Abstract
Background
Multisystem inflammatory syndrome in children (MIS-C), a hyperinflammatory condition associated with SARS-CoV-2 infection, has emerged as a serious illness in children worldwide. Immunoglobulin or glucocorticoids, or both, are currently recommended treatments.
Methods
The Best Available Treatment Study evaluated immunomodulatory treatments for MIS-C in an international observational cohort. Analysis of the first 614 patients was previously reported. In this propensity-weighted cohort study, clinical and outcome data from children with suspected or proven MIS-C were collected onto a web-based Research Electronic Data Capture database. After excluding neonates and incomplete or duplicate records, inverse probability weighting was used to compare primary treatments with intravenous immunoglobulin, intravenous immunoglobulin plus glucocorticoids, or glucocorticoids alone, using intravenous immunoglobulin as the reference treatment. Primary outcomes were a composite of inotropic or ventilator support from the second day after treatment initiation, or death, and time to improvement on an ordinal clinical severity scale. Secondary outcomes included treatment escalation, clinical deterioration, fever, and coronary artery aneurysm occurrence and resolution. This study is registered with the ISRCTN registry, ISRCTN69546370.
Findings
We enrolled 2101 children (aged 0 months to 19 years) with clinically diagnosed MIS-C from 39 countries between June 14, 2020, and April 25, 2022, and, following exclusions, 2009 patients were included for analysis (median age 8·0 years [IQR 4·2–11·4], 1191 [59·3%] male and 818 [40·7%] female, and 825 [41·1%] White). 680 (33·8%) patients received primary treatment with intravenous immunoglobulin, 698 (34·7%) with intravenous immunoglobulin plus glucocorticoids, 487 (24·2%) with glucocorticoids alone; 59 (2·9%) patients received other combinations, including biologicals, and 85 (4·2%) patients received no immunomodulators. There were no significant differences between treatments for primary outcomes for the 1586 patients with complete baseline and outcome data that were considered for primary analysis. Adjusted odds ratios for ventilation, inotropic support, or death were 1·09 (95% CI 0·75–1·58; corrected p value=1·00) for intravenous immunoglobulin plus glucocorticoids and 0·93 (0·58–1·47; corrected p value=1·00) for glucocorticoids alone, versus intravenous immunoglobulin alone. Adjusted average hazard ratios for time to improvement were 1·04 (95% CI 0·91–1·20; corrected p value=1·00) for intravenous immunoglobulin plus glucocorticoids, and 0·84 (0·70–1·00; corrected p value=0·22) for glucocorticoids alone, versus intravenous immunoglobulin alone. Treatment escalation was less frequent for intravenous immunoglobulin plus glucocorticoids (OR 0·15 [95% CI 0·11–0·20]; p<0·0001) and glucocorticoids alone (0·68 [0·50–0·93]; p=0·014) versus intravenous immunoglobulin alone. Persistent fever (from day 2 onward) was less common with intravenous immunoglobulin plus glucocorticoids compared with either intravenous immunoglobulin alone (OR 0·50 [95% CI 0·38–0·67]; p<0·0001) or glucocorticoids alone (0·63 [0·45–0·88]; p=0·0058). Coronary artery aneurysm occurrence and resolution did not differ significantly between treatment groups.
Interpretation
Recovery rates, including occurrence and resolution of coronary artery aneurysms, were similar for primary treatment with intravenous immunoglobulin when compared to glucocorticoids or intravenous immunoglobulin plus glucocorticoids. Initial treatment with glucocorticoids appears to be a safe alternative to immunoglobulin or combined therapy, and might be advantageous in view of the cost and limited availability of intravenous immunoglobulin in many countries.
Funding
Imperial College London, the European Union's Horizon 2020, Wellcome Trust, the Medical Research Foundation, UK National Institute for Health and Care Research, and National Institutes of Health.
Research in context.
Evidence before this study
In the first wave of the COVID-19 pandemic, paediatricians worldwide rapidly identified and described a new inflammatory disorder, causing shock and multi-system failure in children 4–6 weeks after SARS-CoV-2 infection. Faced with this new life-threatening disorder, termed multisystem inflammatory syndrome in children (MIS-C), and with unknown pathophysiological mechanisms, paediatricians and national and international paediatric bodies rapidly adopted treatments that are of benefit in other inflammatory disorders.
Based on the similarity in clinical features of MIS-C to Kawasaki disease, intravenous immunoglobulin, the recognised treatment for Kawasaki disease, was adopted as the most widely used initial treatment, often combined with glucocorticoids and a range of biological agents. In the absence of data from randomised controlled trials, national and international organisations, including WHO, the American College of Rheumatology, and the UK Royal College of Paediatrics and Child Health (RCPCH) produced treatment guidelines recommending intravenous immunoglobulin as initial treatment, combined with glucocorticoids or biological agents for patients who were the most seriously ill or unresponsive.
We searched PubMed for publications in English on treatment of MIS-C (and the alternative name paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2) between April 1, 2020, when the disorder was first recognised, and Nov 1, 2022. In the extensive literature now published on MIS-C, there are many hundreds of observational studies, treatment recommendations, and guidelines based on expert opinion, and reports of outcome after treatment. However, we found no randomised controlled trials, and only four propensity matched comparisons reporting outcomes after specific treatments, only two of which included comparison of glucocorticoids alone and intravenous immunoglobulin, and all were based on relatively small patient cohorts.
Added value of this study
The Best Available Treatment Study (BATS) allowed us to compare treatment of MIS-C with intravenous immunoglobulin alone, glucocorticoids alone, and glucocorticoids plus intravenous immunoglobulin (combined therapy), in over 2000 patients from 39 different countries. To our knowledge, this is the largest study of immunomodulator treatment options in MIS-C, including the largest cohort of patients treated initially with glucocorticoid monotherapy. After correcting for known confounders using propensity score weighting, initial treatment with glucocorticoid monotherapy or combined therapy demonstrated no significant difference to treatment with intravenous immunoglobulin monotherapy in either time to improvement measured on an ordinal clinical severity scale, or in a composite outcome of inotropic support or ventilator support (invasive or non-invasive) from the second day after starting treatment or later, or death. Comparison of glucocorticoid monotherapy with combined therapy suggested a small benefit from combined therapy in time to improvement, but this appeared to be restricted to those who did not require inotropic or ventilatory support at baseline. Combined therapy was associated with faster fever resolution and less escalation of treatment, but with no other notable differences in secondary outcomes. Occurrence and resolution of coronary artery aneurysms was similar in all treatment groups, with the large majority of aneurysms resolving during follow-up.
Implications of all the available evidence
Our study increases confidence that initial treatment of MIS-C with glucocorticoids is associated with similar outcomes to treatment with intravenous immunoglobulin or combined therapy. In the context of all current observational data, there is, at best, only a small benefit in initial therapy combining intravenous immunoglobulin and glucocorticoids compared with monotherapy with intravenous immunoglobulin or glucocorticoids alone. Given the high cost and limited availability of intravenous immunoglobulin in many countries, our evidence supports initial glucocorticoid monotherapy as an acceptable alternative.
Introduction
Since its recognition in April, 2020, multisystem inflammatory syndrome in children (MIS-C), also known as paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2,1, 2, 3, 4 has emerged as a rare but serious post-infectious illness.5, 6, 7, 8 In the absence of evidence from randomised controlled trials, treatment recommendations for the newly recognised disease were developed by clinical consensus in many countries. Based on the similarity of MIS-C to Kawasaki disease, for which intravenous immunoglobulin is the established treatment,9 national and international guidance has recommended intravenous immunoglobulin as initial treatment, with addition of glucocorticoids or other immunomodulatory agents, or both, for patients with severe illness.10, 11
Although there have been no randomised controlled trials comparing treatments for MIS-C published, several observational studies using propensity score methods have suggested that combination treatment with intravenous immunoglobulin and glucocorticoids was associated with improved cardiac outcomes,12, 13, 14 and trials are ongoing.
The Best Available Treatment Study (BATS) was initiated in May, 2020, after first recognition of MIS-C and aimed to provide evidence for treatment recommendations by systematic data collection and analysis of outcomes of treatments chosen by individual paediatricians responsible for patient care. In view of the urgent need for evidence to support treatment recommendations, analysis of the first 614 patients enrolled in BATS was reported in July, 2021.15 No significant differences in outcomes were observed between patients treated with intravenous immunoglobulin alone, glucocorticoids alone, or combination of intravenous immunoglobulin and glucocorticoids, although this finding might have been due to the moderate sample size. Here, we compare the initial treatments for MIS-C in a much larger cohort of children, and also describe the outcomes of cardiac complications.
Methods
Study design and participants
Details of the BATS observational cohort study were described in the initial report.15 In this propensity-weighted cohort study, minor modifications of the data collection procedure and analysis plan were undertaken, which are described here and in the published analysis plan and appendix (p 12–22).
Briefly, paediatricians worldwide were invited to join BATS and upload data from patients with suspected MIS-C onto a web-based Research Electronic Data Capture database,16 from June 14, 2020, to April 25, 2022. Given that the spectrum of post-SARS-CoV-2 inflammatory disease was unknown when BATS was initiated,3, 5, 17, 18, 19 and the reliability of the published criteria for MIS-C was unknown, we invited recruitment of children (aged 0 months to 19 years) with severe inflammatory illness after SARS-CoV-2 infection in addition to those meeting the US Centers for Disease Control (CDC), WHO, or UK case definitions.20, 21, 22 De-identified longitudinal data were collected on presenting features (eg, fever, diarrhoea), demographics (eg, age, sex), laboratory findings (eg, full blood count, inflammatory markers), immunomodulatory (eg, intravenous immunoglobulins, glucocorticoids, or biologicals) and supportive (eg, ventilation or haemodynamic support) treatments. Treatments and daily data were collected by calendar day. Duration of hospital admission, organ support required, and health status on hospital discharge were recorded.
The original BATS case report form recorded no data on coronary artery aneurysms after hospital discharge, and we therefore added an additional follow-up questionnaire regarding coronary artery aneurysm resolution (appendix p 68). Approval for follow-up data collection was provided in the original approval documents.
