Introduction and Importance:
Guillain–Barré syndrome (GBS) is an immune-mediated disorder of the central nervous system presenting as symmetrical, progressive weakness and areflexia. The incidence of GBS is very low during pregnancy, but the risk increases in the postpartum period. The management is done by intravenous immunoglobulin or conservatively.
Case Presentation:
Case of 27 years female with parity 1, living 1, on postpartum day 20 presented to the emergency department (ED) with weakness over legs and hands since 20 days following emergency lower segment cesarean section for her delivery. The weakness prevailed over the lower extremities and progressed to the upper extremities in 4–5 days, affecting her grip strength and ability to stand alone. No history of prior diarrheal or respiratory illness. Cerebrospinal fluid analysis revealed albuminocytologic dissociation. A nerve conduction study showed in-excitable bilateral radial, median, ulnar, and sural nerves. Intravenous immunoglobulin was administered at the rate of 0.4 g/kg once daily for 5 days. Patient was discharged after 2 weeks with regular physiotherapy follow-up.
Conclusion:
GBS in the postpartum period is very rare. There must be a high degree of suspicion among physicians for GBS if a pregnant female or a woman during her postpartum period presents with ascending muscle paralysis, even if there is no recent antecedent history of diarrheal episodes or respiratory illness. An early diagnosis with multidisciplinary supportive measures helps improve the prognosis for both the mother and the fetus.
Keywords: case report, Guillain–Barré syndrome, intravenous immunoglobulin, postpartum
Highlights
Guillain–Barré syndrome (GBS) is an acute paralysis syndrome presenting as symmetrical, progressive weakness, and areflexia.
The patient usually complains of paresthesia and numbness, beginning in the toes and fingertips. It gradually progresses upwards, but generally does not extend beyond the wrists or ankles.
The association between GBS and conditions like bacterial and viral infections, systemic diseases, neoplasia, traumatic injury, autoimmune diseases, immunosuppressive conditions, cancer chemotherapy, and organ transplantation has been successfully established previously.
The incidence of GBS is very low during pregnancy. The risk of GBS increases in the postpartum period.
Evidences suggest favorable outcomes of 70–80% of cases with full recovery treated with plasma exchange and intravenous immunoglobulin.
Our case is unique in that the patient reported to us after 20 days of the postpartum period and was treated successfully with intravenous immunoglobulin.
Our case is also unique in the sense that it is the first reported case of postpartum GBS from our country, Nepal.
Introduction and importance
Guillain–Barré syndrome (GBS), also known as Landry’s paralysis, first came to light in 1916. It is an immune-mediated disorder of the central nervous system and manifests as acute inflammatory polyradiculoneuropathy1. It is an acute paralysis syndrome presenting as symmetrical, progressive weakness and areflexia1–3. It may be associated with abnormal sensory function1. The patient usually complains of paresthesia and numbness, beginning in the toes and fingertips. It gradually progresses upward but generally does not extend beyond the wrists or ankles1,4.
The association of GBS and the conditions like bacterial and viral infections, systemic diseases, neoplasia, traumatic injury, autoimmune diseases, immunosuppressive conditions, cancer chemotherapy, and organ transplant have been successfully established previously1,2. However, the association of GBS with pregnancy is rare, with an incidence of 1.2–1.9 cases per 100 000 population1,2,4. Some studies suggest there is an increase in the risk of GBS in the first 2 weeks following delivery4. Peripartum pre-eclampsia is also proposed as a predisposing factor for GBS in the postpartum period2.
We hereby present the unique case of a 27-year-old woman who landed in the emergency department (ED) on her 20th postpartum day following a cesarean section with weakness of her lower extremities that progressed to her upper extremities. She was diagnosed as having GBS and recovered well with timely management.
This case report has been reported in line with the SCARE Criteria 20205.
