008002. [DOI] [PubMed] [Google Scholar]
- 9.Krawczyk N, Rivera BD, Jent V, Keyes KM, Jones CM, Cerdá M. Has the treatment gap for opioid use disorder narrowed in the US?: A yearly assessment from 2010 to 2019. Int J Drug Policy. 2022;110 doi: 10.1016/j.drugpo.2022.103786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Substance Abuse and Mental Health Services Administration National survey of substance abuse treatment services (N-SSATS): 2020, data on substance abuse treatment facilities. July 14, 2021. https://www.samhsa.gov/data/sites/default/files/reports/rpt35313/2020_NSSATS_FINAL.pdf
- 11.Roberts SK. The politics of stigma and racialization in the early years of methadone maintenance regulation. https://www.nationalacademies.org/documents/embed/link/LF2255DA3DD1C41C0A42D3BEF0989ACAECE3053A6A9B/file/DB9DE4A29281EB740DB6D3CAC55B1EFCB9FDB94AF835?noSaveAs=1
- 12.Substance Abuse and Mental Health Services Administration Federal guidelines for opioid treatment programs. January, 2015. https://store.samhsa.gov/sites/default/files/d7/priv/pep15-fedguideotp.pdf
- 13.Dooling BCE, Stanley L. Extending pandemic flexibilities for opioid use disorder treatment: unsupervised use of opioid treatment medications. 2021. https://minnesotalawreview.org/article/extending-pandemic-flexibilities-for-opioid-use-disorder-treatment-authorities-and-methods/
- 14.Calcaterra SL, Bach P, Chadi A, et al. Methadone matters: what the United States can learn from the global effort to treat opioid addiction. J Gen Intern Med. 2019;34:1039–1042. doi: 10.1007/s11606-018-4801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon C, Vincent L, Coulter A, et al. The methadone manifesto: treatment experiences and policy recommendations from methadone patient activists. Am J Public Health. 2022;112:S117–S122. doi: 10.2105/AJPH.2021.306665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goedel WC, Shapiro A, Cerdá M, Tsai JW, Hadland SE, Marshall BDL. Association of racial/ethnic segregation with treatment capacity for opioid use disorder in counties in the United States. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank D, Mateu-Gelabert P, Perlman DC, Walters SM, Curran L, Guarino H. “It's like ‘liquid handcuffs’”: the effects of take-home dosing policies on methadone maintenance treatment (MMT) patients' lives. Harm Reduct J. 2021;18:88. doi: 10.1186/s12954-021-00535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legislative Analysis and Public Policy Association Telehealth and substance use disorder services in the era of COVID-19: review and recommendations. June 22, 2022. https://legislativeanalysis.org/telehealth-and-substance-use-disorder-services-in-the-era-of-covid-19-review-and-recommendations-2/
- 19.Substance Abuse and Mental Health Services Administration FAQs: provision of methadone and buprenorphine for the treatment of Opioid Use Disorder in the COVID-19 emergency. April 21, 2020. https://www.samhsa.gov/sites/default/files/faqs-for-oud-prescribing-and-dispensing.pdf
- 20.National Archives Executive Order 12866 of September 30, 1993. Oct 4, 1993. https://www.archives.gov/files/federal-register/executive-orders/pdf/12866.pdf
- 21.Kellermeyer L, Harnke B, Knight S. Covidence and Rayyan. J Med Libr Assoc. 2018;106:580. [Google Scholar]
- 22.GovInfo Title 5—government organization and employees. 2021. Section 553, 72–73. https://www.govinfo.gov/content/pkg/USCODE-2011-title5/pdf/USCODE-2011-title5-partI-chap5-subchapII.pdf
- 23.Nobles AL, Johnson DC, Leas EC, et al. Characterizing self-reports of self-identified patient experiences with methadone maintenance treatment on an online community during COVID-19. Subst Use Misuse. 2021;56:2134–2140. doi: 10.1080/10826084.2021.1972317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris MTH, Lambert AM, Maschke AD, Bagley SM, Walley AY, Gunn CM. “No home to take methadone to”: experiences with addiction services during the COVID-19 pandemic among survivors of opioid overdose in Boston. J Subst Abuse Treat. 2022;135 doi: 10.1016/j.jsat.2021.108655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarker A, Nataraj N, Siu W, et al. Concerns among people who use opioids during the COVID-19 pandemic: a natural language processing analysis of social media posts. Subst Abuse Treat Prev Policy. 2022;17:16. doi: 10.1186/s13011-022-00442-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman KA, Foot C, Levander XA, et al. Treatment retention, return to use, and recovery support following COVID-19 relaxation of methadone take-home dosing in two rural opioid treatment programs: a mixed methods analysis. J Subst Abuse Treat. 