BATS was designed by the study team at Imperial College London (appendix p 3). Patient data were collected by local investigators (consortium members; appendix pp 3–11). The updated statistical analysis plan was developed by the study management team and international advisory board, and analysis was done by the statistical group (appendix p 3). The study was approved by the UK Research Ethics Committee (20/HRA/2957). Participating centres obtained ethics approval based on requirements in each country. Informed consent was not required in countries where the ethics committee gave permission for the use of routinely collected hospital data that did not include patient-identifiable information. For countries where informed consent was required, each site received local approvals for data collection and use.
Procedures and outcomes
The first calendar day of immunomodulatory treatment was defined as day 0, and subsequent treatment and outcomes were defined relative to this definition. Primary treatment was defined as the immunomodulatory agent(s) initiated on day 0. Three primary treatment groups were large enough for weighted comparison according to our predefined sample-size estimations (appendix p 69): intravenous immunoglobulin alone, glucocorticoids alone, or intravenous immunoglobulin plus glucocorticoids. Two other groups were predefined for additional analyses: those receiving other immunomodulator treatments (including in combination with intravenous immunoglobulin or glucocorticoids, or both), or no immunomodulator treatments.
Primary outcomes were modified from the previous analysis. The first primary outcome remained a composite of inotropic support or ventilator support (invasive or non-invasive) on day 2 or later, or death. However, the second primary outcome was altered from improvement on the ordinal severity scale by day 2, to time to improvement of at least one level on the ordinal clinical severity scale (the level of disease severity from worst to least were as follows: receiving ventilation and inotropic support; receiving ventilation alone; receiving inotropic support alone; receiving oxygen alone; no supportive therapy stratified by C-reactive protein concentration (≥50 mg/L, <50 mg/L, or missing) and discharged; appendix p 14). This modification was justified by the greater clinical relevance and additional statistical power of the time to event analysis.
Secondary outcomes were immunomodulator escalation (any additional immunomodulator, a second dose of intravenous immunoglobulin if primary treatment included intravenous immunoglobulin, and if primary treatment included glucocorticoids, an increment of 5 mg/kg equivalent daily-dose of prednisolone);23 fever from day 2 onwards; individual components of the first primary outcome (death, or inotropic or ventilator support from day 2); coronary artery aneurysm occurrence and resolution after treatment (coronary artery Z-score ≥2·5 or aneurysm documented);24 left ventricular dysfunction on echocardiography from day 2 onwards; no improvement in clinical severity scale at day 2; any increase in cardiorespiratory supportive therapy after day 0; therapeutic complications; and temporal dynamics of blood markers of inflammation and organ damage.
Statistical analysis
We applied inverse probability of treatment weighting using covariate-balancing propensity scores25 to account for baseline differences between the three primary treatment groups. Confounding covariates were selected by expert consensus before analysis and were used in covariate balancing and treatment effect estimation to produce doubly robust estimates (appendix pp 18–20). As specified in the analysis plan, intravenous immunoglobulin alone was the reference treatment group. Weighted quasibinomial logistic regression was used for dichotomous outcomes and weighted Cox-regression for time-to-event analyses. Outcomes were reported as adjusted odds ratios (ORs) or average hazard ratios (HRs) with 95% CIs and p values. p value correction for multiple hypothesis testing was performed for the two primary outcomes and two treatment-group comparisons with the Bonferroni-Holm procedure with a corrected two-sided α of 2·5% (appendix p 20).
All clinician-diagnosed MIS-C cases were included in our analysis, and more restrictive definitions were evaluated in prespecified subgroup or sensitivity analyses. Subgroup analysis included restricting the analysis to patients meeting the WHO MIS-C criteria,22 and to those meeting Kawasaki disease criteria; we also stratified by subgroups of age category (<6 years, 6–11 years, and >11 years) and baseline inflammation measured by peak C-reactive protein tertiles. We performed planned sensitivity analyses using propensity score matching rather than inverse probability weighting, and by defining primary treatment according to the treatments received on days 0 and 1. A full list of subgroup and sensitivity analyses is in the appendix (pp 21–22).
Inflammatory markers were plotted as percentages of each patient's peak value by hospital admission day relative to treatment initiation. Smoothed curves with 95% CIs were weighted by the same approach and fitted using the generalised additive model method (appendix p 18).
This study was registered with the ISRCTN registry, ISRCTN69546370.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
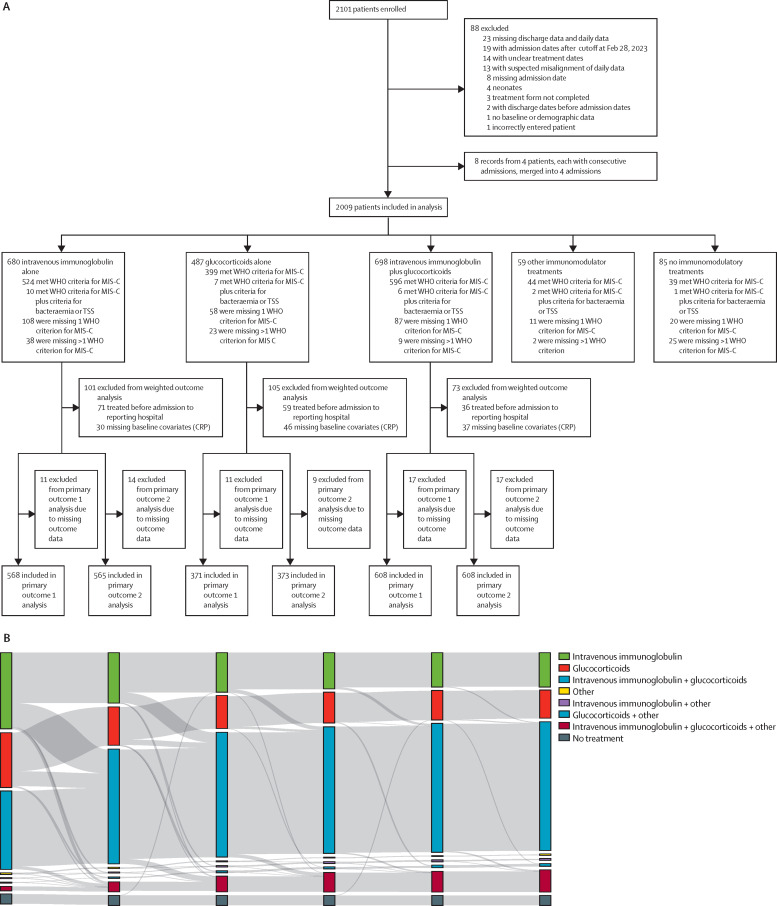
From June 20, 2020, to April 25, 2022, data from 2101 patients with MIS-C from 39 countries and 121 sites were uploaded to BATS (appendix pp 48–51). 92 records were excluded, including four neonates and those with incomplete data, duplicate entries, or admission after the recruitment deadline (figure 1A ). Of 2009 patients (median age 8·0 years ([IQR 4·2–11·4], 1191 [59·3%] male and 818 [40·7%] female, and 825 [41·1%] White) included for analysis, 680 (33·8%) received primary treatment with intravenous immunoglobulin alone, 487 (24·2%) received glucocorticoids alone, 698 (34·7%) received intravenous immunoglobulin plus glucocorticoids, 59 (2·9%) received other immunomodulators, and 85 (4·2%) received no immunomodulators (figure 1A). In the three main primary treatment groups, 579 (31·0%) of 1865 patients received additional immunomodulators by day 2, with 953 (51·1%) patients receiving secondary agents overall. Treatment trajectories are shown in detail in figure 1B and in the appendix (p 23).
Figure 1.
Study flowchart and treatments received by patients at days 0–5 after initiation of immunomodulator treatment
(A) The study flowchart gives an overview of the total number of patients enrolled, excluded, and included for analyses. Patients who met the inclusion criteria are categorised by treatment groups (intravenous immunoglobulin alone, glucocorticoids alone, intravenous immunoglobulin plus glucocorticoids, other immunomodulator treatments [including, anti-tumour necrosis factor, anti-interleukin 1, anti-interleukin 6] and no immunomodulator treatments) and subdivided by our data-driven classification according to the WHO MIS-C criteria. (B) The Sankey diagram shows the number of patients who received cumulative therapies from days after initiation of immunomodulator treatment. Each vertical stack represents a different day in the patients' admission relative to starting immunomodulatory treatment (days 0 to 5), with day 0 representing the first day of immunomodulator treatment. The grey bands represent movement of patients between treatment groups from days 0 to 1, days 1 to 2, days 2 to 3, days 3 to 4, and days 4 to 5. The width of the grey bands is proportional to the number of patients (flow). The flow of patients is independent between time intervals; there is no continuous correspondence across days 1 to 5. Glucocorticoids include intravenous and oral glucocorticoids (appendix p 41). Other includes one or more other immunomodulatory treatments given alone or in combination with glucocorticoids or intravenous immunoglobulin, or both. Other immunomodulatory treatments include anti-interleukin 1, anti-interleukin 6, anti-tumour necrosis factor, cytokine adsorber (CytoSorb), granulocyte colony stimulating factor, colchicine, mesenchymal stem cells, convalescent plasma, cyclophosphamide, plasmapheresis, and hydroxychloroquine. MIS-C=multisystem inflammatory syndrome in children. TSS=toxic shock syndrome.
Baseline clinical and laboratory findings showed some differences between primary treatment groups (table , appendix pp 24–25). Patients who received no immunomodulators had substantially less derangement in laboratory markers of inflammation and organ dysfunction, whereas those who received intravenous immunoglobulin plus glucocorticoids and those who received other immunomodulator treatments had the highest level of derangement overall (appendix p 52). A higher proportion of patients treated with intravenous immunoglobulin plus glucocorticoids and those treated with other immunomodulators received inotropes or ventilation on day 0 than did those treated with intravenous immunoglobulin alone or glucocorticoids alone (appendix p 55). Considering treatment received by day 2, a higher proportion of those treated with intravenous immunoglobulin plus glucocorticoids or those in whom biological agents were added received inotropes or ventilation at baseline (appendix p 55), but there were no major differences in blood markers between these groups (appendix p 53).
