HIGHLIGHTS
-
•
A side effect of cannabidiol is drowsiness, which could impact driving.
-
•
This pilot trial investigated oral cannabidiol's impact on simulated driving.
-
•
Slight detriments to performance were noted.
-
•
The study was statistically underpowered.
-
•
Additional research is needed to determine whether cannabidiol impacts driving.
Keywords: Cannabidiol, cannabis, driving performance, simulation
Abstract
Introduction
A common side effect of cannabidiol is drowsiness, which could impact safe driving. This study's purpose was to determine the feasibility and whether cannabidiol impacts simulated driving performance.
Methods
This was a randomized, parallel-group, sex-stratified, double-blind, pilot trial that consisted of a volunteer sample of healthy, currently driving college students. Participants were randomized and allocated to receive a placebo (n=19) or 300 mg cannabidiol (n=21) by oral syringe. Participants completed a ∼40-minute driving simulation. A post-test survey assessed acceptability. The primary outcomes were mean SD of lateral position, total percent time the individual drove outside travel lanes, total collisions, time to initial collision, and mean brake reaction time. Outcomes were compared between groups using Student's t-tests and Cox proportional hazards models.
Results
None of the relationships were statistically significant, but the study was underpowered. Those receiving cannabidiol experienced slightly more collisions (0.90 vs 0.68, p=0.57) and had slightly higher mean SD of lateral position and slower brake reaction times (0.60 vs 0.58 seconds, p=0.61) than those who received placebo. Participants were satisfied with their experiences.
Conclusions
The design was feasible. Larger trials may be warranted because it is unclear whether the small differences in performance seen in the cannabidiol group were clinically relevant.
INTRODUCTION
The U.S. Food and Drug Administration approved Epidiolex, which is a prescription cannabidiol (CBD) oil, for the treatment of Dravet and Lennox‒Gastaut syndromes and tuberous sclerosis complex in children.1,2 A legislative change in 2018 allowed nonprescription CBD oil to be sold over the counter, and it is being added to numerous products that target the general consumer.3 Nonprescription products tend to contain lower amounts of CBD than prescription.4 Published RCTs of CBD's effectiveness are limited and are predominately focused on clinical populations suffering from neurologic or neuropsychiatric conditions.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 A common side effect of CBD is drowsiness, which could impact driving performance.25, 26, 27, 28 However, only 1 published RCT investigating the effects of smoked nonprescription CBD on driver performance exists.29 In that study, the primary outcome was the SD of lateral position (SDLP), which measures weaving and is a common indicator of impairment.30,31 That study found that SDLP did not differ between CBD-treated and placebo-treated groups nor did their cognitive test performance.29 Previous studies that investigated CBD's effects on cognition or psychomotor function in healthy adults have also found limited effects.32, 33, 34, 35, 36, 37, 38, 39, 40 Given the lack of RCTs in healthy adults, the primary aims of this study were to assess the feasibility/acceptability of an RCT using ingested nonprescription CBD oil and compare measures of simulated driving performance among participants randomized to CBD versus placebo.
METHODS
Study Design and Participants
This study was a randomized, parallel-group, double-blind, 2-arm, pilot feasibility trial. A parallel design was chosen over a crossover design to maximize participant retention and minimize missing data.41 Eligibility criteria were (1) enrolled as a student, (2) aged 18–30 years, (3) possessed a valid driver's license, (4) driven ≥1 time in the past month, (5) could read English, (6) willing to take a urine drug test and complete a test drive to ensure the absence of simulation sickness, (7) not taking any prescription medications (excluding birth control), (8) not diagnosed with any serious chronic disease, and (9) had an individual willing to drive them home after testing. Participants were excluded if they used tobacco, used CBD in the past 7 days, used illegal drugs in the past month, or were pregnant/lactating. The study took place at West Virginia University between April 2021 and January 2022. The study was approved by West Virginia University's IRB and registered on www.clinicaltrials.gov (NCT04590495).
Sample Size
The sample size was calculated using GPower 3.1 a priori.42 Because 1 outcome was between-group differences in mean SDLP, an omnibus 1-way ANOVA test was chosen. Assuming a large effect size (0.50), α=0.05, and 80% power, the analysis recommended 34 participants. Accounting for 15% attrition, 40 participants were targeted. Because no published data regarding CBD's effects on driving existed at conceptualization and because previous trials documented anxiolytic effects starting at 300 mg in healthy adults,38,43,44 we initially posited that participants receiving CBD would show larger differences in performance than those receiving placebo.