2022;141 doi: 10.1016/j.jsat.2022.108801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levander XA, Hoffman KA, McIlveen JW, McCarty D, Terashima JP, Korthuis PT. Rural opioid treatment program patient perspectives on take-home methadone policy changes during COVID-19: a qualitative thematic analysis. Addict Sci Clin Pract. 2021;16:72. doi: 10.1186/s13722-021-00281-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suen LW, Castellanos S, Joshi N, Satterwhite S, Knight KR. “The idea is to help people achieve greater success and liberty”: a qualitative study of expanded methadone take-home access in opioid use disorder treatment. Subst Abus. 2022;43:1143–1150. doi: 10.1080/08897077.2022.2060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treitler PC, Bowden CF, Lloyd J, Enich M, Nyaku AN, Crystal S. Perspectives of opioid use disorder treatment providers during COVID-19: adapting to flexibilities and sustaining reforms. J Subst Abuse Treat. 2022;132 doi: 10.1016/j.jsat.2021.108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunter SB, Dopp AR, Ober AJ, Uscher-Pines L. Clinician perspectives on methadone service delivery and the use of telemedicine during the COVID-19 pandemic: a qualitative study. J Subst Abuse Treat. 2021;124 doi: 10.1016/j.jsat.2021.108288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madden EF, Christian BT, Lagisetty PA, Ray BR, Sulzer SH. Treatment provider perceptions of take-home methadone regulation before and during COVID-19. Drug Alcohol Depend. 2021;228 doi: 10.1016/j.drugalcdep.2021.109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brothers S, Viera A, Heimer R. Changes in methadone program practices and fatal methadone overdose rates in Connecticut during COVID-19. J Subst Abuse Treat. 2021;131 doi: 10.1016/j.jsat.2021.108449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saloner B, Krawczyk N, Solomon K, et al. Experiences with substance use disorder treatment during the COVID-19 pandemic: findings from a multistate survey. Int J Drug Policy. 2022;101 doi: 10.1016/j.drugpo.2021.103537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacka BP, Janssen T, Garner BR, et al. Impacts of the COVID-19 pandemic on healthcare access among patients receiving medication for opioid use disorder. Drug Alcohol Depend. 2021;221 doi: 10.1016/j.drugalcdep.2021.108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joseph G, Torres-Lockhart K, Stein MR, Mund PA, Nahvi S. Reimagining patient-centered care in opioid treatment programs: lessons from the Bronx during COVID-19. J Subst Abuse Treat. 2021;122 doi: 10.1016/j.jsat.2020.108219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amram O, Amiri S, Panwala V, Lutz R, Joudrey PJ, Socias E. The impact of relaxation of methadone take-home protocols on treatment outcomes in the COVID-19 era. Am J Drug Alcohol Abuse. 2021;47:722–729. doi: 10.1080/00952990.2021.1979991. [DOI] [PubMed] [Google Scholar]
- 37.Amram O, Amiri S, Thorn EL, Lutz R, Joudrey PJ. Changes in methadone take-home dosing before and after COVID-19. J Subst Abuse Treat. 2022;133 doi: 10.1016/j.jsat.2021.108552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McIlveen JW, Hoffman K, Priest KC, Choi D, Korthuis PT, McCarty D. Reduction in Oregon's medication dosing visits after the SARS-CoV-2 relaxation of restrictions on take-home medication. J Addict Med. 2021;15:516–518. doi: 10.1097/ADM.0000000000000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levander XA, Pytell JD, Stoller KB, Korthuis PT, Chander G. COVID-19-related policy changes for methadone take-home dosing: a multistate survey of opioid treatment program leadership. Subst Abus. 2022;43:633–639. doi: 10.1080/08897077.2021.1986768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldsamt LA, Rosenblum A, Appel P, Paris P, Nazia N. The impact of COVID-19 on opioid treatment programs in the United States. Drug Alcohol Depend. 2021;228 doi: 10.1016/j.drugalcdep.2021.109049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kidorf M, Brooner RK, Dunn KE, Peirce JM. Use of an electronic pillbox to increase number of methadone take-home doses during the COVID-19 pandemic. J Subst Abuse Treat. 2021;126 doi: 10.1016/j.jsat.2021.108328. 108328.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walters SM, Perlman DC, Guarino H, Mateu-Gelabert P, Frank D. Lessons from the first wave of COVID-19 for improved medications for opioid use disorder (MOUD) treatment: benefits of easier access, extended take homes, and new delivery modalities. Subst Use Misuse. 