Table.
Clinical and demographic features in all treatment groups
| All patients (n=2009) | Intravenous immunoglobulin (n=680) | Glucocorticoids (n=487) | Intravenous immunoglobulin plus glucocorticoids (n=698) | Other immunomodulator (n=59) | No immunomodulator treatment (n=85) | ||
|---|---|---|---|---|---|---|---|
| Median age, years | 8·0 (IQR 4·2–11· 4) | 6·8 (IQR 3·6–10· 4) | 8·8 (IQR 5·1–12·1) | 8·4 (IQR 4·5–11·3) | 10·9 (IQR 6·1–13·1) | 7·3 (IQR 3·3–12·2) | |
| Sex | |||||||
| Male | 1191 (59·3%) | 416 (61·2%) | 288 (59·1%) | 410 (58·7%) | 44 (74·6%) | 33 (38·8%) | |
| Female | 818 (40·7%) | 264 (38·8%) | 199 (40·9%) | 288 (41·3%) | 15 (25·4%) | 52 (61·2%) | |
| Weight (age-adjusted z score ≥2) | 299 (14·9%) | 91 (13·4%) | 70 (14·4%) | 120 (17·2%) | 10 (16·9%) | 8 (9·4%) | |
| Ethnicity | |||||||
| White | 825 (41·1%) | 290 (42·6%) | 210 (43·1%) | 272 (39·0%) | 27 (45·8%) | 26 (30·6%) | |
| Latino | 518 (25·8%) | 161 (23·7%) | 94 (19·3%) | 222 (31·8%) | 9 (15·3%) | 32 (37·6%) | |
| Black | 212 (10·6%) | 81 (11·9%) | 34 (7·0%) | 75 (10·7%) | 13 (22·0%) | 9 (10·6%) | |
| Asian | 131 (6·5%) | 55 (8·1%) | 36 (7·4%) | 30 (4·3%) | 4 (6·8%) | 6 (7·1%) | |
| Other or not known | 323 (16·1%) | 93 (13·7%) | 113 (23·2%) | 99 (14·2%) | 6 (10·2%) | 12 (14·1%) | |
| Significant comorbidity* | 108 (5·4%) | 30 (4·4%) | 32 (6·6%) | 33 (4·7%) | 4 (6·8%) | 9 (10·6%) | |
| SARS-CoV-2 PCR-positive† | 415 (20·7%) | 131 (19·3%) | 97 (19·9%) | 148 (21·2%) | 13 (22·0%) | 26 (30·6%) | |
| SARS-CoV-2 antibody-positive† | 1321 (65·8%) | 412 (60·6%) | 344 (70·6%) | 492 (70·5%) | 43 (72·9%) | 30 (35·3%) | |
| Baseline requirement for ventilation, inotropes, or ECMO | 535 (26·6%) | 117 (17·2%) | 127 (26·1%) | 252 (36·1%) | 29 (49·2%) | 10 (11·8%) | |
| Clinical features during hospital admission | |||||||
| Fever | 1863 (92·7%) | 653 (96·0%) | 439 (90·1%) | 649 (93·0%) | 52 (88·1%) | 70 (82·4%) | |
| Sore throat | 464 (23·1%) | 159 (23·4%) | 104 (21·4%) | 175 (25·1%) | 11 (18·6%) | 15 (17·6%) | |
| Cough | 404 (20·1%) | 125 (18·4%) | 120 (24·6%) | 131 (18·8%) | 16 (27·1%) | 12 (14·1%) | |
| Respiratory distress | 258 (12·8%) | 70 (10·3%) | 57 (11·7%) | 112 (16·0%) | 13 (22·0%) | 6 (7·1%) | |
| Abdominal pain | 1211 (60·3%) | 408 (60·0%) | 289 (59·3%) | 438 (62·8%) | 37 (62·7%) | 39 (45·9%) | |
| Diarrhoea | 882 (43·9%) | 290 (42·6%) | 195 (40·4%) | 340 (48·7%) | 23 (39·0%) | 34 (40·0%) | |
| Vomiting | 1057 (52·6%) | 330 (48·5%) | 251 (51·5%) | 408 (58·5%) | 34 (57·6%) | 34 (40·0%) | |
| Headache | 592 (29·5%) | 199 (29·3%) | 155 (31·8%) | 203 (29·1%) | 21 (35·6%) | 14 (16·5%) | |
| Irritability | 355 (17·7%) | 127 (18·7%) | 69 (14·2%) | 135 (19·3%) | 10 (16·9%) | 14 (16·5%) | |
| Lethargy | 655 (32·6%) | 211 (31·0%) | 186 (38·2%) | 215 (30·8%) | 23 (39·0%) | 20 (23·5%) | |
| Proportion meeting Kawasaki disease criteria‡ | 629 (31·3%) | 265 (39·0%) | 119 (24·4%) | 225 (32·2%) | 12 (20·3%) | 8 (9·4%) | |
| Blood results on hospital admission | |||||||
| Lymphocytes, 109/L | 1·2 (0·7–2·0) | 1·3 (0·8–2·2) | 1·2 (0·7–1·8) | 1·1 (0·7–1·9) | 0·9 (0·5–1·6) | 1·8 (1·1–2·9) | |
| Troponin, ng/L | 25·0 (6·1–80·5) | 13·0 (5·0–43·2) | 31·2 (9·8–103·0) | 40·0 (10–110·0) | 48·0 (10–270·0) | 10·0 (2·0–38·0) | |
| C-reactive protein, mg/L | 150 (85–220) | 150 (85–210) | 160 (75–220) | 160 (90–230) | 180 (97–280) | 85 (23–180) | |
| Ferritin, μg/L | 440 (230–860) | 370 (210–650) | 480 (260–970) | 520 (260–960) | 560 (340–1700) | 280 (140–460) | |
| Albumin, g/L | 32 (28–37) | 34 (28–39) | 32 (27–36) | 32 (27–36) | 32 (27–36) | 35 (30–41) | |
ECMO=extracorporeal membrane oxygenation. Data are median (IQR) and n (%).
Significant comorbidity was defined as any of the following: primary or secondary immunodeficiency, including HIV infection; autoimmune disease; chronic lung disease; congenital heart disease; juvenile idiopathic arthritis; chronic neurological disorders; and malignancy.
SARS-CoV-2 PCR and antibody tests done during admission to hospital.
Proportion of patients meeting Kawasaki disease criteria according to American Heart Association criteria. Patients with coronary artery aneurysms met the definition of Kawasaki disease with less than four Kawasaki disease clinical features. Missing data (where applicable) are available in the appendix (p 24).
1602 (79·7%) of 2009 patients met WHO MIS-C criteria (appendix pp 26–27). The most common missing criterion was evidence of SARS-CoV-2 exposure (appendix p 56). Of 2009 patients, SARS-CoV-2 antibody measurements were not tested in 406 (20·2%) patients, and negative results were reported in 259 (12·9%) patients. Bacteria were cultured in the blood of a small proportion of patients (appendix p 26). 629 (31·3%) of 2009 patients, and 544 (34·0%) of 1602 patients who met WHO MIS-C criteria, also met the American Heart Association (AHA) definitions for complete Kawasaki disease (appendix pp 28, 57).
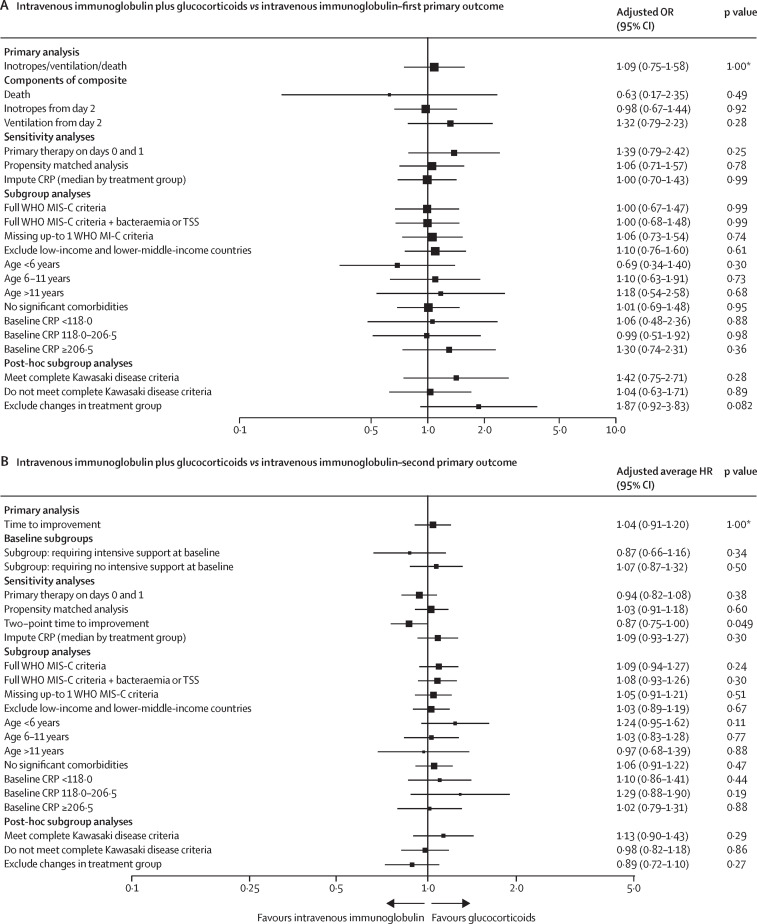
Of 1865 patients in the three main treatment groups, 166 (8·9%) patients received immunomodulators before transfer to the reporting hospital and an additional 113 patients (6·1%) were missing baseline covariates, with 1586 (85·0%) patients considered for our primary weighted analyses (figure 1A). Acceptable covariate balance was achieved for all inverse probability of treatment weighting outcome analyses (appendix pp 60–61, 66). For the first primary outcome (ie, receipt of inotropic support or ventilation on day 2 or later, or death), the adjusted ORs for patients who received primary treatment with intravenous immunoglobulin plus glucocorticoids or glucocorticoids alone compared with intravenous immunoglobulin alone were 1·09 (95% CI 0·75–1·58; corrected p value=1·00) and 0·93 (0·58–1·47; corrected p value=1·00; figure 2A, C , appendix p 29).