Recruitment, Screening, and Enrollment
E-mail advertisements were sent to all students. Using a standardized checklist, 58 individuals were screened and scheduled for testing. All participants received previsit instructions. At the laboratory, personnel rescreened participants; if participants did not follow previsit instructions, their appointment was rescheduled. Written consent was obtained. After consenting, participants provided urine samples, which were immediately analyzed. If a participant's sample tested negative, they completed a 10-minute practice drive on the simulator, which provided practice and screening for simulation sickness.45 If sickness occurred, the individual was ineligible. A total of 40 individuals completed the consent process and enrolled.
Study Procedure Overview
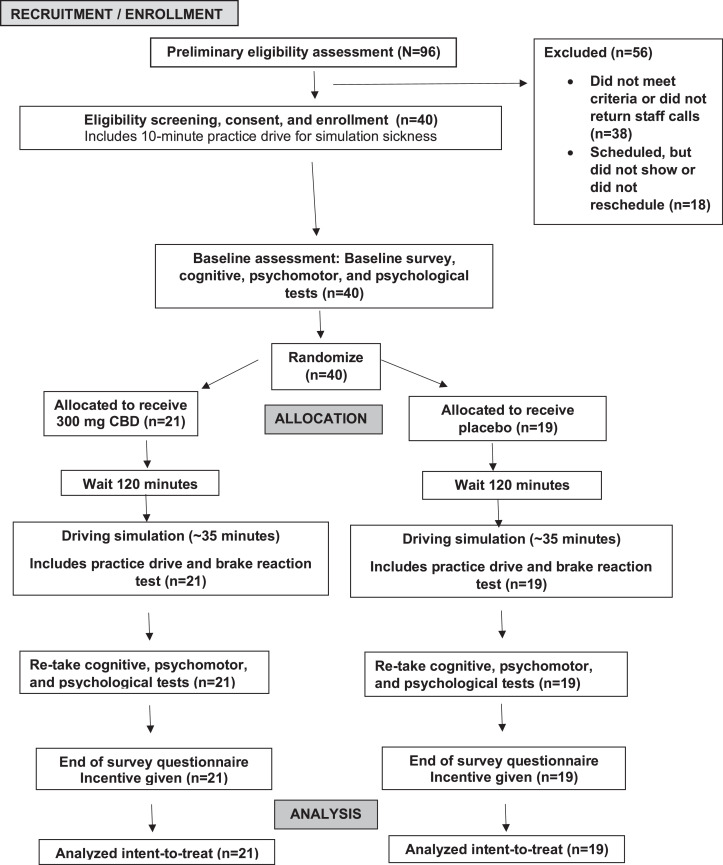
After enrollment, all participants completed a standardized, demographic survey and 6 cognitive/psychomotor tests (Appendix, available online). Participants were then randomized and allocated to a treatment group. Participants were provided a standardized breakfast and then waited for 120 minutes for absorption, which was chosen on the basis of the pharmacokinetics of CBD along with the consideration of participant burden.28,46 Participants completed the driving simulation and were readministered the cognitive/psychomotor tests. Finally, participants received compensation after completing a questionnaire that inquired about the acceptability of procedures and a test of blinding. The procedure lasted for 4–4.5 hours (Figure 1).
Figure 1.
Study flow diagram. An overview of study procedures is shown.
Randomization, Treatment Allocation, and Blinding
Participants were randomized to receive either CBD (n=21) or placebo (n=19) using a 1:1 stratified randomly varying block technique47; the stratification variable was participants’ sex as driving behaviors and risk tolerances differ between the sexes.48,49 The study statistician, who had no contact with participants, prepared the randomization schedule. After this schedule, staff sequentially placed small cards labeled A or B into opaque envelopes, sealed them, and stacked them. When a participant was enrolled, the top envelope was removed. Both the principal investigator, staff delivering the treatment, those assisting with data collection, and participants were blinded to treatment assignment.