2022;57:1144–1153. doi: 10.1080/10826084.2022.2064509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krawczyk N, Bunting AM, Frank D, et al. “How will I get my next week's script?” reactions of Reddit opioid forum users to changes in treatment access in the early months of the coronavirus pandemic. Int J Drug Policy. 2021;92 doi: 10.1016/j.drugpo.2021.103140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Bassel N, Hochstatter KR, Slavin MN, Yang C, Zhang Y, Muresan S. Harnessing the power of social media to understand the impact of COVID-19 on people who use drugs during lockdown and social distancing. J Addict Med. 2022;16:e123–e132. doi: 10.1097/ADM.0000000000000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunn KE, Brooner RK, Stoller KB. Technology-assisted methadone take-home dosing for dispensing methadone to persons with opioid use disorder during the Covid-19 pandemic. J Subst Abuse Treat. 2021;121 doi: 10.1016/j.jsat.2020.108197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ezie C, Badolato R, Rockas M, et al. COVID 19 and the opioid epidemic: an analysis of clinical outcomes during COVID 19. Subst Abuse. 2022;16 doi: 10.1177/11782218221085590. 11782218221085590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welsh C, Doyon S, Hart K. Methadone exposures reported to poison control centers in the United States following the COVID-19-related loosening of federal methadone regulations. Int J Drug Policy. 2022;102 doi: 10.1016/j.drugpo.2022.103591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones CM, Compton WM, Han B, Baldwin G, Volkow ND. Methadone-involved overdose deaths in the US before and after federal policy changes expanding take-home methadone doses from opioid treatment programs. JAMA Psychiatry. 2022;79:932–934. doi: 10.1001/jamapsychiatry.2022.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bart G, Wastvedt S, Hodges JS, Rosenthal R. Did drug use increase following COVID-19 relaxation of methadone take-out regulations? 2020 was a complicated year. J Subst Abuse Treat. 2022;133 doi: 10.1016/j.jsat.2021.108590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larance B, Degenhardt L, Lintzeris N, Winstock A, Mattick R. Definitions related to the use of pharmaceutical opioids: extramedical use, diversion, non-adherence and aberrant medication-related behaviours. Drug Alcohol Rev. 2011;30:236–245. doi: 10.1111/j.1465-3362.2010.00283.x. [DOI] [PubMed] [Google Scholar]
- 51.Krawczyk N, Maniates H, Hulsey E, et al. Shifting medication treatment practices in the COVID-19 pandemic: a statewide survey of Pennsylvania opioid treatment programs. J Addict Med. 2022;16:645–652. doi: 10.1097/ADM.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Figgatt MC, Salazar Z, Day E, Vincent L, Dasgupta N. Take-home dosing experiences among persons receiving methadone maintenance treatment during COVID-19. J Subst Abuse Treat. 2021;123 doi: 10.1016/j.jsat.2021.108276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stanley L, Dooling BCE. Methadone's regulatory thicket. Ann Health Law Life Sci (in press).
- 54.Pessar SC, Boustead A, Ge Y, Smart R, Pacula RL. Assessment of state and federal health policies for opioid use disorder treatment during the COVID-19 pandemic and beyond. JAMA Health Forum. 2021;2 doi: 10.1001/jamahealthforum.2021.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.The Pew Charitable Trusts Most states eased access to opioid use disorder treatment during the pandemic. June 1, 2022. https://www.pewtrusts.org/en/research-and-analysis/articles/2022/06/01/most-states-eased-access-to-opioid-use-disorder-treatment-during-the-pandemic
- 56.Andraka-Christou B, Bouskill K, Haffajee RL, et al. Common themes in early state policy responses to substance use disorder treatment during COVID-19. Am J Drug Alcohol Abuse. 2021;47:486–496. doi: 10.1080/00952990.2021.1903023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kleinman RA, Sanches M. Methadone-involved overdose deaths in the United States before and during the COVID-19 pandemic. Drug Alcohol Depend. 2023;242 doi: 10.1016/j.drugalcdep.2022.109703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adams Z, Krawczyk N, Simon R, Sue K, Suen L, Joudrey P. To save lives from opioid overdose deaths, bring methadone into mainstream medicine. May 27, 2022. https://www.healthaffairs.org/do/10.1377/forefront.20220524.911965/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.