Figure 2.
Forest plots summarising point estimates and 95% CIs for primary analyses, including all subgroup and sensitivity analyses
Outcomes for patients with suspected MIS-C who received intravenous immunoglobulin plus glucocorticoids (A, B) or glucocorticoids alone (C, D), compared with those who received intravenous immunoglobulin alone (reference group, indicated by an OR, or average HR, of 1·00). (A, C) The first primary outcome analyses, risk of inotropes, ventilation or death, and values to the right of the dotted line indicate superiority of intravenous immunoglobulin alone. (B, D) The second primary outcome analyses, time to improvement in ordinal clinical severity score, with values to the left indicating superiority of intravenous immunoglobulin alone. CRP=C-reactive protein. HR=hazard ratio. MIS-C=multisystem inflammatory syndrome in children. OR=odds ratio. *p-values corrected for multiple hypothesis testing using the Bonferroni-Holm procedure, observed p value ×4. Absolute numbers of patients included in each analysis can be found in the appendix (pp 29–32).
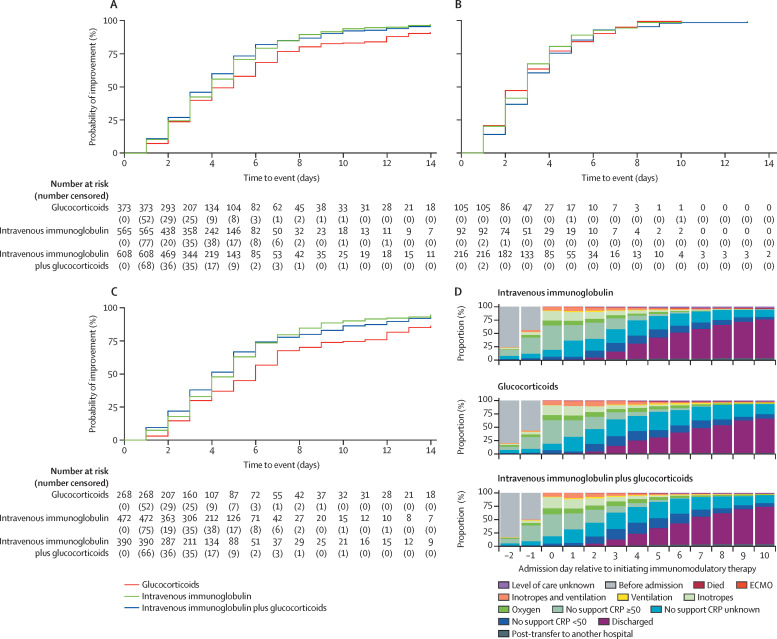
For the second primary outcome (ie, time to improvement on the ordinal clinical severity scale), the adjusted average HR for patients who received intravenous immunoglobulin plus glucocorticoids, compared with intravenous immunoglobulin, was 1·04 (95% CI 0·91–1·20; corrected p value=1·00), and for patients who received glucocorticoids alone compared with intravenous immunoglobulin alone the adjusted average HR was 0·84 (0·70–1·00; corrected p value=0·22; figure 2D, figure 3A , appendix p 29), suggesting slower improvement in the glucocorticoid alone group. Subgroup analyses of time to improvement in children who were severely ill (ie, requiring ventilatory or inotropic support at baseline) and those not requiring intensive support showed that slower improvement in those receiving glucocorticoids alone, compared with intravenous immunoglobulin alone, was confined to patients who were less severely ill (adjusted average HR 1·06 [95% CI 0·75–1·49], p=0·75, in patients who required ventilatory or inotropic support at baseline vs 0·83 [0·62–1·11], p=0·21, in patients who did not require intensive support; figure 2B, D, figure 3B–C, appendix p 34).
Figure 3.
Weighted clinical improvement over time
(A–C) Kaplan-Meier curves for the three main primary treatment groups showing time to one-point improvement in clinical severity on ordinal scale weighted by inverse probability of treatment, for all patients (A), a subgroup of patients needing at least one of inotropes or ventilation at baseline (B), and a subgroup of patients not requiring inotropes or ventilation at baseline (C). Tables below the Kaplan-Meier curves show the numbers at risk at the start of each day, and the number censored at this specific time point. (D) Clinical severity on ordinal scale, shown as proportional column charts from 2 days before treatment to 10 days after treatment, separated by primary treatment group, and weighted by inverse probability of treatment. Additional groups have been added for graphical purposes. CRP=C-reactive protein. ECMO=extracorporeal membrane oxygenation.
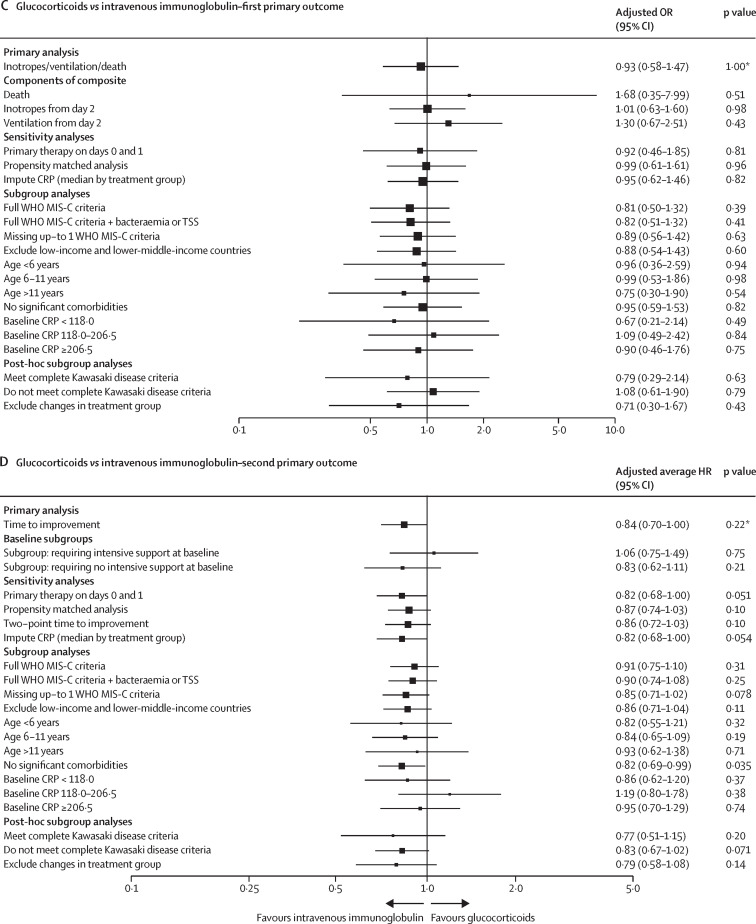
All sensitivity and subgroup analyses, including restricting the analysis to patients meeting WHO MIS-C criteria, showed no significant difference in the first primary outcome for the comparisons of intravenous immunoglobulin plus glucocorticoids or glucocorticoids alone with intravenous immunoglobulin alone (figure 2A, C, appendix p 32). For the second primary outcome, in the subgroup of patients without significant comorbidities (eg, immune deficiency, chronic heart disease, chronic neurological disease, or malignancy), the time to improvement was slower in patients treated with glucocorticoids alone compared with intravenous immunoglobulin alone (adjusted average HR 0·82 [95% CI 0·69–0·99]; p=0·035; figure 2D, appendix p 34) and the two-point time-to-improvement was slower in those treated with intravenous immunoglobulin plus glucocorticoids compared with intravenous immunoglobulin alone (0·87 [0·75–1·00]; p=0·049; figure 2B). All other planned sensitivity and subgroup analyses showed no significant difference in time to improvement for the comparisons of intravenous immunoglobulin plus glucocorticoids or glucocorticoids alone with intravenous immunoglobulin alone.
Escalation of immunomodulator treatment was less common in patients treated with intravenous immunoglobulin plus glucocorticoids (OR 0·15 [95% CI 0·11–0·20]; p<0·0001) and glucocorticoids alone (0·68 [0·50–0·93]; p=0·014), compared with intravenous immunoglobulin alone (appendix p 58). Persistent fever from day 2 was less common in patients treated with intravenous immunoglobulin plus glucocorticoids compared with either intravenous immunoglobulin alone (OR 0·50 [95% CI 0·38–0·67]; p<0·0001) or glucocorticoids alone (0·63 [0·45–0·88]; p=0·0058), with no significant difference between the glucocorticoid alone group and intravenous immunoglobulin alone group. In a post-hoc sensitivity analysis, there was no significant difference in persistent fever from day 3 between patients treated with intravenous immunoglobulin plus glucocorticoids and intravenous immunoglobulin alone. Individual components of the composite outcome showed no significant differences between treatments (figure 2A, C, appendix p 30).
Of 1918 patients with reported echocardiograms, 236 (12·3%) had coronary artery aneurysm at any time (89 [13·5%] of 660 intravenous immunoglobulin recipients, 40 [8·7%] of 458 glucocorticoid recipients, 88 [12·9%] of 680 intravenous immunoglobulin plus glucocorticoid recipients, 11 [26·8%] of other combination [including intravenous immunoglobulin] recipients, six [40·0%] of 15 other combination [excluding intravenous immunoglobulin] recipients, and zero [0%] of 57 who received no immunomodulatory treatment; appendix p 36), with the largest disparity in aneurysm detection before starting immunomodulatory treatment (appendix p 36). In the 705 patients with inpatient echocardiograms before and after treatment initiation (appendix p 31), 50 (7·1%) had coronary artery aneurysm present on the final echocardiogram before discharge, with no statistically significant difference between groups after inverse probability of treatment weighting analysis, including for post-hoc analyses restricted to patients who did and did not meet complete Kawasaki disease criteria (appendix p 58).