Intervention
Study drugs. Treatment A (i.e., placebo) consisted of avocado oil, whereas Treatment B was 300 mg of CBD oil. This dosage was chosen because it was used and is well tolerated in other studies.24,38,40,43 Both treatments were identically flavored before dispensing. The CBD was purchased from Zatural (Idaho) (ID). Because previous studies found that nonprescription CBD products are often mislabeled or contain high levels of delta-9 tetrahydrocannabinol (THC),50 the product was tested by an independent third-party laboratory before use (e.g., Botanacor Laboratories, Denver, Colorado). Testing revealed that the labeling was accurate with virtually no THC present (i.e., 0.006%).
Driving simulation. All participants (N=40) received instructions and underwent an identical driving simulation using the STISIM Drive M1000 simulator. The simulator was equipped with 1 screen, steering wheel, controls, brake, and accelerator pedals. Participants completed a practice drive (∼5 minutes), brake reaction test (∼5 minutes), and primary study drive (∼25 minutes), which included urban, suburban, and rural highway segments that entailed adjustments to speed, avoidance of objects, turns, and navigational instructions.
Measures of feasibility, acceptability, and blinding. At study completion, participants completed a questionnaire that contained structured and open-ended questions to assess the acceptability of procedures. An additional question asked what treatment they thought they received. The number of adverse events was used to gauge the acceptability of procedures. Participants were contacted twice within 24 hours after testing to discern adverse event occurrences.
Measures of driving performance. Five primary outcomes and 3 secondary outcomes were collected. The primary outcome was SDLP, which was measured in 2 separate segments of the main study drive (e.g., SDLP#1 occurred earlier in the drive, and SDLP#2 occurred at the end). SDLP is calculated by taking the SD of the vehicle's lateral position, which is the distance in feet between the vehicle's center with respect to the roadway's center line. Greater SDLP is associated with more impaired driving.30,31 The second outcome was the total percent time the participant spent driving beyond the roadway's center line or shoulder. The third outcome was total collisions. The time to initial collision (i.e., fourth outcome) was the time that elapsed from the beginning of the simulation to the time of the first collision. The fifth outcome was mean brake reaction time, which was the average time (seconds) it took the participant to hit the brake after being exposed to stimuli. Secondary outcomes included the percentage of time that the participant spent driving over the designated speed limit, the total time it took the participant to complete the study drive, and turn signal performance. This was the proportion of good turn signal use out of the total possible turn signal maneuvers. The simulator considered good performance instances when the driver correctly signaled for a turn/lane change in advance. This value ranged from 0 to 1, with values closer to 1 indicating better performance.
Statistical Analyses
All quantitative analyses were performed using SAS, Version 9.4. Only an intent-to-treat analysis was performed because no enrolled participants failed to complete the study. Demographic characteristics and tests of blinding were compared between groups using chi-square, Fisher's exact, or Student's t-tests. To compare between-group differences in driving outcomes and study satisfaction, the data were analyzed using a negative binomial regression test, Student's t test, or Mann‒Whitney U test. To compare the time until the first collision between treatment groups, Cox proportional hazards models were employed; Schoenfeld residuals were analyzed to ensure that the proportional hazards assumptions were not violated.51 A Kaplan‒Meier curve was plotted along with a log-rank test to compare treatment groups.52 Free-text responses were analyzed through qualitative content analyses.53 All analyses utilized 2-tailed hypothesis tests with α=0.05. Effect sizes were calculated using Cohen's d.54
RESULTS
Between April 15, 2021 and November 30, 2021, 96 individuals were prescreened by research personnel. A total of 40 individuals were enrolled and completed the study. There were no missing data (Figure 1).
Demographic characteristics were similar between CBD and placebo groups (Table 1). Overall, the participants' average age was 21.2±2.7 years, 48% were male, and 85% were non-Hispanic White.
Table 1.