Case presentation
Case of 27 years Mrs Rai, parity 1, living 1, on postpartum day 20, nonsmoker, nonalcohol consumer with no prior comorbidities presented to the ED of our health institution with weakness over legs and hands since 20 days following emergency lower segment cesarean section for her delivery. The weakness prevailed over the lower extremities and progressed to the upper extremities in 4–5 days, affecting her grip strength and ability to stand alone. No history of prior diarrheal or respiratory illness. No prior history of focal sensory, motor, or autonomic deficits exists. On examination in the ED, the patient was well oriented, vitals were within normal range, the general condition seemed fair, and systemic examination of the cardiovascular system, chest, and abdomen were normal. In addition to the neurological examination, the motor examination revealed normal muscle bulk, flaccid muscle tone, diminished deep tendon reflexes, and a muscle power grade of three out of five (medical research council grading) in bilateral upper and lower limbs. Higher mental functions, cranial nerve examination, and sensory functions were normal, and no signs of meningeal irritation or cerebellar dysfunction were found. Baseline investigations were sent, including cerebrospinal fluid (CSF) analysis, computed tomography of the head, and nerve conduction velocity test values, which are tabulated below in Table 1.
Table 1.
Baseline investigations including CSF analysis
| Test | Result | Reference range |
|---|---|---|
| Complete blood count | ||
| Hemoglobin (g/dl) | 10.1 | 11–16 |
| PCV (%) | 31.4 | 37–48 |
| TLC | 8000 cells/mm3 | 4000–11 000 cells/mm3 |
| Neutrophil (%) | 80 | 40–75 |
| Lymphocyte (%) | 13 | 20–45 |
| Monocyte (%) | 05 | 2–10 |
| Eosinophil (%) | 02 | 1–6 |
| Platelet count | 357 000 cells/mm3 | 150 000–400 000 cells/mm3 |
| Biochemistry | ||
| Glucose (random) | 93 mg/dl | <140 mg/dl |
| Urea | 52 mg/dl | 10–50 mg/dl |
| Creatinine | 0.4 mg/dl | 0.3–1.2 mg/dl |
| Total protein | 7.3 g/dl | 6.0–8.3 g/dl |
| Albumin | 4.3 g/dl | 3.5–5.0 g/dl |
| Total bilirubin | 0.4 mg/dl | 0.2–1.2 mg/dl |
| Conjugated bilirubin | 0.2 mg/dl | 0.0–0.2 mg/dl |
| Ionized calcium | 1.37 mmol/l | 1.05–1.27 mmol/l |
| Total calcium accent | 10.8 mg/dl | 8.6–10.3 mg/dl |
| Troponin I | Troponin I | Troponin I |
| CK (NAC) accent | CK (NAC) accent | CK (NAC) accent |
| CK MB accent | CK MB accent | CK MB accent |
| Serum electrolytes (mmol/l) | ||
| Sodium | 147 | 136–145 |
| Potassium | 4.6 | 3.5–5.0 |
| Serology | ||
| HBsAg, HCV, HIV | Negative | |
| CSF analysis | ||
| Glucose | 55 mg/dl | 40–65 mg/dl |
| CSF protein | 398 mg/dl | 15–45 mg/dl |
| TLC | Nil (cells/mm3) | |
| Granulocyte | Nil (cells/mm3) | |
| Nongranulocyte | Nil (cells/mm3) | |
| RBC | Nil (cells/mm3) | |
| Urine RE/ME | ||
| Protein | Negative | |
| Sugar | Negative | |
| WBC | 0–2/HPF | |
| RBC | Not seen | |
| Epithelial cells | Not seen | |
| Ketone crystals | Absent | |
CSF indicates cerebrospinal fluid; HCV, hepatitis C virus; HPF, high-power field; ME, microscopic examination; PCV, packed cell volume; RBC, red blood cells; RE, routine examination; TLC, total leukocytic count; WBC, white blood cells.
The bold number and letters are showed that they are deranged from their reference values.
The serum potassium level was normal (4.6 mmol/l). CSF analysis showed raised CSF protein (398 mg/dl) and a nil cell count, suggestive of albuminocytologic dissociation. The findings of the computed tomography scan were suggestive of mild cerebral atrophy.
In nerve conduction study, the compound mixed motor study showed in-excitable bilateral radial nerves and sensory study showed in-excitable bilateral radial, median, ulnar, and sural nerves as shown in Figure 1.
Figure 1.