Follow-up echocardiogram data were available in 196 (83·1%) of 236 patients with coronary artery aneurysm during hospital admission. Most cases of coronary artery aneurysm resolved during follow-up (182 [92·9%] of 196 cases), with similar rates among primary treatment groups (appendix p 36). Similar rates of resolution were seen in all three primary treatment groups when restricted to patients with follow-up by 6-weeks and 12-weeks (appendix p 37).
To establish if patients who did not receive intravenous immunoglobulin were at a greater risk of coronary artery aneurysm, or had different rates of resolution, we explored the incidence of coronary artery aneurysm in the patients treated with glucocorticoids alone. 17 (7·1%) of 239 patients who never received subsequent intravenous immunoglobulin had coronary artery aneurysm detected at any time during admission, compared with 24 (10·9%) of 221 patients who received subsequent intravenous immunoglobulin later during admission. Coronary artery aneurysms were present at hospital discharge in five (2·1%) of 239 patients who received subsequent intravenous immunoglobulin and nine (4·1%) of 221 patients who received subsequent intravenous immunoglobulin, with more than 93% of coronary artery aneurysms resolving in both groups on reported follow-up (appendix p 38). No significant differences were shown between treatment groups regarding the severity of coronary artery aneurysm, as judged by the distribution of z-scores (appendix p 39). Larger z-scores were reported in younger patients (appendix p 39).
Left ventricular dysfunction was reported in 202 (13·4%) of 1512 patients with echocardiograms from day 2 onwards, with no significant difference between groups (appendix pp 31, 58). No significant differences were reported between patients treated with intravenous immunoglobulin plus glucocorticoids or glucocorticoids alone versus intravenous immunoglobulin alone for the secondary outcomes of no improvement by day 2 or increase in level of support after initiation of primary treatment. Death occurred in eight (1·1% unadjusted) patients treated with intravenous immunoglobulin plus glucocorticoids, ten (2·1%) patients treated with glucocorticoids alone, and five (0·7%) patients treated with intravenous immunoglobulin alone .
Drug complications were reported in 59 (3·6%) of 1623 patients who received any glucocorticoids and in 25 (1·5%) of 1658 patients who received intravenous immunoglobulin (appendix p 40). Glucocorticoid-associated complications were predominantly hypertension (n=23) and hyperglycaemia (n=14; appendix p 40).
A planned secondary analysis that compared treatment with glucocorticoids alone and combined intravenous immunoglobulin plus glucocorticoids showed no significant difference in the first primary outcome, but a faster time to improvement for the intravenous immunoglobulin plus glucocorticoids group (adjusted average HR 1·25 [95% CI 1·05–1·48]; p=0·012; appendix p 59). This faster time to improvement was predominantly seen from days 5–7 after treatment onwards, and in those patients not requiring intensive support at baseline (figure 3A–D). Secondary outcomes for this comparison showed that escalation of primary therapy and persistence of fever from day 2 were more common in patients treated with glucocorticoids alone compared with intravenous immunoglobulin plus glucocorticoids (appendix pp 30, 59).
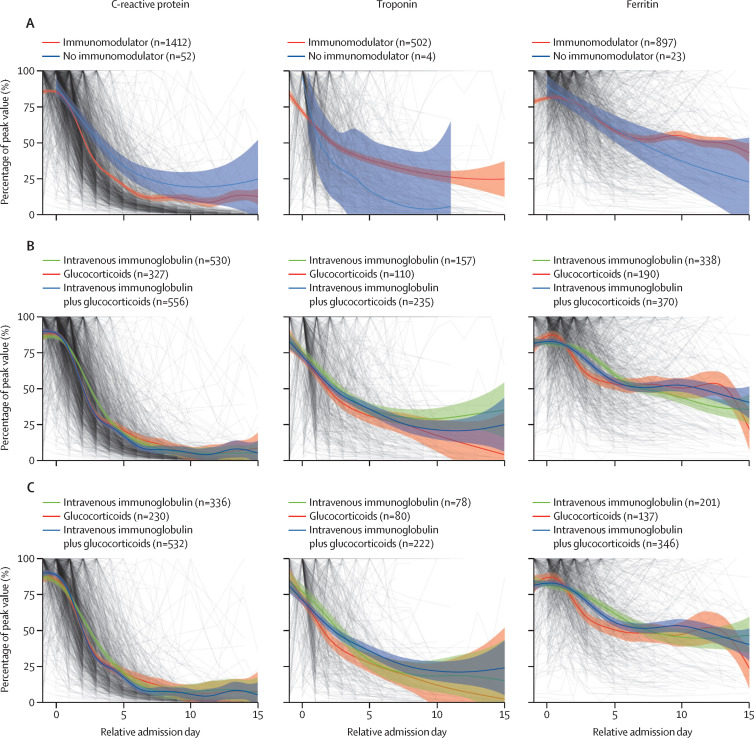
C-reactive protein concentration decreased more rapidly in patients treated with immunomodulators than that in untreated patients (figure 4A ). C-reactive protein concentration declined more rapidly in those treated with glucocorticoids alone and intravenous immunoglobulin plus glucocorticoids than that in patients treated with intravenous immunoglobulin alone (figure 4B). There was a suggestion of more rapid decline in troponin and ferritin in patients treated with glucocorticoids and intravenous immunoglobulin plus glucocorticoids, with a similar trend when restricting to those who did not receive additional treatment between days 0 and 2 (figure 4C). Timecourse plots of other blood markers showed similar dynamics of blood markers between groups (appendix p 62).
Figure 4.
Change in CRP, troponin, and ferritin over time
Each of three key markers of inflammation (CRP, troponin, and ferritin) is plotted as a line and weighted by the covariate balancing propensity score. The levels are shown as a percentage of each patient's peak value, plotted by day relative to starting treatment. A generalised additive model was used to fit the curves. For each plot patients are only included if they had blood results available both before and after treatment initiation, and only if their last value up to treatment initiation was abnormal (CRP ≥8 mg/L, troponin ≥14 ng/L, and ferritin ≥50 μg/L). (A) Fitted curves for the three measures in patients who received any immunomodulators, compared with those who did not receive immunomodulators, using day of admission as relative admission day for patients not receiving immunomodulator treatment, and curves for troponin in (A) were fitted using a loess model due to small sample numbers. (B) Fitted curves for patients who received intravenous immunoglobulin alone, intravenous immunoglobulin plus glucocorticoids, and glucocorticoids alone as their primary treatment. (C) Fitted curves for the three treatments combined in the patients whose primary treatment did not change between treatment initiation (day 0) and day 2. CRP=C-reactive protein.
To investigate whether inadvertent inclusion of children with Kawasaki disease might have influenced treatment responses, we explored changes in blood markers separately in children most resembling Kawasaki disease. Given that Kawasaki disease is generally a disease in children aged 5 years and younger, and MIS-C is often reported in older children, we compared those meeting AHA criteria for Kawasaki disease, and all children younger than 6 years with AHA-defined characteristics of Kawasaki disease, with the remaining patients with MIS-C.
The rate of decline in C-reactive protein concentration was similar between children younger than 6 years and those older than 6 years, and those fulfilling Kawasaki disease criteria treated with intravenous immunoglobulin, with a suggestion of a more rapid decline in C-reactive protein in the patients without AHA-defined Kawasaki disease characteristics treated with glucocorticoids alone (appendix p 63).
Discussion
Our comparison of treatment outcomes in an international cohort of 2009 children with MIS-C shows that treatment with glucocorticoids alone, or intravenous immunoglobulin plus glucocorticoids, is not associated with significant differences in primary outcomes (ie, requirements for inotropic support, ventilation on day 2 or later, or death; or rate of improvement on the ordinal severity scale) in comparison with intravenous immunoglobulin alone. The findings are consistent with our preliminary report of 614 children.15 However, the larger number of patients in each treatment group increases the confidence in our findings. A non-significant trend towards a slower rate of improvement was shown in patients treated with glucocorticoids alone versus intravenous immunoglobulin, but this comparison was confined to those with less severe illness at presentation. Reassuringly, we found no significant difference in coronary artery aneurysm outcomes between primary treatment groups, with resolution in the majority of patients.
Our planned secondary analysis that compared glucocorticoids alone with combined intravenous immunoglobulin plus glucocorticoids showed no significant difference in the requirements for inotropic support, ventilation on day 2 or later, or death, but a faster time to improvement for those treated with intravenous immunoglobulin plus glucocorticoids. This comparison was not adjusted for multiple hypothesis testing, and the effect appears confined to those patients not requiring intensive support at baseline. Other secondary endpoints, and thus also not corrected for multiple hypothesis testing, showed lower rates of treatment escalation and lower rates of fever on day 2 in patients treated with intravenous immunoglobulin plus glucocorticoids.
A key question for clinicians is whether the potential incremental benefits of intravenous immunoglobulin plus glucocorticoids to reduce severity of illness and accelerate resolution of fever are sufficient to justify the use of both agents. We note that the primary outcomes (progression or recovery from organ support) were chosen to select the most clinically important outcomes, whereas the secondary outcomes might detect less clinically important findings. Furthermore, we suggest that the finding of more common escalation of treatment for those who received single agents, which was also observed in earlier studies,12, 13 might be biased by greater clinician readiness to add other treatments for patients who are seriously ill and who do not rapidly improve on monotherapy, whereas options to escalate treatment are fewer in patients treated with primary combination intravenous immunoglobulin plus glucocorticoids.
This question of whether combined intravenous immunoglobulin plus glucocorticoids is beneficial compared with glucocorticoids alone is relevant to both resource-rich countries where intravenous immunoglobulin is readily available and countries where intravenous immunoglobulin has limited availability or cost imposes limitations in its use. For resource-limited settings, our data suggest that primary treatment with glucocorticoids alone is a safe alternative to intravenous immunoglobulin alone or combined with glucocorticoids, with intravenous immunoglobulin reserved for patients who do not improve with glucocorticoids alone. For countries where the cost of intravenous immunoglobulin is less prohibitive, the limited supply of intravenous immunoglobulin and potential for combined treatments to have more side-effects than single agents would make the argument for initial treatment with a single agent, and addition of second agents only in those who do not improve.