Demographics of Study Participants by Treatment Group (N=40)
| Characteristics | Overall (N=40) | CBD (n=21) | Placebo (n=19) | p-Value |
|---|---|---|---|---|
| Age in years, mean (SD)a | 21.2 (2.7) | 21.4 (2.8) | 21.0 (2.7) | 0.57 |
| Male, n (%)b | 19 (47.5) | 10 (47.6) | 9 (47.4) | 0.99 |
| White, n (%)c | 34 (85.0) | 18 (85.7) | 16 (84.2) | 1.00 |
| BMI, mean (SD)d | 26.0 (5.2) | 26.3 (6.3) | 25.6 (3.6) | 0.70 |
| Undergraduate student, n (%)b | 28 (70.0) | 13 (61.9) | 15 (79.0) | 0.24 |
| Employed full or part-time, n (%)b | 21 (52.5) | 11 (52.4) | 10 (52.6) | 0.99 |
| Hours spent playing video games per weeka | 7.1 (10.2) | 5.6 (5.1) | 8.6 (13.8) | 0.66 |
| Miles driven per week, mean (SD)a | 49.5 (52.7) | 49.9 (60.5) | 48.9 (43.7) | 0.60 |
| Risky driving score, mean (SD)d | 8.4 (6.4) | 6.8 (6.5) | 10.2 (5.9) | 0.09 |
| Ever used CBD, n (%)c | 9 (23.1) | 4 (19.1) | 5 (27.8) | 0.71 |
p-Value calculated with Mann‒Whitney U test comparing the CBD with the placebo group.
p-Value calculated with chi-square test comparing the CBD with the placebo group.
p-Value calculated with Fisher's exact test owing to small cell counts comparing the CBD with the placebo group.
p-Value calculated with Student's t test comparing the CBD with the placebo group.
CBD, cannabidiol.
Primary and secondary study outcomes are shown by group in Table 2. None of the relationships were statistically significant. However, the CBD group performed slightly worse on all primary and secondary outcomes. Those receiving CBD drove slower and spent less time speeding. Calculated effect sizes ranged from 0.03 to 0.36.
Table 2.
Primary and Secondary Performance Outcomes by Treatment Group
| Outcomes | CBD (n=21) | Placebo (n=19) | p-Value | ESa |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Total collisionsb | 0.90 (1.09) | 0.68 (0.95) | 0.52 | 0.23 |
| Percentage time out of lanec | 4.41 (2.44) | 4.34 (2.09) | 0.95 | 0.03 |
| SDLP#1c | 4.37 (2.22) | 3.88 (2.49) | 0.90 | 0.20 |
| SDLP #2c | 1.07 (0.77) | 0.98 (0.44) | 0.63 | 0.20 |
| Percentage time speedingc | 5.83 (6.02) | 8.27 (9.58) | 0.27 | 0.25 |
| Turn signal performanced,e | 0.51 (0.13) | 0.54 (0.10) | 0.54 | 0.30 |
| Drive time (seconds)d | 1,309 (58) | 1,281 (78) | 0.20 | 0.36 |
| Mean brake reaction timec | 0.60 (0.11) | 0.58 (0.12) | 0.61 | 0.17 |
ESs were calculated by subtracting the mean of the placebo group from the mean of the CBD group and dividing this difference by the placebo group's SD (i.e., Cohen's d).
p-value obtained from negative binomial regression comparing CBD with placebo.
p-values obtained from Mann‒Whitney U test comparing CBD with placebo.
p-values obtained by Student's t tests comparing CBD with placebo.
Turn signal performance was the proportion of good turn signal usage out of total possible turn signal maneuvers. This value ranges from 0 to 1. Values closer to 1 indicate better performance.
#, number; CBD, cannabidiol; ES, effect size; SDLP, SD of lateral position.
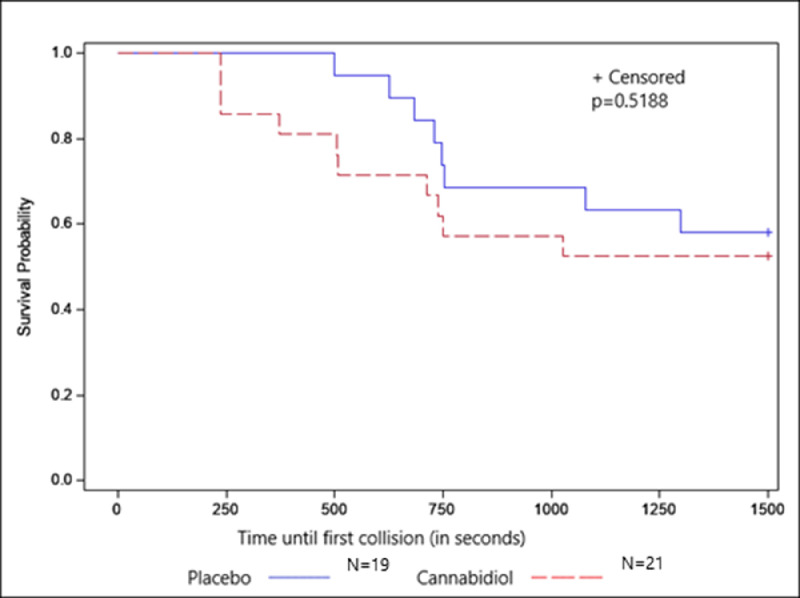
Although not statistically significant, the survival analyses (shown in this study) determined that participants who received CBD were 35% more likely to experience a collision than those who received a placebo (hazard ratio=1.36; 95% CI=0.54, 3.44). The Kaplan‒Meier curve is shown in Figure 2.