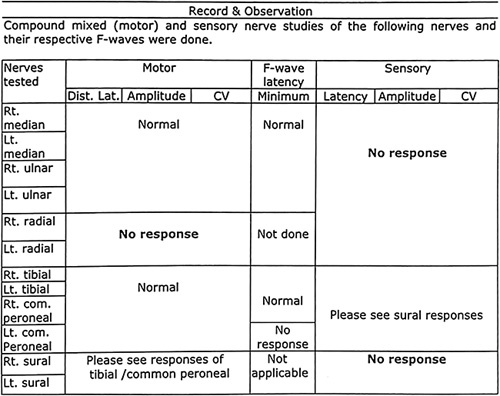
In-excitable bilateral radial, median, ulnar, and sural nerves.
Treatment
She was kept under observation with 6-hourly vitals monitoring, oxygen supplementation, input/output charting, and daily physiotherapy for the limb weakness. Intravenous immunoglobulin (IVIg) was administered at the rate of 0.4 g/kg once daily for 5 days. No respiratory complications were detected. Patient was discharged after 2 weeks with regular physiotherapy follow-up.
Clinical discussion
GBS is an acute paralysis syndrome which presents as symmetrical, progressive weakness, and areflexia. It may be associated with abnormal sensory function. Patients usually complain of paresthesia and numbness that begins in the toes and fingertips and progresses upward but generally does not extend beyond the wrists and ankles. The incidence of GBS is very low during pregnancy1,4,6. The risk of GBS increases in the postpartum period7. Our patient presented with lower limb weakness, which gradually progressed to the upper limbs in 4–5 days. A study reported patients presenting with pain in bilateral knee joints below the knee joint. However, other studies reported patients presenting with pain most severe in the shoulder girdle, back, buttocks, and thighs1. GBS can affect the people of any age group and is equally vulnerable to both male and female. The exact cause of GBS is unknown; however, many researchers found that around 60% of GBS cases usually follow a lung infection or a gastrointestinal infection1. GBS is strongly associated with organisms such as Campylobacter jejuni, influenza virus, Cytomegalovirus, Epstein–Barr virus, Mycoplasma, and HIV8. The syndrome may be triggered in patients who have underwent surgery and anesthesia. Cases are also reported due to vaccination9. The signs and symptoms of GBS usually remains over a period of hours, days, or weeks with most of the patients reaching the stage of highest weakness within the first 2 weeks after the symptoms have started and 90% of patients getting rid of symptoms by third week of illness. The most typical manifestation of the disease begins with ascending paralysis10. The weakness usually starts in the feet and migrates upwards towards the trunk, while some subtypes present with changes in sensation or pain and dysfunction of the autonomic nervous system. The disease can be life-threatening if the autonomic nervous system or respiratory muscles are involved. In severe cases, loss of autonomic function with wide fluctuations of blood pressure and sinus tachycardia, even cardiac arrhythmia, are found – none of which was found in our article. To confirm the diagnosis, a nerve conduction study and lumbar puncture are done, which shows a higher level of protein with a normal cellular count1. The same was done in our case. The risk of maternal mortality increases up to 7% if GBS occurs during pregnancy or the postpartum period, with 20% of patients becoming disabled within a period of 1 year11. After childbirth, there is an increase in cellular immunity and a decrease in humoral immunity, which is due to the proinflammatory cytokine surge in the postpartum period, which could be the reason for the causation of the disease12. A study conducted by Fernando et al.13 showed the worsening condition of GBS in the postpartum period due to increase in delayed type of hypersensitivity. A similar study was conducted, which showed a case of GBS diagnosed at 15 weeks of pregnancy and aggravated in the postpartum period. Evidences suggest favorable outcomes of 70–80% of cases with full recovery treated with plasma exchange and IVIg14. The role of IVIg in patients with GBS was reported in 1988, which led to the first randomized-controlled trial suggesting higher efficacy and cost effectiveness with fever side effects of IVIg therapy, making it preferable over plasma exchange therapy1. A study conducted by Bahadur and colleagues reported a 25-year-old multigravida at 21 weeks of gestation with successful maternal and fetal outcomes13. Our patient also responded favorably to IVIg and was discharged after 2 weeks with regular physiotherapy follow-up, as was managed in other cases discussed in the above articles.
Conclusion
Our case is unique in that the patient reported to us after 20 days of the postpartum period and was treated successfully with IVIg. There must be a high degree of suspicion among physicians for GBS if a pregnant female or a woman during her postpartum period presents with ascending muscle paralysis, even if there is no recent antecedent history of diarrheal episodes or respiratory illness. An early diagnosis with multidisciplinary supportive measures helps improve the prognosis for both the mother and the fetus. Our case is also unique in the sense that it is the first reported case of postpartum GBS from our country Nepal.