A higher proportion of patients treated with intravenous immunoglobulin plus glucocorticoids as primary treatment received inotropes or ventilation at day 0, and had more deranged blood markers, suggesting more patients who were severely ill might have received intravenous immunoglobulin plus glucocorticoids. Importantly, key differences between treatment groups were adjusted for in the propensity score analysis. Children treated with intravenous immunoglobulin plus glucocorticoids had more rapid resolution of fever than did children treated with intravenous immunoglobulin alone or glucocorticoids alone. However, no other clinically significant findings were more frequent in patients treated with intravenous immunoglobulin plus glucocorticoids in comparison with either of the single-agent treatment groups.
Patients who were initially treated with glucocorticoids or intravenous immunoglobulin alone and then received additional treatment by day 2 were more likely to receive inotrope or ventilatory support at baseline. However, there was minimal difference across a wide range of baseline biomarkers between patients who received additional treatments by day 2 and those who did not. This finding suggests that treatment with inotrope or ventilatory support influenced the clinical decision for administration of additional treatment. We included adjustment for both baseline inotrope and ventilatory support in our inverse probability of treatment weighting analysis.
The use of intravenous immunoglobulin as treatment for MIS-C has largely been driven by the similarity of MIS-C to Kawasaki disease, for which intravenous immunoglobulin is the established treatment to reduce risk of coronary artery aneurysm.9 Given that coronary artery aneurysms are observed in 10–20% of patients with MIS-C,13, 15, 26 there has been concern that failure to include intravenous immunoglobulin in initial treatment would be associated with increased risk of coronary artery aneurysm. We found that the incidence of coronary artery aneurysm in patients who received glucocorticoids as initial treatment was similar to the incidence of coronary artery aneurysm in recipients of intravenous immunoglobulin (either intravenous immunoglobulin or intravenous immunoglobulin plus glucocorticoids). Furthermore, the severity of coronary artery aneurysm (as measured by z-score) and the proportion of patients who had complete resolution of coronary artery aneurysm by time of discharge, or at follow-up, was similar in the group treated with glucocorticoid alone and in the groups treated with intravenous immunoglobulin and intravenous immunoglobulin plus glucocorticoids, including post-hoc analysis restricted to patients who never received intravenous immunoglobulin. Our study thus provides reassurance that initial therapy with single-agent glucocorticoids is not associated with increased risk of long-term coronary artery damage in patients with MIS-C.
The American College of Rheumatologists currently recommends combined treatment with intravenous immunoglobulin and glucocorticoids for MIS-C,11 on the basis of limited evidence of benefit from propensity-matched studies undertaken in the USA and France,12, 13 which showed lower rates of treatment escalation and improved cardiac function detected by echocardiogram with combined intravenous immunoglobulin and glucocorticoid therapy. Neither of these studies included a group treated with glucocorticoids alone, and both were substantially smaller than our current analysis.
We observed a more rapid decline in C-reactive protein concentration in all three main treatment groups compared with patients who did not receive immunomodulators. Although the curves for each treatment overlapped, there was a non-significant trend to a more rapid decline in C-reactive protein concentration, ferritin, and troponin in the glucocorticoid-containing groups.
Our study has several limitations. A key concern is the extent to which a retrospective comparison of outcomes after non-randomised choice of treatment can be used to guide clinical practice. We applied two different propensity score methods (weighting and matching), to minimise bias caused by differences in severity, demographics, or resource setting. We achieved good covariate balance between comparator groups using both approaches. However, other unmeasured differences might influence the results, and a large randomised controlled trial would be the preferred approach to provide definitive answers. Additionally, there is a risk of bias from the voluntary nature of data collection, given that not all cases of MIS-C from each site were necessarily included in the study.
A second potential limitation is our use of the broad inclusion criteria of clinician-diagnosed MIS-C. At the time BATS was initiated the accuracy of the published diagnostic criteria was unknown, and there were differences between the WHO, CDC, and Royal College of Paediatrics and Child Health criteria. Furthermore, availability of antibody testing for SARS-CoV-2 was limited in many countries. We therefore chose to include patients whose responsible clinicians considered them to have MIS-C, and in whom alternative diagnoses had been excluded. As we expected, our data supports that the most commonly missed criteria to meet the WHO or CDC definitions of MIS-C was the presence of evidence of SARS-CoV-2 exposure. Notably, as the pandemic has evolved, and a high proportion of children have become SARS-CoV-2 antibody-positive through natural infection or vaccination, the value of antibodies against SARS-CoV-2 as evidence of recent infection has reduced. In view of the high rates of SARS-CoV-2 infection in schools, and the high proportion of asymptomatic childhood infection, a history of exposure to infection is of little value in diagnosis of MIS-C, and the WHO and CDC criteria might need to be re-evaluated. Despite these concerns, the majority of patients in BATS did meet the WHO criteria, with only small differences in proportions from each of the primary treatment groups. Our subgroup and sensitivity analyses did not find any significant difference in outcome when restricted to those meeting the WHO criteria, or the group with features overlapping Kawasaki disease.
An additional concern might be that the nature, severity, and epidemiology of MIS-C has changed over time, and with successive SARS-CoV-2 variants and introduction of childhood vaccinations against SARS-CoV-2. The disorder appears to have become less common in many countries as a high proportion of children have previous infection, and both natural infection and vaccination might reduce the incidence of MIS-C.27 However, with SARS-CoV-2 now increasing in the previously unexposed population of China, there is likely to be a new wave of MIS-C and the findings reported here might be of considerable help to the clinicians experiencing this disease for the first time.
Other limitations include the variety of glucocorticoid dosing regimens used, and the large number of patients in whom additional treatments were added after the primary treatment. Although we have attempted to compare those remaining on a single agent, this group might have been less severely ill and therefore not representative of the treatment group overall. Additionally, after excluding patients with incomplete baseline covariates from the inverse probability of treatment weighting analysis, the final numbers of patients used for primary analyses were marginally below those stated in our sample-size calculations. However, the suggested effect sizes in these calculations are relatively arbitrary. More important is the final width of CIs for treatment effects, which were generally small for our primary analyses. An additional limitation is the use of a composite primary outcome. This outcome was necessitated by the relatively small numbers of patients with individual outcomes, and our aim to capture effects of treatment in patients across a wide spectrum of severity. As mitigation, we evaluated the individual components of the composite score as secondary analyses. The time-to-improvement outcome also incurs the possibility of built-in selection bias,28 although we have attempted to isolate known factors that could incur such bias through extensive subgroup analyses. This limitation is relevant to all survival analyses, and would not be avoidable even for randomised controlled trials using the same outcome. Finally, we are not able to detect rare or longer term effects of either intravenous immunoglobulin or glucocorticoid administration.
The absence of significant differences between treatment groups poses several questions on the mechanisms underlying MIS-C. As intravenous immunoglobulin and glucocorticoids have different possible modes of action in MIS-C,29, 30 the lack of difference between them, and the fact that combination therapy was not superior to single-agent therapy is puzzling. One possible explanation might be different underlying disease processes in MIS-C, some of which respond to intravenous immunoglobulin and some to glucocorticoids. If so, we would have expected that the combination treatment of intravenous immunoglobulin plus glucocorticoids would be superior to each treatment individually. Alternatively, glucocorticoids and intravenous immunoglobulin might act at different points in the same causal pathway and with equal efficacy. This explanation would give reasons for the similar outcomes and lack of additive effect. A final possibility is that neither treatment has a significant effect on the disease process. Given that the number of patients who received no immunomodulator treatment was small and phenotypically distinct from those receiving immunomodulator treatment, we did not have an adequately sized group who received no immunomodulator treatment to evaluate this possibility. However, the more rapid decline in C-reactive protein concentration in the treated group versus untreated group supports a beneficial effect of all three treatment regimes.
In addition to intravenous immunoglobulin and glucocorticoids, several other immunomodulatory agents were administered, including anti-interleukin 1, anti-interleukin 6 and anti-tumour necrosis factor agents. The numbers of patients who received these agents were too low to enable inverse probability of treatment weighting comparison between them, or with intravenous immunoglobulin alone, glucocorticoids alone, and intravenous immunoglobulin plus glucocorticoids. Biologicals tended to be administered in combination with intravenous immunoglobulin and glucocorticoids, and to patients who were more severely ill.
The key question in interpreting clinical significance of this analysis is whether the findings are sufficiently robust to enable glucocorticoids to replace intravenous immunoglobulin as primary treatment of MIS-C. The lack of significant difference in outcomes between patients treated with glucocorticoids as primary treatment, and those receiving intravenous immunoglobulin or intravenous immunoglobulin plus glucocorticoids, and in particular the absence of difference in coronary artery aneurysm severity, frequency, or resolution, suggests that initial treatment with glucocorticoids is a safe alternative to intravenous immunoglobulin. A concern in adopting this approach is the difficulty in distinguishing MIS-C from Kawasaki disease, particularly in patients younger 6 years, and the possibility that intravenous immunoglobulin will be withheld from children with Kawasaki disease because they are thought to have MIS-C. This concern highlights the need for a rapid diagnostic test to distinguish MIS-C from Kawasaki disease, as well as the need for urgent cardiology assessment in patients presenting with a suspected diagnosis of either disease. It also suggests that when clinical features closely resemble Kawasaki disease, particularly in younger children, retaining intravenous immunoglobulin as a component of initial therapy is prudent.
MIS-C has emerged as an important childhood problem in low-income and middle-income countries.26, 31 Given that intravenous immunoglobulin is costly32 and has limited availability in many countries, its use in preference to cheaper anti-inflammatory agents, such as glucocorticoids, should be supported by sound evidence. We did not find significant differences in outcome between treatment with glucocorticoids or intravenous immunoglobulin as single agents or between the single-agent and dual-agent primary treatments. Our findings suggest that glucocorticoids are not inferior to intravenous immunoglobulin or intravenous immunoglobulin plus glucocorticoids as primary treatment of MIS-C, and their wide availability and lower cost would support their choice as initial treatment for MIS-C.