Figure 2.
Time until the first collision by treatment group. Differences between those receiving cannabidiol and placebo were compared using the log-rank test.
As for the acceptability/feasibility of the study, the results of the qualitative analysis are shown in Appendix Table 1 (available online). Most participants enjoyed the way the study was designed or how it was structured (85%). Most participants were highly satisfied with their experience irrespective of group assignment (Appendix Table 2, available online). Only 26% of those who received a placebo and 48% who received CBD correctly identified their group allocation (Appendix Table 3, available online).
DISCUSSION
The findings showed that the protocol was safe, feasible, and acceptable to participants. No enrolled participants were lost to attrition, and no adverse events were reported.
This study also found that primary and secondary driving performance outcomes did not differ statistically between the placebo and CBD groups. These findings were similar to Arkel et al.’s29 crossover RCT that investigated the effect of smoked CBD on driver performance. However, this study's findings need to be interpreted with caution for 2 important reasons. First, this study was statistically underpowered. The study was initially powered at 0.5, but effect sizes were found to range from 0.03 to 0.36. Secondly, the CBD group performed slightly worse across all outcomes than the placebo group. It is unclear whether these small performance deficits would be clinically relevant and result in impaired driving. Impaired drivers typically have problems maintaining lane position and speed, commit more errors, and have slower reaction times than those not impaired.55
Nevertheless, these findings have public health implications. CBD is being added unscrupulously to food and hygienic products, but its effects are understudied. Previous research has shown that many CBD products are mislabeled and contain more THC than allowed by law, and THC alone can negatively impact safe driving.29,50 Even if CBD's effects are small, it is unclear whether compensatory measures such as getting more rest or using caffeine after consumption is enough to counterbalance CBD's effects. Additional research clearly is needed.
Limitations
Only 1 dosage of CBD was utilized, and this may not reflect the normal use. Participants were recruited from a university, so results may not be generalizable to the general population. Although a placebo group was utilized, a positive control group was not used. Finally, CBD's maximum absorption occurs 2–5 hours after consumption.28,46 To balance participant burden, a 2-hour waiting period between dosing and simulation was chosen. It is possible that the full effect of the drug was not reached among some participants.
CONCLUSIONS
This study found that an RCT of CBD is feasible. Although no relationships were statistically significant, the study was underpowered. Those randomized to CBD performed slightly worse on all study outcomes, and it is unclear whether these deficits indicate impairment. Larger RCTs are warranted.
CRediT authorship contribution statement
Toni Marie Rudisill: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. Karen (Kim) Innes: Methodology, Supervision, Writing – original draft, Writing – review & editing. Sijin Wen: Formal analysis, Methodology, Supervision, Writing – original draft. Treah Haggerty: Methodology, Supervision, Writing – original draft, Writing – review & editing. Gordon S. Smith: Methodology, Supervision, Writing – original draft, Writing – review & editing.
Declarations of interest
None.
ACKNOWLEDGMENTS
The study sponsor did not have any role in the study design; collection, analysis, interpretation of data; writing of the report; and the decision to submit the report for publication.
Research reported in this publication was supported by the National Institute of General Medical Sciences of NIH under Award Number 5U54GM104942-05. The study was approved by West Virginia University's IRB (Identification Number 2007073792).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.focus.2022.100053.
Appendix. Supplementary materials
REFERENCES
- 1.FDA and cannabis: research and drug approval process. U.S. Food and Drug Administration. https://www.fda.gov/news-events/public-health-focus/fda-and-cannabis-research-and-drug-approval-process. Updated October 1, 2020. Accessed October 20, 2022.