Ethical approval
Ethical approval is not required for case report.
Patient consent
Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request. We also ensure that none of the identifying characteristics are included in the case report.
Sources of funding
Since we are medical students under supervision, we do not have any financial support for our research.
Author contribution
A.B. and A.S.: literature review, follow-up the patient, writing the manuscript, and final approval of the manuscript. V.M.: literature review, writing the manuscript, follow-up, and final approval of the manuscript. S.B.: literature review, writing the manuscript, follow-up of the patient, and final approval of the manuscript. Dr P.S.: literature review, nerve conduction study, and final approval of the manuscript. Dr A.K.Y.: supervisor, literature review, nerve conduction study, and final approval of the manuscript.
Conflicts of interest disclosure
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Research registration unique identifying number (UIN)
None.
Guarantor
Amrit Bhusal.
Provenance and peer review
Not commissioned, externally peer reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 6 February 2023
Contributor Information
Amrit Bhusal, Email: amritbhusal51@gmail.com.
Anu Shrestha, Email: anushrestha845@gmail.com.
Vitasta Muskan, Email: prapsdm@gmail.com.
Sashank Bhattarai, Email: sankbht@gmail.com.
Priza Subedi, Email: priza.subedi@bpkihs.edu.
Ajay K. Yadav, Email: ajay.yadav@bpkihs.edu.
References
- 1. Sharma S. Guillain–Barré syndrome during post-partum period: a rare entity. J Neurol Nuerosci 2021;12:389. [Google Scholar]
- 2. Paneyala S, Chandrashekaraiah NS, Sundaramurthy H, et al. ‘Double’ trouble in postpartum state. J SAFOG 2021;13:138–43. [Google Scholar]
- 3. Gupta A, Patil M, Khanna M, et al. Guillain-Barre syndrome in postpartum period: rehabilitation issues and outcome – three case reports. J Neurosci Rural Pract 2017;8:475–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kachru C, Bahadur A, Dagar M, et al. Diagnosing Gullian Barre syndrome in the post-partum period: a case report. J Med Sci Heal 2015;01:21–3. [Google Scholar]
- 5. Agha RA, Franchi T, Sohrabi C, et al. The SCARE 2020 Guideline: updating Consensus Surgical CAse REport (SCARE) Guidelines. Int J Surg 2020;84:226–30. [DOI] [PubMed] [Google Scholar]
- 6. Vijayaraghavan J, Vasudevan D, Sadique N, et al. A rare case of Guillain-Barre syndrome with pregnancy. J Indian Med Assoc 2006;104:269–70. [PubMed] [Google Scholar]
- 7. Sharma SR, Sharma N, Masaraf H, et al. Guillain-Barré syndrome complicating pregnancy and correlation with maternal and fetal outcome in North Eastern India: a retrospective study. Ann Indian Acad Neurol 2015;18:215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Syndrome B, Tam CC, Brien SJO, et al. Influenza, campylobacter and Mycoplasma infections, and hospital admissions for guillain-Barré syndrome, England. Emerg Infect Dis 2006;12:1880–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ullah MW, Qaseem A, Amray A. Post vaccination Guillain Barre syndrome: a case report. Cureus 2018;10:6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dimachkie MM, Barohn RJ. ´ Syndrome and Guillain-Barre variants. Neurol Clin NA [Internet] 2013;31:491–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh N, Perfect JR. Immune reconstitution syndrome and exacerbation of infections after pregnancy. Clin Infect Dis. 2007;45:1192–9. [DOI] [PubMed]
- 12. Campos F, Moraes G De, Ubirajara R, et al. Guillain-Barré syndrome in pregnancy: early diagnosis and treatment is essential for a favorable outcome. Gynecol Obstet Invest 2009;030:236–7. [DOI] [PubMed] [Google Scholar]
- 13. Fernando TNMS, Ambanwala AMAS, Ranaweera P, et al. Guillain–Barré syndrome in pregnancy: a conservatively managed case. J Family Med Prim Care 2016;5:688–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van DPA, Kuitwaard K, Walgaard C, et al. IVIG treatment and prognosis in Guillain–Barré syndrome. J Clin Immunol 2010;30:74–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