Data sharing
De-identified clinical and laboratory data and response to treatment data for the cohort included in this study will be made available to legitimate researchers and clinicians from medical and academic institutions, for academic and clinical research, on request to the corresponding author. Approval for data sharing will need to be obtained from the consortium and partner institutions for each request. The study handbook and statistical analysis plans are available at the ISRCTN registry (https://doi.org/10.1186/ISRCTN69546370).
Declaration of interests
AT has provided unpaid consultancy work for Janssen Pharmaceuticals. DM has received grant support from the British Embassy in Moscow (StopCOVID Cohort: Clinical Characterisation of Russian Patients) and from UK Research and Innovation/National Institute for Health and Care Research (NIHR; Long COVID Core Outcome Set [PC-COS] project), and holds the following unpaid positions: Co-Chair of International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) Global Paediatric Long COVID Working Group, Member of ISARIC working group on long-term follow-up in adults, Co-lead of the PC-COS project aiming to define the Core Outcome Set for Long-COVID, in collaboration with the WHO. MJC reports a personal fee from Biotest for speaking at the BioTest Immunology Forum 2022, Royal Society. EW holds the following unpaid positions: member of the paediatric steering committee for the RECOVERY trial; Paediatric Representative for NHS England working on the National paediatric virtual advisory network and expert advisory group for COVID treatment, and independent advisory group for COVID monoclonal antibodies; and Co-lead for the pan-London Post-COVID service for children. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
The BATS writing group receive salary support from Imperial College London and the European Union's Horizon 2020 programme (under GA number 848196 DIAMONDS). Support for individual investigators was received from the Wellcome Trust (AJM, CB, and CW, Imperial College—Wellcome Trust 4i Clinical PhD programme; MK, fellowship 206508/Z/17/Z), the Medical Research Foundation (MK, MRF-160–0008-ELP-KAFO-C0801), UK NIHR (RGN, RGN ACL-2018–021–007; MJC, ACL-2018) and NIH (ML, GA5R01AI128765). Infrastructure support for this research was provided by the NIHR Imperial Biomedical Research Centre.
Contributors
ML, AJM, OV, AJC, EW, JAH, MK, HP, PS, CW, RGN, TD, and CH conceived the original study. OV, SC-W, CB, EGS, HP, GD'S, and IK undertook data checks and quality control. OV, SC-W, and ML accessed and verified the underlying data reported in the manuscript. SC-W, OB, AJM, and ML conceived the current statistical analysis plan, which was reviewed by all co-authors prior to publication. SC-W, EGS, EP, and HP undertook the current analysis. AT, DM, and RU-G formed our international advisory board and reviewed all aspects of the study, including design, analysis plan, and each version of the manuscript. MJC and PR were heavily involved in revision of the analysis plan for this particular study, as well as data collection and development of the manuscript. SC-W and ML prepared the initial manuscript, which was developed by all listed authors. All authors had full access to all the data in the study, vouching for the completeness and accuracy of data, and for fidelity to the protocol and analysis plan. All authors read and approved the final manuscript and had final responsibility for the decision to submit for publication.
Contributor Information
Best Available Treatment Study (BATS) consortium:
Mohamed Chouli, Nacera Hamadouche, Mohamed Samir Ladj, Jorge Agrimbau Vázquez, Rodrigo Carmona, Adrian Gustavo Collia, Alejandro Ellis, Diego Natta, Laura Pérez, Mayra Rubiños, Natalia Veliz, Silvana Yori, Philip N. Britton, David P. Burgner, Emma Carey, Nigel W. Crawford, Hayley Giuliano, Alissa McMinn, Shirley Wong, Nicholas Wood, Wolfgang Holter, Matthias Krainz, Raphael Ulreich, Christoph Zurl, Joke Dehoorne, Filomeen Haerynck, Levi Hoste, Petra Schelstraete, Kristof Vandekerckhove, Jef Willems, Camila Giuliana Almeida Farias, Flávia Jacqueline Almeida, Izabel Alves Leal, André Ricardo Araujo da Silva, Anna Esther Araujo e Silva, Sabrina T.A. Barreiro, Daniella Gregória Bomfim Prado da Silva, Maria Celia Cervi, Mirian Viviane dos Santos Naja Cardoso, Cristiane Henriques Teixeira, Daniel Jarovsky, Julienne Martins Araujo, Eitan Naaman Berezin, Marco Aurélio Palazzi Sáfadi, Rolando Andres Paternina-de la Ossa, Cristina Souza Vieira, Anna Dimitrova, Margarita Ganeva, Stefan Stefanov, Albena Telcharova-Mihaylovska, Catherine M. Biggs, Alison Lopez, Rosie Scuccimarri, Ryan Tan, Sam Wasserman, Davinia Withington, Camila Ampuero, Javiera Aravena, Raul Bustos B, Daniel Casanova, Pablo Cruces, Franco Diaz, Tamara García-Salum, Loreto Godoy, Rafael A. Medina, Gonzalo Valenzuela Galaz, Germán Camacho-Moreno, María L. Avila-Aguero, Helena Brenes-Chacón, Kattia Camacho-Badilla, Gabriela Ivankovich-Escoto, Gabriela Naranjo-Zuniga, Alejandra Soriano-Fallas, Rolando Ulloa-Gutierrez, Adriana Yock-Corrales, Maysa Abbas Amer, Yasmine Abdelmeguid, Yomna H.H.Z. Ahmed, Adham Badib, Karim Badreldin, Yara Elkhashab, Hassan Heshmat, Amna Hussein, Amna Hussein Mohamed Hussein, Sandra Ibrahim, Walaa Shoman, Radwa M Yakout, Santtu Heinonen, François Angoulvant, Alexandre Belot, Naïm Ouldali, Florian Beske, Axel Heep, Katja Masjosthusmann, Karl Reiter, Ingeborg van den Heuvel, Ulrich von Both, Aikaterini Agrafiotou, Charalampos Antachopoulos, Konstantina Charisi, Irini Eleftheriou, Evangelia Farmaki, Lampros Fotis, Dimitrios Kafetzis, Patra Koletsi, Katerina Kourtesi, Stavroula Lampidi, Theodota Liakopoulou, Despoina Maritsi, Elisa Michailidou, Maria Milioudi, Ioanna Mparmpounaki, Eleni Papadimitriou, Vassiliki Papaevangelou, Emmanuel Roilides, Olga Tsiatsiou, Georgios Tsolas, Maria Tsolia, Petrina Vantsi, Linda Yajeira Banegas Pineda, Karla Leversia Borjas Aguilar, Edwin Mauricio Cantillano Quintero, Patrick Ip, Mike Yat Wah Kwan, Janette Kwok, Yu Lung Lau, Kelvin To, Joshua Sung Chih Wong, Mate David, David Farkas, Szofia Kalcakosz, Klaudia Szekeres, Borbala Zsigmond, Nadeem Aslam, Anthony Luder, Laura Andreozzi, Francesco Bianco, Valentina Bucciarelli, Danilo Buonsenso, Rolando Cimaz, Maia De Luca, Rosa Maria Dellepiane, Marianna Fabi, Emanuele Filice, Marcello Lanari, Andrea Lo Vecchio, Maria Vincenza Mastrolia, Angela Mauro, Angelo Mazza, Mario Virgilio Papa, Lorenza Romani, Sara Maria Scarano, Gabriele Simonini, Vincenzo Tipo, Lucio Verdoni, Anne-Marie Macharia, Grace Musiime, Bhupi Reel, Frederick Wangai, David Pace, Paul Torpiano, Nancy Anaya-Enriquez, Juan Manuel Carreon-Guerrero, Enrique Chacon-Cruz, Mariana Cheung López, Enrique Faugier Fuentes, Marisol Fonseca Flores, Miguel García-Domínguez, Ana Luisa Giron Vargas, Ivan Lopez-Delgado, Liliana Lopez Hernández, Hector F. Menchaca Aguayo, Jesus Gilberto Montaño-Duron, Giordano Pérez-Gaxiola, Pamela Ramos Tiñini, Edgardo Tostado-Morales, Julio Valadez, Christopher Inchley, Sjur Klevberg, Per Kristian Knudsen, Per Helge Måseide, Jose Manuel Carrera, Elizabeth Castaño, Carlos Alberto Daza Timana, Tirza De Leon, Dora Estripeaut, Jacqueline Levy, Ximena Norero, Javier Record, Magda Rojas-Bonilla, Mayra Wong, Ricardo Iramain, Roger Hernandez, Gian Huamán, Manuel Munaico, Carlos Peralta, Diego Seminario, Elmer Hans Zapata Yarlequé, Justyna Gadzinska, Kamila Ludwikowska, Joanna Mandziuk, Magdalena Okarska-Napierała, Zalina A. Alacheva, Ekaterina Alexeeva, Petr V. Ananin, Margarita Antsupova, Maya D. Bakradze, Anna Berbenyuk, Polina Bobkova, Svetlana Borzakova, Irina L. Chashchina, Yasmin El-Taravi, Andrey P. Fisenko, Marina S. Gautier, Anastasia Glazyrina, Cyrill Gorlenko, Mariia Grosheva, Herman Kiselev, Elena Kondrikova, Evgeniya Korobyants, Anatoliy A. Korsunskiy, Karina Kovygina, Ekaterina Krasnaya, Seda Kurbanova, Maria K. Kurdup, Anna V. Mamutova, Lyudmila Mazankova, Ilya L. Mitushin, Daniel Munblit, Anzhelika Nargizyan, Yanina O. Orlova, Ismail M. Osmanov, Anastasia S. Polyakova, Anna Pushkareva, Olga Romanova, Elmira Samitova, Anastasia Shvedova, Anna Sologub, Ekaterina Iakovleva, Rustem F. Tepaev, Anna A. Tkacheva, Margarita Yegiyan, Valeriya Yusupova, Elena Zholobova, Carlos Daniel Grasa, Cristina Epalza, Nuria Lopez Segura, Federico Martinon-Torres, Susana Melendo, Ana Mendez-Echevarria, Juan Miguel Mesa Guzmán, Jorge Roberto Palacios Argueta, Irene Rivero-Calle, Jacques Rivière, Moisés Rodríguez-González, Pablo Rojo, Judith Sanchez Manubens, Pere Soler-Palacin, Antoni Soriano-Arandes, Alfredo Tagarro, Serena Villaverde, Maria Altman, Petter Brodin, AnnaCarin Horne, Karin Palmblad, Barbara Brotschi, Patrick Meyer Sauteur, Jana Pachlopnik Schmid, Seraina Prader, Christa Relly, Luregn J. Schlapbach, Michelle Seiler, Sophie Strasser, Johannes