- 2.FDA approves new indication for drug containing an active ingredient derived from cannabis to treat seizures in rare genetic disease. U.S. Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-new-indication-drug-containing-active-ingredient-derived-cannabis-treat-seizures-rare#:∼:text=Today%2C%20the%20U.S.%20Food%20and,year%20of%20age%20and%20older. Updated July 31, 2020. Accessed 20 October 2022.
- 3.United States Congress. Agriculture improvement act of 2018. Public Law 115–334. Washington DC: United States Congress. https://www.congress.gov/115/plaws/publ334/PLAW-115publ334.pdf. Published December 20, 2018. Accessed October 19, 2022.
- 4.Shea LA, Leeds M, Bui D, et al. Over-the-counter” cannabidiol (CBD) sold in the community pharmacy setting in Colorado. Drugs Ther Perspect. 2020;36(12):573–582. doi: 10.1007/s40267-020-00781-3. [DOI] [Google Scholar]
- 5.Peres FF, Lima AC, Hallak JEC, Crippa JA, Silva RH, Abílio VC. Cannabidiol as a promising strategy to treat and prevent movement disorders? Front Pharmacol. 2018;9:482. doi: 10.3389/fphar.2018.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Premoli M, Aria F, Bonini SA, et al. Cannabidiol: recent advances and new insights for neuropsychiatric disorders treatment. Life Sci. 2019;224:120–127. doi: 10.1016/j.lfs.2019.03.053. [DOI] [PubMed] [Google Scholar]
- 7.Prud'homme M, Cata R, Jutras-Aswad D. Cannabidiol as an intervention for addictive behaviors: a systematic review of the evidence. Subst Abuse. 2015;9:33–38. doi: 10.4137/SART.S25081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rohleder C, Müller JK, Lange B, Leweke FM. Cannabidiol as a potential new type of an antipsychotic. A critical review of the evidence. Front Pharmacol. 2016;7:422. doi: 10.3389/fphar.2016.00422. (NOV) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soares VP, Campos AC. Evidences for the anti-panic actions of cannabidiol. Curr Neuropharmacol. 2017;15(2):291–299. doi: 10.2174/1570159x14666160509123955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turna J, Syan SK, Frey BN, et al. Cannabidiol as a novel candidate alcohol use disorder pharmacotherapy: a systematic review. Alcohol Clin Exp Res. 2019;43(4):550–563. doi: 10.1111/acer.13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuardi AW, Crippa JA, Hallak JE, et al. A critical review of the antipsychotic effects of cannabidiol: 30 years of a translational investigation. Curr Pharm Des. 2012;18(32):5131–5140. doi: 10.2174/138161212802884681. [DOI] [PubMed] [Google Scholar]
- 12.Bitencourt RM, Takahashi RN. Cannabidiol as a therapeutic alternative for post-traumatic stress disorder: from bench research to confirmation in human trials. Front Neurosci. 2018;12:502. doi: 10.3389/fnins.2018.00502. (JUL) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blessing EM, Steenkamp MM, Manzanares J, Marmar CR. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics. 2015;12(4):825–836. doi: 10.1007/s13311-015-0387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burstein S. Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg Med Chem. 2015;23(7):1377–1385. doi: 10.1016/j.bmc.2015.01.059. [DOI] [PubMed] [Google Scholar]
- 15.Celius EG, Vila C. The influence of THC:CBD oromucosal spray on driving ability in patients with multiple sclerosis-related spasticity. Brain Behav. 2018;8(5):e00962. doi: 10.1002/brb3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Mello Schier AR, de Oliveira Ribeiro NP, Coutinho DS, et al. Antidepressant-like and anxiolytic-like effects of cannabidiol: a chemical compound of cannabis sativa. CNS Neurol Disord Drug Targets. 2014;13(6):953–960. doi: 10.2174/1871527313666140612114838. [DOI] [PubMed] [Google Scholar]
- 17.Schier AR, Ribeiro NP, Silva AC, et al. Cannabidiol, a Cannabis sativa constituent, as an anxiolytic drug. Braz J Psychiatry. 2012;34:S104–S110. doi: 10.1590/s1516-44462012000500008. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 18.Devinsky O, Cilio MR, Cross H, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia (Series 4). 2014;55(6):791–802. 10.1111/epi.12631. [DOI] [PMC free article] [PubMed]
- 19.Silva TB, Balbino CQ, Weiber AF. The relationship between cannabidiol and psychosis: a review. Ann Clin Psychiatry. 2015;27(2):134–141. [PubMed] [Google Scholar]
- 20.Hermann D, Schneider M. Potential protective effects of cannabidiol on neuroanatomical alterations in cannabis users and psychosis: a critical review. Curr Pharm Des. 2012;18(32):4897–4905. doi: 10.2174/138161212802884825. [DOI] [PubMed] [Google Scholar]
- 21.Lattanzi S, Brigo F, Cagnetti C, Trinka E, Silvestrini M. Efficacy and safety of adjunctive cannabidiol in patients with Lennox–Gastaut syndrome: a systematic review and meta-analysis. CNS Drugs. 2018;32(10):905–916. doi: 10.1007/s40263-018-0558-9. [DOI] [PubMed] [Google Scholar]
- 22.Lattanzi S, Brigo F, Trinka E, et al. Efficacy and safety of cannabidiol in epilepsy: a systematic review and meta-analysis. Drugs. 2018;78(17):1791–1804. doi: 10.1007/s40265-018-0992-5. [DOI] [PubMed] [Google Scholar]
- 23.Osborne AL, Solowij N, Weston-Green K. A systematic review of the effect of cannabidiol on cognitive function: relevance to schizophrenia. Neurosci Biobehav Rev. 2017;72:310–324. doi: 10.1016/j.neubiorev.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Chagas MHN, Eckeli AL, Zuardi AW, et al. Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson's disease patients: a case series. J Clin Pharm Ther. 2014;39(5):564–566. doi: 10.1111/jcpt.12179. [DOI] [PubMed] [Google Scholar]
- 25.Iffland K, Grotenhermen F. An update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017;2(1):139–154. doi: 10.1089/can.2016.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhornitsky S, Potvin S. Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals (Basel) 2012;5(5):529–552. doi: 10.3390/ph5050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergamaschi MM, Queiroz RHC, Zuardi AW, Crippa JAS. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. 2011;6(4):237–249. doi: 10.2174/157488611798280924. [DOI] [PubMed] [Google Scholar]
- 28.Taylor L, Gidal B, Blakey G, Tayo B, Morrison G. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs. 2018;32(11):1053–1067. doi: 10.1007/s40263-018-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arkell TR, Vinckenbosch F, Kevin RC, Theunissen EL, McGregor IS, Ramaekers JG. Effect of cannabidiol and Δ9-tetrahydrocannabinol on driving performance: a randomized clinical trial. JAMA. 2020;324(21):2177–2186. doi: 10.1001/jama.2020.21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaspar JG, Brown TL, Schwarz CW, Lee JD, Kang J, Higgins JS. Evaluating driver drowsiness countermeasures. Traffic Inj Prev. 2017;18:S58–S63. doi: 10.1080/15389588.2017.1303140. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 31.Owens JM, Dingus TA, Guo F, et al. Prevalence of drowsy driving crashes: estimates from a large-scale naturalistic driving study (research brief). Washington, DC: American Automobile Association Foundation for Traffic Safety. https://aaafoundation.org/prevalence-drowsy-driving-crashes-estimates-large-scale-naturalistic-driving-study/. Published February 2018. Accessed August 23, 2021.