Trück, Kathrin Weber, Daniela Wütz, Alaa Hamdan, Ibrahim Melhem, Ahmed Moussa, Joke Dunk, Naomi Ketharanathan, Clementien Vermont, Esra Akyüz Özkan, Benhur Sirvan Cetin, Emine Hafize Erdeniz, Irfan Oğuz Şahin, Galina Borisova, Oksana Boyarchuk, Lidiya Boychenko, Yaryna Boyko, Nadiia Diudenko, Olha Dyvonyak, Olexandr Kasiyan, Kostiantyn Katerynych, Larysa Kostyuchenko, Marina Mamenko, Kateryna Melnyk, Nelia Miagka, Liliya Nazarenko, Iryna Nezgoda, Stanislava Rykova, Olga Svyst, Maria Teslenko, Mykola Trykosh, Nataliya Vasilenko, Alla Volokha, Charlotte Adams, Toju Akomolafe, Eslam Al-Abadi, Nele Alders, Styliani Alifieraki, Hareef Ansumanu, Emily Aston, Paula Avram, Alasdair Bamford, Millie Banks, Robin Basu Roy, Thomas Beattie, Olga Boleti, Abbey Bracken, Jonathan Broad, James Cai, Enitan D. Carrol, Michael Carter, Anchit Chandran, James Charlesworth, Jaya Chawla, Hannah Cooper, Samantha Cooray, Patrick Davies, Francesca Davis, Simon B. Drysdale, Ella Dzora, Marieke Emonts, Ceri Evans, Katy Fidler, Caroline Foster, Chen Gong, Berin Gongrun, Carmen Gonzalez, Berin Gorgun, Louis Grandjean, Karlie Grant, Jonathan Guo, Yael Hacohen, Jack Hall, Hytham K.S. Hamid, Jane Hassell, Christine Hesketh, Jessica Hewlett, Ahmad Hnieno, Hannah Holt-Davis, Aleena Hossain, Shiying Hu, Lee D. Hudson, Sharon Jheeta, Mae Johnson, Sarah Johnson, Deepthi Jyothish, Beate Kampmann, Akhila Kavirayani, Deborah Kelly, Arangan Kirubakaran, Filip Kucera, Daniel Langer, George Lawson, Emily A Lees, Rebecca Lenihan, Jon Lillie, Katherine Longbottom, Hermione Lyall, Niamh Mackdermott, Sarah Maltby, Thomas Mclelland, Anne-Marie McMahon, Danielle Miller, Mariana Miranda, Luwaiza Mirza, Zoe Morrison, Karyn Moshal, Jennifer Muller, Phoebe Musuka, Evangelia Myttaraki, Simon Nadel, Sreedevi Nair, Luke Nuttall, Oyinkansola Oremakinde, Daniella Osaghae, Fatima Osman, Anna Ostrzewska, Davide Paccagnella, Mrinalini Panthula, Eleni Papachatzi, Charalampia Papadopoulou, Fahim Patel, Harsita Patel, Helen Payne, Justin Penner, Shervin Polandi, Andrew J. Prendergast, Padmanabhan Ramnarayan, Lasith Ranasinghe, Muthukumaran Ravichandran, Sophie Rhys-Evans, Andrew Riordan, Charlene M.C. Rodrigues, Lauren Roe, Sam Romaine, Nina Schobi, James Seddon, Delane Shingadia, Oishi Sikdar, Anand Srivastava, Siske Struik, Thomas Sun, Rachel Wei Tan, Alice Taylor, Amanda Taylor, Andrew Taylor, Steven Tran, Stavros Tsagkaris, Gareth Tudor-Williams, Sarah van den Berg, Fabian van der Velden, Lyn Ventilacion, Paul A. Wellman, Joseph Withers Green, Michael P. Yanney, Shunmay Yeung, Aditya Badheka, Sarah Badran, Dwight M. Bailey, Anna Kathryn Burch, Jane C. Burns, Catherine Cichon, Blake Cirks, Michael D. Dallman, Dennis R. Delany, Mary Fairchok, Samantha Friedman, Jennifer Geracht, Allison Langs-Barlow, Kelly Mann, Amruta Padhye, Alexis Quade, Kacy Alyne Ramirez, John Rockett, Imran Ali Sayed, Roberto P. Santos, Amr A. Shahin, Adriana Tremoulet, Samuel Umaru, Rebecca Widener, Hilda Angela Mujuru, and Gwendoline Kandawasvika
Supplementary Material
References
- 1.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viner RM, Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395:1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325:1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godfred-Cato S, Bryant B, Leung J, et al. COVID-19-associated multisystem inflammatory syndrome in children—United States, March–July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin M. Childhood multisystem inflammatory syndrome—a new challenge in the pandemic. N Engl J Med. 2020;383:393–395. doi: 10.1056/NEJMe2023158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 10.Harwood R, Allin B, Jones CE, et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc Health. 2021;5:133–141. doi: 10.1016/S2352-4642(20)30304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology Clinical Guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 1. Arthritis Rheumatol. 2020;72:1791–1805. doi: 10.1002/art.41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouldali N, Toubiana J, Antona D, et al. Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA. 2021;325:855–864. doi: 10.1001/jama.2021.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Son MBF, Murray N, Friedman K, et al. Multisystem inflammatory syndrome in children—initial therapy and outcomes. N Engl J Med. 2021;385:23–34. doi: 10.1056/NEJMoa2102605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villacis-Nunez DS, Jones K, Jabbar A, et al. Short-term outcomes of corticosteroid monotherapy in multisystem inflammatory syndrome in children. JAMA Pediatr. 2022;176:576–584. doi: 10.1001/jamapediatrics.2022.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McArdle AJ, Vito O, Patel H, et al. Treatment of multisystem inflammatory syndrome in children. N Engl J Med. 2021;385:11–22. doi: 10.1056/NEJMoa2102968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaRovere KL, Riggs BJ, Poussaint TY, et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78:536–547. doi: 10.1001/jamaneurol.2021.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tullie L, Ford K, Bisharat M, et al. Gastrointestinal features in children with COVID-19: an observation of varied presentation in eight children. Lancet Child Adolesc Health. 2020;4:e19–e20. doi: 10.1016/S2352-4642(20)30165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention Information for healthcare providers about multisystem inflammatory syndrome in children (MIS-C) 2020. https://www.cdc.gov/mis/mis-c/hcp/index.html
- 21.Royal College of Paediatrics and Child Health Paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS)—guidance for clinicians. 2020. https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance
- 22.World Health Organization Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. Scientific brief. May 2020. https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19
- 23.National Institute for Health and Care Excellence Equivalent anti-inflammatory doses of oral corticosteroids. June 2020. https://cks.nice.org.uk/topics/corticosteroids-oral/background-information/equivalent-anti-inflammatory-doses/
- 24.Lopez L, Colan S, Stylianou M, et al. Relationship of echocardiographic Z scores adjusted for body surface area to age, sex, race, and ethnicity: the Pediatric Heart Network Normal Echocardiogram Database. Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.117.006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imai K, Ratkovic M. Covariate balancing propensity score. J R Stat Soc Series B Stat Methodol. 2014;76:243–263. [Google Scholar]
- 26.Sood M, Sharma S, Sood I, Sharma K, Kaushik A. Emerging evidence on multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection: a systematic review with meta-analysis. SN Compr Clin Med. 2021;3:38–47. doi: 10.1007/s42399-020-00690-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouldali N, Bagheri H, Salvo F, et al. Hyper inflammatory syndrome following COVID-19 mRNA vaccine in children: a national post-authorization pharmacovigilance study. Lancet Reg Health Eur. 2022;17 doi: 10.1016/j.lanepe.2022.100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernán MA. The hazards of hazard ratios. Epidemiology. 2010;21:13–15. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu YP, Shamie I, Lee JC, et al. Immune response to intravenous immunoglobulin in patients with Kawasaki disease and MIS-C. J Clin Invest. 2021;131 doi: 10.1172/JCI147076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel H, McArdle A, Seaby E, Levin M, Whittaker E. The immunopathogenesis of SARS-CoV-2 infection in children: diagnostics, treatment and prevention. Clin Transl Immunology. 2022;11 doi: 10.1002/cti2.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irfan O, Muttalib F, Tang K, Jiang L, Lassi ZS, Bhutta Z. Clinical characteristics, treatment and outcomes of paediatric COVID-19: a systematic review and meta-analysis. Arch Dis Child. 2021;106:440–448. doi: 10.1136/archdischild-2020-321385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson SC, Williams DC, Brinton D, Chew M, Simpson A, Andrews AL. A cost comparison of infliximab versus intravenous immunoglobulin for refractory Kawasaki disease treatment. Hosp Pediatr. 2021;11:88–93. doi: 10.1542/hpeds.2020-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified clinical and laboratory data and response to treatment data for the cohort included in this study will be made available to legitimate researchers and clinicians from medical and academic institutions, for academic and clinical research, on request to the corresponding author. Approval for data sharing will need to be obtained from the consortium and partner institutions for each request. The study handbook and statistical analysis plans are available at the ISRCTN registry (https://doi.org/10.1186/ISRCTN69546370).