- 32.Babalonis S, Haney M, Malcolm RJ, et al. Oral cannabidiol does not produce a signal for abuse liability in frequent marijuana smokers. Drug Alcohol Depend. 2017;172:9–13. doi: 10.1016/j.drugalcdep.2016.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belgrave BE, Bird KD, Chesher GB, et al. The effect of cannabidiol, alone and in combination with ethanol, on human performance. Psychopharmacology. 1979;64(2):243–246. doi: 10.1007/BF00496070. [DOI] [PubMed] [Google Scholar]
- 34.Consroe P, Kennedy K, Schram K. Assay of plasma cannabidiol by capillary gas chromatography/ion trap mass spectroscopy following high-dose repeated daily oral administration in humans. Pharmacol Biochem Behav. 1991;40(3):517–522. doi: 10.1016/0091-3057(91)90357-8. [DOI] [PubMed] [Google Scholar]
- 35.Das RK, Kamboj SK, Ramadas M, et al. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology. 2013;226(4):781–792. doi: 10.1007/s00213-012-2955-y. [DOI] [PubMed] [Google Scholar]
- 36.Englund A, Morrison PD, Nottage J, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27(1):19–27. doi: 10.1177/0269881112460109. [DOI] [PubMed] [Google Scholar]
- 37.Haney M, Malcolm RJ, Babalonis S, et al. Oral cannabidiol does not Alter the Subjective, Reinforcing or cardiovascular Effects of Smoked cannabis. Neuropsychopharmacology. 2016;41(8):1974–1982. doi: 10.1038/npp.2015.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linares IMP, Guimaraes FS, Eckeli A, et al. No acute effects of cannabidiol on the sleep-wake cycle of healthy subjects: a randomized, double-blind, placebo-controlled, crossover study. Front Pharmacol. 2018;9:315. doi: 10.3389/fphar.2018.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoedel KA, Szeto I, Setnik B, et al. Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: a randomized, double-blind, controlled trial. Epilepsy Behav. 2018;88:162–171. doi: 10.1016/j.yebeh.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 40.Zuardi AW, Cosme RA, Graeff FG, Guimarães FS. Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharmacol. 1993;7(1):82–88. doi: 10.1177/026988119300700112. (suppl) [DOI] [PubMed] [Google Scholar]
- 41.Matthews JNS, Henderson R. Two-period, two-treatment crossover designs subject to non-ignorable missing data. Biostatistics. 2013;14(4):626–638. doi: 10.1093/biostatistics/kxt009. [DOI] [PubMed] [Google Scholar]
- 42.Faul F, Erdfelder E, Lang AG, G*Power Buchner A. 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 43.de Faria SM, de Morais Fabrício D, Tumas V, et al. Effects of acute cannabidiol administration on anxiety and tremors induced by a simulated public speaking test in patients with Parkinson's disease. J Psychopharmacol. 2020;34(2):189–196. doi: 10.1177/0269881119895536. [DOI] [PubMed] [Google Scholar]
- 44.Linares IM, Zuardi AW, Pereira LC, et al. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Braz J Psychiatry. 2019;41(1):9–14. doi: 10.1590/1516-4446-2017-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Classen S, Bewernitz M, Shechtman O. Driving simulator sickness: an evidence-based review of the literature. Am J Occup Ther. 2011;65(2):179–188. doi: 10.5014/ajot.2011.000802. [DOI] [PubMed] [Google Scholar]
- 46.Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42(4):327–360. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- 47.Vickers AJ. How to randomize. J Soc Integr Oncol. 2006;4(4):194–198. doi: 10.2310/7200.2006.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cordellieri P, Baralla F, Ferlazzo F, Sgalla R, Piccardi L, Giannini AM. Gender effects in young road users on road safety attitudes, behaviors and risk perception. Front Psychol. 2016;7:1412. doi: 10.3389/fpsyg.2016.01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yagil D. Gender and age-related differences in attitudes toward traffic laws and traffic violations. Transp Res F. 1998;1(2):123–135. doi: 10.1016/S1369-8478(98)00010-2. [DOI] [Google Scholar]
- 50.Freedman DA, Patel AD. Inadequate regulation contributes to mislabeled online cannabidiol products. Pediatr Neurol Briefs. 2018;32:3. doi: 10.15844/pedneurbriefs-32-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harrell FE. In: Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Harrell JFE, editor. Springer International Publishing; Cham, Switzerland: 2015. Cox proportional hazards regression model; pp. 475–519. [DOI] [Google Scholar]
- 52.Rich JT, Neely JG, Paniello RC, Voelker CCJ, Nussenbaum B, Wang EW. A practical guide to understanding Kaplan-Meier curves. Otolaryngology. Head Neck Surg. 2010;143(3):331–336. doi: 10.1016/j.otohns.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 54.Cohen J. Routledge; London, United Kingdom: 2013. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 55.National Highway Traffic Safety Administration. The visual detection of DWI motorists. Washington, DC: United States Department of Transportation, National Highway Traffic Safety Administration. https://www.nhtsa.gov/staticfiles/nti/pdf/808677.pdf. Published March 2010. Accessed Sep\tember 9, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.